方案详情
文
酶解对鲁冰花(黄羽扇豆)蛋白溶解度和乳化性能的影响Effect of Enzymatic Hydrolysis on Solubility and Emulsifying Properties of Lupin Proteins (Lupinus luteus)
方案详情

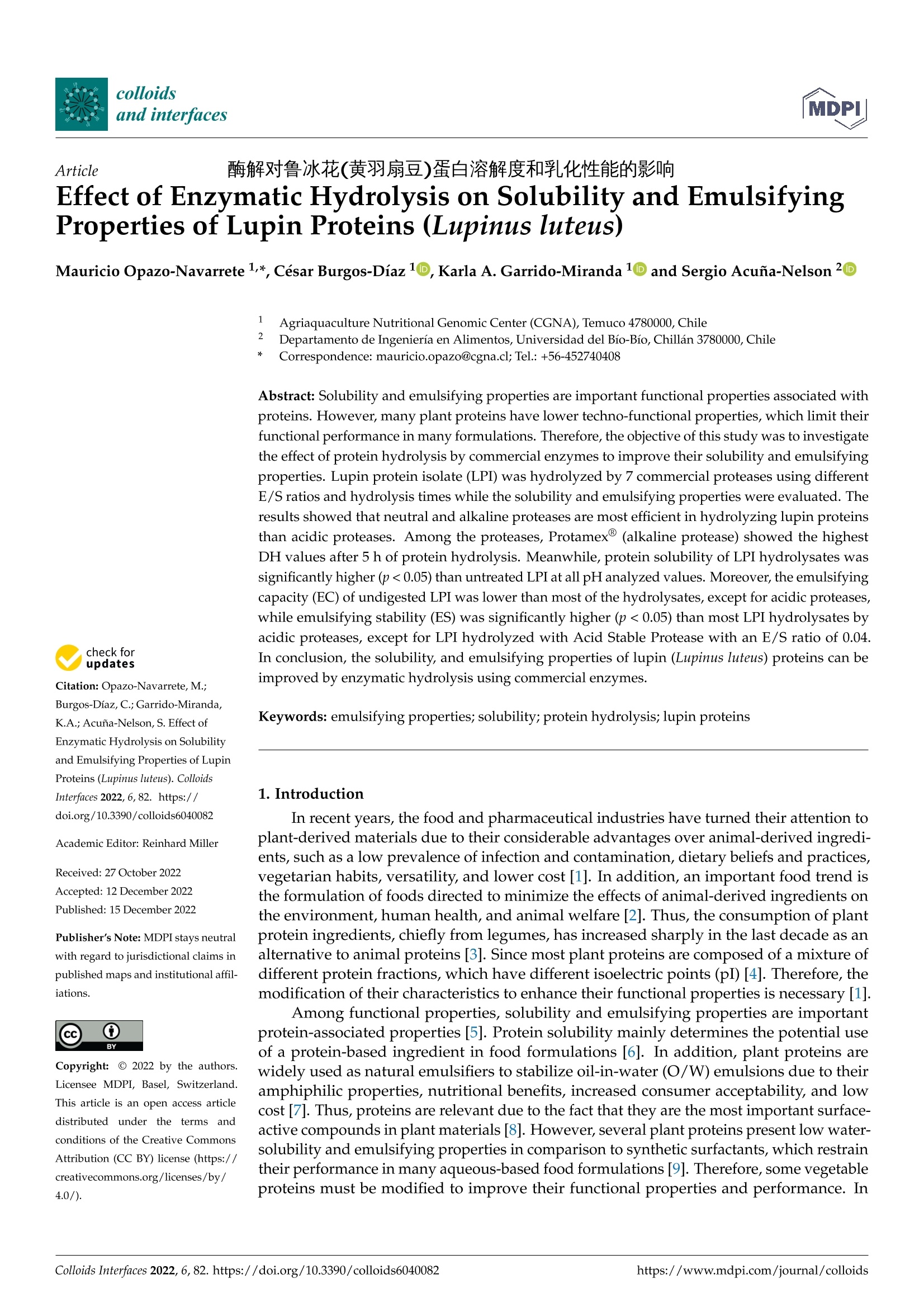
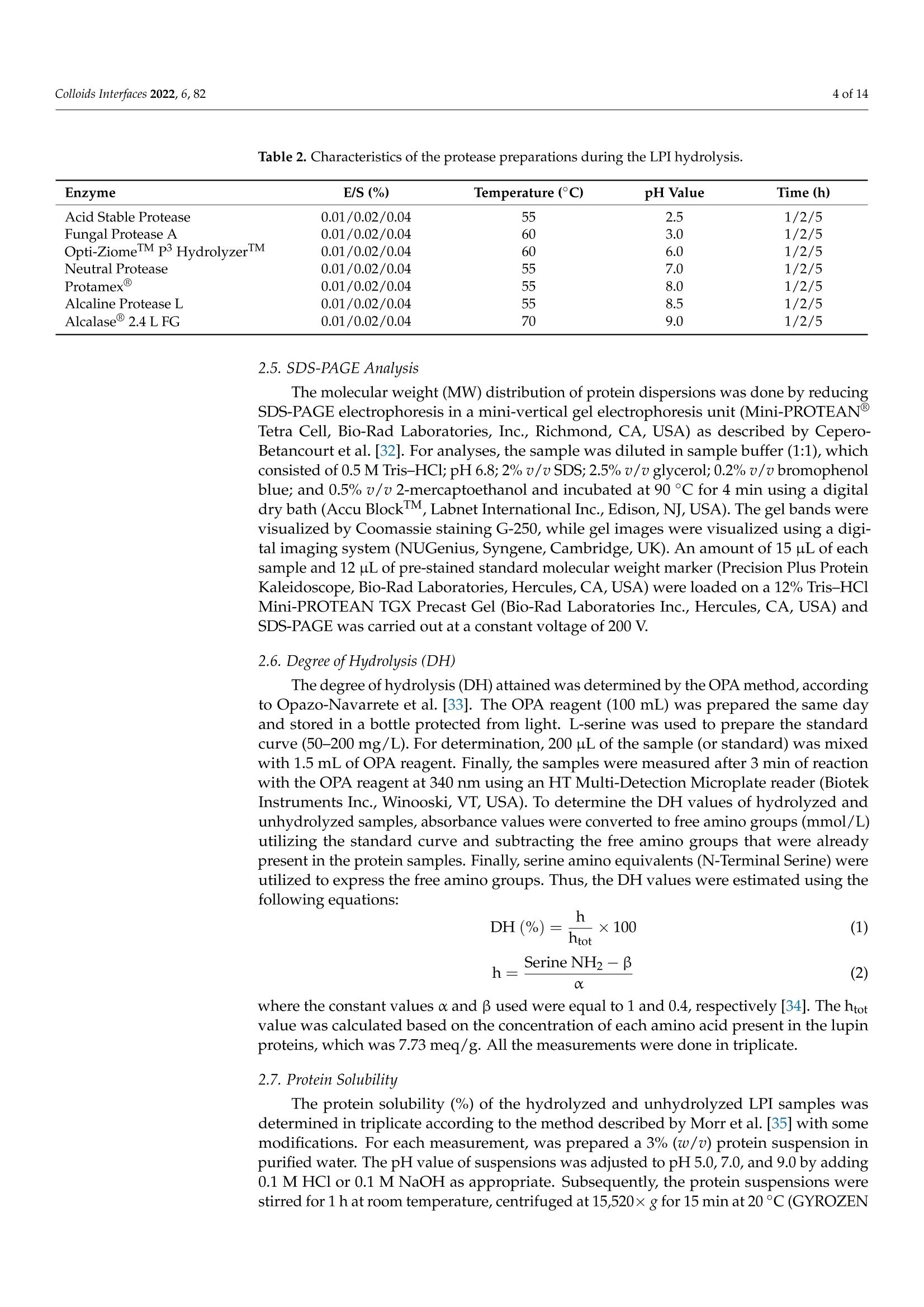
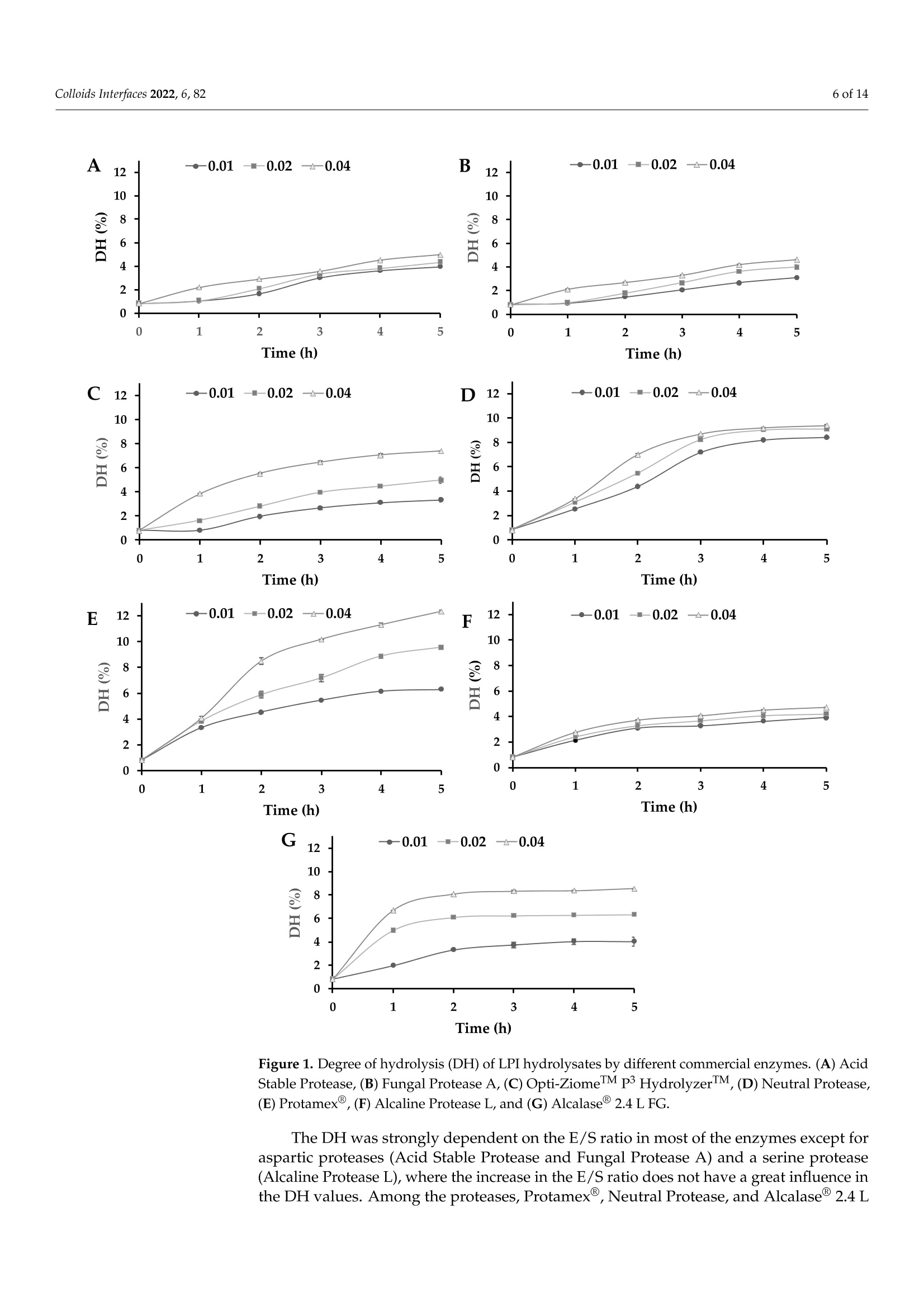
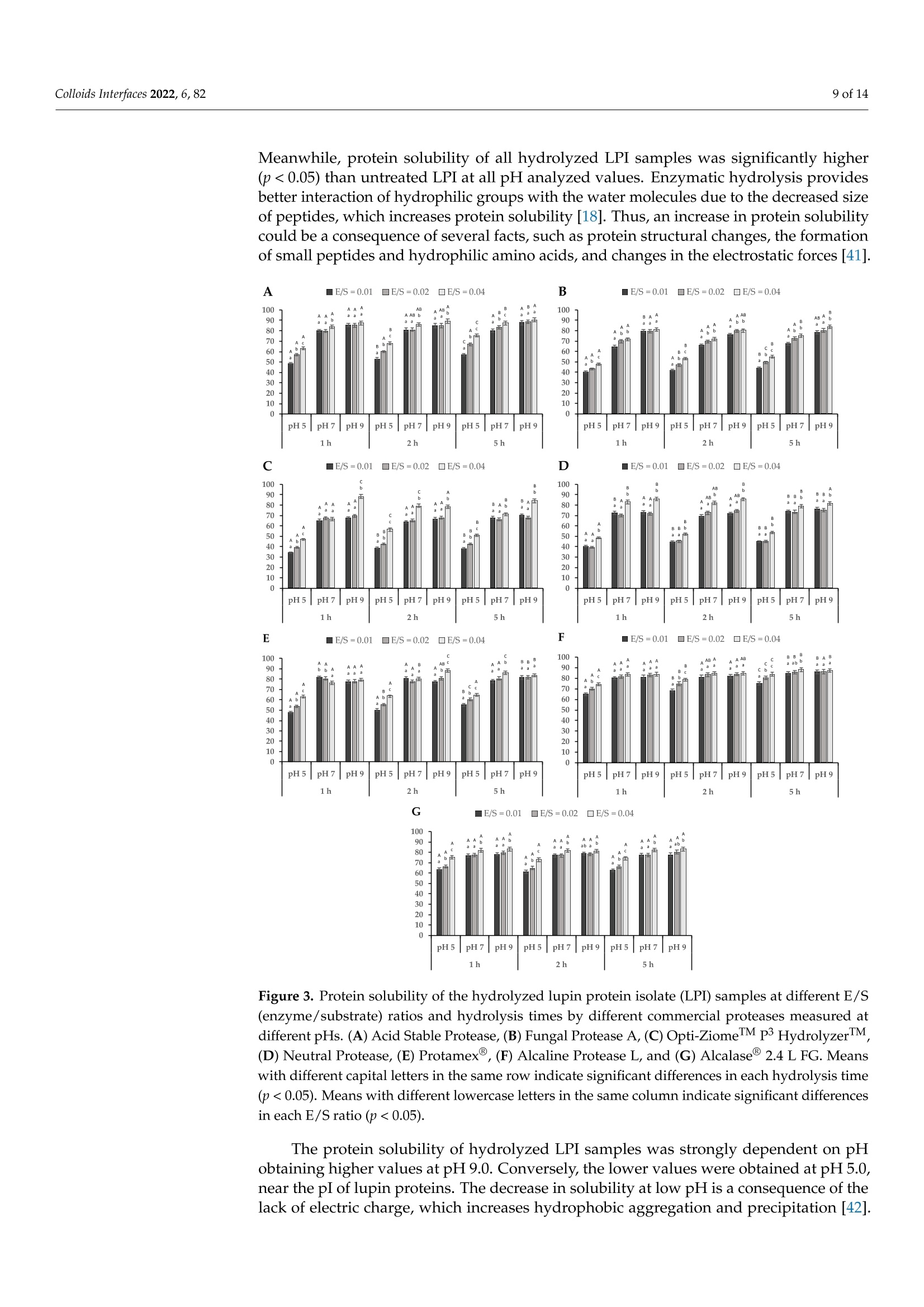
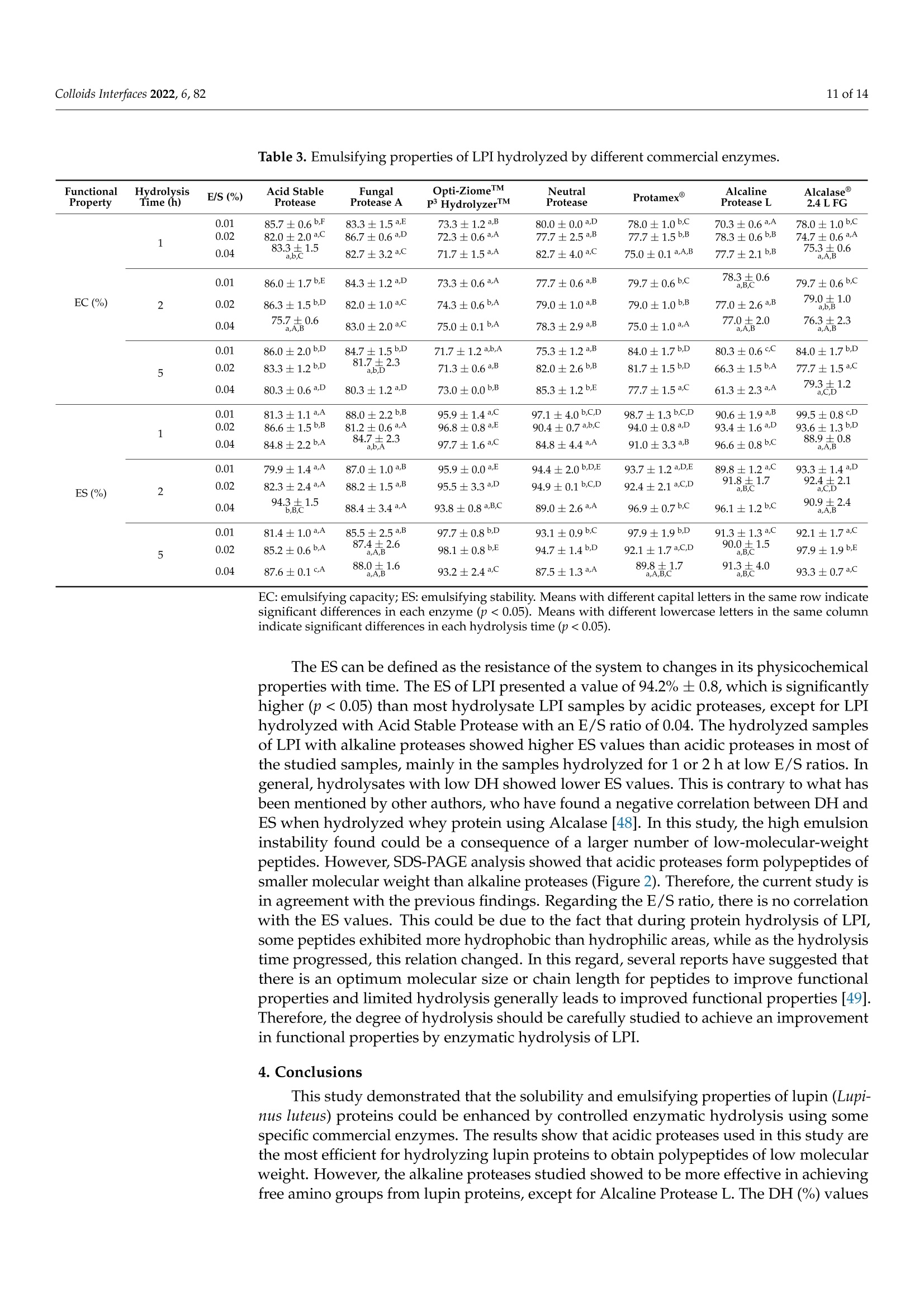
酶解对鲁冰花(黄羽扇豆)蛋白溶解度和乳化性能的影响Effect of Enzymatic Hydrolysis on Solubility and Emulsifying Properties of Lupin Proteins (Lupinus luteus)colloidsand interfaces 2 of 14Colloids Interfaces 2022, 6, 82 Academic Editor: Reinhard Miller Received: 27 October 2022 Accepted: 12 December 2022 Published: 15 December 2022 Publisher's Note: MDPI stays neutral with regard to j u risdictional claims in published maps and institutiona l af fi l -iations. Copyright: @ 2022 by the authors.L i censee MDPI, Basel, Switzerland.This article is an open access article distr i buted under the terms and conditions of the Creative Commons Attribution (CC BY ) l icense (https://creativecommons.org/licenses/by/4.0/) Effect of Enzymatic Hydrolysis on Solubility and Emulsifying Properties of Lupin Proteins (Lupinus luteus) Mauricio Opazo-Navarrete 1*, Cesar Burgos-Diaz1D ,Karla A. Garrido-Miranda 1 and Sergio Acufia-Nelson 2iD Agriaquaculture Nutr i tiona l Genomic Center (CGNA), Temuco 4780000, Chile 2 Departamento de Ingenieria en Al i mentos, Universidad del Bio-Bio, Chi l lan 3780000,Chile Correspondence: mauricio.opazo@cgna.cl ; Tel.: +56-452740408 Abstract: Solubility and emulsifying properties are important functional properties associated with proteins. However, many plant proteins have lower techno-functional properties, which limit their func t ional performance i n many formulations. Therefore, the objective of this study was to investigate the effect of protein hydrolysis by commercial enzymes to improve their solubility and emulsifying properties. Lupin protein isolate (LPI ) was hydrolyzed by 7 commercial proteases using different E/S ratios and hydrolysis times while the solubility and emulsifying properties were evaluated. The results showed that neutra l and alkaline proteases are most efficient i n hydrolyzing lupin proteins than acidic proteases. Among the proteases, Protamex(alkaline protease) showed the highest DH values after 5 h of protein hydrolysis. Meanwhile, protein solubi li ty of LPI hydrolysates was significantly higher (p<0.05) than untreated LPI at all pH analyzed values. Moreover, the emulsifying capacity (EC) of undigested LPI was lower than most of the hydrolysates, except for acidic proteases,while emulsifying stability (ES) was significantly higher (p<0.05) than most LPI hydrolysates by acidic proteases, except for LPI hydrolyzed with Acid Stable Protease with an E/S ratio of 0.04.In conclusion, the solubility, and emulsifying properties of lupin (Lupinus luteus) proteins can be improved by enzymatic hydrolysis using commercial enzymes. Keywords: emulsifying propert i es; solubility; protein hydrolysis; lupin proteins 1. Introduction In recent years, the food and pharmaceutical industries have turned their attention to plant-derived materials due to their considerable advantages over animal-derived ingredi-ents, such as a low prevalence of infection and contamination, dietary bel i efs and practices,vegetarian habits, versatility, and lower cost [1]. In addi t ion, an important food trend is the formulation of foods directed to minimize the effects of animal-derived ingredients on the environment, human health, and animal wel f are [2]. Thus, the consumption of plant protein ingredients, chiefly from legumes, has increased sharply in the last decade as an alternative to anima l proteins [3]. Since most plant proteins are composed of a mixture of different protein fractions, which have different isoelectric points (pI) [4]. Therefore, the modification of their characteristics to enhance their functional properties is necessary [1]. Among func t ional properties, solubility and emulsifying properties are important protein-associated properties [5]. Protein solubility mainly determines the potential use of a protein-based ingredient in food formulations [6]. In addition, plant proteins are widely used as natural emulsifiers to stabilize oil-in-water (O/W) emulsions due to their amphiphilic properties, nutritional benefits, increased consumer acceptability, and low cost [7]. Thus, proteins are relevant due to the fact that they are the most important surface-active compounds in plan t materials [8]. However, several plant proteins present low water-solubility and emulsifying properties in comparison to synthetic surfactants, which restrain their performance in many aqueous-based food formulations [9]. Therefore, some vegetable proteins must be modified to improve their functional properties and performance. In general , protein modifications can be reached by physical , chemical, and biologica l 1 m m ethods.To date, some studies have addressed extensive or mild protein hydrolysis as a way to tailor the f unctional properties of plant proteins [10-19]. Liu et al. [11] found that mild hydrolysis of rice protein i solates resulted in the enhancement of emulsifying properties. In addition,Ecker t et al. [14] showed that the extensive hydrolysis of fava bean proteins enhanced their solubili t y, foaming, and emulsifying properties. However, the plant protein modifications depend on the processing type, pH, concentration, and original structure [20]. The enzymatic hydrolysis by proteases stands out to be the most auspicious method for protein