具有不同多糖结构的乳蛋白浓缩分散体的半动态体外消化研究A semi dynamic in vitro digestion study of milk protein concentrate dispersions structured with different polysaccharides
丹麦奥尔胡斯大学
IFF-杜邦丹尼斯克营养生物科技公司
方案详情

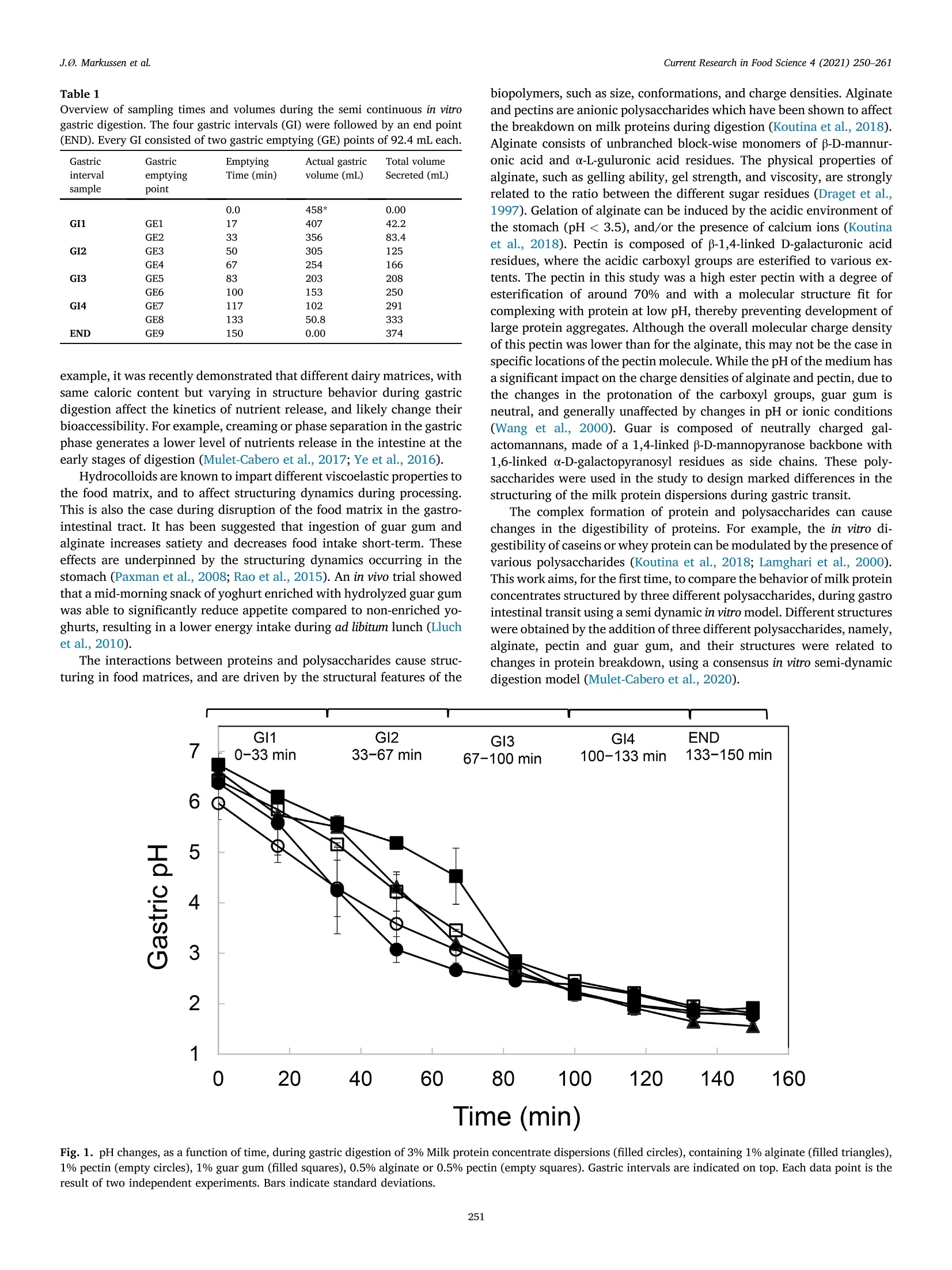
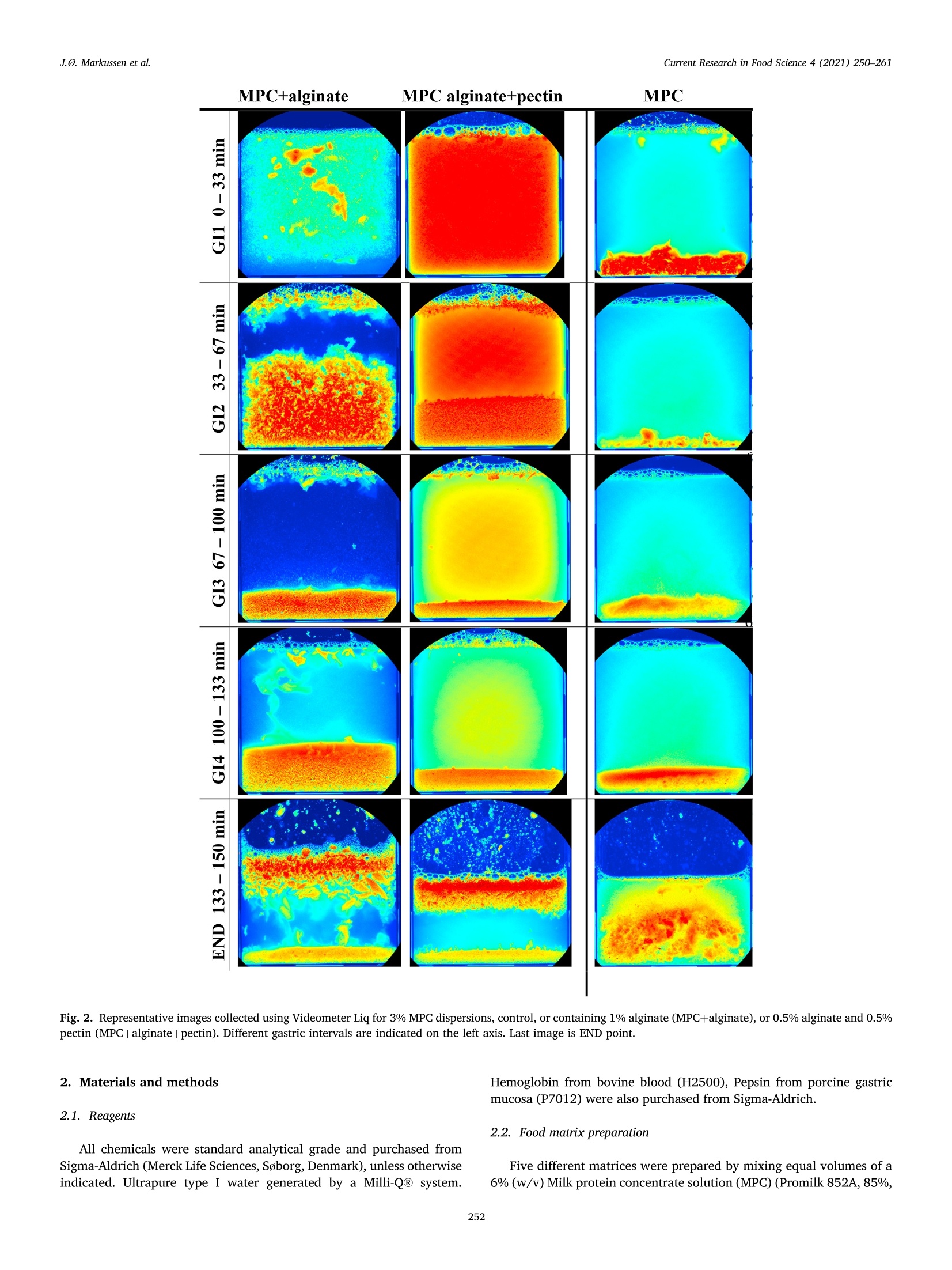
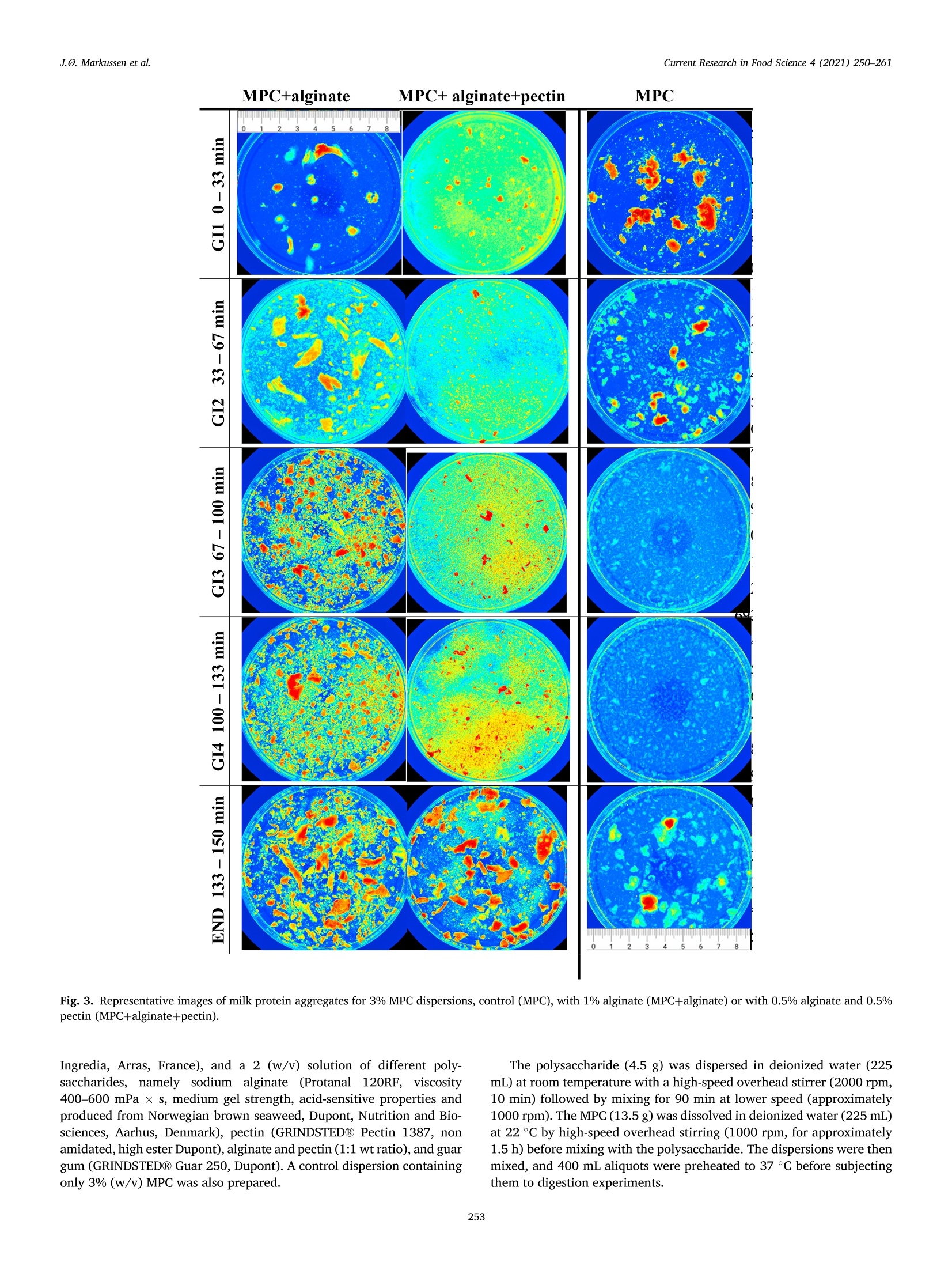
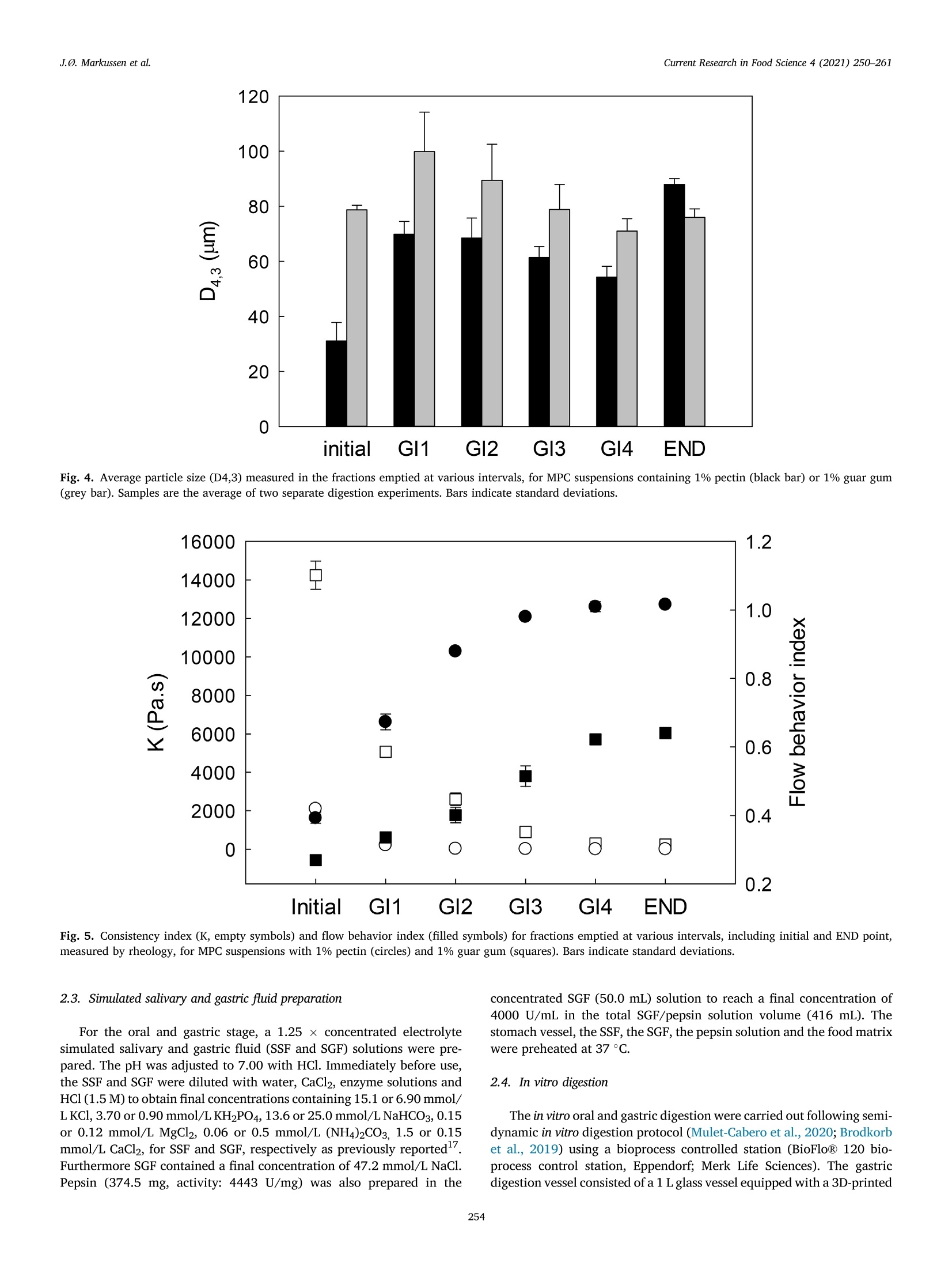
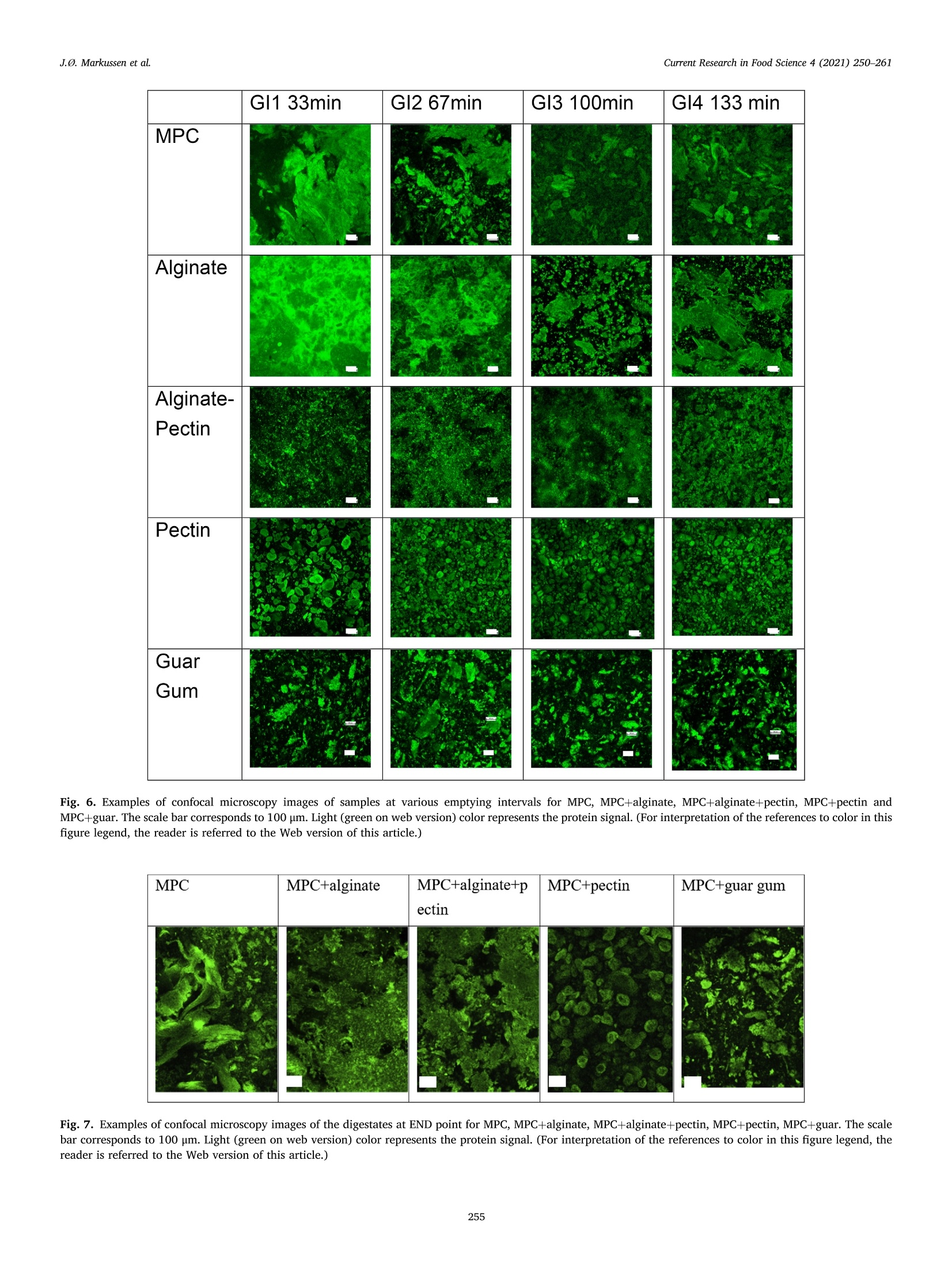
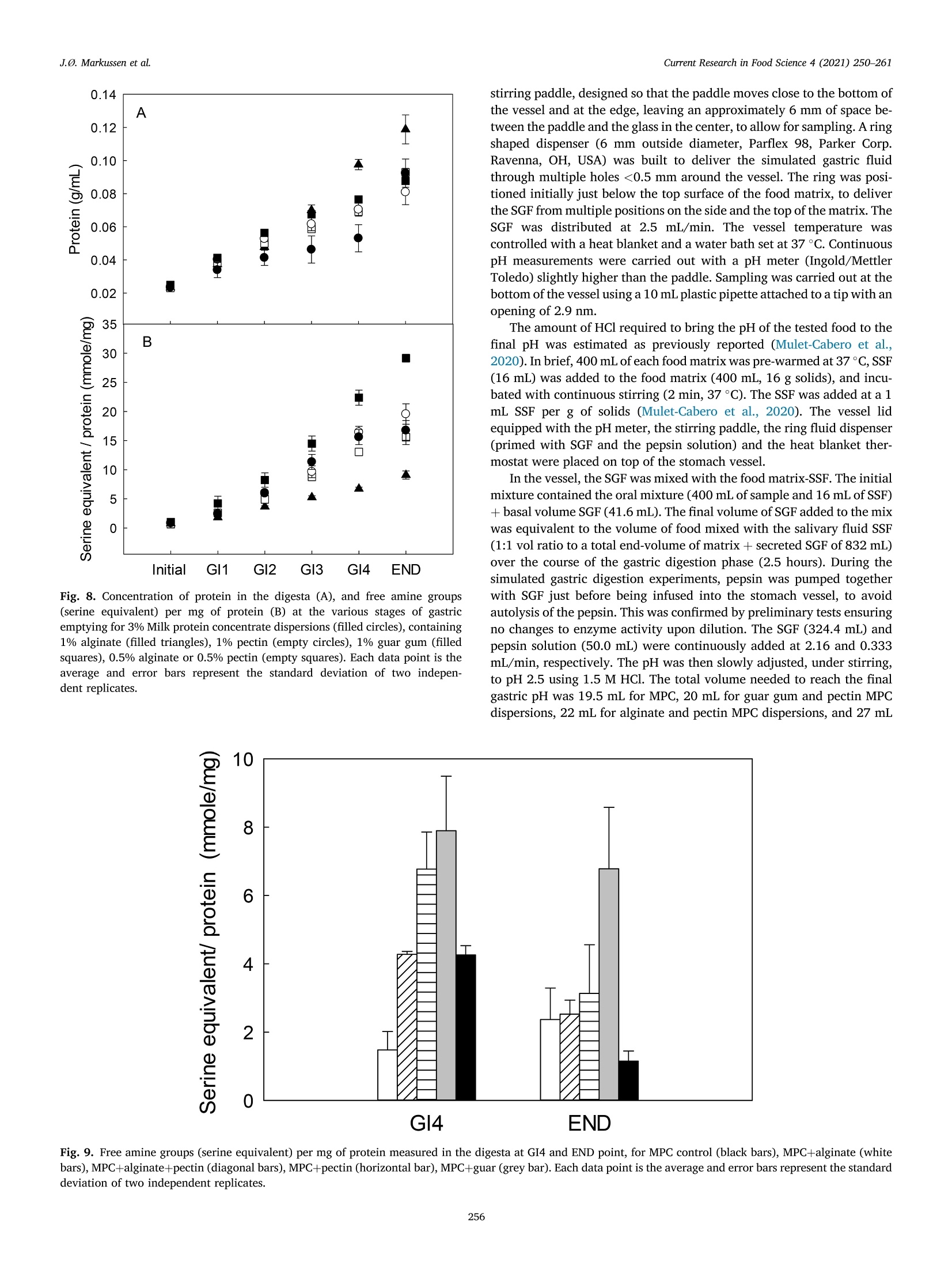
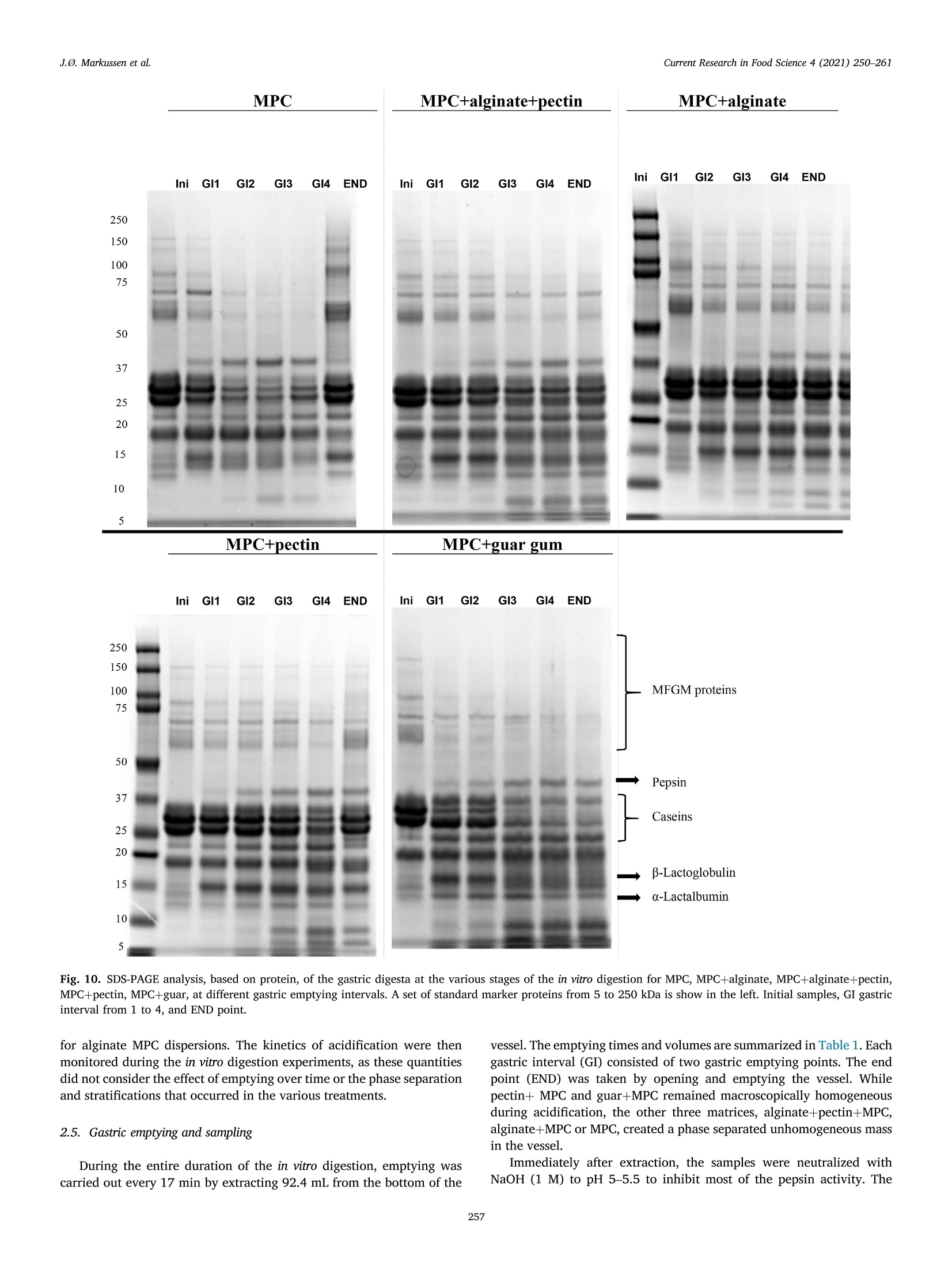
具有不同多糖结构的乳蛋白浓缩分散体的半动态体外消化研究A semi dynamic in vitro digestion study of milk protein concentrate dispersions structured with different polysaccharides丹麦奥尔胡斯大学 IFF-杜邦丹尼斯克营养生物科技公司CurrentResearchinFoodScience4(2021)250–261 J.Ø.Markussenetal.CurrentResearchinFoodScience4(2021)250–261 Contentslistsavailableat ScienceDirect CurrentResearchinFoodScience ELSEVIER journalhomepage:www.editorialmanager.com/crfs/ ResearchPaper 具有不同多糖结构的乳蛋白浓缩分散体的半动态体外消化研究 Asemidynamicinvitrodigestionstudyofmilkproteinconcentrate dispersionsstructuredwithdifferentpolysaccharides JacobØstergaardMarkussen a ,b ,FinnMadsen b ,1,JetteFeveileYoung a ,MilenaCorredig a ,*,1 丹麦奥尔胡斯大学 aDepartmentofFoodScience,CiFoodMultidisciplinaryCenter,AarhusUniversity,48AgroFoodPark,AarhusN,8200,Denmark bIFFR&DBraband,DuPontNutritionBiosciencesApS,EdwinRahrsVej38,8220,Brabrand,DenmarkIFF-杜邦丹尼斯克营养生物科技公司 ARTICLEINFO ABSTRACT Keywords: Hydrocolloidsareoftenaddedasfunctionalingredientsinfoods,tobetterdesignthestructureofthematrixand Hydrocolloids ensurefoodqualityandoptimalsensoryproperties.However,muchlessisknownabouttheirinluenceonthe Invitrodigestion physicalandchemicalchangesduringgastricdigestion.Inthisstudy,semi-continuousinvitrogastricdigestionSemi-dynamic wasappliedonamodelfoodsystem,preparedwithmilkproteinconcentrate(MPC)(3%w/v)and1%alginate,Milkproteinconcentrate Foodmatrixpectin,guargum,aswellasa1:1mixtureofalginateandpectin.Thedynamicsduringsimulatedgastricdigestionwereobservedbymeasuringparticlesizedistributions,structuringatvariouslengthscales,aswellasbyeval-uatingdifferencesinproteinbreakdown.Immediatelyaftercontactwiththesimulatedgastricluids,allsamples showedextensiveaggregationandformationofdifferentstructures.MPCcontroldispersions(nopolysaccharide)andMPCcontainingalginateformedlargeinhomogeneousaggregates.Thelackofstructuralhomogeneity affectedthesimulatedgastricemptying:thereweremarkeddifferencesinthetypeofaggregatespresentat varioustimesofemptyingdependingonthehydrocolloidpresentinthemixture.MPCcontainingpectinorguar gumformedmacroscopicallyhomogeneousdispersion,withrathersmallproteinaggregatesshowingalarge populationofparticlesbetween60and100umofdiameter,withmarkeddifferencesinmicrostructure.Pectin createdlargecoacervates,whileguarmicroscopicphaseseparatedsystems.Thesedispersionsshowedahigher extentofproteindigestion,duetothelargersurfaceareacreatedforenzymeactivitycomparedtothemacro-scopicallyphaseseparatedmatrices.Inallcases,therewasalargeundigestedfractionattheendpointof140min.SDSPAGEdemonstrateddifferencesinthecaseinpeptidesdistributiondependingonthetypeofpolysaccharide presentduringsimulatedgastricemptying.Thisinspiteofsimilaritiesincumulativeproteinemptied.Itwas concludedthatinthissemi-continuousinvitrogastricdigestionmodel,structuringwithpolysaccharideshasa signiicantimpactongastricemptyingandproteindigestionkinetics. 1.Introduction Characterizationofthebehaviorandstructureofvariousfoodsduring gastrointestinaltransitisimportanttoclarifytheroleoffoodinhuman nutrition.Itisbecomingincreasinglyclearthatthestructureofthefood regulatesitsdisruptionduringdigestion,affectinggastricempting,hor-monalresponsesandabsorptionofthenutrients(Marcianietal.,2013;Mulet-Caberoetal.,2019).Mechanisticinvestigationsontherelation-shipsbetweenthephysicalandchemicalpropertiesofthefoodmatrix,anditssubsequentbehaviorinthegastrointestinaltract,areneeded.Suchdatacanhelpenlightenhowtodevelopfoodproductswithtargeted nutritionalproperties,forexample,tocreatesatietypromotingfunctions, ortoimprovedeliveryandabsorptionofnutrients. Thestructuringoffoodduringdigestionimpactsnutrientabsorption (Boutrouetal.,2013;Fardetetal.,2019;Guoetal.,2020).Studies showedthatasteadyreleaseofnutrientsduringgastricemptyingcan affectsatietydifferentlythanthesamemealconsumedinasolidstate (Marcianietal.,2012)causingdelayedgastricemptyinginhealthy subjects.Thephysico-chemicalmechanismsbehindstructuringofthe foodmatrixinthegastricphase,modifyingthekineticsofnutrient releasearenotfullyrevealedbyinterventionstudies.Reliabletoolsare neededtoenabletheseinvestigations. Usingsemi-dynamicinvitromodelsmakesitpossibletofollowthe fateofthenutrientsduringgastrictransit(Mulet-Caberoetal.,2020).For 1Contributedequallytotheresearch. https://doi.org/10.1016/j.crfs.2021.03.012 Received9November2020;Receivedinrevisedform28March2021;Accepted29March20212665-9271/©2021TheAuthors.PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/). Table1 Overviewofsamplingtimesandvolumesduringthesemicontinuousinvitro gastricdigestion.Thefourgastricintervals(GI)werefollowedbyanendpoint (END).EveryGIconsistedoftwogastricemptying(GE)pointsof92.4mLeach. Gastricinterval sample Gastric emptying