
方案详情
文
The impact of a-trifluoromethanesulfonyl groups on the chemistry of various carbonyl groups is reported. Allylic a,a-dialkylated-a-trifluoromethanesulfonyl esters readily underwent decarboxylative allylation. a Trifluoromethylsulfonyl esters, ketones, and amides were all methylated in the presence of trimethylsilyldiazomethane. Esters afforded a mixture of O- and C-methylation; however, ketones and amides offered exclusively O-methylation, with varying degrees of E/Z selectivity, thus affording ambiphilic alkenes. a-Trifluoromethanesulfonyl ketones also exhibited keto-enol tautomerism.
方案详情

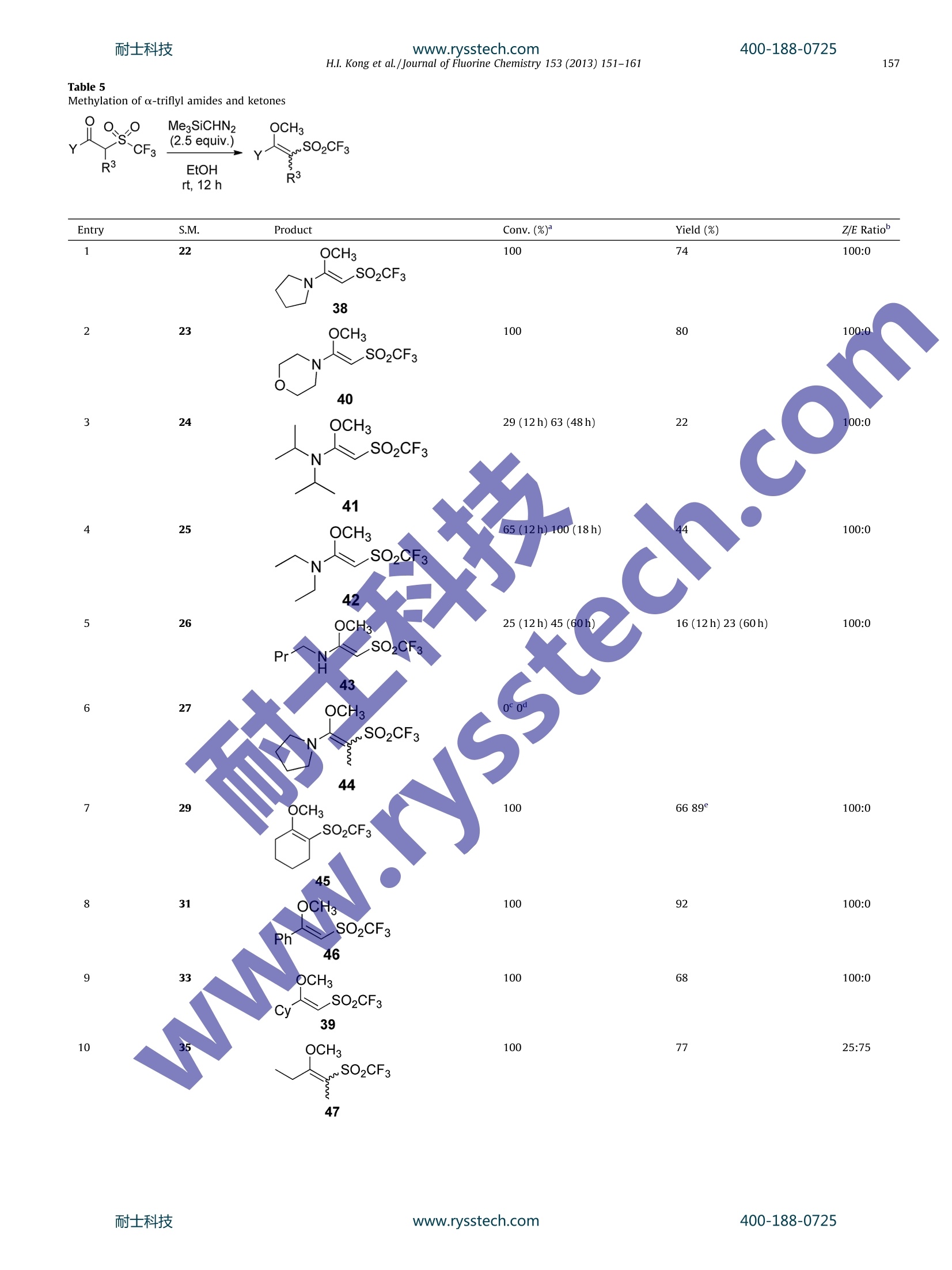
耐士科技400-188-0725www.rysstech.comJournal of Fluorine Chemistry 153 (2013)151-161Contents lists available at SciVerse ScienceDirectJournal of Fluorine Chemistry 400-188-0725耐士科技www.rysstech.com152H.I. Kong et al. /Journal of Fluorine Chemistry 153 (2013) 151-161 journal homepage: www.elsevier.com/locate/fluor Reactivity of a-trifluoromethanesulfonyl esters, amides and ketones:Decarboxylative allylation, methylation, and enol formation Han Il Kong, Monica A. Gill1, Amy H. Hrdina,Jennifer E. Crichton, Jeffrey M.Manthorpe*Carleton University, Department of Chemistry, 1125 Colonel By Drive,203 Steacie Building, Ottawa, ON K1S 5B6, Canada ARTICLEINFO ABSTRAC T Article history:Received 7 February 2013Received in revised form 18 March 2013Accepted 26 March 2013 Available online 6 April 2013 The impact of a-trifluoromethanesulfonyl groups on the chemistry of various carbonyl groups isreported.Allylic a,a-dialkylated-a-trifluoromethanesulfonyl esters readily underwent decarboxylativeallylation. a-Trifluoromethylsulfonyl esters, ketones, and amides were all methylated in the presence oftrimethylsilyldiazomethane. Esters afforded a mixture ofO-and C-methylation; however, ketones andamides offered exclusively O-methylation, with varying degrees of E/Z selectivity, thus affordingambiphilic alkenes. a-Trifluoromethanesulfonyl ketones also exhibited keto-enol tautomerism. Keywords: TriflonesDecarboxylative allylationKeto-enol tautomerismDiazomethaneTrimethylsilyldiazomethaneAmbiphilic alkenesO-alkylation of enolates Beginning in the 1970s, the Hendrickson research groupexplored the synthesis and reactivity of trifluoromethyl sulfones(triflones) in alkylation, conjugate addition, and cycloadditionreactions [1]. Several reagents, both nucleophilic and electrophilic,have been reported for the construction of thiotrifluoromethylgroups in various oxidation states [2-8]. Metal-catalyzed decarboxylative allylation has proven to be ahighly efficient