modification to formulate tailor-made foods, particularly due to reactants and by-products being innocuous [1,21]. Also, this type of modi f ication presents some advan-tages over other modification methods, such as i t can be reached by moderate condi t ions with only a few by-products, the chemical composition of the protein being preserved,and the fast enzyme reaction time and specificity [1]. Proteases based on characteristic mechanistic features are classified i nto six groups: cysteine, aspartate, glutamate, serine,metallo, and threonine [22]. There is a wide variety of proteases in commercial use, which range from detergent additives to therapeutics [23]. However, proteases today are be-coming increasingly used to improve the functional properties of proteins. Concerning improvements in the emulsifying capacity of enzymatic hydrolysis derivatives of proteins,it has been found to depend on the protein source and hydrolysis conditions, such as the degree of hydrolysis, the type of enzyme, and the pH of the medium [24]. Currently,some research has analyzed the emulsifying properties of different proteins hydrolyzed with enzymes. For instance, Xu et al . [25] showed that partially hydrolyzed rice protein enhanced its emulsion stability. Also, Padial-Dominguez et al. [24] found that partially hydrolyzed soy protein presented improved emulsifying properties than i ts counterpart.Otherwise, Avramenko et al . [7] found t hat partially hydrolyzed lentil protein reduced t heir solubility and emulsifying properties as a function of the degree of hydrolysis (%). Consequently, this study aims to research the effect of enzymatic modifications of lupin proteins on the solubility and emulsifying properties produced by some commercial enzymes. Thus, enzymatic hydrolysis of lupin proteins could enhance their emulsifying properties to be used as a natural emulsifier of O/W emulsions for food formulations. It is important to mention that the effect of protein modifications of the lupin protein-rich variety AluProt-CGNA has not been previously investigated. Therefore, t he knowledge acquired from this research could be utilized to select the appropriate enzyme and hydrolysis conditions for a specific food application. 2. Materials and Methods 2.1. Materials Dehulled yellow l upin seeds (Lupinus luteus) variety AluProt-CGNA were provided by CGNA (Agriaquaculture Nutritional Genomic Center, Temuco, Chile). Analytical grade chemicals such as hydrochloric acid (HCl), sodium hydroxide (NaOH), Tris, glycerol,glycine, sodium dodecyl sulphate (SDS), bromophenol blue (BPB), and 2-mercaptoethanol were acquired from Sigma (St. Louis, MO, USA). The i nformation about commercial enzymes is l isted in Table 1. Table 1. Sources and properties of the commercial enzymes used. Enzyme Type Biological Source Supplier Activity (Under Optimal Conditions) Acid Stable Protease Aspartic endopeptidase Aspergillus niger Bio-Cat (Troy, VA,USA) 4000 SAP/g Fungal Protease A Aspartic exo-and endopeptidase Aspergillus oryzae Bio-Cat (Troy, VA,USA) 1,000,000 HUT/g Opti-ZiomeTM P3 HydrolyzerIMM NeutraI Protease Aspartic exo-and Aspergillus oryzae, Bio-Cat (Troy, VA,USA) 130,000 HUT/g endopeptidase Aspergillus melleus Metallo endopeptidase Bacillus subtilis Bio-Cat (Troy, VA,USA) 2,000,000 PC/g Protamex Serine endopeptidase Bacillus licheniformis, Bacillus amyloliquefacies Novozymes A/S (Bagsvaerd, Denmark) 1.5AU-N/g Alcaline Protease L Serine endopeptidase Bacillus licheniformis Bio-Cat (Troy, VA,USA) 625,000 DU/g Alcalase2.4L FG Serine endopeptidase Bacillus licheniformis Novozymes A/S (Bagsvaerd, Denmark) 2.4AU-A/g 2.2. Preparat i on of Lupin Protein Isolate (LPI) Lupin protein isolate (LPI) from lupin seeds was obtained according to the procedure described by Burgos-Diaz et al. [31] with minor modifications. The lupin seeds were milled using a rotor mill (Fri t sch Mi l l Pulverisette 14, Indar-Oberstein, Germany) at 10,000 rpm and sieved to 200 um size to turn into flour. Subsequently, the flour was suspended i n a puri f ied water ratio of 1:10 (w/v), and the pH was adjusted to 9.0 ±0.1 by the addition of 1 M NaOH and stirred for 1 h at room temperature. Later, the suspension was centrifuged using a laboratory-scale centrifuge (GYROZEN 1580R, Daejeon, Korea) at 3500 rpm for 15 min at 20 °C. The supernatant containing the extracted proteins was shifted to the isoelectric point (pI) at pH 4.6 ±0.1 using 1M HCl to precipitate proteins and stirred for 1 h at room temperature. Afterwards, the suspension was subjected to centrifugation (GYROZEN 1580R, Daejeon, Korea) to separate the proteins under the same conditions mentioned above. The precipitated protein was neutralized by re-suspending it i n purified water (1:5, w/v), bringing the pH to a value of 7.0±0.1 using 1M NaOH and frozen to subsequently freeze-dried using a lyophilizer (Liobras, Liotop LP1280, Sao Carlos, Brazil).Finally, the LPI powder was kept in a plastic bag and stored a t room temperature until later use. 2.3. Chemical Analysis of LPI The chemical analysis of LPI samples was carried out through the use of a Dumas Nitrogen Analyser (DumathermN Pro, Konigswinter, Germany) to determine the nitrogen content of LPI and then multiplied by a conversion factor of 6.25. The moisture was measured using the gravimetric method according to NCh 841 of 78. 