
The syntheses of a diverse set of 2-aryl-4 quinolone derivatives were achieved by exposing corresponding acylated 20-aminoacetophenones to microwave irradiation in the presence of NaOH. The microwave accelerated cyclizations were complete within 10–22 min at 120 C giving 57–95% isolated yields.
方案详情

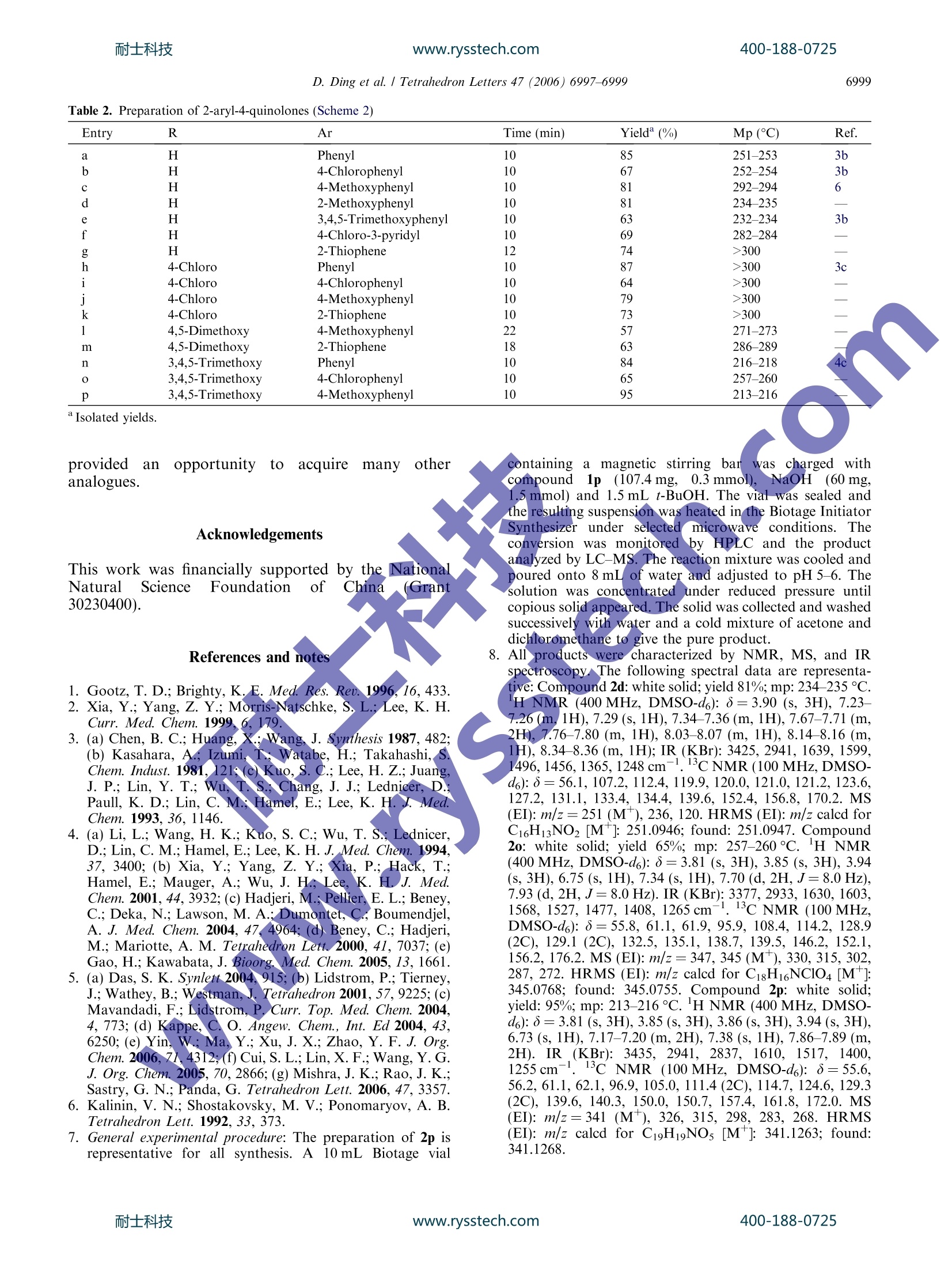
www.rysstech.comAvailable online at www.sciencedirect.com耐士科技400-188-0725ScienceDirectTetrahedronLettersTetrahedron Letters 47 (2006) 6997-6999 耐士科技400-188-0725www.rysstech.comD. Ding et al. / Tetrahedron Letters 47 (2006)6997-69996998 0 Microwave-assisted rapid and straightforward synthesis of2-aryl-4-quinolones from acylated 2-aminoacetophenones Derong Ding, Xin Li,* Xin Wang, Yongli Du and Jingkang Shen* State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Shanghai Institutes for BiologicalSciences, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, PR China Received 28 May 2006; revised 22 July 2006; accepted 25 July 2006 mo Abstract-The syntheses of a diverse set of 2-aryl-4-quinolone derivatives were achieved by exposing corresponding acylated 2'-aminoacetophenones to microwave irradiation in the presence of NaOH. The microwave accelerated cyclizations were completewithin 10-22 min at 120°C giving 57-95% isolated yields. 4-Quinolone derivatives are an important class of anti-bacterial agents.Numerous analogues have been synthe-sized to evaluate their antibacterialpropertiesaandantibacterial spectrum. Recent studies alsoi revealedthat 2-aryl-4-quinolones possessed antitumor activitiesowing to their ability of inhibiting tubulin polymeriza-tion. To construct this intriguing 2-aryl-4-quinolonescaffold, several synthesis methods have been developedfrom different starting materials.,3Among them, cycliza-tion of acylated 2-aminoacetophenones attracts moreattention because diverse starting materials are readilyavailable and the reaction conditions are relatively mild,at 70-80C in the presence of potassium tert-butoxide(t-BuOK). However, long reaction time, 10-24 h, isalways needed for reaction completion, and low yieldsare sometimes encountered.+ The introduction of microwave heating has greatly im-pacted many aspects of chemical synthesis. There aresome excellent reviews and reports on the broad use ofmicrowave irradiation in organic synthesis. It has beendemonstrated that the: use of microwave heating candramatically cut down reaction time, increase productpurity and yields, and allow precise control of reactionconditions, all of which make it suited to meet theincreased demands of high throughput