方案详情
文
膜浓度诱导酪蛋白胶束结构和胶体变化及其对环境交换条件的关系Structural and colloidal changes of casein micelles induced by membrane concentration and their dependence on the milieu exchange conditions
使用格哈特公司杜马斯定氮仪检测脱脂乳中酪蛋白含量和总蛋白含量
Casein content of retentates:
Total protein contents of skim milk, UF and MF retentates, and their centrifugal supernatants were determined by nitrogen analysis using a Gerhardt Dumatherm (C. Gerhardt GmbH & Co.KG, K¨
onigswinter, Germany) and a conversion factor of N × 6.38. The protein contents referring to casein micelles (PCM) in skim milk and retentates were
calculated by subtracting the protein content of their centrifugal supernatants (PSN) from the total protein contents (Ptotal):
PCM = Ptotal − PSN
方案详情

Colloids and Surfaces A: Physicochemical and Engineering Aspects 670 (2023) 131580 Contents lists available at ScienceDire c t CO LO Colloids and Surfaces A: Physicochemical and Engineering Aspects journal homepage: www.e l sev i er .com/l ocate/col s ur f a 膜浓度诱导酪蛋白胶束结构和胶体变化及其对环境交换条件的关系 Structural and colloidal changes of casein micelles induced by membrane concentration and their dependence on the milieu exchange conditions Norbert Raak =,*, Jan Skov Pedersen,Milena Corredig a Department of Food Science and ciFOOD Centre for Innovative Food Research, Aarhus University, Agro Food Park 48, 8200 Aarhus N, Denmark b Department of Chemistry and iNANO I nterdisciplinary Nanoscience Centre, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus C, Denmark HIGHLIGHTS ·Concentrated caseinnmicelles were studied under different diafiltration treatments. ·Casein micelle structure was assessed by fi t ting small-angle X-ray scattering data. ·Diafiltration with water caused dissoci-ation of caseins into the serum phase. · Diafiltration with water caused changes in the internal structure of the micelles. ·Diafiltration with permeate preserved the structure of the casein micelles. ARTICLEINFO Keywords:Membrane f il t rat i on Microfilt r ation Diaf i ltration Milk protein concentrate Small-angle X-ray scattering Case i n mice l le struc t ure ABSTRACT Casein micel l es are colloidal composite particles composed of several thousand casein molecules (referred to as os1-, asz-, -, and k-casein) and inorganic calcium and phosphate, which change their supramolecular structure depending on external influences such as t emperature, pH,ionic equilibrium, or volume fraction [6]. Casein is t he major protein fraction in milk from most mammals, including bovine mi l k, where whey proteins are the minor protein fraction with approx. 20%[14]. Micel l ar casein concentrates (MCCs) are milk-derived i ngredients with an i ncreased ratio of casein/whey proteins and typically produced via microfi l tration (MF) combined with a milieu exchange, which is commonly referred to as diafiltration (DF) [7]. In MF, skim mi l k i s passed over a semi-permeable membrane with typical pore sizes of~0.08-0.2 um, which retains and concentrates the casein micelles, whi l e water, minerals, lactose, and major whey proteins (i.e., o-lactalbumin,B-lactoglobulin) are partially removed. The purity of the casein f raction in MCCs can be further raised by DF, where water or other media are added during the process to wash out undesired constituents while maintaining flux and separation efficiency [55]. Even though the concept to produce MCCs i s well understood and has been extensively studied in the past, the relationships between pro-cessing history (e.g., concentration f actor, DF extent, DF medium),structural changes of t he casein micelles (e.g., rearrangements on the micelle surface, dissociation of caseins and minerals into t he dispersion phase), and functional properties of final MCCs (e.g., heat stabili t y,gelation properties) are still largely unknown [9]. Previous studies showed that concentration and, especially, DF of milk result in dissociation of some casein molecules from the micelles into the dispersion phase of the retentates [11]. These caseins were hypothesised to form smal l , soluble aggregates, which cannot be sedi -mented by centrifugation and are likely to i nteract with the whey pro-teins during heat treatment . As the dissociation leads to a different composition of the dispersion phase compared to that of the original milk, i.e., with more soluble os- and p-caseins [34,59], this can have important technological implications. For instance, the presence of non-sedimentable caseins has been reportedto hamper the rennet -i nduced coagulation of casein micel l es, an important functional property of MCCs, due to re-adsorption of the caseins on t he micellar surface and their calcium chelating effect [17,29]. Remarkably, the amount of casein released from the micelles dur i ng concentration up to 3 × volume reduction is lower when using membrane f iltration compared to using osmotic stressing, which i s a no-shear, very gent l e process based on osmotic pressure differences [28]. This was ascribed by the authors to shear- and/or pressure-induced rearrangements of the casein micelle structure during concentration via membrane filtration.These possible structural changes may be further exacerbated by milieu exchanges such as DF processes, as t his wi l l disrupt t he important i onic equi l ibrium between the colloidal calcium phosphate i n the micelles and ions in t he dispersion phase. Due to the dependence of these colloida l particles on environmental conditions, structural modifications of casein micelles during milk pro-tein concentration processes have to be studied using non-disruptive analyses of undiluted systems to f ully preserve particle-particle i n-teractions. Small-angle X-ray and neutron scattering (SAXS and SANS)can provide i nformation across a large range of l ength scales f rom nano-to micrometres and have been proven useful techniques to evaluate structural changes in colloidal protein particles such as casein micelles [19]. Besides the character i sation of native casein micelles [12,22,25,53,63], SAXS and SANS have been widely applied i n studies related to milk protein ingredients and their manufacture. Bouchoux et al . [5]studied the changes of the internal structure of casein micelles during concentrating a casein suspension up to 400 g/L via osmotic stressing and concluded from the SAXS data that casein micelles get compressed,lose water, and shrink above protein concentrations of 150 g/L. They further observed that this deformation is not homogeneous due to a heterogeneous structure of hard regions and voids. More recently,Christiansen et al. [8] suggested from the slopes of the SAXS curves that casein micelles acquire elongated shapes when concentrat i ng pas-teurised skim milk, and that the surface of the casein micelles becomes smoother in pasteur i sed skim milk concentrates. Gebhardt [18] studied casein micelles on porous f i lter layers and concluded from X-ray scat-tering a stretching along the f il tration flow as a consequence of elon-gation forces at moderate pressure gradients, whereas a compression along the filter l ayer was observed at higher pressure gradients. This compression of casein micelles accumulated in deposit layers on the membrane surfaces seems to be partially reversible, as indicated by other studies [13,35]. It i s believed that the colloidal calcium phosphate nanoclusters result in a characteristic shoulder in the scattering of casein micelles, as this shoulder disappeared after removal of calcium from t he casein micelles by acidi f ication or demineral i sation [1,43,45,66]. However, t his f eature has recently been attributed to the contribution of both protein and colloidal calcium phosphate to t he scattering intensity [25,53]. The present study is a detailed comparison between different DF treatments during concentration of fresh casein micelles using MF,aiming at providing insights on the i mpact of t he milieu exchange conditions on the casein micelle structure and the relationship between structural changes and protein dissociation from the casein micelles.MCCs at 4 × volume concentration factor (i.e., based on volume reduction) were prepared using MF combined with continuous or discontinuous DF, using either water or ultrafil t ration (UF) permeate as DF medium. An i n-depth analysis of the composi t ion of the dispersion phases of the various concentrates was car r ied out, and detailed infor-mation on the casein micelle structure in the MCCs was obtained by applying a recently developed SAXS model for bovine casein micelles [53]. 2. Materials and methods 2.1. Membrane filtration Pasteurised skim milk (72°C, 15 s) was provided by Arla Foods amba (Viby, Denmark) and treated with 0.2 g/kg sodium azide for preserva-tion. A Vibro-Lab3500 system was equipped with either a 30 kDa PESH (0.35 m’membrane area) or an 800 kDa PDVF (0.35 m’ membrane area) plate-and-frame membrane uni t (volume of membrane housing was 1.7 L) for UF and MF, respectively (Sani Membranes ApS, Al l erod,Denmark). The system was operated in vibration mode, i.e., membrane plates were osci l lated relative to the media with an osci l lation frequency of 24 Hz to minimise fouling and allow an efficient separation of whey proteins. Skim milk was kept i n an ice bucket during membrane f iltra-tion to maintain a temperature of approx. 10°℃. First, skim milk was processed by UF to reach a two t imes concen-tration (based on volume reduction, referred to as 2 ×). A 9 L stainless steel pressurised container was used as feed tank, and t he filtration was driven by pressurised air at 0.2 MPa. The retentate valve opening was controlled manually to achieve similar flow rates for permeate and retentate, and the concentrate within the membrane chamber was cont i nuously circulated by an external pump (RLFP122202, Sani Membranes ApS). The resulting 2 × UF retentate was kept at 4℃overnight before further concentrat i on using MF in combination with DF, to reach a f ina l concentration factor of four (4 ×; based on volume reduction). The 2 × concentrate was fed into the MF membrane module by a pump (Masterflex P/S Easy Load II, Thermo Fisher Scientific,Waltham, MA, USA), and the retentate was recirculated into the feed tank. The DF was carried out either in discontinuous or continuous mode,using two DF media: demineralised water or UF permeate (collected from t he initial UF step), as depicted in Fi g . 1. T he total volume of added DF medium corresponded to 0.5 t i mes the volumes of original mi l k (i.e.,1 volume of UF retentate), and the total volume of permeate obtained Fig. 1. Schematic illustration of the change in volume concentration f actor (closed symbols) and amount of added diafiltration (DF) medium (open sym-bols) during discontinuous (A) and continuous DF (B), starting from 2 × u1-trafiltration retentate. The x-axes i ndicate the progress of membrane filtration as total volume of permeate obtained in microf i ltration (MF) relative to the initial volume of skim milk. The vertica l grey dashed l i nes indicate the times i n discont i nuous DF when 4 × volume concentration factor was reached and the DF medium was added. during the MF stage was 0.75 times the volume of the original skim mi l k (i .e., 1.5 volume of the 2×UF retentate). During discontinuous DF, the 2×UF retentate was concentrated to a final concentration factor of 4× (based on volume reduction) and subsequently diluted back to 2 × using DF medium. This procedure was repeated twice, and the f i nal retentate was col l ected after reaching 4 ×concentration (Fig . 