2.4. Enzymatic Hydrolysis ofLPI LPI samples were enzymatically hydrolyzed in a 0.5 L bioreactor with pH-and temperature-adjusted to the optimal conditions of each commercial protease (Table 2).LPI suspensions were prepared by diluting LPI in purified water (1.5:10, w/v) and stirring unti l complete hydration. Three enzyme-to-substrate ratios (E/S) were chosen (0.01, 0.02,and 0.04% mg enzyme/g protein). Later, the enzyme was added to protein suspension and stirred while temperature and pH were kept constant. The proteins were hydrolyzed from 1to 5 h and, after hydrolysis, heated at 90 °C for 5 min for enzyme i nactivation. Subsequently,the protein hydrolysates were cooled and brought to neutral pH (7.0 ±0.1) for neutral-ization. Finally, the hydrolysates samples were lyophi l izer (Liobras, Liotop LP1280,Sao Carlos, Brazil) and milled using a rotor mill (Fritsch Mill Pulverisette 14, Indar-Oberstein,Germany) at 10,000 rpm and sieved to 200 um size to turn into flour. The control samples used in this study were treated under the same processing conditions without the addition of the enzymes. The hydrolysis and control samples were performed in triplicate. Table 2. Characteristics of the protease preparat i ons during the LPI hydrolysis. Enzyme E/S(%) Temperature (°C) pH Value Time (h) Acid Stable Protease 0.01/0.02/0.04 55 2.5 1/2/5 Fungal Protease A 0.01/0.02/0.04 60 3.0 1/2/5 Opti-ZiomeTM p3 HydrolyzerTM 0.01/0.02/0.04 60 6.0 1/2/5 Neutral Protease 0.01/0.02/0.04 55 7.0 1/2/5 Protamex@ 0.01/0.02/0.04 55 8.0 1/2/5 Alcaline Protease L 0.01/0.02/0.04 55 8.5 1/2/5 Alcalase2.4 L FG 0.01/0.02/0.04 70 9.0 1/2/5 2.5. SDS-PAGE Analysis The molecular weight (MW) distribution of protein dispersions was done by reducing SDS-PAGE electrophoresis in a mini-vertical gel electrophoresis unit (Mini-PROTEANW Tetra Cell, Bio-Rad Laboratories, Inc., Richmond, CA, USA) as described by Cepero-Betancourt et al. [32]. For analyses, the sample was di l uted in sample buffer (1:1), which consisted of 0.5 M Tris-HCl; pH 6.8;2% v/v SDS; 2.5% v/v glycerol; 0.2% v/v bromophenol blue; and 0.5% v/v 2-mercaptoethanol and incubated at 90 °℃ for 4 min using a digital dry bath (Accu BlockTM, Labnet International Inc., Edison, NJ, USA). The gel bands were visualized by Coomassie staining G-250, while gel images were visualized using a digi-tal imaging system (NUGenius, Syngene, Cambridge, UK). An amount of 15 uL of each sample and 12 uL of pre-stained standard molecular weight marker (Precision Plus Protein Kaleidoscope, Bio-Rad Laboratories, Hercules, CA, USA) were loaded on a 12% Tris-HC1Mini-PROTEAN TGX Precast Gel (Bio-Rad Laboratories Inc., Hercules, CA, USA) and SDS-PAGE was carried out at a constant voltage of 200 V. 2.6. Degree of Hydrolysis (DH) The degree of hydrolysis (DH) attained was determined by the OPA method, according to Opazo-Navarrete et al. [33]. The OPA reagent (100 mL) was prepared the same day and stored i n a bottle protected from l ight. L-serine was used to prepare the standard curve (50-200 mg/L). For determination, 200 puL of the sample (or standard) was mixed with 1.5 mL of OPA reagent. Finally, the samples were measured after 3 min of reaction with the OPA reagent at 340 nm using an HT Mul t i-Detection Microplate reader (Biotek Instruments Inc., Winooski, VT, USA). To determine the DH values of hydrolyzed and unhydrolyzed samples, absorbance values were converted to f ree amino groups (mmol/L)utilizing the standard curve and subtracting the free amino groups that were already present in the protein samples. Finally, serine amino equivalents (N-Terminal Serine) were utilized to express the free amino groups. Thus, the DH values were estimated using the following equations: where t he constant values a and B used were equal to 1 and 0.4, respectively [34]. The htot value was calculated based on the concentration of each amino acid present in the lupin proteins, which was 7.73 meq/g. All the measurements were done in triplicate. 2.7. Protein Solubility The protein solubility (%) of the hydrolyzed and unhydrolyzed LPI samples was determined in triplicate according to the method described by Morr et al. [35] with some modifications. For each measurement, was prepared a 3% (w/u) protein suspension in purified water. The pH value of suspensions was adjusted to pH 5.0, 7.0, and 9.0 by adding 0.1 M HC l or 0.1 M NaOH as appropriate. Subsequently, the protein suspensions were stirred for 1 h a t room temperature, centrifuged at 15,520× g for 15 min at 20°C (GYROZEN 1580R, Daejeon, Korea), and the supernatant was transferred to another tube to separate from the non-dissolved fraction. The supernatant was frozen at -20 °C and freeze-dried using a lyophilizer (Liobras, Liotop LP1280, Sao Carlos, Brazil). Finally, the freeze-dried sample was weight, and the protein content was determined by Dumas according to the methodology described above (Section 2.3), while protein solubility was estimated as follows: sample mass supernatant (mg) x protein content supernantant t((%)Protein solubility (%)=Sai -×100 (3)sample mass (mg) × protein content(%) 2.8. Emulsifying Capacity (EC) and Emulsion Stability (ES) The emulsifying capacity (EC) and emulsion stability (ES) were determined according to Burgos-Diaz et al . [36] with minor modifications. Thus, 0.2 g of LPI powder was added to 20 mL of distilled water (1%, w/v) in a 50-mL Falcon tube and shaken for 1 h at room temperature through constant stirring using an orbital shaker (Multi Reax, Heidolph Instruments, Schwabach, Germany). Subsequently, the sample was kept at 4C overnight.Further, the sample was adjusted to pH 7, 20 mL of sunflower oil was added (1:1), and the sample was homogenized for 2.5 min at 10,000 rpm uti l izing an Ultra-Turrax (IKA-Werke GmbH & Co. KG, Staufen, Germany). After, the sample was allowed to stand at room temperature for 1 h. The emulsions were transferred to the test tubes, while total and emulsion layer height was measured at 0 and 24 h. The EC24 was estimated as the relation between the height of the emulsion layer at 24 h (HeL) and the total height of the sample (HT). For its part, the ES was calculated by dividing the EC24 by the EC at the start time (EC0). 