
方案详情
文
We report herein a method that allows for the formation of a CeN bond between the Ce3 carbon of alactams and the nitrogen atom of indoles. A general procedure for the coupling of indoles and a-lactams in only 25 min with high yield is reported. The scope of the reactionwas extended by the development of a method for the in situ generation of less stable phenyl-substituted a-lactams. The developed method
provides an atom-economical method for the formation of substituted a-amino amides that are found in a variety of biologically-active compounds.
方案详情

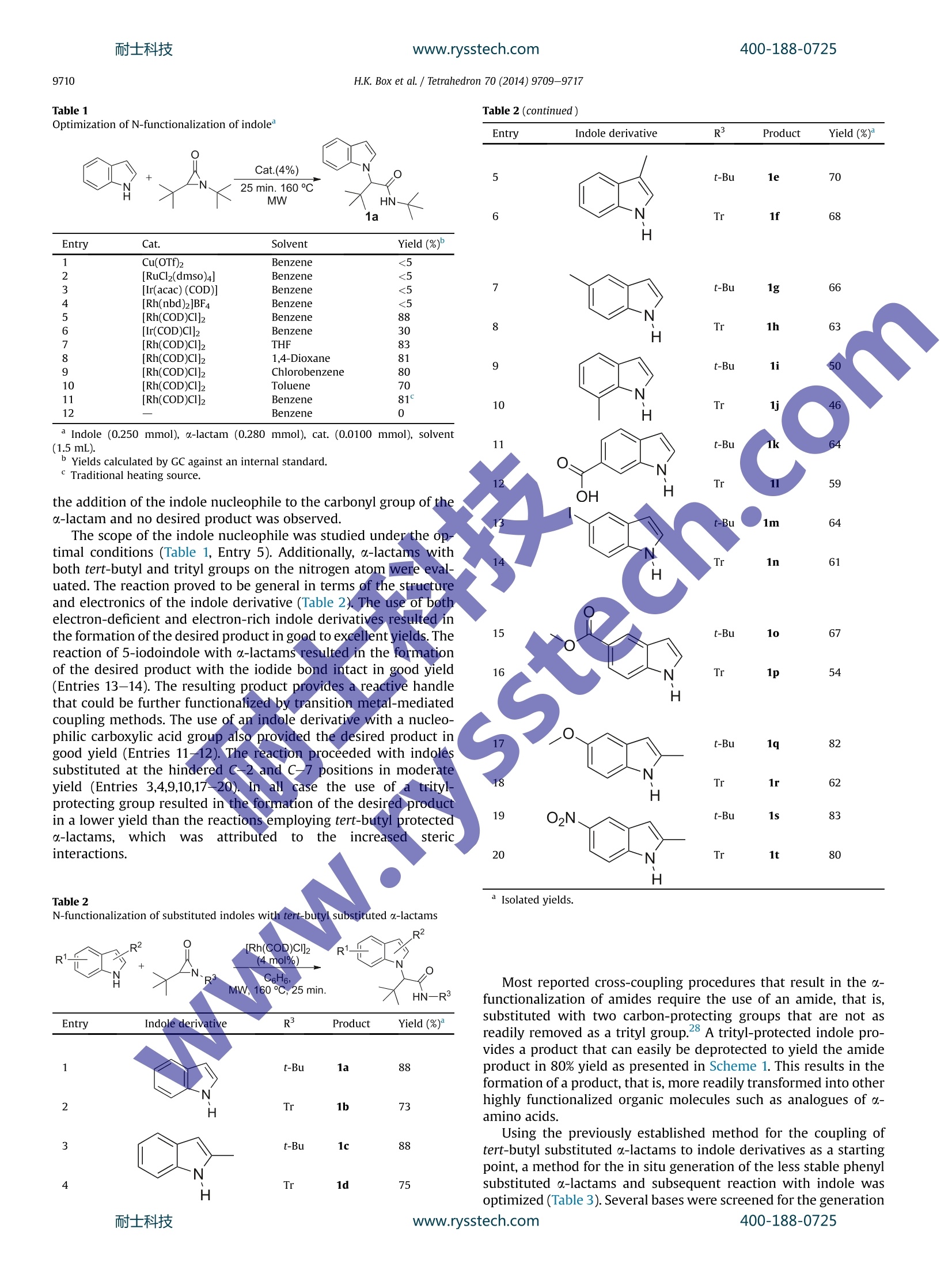
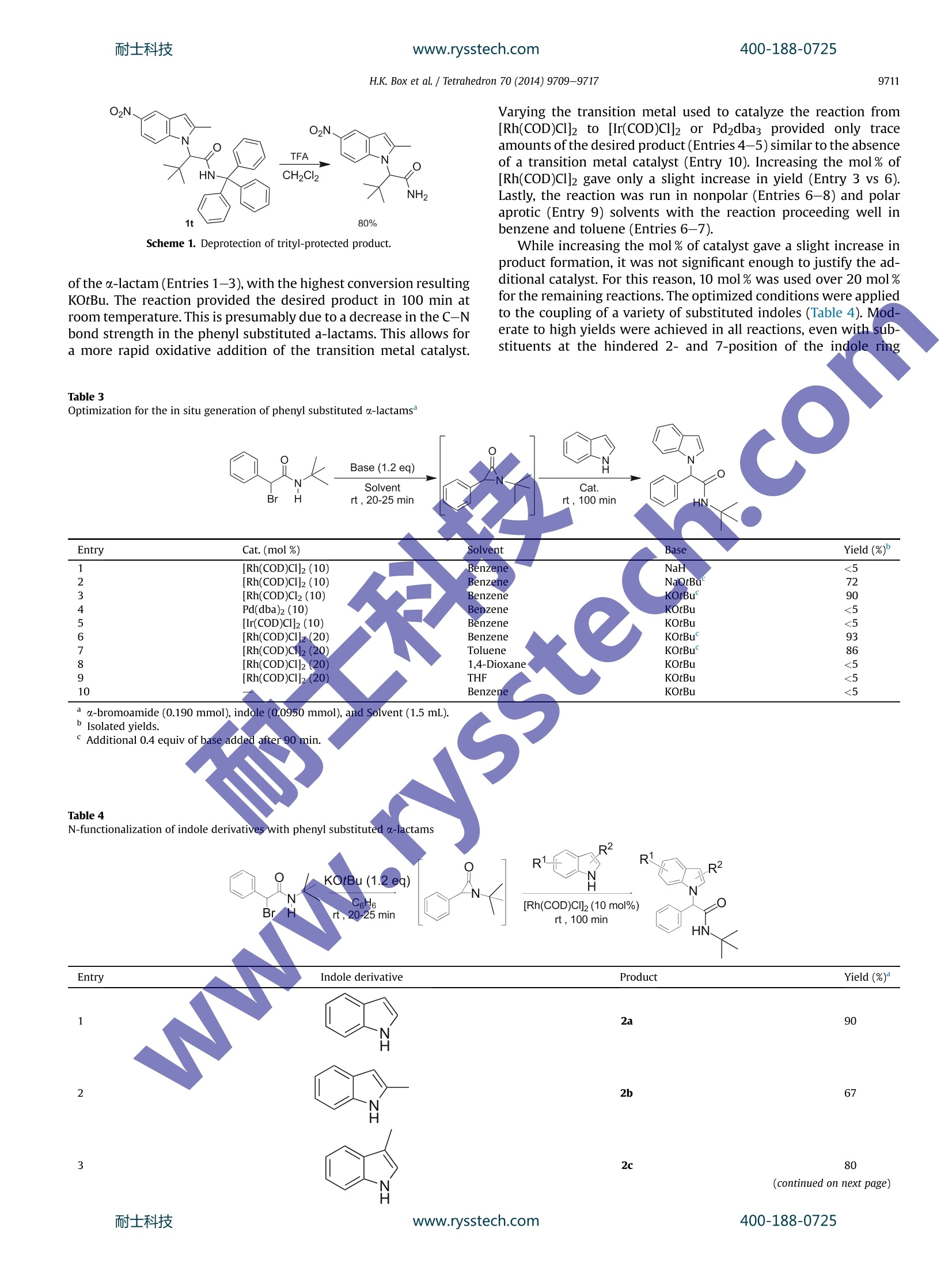
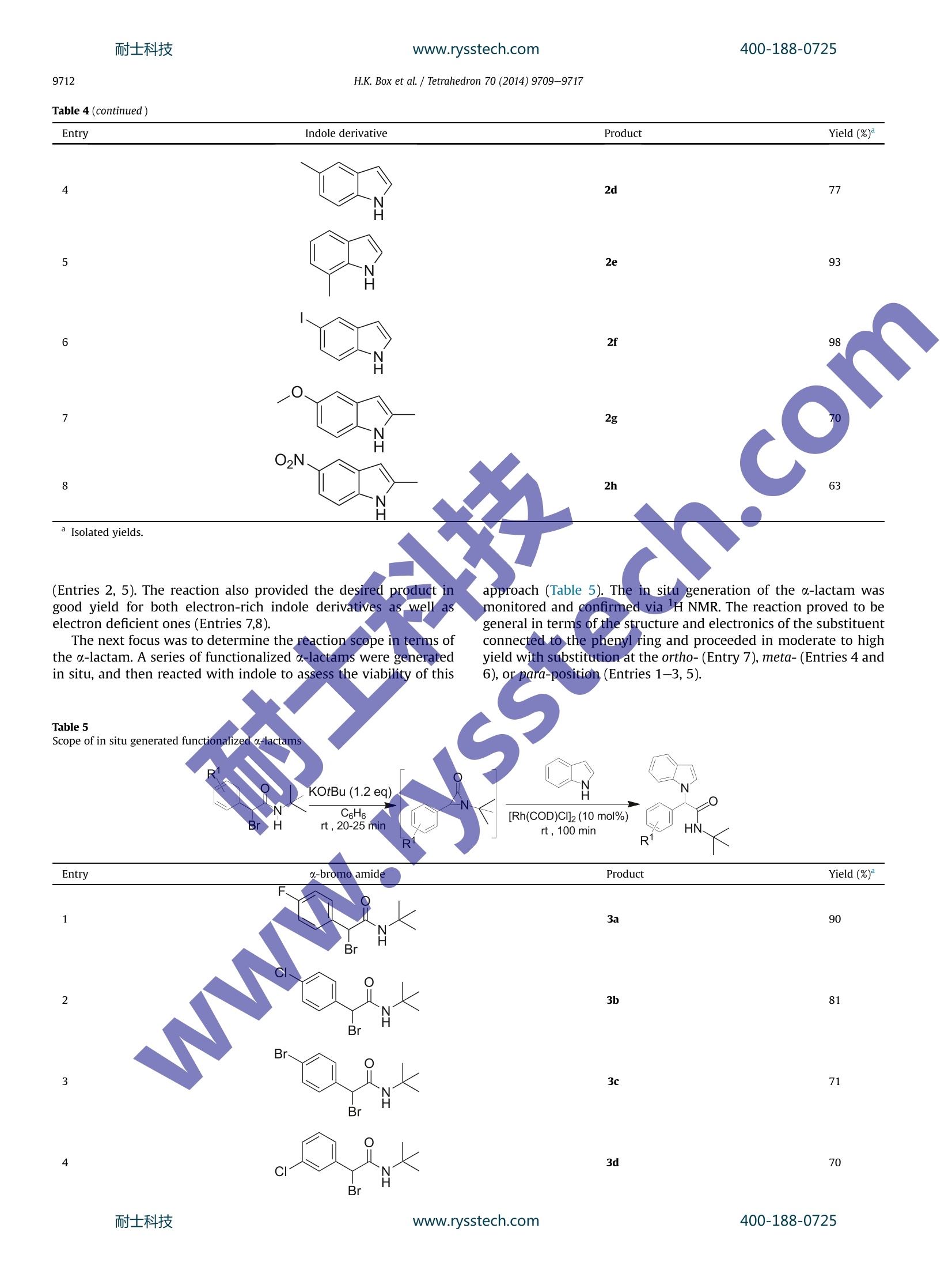
耐士科技400-188-0725www.rysstech.comTetrahedron 70 (2014)9709-9717 400-188-0725耐士科技www.rysstech.comH.K. Box et al./ Tetrahedron 70 (2014)9709-97179710 Tetrahedron ELSEVIER journalhomepage: www.elsevier.com/locate/tet Rhodium-catalyzed coupling of a-lactams with indole derivatives CrossMark Hannah K. Boxat, K.G. Upul Kumarasinghe a t, Radhika R. Nareddy,Gopalakrishna Akurathi, Amarraj Chakraborty, Babatunde Raji,Gerald B. Rowlanda,b,* Department of Chemistry, Mississippi State University, MS 39762, USA Department of Chemistry and Biochemistry, The University of Mississippi, MS 38677, USA ARTICLEINF O ABSTRACT Article history:Received 8 August 2014Received in revised form 22 October 2014Accepted 23 October 2014 Available online 7 November 2014 We report herein a method that allows for the formation of a C-N bond between the C-3 carbon of a-lactams and the nitrogen atom of indoles. AAgeneral procedure for the coupling of indoles and a-lactamsin only 25 min with high yield is reported. The scope of the reaction was extended by the development ofa method for the in situ generation of less stable phenyl-substituted a-lactams. The developed methodprovides an atom-economical method for the formation of substituted a-amino amides that are found ina variety of biologically-active compounds. Recent reaction development has focused on methods devotedto either the synthesis or utilization of strained heterocycles, suchas,epoxides and aziridines. Aziridinones (a-lactams) are anotherclass of strained heterocycles thatwere first reported as;in-termediates in the reaction of diazomethane with isocyanates in1949 by Sheehan.Since their initial discovery, limited research hasbeen performed on this class of compounds. The presence ofa carbonyl group adds additional strain to the molecule as compared to epoxides or aziridines, making them highly reactive. Thenucleophilic ring opening of o-lactams with strong nucleophilesresults in the product formation from nucleophilic C-2 addition,while the addition of weak nucleophiles results in C-3 addition.4In 1984, Alper and co-workers reported the ring-expansion of a-lactams in the presence of Rh and Co catalysts yielding azetidine-2,4-diones. More recently, Jeffrey and co-workers were able toprovide the first example of [4+3] cycloaddition involving a-lactams.7-9 To our knowledge, these are the only two examples inthe literature of the ring-expansion of a-lactams. The indole framework is found in a variety of biologically activecompounds and natural products.10-14 For this reason, the de-velopment of catalytic methods for selective functionalization ofindole has drawn much attention.15-17 The preparation of indoles ( * Corresponding author. E-mail address: r owland@olemiss.edu (G . B. Rowland). T Both authors contributed equally. ) bearing amino acid derivatives would provide a new class ofcompounds to screen for their biologically-active properties. Whilethe majority of reactions are C-3 functionalization, severalmethods have been developed for the N-functionalization of theindole ring.18-20 The formation of C-N bonds has played an in-creasing role in the development of new pharmaceutical com-pounds over the last 15 years.21.22 Since initial reports of practicalmethods for C-N bond formation by Buchwald and Hartwig, thisprocess has been one of the most utilized transformations in or-ganic synthesis.23-27 Herein we report a Rh-catalyzed C-3-N-1ringopening reaction of a-lactams in the formation of a C-N bondwith indoles. 