1A). In cont i nuous DF, the same quantity of water or UF permeate as for the discontinuous DF was utilised, but added cont i nuously at the same rate as permeate flow to maintain the original volume of the 2 ×UF retentate throughout t he f iltration. At t he end of the DF, the retentate was further t reated t o reach a final volume con-centration factor of 4 × without adding DF medium (see Fig . 1B). It was hypothesised that t he two different t reatments would result i n differences in casein micelles dissociation, due to the different inter-particle interactions at 2×and4× concentration. 2.2. Separation of colloidal and dispersion phases The dispersion phases of mi l k and milk protein concentrates were obtained by ultracentri f ugation (Opt i ma L-80 XP, Beckman Coulter,Inc., Brea, CA, USA; rotor type Ti70; 23 mL t ube volume) at 100,000g for 1 h at 21°C [30,11]. 2.3. Protein analysis 酪蛋白 2.3.1. Casein content of retentates Total protein contents of skim milk, UF and MF retentates, and their centrifugal supernatants were determined by nitrogen analysis using a Gerhardt Dumatherm (C. Gerhardt GmbH & Co.KG, Konigswinter,Germany) and a conversion factor of N ×6.38. The protein contents referring to casein micelles (PcM) in skim mi l k and retentates were calculated by subtracting the protein content of their centrifugal su-pernatants (Psn) from the t otal protein content s (Protal): 2.3.2. Total protein content of permeates and milk serum Protein contents of permeate samples and milk serum were deter-mined using the Lowry method [36] with some modifications according to Markwell et al. [41]. Samples were diluted 1:100 (v/v) wi t h 0.1 mol/L NaOH and subsequent l y mixed 1:4 (v/v) with a freshly pre-pared solution of 20 g/L Na2CO3, 4g/L NaOH, 1.6 g/L sodium t artrate,10 g/L sodium dodecyl sulphate and 0.4 g/L CuSo4 × 5H2O. After i n-cubation at ambient temperature (~ 21 *C) for 30 min, 267 uL of sample were mixed with 20 uL of 50 % Fol i n-Ciocalteu phenol reagent and incubated for 45 min. Finally, the absorbance at 750 nm was measured using a Synergy 2 Multi-Detection Microplate Reader (BioTek In-struments, Inc., Winooski, VT , USA). A calibration curve was generated us i ng 10-100 ug/mL bovine serum albumin in 0.1 mol/L NaOH. 2.3.3. Reverse-phase high performance l iquid chromatography (RP-HPLC) RP-HPLC was carried out as described previously by Coskun et al.[11] to identify the ratios between the different casein and whey protein fractions in permeates and dispersion phases. A Jupiter 5u C4 column (Phenomenex Ltd., Aschaffenburg, Germany) was coupled to an Agi l ent 1260 Inf i nity II System (Agilent Technologies, Santa Clara, CA, USA)consisting of a binary pump including degasser (G7112B), an auto-sampler (G7129A), a column oven (G7116A) and a DAD detector (G7117C). 150 uL of sample were blended with 450 uL working solution (6 mol/L guanidine hydrochloride and 5.37 mmol/L sodium citrate dissolved in 0.1 mol/L Bis-Tris-propane buffer at pH 6.8) and 12 uL of 1 mol/L dithioerythritol. The mixture was incubated at room tempera-ture for 1 h and subsequently centri f uged at 18,000g and 7 °C for 10 min (Eppendorf centri f uge 5417R, rotor t ype 26814, Eppendorf AG,Hamburg, Germany) and filtered (Whatman Mini-UniPrep syringeless fi l ters, PTFE 0.2 um, GE Healthcare UK Limited, Little Chalfont, UK).20 uL of sample were injected and separated at 40°C and a flow rate of 0.7 mL/min using 0.5 mL/L t rifluoro acetic acid (TFA) in Milli-Q water and 0.5 mL/L TFA in acetonitrile as elution buffers, where t he ratio of the latter was raised from 33 % to 50 % within 25 min and t hen lowered from 50 % to 33% within 1 min. The eluted protein fractions were detected at 214 nm, and the peaks were assigned to the different casein types (glycosylated k-casein, non-glycosylated k-casein, os2-casein,B-casein, asi-casein) and whey proteins (B-lactoglobulin, o-lactalbumin)as was done previously [11]. The peaks were integrated using Chem-station (Rev. B.04.03; Agilent Technologies), and ratios between peak areas of different fractions were calculated. 2.3.4. Characterisation of non-sedimentable casein aggregates Cent r ifugal supernatants of mi l k and milk protein concentrates were subjected to size exclusion chromatography. A self-packed column (XK 16, Amersham Biosciences, Amersham, UK) containing a Sephacryl S-500 high resolution r esin (GE Heal t hcare, Uppsala, Sweden) at a bed height of 60 cm was integrated i nto an AKTA purifier system consisting of a P-900 pump, an IN-907 injection valve, and a UV-900 UV detector (GE Healthcare). The elution buffer contained 20 mmol/L Bis-Tris-propane and 0.2 g/L sodium azide at pH 7.15, and the samples were injected using a 1 mL sample loop. The separation was carried out at ambient temperature (~ 21°C) and an isocrati c flow rate of 1.0 mL/min. The eluting protein was detected at 280 nm, and t he data was collected and processed using the UNICORN software (v5.31; GE Healthcare). 2.4. Determination of total and soluble calcium Calcium analysis was performed according to Coskun et al. [11].Briefly, samples were diluted in 1 mol/L HCl (1:2 (v/v) for 4× con-centrates, 2:1(v/v) for all other samples), incubated a t 60°C for 1 h,and centrifuged at 10.000 ×g and 5 C for 10 min (Eppendorf centrifuge 5417R, rotor type 26814, Eppendorf AG). The clear supernatant was filtered and subjected to ion chromatography (DionexTM ICS-5000,Thermo Fischer Scientific, Waltham, MA, USA) equipped with cation exchange column (DionexTM Ionpoc CS16 3 × 250 mm) i n combination with a guard column (DionexTM Ionpoc CG16 RFIC 3 × 50 mm), a con-ductivity detector and an eluent generator (EGC 500 MSA). The eluent consisted of 48 mmol/L methanesulfonic acid and was run at 0.36 mL/min and 40 °C. The system was operated using a CERS-2mm suppressor at 51 mA. Calcium concentration was measured for skim milk and milk protein concentrates, their centrifugal supernatants, as well as for all permeate samples obtained f rom UF and MF. Colloidal calcium (i.e., calcium in the casein micelles; CacM) was calculated as the difference between t otal calcium (Catotal ) of skim milk or milk protein concentrates and calcium concentration of the respective supe r natants (Casn): 2.5. Static and dynamic light scattering Light scattering measurements were carried out using an ALV/LSE-5004 instrument (ALV-Laser Vertriebsgesellschaft mbH, Langen, Ger-many). Milk, 2× concentrates, and 4× concentrates were diluted 1:100, 1:200, and 1:400 (v/v) with filtered (0.45 um nylon; Frisenette ApS, Knebel , Denmark) UF permeate and transferred to borosilicate glass tubes (Lab Science ApS Danmark, Valby, Denmark), which were placed in a toluene bath at T=20°C and exposed to laser l ight at a=632.8 nm. Al l data was collected us i ng the ALV-Correlator Software (v3.0; ALV-Laser Vertriebsgesellschaft mbH). In dynamic l ight scattering (DLS), t he scattering intensity signal was recorded in cross-correlation mode at an angle 0=90°over a period of 60 s. To obtain the di f fusion coefficient Dr, the correlation function g(r) -1 was f itted assuming a single Schulz distribution as described by Mailer et al. [39]. The hydrodynamic radius (Rh) of the casein micelles was calculated according to the Stokes-Einstein-equation using a solvent viscosity of 1.14 mPa s (DHR 20, TA Instruments, New Castle, DE, USA;y =10/s) and a refractive index of 1.34 [4]. In static l ight scattering (SLS), the scattering intensity was measured at 0=50-150° in 10 ° intervals for 10 s per angle. Data were only used when three subsequent measurements deviated l ess than 5%. The radius of gyration (Rg) was obtained from the slope of the Guinier plot [21]. SLS data was treated with ALV/Static and Dynamic Fit and Plot (v4.42; ALV GmbH) to obtain the Rayleigh ratio as a function of the scattering vector modulus. 2.6. Small-angle X-ray scattering (SAXS) SAXS measurements were conducted using a NanoSTAR (Bruker AXS GmbH, Karlsruhe, Germany), modified according to Pedersen [51]. The instrument is a pinhole camera with two Gobel mirrors for mono-chromat i sing and focusing the beam, and a two-dimensional position--sensitive gas detector (Vantec 500) serves for data collection. The instrument was optimised to obtain a higher f lux and had a scatterless pinhole i n front of the sample [37]. The modif i ed camera i s installed on a powerful rotating-anode X-ray source (MacScience 6kW Cu with 0.1 ×0.1 effective source size operated at 4kW). The samples were analysed at two different sample-detector distances to cover a broad range of scattering vectors (q= 4nsin0/a, where 20 i s the scattering angle and is the X-ray wavelength): 1) 106 cm for q=4.0×10-3-2.3 ×10-1 A-1, acquisition t ime 20 min; and 2) 26 cm for q=1.1×10-2-9.7×10-1 A-1, acquisition time 10 min. T he raw spectra of skim milk, UF retentate, MF retentates, and centrifugal were corrected for the background signals following standard procedures [46]. For mi l k and retentates, the scattering from the corresponding centrifugal supernatants was subtracted, and for the centri f ugal super-natants, the scatter i ng from water was subtracted. The intensities were converted to an absolute scale and corrected for variations i n detector efficiency by normalising to the scattering of pure water [51]. The data from sett i ng 1 were scaled to that from set t ing 2 using a home-written program t that uses l i near interpolations and d a aweighted linear least-squares procedure for optimising the agreement for the overlap region within a scale factor and a constant. The same programme was used for scaling the data sets for di f ferent samples for comparisons. The data from a certain q-value and beyond were used for the scaling, as the di f ference occurs at low and i ntermediate q. When varying the value, a plateau is observed with a low value of the reducedx2 when the two data sets agree beyond this value. The reduced x? i s a measure of the devi -ation between a model fi t and the experimental data and close to unity for a perfect fit [50]. The q-value a t the start of the plateau was chosen for the scaling. 