2.9. Statistical Analysis Statistical analysis was performed using Statgraphics Centurion XVI version 16.1.11software (Statistical Graphics Corp., Herndon, VA, USA). The data were subjected to a one-way analysis of variance (ANOVA) and Duncan's multiple range test (DMRT) to determine significance differences (p<0.05). All results in this research were expressed as mean values ± standard deviations. 3. Results and Discussions 3.1. Enzymatic Hydrolysis of Lupin Proteins The Dumas analysis of LPI and their hydrolysates presented a protein content of 94.3% ±0.2, and the dry matter was 98.3%..T The degree of hydrolysis (DH) analyzed by the OPA method showed that LPI samples without adding enzymes presented a DH value of 0.82% ±0.01. DH values differed by every enzyme treatment according to their protease specificity. The DH values of all enzymes progressed with the advancement of the hydrolysis t ime (Figure 1). Meanwhile, the Neutral Protease showed a fast hydrolysis rate during the first 3 h, and the rate subsequently decreased until reaching a stationary.This i i s s i typical behaviour of enzymatic reactions where the rate of hydrolysis decreases,which is due to a decrease of peptide bonds, causing the proteases and their substrates to reach a saturation state [37]. However, acidic proteases and Alcaline Protease L showed a low hydrolysis rate with a linear behaviour. Therefore, different results within the same protease family might be due to substrate specificity. Figure 1. Degree of hydrolysis (DH) of LPI hydrolysates by different commercial enzymes. (A) Acid Stable Protease, (B) Fungal Protease A, (C) Opti-ZiomeM p3 HydrolyzerM,(D) Neutral Protease,(E) Protamex@, (F) Alcaline Protease L, and (G) Alcalase@ 2.4 L FG. The DH was strongly dependent on the E/S ratio i n most of the enzymes except for aspartic proteases (Acid Stable Protease and Fungal Protease A) and a serine protease (Alcaline Protease L), where the increase in the E/S ratio does not have a great i nf l uence in the DH values. Among the proteases, Protamex @,Neutral Protease, and Alcalase@2.4L FG showed higher DH values after 5 h of protein hydrolysis. This result i s different from those previously found i n hydrolyzed pea protein isolate (PPI) using Protamex at an E/S ratio of 0.5% [21]. In the mentioned study, after 2 h of hydrolysis reached a DH value of 4.15%, which is lower than those found in our study (8.51%) using an E/S ratio of 0.04%.In addition, Garcia Arteaga et al. [21] showed a higher DH value for PPI hydrolyzed by Alcalase 2.4 L FG (9.24%) for 2 h at an E/S ratio of 0.5%, which i s a 12.5-times higher E/S ratio that used in this study. However, the protein hydrolysis of LPI by Alcalase@ 2.4 L FG showed to be strongly dependent on the E/S ratio. Thus, higher DH values could be expected using the same E/S ratio. On the other hand, acidic proteases showed lower DH values in comparison with alkaline and neutral proteases. Shu et al. [38] indicated that alkaline protease-assisted reactions exhibited higher DH values compared to neutral or acid enzymes from microbial,animal, or plant origin. However, the Alcaline Protease L exhibited lower DH values than the other alkaline enzymes. This could indicate a lower activity of Alcaline Protease L to hydrolyze lupin proteins. Meanwhile, the lower DH values of the acid proteases could be a consequence of the pHs utilized to hydrolyze lupin proteins with these enzymes (2.5and 3.0) near the pI of the lupin proteins (4.5), which could resul t in a certain degree of aggregation and masking of cleavage sites. This phenomenon was previously described by Abdel-Hamid et al. [39] i n a study on buffalo milk proteins. Nevertheless, Alcaline Protease L seems to be an exception due to the LPI hydrolyzed by this enzyme exhibiting lower DH values in comparison with other alkaline proteases, which could be a consequence of a lower activity at these E/S relations in comparison with the other alkaline proteases used in this study. 3.2. Molecular Weight Distributions (SDS-PAGE) According to the SDS-PAGE results, the E/S ratios seem to be an effect mainly in α-and B-conglutin frac t ions, while the time had an effect i n the increase of the hydrolysis of the same fractions, which i s evidenced by the faded of these bands. However, in this study,clear differences were observed among the different E/S ratios and hydrolysis times, which cannot be correlated with the DH results. These results are important because they could potentially be used to predict some functional properties of proteins. Figure 2. Molecular weight (kD) profiles of LPI hydrolysates by different commercial proteases at different E/S ratios and hydrolysis times as determined by SDS-PAGE under reducing conditions.