point Emptying Time(min) Actualgastric volume(mL) Totalvolume Secreted(mL) GI1 0.0 458* 0.00 GE1 17 407 42.2 GE2 33 356 83.4 GI2 GE3 50 305 125 GE4 67 254 166 GI3 GE5 83 203 208 GE6 100 153 250 GI4 GE7 117 102 291 GE8 133 50.8 333 END GE9 150 0.00 374 example,itwasrecentlydemonstratedthatdifferentdairymatrices,with samecaloriccontentbutvaryinginstructurebehaviorduringgastric digestionaffectthekineticsofnutrientrelease,andlikelychangetheir bioaccessibility.Forexample,creamingorphaseseparationinthegastric phasegeneratesalowerlevelofnutrientsreleaseintheintestineatthe earlystagesofdigestion(Mulet-Caberoetal.,2017;Yeetal.,2016). Hydrocolloidsareknowntoimpartdifferentviscoelasticpropertiesto thefoodmatrix,andtoaffectstructuringdynamicsduringprocessing.Thisisalsothecaseduringdisruptionofthefoodmatrixinthegastro-intestinaltract.Ithasbeensuggestedthatingestionofguargumand alginateincreasessatietyanddecreasesfoodintakeshort-term.These effectsareunderpinnedbythestructuringdynamicsoccurringinthe stomach(Paxmanetal.,2008;Raoetal.,2015).Aninvivotrialshowed thatamid-morningsnackofyoghurtenrichedwithhydrolyzedguargum wasabletosigniicantlyreduceappetitecomparedtonon-enrichedyo-ghurts,resultinginalowerenergyintakeduringadlibitumlunch(Lluch etal.,2010). Theinteractionsbetweenproteinsandpolysaccharidescausestruc-turinginfoodmatrices,andaredrivenbythestructuralfeaturesofthe biopolymers,suchassize,conformations,andchargedensities.Alginate andpectinsareanionicpolysaccharideswhichhavebeenshowntoaffect thebreakdownonmilkproteinsduringdigestion(Koutinaetal.,2018).Alginateconsistsofunbranchedblock-wisemonomersofβ-D-mannur-onicacidandα-L-guluronicacidresidues.Thephysicalpropertiesof alginate,suchasgellingability,gelstrength,andviscosity,arestrongly relatedtotheratiobetweenthedifferentsugarresidues(Dragetetal.,1997).Gelationofalginatecanbeinducedbytheacidicenvironmentof thestomach(pH<3.5),and/orthepresenceofcalciumions(Koutina etal.,2018).Pectiniscomposedofβ-1,4-linkedD-galacturonicacid residues,wheretheacidiccarboxylgroupsareesteriiedtovariousex-tents.Thepectininthisstudywasahighesterpectinwithadegreeof esteriicationofaround70%andwithamolecularstructureitfor complexingwithproteinatlowpH,therebypreventingdevelopmentof largeproteinaggregates.Althoughtheoverallmolecularchargedensity ofthispectinwaslowerthanforthealginate,thismaynotbethecasein speciiclocationsofthepectinmolecule.WhilethepHofthemediumhas asigniicantimpactonthechargedensitiesofalginateandpectin,dueto thechangesintheprotonationofthecarboxylgroups,guargumis neutral,andgenerallyunaffectedbychangesinpHorionicconditions (Wangetal.,2000).Guariscomposedofneutrallychargedgal-actomannans,madeofa1,4-linkedβ-D-mannopyranosebackbonewith 1,6-linkedα-D-galactopyranosylresiduesassidechains.Thesepoly-saccharideswereusedinthestudytodesignmarkeddifferencesinthe structuringofthemilkproteindispersionsduringgastrictransit. Thecomplexformationofproteinandpolysaccharidescancause changesinthedigestibilityofproteins.Forexample,theinvitrodi-gestibilityofcaseinsorwheyproteincanbemodulatedbythepresenceof variouspolysaccharides(Koutinaetal.,2018;Lamgharietal.,2000).Thisworkaims,fortheirsttime,tocomparethebehaviorofmilkprotein concentratesstructuredbythreedifferentpolysaccharides,duringgastro intestinaltransitusingasemidynamicinvitromodel.Differentstructures wereobtainedbytheadditionofthreedifferentpolysaccharides,namely,alginate,pectinandguargum,andtheirstructureswererelatedto changesinproteinbreakdown,usingaconsensusinvitrosemi-dynamic digestionmodel(Mulet-Caberoetal.,2020). 工 a Fig.1.pHchanges,asafunctionoftime,duringgastricdigestionof3%Milkproteinconcentratedispersions(illedcircles),containing1%alginate(illedtriangles),1%pectin(emptycircles),1%guargum(illedsquares),0.5%alginateor0.5%pectin(emptysquares).Gastricintervalsareindicatedontop.Eachdatapointisthe resultoftwoindependentexperiments.Barsindicatestandarddeviations. MPC+alginate MPC alginate+pectin MPC Fig.2.RepresentativeimagescollectedusingVideometerLiqfor3%MPCdispersions,control,orcontaining1%alginate(MPCþalginate),or0.5%alginateand0.5%pectin(MPCþalginateþpectin).Differentgastricintervalsareindicatedontheleftaxis.LastimageisENDpoint. 2.Materialsandmethods Hemoglobinfrombovineblood(H2500),Pepsinfromporcinegastric mucosa(P7012)werealsopurchasedfromSigma-Aldrich. 2.1.Reagents 2.2.Foodmatrixpreparation Allchemicalswerestandardanalyticalgradeandpurchasedfrom Sigma-Aldrich(MerckLifeSciences,Søborg,Denmark),unlessotherwise indicated.UltrapuretypeIwatergeneratedbyaMilli-Q®system. Fivedifferentmatriceswerepreparedbymixingequalvolumesofa 6%(w/v)Milkproteinconcentratesolution(MPC)(Promilk852A,85%, MPC+alginate MPC+ alginate+pectin MPC Ingredia,Arras,France),anda2(w/v)solutionofdifferentpoly-saccharides, namely sodium alginate (Protanal 120RF, viscosity400–600mPa, s,mediumgelstrength,acid-sensitivepropertiesand producedfromNorwegianbrownseaweed,Dupont,NutritionandBio-sciences,Aarhus,Denmark),pectin(GRINDSTED®Pectin1387,non amidated,highesterDupont),alginateandpectin(1:1wtratio),andguar gum(GRINDSTED®Guar250,Dupont).Acontroldispersioncontaining