2. Results/discussion A variety of transition metal catalysts were screened and gave<5% of the product (Entries 1-4), however, the reaction proceededsmoothly in the presence of either [Rh(COD)Cl]2 or [Ir(COD)Cl]2(Entries 5-6). Product formation occurs in both non-polar (Entries5, 7,9,10) and polar aprotic (Entry 8) organic solvents. The desiredproduct was obtained in 88% yield using [Rh(COD)Cl]2, benzene asthe solvent,and a microwave reactor as the heat source (Table 1,Entry 5). While a traditional heat source provides the desiredproduct in high yield, the reaction time increases from 25 min to48 h (Entry 11). The reaction did not proceed in the absence of[Rh(COD)Cl]2 (Entry 12). To exclude the possibility of a nucleophilicsubstitution mechanism, a-lactam was reacted with an indole an-ion generated by potassium tert-butoxide. The reaction resulted in Table 1Optimization of N-functionalization ofindole Entry Cat. Solvent Yield (%) 1 Cu(OTf)2 Benzene <5 2 [RuCl2(dmso)4] Benzene <5 3 [Ir(acac) (COD)] Benzene <5 4 [Rh(nbd)2]BF4 Benzene <5 5 [Rh(COD)Cl]2 Benzene 88 6 [Ir(COD)Cl]2 Benzene 30 7 [Rh(COD)Cl]2 THF 83 8 [Rh(COD)Cl]2 1,4-Dioxane 81 9 [Rh(COD)Cl]2 Chlorobenzene 80 10 [Rh(COD)Cl]2 Toluene 70 11 [Rh(COD)Cl]2 Benzene 819 12 一 Benzene 0 Indole (0.250 mmol), a-lactam (0.280 mmol), cat. (0.0100 mmol), solvent(1.5 mL). Yields calculated by GC against an internal standard. Traditional heating source. the addition of the indole nucleophile to the carbonyl group of theo-lactam and no desired product was observed. The scope of the indole nucleophile was studied under the op-timal conditions (Table 1, Entry 5). Additionally, a-lactams withboth tert-butyl and trityl groups on the nitrogen atomwere eval-uated. The reaction proved to be general in terms of the structureand electronics of the indole derivative (Table 2). The use of bothelectron-deficient and electron-rich indole derivatives resulted inthe formation of the desired product in good to excellent yields.Thereaction of 5-iodoindole with a-lactams resulted in the formationof the desired product with the iodide bond intact in good yield(Entries 13-14). The resulting product provides a reactive handlethat could be further functionalized by transition metal-mediatedcoupling methods. The use of an indole derivative with a nucleo-philic carboxylic acid group also provided the desired product ingood yield (Entries 11-12).The reaction proceeded with indolessubstituted at the hinderedC-2 andC--771positions in moderateyield (Entries 3,4,9,10,17-220).. In alcase the use of a trityl-protecting group resulted in the formation of the desired productin a lower yield than the reactions employing tert-butyl protecteda-lactams, whichWasattributed to the increasedstericinteractions. Table 2N-functionalization of substituted indoles with tert-butyl substituted a-lactams Entry Indole derivative R3 Product Yield (%) Isolated yields. Most reported cross-coupling procedures that result in the a-functionalization of amides require the use of an amide, that is,substituted with two carbon-protecting groups that are not asreadily removed as a trityl group.A trityl-protected indole pro-vides a product that can easily be deprotected to yield the amideproduct in 80% yield as presented in Scheme 1. This results in theformation of a product, that is, more readily transformed into otherhighly functionalized organic molecules such as analogues of a-amino acids. Using the previously established method for the coupling oftert-butyl substituted a-lactams to indole derivatives as a startingpoint, a method for the in situ generation of the less stable phenylsubstituted a-lactams and subsequent reaction with indole wasoptimized (Table 3). Several bases were screened for the generation of the o-lactam (Entries 1-3), with the highest conversion resultingKOtBu. The reaction provided the desired product in 100 min atroom temperature. This is presumably due to a decrease in the C-Nbond strength in the phenyl substituted a-lactams. This allows fora more rapid oxidative addition of the transition metal catalyst. Varying the transition metal used to catalyze the reaction from[Rh(COD)Cl]2 to [Ir(COD)Cl]2 or Pd2dba3 provided only traceamounts of the desired product (Entries 4-5) similar to the absenceof a transition metal catalyst (Entry 10). Increasing the mol% of[Rh(COD)Cl]2 gave only a slight increase in yield (Entry 3 vs 6).Lastly, the reaction was run in nonpolar (Entries 6-8) and polaraprotic (Entry 9) solvents with the reaction proceeding well inbenzene and toluene (Entries 6-7). While increasing the mol% of catalyst gave a slight increase inproduct formation, it was not significant enough to justify the ad-ditional catalyst. For this reason, 10 mol% was used over 20 mol%for the remaining reactions. The optimized conditions were appliedto the coupling of a variety of substituted indoles (Table 4). Mod-erate to high yields were achieved in all reactions, even with sub-stituents at the hindered 2- and 7-position of the indole ring Table3 Optimization for the in situ generation of phenyl substituted u-lactams° Base (1.2 eq) Solvent Cat. Br H rt, 20-25 min rt , 100 min Table 4 H.K. Box et al./ Tetrahedron 70 (2014)9709-9717 Table 4 (continued) Entry Indole derivative Product Yield (%) 4 2d 77 5 2e 93 6 2f 98 O、 7 2g O2N、 8 2h H Isolated yields. 