(A) Acid Stable Protease, (B) Fungal Protease A, (C) Opti-ZiomeTM p3 HydrolyzerM,(D) Neutral Protease, (E) Protamex@, (F) Alcaline Protease L, and (G) Alcalase@2.4 L FG. 3.3. Functional Properties 3.3.1. Protein Solubility The solubility of each LPI hydrolyzed at different E/S ratios was estimated as a func t ion of pH at values of 5.0, 7.0, and 9.0 (Figure 3). Untreated LPI samples showed solubility of 24.4%±0.5 at pH 5.0, 50.1%±0.8 at pH 7.0, and 78.0%±1.5 at pH 9.0. Meanwhile, protein solubility of all hydrolyzed LPI samples was significantly higher (p<0.05) than untreated LPI at al l pH analyzed values. Enzymatic hydrolysis providesb etter interaction of hydrophilic groups with the water molecules due to the decreased size of peptides, which increases protein solubility [18]. Thus, an increase in protein solubility could be a consequence of several facts, such as protein structural changes, the formation of small peptides and hydrophilic amino acids, and changes in the electrostatic forces [41]. Figure 3. Protein solubility of the hydrolyzed lupin protein isolate (LPI) samples at di f ferent E/S (enzyme/substrate) ratios and hydrolysis times by different commercial proteases measured at different pHs. (A) Acid Stable Protease, (B) Fungal Protease A, (C) Opti-ZiomeM p3HydrolyzerM,(D) Neutral Protease,(E) Protamex @, (F) Alcaline Protease L, and (G) Alcalase@ 2.4 L FG. Means with different capital letters i n t he same row indicate significant differences i n each hydrolysis time (p<0.05). Means with different lowercase letters i n the same column i ndicate significant differences in each E/S ratio (p<0.05). The protein solubility of hydrolyzed LPI samples was strongly dependent on pH obtaining higher values at pH 9.0. Conversely, the lower values were obtained a t pH 5.0,near t he pI of l upin proteins. The decrease in solubility a t low pH is a consequence of the lack of elec t ric charge, which i ncreases hydrophobic aggregation and precipitation [42]. The same was found by Schlegel et al. [19], where the LPI hydrolyzed by various proteases (Neutrase 0.8L, Corolase 7089, Papain, and Alcalase@ 2.4 L FG) exhibited higher protein solubility at pH 9.0 and lower at pH 5.0. The increase in protein solubility of hydrolyzed LPI in acidic conditions compared to unhydrolyzed LPI can be attributed to the protein hydrolysis generating low molecular weight soluble peptides [43].Meanwhile, Schlegel et al. [19] indicated that the main influence that affects the protein solubility characteristics corresponds to hydrophobic interactions, which increase protein-protein interactions and decrease solubility. Protein solubility is an important property because an increase in the water-solubility of plant proteins is commonly correlated with some functional properties such as emulsifying, gelling, and foaming [11]. In addition, protein solubility at pH 5.0 was significantly dependent (p<0.05) on the E/S ratio, proteolysis time, and commercial enzyme used to hydrolyze the LPI. Meanwhile,protein solubility at pH 7.0 for all hydrolyzed LPI samples varied between 64-67% by Fungal Protease A (E/S=0.01) to 83-88% by Alcaline Protease L (E/S=0.04). These results are higher than those previously found at neutral pH on faba bean protein (24.7%), chickpea (47%), and pea (63-75%)[14]. Finally, the protein solubility at pH 9.0 exhibited higher values reaching values near 90% for LPI hydrolyzed by Acid Stable Protease (E/S=0.04). Hydrolysis by Acid Stable Protease produced polypeptides with molecular weight >20 kD (Figure 2). These results are higher than those found on rice proteins hydrolyzed by Alcalase and Papain, where values close to 50% were reached [11]. 3.3.2. Emulsion Capac i ty (EC) and Emulsion Stability (ES) of Hydrolyzed LPI Emulsification is one of the most important processes in the manufacturing of for-mulated foods. The oil-in-water (O/W) emulsions combining proteins and lipids with aqueous solutions are the most commonly used in the food industry [19]. The effect of protein hydrolysis on EC and ES of LPI is shown in Table 3. The EC of undigested LPI was 81.0% ±1.7, which was lower than most of the hydrolysates, except for acidic pro-teases Acid Stable Protease and Fungal Protease A. Both acidic proteases showed lower DH values in comparison with the other proteases (Figure 1), which could be indicative that these enzymes could release more hydrophobic groups. Liu et al. [44] indicated that greater exposure by the hydrophobic groups of proteins could improve their emulsifying properties, mainly due to the enhancement of the electrostatic repulsive force between emulsion droplets. Shen et al. [45] reported that hydrophobic interactions enhanced inter-actions between proteins and then produced large aggregates, which increased in particle size. Furthermore, Zhang et al. [46] demonstrated that the formation of aggregates might decrease the flexibility of proteins to adsorb at the surface of l ipid droplets, thus decreasing emulsifying properties. Pan et al. [47] l inked the decrease in emulsifying properties to the particle size, where a larger particle size had a negative influence on EC and ES. In this study, there is an inverse correlation between the EC of proteins and protein solubi l ity in hydrolyzed LPI samples, where less soluble samples showed a higher EC. These results are opposed to those found by Schlegel et al. [19], where a direct correlation was found between EC and protein solubility. They indicated that this behaviour was a consequence of a greater dissolution of proteins in the emulsion, which means that a higher amount of proteins is found in the oil-in-water interface during emulsification. The effect of the E/S ratio on EC did not show great changes after 2 h of hydrolysis. However, after 5 h of protein hydrolysis, an increase in E/S ratio presented a significant decrease (p<0.05) in EC values, except for the hydrolyzed LPI samples with Opti-ZiomelM p3 