only3%(w/v)MPCwasalsoprepared. Thepolysaccharide(4.5g)wasdispersedindeionizedwater(225mL)atroomtemperaturewithahigh-speedoverheadstirrer(2000rpm,10min)followedbymixingfor90minatlowerspeed(approximately 1000rpm).TheMPC(13.5g)wasdissolvedindeionizedwater(225mL)at22m)Cbyhigh-speedoverheadstirring(1000rpm,forapproximately 1.5h)beforemixingwiththepolysaccharide.Thedispersionswerethenmixed,and400mLaliquotswerepreheatedto37. Cbeforesubjecting themtodigestionexperiments. initial END Fig.4.Averageparticlesize(D4,3)measuredinthefractionsemptiedatvariousintervals,forMPCsuspensionscontaining1%pectin(blackbar)or1%guargum (greybar).Samplesaretheaverageoftwoseparatedigestionexperiments.Barsindicatestandarddeviations. Fig.5.Consistencyindex(K,emptysymbols)andlowbehaviorindex(illedsymbols)forfractionsemptiedatvariousintervals,includinginitialandENDpoint,measuredbyrheology,forMPCsuspensionswith1%pectin(circles)and1%guargum(squares).Barsindicatestandarddeviations. Fortheoralandgastricstage,a1.25x concentratedelectrolyte simulatedsalivaryandgastricluid(SSFandSGF)solutionswerepre-pared.ThepHwasadjustedto7.00withHCl.Immediatelybeforeuse,theSSFandSGFweredilutedwithwater,CaCl2,enzymesolutionsandHCl(1.5M)toobtaininalconcentrationscontaining15.1or6.90mmol/LKCl,3.70or0.90mmol/LKH2PO4,13.6or25.0mmol/LNaHCO3,0.15or0.12mmol/LMgCl2,0.06or0.5mmol/L(NH4)2CO3,1.5or0.15mmol/LCaCl2,forSSFandSGF,respectivelyaspreviouslyreported17.FurthermoreSGFcontainedainalconcentrationof47.2mmol/LNaCl.Pepsin(374.5mg,activity:4443U/mg)wasalsopreparedinthe concentratedSGF(50.0mL)solutiontoreachainalconcentrationof 4000U/mLinthetotalSGF/pepsinsolutionvolume(416mL).The stomachvessel,theSSF,theSGF,thepepsinsolutionandthefoodmatrixwerepreheatedat37 °C. 2.4.Invitrodigestion Theinvitrooralandgastricdigestionwerecarriedoutfollowingsemi-dynamicinvitrodigestionprotocol(Mulet-Caberoetal.,2020;Brodkorb etal.,2019)usingabioprocesscontrolledstation(BioFlo®120bio-processcontrolstation,Eppendorf;MerkLifeSciences).Thegastric digestionvesselconsistedofa1Lglassvesselequippedwitha3D-printed Fig.6.ExamplesofconfocalmicroscopyimagesofsamplesatvariousemptyingintervalsforMPC,MPCþalginate,MPCþalginateþpectin,MPCþpectinandMPCþguar.Thescalebarcorrespondsto100im.Light(greenonwebversion)colorrepresentstheproteinsignal.(Forinterpretationofthereferencestocolorinthis igurelegend,thereaderisreferredtotheWebversionofthisarticle.) Fig.7.ExamplesofconfocalmicroscopyimagesofthedigestatesatENDpointforMPC,MPCþalginate,MPCþalginateþpectin,MPCþpectin,MPCþguar.Thescalebarcorrespondsto100um.Light(greenonwebversion)colorrepresentstheproteinsignal.(Forinterpretationofthereferencestocolorinthisigurelegend,the readerisreferredtotheWebversionofthisarticle.) A 本 日 B ■ OH0EH 白 本 盒 stirringpaddle,designedsothatthepaddlemovesclosetothebottomof thevesselandattheedge,leavinganapproximately6mmofspacebe-tweenthepaddleandtheglassinthecenter,toallowforsampling.Aring shapeddispenser(6mmoutsidediameter,Parlex98,ParkerCorp.Ravenna,OH,USA)wasbuilttodeliverthesimulatedgastricluid throughmultipleholes<0.5mmaroundthevessel.Theringwasposi-tionedinitiallyjustbelowthetopsurfaceofthefoodmatrix,todeliver theSGFfrommultiplepositionsonthesideandthetopofthematrix.The SGFwasdistributedat2.5mL/min.Thevesseltemperaturewascontrolledwithaheatblanketandawaterbathsetat37emC.Continuous pHmeasurementswerecarriedoutwithapHmeter(Ingold/Mettler Toledo)slightlyhigherthanthepaddle.Samplingwascarriedoutatthe bottomofthevesselusinga10mLplasticpipetteattachedtoatipwithan openingof2.9nm. Fig.8.Concentrationofproteininthedigesta(A),andfreeaminegroups (serineequivalent)permgofprotein(B)atthevariousstagesofgastric emptyingfor3%Milkproteinconcentratedispersions(illedcircles),containing 1%alginate(illedtriangles),1%pectin(emptycircles),1%guargum(illed squares),0.5%alginateor0.5%pectin(emptysquares).Eachdatapointisthe averageanderrorbarsrepresentthestandarddeviationoftwoindepen-dentreplicates. Inthevessel,theSGFwasmixedwiththefoodmatrix-SSF.Theinitial mixturecontainedtheoralmixture(400mLofsampleand16mLofSSF)þbasalvolumeSGF(41.6mL).The0nalvolumeofSGFaddedtothemix wasequivalenttothevolumeoffoodmixedwiththesalivaryluidSSF(1:1volratiotoatotalend-volumeofmatrixþsecretedSGFof832mL)overthecourseofthegastricdigestionphase(2.5hours).Duringthe simulatedgastricdigestionexperiments,pepsinwaspumpedtogether withSGFjustbeforebeinginfusedintothestomachvessel,toavoid autolysisofthepepsin.Thiswasconirmedbypreliminarytestsensuring nochangestoenzymeactivityupondilution.TheSGF(324.4mL)and pepsinsolution(50.0mL)werecontinuouslyaddedat2.16and0.333mL/min,respectively.ThepHwasthenslowlyadjusted,understirring,topH2.5using1.5MHCl.Thetotalvolumeneededtoreachtheinal gastricpHwas19.5mLforMPC,20mLforguargumandpectinMPC dispersions,22mLforalginateandpectinMPCdispersions,and27mL GI4 END Fig.9.Freeaminegroups(serineequivalent)permgofproteinmeasuredinthedigestaatGI4andENDpoint,forMPCcontrol(blackbars),MPCþalginate(whitebars),MPCþalginateþpectin(diagonalbars),MPCþpectin(horizontalbar),MPCþguar(greybar).Eachdatapointistheaverageanderrorbarsrepresentthestandard deviationoftwoindependentreplicates. Ini GI1 GI2 GI3 GI4 END Ini GI1 GI2 GI3 GI4 END Fig.10.SDS-PAGEanalysis,basedonprotein,ofthegastricdigestaatthevariousstagesoftheinvitrodigestionforMPC,MPCþalginate,MPCþalginateþpectin,MPCþpectin,MPCþguar,atdifferentgastricemptyingintervals.Asetofstandardmarkerproteinsfrom5to250kDaisshowintheleft.Initialsamples,GIgastric intervalfrom1to4,andENDpoint. foralginateMPCdispersions.Thekineticsofacidiicationwerethen monitoredduringtheinvitrodigestionexperiments,asthesequantities didnotconsidertheeffectofemptyingovertimeorthephaseseparation andstratiicationsthatoccurredinthevarioustreatments. 