chemistry. In thisletter, we report an efficient and good yielding condition ( Keywords: Microwave-assisted synthesis; 2-Aryl-4-quinolone; Acylated 2'-aminoacetophenone. ) ( *Corresponding authors. Tel.: + 86 2 1 50806896; fax: + 86 21 50807088(J.S.); e-mail: J k s h en@mail. s hcnc.ac.cn ) for synthesis of 2-aryl-4-quinolones from acylated 2'-' aminoacetophenones with the aid of microwave irradia-tion. All microwave experiments were performed in aself-tuningsingle mode Biotage Initiator MicrowaveSynthesizer.7 To optimize the reaction conditions, cyclization of N-(2-acetyl-phenyl)-benzamide 1a was selected as model reac-tion (Scheme 1). A mixture of 1a and 5 equiv of t-BuOKin t-BuOH was initially exposed to microwave irradia-tion at 80 °C for 10 min. TLC test indicated the reactionasuncompleted. On increasing the temperature to120 °C, the process of conversion of 1a was completedgiving 88% of desired product, 2-phenyl-4-quinolone2a (Table 1, entry 1). Under this condition, other fourcommonly used bases were employed to investigate theirinfluence on the cyclization (Table 1, entries 2-5). Inter-estingly, 95% yield of product could be afforded underthe effect of NaOH, whereas no product was detectablewith K2COs due to its weak basicity. More experimentswere carried out to evaluate the influence of temperature Scheme 1. ( 0040-4039/$- see f r ont matter @ 20 0 6 Elsevier Ltd. All rights reserved. doi:10.1016/j.tetlet.2006.07.117 ) Table 1. Reaction conditions on the cyclization of 2'-aminoacetoph-enone benzamide (Scheme 1) Entry Microwave conditions Conv.(%) Yields(%) 1 t-BuOK, 120°C, 10 min 100 88 2 NaOEt, 120°C, 10 min 100 79 3 KOH, 120°C, 10 min 100 82 4 NaOH, 120 °C, 10 min 100 95 5 K2CO3, 120°C, 10 min 0 0 6 NaOH, 130°C, 10 min 100 84 7 NaOH, 110°C, 10 min 98 76 8 NaOH, 100°C, 10 min 75 57 9 NaOH, 90°C, 10 min 60 44 10 NaOH, 80 C, 10 min 40 25 Determined by HPLC at 215 nm and LC-MS. on microwave assisted cyclization of benzamide 1a (Ta-ble 1). With the decrease of the reaction temperature,both conversions and yields dropped (Table 1, entries4and 6-10). Although the starting material disappearedat 130°C for 10 min, only 84% yield of expected productwas gained, which possibly suggested that higher tem-perature might result in more by-products. It is accepted that both thermal effect and specific micro-wave effect may induce the acceleration of some reaac-tions somehow on irradiating with microwave. Inorder to testify if the microwave speeds upthe cycliza-vastion, the model reaction was conductedina sealedthick-walled tube under an identical