HydrolyzerTM and Neutral Protease. T able 3. Emulsifying properties of LPI hydrolyzed by different commercial enzymes. Functional Property Hydrolysis Time (h) E/S(%) Acid Stable Protease Fungal Protease A Opti-ZiomeTM P HydrolyzerM Neutral Protease Protamex Alcaline Protease L Alcalase 2.4 L FG 1 0.01 0.02 85.7±0.6b,F 82.0±2.0a.C 83.3±1.5a,E 86.7±0.6a,D 73.3±1.2a,B 72.3±0.6a.A 80.0±0.0a,D 77.7±2.5a,B 78.0±1.0b,C 77.7±1.5b,B 70.3±0.6a.A 78.3±0.6b,B 78.0±1.0b,C 74.7±0.6a.A 0.04 83.3±1.5 a,b,C二 82.7±3.2a,C 71.7±1.5a.A 82.7±4.0a.C 75.0±0.1a.A,B 77.7±2.1b,B 75.3±0.6 a.A,B EC(%) 0.01 86.0±1.7b,E 84.3±1.2a,D 73.3±0.6a.A 77.7±0.6a,B 79.7±0.6b,C 78.3±0.6 a,B,C 79.7±0.6b,C 2 0.02 86.3±1.5b,D 82.0±1.0a.C 74.3±0.6b,A 79.0±1.0a,B 79.0±1.0b,B 77.0±2.6a,B 79.0±1.0 a,b,B 0.04 75.7±0.6 a,A,B 83.0±2.0a.C 75.0±0.1b,A 78.3±2.9a,B 75.0±1.0a.A 77.0±2.0 a,A,B 76.3±2.3 a.A.B 0.01 86.0±2.0b,D 84.7±1.5b,D 71.7±1.2a,b,A 75.3±1.2a,B 84.0±1.7b,D 80.3±0.6CC 84.0±1.7b,D 5 0.02 83.3±1.2b,D 81.7±2.3 a,b,D5 71.3±0.6a,B 82.0±2.6b,B 81.7±1.5b,D 66.3±1.5b.A 77.7±1.5a.C 0.04 80.3±0.6a,D 80.3±1.2a,D 73.0±0.0b,B 85.3±1.2b,E 77.7±1.5a,C 61.3±2.3a.A 79.3±1.2 a,C,D 1 0.01 0.02 81.3±1.1a,A 86.6±1.5b,B 88.0±2.2b,B 81.2±0.6a.A 95.9±1.4a,C 96.8±0.8a,E 97.1±4.0b,C,D 90.4±0.7 a,b,C 98.7±1.3b,C,D 94.0±0.8aD 90.6±1.9a,B 93.4±1.6a,D 99.5±0.8CD 93.6±1.3b,D 0.04 84.8±2.2b,A 84.7±2.3 a,b,A 97.7±1.6a.C 84.8±4.4a.A 91.0±3.3a,B 96.6±0.8b,C 88.9±0.8 a,A,B ES(%) 0.01 79.9±1.4a.A 87.0±1.0a,B 95.9±0.0a.上 94.4±2.0b,D,E 93.7±1.2a.D,E 89.8±1.2a.,C 93.3±1.4a.D 2 0.02 82.3±2.4a.A 88.2±1.5a.B 95.5±3.3a,D 94.9±0.1 b,C,D 92.4±2.1 a,C,D 91.8±1.7 aB.C 92.4+2.1 0.04 94.3±1.5 b,B.C 88.4±3.4a.A 93.8±0.8a,B,C 89.0±2.6a.A 96.9±0.7b,C 96.1±1.2b,C 90.9±2.4 a,A,B 87.4±2.6 0.01 81.4±1.0a.A 85.5±2.5a,B 97.7±0.8b,D 93.1±0.9b,C 97.9±1.9b,D 91.3±1.3a.C 92.1±1.7a.C 5 0.02 85.2±0.6b,A a,A,B3 98.1±0.8b,E 94.7±1.4b,D 92.1±1.7a,C,D 90.0±1.5 a,B.C 97.9±1.9b,E 0.04 87.6±0.1C.A 88.0±1.6 a,A,B 93.2±2.4a.C 87.5±1.3a.A 89.8±1.7 aABC 91.3±4.0 a,B,C 93.3±0.7a.C EC: emulsifying capac i ty; ES: emulsifying stabil i ty. Means with different capita l l etters i n the same row indicate significant differences in each enzyme (p <0.05). Means with different lowercase letters in the same column indicate significant di f ferences i n each hydrolysis t i me (p<0.05). The ES can be defined as t he resistance of the system to changes in its physicochemical properties with time. The ES of LPI presented a value of 94.2% ± 0.8, which is significantly higher (p<0.05) than most hydrolysate LPI samples by acidic proteases, except for LPI hydrolyzed with Acid Stable Protease with an E/S ratio of 0.04. The hydrolyzed samples of LPI with alkaline proteases showed higher ES values than acidic proteases in most of the studied samples, mainly in the samples hydrolyzed for 1 or 2 h at low E/S ratios. In general, hydrolysates with low DH showed lower ES values. This is contrary to what has been mentioned by other authors, who have found a negative correlation between DH and ES when hydrolyzed whey protein using Alcalase [48]. In this study, the high emulsion instability found could be a consequence of a larger number of low-molecular-weight peptides. However, SDS-PAGE analysis showed that acidic proteases form polypeptides of PeP smaller molecular weight than alkaline proteases (Figure 2). Therefore, t he current study is in agreement wi t h the previous findings. Regarding the E/S ratio, there is no correlation with the ES values. This could be due to the fact t hat during protein hydrolysis of LPI,some peptides exhibited more hydrophobic than hydrophilic areas, while as the hydrolysis time progressed, this relation changed. In this regard, several reports have suggested that there is an optimum molecular size or chain length for peptides to improve functional properties and limited hydrolysis generally leads to improved func t ional properties [49].Therefore, the degree of hydrolysis should be carefully studied to achieve an improvement in functional properties by enzymatic hydrolysis of LPI. 4. Conclusions This study demonstrated that t he solubility and emulsifying properties of l upin (Lupi-nus luteus) proteins could be enhanced by controlled enzymatic hydrolysis using some specific commercial enzymes. The results show that acidic proteases used i n this study are the most efficient for hydrolyzing lupin proteins to obtain polypeptides of low molecular w eight. However, the alkaline proteases studied showed to be more effective in achieving free amino groups f rom l upin proteins, except for Alcaline Protease L. The DH (%) values increased with the hydrolysis time, and the highest DH values were logged with Neutral Protease for the E/S ratio of 0.01 and Protamex@ for E/S ratios of 0.02 and 0.04, which indicates t hat Protamex has a greater ability to hydrolyze lupin proteins. The obtained results indicated that hydrolysis of LPI using the commercial acidic proteases studied can be used to i mprove the emulsifying capacity (EC) of lupin proteins. However, the protein hydrolysis by acidic proteases decreases the emulsifying stability (ES). These findings demonstrate that enzymatic hydrolysis by commercial enzymes under controlled conditions is an effective way to improve the functional properties of lupin pro-teins and thus increase the potential use of lupin protein ingredients in food formulations. Author Contributions: Conceptualization,M.O.-N. and C.B.-D.; formal analysis, M.O.-N., C.B.-D.,K.A.G.-M. and S.A.-N.; funding acquisition, M.O.-N.; investigation, M.O.-N., C.B.-D., K.A.G.-M.and S.A.