70 63 (Entries 2, 5). The reaction also provided the desired productt ingood yield for both electron-rich indole derivatives as well aselectron deficient ones (Entries 7,8). The next focus was to determine the reaction scope in terms ofthe a-lactam. A series of functionalized a-lactams were generatedin situ, and then reacted with indole to assess the viability of this approach (Table 5). The in situ generation of the c-lactam wasmonitored and confirmed via H NMR. The reaction proved to begeneral in terms of the structure and electronics of the substituentconnected to the phenyl ring and proceeded in moderate to highyield with substitution at the ortho-(Entry 7), meta-(Entries 4 and6), or para-position (Entries 1-3,5). Entry a-bromo amide Product Yield (%) 5 0 3e 60 Br 6 F3C H Br 3f 63 7 3g 75 Br °Isolated yields. 3. Conclusions We report a novel Rh-mediated method for the coupling of in-doles with a-lactams. The developed method results in the for-mation of the desired product in good to excellent yield. Thereaction was found to be tolerant of functional groups around theindole ring. The use of trityl protecting groups results in a slightdecrease in the yield of the desired product but can easily bedeprotected to provide an a-amino amide analogue. The scope ofthis methodology was extended to include the less stable phenylsubstituted a-lactams by in situ generation. Future work will focuson the development of a more general reaction in terms of lf bothnucleophiles and a-lactams. 4. Experimental 4.1. General methods The following reagents were purchased and used as received:indole derivatives (Sigma Aldrich), benzene (Sigma Aldrich; anhy-drous), and chloro(1,5-cyclooctadiene)rhodium(I) dimer (Strem).Unless otherwise noted, reactions were conducted with stirring inoven-dried glassware under an inert atmosphere.Reactions wereperformed in the Biotage Initiator microwave reactor and flashcolumn chromatography was performed on a Biotage Isolera Onesystem using silica gel. Mass spectrometry was performed ona Bruker UHPLC-Micro-Q/T ESI-MS in positive mode. All NMRspectra were taken on a Bruker ADVANCE III 600 MHz spectrometer. 4.2. Synthesis of o-lactams 4.2.1. 1,3-Di-tert-butylaziridin-2-one.2-Bromo-N-(tert-butyl)-3,3-dimethylbutanamide (0.0600 mol) was dissolved in anhydrousEt20 (60 mL), cooled to 0°℃, and KOtBu (0.0900 mol) was added tothe reaction. The reaction stirred for 10 min at 0 °C. The mixturewas then filtered through a bed of Celite and concentrated underreduced pressure. With no further purification, an oil was re-covered (8.00g78%)The spectroscopic data matches previouslyported literature.29-32 4.2.2. 3-(tert-Butyl)-1-tritylaziridin-2-one. 2-Bromo-3,3-dimethyl-N-tritylbutanamide (0.0110 mol) was dissolved in Et20 (80 mL),cooled to 0°C, and NaOtBu (0.0210 mol) was added to the reaction.The reaction warmed to room temperature and proceeded 2 h. Thereaction was washed with water (1 x 80 mL) and brine (1x80 mL),dried with Na2SO4, and concentrated under reduced pressure. The solid was washed with pentane and dried under high vacuum. Withno further purification, a white solid was recovered (2.50 g,68%). TheSIectroscopic data matches previously reported literature.29,30,33 4.3. General procedure for 1a-t In a glove box, a microwave vialwas charged with a-lactam(0.250 mmol), indole (0.280 mmol), and [RhCODCl]2(0.0100 mmol).The vial was closed with a cap fitted with a septum and benzene(1.5 mL) was added via syringe. The reaction was removed from theglove box and heated in the microwave reactor for 25 min at 160°C.The reaction mixture cooled to room temperature and was removedfrom reactor. The mixture was exposed to air, filtered through a bedof silica, and washed with CH2Cl2. Compounds were purified usingflash column chromatography (hexanes/CH2Cl2). 4.3.1. N-(tert-Butyl)-2-(1H-indol-1-yl)-3,3-dimethylbutanamide(1a). The compound was obtained as a white solid, 62.6 mg, 88%yield, mp 58-60°C; 1H NMR(CDCl3, 600 MHz) ; 7.68-7.65(m,2H),7.36 (d, 1H,J=8.2 Hz), 7.23 (t, 1H,J=15.1Hz), 7.13 (t,1H,J=14.8Hz),6.57 (d,1H,J=3.3 Hz), 5.36 (s, 1H), 4.49 (s, 1H), 1.30 (s,9H), 1.13 (s,9H); 13cNMR (CDCl3, 125 MHz) 6 166.2,131.9,131.1,130.6,129.7,124.0,122.2,112.0,104.6,69.8,54.3,39.7,31.2,30.3; HRMS (ESI) m/zcalculated for C18H26N2NaO [M+Na]+: 309.1943, found 309.1953. 4.3.2. 2-(1H-Indol-1-yl)-3,3-dimethyl-N-tritylbutanamide (1b). Thecompound was obtained as a white solid, 87.0 mg, 73% yield,mp56-59°C; HNMR(CDCl3,600MHz)ò 7.68(d,1H,J=7.8 Hz), 7.45 (s,1H), 7.42 (d, 1H, J=8.2 Hz), 7.32 (t, 1H,J=14.9 Hz), 7.24-7.22 (m,10H), 7.16(t,1H,J=14.7 Hz), 7.07-7.06(m,5H), 6.68 (s, 1H), 6.59(d,1H, J=3.1 Hz), 4.70 (s, 1H), 1.09 (s,9H); 1C NMR (CDCl3, 125 MHz); 160.8, 146.7, 131.2,131.1,130.7,130.6,129.8,129.7,124.4, 123.7,122.4, 105.1,73.2,39.7,30.3,29.8, 21.1; HRMS (ESI) m/z calculatedfor C33H32N2NaO [M+Na]+: 495.2412,found 495.2368. 