2.5.Gastricemptyingandsampling Duringtheentiredurationoftheinvitrodigestion,emptyingwas carriedoutevery17minbyextracting92.4mLfromthebottomofthe vessel.Theemptyingtimesandvolumesaresummarizedin Table1.Eachgastricinterval(GI)consistedoftwogastricemptyingpoints.Theend point(END)wastakenbyopeningandemptyingthevessel.WhilepectinþMPCandguarþMPCremainedmacroscopicallyhomogeneous duringacidiacation,theotherthreematrices,alginateþpectinþMPC,alginateþMPCorMPC,createdaphaseseparatedunhomogeneousmass inthevessel. Immediatelyafterextraction,thesampleswereneutralizedwith NaOH(1M)topH5–5.5toinhibitmostofthepepsinactivity.The sampleswerenotneutralizedcompletelytoavoidthedisruptionof structures.Thesamplesforstructurecharacterizationwerekeptrefrig-erated(4heC)andrheology,confocallaserscanningmicroscopyand particlesizeanalyseswereperformedwithin48hfromdigestion. Forproteinanalysis,representativesamplesfromthefourgastric intervalswereneutralizedwithNaOH(1M)toapHabove8,toinacti-vatepepsin,andthesampleswerehomogenized(25000rpm,~30s,T25Ultra-Turrax®,IKA®)andimmediatelyfrozenandkeptatm2,30 C.The sameprocedurewasperformedwithundigested(initial)samples. 2.6.Visualobservations VisualanalysisofthegastricsampleswascarriedoutusingVideo-meterLiqandVideometerLabmultispectralimaginginstruments(Vid-eometerA/S,Herlev,DK),shortlyafterthegastricdigestionexperiment.Thedigestateswerelefttosettleinlasks(SarstedtT-75CellCulture Flask)andthenobservedwithaVideometerLiq(Videometer)at365nm.Theywerealsoplacedonpetridishesandgentlydrained,andthen suspendedinpuriiedwater.Inthiscase,thesedimentswereobservedwithaVideometerLab(Videometer)at375nm.SamplesofMPCþpectin andMPCþguardidnotshowphaseseparationandthereforewerenot analyzedwiththistechnique,butbyusingintegratedlightscattering.Therawpictureswerecoloredwitha“jetcolorscale”,colorsfromblueto red,illustratingincreaseinaggregatedensity. 2.7.Confocallaserscanningmicroscopy(CLSM) Themicrostructuresoftheinitialanddigestedsampleswereanalyzed byconfocallaserscanningmicroscopy(CLSM)usingaNikonmotorized spectralconfocalmicroscopemodelTi-E(DFAinstruments,Glostrup,DK).Theproteinfractioninthedigestatewaslabeledwithluorescein isothiocyanate(FITC,MerksBiosciences).TheFITCwasdissolvedin acetone,andsmearedevenlyoveramicroscopeglassandallowedtodry.Thesamplewasgentlyplacedonthemicroscopeglassandthedyewas allowedtoabsorbintothesampleforminimum30minutesbeforemi-croscopy.Alltheimagesweretakenusinga1030objectiveandanArgon laseratanexcitationwavelengthof488nm. 2.8.Particlesizedistribution Particlesizedistributionofthegastricdigestateswasmeasuredusing laserlightscattering(Mastersizer,MalvernInstruments,Malvern,UK).Themeasurementswereonlyperformedonvisuallyhomogeneous matrices(MPCþpectinandMPCþguarsuspensions).Particlesizedistri-butionswererecordedasvolumeweighted(d4,3)means.Eachmea-surementwascarriedoutinduplicate. 2.9.Rheologicalanalysis InthecaseofMPCþpectinandMPCþguardispersions,itwaspossible tomeasuretheirrheologicalproperties,asallemptyingpointsappeared homogeneous.InitialdispersionsandthedigestatesatthevariousGIweregentlyloadedinacup/bobgeometry(CylinderB-CC27,Physica MCR301,AntonPaar)andmeasuredat37 (Cusingarheometer(Physica MCR301,AntonPaar).Ashortstrainsweep(20s)wasconductedatvery lowstrainpercentages(0.1–1%),followedbyalowcurvemeasurement,calculatingviscositiesoverabroadrangeofshearrates(0.01–100sm1e).Usingtheobtainedlowcurvedata,theviscosity(η)wasplottedasafunctionofshearrate(γ_)onlogarithmicscales(logðηÞvs.logðγ_Þplotfor eachGI-sample).Linearregressionsweremadeonthelinearpartoftheselogarithmicplotsaccordingtothepowerlawviscositymodel,tocalculate thelowbehaviorindex(n)andtheconsistencyindex(K). 2.10.Degreeofproteolysis usingtheo-phtaldialdehyde(OPA)assay(Nielsenetal.,2001).Abuffer containingNa3PO4⋅12H2O(15g/L)andCH3(CH2)11OSO3Na(SDS,1g/L)waspreparedindeionizedwaterandadjustedtopH11withHCl.A o-phtaldialdehyde(OPA)solutionwaspreparedbydissolving0.04g/mL inEtOH.ADTT-solutioncontainingdithiothreitolat0.044g/mLwas preparedindeionizedwater.Atthetimeofuse,anOPA-reagent-solution (50mL)waspreparedbymixingtheOPA-solution(1mL)andthe DTT-solution(1mL)withtheSDSPhosphatebuffer(48mL).The OPA-reagent-solutionwasprotectedfromlightandusedimmediately. Aliquotsofgastricsamples(850uL)werepretreatedwith150uLof 20%trichloroaceticacidsolution,tocauseproteinprecipitation.Thesampleswerecentrifuged(17000g,20min,22usC)andthesupernatant wasremoved,andcentrifugedagain(17000g,5min,222°C).Superna-tants(25uL)weremixedwiththeOPA-reagent-solution(175uL)ina microtiterplate,andafter50minofincubation,theabsorbancewasmeasuredat340nmandsubtractedfromcontrolspreparedwithwater.ThedataweremeasuredagainstastandardcurvepreparedwithSerine(0.07–4.7mM)andreportedasreleasedserineequivalentspermgof protein. TheproteinconcentrationwasmeasuredusingtheDumasmethod usingaDUMATHERM®nitrogenanalyzer(GerhardtAnalyticalSystems,K€onigswinter,Germany).Aconversionfactorof6.38wasusedtoobtain theproteincontentfromthenitrogencontent. 