and popular method for the construction ofcarbon-carbon and carbon-heteroatom bonds in both inter- andintramolecular reactions [9]. One of the key requirements formetal-catalyzed decarboxylative allylation is an electron with-drawing group to stabilize the intermediate anion. Given theexceptional electron withdrawing character of triflones, severalyears ago we initiated a researchprogram exploring their viabilityin decarboxylative allylation, with an eye toward using them asalkane synthons via reductive excision of the sulfone group. Duringthe course of our studies, Tunge and co-workers reported thisprocess using phenyl sulfones at high temperature [10]. Herein wewish to report our explorations of this process utilizing the much ( * Corresponding author. Tel.: + 1 613 520 2600x1711; fax: +1 613 520 3749. E-mail address: jeffre y _ manthorpe@carleton.ca (J.M. Manthorpe).T h ese authors co n tributed equally to th i s article. ) more electron deficient trifluoromethyl sulfones and detail howthis work led us to develop a process for preparing ambiphilicalkenes, including the first sulfonyl-substituted ambiphilic alkenesderived from amides. 2. Results and discussion Our initial work focused on allyl a-triflylacetate 2 (Scheme 1),which was prepared via reaction of allyl chloroacetate with sodiumiodide and the Langlois reagent, sodium trifluoromethanesulfinate1[2]. Palladium-catalyzed decarboxylative allylation was exploredusing dipalladium tris(dibenzylideneacetone) and triphenylpho-sphine. Consumption of the starting material was very rapid atroom temperature but afforded a mixture of polyallylationproducts. This prompted us to prepare an a,a-disubstitutedcompound. When initial attempts at a seemingly straightforward alkylationapproach did not meet with success (vide infra), we opted for a one-potorganolithiumapproach.Hendrickson reported that the additionoforganolithiums to N-phenyltriflimide resulted in formation ofthecorresponding triflone in good yield. However, other electrophilictriflyl sources, such as Tf20 afforded bis-triflones [4]. We thereforeexamined the reaction of sec-butyllithium with PhNTfz (Scheme 2).It is important to note that after the initial (rate-limiting)nucleophilic attack by the organolithium, a second equivalent oforganolithium acts as a base to deprotonate a to the newly formed Scheme 1. Synthesis and decarboxylative allylation of 2. PhNTf, CI (0.95 equiv.) .S F3C 0 R + F3C 3a R=H 62% moc3b R=CH2CH Ph 40% Scheme 2. Preparation of allylic a,a-dialkylated a-triflylaceates. triflone,affording carbanion 6. We surmised that 6 could