2.7. Small-angle scattering data modelling The SLS data was merged with the SAXS data using a scaling factor to extend the q-range. The data was f itted using the model recently intro-duced by Pedersen et al. [53], which extends previous models [22,27,64] over a broader q-range and expresses the scattering on an absolute scale and uses the concentrations of casein micelles and colloidal cal-cium phosphate as constraints. In brief, the "dirty snowball"model, i.e.,a large sphere embedding numerous smaller spheres (Fi g .S1), served as the starting point. Both the overal l casein micelle structure and the i n-termediate size variations, related to protein-dense clusters, are modelled after polydisperse spheres following Schultz distributions. On smaller length scales, the features attributed to the col l oidal calcium phosphate nanoc l usters are described as the scattering of oblate ellip-soids, whereas protein heterogenei t ies are modelled as six-armed star polymers with their intercorrelations taken i nto account using a partial structure factor [53]. At very high-q, another shoulder, att r ibuted to the correlations between polypeptide chains, i s fitted with a Lorentzian term. To describe hard-sphere interparticle correlations, an effective hard-sphere structure factor S(q) is i ncluded within a “local mono-disperse approximation"[49]. Although the effects of high concentra-t ions clearly appeared as a down-turn at t he low-q end of the SAXS data,only a few data points were influenced, which do not provide sufficient information for optimising the values of the effective volume f raction of the casein micelles through fit ti ng. Therefore, the effective volume fraction of the casein micelles was kept fixed at 0.15, 0.25, and 0.4 for skim mi l k, 2×UF retentate, and 4× MF retentates, respectively, as done in a recent study [63] and based on theoretical considerations.Instrumental smearing influenced the low-q data, and the model was therefore convoluted using ga a resolution function. The complete desc r iption of the model, smearing and fitting procedure can be found elsewhere [53]. The model depends on a number of parameters. In br i ef, the overall size of the casein micelles i s described by the number-averaged radius (Rz), i ts relative polydispersity (oz), and i ts effective volume fraction.The intermediate structures are described by their number-averaged radius (Rint), their relative polydispersity (Gint ), and the r atio of the overall forward scattering to that of the intermediate structures (nint ).The protein heterogenei t ies/particles are described by thei r molar mass (M(PP)), the contour l ength (L) and number (na) of the arms in t he polymer star structure, as well as a cross-section radius (Rys). The colloidal calcium phosphate nanoclusters are described by oblate ellip-soids with radius R, axis ratio e, and an expansion factor that describes the ratio between the actual density and a reference value taken from Ingham et al. [25]. Additionally, the parameter nsub describes the ratio of the overall forward scattering t o that of the subparticles. The correla-t ions between the protein particles and colloidal calcium phosphate nanoclusters are described by effective hard-sphere volume fractions and interaction radii. 2.8. Statistical analysis Al l samples were produced in three biological replicates. Six indi -vidual fresh mi l k batches from different weeks were used, meaning that two different sets of samples were produced from each batch of milk. Data were statistically evaluated using two-way analysis of variance and Tukey’s post hoc test using SigmaPlot v14.0.3.192 (Systat Sof t ware Inc., San Jose, CA, USA) to identify significant effects of DF t reatments (i.e., DF medium + DF modus) and DF stage. The statistical acceptance level was p < 0.05. 3.1. General characterisation of the concentrates The protein content referring to casein micelles was 28 ±1 g/kg for skim milk, 57 ±4 g/kg for 2× UF retentate, and in the r ange of 87±8-91 ±7 g/kg for the f i nal 4× MF retentates after complete DF,which is comparable to previous studies [10,59]. The concentration of casein micelles i n the final 4 × MF retentates was lower t han expected by a 4× volume reduction, i ndicating that foul i ng could indeed not be fully avoided by the oscillation movement of the chamber and some casein micel l es formed a gel layer on the membrane surface due to high viscosity and low shear forces at the high volume fraction. Furthermore,a higher ratio of non-sedimentable caseins (i.e., i n the centri f ugal su-pernatant) to casein micelles might be another cause for the l ow con-centration of casein micelles in the retentates. In fact, 23 ± 2-28±1 g/kg non-sedimentable proteins were f ound i n the f i nal 4×MF retentates after complete DF, and the peak area ratios observed i n RP-HPLC suggest that approx. 40-60 % of these proteins might be ca-seins. This would add up to approx. 100-104 g/kg casein in the final 4×MF retentates, meaning that approx. 10 % of the micellar casein disso-ciated into non-sedimentable casein aggregates, while another 10 %remained in the gel layer on the membrane. Tab le 1 summar i ses the apparent size (Rh and R) of casein micelles for the various treatments. It is important to note that, in spite of the di f ferent DF media, the samples were al l diluted i n UF permeate to allow measurements under comparable ionic milieu conditions. Rh, which corresponds to the radius of a sphere with equal di f fusion coef f icient, did not significantly differ between the treatments, consistent with previous reports [56,60,11]. Additionally to Rh, the Rg was determined, which is defined by the mass dist r ibution within a particle and is thus, in case of a sphere, smaller than i t s geometric radius. The ratio of Re to Rh charac-terises the shape of a part i cle, with 0.775 corresponding to i deal solid spheres and 1-2 to linear random coils [57]. Neither Rg nor Re/Rh were significantly affected by the concentration and milieu exchange, indi-cating that there was no irreversible change in the overall shape of the casein micelles. Rg/Rhn was ~ 0.85 and thus close to the theoret i cal value for solid spheres. In a field flow fractionation study using a calcium containing i midazole buffer as mobile phase, Glantz et al. [20] observed an Re/Rh of ~ 0.77 for the majority of casein micelles, with a small population showing greater values. However, it is important to note that caseins are calcium sensi t ive proteins, and that their shape and size will be affected by the calcium concentration in the dispersion phase. The difference to the present study can thus be attributed to t he different buffer system as wel l as the fractionat i on prior to the light scattering analysis. 3.2. Protein transmission during diafiltration 3.2.1. Whey protein depletion It was hypothesised that structural changes and protein dissociation may occur during concentration of t he casein micelles depending on the mi l ieu exchange conditions (i.e., DF medium and/or mode) and the volume fraction of the casein micelles. Hence, the protein concentration in the permeate was analysed, as whey proteins and some p-casein transmit through the membrane during the MF/DF process. T he proteins in the permeates were mainly o-lactalbumin and p-lactoglobulin with some p-casein also present, as concluded from RP-HPLC analyses (F i g . S2). There were only few statistically signi f icant differences be-tween the protein contents of permeates obtained at the different stages and from the different DF conditions (F i g.2). In general, t he permeate with the highest protein content was collected during discontinuous DF after 4 × concentration factor was reached for the first time; this was followed by a sl i ght but gradual decrease i n protein concentration with further treatment. In contrast, the protein concentration i n permeates from continuous DF remained stable during the process, wi t h a slight minimum before the beginning of the concentration step (MF permeate/skim milk ~ 0.5). Neither the DF mode (continuous or discont i nuous)nor the DF medium (water or UF permeate) had statistically significant effects on the protein contents of t he permeates. These results are in agreement with previous reports [58], who observed comparable re-ductions in p-lactoglobulin during DF wi t h di f ferent media, namely,demineralised water , softened water, tap water, or simulated mi l k ul-trafiltrate with lactose. F i g. 3 illustrates the protein concentration measured in the super-natants of skim milk and the different retentate samples after removing the colloidal fraction by centrifugation. This non-sedimentable fraction contains whey proteins as wel l as dissociated caseins [10,11] and was 19-24% of total protein for 2 × MF retentates and 20-30 % of total protein for 4× MF retentates, depending on the DF stage. The depletion of whey proteins during MF and the partial dissociation of casein mi-celles might compensate each other, leading to only smal l changes or even an increase in the concentration of non-sedimentable protein during the DF process. Assuming complete transmission of whey pro-teins during microfiltrat i on and no dissociation of the casein micel l es,the protein concentration of the dispersion phase of the retentates would remain constant during the concentration step and equal to that of the permeate, and then decrease with the addition of DF medium due to the dilution. Fig . 2 and Fig . 3 clearl y show that this was not the case, as the protein concentration in the non-sedimentable phase of the retentates was higher than that of the permeate fractions. This suggests that the whey protein transmission was l i mited due to concentration polarisation and fouling on the membrane, but also that protein aggregates too large to transmit the membrane were present in the dispersion phase, possibly resulting from the partial dissociation of the casein micelles. Neverthe-less, the protein concentration in the cent r ifugal supernatant slightly decreased with DF due to further transmission of proteins (F ig . 3). The relative depletion of whey proteins from skim milk was esti-mated from the RP-HPLC analysis by relating the peak areas found i n the centrifugal supernatants of the 4× MF retentates to those f ound i n the Table 1 Hydrodynamic radius (Rh), radius of gyration (R,) and ratio of Rg to Rh of casein micelles obtained f rom dynamic and static l ight scattering measurements for skim mi l k, ultrafiltration (UF) retentate, and final microfiltration (MF) retentates, after di f ferent diafil t ration (DF) treatments. Values represent averages of three replicates and their standard deviations. Di f ferent letters correspond to significant differences (p < 0.05) within the same row. Skim milk UF retentate MF retentate Demineralised water UF permeate Discontinuous DF Continuous DF Discontinuous DF Continuous DF Rh 96±2AB 95±2AB 95±3^ 97.±1^ 102±6B 101±7AB Rg 82±2^ 81±2^ 87±5^ 82±2^ 85±3^ 84±5^ Rg/Rn 0.85±0.04^ 0.85±0.03^ 0.92±0.08^ 0.84±0.01^ 0.84±0.07^ 0.83±0.06^ MF Permeate / Skim Milk (vol/vol) Fig. 2. Protein concentration i n microfiltration (MF) permeates at different stages of discontinuous (A) or continuous diafiltration (B) with demineralised water (i) or UF permeate (ii ). Values represent averages of three replicates and their standard deviations. Different letters correspond to significant differences (p <0.05) be-tween al l treatments at the same stage of diafiltration (capital letters) and along the filtration process within a treatment (lower case letters). original skim milk, after correct i ng for the volume concentration factor.Al l final 4 × MF retentates showed a whey protein depletion of approx.55%, regardless of the treatment. This value is lower than what was reported in previous studies using ceramic membranes, where 95-98%protein depletion could be reached using DF medium corresponding t o 1.33 volumes of the original milk in t wo steps of discontinuous DF of 3×concentrated skim milk [24,42]. However, a case study by Zulewska et al. [68] indicated that polymeric membranes may allow for a much lower whey protein transmission compared to ceramic membranes. The present work showed a higher whey protein depletion compared to another study using spiral-wound PVDF membranes, where a maximum reduction in whey proteins of approx. 