2.11.ProteindigestionmeasuredbySDS-PAGE SDS-PAGEanalysiswasconductedonthevariousdigestasamplesas wellastheinitialundigestedmatrices,afterdilutionofallsamplesto 0.5%proteininmilliQwaterandthenfurtherdiluted1:2in0.1mol/LNa-phosphatebuffer,pH7.0.Thesampleswerethenmixed1:1with2/L Laemmlisamplebuffer(20pL)containingDTT(350mmol/L)andplacedonaheatblock(80r C,4min).Proteinstandards(10mL,PrecisionPlus Protein™DualXtra#1610377,BIO-RAD)andthetestsamples(20uL)wereloadedontoaNuPAGE™4–12%Bis-Trisgel(10well,1.0mm,Invitrogen™).Dithiothreitol99%(DTT,457779,Sigma-Aldrich),2x LaemmliSampleBuffer(BIO-RAD,#1610737),NuPAGE™4–12%Bis-Trisgel10well1.0mm(Invitrogen™),NuPAGE™MOPSSDSRunning Buffer(20x,Invitrogen™),PrecisionPlusProtein™DualXtra(BIO-RAD,#1610377),SimplyBlue™SafeStain(Invitrogen™).Theelectrophoresis (40min,200V,120mA[240mAfor2gels],25W)wasperformedusing NuPAGE™MESSDSrunningbuffer.Thegelwasrinsedtwotimesin water,followedbystainingwithInvitrogen™SimplyBlue™SafeStain(60min,22llC).Thegelwasde-stainedinwater(100mL,1hour).ANaCl solution(20%w/v,20mL)wasaddedtothede-stainingwaterandthe gelwasincubatedovernight.GelImagingwereperformedonaGelDoc™EZSystem(BIO-RAD)usingImageLab™software. 3.Resultsanddiscussion 3.1.Structureformationduringgastricdigestion Theamountoforalandgastricjuicestobeaddedtothemixturewas derivedfromtheINFOGESTrecommendations,wherebya1:1ratioof SSFwasaddedbasedonsolids,andthen,thismixtureenteredthegastric vesselalreadycontaining10%ofthetotalSGFvolume.ThepHwillthen decreasegraduallyfromaninitiallyhighlevel,duetothebufferingca-pacityoftheprotein,toainalpHof2.DifferentamountsofHClwere addedtothemixturestoaccountforthedifferencesinbufferingcapacity ofthepolysaccharidemolecules.ThepHproilesofthevariousmixtures areshownin Fig.1.Thereweresomedifferencesintheirstpartofthe digestion,forthemixturescontainingguargum(Fig.1,illedsquares),as wellasthosecontainingalginate,andalginateandpectin.Thehigher valuesforguargumattheinitialstagesofmixingwereduetothemore homogeneousproteindistributioninthemixtureandthehigherviscosity ofthecontinuousphasecomparedtotheothersystems,slowingdown migrationofacidtothepHelectrodethroughthemixing. InMPCcontroldispersions(Fig.1,illedcircles),valuesdroppedto pHaround3after45minofdigestion.Thiswasduetotheinhomoge-neousstructure,whichcausedahighdischargeofproteinattheinitial stagesofgastricemptying(smallparticles,precipitatedatthebottomof thevessel)incombinationwiththecontinuousadditionofSGFinthe vessel.ThepresenceofinhomogeneousaggregatedstructuresinMPC conirmedpriorinvivoobservations(Fletcheretal.,2001;Hilaetal.,2006). Thevisualappearanceofthesesuspensionsatvariousstagesofgastric emptyingareshownin Figs.2and3.Fig.2illustratesdifferenceinthe natureoftheprecipitatespresentatthevariousgastricinterval(GI)points,while Fig.3comparesthesizeofthelargeaggregatespresentinMPC,andMPCþalginateandMPCþalginateþpectindigestates,atthe variousemptyingstages.Thesamplescontainingguarandpectinarenot shown,astheywerehomogeneousandshowedverysmallaggregates,whichwereinsteadcharacterizedbyintegratedlightscattering(Fig.4). TheMPCþalginatecontainedthelargestprecipitates,comparedtoMPCcontrolorMPCþpectinþalginate.Theeffectofalginateonthe presenceoflarge,undigestedaggregatesisobviouslookingattheENDpointsin Figs.2and3.AlsointhecaseofMPCdispersionswithalginate andalginateþpectin,thedispersionsenteredthevesselaspourable,homogeneousmatrices,andimmediatelyaggregateduponcontactwiththeSGF.Inthiscase,theaggregationwasnotonlymediatedbyacidand pepsin,butalsothepresenceofcalciumions,presentintheSGF,aswellasreleasedbythecaseinmicellesduringacidiication(LiandCorredig,2019).Thestructuresweregraduallydisruptedwithintheirst60minofinvitrodigestion,duetodilutionwiththeSGFandmixing.Attheirst emptyingpoint(GI1)theMPCþalginatematrixappearedtohavesome largeaggregatessuspendedinaviscousmatrix(Fig.2),atthistime,mostaggregateswerestilltoolargetopassthroughtheinvitroemptyingstage.Subsequentemptyingpointsshowedanincreaseintheprecipitation,withagradualdecreaseintheaggregatesize,asshownin Fig.3.The MPCþalginateþpectinsuspensionsformedsmallerstructuresthanthoseofMPCþalginate(Figs.2and3).Inallcases,theENDpointsstillpre-sentedlargeinhomogeneousstructures. UnliketheMPCcontroldispersion,andthosecontainingalginate,the 3%MPCwithpectinorguargumhadamacroscopicallyhomogenousappearance.Itwasthenpossibletoanalyzetheirparticlesizebylight scattering,asshownin Fig.4.Theaverageapparentparticlediameterincreasedfrominitialtotheirstgastricemptyingpoint(GI1)inboth treatments.Ingeneral,MPCþpectinshowedasmalleraverageparticlesizethanMPCþguar,and,inthecaseofguargum,therewasadecrease inparticlesizeovergastricemptyingtime.BothsuspensionsattheENDpointshowedanaverageparticlediameter(D4,3ofabout80um). Duetothehomogeneousappearanceofthesetwotreatments,the