4.3.3. N-(tert-Butyl)-3,3-dimethyl-2-(2-methyl-1H-indol-1-yl) buta-namide (1c). The compound was obtained as a white solid, 66.2 mg,88% yield, mp 100-102 ℃;lH NMR (CDCl3, 600 MHz) 6 7.51 (d,1H,J=7.3 Hz), 7.45(d,1H,J=7.8 Hz), 7.10-7.06(m,2H), 6.34(s,1H), 5.20(s,1H), 4.52 (s, 1H), 2.47 (s, 3H), 1.19 (S, 9H), 1.17(S,9H); 13C NMR(CDCl3, 125 MHz) 168.3, 137.5,135.7,128.8,120.4,119.9,119.6,113.9, 102.2, 65.7,51.2,38.3,29.2, 28.4, 14.2; HRMS (ESI) m/z cal-culated for C19H28N2NaO[M+Na]+: 323.2099, found 323.2116. 4.3.4. 3,3-Dimethyl-2-(2-methyl-1H-indol-1-yl)-N-trityl butanamide(1d). The compound was obtained as a white solid, 91.4 mg, 75%400-188-0725 yield, mp 60-64 °C; H NMR (CDCl3, 600 MHz) 7.51 (d, 1H,J=7.9 Hz), 7.46 (d, 1H, J=8.4 Hz), 7.33-7.16 (m, 10H), 7.11-7.06(m,2H),7.01-6.98 (m,5H), 6.62(s,1H), 6.35 (s,1H),4.60(s,1H), 2.49 (s,3H), 1.16 (s,9H); 13C NMR (CDCl3, 125 MHz) 8 170.2, 146.9, 139.7,131.1,131.0,130.6,130.5,129.5,123.5,122.6,122.2,117.0,105.2,69.6,40.7, 31.7,29.8, 17.1; HRMS (ESI) m/z calculated for C34H34N2NaO[M+Na]+: 509.2569, found 509.2525. 4.3.5. N-(tert-Butyl)-3,3-dimethyl-2-(3-methyl-1H-indol-1-yl) buta-namide(1e). The compound was obtained as a white solid, 52.8 mg,70% yield, mp 125-127°C; H NMR (CDCl3, 600 MHz) 6 7.59 (d,1H,J=7.8 Hz), 7.42 (S,1H), 7.31 (d,1H,J=8.2Hz), 7.22 (t, 1H,J=15.0 Hz),7.13 (t, 1H,J=14.7 Hz),5.37 (s, 1H), 4.44(s,1H), 2.35(s,3H), 1.30 (s,9H), 1.12 (s, 9H); 13C NMR(CDCl3, 125 MHz) 6 168.5, 137.7,128.4,125.1,121.3,119.0,118.8,111.0,109.1,67.1,51.6,37.0,28.6,27.7,9.7;HRMS (ESI) m/z calculated for C19H28N2NaO [M+Na]+: 323.2099,found 323.2096. 4.3.6. 3,3-Dimethyl-2-(3-methyl-1H-indol-1-yl)-N-trity lbutana-mide (1f). The compound was obtained as a white solid, 84.0 mg,68% yield, mp 53-56C; HNMR (CDCl3, 600 MHz) 6 7.61 (d,1H,J=7.8 Hz),7.38 (d, 1H,J=8.2 Hz), 7.34-7.15 (m,11H), 7.06-7.05(m,5H), 6.66 (s,1H), 4.66 (s, 1H), 2.35 (S, 3H), 1.12 (s, 9H); 13C NMR(CDCl3, 125 MHz) ; 170.9, 146.7,131.3,131.1,130.7,130.5,129.8,129.6,124.4,121.9,121.8,114.3,105.2,73.1,39.4, 30.4,29.8,12.3;HRMS (ESI) m/z calculated for C34H34N2NaO [M+Na]+:509.2569,found 509.2503. 4.3.8. 3,3-Dimethyl-2-(5-methyl-1H-indol-1-yl)-N-trityl butanamide(1h). The compound was obtained as a white solid, 77.4 mg, 63%yield, mp 52-55°C;1H NMR (CDC3,600 MHz) ; 7.47 (s, 1H), 7.40(m,1H), 7.34-7.23 (m,11H),7.09-7.07 (m,6H), 6.70(s,1H), 6.51(d,1 Hz,J=3.0 Hz), 4.66 (s, 1H), 2.49 (s, 3H), 1.09 (s, 9H);C NMR(CDCl3, 125 MHz) ; 170.7,146.7,131.6,131.3,131.1,130.7,130.6,129.8,129.6,125.9,123.4,104.6,73.2,39.7, 30.3,29.8,23.9, 16.7; HRMS(ESI) m/z calculated for C34H34N2NaO [M+Na]: 509.2569, found509.2516. 4.3.9. N-(tert-Butyl)-3,3-dimethyl-2-(7-methyl-1H-indol-1-yl) buta-namide (1i). The compound was obtained as a white solid, 37.6 mg,50% yield, mp53-54 °C; H NMR (CDC3, 600 MHz) 6 7.53 (d, 1H,J=7.7 Hz), 7.49 (d,1H,J=3.3 Hz), 7.04 (t, 1H,J=14.9 Hz), 6.97 (d,1H,J=7.0 Hz), 6.60 (d,1H, J-3.3 Hz), 5.41 (s, 1H), 5.21 (s, 1H), 2.75 (s,3H), 1.30 (s, 9H), 1.12 (s,9H); CNMR (CDCl3, 125 MHz) 6 171.8,138.9,131.4,130.1,128.6,123.0,122.5,122.2,106.0,70.4,54.2,39.7,31.3, 30.7, 24.5; HRMS(ESI) m/z calculated for C19H28N2NaO[M+Na]+:323.2099,found 323.2113. ( 4.3.10. 3,3-Dimethyl-2-(7-methyl-1H-indol-1-yl)-N-trityl butana- mide (1j). The compound was obtained as a white solid, 56.2 mg, 46% yield, mp 112-115 °C; 1H NMR(CDCl3, 600 MHz) ; 7.54-7.50 (m,1H), 7.44(m,1H),7.32-7.22(m,10H), 7.07-6.99(m,7H), 6.77 (s, 1H), 6.63 (m, 1H), 4.11 (s, 1H), 2.75 (s , 3H) , 1.11 (s, 9H ) ; 1 3 C NMR (CDCl3, 125 MHz) ; 171.4, 146.9,131.2,131.0,130.6, 1 29.8, 1 29.6, 128.9, 122.8 , 122.4,106.7,70.9,39.6,38.5,30.8,29.5, 24.5; H RMS ) (ESI) m/z calculated for C34H34N2NaO [M+Na]+: 509.2569, found509.2504. ( 4.3.11 . 1-(1-(tert-Butylamino)-3,3-dimethyl-1-oxobutan-2-yl)-1H- indole-6-carboxylic acid (1k). T h e compound was o btained as a white solid, 53 . 1 mg, 64% yield, mp 226-227°C; HNMR(CD30D, 600 MHz) ; 8 .22 (s, 1 H ), 7.77-7.76 (m,1H) , 7.64 (d, 1H , J=8.1 Hz) , 7.48-7.47 (m,2H), 6.56-6.55 (m, 1H), 4.80 (s,1H), 1.38 (s, 9H), 1.17 (s,9H);13CNMR(CD3OD, 125MHz)6 169.1,167.3,135.4,132.1,128.3, 122.0, 119.6, 119.5,113.4,101.5,80.9,51.0,33.6 , 27.48, 25.5 ; HRMS (ESI) m/z calculate d for C19H26N2NaO3[M+Na]+: 353.1841, found 353.1892. ) ( 4.3.12. 1-(3,3-Dimethyl-1-0x0-1-(tritylamino)butan-2-yl)-1H-in- dole-6-carboxylic acid (1l ) . Th e compound w as obtained as a w hite solid, 76.5 mg, 59% yiel d , m p 119-121 ℃ ; 1 H NMR (CDCl3, 600 MHz) 88.65(s,1H),8.23(s,1H), 7.86-7.85(m,1H), 7.68(d,1H , J = 8 .2 Hz), 7.38-7.37(m,1H), 7.33(s,1H), 7.22-7.19(m,1 5 H), 6.6 3 (s, 1H), 5.19 (s, 1H), 1.0 9 (s, 9H); ’C NMR (CDCl3, 12 5 M H z) 16 7 .6, 166.5, 144.4,135.1,132.1,128.5,128.1,127.9, 1 26.9, 12 2.5 , 1 2 0.6,120.5,113.9,103.0,81.3,70.1,34.8,26.5; HRMS (E S I) m/z cal c ulated for C 3 4H32N2NaO3[M+Na]+:539.2311, found 5 39.2243. ) 4.3.14. 