20 % was obtained by one DF step of 3× concentrated skim milk [60]. The higher depletion values in the present study are the result of the different membrane system used, a vibrating plate-and-frame membrane module, which clearly allows for greater t ransmission of whey proteins. The oscillatory agitation of the filtration equipment decreased the concentration polarisation and fouling. I t is also important to note that Hartinger and Kulozik [23] reported a more ef f icient separation of whey proteins when using a 2 × milk protein concentrate as a starting material for the MF process, compared to using skim mi l k, as was done by Renhe et al. [60]. 3.2.2. Protein composition of the microfiltration permeates Differences in the protein composi t ion of t he permeates were measured using RP-HPLC. F ig. 4 shows the relative peak area r atios of β-lactoglobulin (circles) and p-casein (diamonds) to total protein. This information can help understanding whether different interactions occurred between whey proteins and caseins and which role the DF conditions played with regard to their transmission. β-lactoglobulin accounted for approx. 75 % of all protein i n the permeates (Fi g . 4), which is in good agreement with t he relative amounts of B-lactoglobulin the dispersion phase of bovine milk [14]. The relative peak area of β-lactoglobulin in the permeate showed a signifi-cant increase along the DF process, wi t h l i t tle to no stat i st i cally signif-icant differences between the DF t reatments. This means that the transmission of β-lactoglobulin relative to that of other proteins (i.e.,o-lactalbumin and B-casein) i ncreased along the DF process regardless of the DF conditions. Fi g. 4 also shows t hat an average of approx. 3-5% of t he t otal pro-tein area in the permeates was B-casein. Since the MF was carried out at 10 °C, some of the B-casein was dissociated from the casein micelles and separated from the retentates [3]. The extent of t ransmission of B-casein in the present study was lower compared to a previous study using skim milk as initial substrate [11]. This may be due to the l ower extent of DF,as wel l as the higher casein concentration in 2 ×UF retentate, creating a higher ratio of associated to monomer i c β-casein during cold storage prior to MF, even at temperatures below 10 °C [31]. In the l ast stage of DF, there was a significantly lower ratio of p-casein in the permeates obtained f rom continuous DF regardless of the DF medium, which might indicate a different gel layer formation on the membrane depending on the DF mode. 3.3. Formation of non-sedimentable casein aggregates in relation to the calcium equilibrium 3.3.1. Colloidal calcium Fi g. 5 shows the concentrations of colloidal calcium at each filtration step, calculated as the difference between the total calcium content and that in the centri f ugal supernant . When concentrat i ng skim milk (open symbol) to 2 × volume concentration factor by UF (MF permeate/skim MF Permeate / Skim Milk (vol/vol) Fig. 3. Protein concentration in centrifugal supernatants of microfiltration (MF) retentates at different stages of discontinuous (A) or continuous diafiltration (B) with demineralised water (i) or UF permeate (ii). Open circles refer to the protein concentration in the supernatant of skim milk. Values represent averages of three repl i cates and their standard deviations. Di f ferent letters correspond to signi f icant differences (p <0.05) between all treatments at the same stage of diafiltration (capital letters) and along the fil t ration process within a t reatment (lower case let t ers). milk=0.0), the colloidal calcium increased by a factor of two. Further f i ltration to 4× volume concentration factor (MF permeate/skim milk = 0.25) increased t he amount of colloidal calcium significantly,but to a lesser extent as what would be expected based on volume reduction. However, this is in agreement with the lower content of micellar casein i n the 4 × MF retentates (Sec t i on 3.1). The colloidal calcium relative to the protein content referred to casein micelles was not signi f icantly different between the 2× UF retentate (34±3 mg/g) and the f i nal 4×MF retentates (after DF) (32±10 mg/g-39±6 mg/g). These values differed f rom previous studies reporting a decreased calcium-to-casein ratio after DF with water [1,32,11], although Ferrer et al. [15] did not f ind an effect of DF on colloidal calcium either. I n addition, Gaber et al. [16] reported recently that DF of MCCs at a protein concentration of 80 g/kg did not change the total calcium content of the retentate. However, no information was given on the r atio between colloidal and soluble calcium. Since the dissolution of colloidal calcium phosphate is f acilitated at low temperatures, and the milk was stored and processed below 10°℃[26], it might be hypothesised that the effect of DF on colloidal calcium was low compared to that of the temperature. However, Coskun et al.[11] showed a decrease in colloidal calcium per casein upon DF with three times the volume of the initial milk, whereas only 0.5 volumes were used in the present study. This indicates that the extent of DF may have an effect on the colloidal calcium. 3.3.2. Soluble and permeable calcium F i g.6 il l ustrates the changes in soluble and permeable calcium along the filtrat i on process, expressed as calcium concentrations of t he cen-trifugal supernatants and permeates, respectively. The concentration of calcium in the permeate gradually decreased during DF with water due to the mil i eu exchange, whereas it remained relat i vely stable f or DF with UF permeate. If all calcium present in the dispersion phase could pass the membrane, the concentration would be the same for soluble and permeable calcium throughout the concentration of casein micelles.However, the concentration of soluble calcium i ncreased approx. 1.5times upon concentration from 2 × to 4 × volume concentration factor,indicating the retention of some soluble calcium that is bound to non-permeable, non-sedimentable proteins, as was also reported i n previ-ous studies [67,11]. Redilution to 2 × volume concentration factor in both steps of the discontinuous DF reduced the concentration of soluble calcium by a factor of two i n case of water addition (Fi g . 6Ai ), and to a level between the calcium content of the UF permeate and the soluble calcium concentration of 4× retentate when adding UF permeate (F ig . 6Ai i ). 3.3.3. Formation of non-sedimentable casein aggregates during diafiltration MF Permeate / Skim Milk (vol/vol) equal (0.85 L/(g cm) and 1.046 L/(g cm), respectively [65,38]), the peak maxima as wel l as peak area ratios give a good i ndication for the changes in the composition of the dispersion phase. The peak intensity of the casein aggregates increased f rom ~ 15 mAU in 2× UF retentate (F ig . S 3) to ~ 80 mAU upon the f i rst concentration to 4× in discont i nuous DF (F ig. 7A, f ull orange line;Fi g . S 3A), more than twice of what would be expected from a two-fold volume reduction. These results demonstrated that caseins dissociated from the micelles and were associated with calcium, as suggested by the increase in soluble calcium concentration (F ig . 6A ). Remarkably, the peak ratio of caseins to whey proteins was higher for the centrifugal supernatants of the 4 ×MF r etentates than for those of the corresponding 2× MF retentates after adding the DF medium (Fi g . 7A), which was in agreement with the respective peak area ratios obtained from RP-HPLC (data not shown). This means that di l ution of the 4× MF retentate to 2 × volume concentration f actor with both water and UF permeate caused a reduction i n the amount of the non-sedimentable casein. As l it t le to no casein could be detected in the MF permeates by RP-HPLC (F ig . S2), this would suggest a reassembly of the non-sedimentable caseins with the casein micelles rather than dissoci-ation into individual molecules that would pass the MF membrane. After concentrating the diluted MF retentates once again to 4×volume concentration f actor, the height of the casein peak (75-90 min)increased by almost a factor of two compared to the f irst concentration step in the case of discontinuous DF with water (Fi g . 7Ai; F i g . S 3A i ),whereas this was not the case for discontinuous DF with UF permeate (F ig . 7Aii ; Fi g . S3Ai i ). This confirms previous studies showing that a decrease in ionic strength t hrough milieu exchange enhances the dissociation of casein micelles upon concentration [33]. In contrast, the casein micelles were more resistant against disruption when the ionic equilibrium was maintained. The final concentration step did not further increase the intensity of the aggregate peak, which i s consistent with a previous study, where DF was carried out at 2×volume concentration factor [11]. The increase in pH upon DF with water (T a b le S1) might be an additional cause for the dissociation of caseins i nto t he dispersion phase [2]. However , the strong increase in non-sedimentable caseins with increasing volume concentration factor from 2 × to 4 × without changes in pH indicates that the concentration process i t self also con-tributes to the partial disintegration of the casein micelle. The diafi l t r ation mode did not affect the formation of non-sedimentable aggregates in case of DF wi t h UF permeate, whereas there were differences between continuous and discontinuous DF with water. During continuous DF at 2× volume concentration factor, the aggregate peak barely changed compared to that of the 2 ×UF retentate.After the final concentration step, i ts height increased to ~ 80 mAU and ~ 50 mAU for DF with water and UF permeate, respectively (F i g. 7B;Fi g. S3B ). Noteworthy, the levels of soluble and permeable calcium at the various stages of the fi l tration did not differ between the DF modes,meaning that the differences in the aggregate peak were not due to different calcium equilibria. I t was concluded that continuous DF with water at a low volume concentration factor caused less disruption of the casein micelles compared to discontinuous DF. 3.3.4. Composition of non-sedimentable casein aggregates The composition of the non-sedimentable casein f raction was ana lysed by RP-HPLC as area ratios from the respective peaks of os1-, αs2-, MF Permeate / Skim Milk (vol/vol) p-, and total and glycosylated k-casein (F i g.8). All casein t ypes were present in this fraction, with no significant differences in the area ratio between DF with water or permeate. However, compared to the overall casein composition of the skim milk used, a higher ratio of k-casein and a lower ratio of osi-casein was found. This could be explained by a partial collapse of the k-casein layer of the casein micel l es due to the close in-teractions at high volume fractions and a subsequent release of some k-casein into the dispersion phase. On t he other hand, asi-casein is known to be more abundant in the interior of the casein micelles through interactions with colloidal calcium phosphate, and might t hus dissociate less into the dispersion phase compared to p-and k-casein. These data are in good agreement with those reported previously for MF retentates at 4 × volume concentrat i on factor after extensive DF with water [11],but demonstrate that the DF medium has no effect on the casein composition in the dispersion phase. 