viscositywasalsomeasuredintheMPCdispersionscontainingpectinor guargum,atvariousstagesofinvitrogastricemptying,asshownin Fig.5.Asshownbytheinitialvaluesoftheconsistencyindex,bothmatrices enteredthestomachasviscousliquids,withthesuspensioncontaining guargummarkedlymoreviscousthanthatcontainingpectin.The MPCþpectindispersionshowedasharpdropinconsistencyindex,immediatelyaftergastricdilutionandmixing,withacontinuousdecreaseovertheinvitrodigestion.Incontrast,theMPCþguarmixture displayedamoregradualdeclineinconsistencyindex(duetothegradualdilutionwithSGF),showingasigniicantlyhigherviscositythroughout,comparedtotheMPCþpectinsuspension.Inbothsuspensions,thehow behaviorindexshowedamarkedincreaseovertime,withpectinshowingamorenewtonianluidbehaviorinthelastphasesofgastricdigestion.Ontheotherhand,MPCþguarsuspensionsshowedaowbehaviorin-dexes<0.7evenattheENDpoint,indicatingashearthinningstructurethroughoutgastricdigestion. 3.2.Microstructuralchangesduringinvitrodigestion Examplesofthemicrostructuresobservedforthedifferentemptying timefractions,areshownin Fig.6.MPCcontroldispersionsshowedlarge aggregatedparticles,decreasinginsizeovertime.Aggregateslargerthan100umwerestillobservedinthelastgastricemptyingpoint.Both MPCþalginateandMPCþalginateþpectinwerecharacterizedbyaclear proteinnetworkthroughouttheieldofview,andmixturescontaining bothalginateandpectinsshowedsmalleraggregatesthanthosecon-tainingonlyalginate,orMPCcontrol.Conirmingvisualobservations,theMPCdispersionscontainingpectinorguargumshowedaggregation microscopically,butahomogeneousdispersionmacroscopically,with proteinaggregatesmarkedlydifferentinstructure. Theirstfractionemptiedafter30minofinvitrodigestion,showeddrasticdifferencesbetweentreatments,intermsofsizeandmicrostruc-ture.Duringdigestion,MPCþalginatesuspensionprimarilyformedlarge,low-density,porousstructures.MPCcontrolinitiallyaggregatedintolargenetworkstructures,andthegelgraduallywasdisruptedovertime.TheinitialgelstructureofMPCþalginatewaslargelypreservedinGI1andGI2.ThepresenceofpectinintheMPCþalginateþpectinmixtures causedaggregationinsigniicantlysmallerparticlesandinainergelstructurescomparedtoMPCþalginate.InMPCmixturescontaining pectinorguargum,smallermicro-phaseseparatedaggregateswerepresentintheinitialstagesofgastricemptyingandsizesslightly decreasedovertime.Theaggregateswerelessthan100uminsize,inagreementwiththelightscatteringresultsreportedin Fig.4. Theconfocalobservationsconirmedwhatexpectedfromthefunc-tionalityofthispectin(suitedtostabilizeacidiiedproteindrinks),namelytheabilitytolimittheaggregatessize,byhinderingtheinter-actionbetweenthesmallaggregates.AsthepHofthecaseinsdecreases,thenegativechargesofthepectininteractwiththepositivemoieties presentonthecaseins,formingprotein-polysaccharideaggregates.These coacervatesformedbetweentheproteinaggregatesandpectinsterically stabilizedthesestructuresandmaintainedamacroscopicallyhomoge-neusdispersion,whichgraduallydecreasedovertime. IntheMPCþguarmixture,guargumcausedmicroscopicphasesep-aration,duetothethermodynamicincompatibilitybetweentheguargummoleculesandthecaseinproteins,formingaggregates.Hence,small proteinparticleaggregatesformedintheproteinrichphases.Inthiscaseitwasshownthattheparticlesizedecreasedovertime,alsoduetoa contractionofthestructuresduringfurtheracidiication,andincreasedhydrolysis.ThesedifferencesinmicrostructurebetweenMPCþpectin andMPCþguarwerewellalignedwiththerowbehaviorcharacteristics shownin Fig.5. Fig.7comparesalltheENDpointsfortheinvitrogastricdigestion.In allcases,largeproteinaggregateswerestillpresent.Duetothehomo-geneousdistributionwithinthegastricvessel,theENDpointsforMPC dispersionscontainingpectinorguargumseemedtobesimilartothose shownin Fig.6forGI4. 3.3.Proteindigestion Fig.8showsthecumulativeamountofproteinmeasuredduringthe gastricemptying(A)andthefreeaminesreleasedpermgofprotein(B), relectingtheextentofgastricproteolysisofMPCasafunctionoftime.In allmatrices,therewasanincreaseintheamountcumulativeamountof proteinemptied,aswellasagradualincreaseinproteolysis. Structuringwithalginate(triangles)causedthehighestlevelofpro-teinmeasuredinthefractionsemptied,butwiththelowestleveloffree aminesinthedigestates,comparedtotheothertreatments.Ontheotherhand,theMPCþguardispersions(olledsquares)showedthehighest levelsoffreeamines,especiallyatGI4andENDpoint.Allothertreat-mentsshowedsimilarvaluesofproteinduringdigestion(Fig.8A)and intermediatelevelsofaminerelease.Whencomparedtotheotherho-mogeneousdispersion,thedatawouldindicatethat,althoughtheho-mogeneityofthesuspensionsandthesmallproteinparticlesizecauseda higherextentofdigestion,thepectin-MPCwaslessaccessedbythegastricenzyme,comparedtoMPCþguardispersion.Thisisexpected,as thecoverageoftheproteinbythepectincausesaloweraccessibilitytothepepsininthecoacervate22.Fig.9showstheamountofserine equivalentmeasuredatGI4andtheENDpointsamples.TheENDshowed signiicantlylowerlevelsoffreeaminespermgofprotein,demonstrating stillthepresenceofundigestedproteinafter2.5hofinvitrodigestion.OnlytheMPCþguarmixtureshowedaconsistentleveloffreeaminesat theEND,comparedtoGI4,showingthehomogeneousnatureofthe matrix.MPCsamples(blackbars),aswellasthosesamplescontaining