condition1 bbut in-stead heating in an oil bath. The time course resultsare plotted in Figure 1. Microwaye heatedreactionscould produce, through all the way of the reactions’pro-cess, higher conversion of the starting material and high-er yield of the product than when heated in traditionaloil bath (Fig. 1A and B)The differences are especiallysignificant within 5 min,90% conversions for microwavebut only 50% for oil-bath heating.And to complete thecyclization, it takesonlyyi10 minby microwave: heating(Fig. 1A), while normally10-24h by previouslyre-ported method. These results may elucidate that micro-wave effect might play a certain role in the cyclization. To expand the scope of this chemistry to more 2-aryl-4-quinolone derivatives syntheses, adiverseset of sub-strates 1a-p were selected and exposed to the irradiation Scheme 2. of microwave under the condition described above(Scheme 2). Because t-BuOK and NaOH exhibited sim-ilar catalytic character (Fig. 1), and NaOH is cheaperand easily obtainable, reactions listed in Table 2 werecarried out using NaOH as the base. The desired prod-ucts 2a-p could be precipitated in water at pH 5-6, andcollected by filtration followed by washing with waterand a cold mixture of acetone and dichloromethane.Good to excellent isolated yields, 57-95%, weregained,which are summarized in Table 2. In general, R1substi-tuent produced minor influence on ithe microwave as-sistedcyclization (Table 2, entriesa-c, g-k, and n-p),but tthere was stilll a vague casethat 4,5-dimethoxysubstituted substratesaffordedrelatively lower yieldseven exposed to irradiation for a prolonged period (Ta-ble 2, entries l and m). In contrast, the properties of Rdisplayed prominent effect on the isolated yields. para-Chloro substitutedbenzamides (Table 2, entries b, i,ithand o) always afforded lower yields when compared withbenzamides (Tablee 2, entries a, h, and n) and substitutedbenzamides containing electron-donating groups (Table2, entries c, d,j, and p). However again a vague case wasobserved in which a low yield was produced from 3,4,5-trimethoxy-benzamide cyclization (Table 2, entry e).Heterocyclic acylated substrates (Table 2, entries f, g,k,andm) could not afford as much product as benz-amides (Table 2, entries a, h, and n). In summary, a rapid and straightforward method wasdeveloped for the synthesis of 2-ary1-4-quinolone deriv-atives from acylated 2'-aminoacetophenones under theirradiation of microwave and in the presence of NaOH.The reaction time was dramatically reduced from 10 to24 h by traditional oil bath heating to 10-22 min. A