3.3.5. Small -angle X-ray scattering of centrifugal supernatants Fi g. 9 shows the SAXS data of the cent r i f ugal supernatant s of skim milk (B), 2× UF retentate (C), and 4× MF retentate after complete discontinuous DF wi t h demineralised water (D) or UF permeate (E) i n the r ange of q=4.0×10-3-2.3×10-1 A-1. Only water and capillary scattering have been subtracted f rom the data, allowing the comparison to the signal of their background, namely UF permeate (A). The scat-tering of all centr if ugal supernatants was clearly different from that of the UF permeate due to the presence of proteins, underlining the importance of subtracting the correct background when studying casein micelles in different colloidal systems by small-angle scattering techniques. The scattering of the supernatants of 2× UF retentate (Fi g . 9C)showed a higher intensity than that of skim milk, whereas the shape of the pattern was very similar. This indicates a higher concentration but similar composition and structure of the scatterers, as 2× volume reduction by UF resulted in a concentration of whey protein i n the dispersion phase with li mited dissociation of the casein micelles [11].In contrast, the scattering patterns of the cent r ifugal supernatants of 4 ×MF retentates (Fi g. 9D + E) showed clearly different shapes in addi t ion to a higher scattering intensity. Due to the greater contribution of non-sedimentable caseins to the scattering, there was a clear shoulder emerging at q~ 0.01-0.02 A-1, similar to what has been reported for sodium caseinate particles in the presence of 11 mmol/L CaCl2 [62] and 250 mmol/L NaCl [54]. This indicates that non-sedimentable caseins were present i n the form of aggregates. The scattering of the centrifugal supernatants was comparable for all 4× MF retentates. However, the shoulder was more pronounced in the case of DF with water (Fi g. 9D)than for DF with UF permeate (F i g. 9E), in agreement wi t h the higher number of non-sedimentable casein found in these supernatants (F ig .7A). 3.4. Effect of the diafiltration conditions on the internal structure of casein micelles I t was hypothesised that concentration and DF will modify the in-ternal structure of casein micelles due to internal rearrangements caused by t heir partial dissociation and demineralisation. Therefore, all reten-tates were studied using SAXS and SLS. In case of SAXS, the scattering patterns were background subtracted for their corresponding centrifugal MF Permeate / Skim Milk (vol/vol) Fig. 6. Calcium concentration of microfi l t ration (MF ) permeates (black closed circles) and centri f ugal supernatants of retentates (grey closed circles) at different stages of discontinuous (A) or continuous diafi lt ration (B) wi t h demineralised water (i) or UF permeate (ii ). Calcium concentration of UF permeate (open black circle,dashed-dotted l ine) and centrifugal supernatant of skim milk (open grey circle) are shown f or compar i son. Values represent averages of three replicates and t heir standard dev i ations. Different l etters correspond to signi f icant differences (p < 0.05) between all treatments at t he same stage of diafil t ration (capital letters) and along the fil t ration process within a treatment (lower case letters). supernatants (see F ig. 9), whereas only water was subtracted in SLS measurements, as the samples were heavi l y di l uted in UF permeate and the scattering of this solvent was negligible compared to t hat of the casein micelles. 3.4.1. Changes in casein micelle scattering during diafiltration Fi gs . 10-12 show the SAXS patterns of the casein micelles i n all UF and MF retentate samples, compared t o t he i ntensity of casein micelles in skim milk (grey symbols) by scaling the scattering curves to that of casein micel l es in skim mi l k. Quali t at i vely, no difference between the scattering of casein micelles in skim milk and 2×UF retentate could be noticed, as shown in F ig . 10. The first concentration to 4 × volume concentration factor without DF did not change the scattering data either (F ig . 11A, F i g. 12A; lower black symbols), except f or slightly lower intensities at low-q (q <0.006 A-1) due to interparticle i n-teractions at the higher volume fraction. Bouchoux et al. [5] reported similar effects, and found changes at q>0.006 A-1 only for casein concentrations of 150 g/L or higher (volume concentration f actor ≥ 6),which were attributed to the deformation and deswelling of casein micelles. Fi g . 11 and Fig . 12 also show t he scattering for the MF retentates prepared with different DF modes and media. The addition of water i n both discontinuous and continuous DF caused the appearance of a bump at intermediate q(~ 0.007-0.02 A -1), which i ncreased further with the second DF step (F ig . 11A,B; blue symbols). In contrast, DF with UF permeate did not alter the qualitative scat t ering pattern at any stage of the process regardless of the DF mode (Fig . 12A ,B). The increased scattering intensity at intermediate q points to modi f ications of the internal structure of the casein micelles at length scales of about 50 nm,which cause a higher contrast between these structural elements and the overal l casein micel l es and hence a higher contribution to the overall scattering. This could be due to a decrease in t he homogeneity of these structures. Remarkably, DF did not affect the shoulder observed at q~0.05-0.08 A -1, which is believed to reflect contributions of scat-tering from both colloidal calcium phosphate and protein heterogene-ities at length scales of approx. 1.5 nm [53]. Changes in t his q-range were previously attributed to modifications of the colloidal calcium phosphate nanoclusters in the casein micelles, usually due t o calcium dissociation [40,45]. T he unaltered scattering pattern i n this q-range is therefore in line with the minimal changes in colloidal calcium observed in the present study (see F ig. 5). Interestingly , Bouchoux et al. [5] compared the SAXS patterns of casein micelles in fresh and pasteurised milk to that of MCC redispersed in UF permeate, and concluded that they exhibit identical structures. It might be possible that the structural changes can be r everted to a certain extent when the original ionic milieu i s re-establ i shed by, e.g.,dispersing MCC powder in UF permeate. This can, however, not be concluded with certainty from the present study and should be investi-gated i n future work. 3.4.2. Modelling of small-angle scattering of casein micelles In order to obtain a more quantitative i nsight into t he changes of the casein micelle structure, the SAXS data of skim mi l k, 2 ×UF retentates,and the 4× MF retentates after complete DF were merged wi t h the respective SLS data using a scale factor and fitted with the newly developed model of Pedersen et al. [53]. F i g. 13 illustrates the SLS and Retention Time (min) Fig.7. Size exclusion chromatograms of cent r ifugal supernatants of microfiltration (MF) retentates (dashed l ines: 2 × volume concentration factor; full lines: 4×volume concentration factor) at different stages of discontinuous (A) or cont i nuous diafiltration (B) with demineralised water (i) or UF permeate (ii). MF permeate/skim mi l k (vol/vol) was 0.25 (orange), 0.5 (blue) or 0.75 (black). Curves are averages of three i ndependent experiments. Fig. 8. Casein composition of skim mi l k (empty bars) and centrifugal super-natants of final 4 × microfiltration retentates produced by discontinuous dia-fi l tration (DF) with demineralised water (hatched bars) or ul t rafiltration permeate (ful l bars) expressed as area ratios of as1-, as2-, β-and k-casein to total casein, and area ratio of glycosylated k-casein to total k-casein. Values represent averages of three replicates and their standard deviations. Different capital letters indicate statistically significant differences i n composi t ion between the samples (p <0.05). SAXS intensities of the six samples as a function of q. The apparent discrepancy between the data obtained from the two techniques i s pri-marily due to the di f ference in concentration, as SLS was obtained f rom very dilute samples, and also due to i nstrumental smearing and beam-stop shadowing of the SAXS equipment at l ow-q. The smearing and beamstop shadowing was corrected i n the fits by inclusion of a r esolu-tion function according to establ i shed procedures [48,51,52]. The fits that include the instrumental smearing are in good agreement with the q(A) Fig. 9. Small-angle X-ray scattering of ul t raf i ltration (UF ) permeate (A) and centri f ugal supernatants of skim milk (B), 2×ultraf i ltration retentate (C), and f inal 4 × microfiltration retentates after discontinuous diafiltration with dem-ineralised water (D) or UF permeate (E). The scattering intensities were cor-rected for water and capi l lary background signal. experimental SAXS data (orange l ines), demonstrating the val i dity of the model. The non-smeared model also f i ts the SLS data well (full blue lines), showing the typical levelling off at low-q in the Guinier region. As SLS was conducted on di l uted samples, the effect of the structure factor (dashed blue lines) was not i ncluded for the SLS data . The apparent j ump in the non-smeared model f it between the SLS and SAXS data is due to the exclusion of the structure factor for SLS and inclusion of i t for SAXS. Four main regions in the scat t ering patterns can be i dentified [53]: Fig. 10. Small-angle X-ray scattering data of bovine casein micelles i n skim milk (grey symbols) and 2 ×ultrafi l tration retentate (blue symbols). Scattering intensities were background corrected for water, capi ll ary, and their centrifugal supernatants, and scaled to that of casein micelles in skim mi l k. Note: For most parts, the size of the symbols in the f igure are larger than the error on the data. 1) q <0.006 A -1: Related the overall casein micelle size; 2)q~0.006-0.03A -1: Related to the intermediate structures, i.e.,protein-rich regions and voids/low-density regions in the casein micelles; 3) q~0.03-0.4 A -1: Related to the “subparticles", i ncluding colloidal calcium phosphate, protein heterogeneities, and their correlations; 4) q>0.4 A-1: A shoulder/broad peak, most likely related to correla-tions between polypeptide chains. While the first two structural levels are in agreement with what was suggested by, e.g., Bouchoux et al. [5] and Ingham et al. [25], the interpretation of the third level as contributions from both colloidal calcium phosphate and protein heterogeneities deviates f rom what has been assumed before, namely that the feature is solely due to colloidal calcium phosphate [5] or protein heterogeneit i es (i.e., interacting pro-teins regions) [12,25]. However, i t has been shown t hat i ndeed both protein and colloida l calcium phosphate contribute signif i cantly to the scattering in thi s region [53]. The fourth region has not been described