-N.; methodology, M.O.-N., C.B.-D. and S.A.-N.; project administration, M.O.-N.; resources,M.O.-N. and C.B.-D.; supervision , M.O.-N.; validation, M.O.-N.; visualization, K.A.G.-M.; writing一 original draft, M.O.-N. and K.A.G.-M.; writing-review and editing, M.O.-N., C.B.-D., K.A.G.-M. and S.A.-N. All authors have read and agreed to the published version of the manuscript. Funding: This research was funded by “Agencia Nacional de Investigacion y Desarrollo"(ANID,Chile) through FONDECYT-REGULAR project N°1210136 and FONDECYT-POSTDOCTORAL 3220459. Institutional Review Board Statement: Not applicable Informed Consent Statement: Not applicable. Data Availabi l ity Statement: Not applicable. Acknowledgments: We also acknowledge the Chilean Agency for Research and Development (ANID)and CGNA's chemical unit for their technical assistance . Conflicts of Interest: The authors declare no conflict of interest. References 1. Nikbakht Nasrabadi , M.; Sedaghat Doost , A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use i n Food Products. Food Hydrocoll. 2021,118,106789.[CrossRef ] 2. McClements, D.J .; Lu, J .; Grossmann, L. Proposed Me t hods for Testing and Comparing the Emulsifying Properties of Proteins from Animal, Plant, and Alternative Sources. Colloids Interfaces 2022, 6,19. [CrossRef ] 3. Burgos-Diaz,C.;Opazo-Navarrete, M.; Wandersleben, T.; Soto-Afual , M.; Barahona, T.; Bustamante, M. Chemical and Nutritional Evaluation of Protein-Rich Ingredients Obtained through a Technologica l Process f rom Yellow Lupin Seeds (Lupinus luteus). Plant Foods Hum. Nutr. 2019, 74,508-517.[CrossRef] [PubMed ] 4. Nikbakht Nasrabadi , M.; Gol i , S.A.H.; Sedagha t Doost, A.; Dewet t inck, K.; Van der Meeren, P. Bioparticles of Flaxseed Protein and Mucilage Enhance the Physical and Oxidative Stability of Flaxseed Oil Emulsions as a Potential Natural Alternative for Synthetic Sur f actants. Colloids Surf . B Biointerfaces 2019,184,110489. [CrossRef ] 5. Hu, G.J.; Zhao, Y.; Gao, Q.; Wang, X.W.; Zhang, J .W.; Peng, X.; Tanokura, M.; Xue, Y .L. Functional Properties of Chinese Yam (Dioscorea opposita Thunb. Cv. Baiyu) Soluble Protein. J. Food Sci . Technol . 2018, 55, 381-388. [CrossRef ] 7 Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013,51, 162-169.[CrossRef ] 8. Burgos-Diaz, C.; Mosi-Roa, Y.;Opazo-Navarrete, M.; Bustamante, M.; Garrido-Miranda, K. Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capac i ty. Foods 2022,11,2514.[CrossRe f 9. McClements, D.J.; Gumus, C.E. Natural Emulsifiers—Biosurfac t ants, Phospholipids, Biopolymers, and Colloidal Particles:Molecular and Physicochemical Basis of Functional Performance. Ado. Colloid Interface Sci . 2016,234,3-26.[CrossRef ] 12. Gomes, M.H.G.; Kurozawa, L.E. Improvement of the Functiona l and Antioxidant Properties of Rice Protein by Enzymatic Hydrolysis for the Microencapsulation of Linseed Oil . J. Food Eng. 2020, 267, 109761. [CrossRef ] 13. Al-Ruwaih, N.; Ahmed,J .; Mulla, M.F; Arfat , Y.A. High-Pressure Assisted Enzymatic Proteolysis of Kidney Beans Protein I solates and Characterization of Hydrolysates by Functional, Structural, Rheologica l and Antioxidant Properties. LWT Food Sci. Technol.2019,100,231-236.[CrossRef ] 14. Eckert, E.; Han, J .; Swallow, K.; Tian, Z.; J arpa-Parra, M.; Chen, L. Effects of Enzymatic Hydrolysis and Ultrafiltration on Physicochemical and Functional Properties of Faba Bean Protein. Cereal Chem. 2019, 96,725-741.[CrossRef ] 15. Bruckner-Guhmann,M.;Heiden-Hecht, T .; Sozer, N.;Drusch, S. Foaming Characteristics of Oat Protein and Modification by Partia l Hydrolysis. Eur. Food Res. Technol. 2018,244,2095-2106.[CrossRef ] 16. Chen, L.; Chen, J; Yu, L.; Wu, K.;Zhao, M. Emulsification Performance and Interfacial Properties of Enzymically Hydrolyzed Peanut Protein Isolate Pretreated by Extrusion Cooking. Food Hydrocoll . 2018, 77,607-616. [CrossRef ] 17. Chen, L.; Chen, J.; Yu, L.; Wu, K. Improved Emulsifying Capabilities of Hydrolysates of Soy Protein Isolate Pretreated with High Pressure Microfluidization. LWT Food Sc i . Technol. 2016,69,1-8. [C rossRef ] 18. Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci . Food Saf. 2016, 15,786-800. [CrossRef ] 19. Schlegel, K.; Sontheimer, K.; Hickisch, A.; Wani, A.A.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic Hydrolysis of Lupin Protein Isolates-Changes i n t he Molecular Weight Distribution, Technofunctiona l Characteristics, and Sensory Attributes. Food Sci. Nutr.2019,7,2747-2759. [CrossRef ] 20. Muhoza, B.; Qi , B.; Harindintwali, J .D.; Farag Koko, M.Y.; Zhang, S.; Li , Y. Combined Plant Protein Modification and Complex Coacervation as a Sustainable Strategy to Produce Coacervates Encapsulating Bioactives. Food Hydrocoll. 2022,124,107239.[CrossRe f 21. C Garcia Arteaga, V.; Apestegui Guardia, M.; Muranyi , I .; Eisner, P.; Schweiggert-Weisz, U. Effect of Enzymatic Hydrolysis on Molecular Weight Distribution, Techno-Functional Properties and Sensory Perception of Pea Protein Isolates. Innov. Food Sci.Emerg. Technol. 2020,65,102449. [CrossRef ] 22. Lopez-Otin, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283,30433-30437.[CrossRef ][PubMed ] Li, Q.; Yi, L.; Marek, P.; Iverson, B.L. Commercial Proteases: Prese 23.nt and Future. FEBS Lett . 