pectinshowedsigniicantlylowerlevelsofserineequivalentsattheEND pointcomparedtoGI4,demonstratinglargeunhomogeneities. Tobetteridentifypossibledifferencesinproteinbreakdowninthe gastricstage,asafunctionofpolysaccharidetype,theproteincomposi-tionatthevariousemptyingpointsduringthegastricphasewasanalyzed bySDS-PAGE(Fig.10).Thegastricdigestawerecomparedatsimilar proteinconcentration,tobetterdifferencesinthedigestionpattern.The MPCsuspension,beforedigestioncontainedallthemajormilkproteins,includingthemilkfatglobulemembraneproteins,caseins,andwhey proteins.Theproteinbandswereclearlydifferentalreadyaftertheirst GIemptyingpoint,inallcases,withhigherratiosofwheyproteinsand caseinspresentintheemptiedfraction.However,thereweremajordif-ferencesinthebreakdownpatterndependingonthetypeofaggregates formedwiththepolysaccharides.Inallcases,β-lactoglobulinseemedto beweaklyaffectedbythegastricdigestioncomparedtotheothermajor proteins.Thisisinagreementwithinvivoandinvitrodatashowingthat theβ-lactoglobulinmainlyemptiesfromthestomachasintactprotein (Mahee etal.,1996;Villaumeetal.,2004). Thecaseins,aswellasthehighmolecularweightbandsassociated withthemilkfatglobulemembraneproteinstendedtoweakengoingfromGI1toGI4,innearlyallmatrices.However,theMPCþalginate showedhighpreservationofthecaseinfromGI1toENDpoint.Inthe MPCcontrol,proteinemptiedattheearlystages,duetotheprecipitation andbreakdown,allowingforamoredilutedsystemoverallatthelater stagesoftheinvitrogastricdigestion,andatGI4pointmuchlessprotein ispresent,indicatingmorehydrolysis,asallbandswereloadedatthesameproteinlevel(aspernitrogenmeasurement).MPCþguaralso showedahigherextentofhydrolysisattheendoftheinvitrodigestion.Theresults,albeitqualitativeinnature,mayalsosuggestdifferencesin thecaseinaccessibility. Theintensitydecreaseofthecaseinbandswereaccompaniedbyanincreaseinsmallmolecularweightpeptides.Markeddevelopmentof smallpeptides(5–10kDa)inGI3,GI4,andEPS,wasobservedforallmatrices.Thelargestincreaseinsmallpeptideswasobservedfor MPCþguarduringthegastricphase.TheleastcaseinbreakdownwasshownforMPCþalginate. 4.Conclusions Gastricstructuringusinghydrocolloidsisavalidstrategytomodulate gastricdigestion,asitmodiiesthemicrostructureoftheaggregatesand maycausedelaysinproteolysis.Differenthydrocolloidswerepurposely choseninthisworkinlinewiththeknownphysico-chemicalbehavior andtheirabilitytointeractwithproteinsasafunctionofpH.Ingeneral, proteolysislevelsdecreasedgoingfromGI4toEND,indicatingtheeffect ofnonhomogeneousdistributionsofproteinandaggregatesizes.The milkproteinspresentintheMPCcontroldispersionsshowedimmediate precipitationinthepresenceofthegastricjuicesduetoacidiicationand pepsinactivity.However,inthepresenceofalginate,largeparticleswere obtainedandthesecoacervatesmadetheproteinlesssusceptibleto pepsinhydrolysis.Macroscopicallyhomogeneoussuspensionswereob-tainedwiththeadditionofpectinorguargumtotheMPCdispersion.Thesesystemsshowedthatmicrostructuraldifferencesinluencedbythe speciicpolysaccharidescanalsomodulateproteolysiskinetics.High esterpectinisknowntomaintaincaseinaggregatesdispersedinacid environments.Inthisstudy,itwasclearlyshownthatthesecoacervates weremoreresistanttoproteolysiscomparedtotheaggregatessuspended inamicrophaseseparatedsystemformedbyusingguargum. Inconclusion,bycarefullydesigningthestructuresusingtheirknown physico-chemicalpropertiesandtheirabilitytointeractwithproteins,it ispossibletomodulatethedigestionbehavioroftheproteinsatthe gastricstage.Thematricesprepareddisplayedlargestructuraldiffer-encesduringgastricdigestion,andthesecouldbedirectlycorrelatedto themeasuredvariationsinproteindigestion.Thepresentedmethodology constitutesapowerfultooltounderstandfoodstructuringandnutrient digestibilitywhichcanhelptoprovideknowledgeaboutthenutritional impactofvariousfoods. CRediTauthorshipcontributionstatement JacobØstergaardMarkussen:Methodology,datacollection,Data curation,Writing–originaldraft.FinnMadsen:Conceptualization,Data curation,advising,writing,Validation,Writing–review&editing.Jette FeveileYoung:advising,Writing–review&editing.MilenaCorredig:Conceptualization,Datacuration,advisingwriting,Validation,Writing–review&editing. Declarationofcompetinginterest Theauthorsdeclarethattheyhavenoknowncompetinginancial interestsorpersonalrelationshipsthatcouldhaveappearedtoinluence theworkreportedinthispaper. References Boutrou,R.,Gaudichon,C.,Dupont,D.,Jardin,J.,Airinei,G.,Marsset-Baglieri,A.,Benamouzig,R.,Tom,e, D.,Leonil,J.,2013.Sequentialreleaseofmilkprotein-derived bioactivepeptidesinthejejunuminhealthyhumans.Am.J.Clin.Nutr.97,1314–1323.https://doi.org/10.3945/ajcn.112.055202. Brodkorb,A.,Egger,L.,Alminger,M.,Alvito,P.,Assunc~ao,R.,Balance,S.,Bohn,T.,Bourlieu-Lacanal,C.,Boutrou,R.,Carrirerre,F.,Clemente,A.,Corredig,M.,Dupont,D.,Dufour,C.,Edwards,C.,Golding,M.,Karakaya,S.,Kirkhus,B.,LeFeunteun,S.,Lesmes,U.,Macierzanka,A.,Mackie,A.R.,Matins,C.,Marze,S.,McClements,D.J.,Mneanard,O.,Minekus,M.,Portmann,R.,Santos,C.N.,Souchon,I.,Singh,R.P.,Vegarud,G.,Wickham,M.S.J.,Weitschies,W.,Recio,I.,2019.INFOGESTstaticin