before, as only very few studies on casein micelles were conducted at such high-q, but without attempt i ng to explain the physical meaning of Fig. 11. Smal l -angle X-ray scattering data of bovine casein micelles i n microfiltration (MF)retentates (blue symbols: 2 × volume concen-tration f actor; black symbols: 4 × volume con-centration factor)at different stages of discont i nuous (A) or continuous diafiltration (B) with demineralised water. Italic numbers refer to the stage of diafi l tration (i .e., MF permeate/skim mi l k (vol/vol)). Scattering i n-tensities were background corrected for water,capil l ary, and their centr i fugal supernatants,scaled to that of casein micelles in skim milk (grey symbols), and pairwise shifted along the i ntensity-axis for better distinction (shift factor a=1, 5, and 25 for MF FI permeate/skim milk = 0.25, 0.50, and 0.75, respec t ively).Note: For most par t s, the size of the symbols i n the figure are larger than the error on the data. q(A) Fig. 12. Small-angle X-ray scattering data of bovine casein micelles in microfiltration (MF)retentates (blue symbols: 2×volume concen-tration factor; black symbols: 4× volume con-centration f actor) at di f ferent stages of discont i nuous (A) or continuous diafiltration (B) with ultrafiltration permeate. Italic numbers refer to the stage of diaf il tration (i .e., MF permeate/skim mi l k (vol/vol)). Scatte r ing i n-tensities were background cor r ected f or water,capil l ary, and their centri f ugal supernatants,scaled to that of casein micelles i n skim milk (grey symbols), and pairwise shi f ted along the intensity-axis for better distinction (a=1,5,and 25 for MF permeate/skim milk =0.25,0.50, and 0.75, respectively). Note: For most parts, the size of t he symbols in t he figure are larger than the error on t he data. the scattering (e.g.,[43,44]). Ta ble 2 summarises the resul t s from f i tt ing the scattering data of the six samples using the model to evaluate potential effects of t he diafil-tration medium and mode. Note that some parameters were fixed during the fitting as indicated i n t he t able. The intensity-weighted, geometric radius of the casein micelles (=√5/3R. for spheres) was relatively s i mi l ar for all samples, although slightly higher values were found for the 4 × MF retentates discontinuously diafi l tered with water. However,it is important to point out that both Rh and R, determined i n DLS and SLS experiments were found not to be affected by the f iltration (Ta bl e 1). The most obvious differences between the samples occurred i n t he values estimated for the intermediate structures. It can be seen that UF of skim milk to 2× volume concent r ation factor did not affect the intensity-weighted radius and nint, meaning that no changes in the i n-ternal structure occurred with this treatment (T ab l e 2). However, the 4 × MF retentates showed larger radii and lower nint , where the changes in nint were greater for DF with demineralised water. The l atter means a greater contribution of the intermediate structures to the overal l scat-tering of the casein micelle, as was also visible from the appearance of a bump at intermediate q in Fi g . 11. As both discontinuous and continuous DF with water caused dissociation of casein molecules into the disper-sion phase (F ig . 7), it is l ikely that this, i n combination with the milieu exchange , resulted in some internal rearrangements of the casein mi -celles, which changed the conformation of the intermediate structures towards larger and perhaps less homogeneous ent it ies. The latter could possibly be due to the removal of low-density material f rom the voids between the protein-rich regions and/or the removal of material from low-density regions in the casein micelle. Interestingly, even though the qualitative scattering patterns did not indicate changes i n the casein micel le structure when DF was conducted with UF permeate (F ig . 12), the results of the modelling suggest modi-fications of the internal structure also when using this medium (T a bl e 2).nint decreased compared to skim milk, but t o a lesser extent than for 4 ×MF retentates diaf i ltered with demineralised water. Furthermore, the intensity-weighted radius was larger for all the 4× MF retentates compared to 2×UF and skim milk. The polydispersity became too large during the f i t compared to what can be considered a reasonable value (i.e., larger than 0.7) and was therefore f ixed to 0.7. These changes in t he casein micelle structure are probably related to the par t ial dissociation of casein micelles with concentration up to 4 × volume concentration q(A) Fig. 13. Shifted scattering data from bovine casein micelles i n skim milk (A; shift factor a=1), 2× ultrafiltration (UF) retentate (B;a=10), and final 4× microfi l tration (MF)retentates (i .e., MF permeate/skim mi l k (vol/vol)=0.75) after discontinuous diaf i ltration with demineralised water (C; a =100), contin-uous diaf i ltration with demineralised water (D;a=2000), discontinuous diafiltration with UF permeate (E; a=40,000), or continuous diafil-tration with UF permeate (F; a=800,000).Black circles show the shi f ted data f rom static light scattering (SLS; q=0.001-0.0025A-1),and smal l -angle X-ray scattering data (SAXS;q =0.004-1.0 A -1) after subtracting the signals from the centrifugal supernatants, water, and capillary; orange curves show the f i t including structure factor and i nstrumental smearing;blue curves show the i deal, non-smeared f it with the structure factor omi t ted in q=0.001-0.0025A -1 (full l ines), or with structure factor extrapolated into the range of the SLS data (dashed l ines). Note: SLS data were obtained on di l uted samples and the structure factor was therefore not included i n the model curve (f ull blue l i ne), leading to a jump between the SAXS and SLS data. For most parts, the size of the symbols in the figure are larger t han the error on the data. factor (Fi g . 7). The value nsub was i n the same range for skim milk, 2×UF retentate,and continuously diaf il tered 4 ×MF retentates, but higher for discon-tinuously diafi l tered MF retentates, with discontinuous DF with water showing t he highest values (T a ble 2), meaning that the subparticles (i.e.,colloidal calcium phosphate and protein heterogeneit i es) contributed less to the overall scattering. As the concentration of colloidal calcium barely changed throughout the fi l tration process (F ig. 5), this was probably rather related to rearrangements of the proteins at length scales of approx. 1.5 nm towards more homogeneous conformations.Even though colloidal calcium phosphate and protein heterogeneities contributed both to the same feature in the scattering patterns, i t was possible to derive some characteristics for each of them through t heo-retical considerations. The colloidal calcium phosphate nanoclusters are described as el l ipsoids, and neither thei r core radius (R) nor their aspect ratio (e) were markedly affected by the fi l tration treatments. However,the expansion of the colloida l calcium phosphate particles relative to the reference value was higher for 2 × UF retentate and 4 ×MF retentate diafi l tered with water than i t was for skim mi l k, whereas i t was lower for 4× MF retentates obtained from DF with UF permeate. The protein heterogeneities were approximated as six-armed star polymers in the scattering model [53], and L and M(PP) were determined for all samples.While L was similar for all samples,M(PP) increased with the increase to 4 × volume concentration factor, and was higher for samples prepared by DF with UF permeate. This may be explained by the proteins in the casein micelles becoming slightly closer to one another at higher volume concentration factor, which was more pronounced for DF with UF permeate due to t he lower extent of casein dissociation compared to the samples diafiltered wi t h demineralised water (see Fig . 7). The Lorentzian f it, which describes the feature at q> 0.4 A-1, did barely differ between the samples with regard to width and position of the shoulder on the q-axis (Ta bl e 2), indicat i ng that the correlations between the polypeptide chains were not affected by any of the treatments. One drawback in the current study is that the low-q data that describe the overall casein micelles could be obtained only from SLS after diluting the samples. While Ingham et al. [25] could fit t heir SAXS data obtained from reconst i tuted n mi l k over a q-range of 0.0015-0.24 A-1 with a model comprised of four components (casein micelles, intermediate structures, colloidal calcium phosphate, protein heterogeneities) by assuming diluted solutions of casein micelles, Smith et al. [63] demonstrated recent l y that this model does not s uf f icientl y f it SAXS data obtained at q~ 0.0003-0.0015 A-1 without additionally including attractive interparticle interactions (“sticky spheres"), which accounts for a higher intensity as q approaches zero. Within the measured range of the SLS data we did not observe a significant devia-tion between the data and our model , and a sticky hard-sphere structure factor was therefore not used. Furthermore, being batch techniques,SAXS and SLS do not allow to evaluate different populations of particles in a sample separately. The polydispersity of the casein micelles [61]was taken into account for the mathematical model, and small proteins and aggregates were eliminated by subtracting the signal f rom the centrifugal supernatants, whereas other supramolecular structures than the casein micelles, as, e.g., observed using separating techniques such as field f low fractionation [20], were not taken into account. However,these structures can be expected to appear i n very low concentrations compared to the casein micelles [20] and were thus neglected in the calculations. In any case, the application of the model has proven to be a powerful tool for characterising the internal structure of the casein mi -celles, which was the primary aim of this study. 