2013, 587,1155-1163. [CrossRef ]Padial-Dominguez, M.;Espejo-Carpio, FJ; Pérez-Galvez, R.; Guadix, A.; Guadix, E.M. Optimization of the Emulsifying Properties of Food Protein Hydrolysates for the Production of Fish Oil-in-Water Emulsions. Foods 2020, 9,636. [CrossRef ] [PubMed ] 25. Xu, X.; Zhong, J; Chen, J.; Liu, C.; Luo, L.; Luo, S.; Wu, L.;McC l ements, D.J . Effec t iveness of Partially Hydrolyzed Rice Glutelin as a Food Emulsifier: Comparison to Whey Protein. Food Chem. 2016,213,700-707. [CrossRef ] [PubMed] 26. Hickisch, A.; Bindl , K.; Vogel, R.F.; Toelstede, S. Thermal Treatment of Lupin-Based Milk Alternatives-Impact on Lupin Proteins and the Network of Respective Lupin-Based Yogurt Alternatives. Food Res . I nt. 2016, 89,850-859. [CrossRef ] 27. Cabello-Hurtado, F; Keller, J.; Ley, J;Sanchez-Lucas, R.; Jorrin-Novo, J.V.; Ainouche, A. Proteomics for Exploiting Diversity of Lupin Seed Storage Proteins and Their Use as Nutraceuticals for Health and Welfare. J. Proteom. 2016,143,57-68.[CrossRef ] 28. Starkute, V.; Bartkiene, E.; Bartkevics, V.;Rusko,J.; Zadeike, D.; Juodeikiene, G. Amino Acids Profile and Antioxidant Activity of Different Lupinus angustifolius Seeds after Solid State and Submerged Fermentations. J. Food Sci. Technol. 2016,53,4141-4148.[CrossRef | 29. Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow Lupin (Lupinus luteus L.) Polysaccharides: Antioxidant,Immunomodulatory and Prebiotic Activities and Their Structural Characterisation. Food Chem. 2018, 267,319-328. [CrossRef ] 30. Burgos-Diaz, C.; Piornos, J.A.; Wandersleben,T.; Ogura, T .; Hernandez, X.; Rubilar, M. Emulsifying and Foaming Properties of Different Protein Fractions Obtained from a Novel Lupin Variety AluProt-CGNA@ (Lupinus luteus). J. Food Sci. 2016, 81,C1699-C1706. [CrossRef ] 31. Burgos-Diaz, C.;Opazo-Navarrete, M.; Soto-Afual , M.;Leal-Calderon, F.; Bustamante,M. Food-Grade Pickering Emulsion as a Novel Astaxanthin Encapsulation System for Making Powder-Based Products: Evaluation of Astaxanthin Stability during Processing, Storage, and Its Bioaccessibility. Food Res. Int. 2020,134,109244.[CrossRef ] [PubMed ] 32. Cepero-Betancourt, Y.;Opazo-Navarrete, M.;Janssen,A.E.M.; Tabilo-Munizaga, G.; Perez-Won, M. Effects of High Hydrostatic Pressure (HHP) on Protein Structure and Digestibility of Red Abalone (Haliotis rufescens) Muscle. Innov. Food Sci . Emerg. Technol .2020,60,102282.[CrossRe f 33. Opazo-Navarrete, M.; Tagle Freire, D.; Boom, R.M.; Janssen, A.E.M. The Influence of Starch and Fibre on In Vitro Protein Digestibility of Dry Fractionated Quinoa Seed (Riobamba Variety). Food Biophys. 2019,14,49-59.[CrossRef ] 34. Nielsen, P.M.; Petersen, D.; Dambmann,C. Improved Method for Determining Food Protein Degree of Hydrolysis. J . Food Sci.2001,66,642-646. [CrossRef ] 35. Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Buren, J.P.V.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985,50,1715-1718. [CrossRef ] 36. Burgos-Diaz, C.; Rubi l ar, M.; Morales, E.; Medina, C.; Acevedo, F.; Marques, A.M.; Shene, C. Naturally Occurring Protein-Polysaccharide Complexes from Linseed (Linum usitatissimum) as Bioemulsifiers. Eur. J. Lipid Sci. Technol. 2016, 118,165-174I CrossRe f 37. Yu, Y; Fan, F; Wu, D.; Yu, C.; Wang, Z.; Du, M. Antioxidant and ACE Inhibitory Activity of Enzymatic Hydrolysates from Ruditapes philippinarum. Molecules 2018, 23, 1189. [CrossRef ] 38. Shu, G.;Huang,J; Bao, C.; Meng, J; Chen, H.;Cao, J. Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Convert i ng Enzyme-Inhibitory Activity i n Goa t and Cow Milk. Biomolecules 2018,8,101. [CrossRef ] 39. Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-Converting Enzyme Inhibitory Activity and Antioxidant Capacity of Bioactive Peptides Derived from Enzymatic Hydrolysis of Buffalo Mi l k Proteins. Int. Dairy J. 2017,66,91-98. [CrossRef ] 40. Goggin, D.E.; Mir, G.; Smith, W.B.; Stuckey, M.; Smith, P.M.C. Proteomic Analysis of Lupin Seed Proteins to Identify Conglutin as an Allergen, Lup an 1. J. Agric. Food Chem. 2008,56,6370-6377.[CrossRef ] 42. Kramer, R.M.; Shende, V.R.; Motl , N.; Pace, C.N.; Scholtz, J .M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J . 2012, 102,1907.[CrossRef ] [PubMed ] 43. Tsumura, K.; Saito, T.; Tsuge, K.; Ashida, H.; Kugimiya, W.; Inouye, K. Functional Properties of Soy Protein Hydrolysates Obtained by Selective Proteolysis. LWT Food Sci. Technol. 2005, 38,255-261. [CrossRef ] 44. Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-Modification by Moderate Oxidation in Hydroxyl Radical-Generating Systems Promote t he Emulsifying Properties of Soy Protein Isolate. Food Struct. 2015,6,21-28. [CrossRef ] 45. Shen, H.; Stephen Elmore,J;Zhao, M.; Sun, W. Effec t of Oxidation on the Gel Properties of Porcine Myofibrillar Proteins and Thei r Binding Abilities with Selected Flavour Compounds. Food Chem. 2020,329, 127032. [CrossRef ] 46. Zhang, C.; Liu, H.; Xia, X.; Sun, F.; Kong, B. Effect of Ultrasound-Assisted Immersion Thawing on Emulsify i ng and Gelling Properties of Chicken Myofibrillar Protein. LWT Food Sci. Technol. 2021, 142,111016. [CrossReff ] 47. Pan, N.; Wan, W.; Du, X.; Kong, B.; Liu, Q.; Lv, H.; Xia, X.; Li, F. Mechanisms of Change in Emulsifying Capacity Induced by Protein Denaturation and Aggregation in Quick-Frozen Pork Patties with Different Fat Levels and Freeze-Thaw Cycles. Foods 2021,11, 44. [CrossRef] 48. Tamm, F.; Gies, K.; Diekmann, S.; Serfert, Y.; Strunskus, T.; Brodkorb, A.; Drusch, S. Whey Protein Hydrolysates Reduce Autoxidation in Microencapsulated Long Chain Polyunsaturated Fatty Acids. Eur. J . Lipid Sc i. Technol. 2015, 117,1960-1970.[CrossRef |
确定








还剩12页未读,是否继续阅读?
中国格哈特为您提供《鲁冰花(黄羽扇豆)蛋白含量以及蛋白质溶解度的检测》,该方案主要用于豆制品中营养成分检测,参考标准《GB 5009.5 食品安全国家标准 食品中蛋白质的测定》,《鲁冰花(黄羽扇豆)蛋白含量以及蛋白质溶解度的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro
推荐专场
相关方案
更多
该厂商其他方案
更多
