2-(5-Iodo-1H-indol-1-yl)-3,3-dimethyl-N-tritylbutanamide(1n). The compound was obtained as a white solid, 90.9 mg, 61%yield, mp 69-73℃; 1H NMR(CDCl3, 600 MHz) 6 8.01 (m, 1H), 7.49(d,1H,J=9.3Hz), 7.42(s,1H),7.34-7.23(m,11H), 7.07-7.06(m,5H),6.66 (s,1H), 6.50(d, 1H,J=3.2 Hz), 4.60 (s, 1H), 1.07 (s,9H);13CNMR(CDCl3,125 MHz); 170.2146.9,146.6,132.6,132.5,131.2,131.1,130.7,130.6,129.8,123.2,104.1,85.7,73.4,39.7,30.1,29.8; HRMS (ESI) m/zcalculated for C33H31IN2NaO [M+Na]+:621.1379, found 621.1311. 4.3.15. Methyl 1-(1-(tert-butylamino)-3,3-dimethyl-1-oxobutan-2-yl)-1H-indole-5-carboxylate (1o). The compound was obtained asa white solid, 57.4 mg, 67% yield, mp 188-189C; H NMR (CDCl3,600 MHz)8 8.41 (s,1H), 7.92(d,1H,J=8.7 Hz), 7.83 (d,1H,J=3.1 Hz),7.36(d,1H,J=8.7Hz), 6.65(d,1H,J=3.1 Hz), 5.47 (s,1H), 4.49(s,1H),3.95 (s, 3H), 1.32 (s, 9H), 1.11 (s, 9H); 13C NMR (CDCl3, 125 MHz) 168.1, 167.7,139.8,129.5,127.6,123.91,123.90,122.7,121.5,109.1,103.1, 67.3,51.8,37.1,28.5, 27.5; HRMS (ESI) m/z calculated forC20H28N2NaO3[M+Na]+:367.1998, found 367.1985. ( 4.3.16. Methyl-1-(3,3-dimethyl-1-ox0-1-(tritylamino)butan-2-yl)- 1H-indole-5-carboxyla t e (1p). T h e compound was o btained a s a white solid, 71. 5 mg, 54% yield, mp 231-233 ℃; 1H NMR(CDCl3, 600 MHz) 8.44 (s, 1H), 7.95 (d, 1H,J=8.2 H z), 7.54 (s, 1H), 7.43 (d, 1H,J=8 . 5 Hz), 7 . 23-7.14(m, 10H), 7.07-7.06 (m, 5H), 6.72 (s, 1H) , 6.66 (s, 1 H ), 4.70 ( s,1H), 3.95 ( s ,3 H ), 1.09 (s, 9H); 13c NMR (CDCl3, 125 MHz); 168.0,167.5,144.0,143.9,128.6,128.4,128.0,127.8,127.2, 127.1, 124.0,123.1,121.9 , 109.1,103.6,70.7,51.9,51.8,37.0, 2 7.4;HRMS (ESI) m/z calculated for C35H34N2NaO3[M+Na]+:553.2467, found 553.2388. ) ( 4.3.17. N-(tert-Butyl)-2-(5-methoxy-2-methyl-1H-indol-1-yl)-3,3-dimethylbutanamide (1q). T he compound was obtained as a white solid, 67.5 mg, 82% yield, mp 101-104C;1HNMR(CDCl3, 600 MHz) o 7.33 (d, 1H, J =9 .0 H z), 6 .98 ( d, 1H, J= 2 .4 Hz), 6 .75 ( dd, 1 H, 400-188-0725 ) J1=9.0 Hz, J2=2.5 Hz), 5.23 (s,1H), 4.47 (s,1H), 3.86 (s,3H), 2.44 (S,3H), 1.19 (s, 9H), 1.15 (s, 9H); 1’c NMR (CDCl3, 125 MHz) ; 168.4,154.0, 138.2, 130.7,129.4,114.6,110.1,102.0,101.5,65.8,55.6,51.2,38.3,29.1, 28.4, 14.2; HRMS (ESI) m/z calculated for C20H30N2Na02[M+Na]+: 353.2205, found 353.2188. 4.3.18. 2-(5-Methoxy-2-methyl-1H-indol-1-yl)-3,3-dimethyl-N-tri-tylbutanamide (1r). The compound was obtained as a white solid,81.1 mg, 62% yield, mp 63-66°C; 1H NMR(CDCl3, 600 MHz) 67.31(d, 1H, J=9.0 Hz), 7.27-7.17 (m, 10H), 7.10 (d, 1H, J=6.9 Hz),7.01-6.99(m, 5H), 6.98 (d,1H,J=2.0 Hz), 6.65-6.63 (m,1H), 6.27 (s,1H), 4.56 (s, 1H), 3.86 (S, 3H), 2.47 (s, 3H), 1.14 (s, 9H); 13c NMR(CDCl3, 125 MHz) ; 172.6, 159.9,146.9,138.6,135.8,132.1,131.0,130.5,129.5,125.3,117.7,113.0,105.0,73.0,69.5,58.4,40.6,31.6,17.1;HRMS (ESI) m/z calculated for C35H36N2Na02 [M+Na]+:539.2674,found 539.2619. 4.3.19. N-(tert-Butyl)-3,3-dimethyl-2-(2-methyl-5-nitro-1H-indol-1-yl)butanamide (1s). The compound was obtained as a yellow solid,71.5 mg, 83% yield, mp 176-178 °C; 1H NMR (CDCl3, 600 MHz) 8.44 (s,1H),8.00 (d,1H,J=9.1 Hz), 7.61(d, 1H,J=9.1 Hz), 6.53 (s,1H), 4.98 (s,1H), 4.48 (s, 1H), 2.52 (s, 3H), 1.20 (S, 9H), 1.17 (s,9H);1cNMR (CDCl3, 125 MHz) 167.1,141.8,141.0,139.1, 127.9, 116.4,116.1, 113.7,104.4, 66.2,51.6,38.3,28.8,28.4,14.4; HRMS (ESI) m/zcalculated for C19H27N3Na03[M+Na]+: 368.1950, found 368.1926. butanamide. 3,3-Dimethyl-2-(2-methyl-5-nitro-1H-indol-1-yl)-N-trityl butanamide (0.0280 mmol) was dissolved in CH2Cl2(0.5 mL)and cooled to 10 C followed bythe slow addition off1TFA(1.30 mmol). The reaction stirred for 3 h. The solution was con-centrated and the crude residue was dissolved in methyl-t-butylether followed by concentration. The crude product was againdissolved in methyl-tert-butyl ether followed by the addition ofhexanes. The compound was obtained as a white solid, 11.0 mg, 80%yield, mp 153.2-155.6°C; 1H NMR (CDCl3, 600 MHz) ; 8.45 (s,1H),8.01(d, 1H,J=9.0 Hz), 7.55(d,1H,J-9.1 Hz),6.54(s,1H), 5.25(s,2H),4.63 (s, 1H), 2.54 (s, 3H), 1.19 (s,9H); 13CNMR (CDCl3, 125 MHz)0 172.7,143.6,141.7,130.7,130.5,119.2,119.0,116.0,107.1,67.9, 40.8,31.4, 17.0; HRMS (ESI) m/z calculated for C19H26N2NaO3 [M+Na]+:312.1324, found 312.1342. 4.5. General procedure for the synthesis of a-bromoamides In air, a two neck oven dried round bottom flask equipped witha reflux condenser capped with a calcium chloride drying tube wascharged with the substituted phenyl acetic acid (1.0 equiv) andphosphorous trichloride (1.0 equiv). Bromine (2.0 equiv) was addedvia syringe and the reaction mixture was heated for 1.5 h at70-90°C. After 1.5 h, excess bromine (0.4-0.6 equiv) was addedand reaction was stirred for another 18 h at 70-90C. The mixturewas cooled to room temperature and poured into 150 mL of icewater. The mixture was extracted using toluene (2×x150 mL), driedover anhydrous NazSO4, and the residual bromine and toluene were removed under reduced pressure. The crude bromo(phenyl)acetylchloride was used directly for the next step. N,N'-Diisopropylethylamine or triethylamine (1.0-2.0 equiv)was charged to a solution of tert-butylamine(1.0-1.1 eq) in CH2Cl2.The reaction mixture was cooled to 0°C and the crude bromo(-phenyl)acetyl chloride wasadded slowly viaasyringe over45-60 min. The reaction warmed to room temperature and stirredfor 3-5 h. Products were purified by flash column chromatography(hexanes/CH2Cl2). 