4. Conclusions This study presents a detailed invest i gation of the effects of milieu Results from mod e lling t he SAXS and SLS data of casein micel l es in skim mi l k, ultrafiltration (UF) retentate, and final microfilt r ation (MF) retentates after different diafiltration (DF) treatments. Values shown are fit results ± standard error; * indicates parameters that were fixed f or t he calculations. Parameter Symbol Skim milk UF retentate MF retentates Demineralised water UF permeate Casein Micelles P(q) Discontinuous DF Continuous DF Discontinuous DF Continuous DF Number-Averaged Radius (nm) Rz 45.5±2.4 42.3±1.6 47.8±4.4 37.3±5.4 33.4±2.3 32.5±2.6 Intensity-Weighted Radius (nm) 117.8 116.2 130.2 123.9 125.4 119.2 Polydispersity (-) OL 0.49±0.02 0.52±0.02 0.52±0.05 0.60±0.07 0.65±0.03 0.64±0.04 *Concentration (mg/mL) 29.8 59.7 91.4 94.2 92.6 95.2 *Relative Protein Content (-) 0.95 0.95 0.95 0.95 0.96 0.96 Casein Micelles S(q) *Scaled Hard Sphere Radius (-) 1.00 1.00 1.00 1.00 1.00 1.00 *Effective Volume Fraction (-) Intermediate Structures 0.15 0.25 0.40 0.40 0.40 0.40 Number-Averaged Radius (nm) Rint 10.4±0.7 9.7±0.4 12.9±0.3 13.8±0.3 7.3±0.2 7.6±0.2 Intensity-Weighted Radius (nm) 21.5 20.7 28.0 29.7 30.4 31.6 Polydispersity (-) Bint 0.41±0.05 0.42±0.03 0.41±0.02 0.42±0.02 *0.7 *0.7 Relative Forward Scattering 刀int 567±64 518±39 162±19 93±15 345±48 268±47 Sub-Particles (= Colloidal Calcium Phosphate + Protein Heterogeneities) Relative Forward Scattering nsub 35,710±2515 33,240±1658 46,100±2947 30,800±1359 39,460±1473 33,230±1879 Sub-Particles Partial Structure Factor S(q) between Proteins and between Protein and Colloidal Calcium Phosphate Hard Sphere Radius (nm) 3.85±0.06 3.90±0.03 3.84±0.04 3.73±0.4 3.88±0.04 3.87±0.03 Effective Volume Fraction (-) 0.25±0.02 0.24±0.01 0.24±0.02 0.22±0.1 0.23±0.02 0.23±0.01 Colloidal Calcium Phosphate Core Radius (nm) R 2.50±0.06 2.38±0.04 2.32±0.06 2.36±0.05 2.39±0.05 2.32±0.04 Aspect Ratio of Ellipsoids (-) e 0.39±0.03 0.41±0.02 0.43±0.03 0.42±0.03 0.43±0.03 0.44±0.02 Expansion (-) 0.76±0.06 0.83±0.05 0.85±0.08 0.83±0.07 0.61±0.04 0.65±0.04 *Density (-) 2.31 2.31 2.31 2.31 2.31 2.31 *Electron Density (-) 0.71 0.71 0.71 0.71 0.71 0.71 Colloidal Calcium Phosphate Partial Structure Factor S(q) *Hard sphere radius (nm) 10.0 10.0 10.0 10.0 10.0 10.0 *Effective volume fraction (-) 0.2 0.2 0.2 0.2 0.2 0.2 Protein Heterogeneities *Number or Arms (-) 6 6 6 6 6 6 Length of Arms (nm) La 5.14±0.35 4.79±0.21 4.58±0.23 4.77±0.20 4.78±0.20 4.66±0.16 *Cross-Section Radius (nm) Rxs 0.7 0.7 0.7 0.7 0.7 0.7 Molar Mass (g/mol) M(PP) 13,940±1667 12,360±662 13,310±809 14,900±805 15,060±909 15,230±648 Lorentzian Fit Scale (×10-3) 1.69±0.16 3.23±0.14 6.14±0.31 6.46±0.27 5.71±0.29 6.00±0.24 Width (A-1) 0.17±0.03 0.16±0.01 0.20±0.02 0.19±0.02 0.16±0.02 0.17±0.01 qCentre (A-1) 0.56±0.01 0.57±0.00 0.55±0.01 0.55±0.00 0.57±0.01 0.57±0.00 Goodness of Fit x(-) 7.72 5.56 6.54 9.60 7.73 7.09 exchange by DF on the structural integrity of casein micelles concen-trated by membrane filtration. The DF conditions were carefully compared by varying them systematically to draw conclusions on the effects of mi l ieu exchange and volume f raction on changes of t he i n-terna l structure of the casein micelles in concentrated systems. The DF medium (water vs. UF permeate) barely affected the transmission of whey proteins during the MF, l eading to the same degree of whey pro-tein depletion for all samples i n spite of the different i onic strengths of the soluble phase. DF with water decreased the amount of permeable calcium, whereas the overall soluble calcium was l ess affected, pointing to its association to non-sedimentable proteins. Due to t he rather low extent of DF, the amount of colloidal calcium bound to the casein mi-celles was barely affected. Non-sedimentable caseins were found i n the dispersion phases of al l MF retentates, but to the greatest extent after discontinuous DF with water, whereas DF with UF permeate caused considerably less dissociation. This demonstrates the i mportance of milieu exchanges for partial dissociation of casein micelles at high vol-ume fractions. Remarkably, evidence was found that non-sedimentable caseins reassociate with the casein micelles upon dilution of the 4 ×concentrates. The smal l-angle scattering patterns of t he casein micelles revealed changes in their internal structure by DF with water, which was conf i rmed by f itting the data with a model t hat describes t he scattering of casein micelles over a broad q-range and on absolute scale. I n contrast, the structure of the casein micelles was much less affected upon DF with UF permeate. Concentration of casein micelles using water as the DF medium to reach high protein/sol i ds ratio affects t he structure and integrity of casein micelles, possibly with consequences on the techno-functional properties of the casein ingredients. CRediT authorship contribution statement Norbert Raak: Conceptualization,Methodology, Validation, Formal analysis, Investigation, Data curation, Visual i zation, Writing-original draft. Jan Skov Pedersen: Methodology, Software, Resources, Writing-review & edi t ing. Milena Corredig: Conceptualization, Methodology,Resources, Supervision, Project administration, Funding acquisition,Writing- review & editing. Funding This work was funded by the Danish Dairy Research Foundation. T he support of Arla Foods amba and the Centre for Innovative Food Research (CiFOOD) at Aarhus University i s also greatly acknowledged. Data were generated through accessing the research i nfrastructure at Aarhus University. Declaration of Competing Interest The authors declare that they have no known compet i ng f i nancial interests or personal relationships that could have appeared to influence the work reported in this paper. Data Availability Data wi l l be made available on request. Acknowledgements The authors would like to acknowledge Mette Bisgaard Jensen, Gi t te Hald Kristensen and Rita Albrechtsen (Department of Food Science,Aarhus University, Denmark), Carsten Pedersen (Department of Chem-istry, Aarhus University, Denmark), and Betina Bianca Hansen and Saeed Rahimi Yazdi (Ar l a Innovation Centre, Arla Foods Amba,Denmark) for their assistance in the experimental work. Appendix A. Supporting information Supplementary data associated with this article can be found i n t he online version at d o i :10.1016/j.c ol s ur f a .2023.131580. References [1] M. Alexander, M.-P. Nieh, M.A. Ferrer, M. Corredig, Changes in the calcium cluster dist r ibution of ult r afil t ered and diafil t ered f resh skim milk as obse r ved by small angle neutron scattering, J. Dairy Res. 78 (2011) 349-356, h t tps ://d o i.or g /10.1017/S O 022029911000409. [2] S.G. Anema, H. Klostermeyer, Heat-induced, pH-dependent di s sociation of casein micelles on heating reconst it uted skim milk at temperatures below 100 °C, J. Agric.Food Chem. 45 (1997) 1108-1115, h t t ps://d o i .o r g/10.1021/j f 960507m. [3] Z. Atamer, A.E . Post, T . Schubert, A. Holder, R.M. Boom, J. Hinr i chs, Bovine B-casein: isolation, properties and f unctionality. A rev i ew, Int. Dairy J. 66 (2017)115-125, htt ps://do i .or g/10.1016/j .i da i ryj .2016.11.010. [4]C.M. Beliciu, A. Sauer, C.I . Moraru, The effect of commercial sterilization r egimens on micellar casein concentrates, J. Dairy Sci . 95 (2012) 5510-5526,h t t p s://doi.org/10.3168/jd s .2011-4875. [5] A . Bouchoux, G . Gesan-Guiziou, J. Pé r ez, B. Cabane, How to squeeze a sponge :case i n micelles under osmotic stress, a SAXS study, Biophys. J. 99 (2010)3754-3762, h t t ps ://do i .or g/10.1016/j.b pj.2010.10.019. [6]C. Broyard, F. Gaucheron, Modificat i ons of st r uctures and f unctions of caseins: a scient if ic and technological challenge, Dairy Sci. Technol. 95 (2015) 831-862,htt ps://d o i .o r g/10.1007/s 13594-015-0220-y . [7] B.G. Carter, N . Cheng, R. Kapoor, G.H. Meletharay i l , M.A. Drake, MF -derived casein and whey proteins from milk, J. Dairy Sci. 104 (2021) 2465-2479, h tt ps ://doi .org /10.3168/j d s .2020-18811. [8]M.V. Christiansen, G.N. Smith, E.S. Brok, M. Schmiele, L. Ahrne, The relationship between ultra-smal l -angle X-ray scattering and viscosity measurements of casein micelles i n skim mi l k concentrates, Food Res. Int. 147 (2021), 110451, h tt p s ://doi.org /10.1016/j .f ood r es.2021.110451. [9] M. Corredig, P. Krishnankutty Nair, Y. Li , H. Eshpar i , z. Zhao, Understanding t he behavior of caseins in mi l k concentrates, J. Dairy Sci. 102 (2019) 4772-4782,h t t ps://d oi.o r g/10.3168/j d s.2018-15943. [10]]O. Coskun, N . Raak, M. Corredig, Heat i nduced i nteractions in whey protein depleted milk concentrates: compar i son of ultraf i ltration and microfiltration, Food Hydrocoll . 137 (2023), 108354, h t t ps://do i .o rg/10.1016/j .food h yd.2022.108354. [11]]O. Coskun, L . Wiking, S. Rahimi Yazdi, M. Corredig, Molecul a r details of t he formation of soluble aggregates during UF, MF combined with DF of skim milk,Food Hydrocoll. 124A (2022),107244, h t t ps://d oi.org/10.1016/j.f o odhyd.2021.107244. [12]C.G. de Kruif , T. Huppe r tz, V.S. Urban, A .V. Petukhov, Casein micelles and their internal structure, Adv. Colloid Interface Sc i . 171-172 (2012) 36-52, h t tps ://d o i.or g/10.1016/j.c is.2012.01.002. [13] F . Doudies, M. Loginov, N . Hengl, M. Karrouch,N. Leconte, F . Garnier-Lambrouin,J. Perez, F. Pignon, G. Gesan-Guiziou, Build-up and r elaxation of membrane f ouling deposits produced during c r ossflow ultrafiltration of casein micel l e dispersions at 12°C and 42 °C probed by i n situ SAXS, J . Membr. Sci . 618 (2021),118700, h t t ps://d oi.or g/10.1016/j .me msci.2020.118700. [14] H.M. Farrel l Jr., R. Jimenez-Flores, G.T. Bleck, E.M. Brown, J.E. Butler, L .K. Creamer, C.L. Hicks, C.M. Hollar, K.F. Ng-Kwai-Hang, H.E. Swaisgood,Nomenclature of the proteins of cows’ milk-sixth r evision, J. Dairy Sci. 87 (2004)1641-1674, h t t ps://d oi.o r g/10.3168/jd s .S0022-0302(04)73319-6. [15] M. Ferrer, M. Alexander , M. Corredig, Changes in the physico-chemical proper t ies of casein micelles during ultrafiltration combined with diaf i ltrat i on, LWT-Food Sci . Technol. 59 (2014) 173-180, h t tps ://d o i .o r g/10.1016/j .l wt .2014.04.037. [17] Z. Gaygadzhiev, V. Massel , M. Alexander, M. Corredig, Addit i on of sodium c aseinate to skim mi l k inhibit s rennet -induced aggregation of casein micelles, Food Hydrocoll . 26 (2012) 405-411, h t tps://doi .org/10.1016/j.foo dh yd .2011.02.015. [18] R. Gebhardt , Effect of fi lt ration f orces on the struc t ure of casein micelles, J. Appl.Crystallogr . 47 (2014) 29-34, h t tps ://d o i .or g /10.1107/S1600576713029841. [19] E.P. Gilbert, Small -angle x-ray and neutron scattering i n food colloids, Curr. Opin.Colloid I nterface Sci . 42 (2019) 55-72, h t t ps://d o i .org/10.1016/j .c o cis .2019.03.005. [20] M. Gl a ntz, A. Hakansson, H. L i ndmark Mansson, M. Paulsson, L. Nilsson, Revealing the size, conformation, and shape of casein micelles and aggregates wi t h asymmet r ical flow f ield-f low fractionation and mul t iangle l ight scattering,Langmui r 26 (2010) 12585-12591, h t t p s://d oi .o r g/10.1021/l a101892x. [21]A. Gu inier , G . Fo u rne t , Small -Ang l e X-Ra y Sc a tt e r i n g , J ohn Wi l e y & Sons , New Y ork , 1955. [22] S. Hansen, R. Bauer, S. Bredsted Lomholt, K.B. Quist , J.S. Pedersen, K. Mortensen,Structure of casein micelles studied by small-angle neutron scattering, Eur.Biophys. J . 24 (1996) 143-147, h t t ps://d o i .o r g/10.1007/B F00180271. [23] M. Hartinger , U. Kulozik, Milk protein fractionation by spiral -wound MF membranes i n DF mode influence of f eed protein concentration and composition on the f i l tration performance , I nt. Dairy J. 102 (2020), 104606, h tt p s://d o i .o r g/10.1016/j .i d ai r yj.2019.104606. [24] E. Hurt , j. Zulewska, M. Newbold, D.M. Barbano, Micellar casein concentrate production with a 3×, 3-stage, uniform transmembrane pressure ceramic membrane process at 50 °C, J. Dairy Sci . 93 (2010) 5588-5600, h t t ps://d o i .org/10.3168/j ds.2010-3169. JOS [25]E B. Ingham, A. Smialowska, G.D. Er l angga, L. Matia-Merino, N.M. Kirby , C. Wang,R.G. Haverkamp, A.J . Carr, Rev i siting t he i nterpretation of casein micelle SAXS data , Soft Matter 12 (2016) 6937-6953, h t tps ://do i .org/10.1039/C6SM 01091A . [26] Y. Jiang, G. Barone, V. Rauh, S.K. Li l levang, L.H. Skibsted, L. Ahrne, Temperature effects on calcium partition kinetics in pasteurised skim milk during storage, Int.Dairy J . 