di-verse range of 2-aryl-4-quinolone derivatives were syn-thesized to demonstrate that the reported method Figure 1. Comparison of the effect of microwave and oil-bath heating on the cyclization: (A) kinetic curves of conversion and (B) kinetic curves ofyield. www.rysstech.com D. Ding et al. /Tetrahedron Letters 47 (2006) 6997-6999 Table 2. Preparation of 2-aryl-4-quinolones (Scheme 2) Entry R Ar Time (min) Yield(%) Mp (C) Ref. a H Phenyl 10 85 251-253 3b b H 4-Chlorophenyl 10 67 252-254 3b c H 4-Methoxyphenyl 10 81 292-294 6 d H 2-Methoxyphenyl 10 81 234-235 e H 3,4,5-Trimethoxyphenyl 10 63 232-234 3b f H 4-Chloro-3-pyridyl 10 69 282-284 g H 2-Thiophene 12 74 >300 h 4-Chloro Phenyl 10 87 >300 3c i 4-Chloro 4-Chlorophenyl 10 64 >300 l 4-Chloro 4-Methoxyphenyl 10 79 >300 k 4-Chloro 2-Thiophene 10 73 >300 4,5-Dimethoxy 4-Methoxyphenyl 22 57 271-273 m 4,5-Dimethoxy 2-Thiophene 18 63 286-289 n 3,4,5-Trimethoxy Phenyl 10 84 216-218 4c 3,4,5-Trimethoxy 4-Chlorophenyl 10 65 257-260 3,4,5-Trimethoxy 4-Methoxyphenyl 213-216 providedan opportunityto aacquire manyy otheranalogues. Acknowledgements This work was financially supported by the NationalNaturallScience Foundation ofChina (Grant30230400). ( References and notes ) ( 1. Gootz, T. D.; Brighty, K . E. Med . Re s . R ev. 1996 , 1 6,433. ) 2. Xia, Y.; Yang, Z. Y.; Morris-Natschke, S. L.; Lee, K. H.Curr. Med. Chem. 1999,6. 79. 3. (a) Chen, B. C.; Huang, Wang, J. Synthesis 1987, 482;(b) Kasahara, A.; Izun Vatabe, H.; Takahashi, S.Chem. Indust. 1981,121 uo,S. C.; Lee, H. Z.; Juang,J. P.; Lin, Y. T.; Wu, hang, J. J.; Lednicer, D.Paull K. D.. Lin, C. M. Hamel, E.; Lee, K. H. I. MedChem. 1993,36,1146. 4. (a) Li, L.; Wang, H. K.; Kuo, S. C.; Wu, T. S.; Lednicer,D.; Lin, C. M.; Hamel, E.; Lee, K. H. J. Med. Chem. 1994,37,3400; (b) Xia, Y; Yang, Z. Y.; Xia, P.; Hack, T.;Hamel, E.; Mauger, A.; Wu, J. H, Lee, K. H. J. Med.Chem. 2001, 44, 3932; (c) Hadjeri, M.; Pellier,E. L.; Beney,C.; Deka, N.; Lawson, M. A.; Dumontet, C., Boumendjel,A. J. Med. Chem. 2004, 47,4964; (d) Beney, C.; Hadjeri,M.; Mariotte, A. M. Tetrahedron Lett. 2000, 41, 7037;(e)Gao, H.; Kawabata, J. Bioorg Med. Chem. 2005, 13, 1661. 5. (a) Das, S. K. Synlett 2004, 915;(b) Lidstrom, P.; Tierney,J.; Wathey, B.; Westman,J. Tetrahedron 2001,57,9225;(c)Mavandadi, F.; Lidstrom, P.Curr. Top. Med. Chem. 2004,4, 773;(d) Kappe, C.O. Angew. Chem., Int. Ed 2004, 43,6250;(e) Yin, W;Ma,Y.; Xu, J. X.; Zhao, Y. F. J. Org.Chem. 2006, 71,4312; (f) Cui, S. L.; Lin, X. F.; Wang, Y. G.J.Org. Chem. 2005,70,2866; (g) Mishra, J. K.; Rao, J. K.;Sastry, G. N.; Panda, G. Tetrahedron