4.5.1. 2-Bromo-N-(tert-butyl)-2-phenylacetamide. Phenyl aceticacid (15.0 mmol),phosphorous trichloride (15.0 mmo), bromine(30.0 mmol, excess 9.00 mmol), tert-butylamine (15.0 mmol),N,N'-diisopropylethylamine (30.0 mmol), CH2Cl2 (75 mL). The com-pound was obtained as a white solid, 3.30 g, 81% yield, mp134-136C; 1H NMR (CDCl3, 600 MHz) ò 7.43 (d, 2H, J 7.2 Hz),7.34-7.29(m, 3H), 6.56 (s, 1H), 5.33 (s, 1H), 1.37 (s,9H), 13cN(CDCl3, 125 MHz); 166.1, 137.8,128.9,128.8,128.2,52.1,51.9,28.4;HRMS (ESI) m/z calculated for C12H17BrNO [M+H]:270.0494;found: 270.0495. 4.5.2. 2-Bromo-N-(tert-butyl)-2-(4-fluorophenyl)acetamide. 2-Bromo-N-(tert-butyl)-2-(4-fluorophenyl)acetamide (4-Fluorophenyl)aceticacid (18.0mmol),phosphorous trichloride(18.00rmmol), bromine (36.0mmol, excess 11 mmol), tert-butyl-amine (18.0 mmol), N,N'-diisopropylethylamine (36.0 mmol),CH2Cl2 (75 mL). The compound was obtained as a white solid,2.90 g,56% yield, mp 133-135C;HNMR(CDCl3,600 MHz) 6 7.41(dd,2H,J=5.4, 8.4 Hz),), 7.02 (t, 2H,J=8.4 Hz), 5.32 (s, 1H), 1.39 (S,9H); 13C NMR (CDCl3, 125 MHz) 6 165.9,163.6(d,J=990 Hz), 133.8(d,J=12 Hz),130.2(d,J=36Hz), 115.9(d,J=84 Hz), 52.2, 50.8, 28.4;HRMS (ESI) m/z calculated for C12H16BrFNO [M+H]+:288.0399;found: 288.0407. 4.5.3. 2-Bromo-N-(tert-butyl)-2-(4-chlorophenyl)acetamide. 2-Bromo-N-(tert-butyl)-2-(4-chlorophenyl)acetamide (4-chlorophenyl)acetic acid (30.0 mmol), phosphorous trichloride (30.0 mmol),bromine((60.0mmol, excess11mmol),tert-butylamine(68.0 mmol), N,N'-diisopropylethylamine (77.0 mmol), CH2Cl2(50 mL). The compound was obtained as a white solid, 6.60 g, 72%yield, mp 156-158 C; 1H NMR (CDCl3, 600 MHz) ò 7.37 (d, 2H,J=8.4 Hz), 7.32 (d, 2H, J=8.4 Hz), 6.48 (s, 1H), 5.28 (s, 1H), 1.39 (s,9H); 13c NMR (CDCl3, 125 MHz) 6 165.6, 136.5,134.9,129.7,129.1,52.3, 50.9, 28.4; HRMS (ESI) m/z calculated for C12H15BrClNNaO[M+Nal+:327.9897; found: 327.9901. 4.5.4. 2-Bromo-2-(4-bromophenyl)-N-(tert-butyl)acetamide. 2-Bromo-2-(4-bromophenyl)-N-(tert-butyl)acetamide (4-bromophenyl)acetic acid (4.90 mmol), phosphorous trichloride (4.90 mmol),bromine (9.80 mmol, excess 2.50 mmol), tert-butylamine(5.10 mmol), triethylamine (7.00 mmol), CH2Cl2 (50 mL). Thecompound was obtained as a white solid,0.500 g, 30% yield, mp177-179°C; 1H NMR(CDC3, 600 MHz) 6 7.49(d, 2H,J=8.4Hz), 7.31(d, 2H,J=8.4 Hz), 6.48 (s, 1H), 5.27 (s, 1H), 1.39 (s, 9H); 1’C NMR(CDCl3, 125 MHz) ; 165.5,137.0,132.1,129.9,123.1,52.3,50.9,28.39;HRMS (ESI) m/z calculated for C12H15Br2NNaO[M+Na]+: 371.9393;found: 371.9394. 4.5.5. 2-bromo-N-(tert-butyl)-2-(3-chlorophenyl)acetamide. 2-bromo-N-(tert-butyl)-2-(3-chlorophenyl)acetamide (3-chlorophenyl)acetic acid (18.0 mmol), phosphorous trichloride (18.0 mmol),bromine (36.0) mmol, excess111.)0 mmol), tert-butylamine(18.0 mmol), N,N'-diisopropylethylamine (36.0 mmol), CH2Cl2(75 mL). The compound was obtained as a white solid, 3.10 g, 56%yield, mp 124-127°C; 1HNMR (CDCl3, 600 MHz) 6 7.41 (s,1H), 7.30(m,3H), 6.56 (s, 1H), 5.26 (s, 1H), 1.40 (s, 9H); 13C NMR (CDCl3,400-188-0725 125 MHz);165.4, 139.7,134.6,130.2,129.1,128.5,126.5,52.3,50.6,28.4; HRMS (ESI) m/z calculated for C12H15BrClNNaO [M+Na]+:327.9897;found: 327.9910. 4.5.6. 2-Bromo-N-(tert-butyl)-2-(4-methylphenyl) acetamide.· Under theegeneralprocedure, (4-methylphenyl)aceticc acid(12.0 mmol), dimethylformamide (2 drops), chloroform (15 mL),phosphorous trichloride (12.0 mmol) were reacted. The mixture wascooled to 0 °C and thionyl chloride (18.0 mmol) was added via sy-ringe. The mixture warmed to room temperature and stirred for 2 h,bromine (14.4 mmol, excess 11.0 mmol) was added, and stirred for16 h at 80°C. N,N'-diisopropylethylamine/triethylamine (24.0 mmol)was charged to a solution of tert-butylamine (18.0 mmol) in CH2Cl2(50 mL). The reaction mixture was cooled to 0 °C and the crudebromo(phenyl)acetyl chloride was added slowly via syringe over45-60 min. The reaction warmed to room temperature and stirredfor 3-5 h. Product purified by flash column chromatography (hex-anes(EtOAc). The compound was obtained as a white solid 0.800 g,24% yield, mp 135-138 ℃; 1HNMR (CDCl3, 600 MHz) 6 7.32 (d, 2H,J=7.8 Hz), 7.17 (d, 2H,J=7.8 Hz), 6.53 (s, 1H), 5.33 (s,1H), 2.33 (s,3H),1.39 (s,9H);13c NMR (CDCl3, 125 MHz)6 166.1,139.0,134.9,129.6,128.1,52.2,52.1,28.4,21.2; HRMS (ESI) m/z calculated forC13H18BrNNaO [M+Na]+: 306.0464; found: 306.0457. with dichloromethane and filtered through plug of silica. The crudereaction mixture was concentrated under reduced pressure andpurified by flash column chromatography (hexanes/CH2Cl2). ( 4.6.1. N-(tert-butyl)-2-(1H-indol-1-yl)-2-phenylacetamide (2a ) . The compound was ob t ained as a w h i te solid, 2 6 .2 m g , 90% yield, mp 174-177 C ; lH NMR (CD C l3, 60 0 MHz ) 6 7. 