137 (2023), 105518, h t t ps://d o i.org /10.1016/j .i da i ryj .2022.105518. [27] M. Keer l , J.S. Pedersen, W. Richtering, Temperature sensitive copolymer microgels with nanophase separated structure, J. Am. Chem. Soc. 131 (2009) 3093-3097,h t t p s ://doi .o r g/10.1021/j a 807367p . [28]F P. Krishnankutty Nair, M. Corredig, Rennet-induced gelation of concentrated milk i n the presence of sodium caseinate: di f ferences between milk concentration using ultrafiltration and osmotic stressing, J. Dairy Sci. 98 (2015) 27-36, htt p s://d o i.o r g/10.3168/j d s.2014-8356. [29] P. Krishnankutty Nair, M. Corredig, Sodium case i nate hinders chymosin-induced aggregation of caseins i n concentrated mi l k: t he r ole of soluble caseins and calcium ions, JDS Commun. 2 (2021) 309-312, h t tp s://d o i .org /10.3168/j dsc.2021-0124. [30] P . Krishnankutty Nair , D.G. Dalgleish, M. Corredig, Colloidal properties of concentrated heated mi l k, Soft Matter 9 (2013) 3815, ht tp s ://doi.or g/10.1039/c2s m 27540f . [31] M. Li, M.A.E. Auty, S.V. Crowley, A.L . Kelly, J.A. O'Mahony, A. Brodkorb, Self-association of bovine p-casein as influenced by calcium chloride , buffer t ype and OVn temperature, Food Hydrocol l . 88 (2019) 190-198, h t t ps ://doi .or g /10.1016/j .food h yd.2018.09.035. [32]Y. Li, M. Corredig, Calcium release from milk concentrated by ul t rafiltration and diafiltration, J. Dairy Sci. 97 (2014)5294-5302, h t tp s://doi.or g/10.3168/j ds.2013-7567. [33] Y . Li, M. Corredig, Acid i nduced gelation behaviour of skim mi l k concentrated by membrane f il t ration, J. Text . Stud. 51 (2020) 101-110, ht t p s ://doi .org /10.1111/jt xs.12492. [34]Y. Li, D. Dalgleish, M. Corredig, Influence of heating treatment and membrane concentration on the formation of soluble aggregates, Food Res. Int . 76 (2015)309-316, ht t p s://doi.o r g/10.1016/j .foo d res.2015.06.016. [35] M. Loginov , F. Doudies, N . Hengl, M. Karrouch,N. Leconte, F. Garnier-Lambrouin,J. Pérez , F . Pignon, G. Gesan-Guiziou, On the reversibi l ity of membrane fouling by deposi t s produced dur i ng crossflow ul t raf i l t ration of casein micelle suspensions,J. Membr. Sci. 630 (2021), 119289, h t tps://d o i .org/10.1016/j . me m s c i .2021.119289. [36]5]O.H . Lowry, N.J . R osebrough , A.L . Farr , R.J. R and al l , P ro t ei n m e as u r e m e nt wi t h the Fo l i n p he n o l r eage n t , J. Bi ol . Ch e m. 193 (1951) 265-275. [37] J . Lyngso, J.S. Pedersen, A high-flux automated laboratory smal l -angle X-ray scattering ins t rument optimized for solution scatter i ng, J. Appl . Chrystallogr. 54(2021) 295-305, ht tps ://doi.o r g/10.1107/S 1600576720016209. [38]I N. Mahmoudi , s. Mehalebi , T. Nicolai , D. Durand, A. Riaublanc, L ight-s cattering study of the st r ucture of aggregates and gels formed by heat-denatured whey protein isolate and p-lactoglobulin at neutral pH, J. Agric. Food Chem. 55 (2007)3104-3111, htt ps://d oi .o r g/10.1021/j f063029g . [39]A.G. Mailer, P.S. Clegg, P.N. Pusey , Particle siz i ng by dynamic l ight scattering: non-l i near cumulant analysis, J. Phys.: Condens. Matter 27 (2015),145102, h t tp s://d oi.o r g/10.1088/0953-8984/27/14/145102. [40] S. Marchin, J.-L. Putaux, F. Pignon, J. Leoni l , Effects of t he environmental f actors on the casein micelle structure studied by cryo t ransmission electron microscopy and smal l -angle x-ray scattering/ultrasmall-angle x-ray scattering, J. Chem. Phys.126(4)(2007),045101, h t t ps ://doi .or g /10.1063/1.2409933. [41] M.A.K. Markwell , S.M. Haas, L.L. Bieber, N.E. Tolber t , A modification of the L owry procedure to simpl i f y protein determination in membrane and l i poprotein samples,Anal. Biochem. 87 (1978) 206-210, htt ps://d o i .or g/10.1016/0003-2697(78)90586-9. [42]E B.K. Nelson, D.M. Barbano, A MF process t o maximize removal of serum proteins from skim mi l k before cheese making, J. Dairy Sc i . 88 (2005) 1891-1900, h t t p s ://doi .o rg /10.3168/j ds.S0022-0302(05)72865-4. [43] M.H. Nogueira, S. Ben-Harb, M. Schmutz, B. Doumert, S. Nasser, A. Derensy,R. Karoui , G. Delaplace, P.P.S. Peixoto, Multiscale quantitati v e characterization of demineralized casein micelles: how t he par ti al exci s ion of nano-clusters l eads to the aggregat i on dur i ng rehydration, Food Hydrocoll. 105 (2020), 105778, h t t p s ://d oi .org/10.1016/j .foo d hyd .2020.105778. [44] M.H. Nogueira, L .A. Scudeler, L. Humblot, B. Doumert, M. Hennetier, F. Violleau,C. Lesur, G. Delaplace, P.P.S. Peixoto, Assessment of structures in phosphocaseinate dispersions by A4F NMR and SAXS: the impact of demineralization and heat treatment on viscosity, Food Hydrocoll.(2022),108366,h t tps ://d o i .org/10.1016/j .f o odh yd.2022.108366. [45] M.H. Noguei r a, L . Humblot , R.P. Singh, E. Dieude-Fauvel , B. Doumert, S. Nasser,C. Lesur, R. Karoui, G. Delaplace, P.P.S. Pe i xoto, The hete r ogeneous substruc t ure of casein micelles evidenced by SAXS and NMR in demineral i zed samples, Food Hydrocoll. 117 (2021), 106653, ht tps://d oi.o r g /10.1016/j .food h yd.2021.106653. [46]C.L.P. Oliv i eira,T. Vorup-Jensen, C.B.F. Andersen, G.R. Andersen, J .S. Pedersen,Discovering new features of protein complexes structure s by small-angle X-ray scattering, i n: M. Gomez, A. Nogales, M. Cruz Garcia-Gutierrez, T.A. Ezquerra (Eds.), Applicat i ons of Synchrotron Light to Scatter i ng and Diffraction in Materials and Life Sciences, Lec t ure Notes in Physic s , 776, Springer, 2009, pp. 231-244,h t tps ://do i.org/10.1007/978-3-540-95968-7_11. [47] E.A. Parker , L . Donato, D.G. Dalgleish, Effects of added sodium caseinate on t he formation of par t icles i n heated skim milk, J. Agric. Food Chem. 53 (2005)8265-8272, htt p s ://doi .o rg/10.1021/jf0512306. [48] J.S. Pedersen, Resolution effects and analysis of small -angle neutron-scattering data, J. Phys. IV 3 (1993) 491-498, h t tp s://d o i .o r g/10.1051/j p 4:19938102. [49] J.S. Pedersen, Determination of size distributions f rom small-angle scattering data f or sys t ems with effec t ive hard-sphere interactions, J . Appl. Crys t allogr. 27 (1994)595-608, ht tp s://doi.org/10.1107/S0021889893013810. [50] J.S. Pedersen, Analysis of small-angle scattering data from colloids and polymer solutions: model i ng and least -square f i t s , Adv. Colloid I nterface Sci. 70 (1997)171-210, ht tps ://d o i.org /10.1016/S0001-8686(97)00312-6. [51] J.S. Pedersen, A flux- and background-optimized version of t he nanoSTAR small-angle X-ray scattering camera f or solut i on scatter i ng, J. Appl. Crystallogr. 37(2004) 369-380, h t t ps://do i .or g/10.1107/S 0021889804004170. [52] J.S. Pedersen, D. Posse l t , K. Mortense n , Analyt i cal t reatment of t he r esolution f unction for small -angle scatter i ng, J . Appl . Crystallogr. 23 (1990) 321-333,https ://d o i.org /10.1107/s o o 21889890003946. [53] J.S. Pede r sen, T.L. Moller, N. Raak, M. Corredig, A model on absolute scale for the small -angle x-ray scattering from bovine casein micelles, Soft Matter 18 (2022)8613-8625, h t t ps ://do i .org /10.1039/D2SM00724J. [54] A. Pi t kowski , D. Durand, T . Nicolai , Structure and dynamical mechanical properties of suspensions of sodium caseinate, J. Colloid I nterface Sci. 326 (2008)96-102, htt ps://d o i .o r g/10.1016/j .jc i s.2008.07.003. [55] N. Raak, M. Corredig, Manufacture of mi l k and whey products: caseins, caseinates and micellar casein, in: P. McSweeney, J. McNamara (Eds.), Encyclopdedia of Dairy Sciences, 5, Elsevier , 2022, pp. 8-17, ht tps ://doi.o rg/10.1016/B978-0-12-818766-1.00135-5. [56] N. Raak, M. Corredig, Kinetic aspect s of casein micelle cross-l inking by transglutaminase at di f ferent volume f ract i ons, Food Hydrocoll. 128 (2022),107603, h t t ps://d oi.o r g /10.1016/j .f oodhyd.2022.107603. [57] N. Raak, R. Abbate , A. Lederer, H. Rohm, D. J aros, Size separation t echniques for the characterisation of cross-l i nked casein: a review of methods and t heir applications, Separations 5 (2018) 14, ht t ps ://doi.or g /10.3390/ se pa rat i ons5010014. [58] M. Reitmaier , I. Bachmann, H.-J . Heidebrecht, U. Kulozik, Effect of changes in ionic composition induced by different DF media on deposited layer properties and separation efficiency in mi l k prote i n f ractionation by MF, I nt. Dairy J. 120 (2021),105089, h t t ps://d oi.o r g/10.1016/j .i dair y j.2021.105089. [59] I.R.T . Renhe, M. Corredig, Effect of partial whey protein depletion during membrane f iltration on thermal stabi l ity of milk concentrates, J . Dairy Sci. 101(2018) 8757-8766, h t t ps://doi .or g /10.3168/j ds .2018-14407. [60] I.R.T . Renhe, Z. Zhao, M. Corredig, A comparison of the heat stability of fresh milk protein concentrates obtained by MF , UF and DF , J. Dairy Res. 86 (2019) 347-353,h t t ps ://d oi .o rg /10.1017/SO 022029919000426. [61] A. Shukla, T. Narayanan, D. Zanchi , Structure of casein micelles and their complexation with tannins, Soft Matter 5 (2009) 2884, h t t ps://d o i .o rg/10.1039/b903103k. [62]A. Smialowska, L. Matia-Merino, B. I ngham, A.J. Carr, Effect of calc i um on t he aggregation behaviour of caseinates, Colloids Surf. A: Physicochem. Eng. Asp. 522 (2017) 113-123,ht tps://d o i .o r g/10.1016/j .colsurfa.2017.02.074. [63]G.N . Smith, E. Brok, M.V. Christiansen, L. Ahrne, Casein micelles in milk as sticky spheres, Soft Matter 43 (2020) 9955-9963, h t t ps ://doi.or g /10.1039/D O S M 01327G . [64] H. Sorensen, J .S. Pedersen, K. Mor t ensen, R. Ipsen, Characterisation of f ractionated skim milk with small -angle X-ray scatter i ng, Int. Dairy J. 33 (2013) 1-9, h t tp s://doi .o r g/10.1016/j .idairyj.2013.05.006. [65] P. Thomar, T. Nicolai , L . Benyahia, D. Durand, Comparat i ve study of the rheology and the structure of sodium and calcium caseinate solut i ons, I nt . Dairy J. 31 (2013)100-106, htt ps ://doi.org/10.1016/j .ida ir y j.2013.02.005. [66] Y. Xu, D. Liu, H. Yang, J. Zhang, X. L i u, J.M. Regenstein, Y. Hemar, P. Zhou, E f fect of calcium sequestration by ion-exchange treatment on t he dissociation of casein micelles in model mi l k protein concentrates, Food Hydrocoll . 60 (2016)59-66,h t t ps ://d oi .o r g /10.1016/j .f ood h yd.2016.03.026. [67] z. Zhao, M. Corredig, Effects of pH-modification on the renne t coagulation of UI concentrated casein micelles suspensions, Food Chem. (2020), 126199, h t tp s://doi.o rg /10.1016/j .foodc h em.2020.126199. [68] J . Zulewska, M. Newbold, D.M. Barbano, Efficiency of serum protein removal from skim mi l k with ceramic and polymeric membranes at 50 °C, J. Dairy Sci. 92 (2009)1361-1377, h t t ps://d o i .o r g/10.3168/j ds.2008-1757.
确定




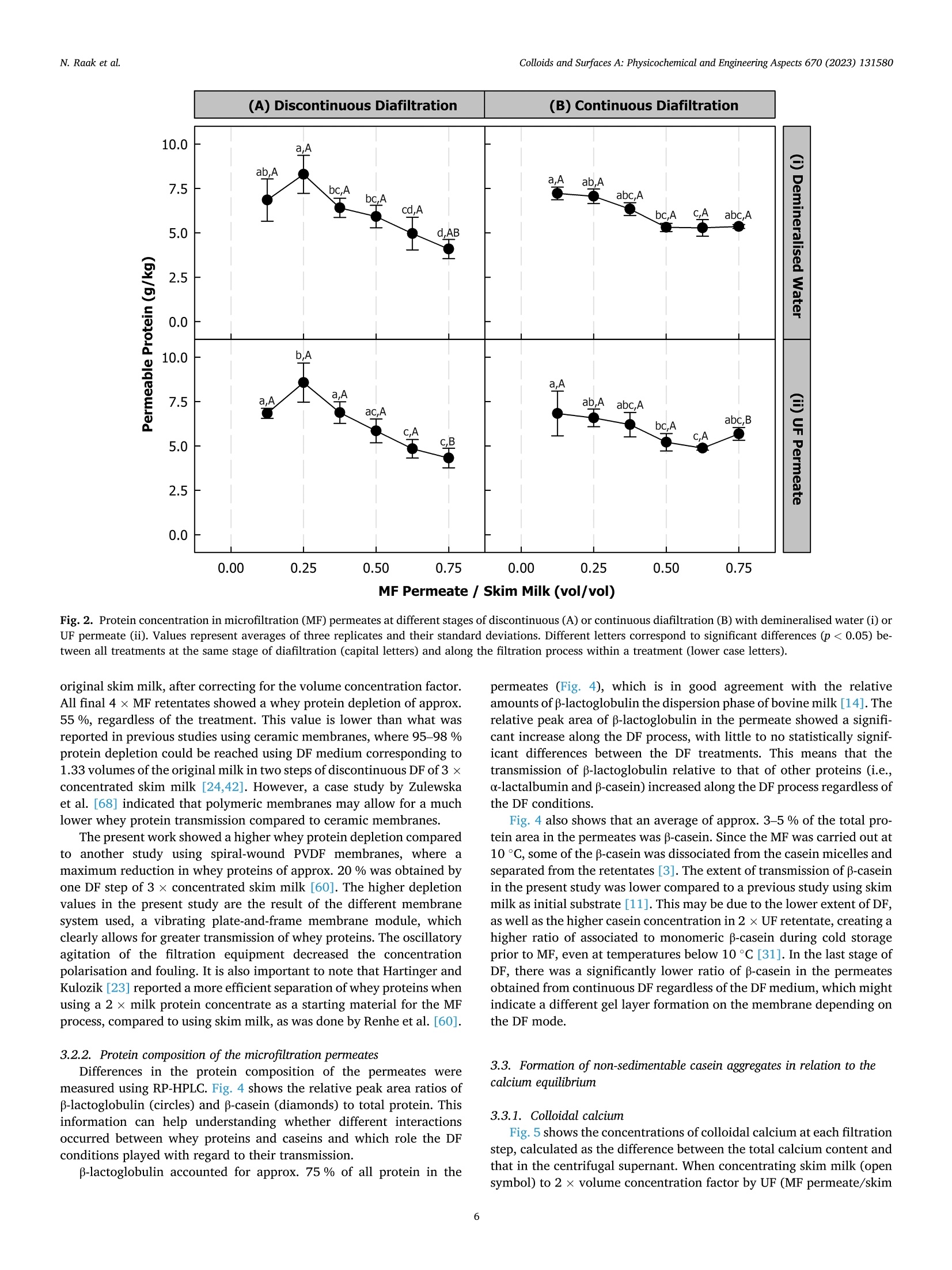
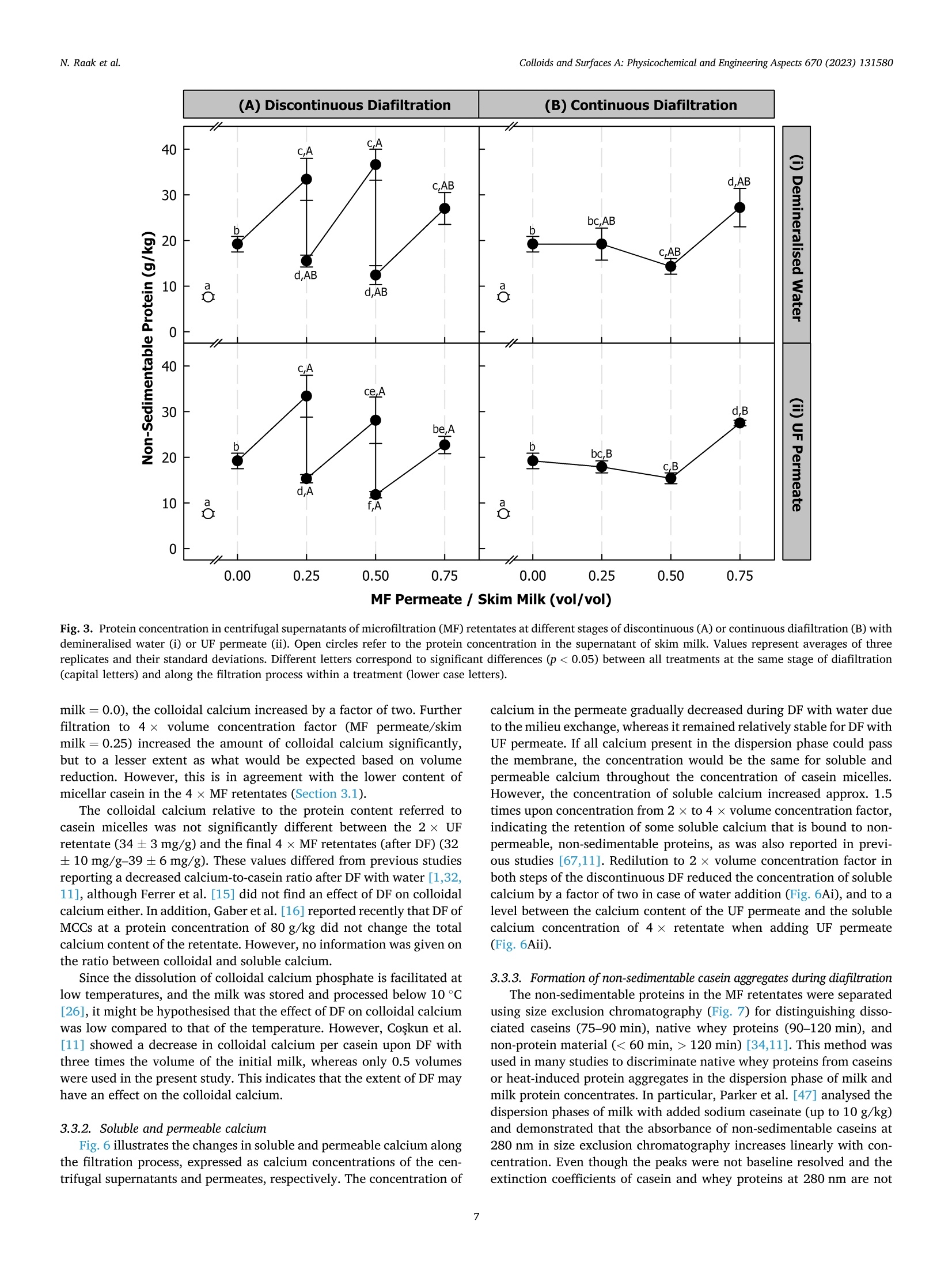
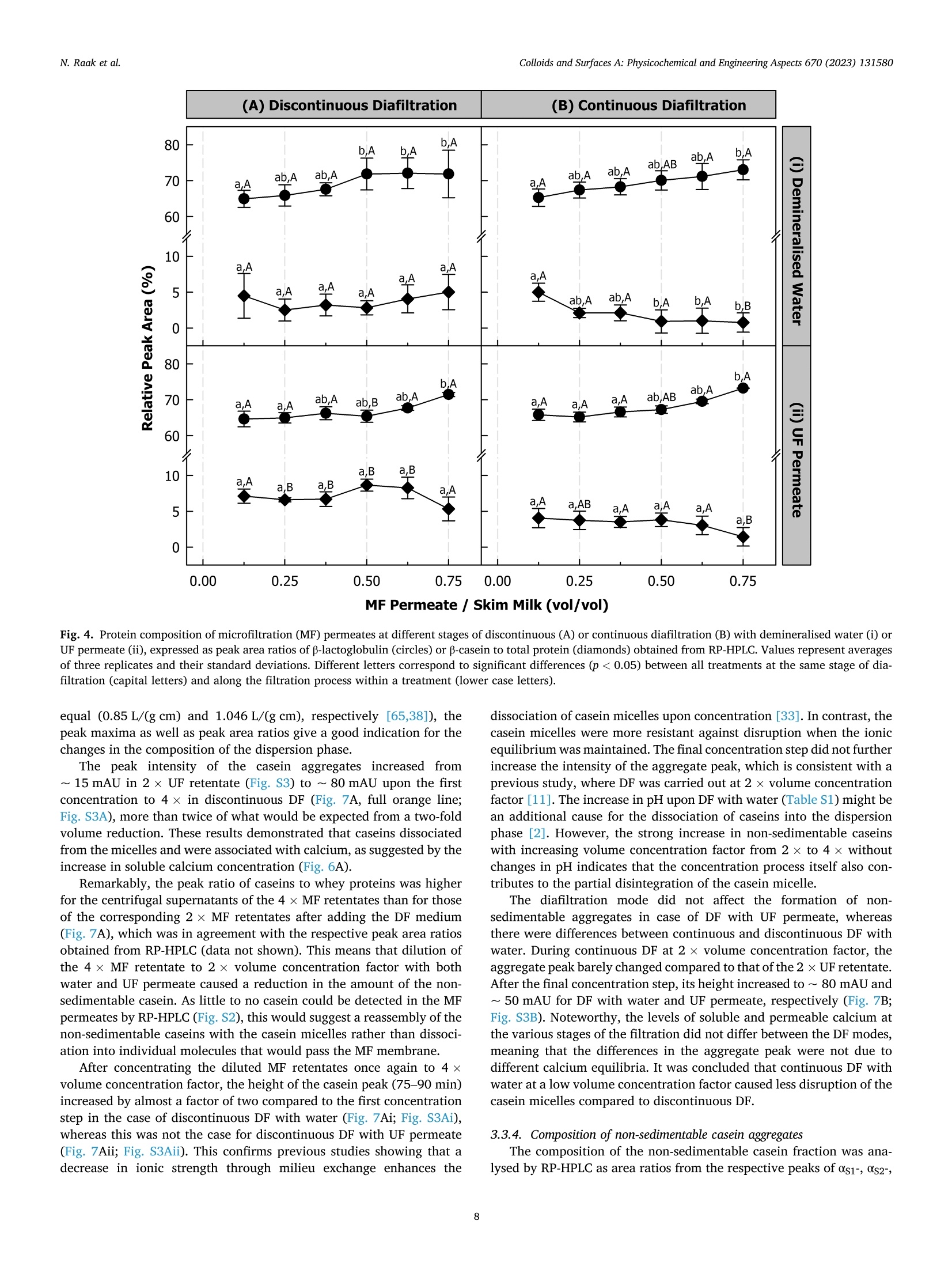
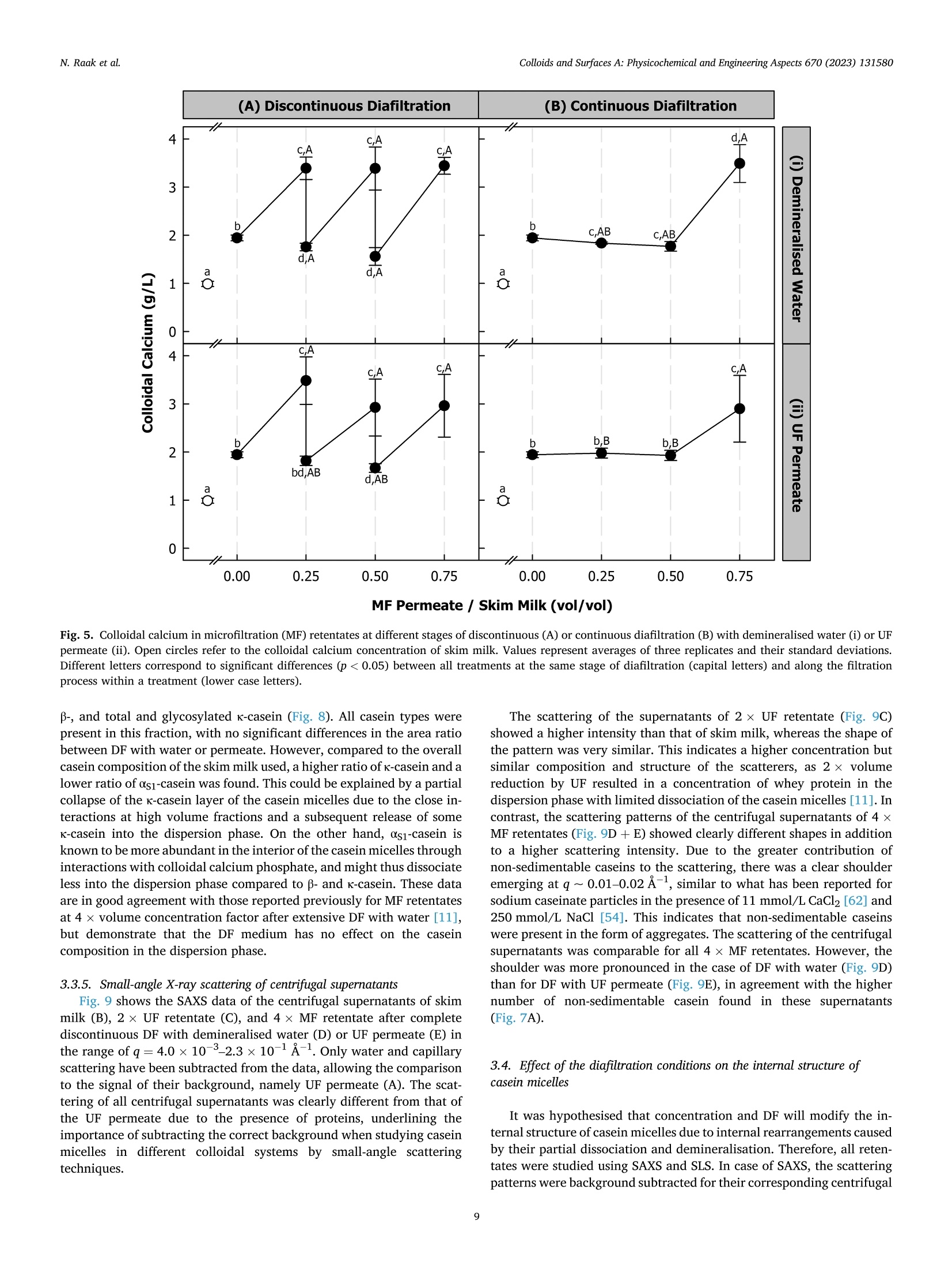
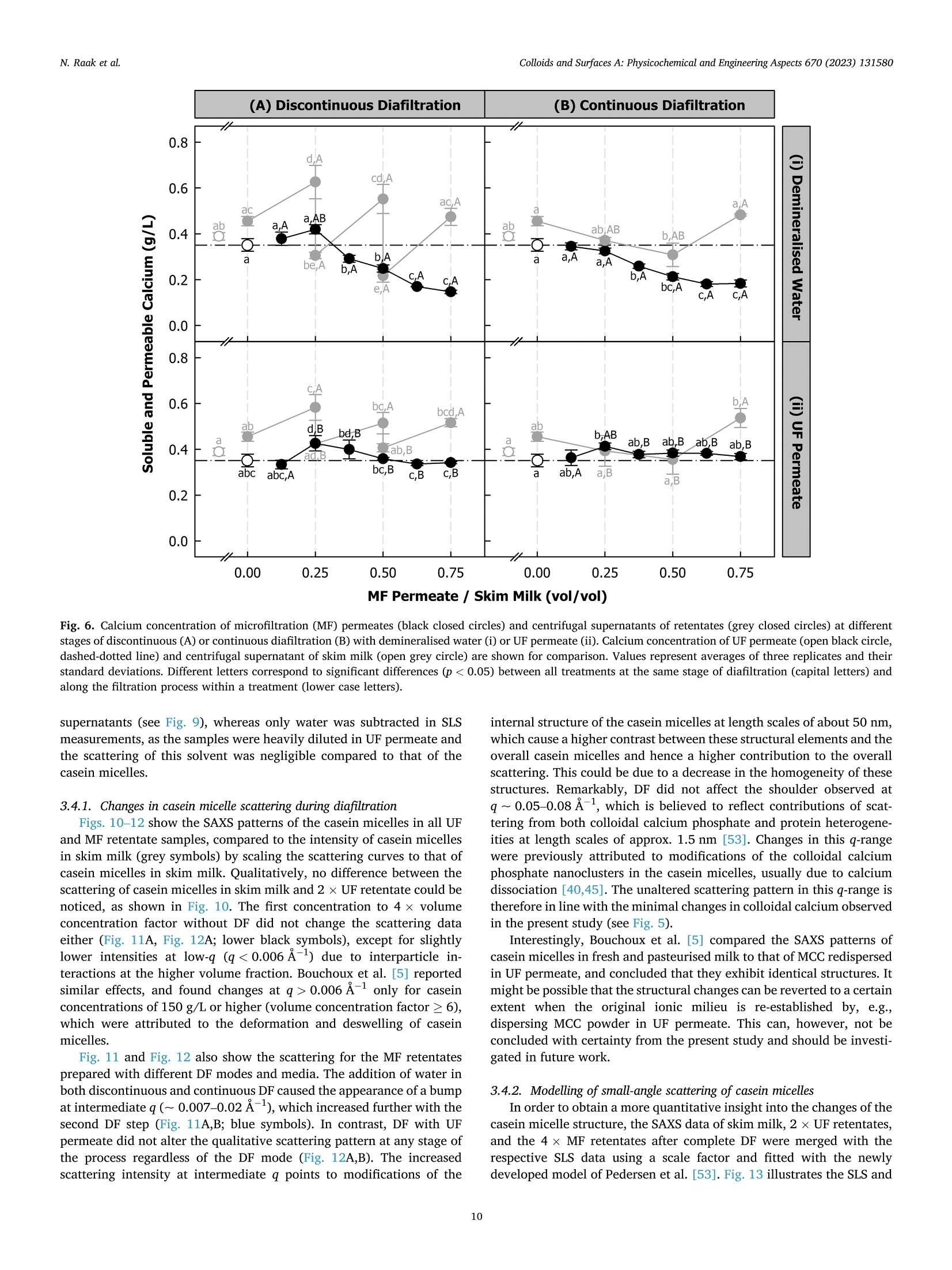
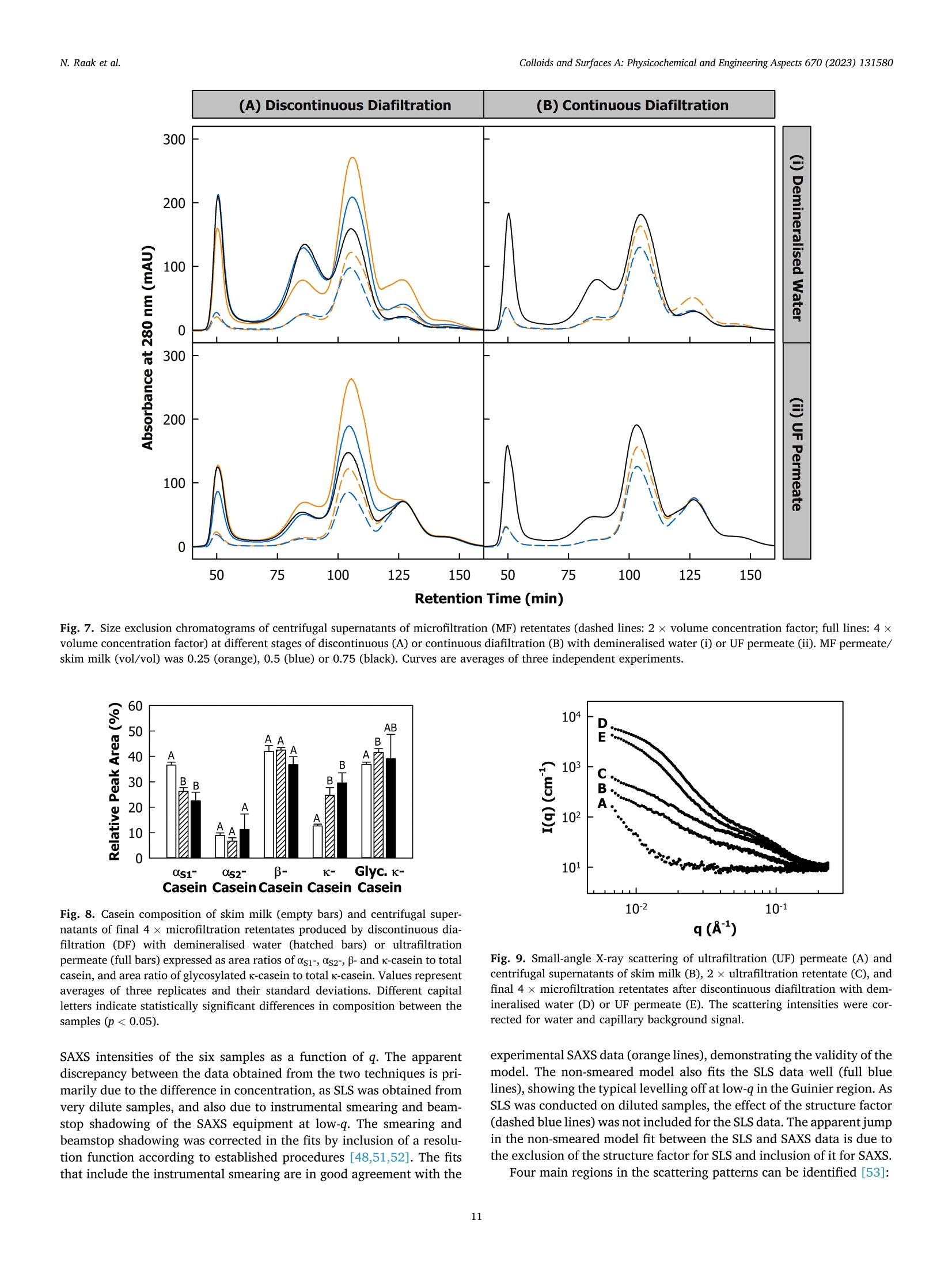
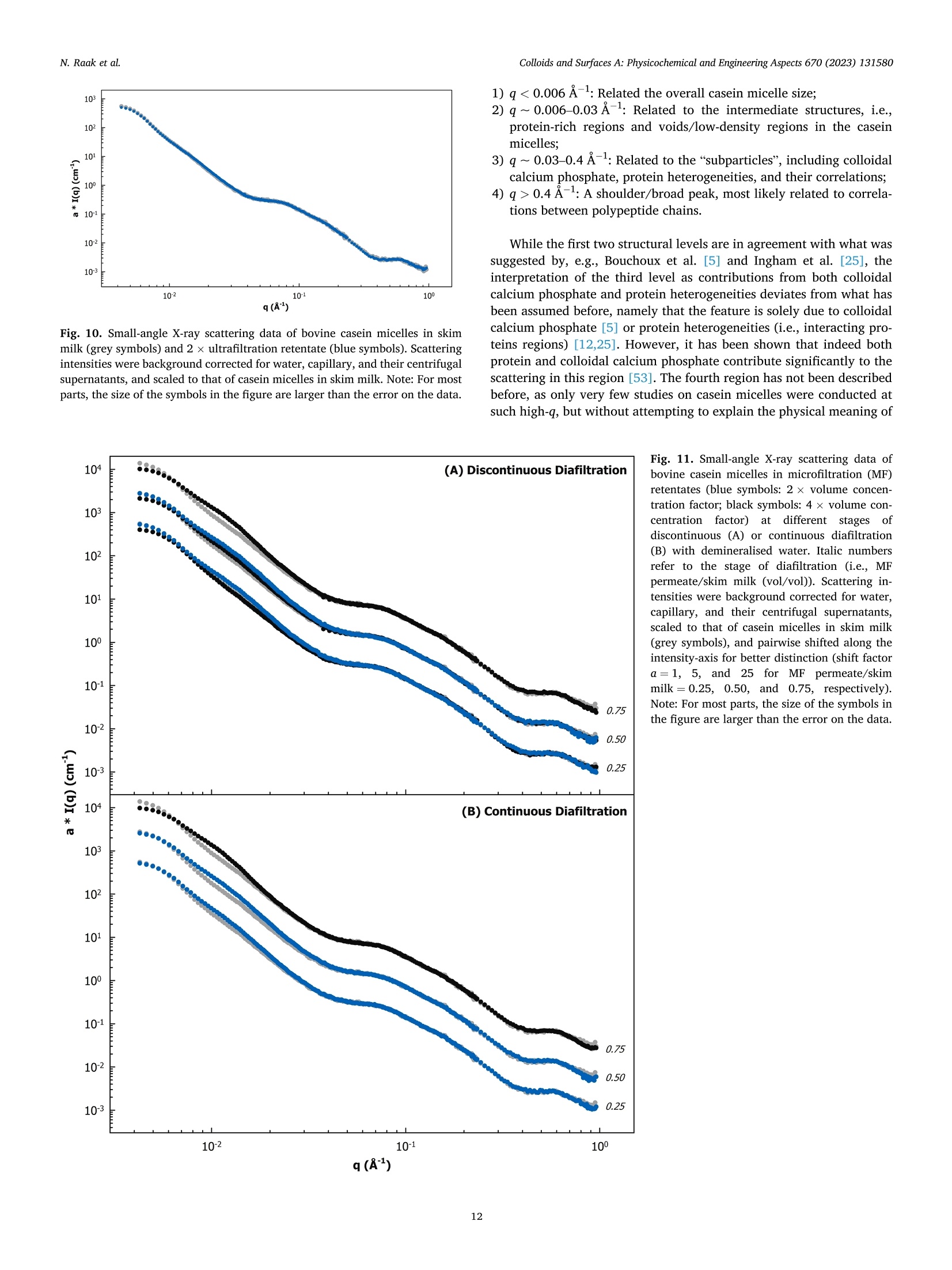
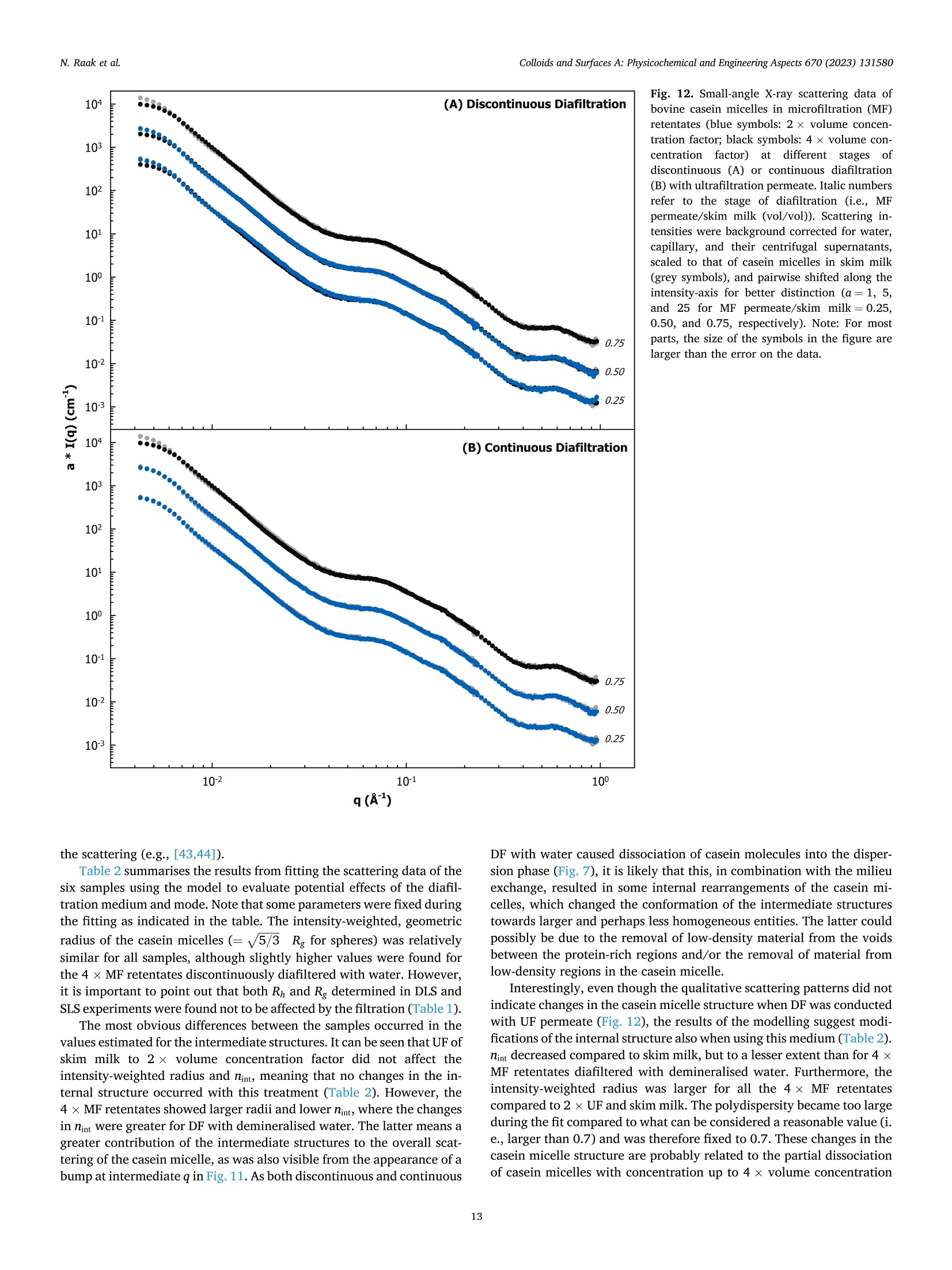
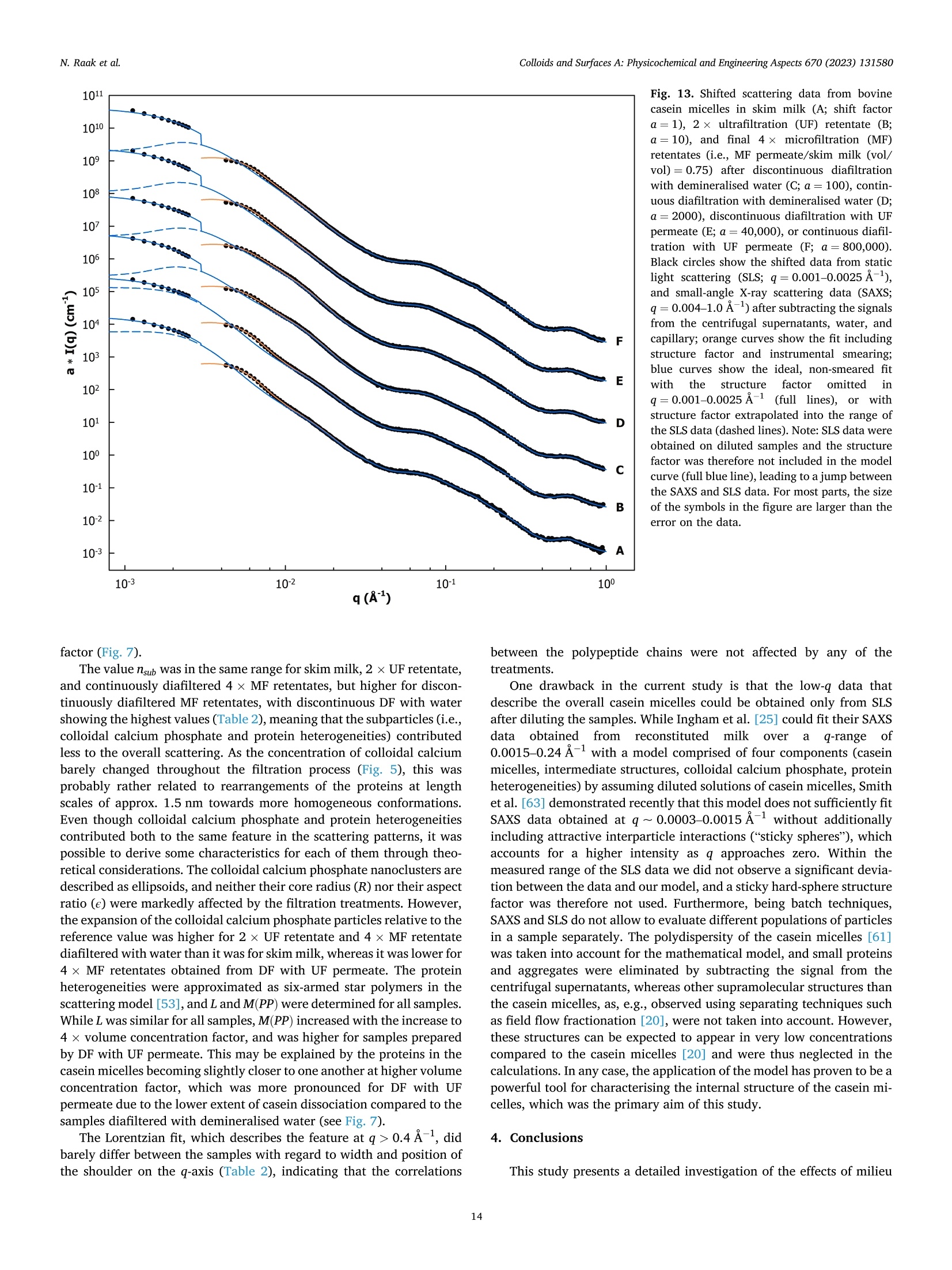
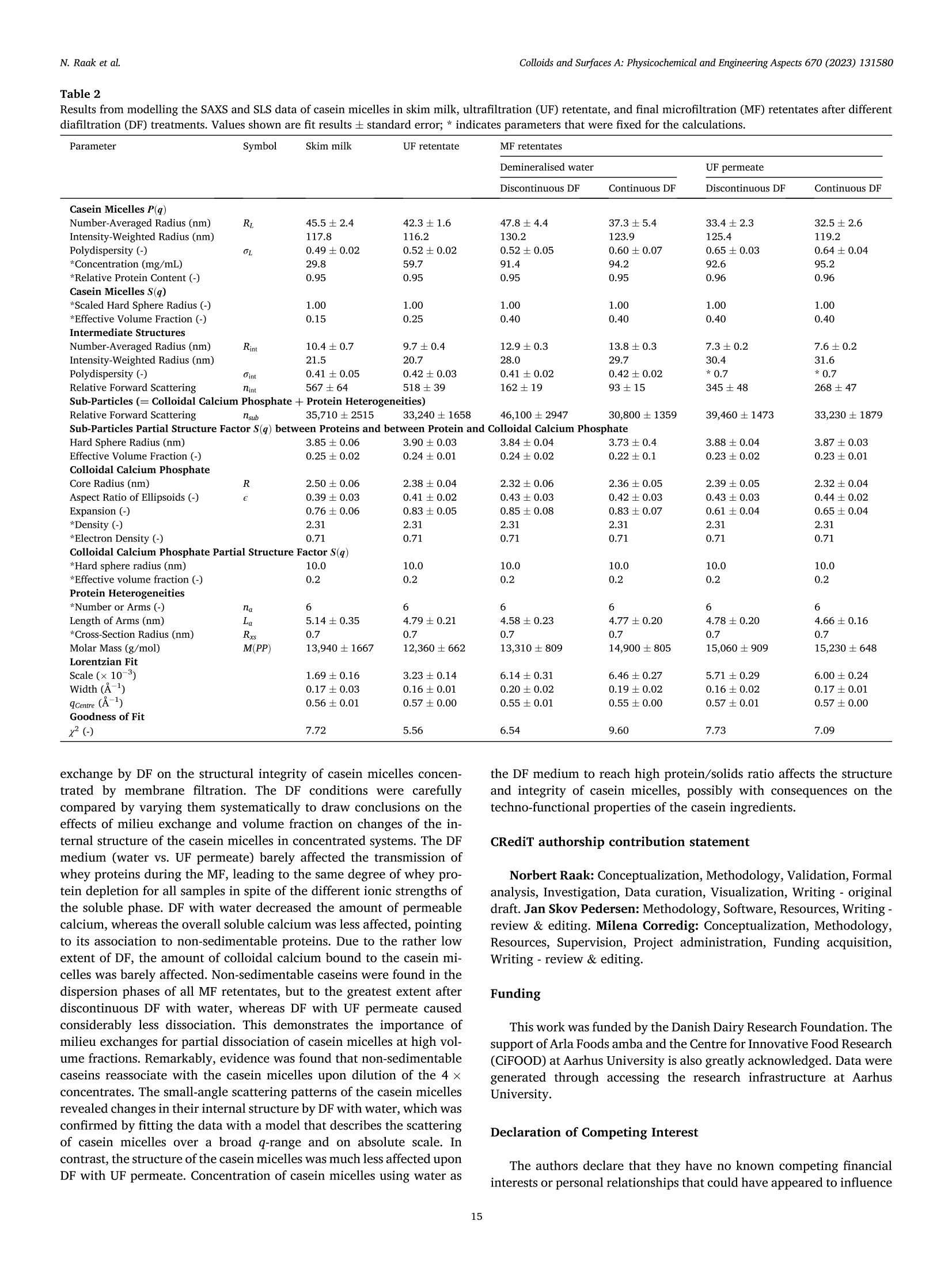


还剩15页未读,是否继续阅读?
中国格哈特为您提供《脱脂乳中酪蛋白含量和总蛋白含量的检测》,该方案主要用于液体乳中营养成分检测,参考标准《GB 5009.5 食品安全国家标准 食品中蛋白质的测定》,《脱脂乳中酪蛋白含量和总蛋白含量的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro
推荐专场
相关方案
更多
该厂商其他方案
更多
