Lett. 2006,47,3357. ( 6. Kalinin, V. N .; Shostakovsky, M. V.; Ponomaryov, A . B. Tetrahedron Lett. 1992,33,373. ) ( 7. G ( eneral e xperimental procedure: T he p r eparation of 2p is Pis representativ e for al l synthesis. A 10 mL B iotage vial ) containingaa magnetic stirring barwass(chargedi withcompound1p((107.4 mg,0.3 mmol),),NaOH (60 mg,1.5 mmol) and 1.5 mL t-BuOH. The vial was sealed andthe resulting suspension was heated in the Biotage InitiatorSynthesizer under selectedmicrowave conditions. Theconversion was monitoredby HPLC and the productanalyzed by LC-MS. The reaction mixture was cooled andpoured onto 8 mL of water and adjusted to pH 5-6. Thesolution was concentrated under reduced pressure untilcopious solid appeared. The solid was collected and washedsuccessively with water and a cold mixture of acetone anddichloromethane to give the pure product. 8. All products were characterized by NMR, MS, and IR spectroscopy. The following spectral data are representa-tive: Compound 2d: white solid; yield 81%; mp: 234-235C.H NMR (400 MHz, DMSO-d6):8=3.90 (s,3H),7.23-7.26(m,1H), 7.29 (s, 1H), 7.34-7.36 (m, 1H), 7.67-7.71 (m,2H), 7.76-7.80 (m, 1H), 8.03-8.07 (m, 1H), 8.14-8.16 (m,1H), 8.34-8.36 (m,1H); IR (KBr): 3425,2941,1639,1599,1496,1456,1365,1248 cm-.1cNMR(100MHz, DMSO-d6): 8=56.1,107.2,112.4,119.9,120.0,121.0,121.2,123.6,127.2,131.1,133.4,134.4,139.6,152.4,156.8,170.2. MS(EI): m/z=251 (M*), 236, 120. HRMS (EI): m/z calcd forC16H13NO2 [M*]: 251.0946; found: 251.0947. Compound2o: white solid; yield 65%; mp: 257-260C. H NMR(400 MHz, DMSO-d6): ;=3.81 (s, 3H), 3.85 (s,3H), 3.94(s, 3H), 6.75 (s, 1H), 7.34 (s, 1H), 7.70 (d, 2H,J=8.0 Hz),7.93 (d,2H,J=8.0 Hz). IR (KBr): 3377,2933,1630,1603,1568, 1527, 1477, 1408,1265 cm=1.13c NMR (100MHz,DMSO-d6):8=55.8,61.1,61.9,95.9,108.4,114.2,128.9(2C), 129.1(2C),132.5,135.1,138.7,139.5,146.2,152.1,156.2, 176.2. MS (EI): m/z=347, 345 (M*), 330, 315,302,287, 272. HRMS (EI): m/z calcd for C18H16NC104 [M*]:345.0768; found: 345.0755. Compound 2p: white solid;yield: 95%; mp: 213-216℃.H NMR (400 MHz, DMSO-d6):=3.81 (s,3H), 3.85 (s,3H), 3.86 (s, 3H), 3.94 (s, 3H),6.73 (s, 1H), 7.17-7.20 (m,2H), 7.38 (s,1H), 7.86-7.89 (m,2H). IR (KBr): 3435,2941,2837,1610,1517,1400,1255 cm-. 13c NMR (10o0o MHz, DMSO-d6): 8=55.6,56.2,61.1,62.1,96.9,105.0,111.4(2C),114.7,124.6,129.3(2C), 139.6,140.3,150.0,150.7,157.4,161.8, 172.0. MS(EI): m/z=341 (M*), 326, 315, 298,283,268. HRMS(EI): m/z calcd for C19H19NO5[M*]: 341.1263; found:341.1268. ww.rysstech.com耐士科技 士科技
确定


还剩1页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