6 4 (d, 1H, J=7.8 Hz), 7.39-7.33(m,6H), 7.23 (t , 1 H ,J=7 . 8 Hz ) , 7.15 (t, 1H,J= 7. 8 Hz), 6.86(d, 1H,J=3.0 Hz), 6.52 (d, 1H,J=3.0 Hz), 6.01 (s, 1H), 5.35 (s , 1 H ), 1.25 ( s , 9H);13CNMR(CDC,125 MHz)6 168.2,136.6,135.6,129.0,128.9,128.8, 128.7,126.0, 1 22.3,121.3, 1 20.5,109.6,103.1,64.7,51.8,28.5;HRMS (ESI)m/z calculated for C20H22N2NaO[M+Na]+: 329.1624; found: 329.1623. ) ( 4.6.2. N-(tert-butyl)-2-(2-methyl-1H-indol-1-yl)-2-phenyl ac e t - amide ( 2b). The compound was obtained as a white solid, 20 . 5 mg, 67% yield , mp 161-164°C; HNMR (CDCl3, 600 MHz) 6 7.53(d,1H, J =7.2 H z), 7.31-7.25 ( m , 3 H ), 7.22 (d, 2 H ,J=7.2 Hz), 7.06 (dd, 1H, J = 7. 8, 6 .6 Hz), 6.99 (dd,1H,J=8 . 4, 6.6 Hz), 6.96(d, 1H,J = 7 . 8 Hz), 6 . 37 (s, 1H), 6.10 (s, 1H), 5.48 ( s, 1H), 2.35 (s, 3 H), 1 .26 ( s ,9 H ); 13 C N M R (CDCl3, 125 MHz);167.4, 137.1,136.3,135.5,128.8, 1 28.6,128.0,12 7 .9,121.2,120.2,120.0,111.0,102.7, 6 2.5,51.8,28.5,13.7;HR M S (ES I ) m/z calculated for C21H25N20[M+H]+:321.1967; f o un d : 321 . 1966. ) 4.6. General procedure for 2a-h and 3a-g In a glove box, a-bromoamide (0.190 mmol) and KOt-Bu(0.230 mmol) were weighed to an oven dried vial while indolederivatives (0.0950 mmol) and [Rh(COD)Cl]2 (0.00950 mmol) weremeasured into a separate vial. Benzene(0.7mL) was then added viasyringe to the mixture of a-bromoamide and KOt-Bu and stirred for20-25 min at room temperature. Benzene (0.50 mL) was added viasyringe to mixture of indole derivative and [Rh(COD)Cl]2 and thentransferred via syringe to the vial containing the in situ generatedaziridinone. Another portion of benzene (0.30 mL) was used torinse the vial and added to the reaction vial. The reaction mixturewas stirred for 70 min at room temperature and excess KOtBu(0.4-0.5 equiv) was added. The reaction mixture stirred for another30 min at room temperature. The reaction mixture was diluted ( 4 .6.3. N -(tert-butyl)-2-(3-methyl-1H-indol-1-yl) - 2 - p henyl acet- amide ( 2 c) . The compound was obtained as a w h ite solid, 24.3 mg, 8 0% y i eld, mp 1 86-189 C; H N MR (CDC3,600 MHz)6 7.57 (d, 1H, =7.8 : Hz), 7 .40-7.36 ( m, 3 H ) , 7. 3 4-7. 3 0 (m, 3 H ), 7 . 23 ( t , 1H, = 7.8Hz ), 7 .16(t, 1H,J=7.8 Hz), 6 . 61 ( s ,1H),5.96(s,1H),5.39(s,1H), 2. 2 6 ( s,3H), 1.25 (s, 9H); 13c NMR (CDCl3,125 M H z) 6 16 8 .5, 137.0, 135.9, 129.3, 1 29.0, 1 2 8.7 , 1 2 8 .6,1 2 3.3, 1 2 2.3,11 9 .8,119.3, 11 2 .3, 1 09.5,64.5,51.7 , 28.5 , 9. 7 ;HRMS (ESI) m/z calculated for C21H25N20 [M+H]+: 32 1.1961; f o und: 3 21.1964. ) 4.6.4. N-(tert-butyD)-2-(5-methyl-1H-indol-1-yl)-2-phenyl acet-amide (2d). The compound was obtained as a white solid, 23.4 mg,77% yield, mp 187-189°C;HNMR(CDCl3, 600 MHz) 6 7.42(s,1H),7.39-7.32 (m,5H), 7.24 (dd, 1H,J=9.6, 1.2 Hz), 7.06 (dd,1H,J=8.4,1.2 Hz), 6.81 (d,1H,J=3.6 Hz), 6.43 (d, 1H,J=3.0 Hz), 5.98 (s,1H),5.37 (s,1H), 2.44 (s, 3H), 1.25 (s, 9H); 13c NMR (CDCl3,125 MHz); 168.3,135.7,135.0,129.7,129.2,129.0,128.7,128.7,126.0,123.9,120.9,109.3,102.6,64.8,51.7,28.5,21.4; HRMS (ESI) m/z calculatedfor C21H24N2NaO [M+Na]+: 343.1781; found: 343.1763. 4.6.5. N-(tert-butyl)-2-(7-methyl-1H-indol-1-yl)-2-phenyl acet-amide (2e). The compound was obtained as a white solid, 28.2 mg,93% yield, mp 170-173 ℃;H NMR(CDCl3, 600 MHz) ; 7.47 (d, 1H,J=7.8 Hz), 7.40-7.36 (m, 3H), 7.32 (d, 2H,J=7.2 Hz), 7.03 (t, 1H,J=7.2Hz), 6.97 (d, 1H,J=7.2 Hz), 6.73 (s, 1H), 6.56 (s, 1H), 6.49 (s,1H), 5.19 (s,1H), 2.75(s,3H), 1.25 (s,9H); 13CNMR(CDCl3,125 MHz); 169.2, 136.6,135.8,129.9,129.0, 128.9, 128.6,126.6,125.7, 121.2,120.6,119.5,103.6,66.3,51.7,28.4, 20.3; HRMS (ESI) m/z calculatedfor C21H25N20[M+H]+: 321.1967; found: 321.1973. 4.6.6. N-(tert-butyl)-2-(5-iodo-1H-indol-1-yl)-2-phenylacetamide(2f). The compound was obtained as a white solid, 40.2 mg, 98%yield, mp 206-208°C; 1H NMR (CDCl3,600 MHz) 7.96 (s,1H), 7.46(d,1H,J=8.4 Hz), 7.39 (d, 3H,J=5.4 Hz), 7.29 (d, 2H,J=6.0 Hz), 7.11(d,1H,J=8.4 Hz), 6.86(d,1H,J=3.0 Hz), 6.44 (d,1H,J=3.0 Hz), 5.94(s, 1H), 5.36 (s, 1H), 1.27 (s,9H); ’CNMR(CDCl3, 125 MHz) 167.6,135.6,135.2,131.4,130.5,130.1,129.1,128.9,128.5,127.0,111.5,102.2,84.0, 64.7, 51.9, 28.5; HRMS (ESI) m/z calculated for C20H21IN2NaO[M+Na]+:455.0596 found: 455.0558. ( 4.6.7. N-(tert-butyl)-2-(2-methyl-5-methoxy-1H-indol-1-yl)-2- phenylacetamide (2g). Th e compound was o btained a s a w hite solid , 23.2 mg, 70% yield, m p 131-13 3 C; H NMR (CDCl3, 400-188-0725 ) 600 MHz) 8 7.31-7.29 (m, 3H), 7.22 (d, 2H,J=7.2 Hz), 6.99 (s, 1H),6.79 (d,1H,J=9.0 Hz), 6.64 (dd,1H,J=7.2, 1.8 Hz), 6.29 (s,1H), 6.04(s, 1H), 5.47 (s,1H), 3.81 (s, 3H), 2.34 (s, 3H), 1.26(s,9H); 13C NMR(CDCl3, 125 MHz) 6 167.4,154.3,137.8,135.6,131.3, 129.4, 128.6,128.0,127.9,111.8,110.8,102.4,102.2,62.6,55.7,51.7,28.6, 13.7;HRMS (ESI) m/z calculated for C22H26N2NaO2 [M+Na]+: 373.1886;found: 373.1877. 