vitrosimulationofgastrointestinalfooddigestion.Nat.Protoc.14,991–1014.https://doi.org/10.1038/s41596-018-0119-1. Draget,K.I.,Skjåk-Bræk,G.,Smidsrød,O.,1997.Alginatebasednewmaterials.Int.J.Biol.Macromol.21,47–55.https://doi.org/10.1016/S0141-8130(97)00040-8. Fardet,A.,Dupont,D.,Riouz,L.E.,Turgeon,S.L.,2019.Inluenceoffoodstructureon dairyprotein,lipidandcalciumbioavailability:anarrativereviewofevidence.Crit.Rev.FoodSci.Nutr.59,1987–2010.https://doi.org/10.1080/10408398.2018.1435503. Fletcher,J.,Wirz,A.,Young,J.,Vallance,R.,McColl,K.E.,2001.Unbufferedhighlyacidic gastricjuiceexistsatthegastroesophagealjunctionafterameal.Gastroenterology 121,775–783.https://doi.org/10.1053/gast.2001.27997. Guo,Q.,Ye,A.,Singh,H.,Rousseau,D.,2020.Destructuringandrestructuringfoods duringgastricdigestion.Compr.Rev.FoodSci.FoodSaf.19,1658–1679.https://doi.org/10.1111/1541-4337.12558. Hila,A.,Bouali,H.,Xue,S.,Knuff,D.,Castell,D.O.,2006.Postprandialstomachcontents havemultipleacidlayers.J.Clin.Gastroenterol.Behav.Skim40,612–617.https://doi.org/10.1097/00004836-200608000-00010. Koutina,G.,Ray,C.A.,Lametsch,R.,Ipsen,R.,2018.Theeffectofprotein-to-alginate ratiooninvitrogastricdigestionofnanoparticulatedwheyprotein.Int.DairyJ.77,10–18.https://doi.org/10.1016/j.idairyj.2017.09.001. Lamghari,E.,Kossori,R.,Sanchez,C.,ElBoustani,E.S.,Maucourt,M.N.,Sauvaire,Y.,Mejean,L.,Villaume,C.,2000.Comparisonofeffectsofpricklypear(Opuntiaicus indicasp)fruit,Arabicgum,carrageenan,alginicacid,locustbeangumandcitrus pectinonviscosityandinvitrodigestibilityofcasein.J.Sci.FoodAgric.80,359–364.https://doi.org/10.1002/1097-0010(200002)80:3<359::AID-JSFA534>3.0.CO;2-8. Li,Y.,Corredig,M.,2019.Acidinducedgelationmilkconcentratedbymembrane iltration.J.TextureStud.51,101–110.https://doi.org/10.1111/jtxs.12492. Lluch,A.,Hanet-Geisen,N.,Salah,S.,Salas-Salvad6o,6J.,L’Heureux-Bouron,D.,Halford,J.C.,2010.Short-termappetite-reducingeffectsofalow-fatdairyproduct enrichedwithproteinandibre.FoodQual.Prefer.21,402–409.https://doi.org/10.1016/j.foodqual.2009.10.001. Mah,e, S.,Roos,N.,Benamouzig,R.,Davin,L.,Luengo,C.,Gagnon,L.,Gaussergeess,N.,Rrautureau,J.,Tomee, D.,1996.Gastrojejunalkineticsandthedigestionof[15N]beta-lactoglobulinandcaseininhumans:theinluenceofthenatureandquantityof theprotein.Am.J.Clin.Nutr.63,546–552.https://doi.org/10.1093/ajcn/63.4.546. Marciani,L.,Hall,N.,Pritchard,S.E.,Cox,E.F.,Totman,J.J.,Lad,M.,Hoad,C.L.,Foster,T.J.,Gowland,P.A.,Spiller,R.C.,2012.Preventinggastricsievingbyblending asolid/watermealenhancessatiationinhealthyhumans.J.Nutr.142,1253–1258.https://doi.org/10.3945/jn.112.159830. Marciani,L.,Pritchard,S.E.,Hellier-Woods,C.,Costigan,C.,Hoad,C.L.,Gowland,P.A.,Spiller,R.C.,2013.Delayedgastricemptyingandreducedpostprandialsmallbowel watercontentofequicaloricwholemealbreadversusricemealsinhealthysubjects:novelMRIinsights.Eur.J.Clin.Nutr.67,754–758.https://doi.org/10.1038/ejcn.2013.78. Mulet-Cabero,A.I.,Rigby,N.M.,Brodkorb,A.,Mackie,A.R.,2017.Dairyfoodstructures inluencetheratesofnutrientdigestionthroughdifferentinvitrogastricbehavior.FoodHydrocolloids67,63–73.https://doi.org/10.1016/j.foodhyd.2016.12.039. Mulet-Cabero,A.I.,Mackie,A.R.,Wilde,P.J.,Fenelon,M.A.,Brodkorb,A.,2019.Structuralmechanismandkineticsofinvitrogastricdigestionareaffectedby process-inducedchangesinbovinemilk.FoodHydrocolloids86,172–183.https://doi.org/10.1016/j.foodhyd.2018.03.035. Mulet-Cabero,A.I.,Egger,L.,Portmann,R.,M eMnard,O.,Marze,S.,Minekus,M.,Le Feunteun,S.,Sarkar,A.,Grundy,M.M.L.,Carrieerre,F.,Golding,M.,Dupont,D.,Recio,I.,Brodkorb,A.,Mackie,A.,2020.Astandardisedsemi-dynamicinvitro digestionmethodsuitableforfood–aninternationalconsensus.FoodFunct.11,1702–1720.https://doi.org/10.1039/C9FO01293A . Nielsen,P.,Petersen,D.,Dambmann,C.,2001.Improvedmethodfordeterminingfood proteindegreeofhydrolysis.J.FoodSci.66,642–646.https://doi.org/10.1111/j.1365-2621.2001.tb04614.x . Paxman,J.R.,Richardson,J.C.,Dettmar,P.W.,Corfe,B.M.,2008.Dailyingestionof alginatereducesenergyintakeinfree-livingsubjects.Appetite51,713–719.https://doi.org/10.1016/j.appet.2008.06.013. Rao,T.P.,Hayakawa,M.,Minami,T.,Ishihara,N.,Kapoor,M.P.,Ohkubo,T.,Juneja,L.R.,Wakabayashi,K.,2015.Post-mealperceivablesatietyandsubsequentenergyintake withintakeofpartiallyhydrolysedguargum.Br.J.Nutr.113,1489–1498.https://doi.org/10.1017/S0007114515000756. Villaume,C.,Sanchez,C.,M ejMean,L.,2004.β-Lactoglobulin/polysaccharideinteractions duringinvitrogastricandpancreatichydrolysisassessedindialysisbagsofdifferent molecularweightcut-offs.Biochim.Biophys.ActaGen.Subj.1670,105–112.https://doi.org/10.1016/j.bbagen.2003.10.017. Wang,Q.,Ellis,P.R.,Ross-Murphy,S.B.,2000.Thestabilityofguarguminanaqueous systemunderacidicconditions.FoodHydrocolloids14,129–134.https://doi.org/10.1016/S0268-005X(99)00058-2. Ye,A.,Cui,J.,Dalgleish,D.,Singh,H.,2016.Formationofastructuredclotduringthe gastricdigestionofmilk:impactontherateofproteinhydrolysis.FoodHydrocolloids 52,478–486.https://doi.org/10.1016/j.foodhyd.2015.07.023.
确定





还剩10页未读,是否继续阅读?
中国格哈特为您提供《浓缩牛奶蛋白( MPC)蛋白质含量的检测》,该方案主要用于液体乳中营养成分检测,参考标准《GB 5009.5 食品安全国家标准 食品中蛋白质的测定》,《浓缩牛奶蛋白( MPC)蛋白质含量的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro、德国移液器MM
推荐专场
相关方案
更多
该厂商其他方案
更多

