4.6.8. N-(tert-Butyl)-2-(2-methyl-5-nitro-1H-indol-1-yl)-2-pheny lacetamide (2h). The compound was obtained as a yellow solid,22.0 mg, 63% yield, mp 73-75 °C; H NMR(CDCl3, 600 MHz) ò 8.45(d, 1H,J=1.8 Hz), 7.91 (dd,1H,J=9.0, 1.8 Hz), 7.35 (d, 3H,J=4.8 Hz),7.18(d,2H,J=4.8 Hz), 7.04(d,1H,J=9.0 Hz), 6.54(s,1H), 6.10(s,1H),5.50(s,1H), 2.41 (s, 3H), 1.32 (s,9H); 13C NMR (CDCl3, 125 MHz)ò 166.3,141.9,140.8,139.5,134.7,129.1,128.7,128.0,127.7, 116.9,116.8,110.9,104.4,63.2,52.3,28.5,13.8; HRMS (ESI) m/z calculatedfor C21H23N3Na03[M+Na]+: 388.1632; found: 388.1623. ( 60% yield, mp 162-165 °C;H NMR(CDCl3, 600 MHz) 6 7.65 (d,1H, J =7.8 H z), 7.37 (d, 1H,J=7.8 Hz), 7.26(d, 3H,J=8.4Hz), 7.21 (d, 2H, 7.8 Hz), 7.16 (t, 1H,J=7.8 H z), 6.86 ( d,1 H ,J=3.0 Hz), 6.52(d, 1H, J =3.0Hz), 5.98 (s,1H), 5.33 (s,1H),2.36(s,3H),1.25(s,9H);13CNMR (CDCl3, 125 MHz) ; 1 6 8.4, 138.7, 1 36.4,132.4,129.7,128.8, 128.6, 125.9,122.2,121.2,120.4,109.6,102.9, 64.4,51.7,28.4,21.2; HRMS (ESI) m/z calculated for C21H24N2NaO [ M+ N a]+: 3 4 3.1781; f ound: 343.1794. ) 4.6.14. N-(tert-Butyl)-2-(1H-indol-1-yl)-2-[4-(trifluoromethyl) phe-nyljacetamide (3f). The compound was obtained as a white solid,22.4 mg, 63% yield, mp 188-191 C; H NMR (CDCl3, 600 MHz)6 7.66 (d,1H,J=7.8 Hz), 7.63 (d, 2H,J=5.4 Hz), 7.52-7.49(m, 2H),7.34(d, 1H,J=7.8 Hz), 7.27 (d, 1H,J=7.2 Hz), 7.18 (t, 1H,J=7.8Hz),6.86 (d,1H,J=3.0 Hz), 6.58 (d, 1H,J=3.0 Hz), 6.06 (s, 1H), 5.37 (s,1H), 1.26 (s, 9H); 13C NMR (CDCl3, 125 MHz) 6 167.3,136.7,136.5,132.0, 131.3 (q,J=132.0 Hz), 129.5,128.9,125.6,125.5,124.7, 122.9,122.7, 121.5,120.8,109.5,103.9,64.0, 52.0, 28.5; HRMS (ESI) m/zcalculated for C21H21F3N2NaO[M+Na]+:397.1498; found:397.1474. ( References and notes ) ( Aziri d i nes and E poxid e s i n Organi c S yn the s is; Wile y -VCH: Weinhei m , 2 006. ) ( S h ee h an, J J . . C.; Iz zo, P. T . J . Am. Chem. Soc. 1949, 7 1 , 4 0 59. ) ( B ac h ,R . D . ; D m itrenko,O. J. Am. Chem. Soc. 2 0 06, 128,45 9 8. ) ( 4. Tant i llo , D.J ; Hou k , K . N. ; Hoffm a n, R . V.;T a o, J . j . Org. C hem. 1 999,64,38 3 0. 5. H o ffm an, R . V . ; Nayyar, N. K.; K l in ek ole, B . W . J . A m. C hem. Soc. 1 9 92, 114, 6 2 62. 6 . R o bert o , D.; Alper, H . Organo m etallics 1 9 84, 3,1 7 67. ) ( A cha ry a, A.; E ickhoff , J. A.; Jeffery, C . S. Synthesis 2013, 4 5,1 8 2 5. ) ( J e f frey, C. S . A . D.; Cars o n , C . R. Org . Lett. 2012 , 14, 5 764. ) ( 9. J e ffrey, C. S .; Barnes, K. L. E . J . A . ; C arson, C . R . J. Am. Chem. S o c. 2 0 11, 13 3 ,7688. 10. d e Sa Alves, F . R . ; Barreir o , E. J ; Fraga, C . A. M . M ini- R ev. M ed. C h em. 20 0 9, 9, 7 8 2 . ) ( 11. F u, L. Top. Heterocycl. Chem. 2 010, 26,433. ) ( 12. Kaushik, N .; K aushik, N.; A ttri, P. ; Kumar, N. ; Kim, C.; Verma, A. ; C h oi, E. Mol- e cules 2013, 18,6620. ) ( 13. S harm a , V.; K u mar , P.; Pathak, D. J. H e terocycl. C h em. 20 1 0, 47, 49 1 . ) 14. Wu, Y.-J.Top. Heterocycl. Chem. 2010, 26,1. ( 15. B andini, M.; E i c h ho l zer, A . A n gew. Chem., Int . E d . 2009, 48 , 9608. ) ( 16. Bandi n i, M .;M e llo n i, A .; Tommasi, S.; Uman i -Ronchi, A . Synlett 2005 , 1 1 99. ) ( 1 7. B andin i , M .; M ell o ni, A.; Umani -R o n chi, A. A n gew. Ch e m., Int. Ed. 2 0 04,43,550 . 18. C ui, H.- L .; F e n g , X.; Pe n g , J ; L e i , J. ; J i ang , K . ; C h en, Y.-C. A n g ew. Chem . , Int. E d . 2009,48, 5 737. ) ( 1 9. A nti l la, J . C . ; K l a pars, A.; Bu c hwald, S . L. J.Am . Chem. Soc. 2002, 124, 1 1684. ) ( 2 0. S evov, C . S . ; Z hou, J; H a r twig, J. F. J. Am. Chem. Soc. 2 014, 1 3 6,32 0 0. ) ( 21. C ( arey,J . S . ; Laffan , D. ; Thomson, C.W.. ,M . T. O rg. Biomol. Chem. 2 006, 4,2337. ) ( 2 2. B ariwal, J; Van d er Eycken, E. C hem. S oc. R ev. 2 013, 42, 9 283. ) ( 23. H a rtw i g,J. F . Acc . Chem. Res. 2008, 41 , 1534 . ) ( 2 4. Ha rtwig, J. F. N ature 2008, 4 5 5, 3 1 4. ) ( 2 5. S urr y ,D . S. B .. , S . L . A n gew. Chem., Int . Ed . 2008, 4 7,6 3 38. ) ( 2 6. B uckw a ld,s. L. M . ., C . ; M i g n ani, G . ; S c ho l z, U . Adv. S yth. Catal . 2006, 348,23. ) ( 27. Y ang, B. H .; B uchwald , S. L . J . Organomet. Chem. 1999,576,1 2 5. ) ( 28. . H a ma, T.; Culkin, D . A.; H artwi g. J.F . J. Am. Chem. Soc. 2006,1 2 8,4976. se v.l a ) ( 2 9. Le ngy el , I .; C easre, V .; K ar r am, H.; Taldon e , T. J . H eterocycl . Chem. 2001, 38, 997. ) ( 3 0. L e n gy el, I.; Ceasre, V . ; T a ldone, T . S ynth. Commun. 2001, 3 1, 2499. 3 1. Y u, H . C hirali ty 2008, 2 0, 6 9. ) ( 3 2. S heeha n , J. C . ; B eeson, J . H . J . Am. C hem. S o c. 19 67, 8 9 , 3 6 2. ) ( 33. L e ng y el, . ; Ceasre, V . ; Chen , S.; T aldon e , T . H e t erocycles 20 0 2, 57, 677. ) Elsevier Ltd. All rights reserved.ww.rysstech.com耐士科技 www.rysstech.com
确定






还剩7页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