beacylatedwith an allylic chloroformate to afford our desired starting material.To our delight, the reaction proceeded as predicted,affording thedesired 3a in 62% yield. However, a critical impurity4 was alsoformed via acylation of the N-phenyltriflamide anion byproduct 5and separation of these two compoundsrequirediudiciouschromatography. Use of other allyl sources, such as allyl cyano-formate [11] or allyl imidazolyl formate [12]did not improve theyield or minimize the formation of 4. Use of 0.95 equivalents ofallylic chloroformate proved optimal. This one-pot synthesis of 3 has several intriguing features. Thefirst is that the acylation of anion 6 proceeds at all. Hendricksonreported extensively studying the acylation of triflone anions andwas only able to provide a single example [13]; however, it isunclear whether the one-pot approach in Scheme 2 was explored.We were also surprised that the acylation of 5and 6 wascompetitive, as the drastic difference in their respective pK values(ca. 18.8 for 6 [14] vs. ca. 4.35 for 5 [15])would lead to theexpectation that the 6 would be far more reactive. We surmise thatboth of these results are due to a combination of aggregation andsolvent effects, as well as the rate of complexation of 5 and 6 withthe chloroformate electrophile. With test substrate 3a in hand, we attempted palladium-catalyzed decarboxylative allylation using 5 mol% Pd2(dba)3 and20 mol% PPh3 (Scheme 3). Gratifyingly, the reaction proceededrapidly and in high yield at room temperature to afford desiredhomoallylic triflone 7a. This result stands in stark contrast to the analogous phenyl sulfone system [10], where the same catalystsystem had to e heated to 95℃ to induce allylation andimportantly, a protonated side-product was produced in a 1:1ratio with the desired product. Here, the protonation side-product8 was not observed. A solvent and catalystloading screen was performed and it wasfound that the reaction yield was largely independent of solvent.Reduction of the catalyst loading to 2 mol% still afforded 7a in goodyield, though lowering the temperature to 0℃ did result in anoticeable yield attenuation (Table 1). We then turned our attention to the preparation of additionalsubstrates. Variation of the chloroformate proved to be feasibleand afforded 3b in moderate yield. The subsequent decarbox-ylative allylation proceeded smoothly to afford 7b in 77% yield(Scheme 4). Again, protonation product 8 was not observed. The final step in our process was to demonstrate the viability ofour triflones as alkane synthons by reductive cleavage of thetriflone. Magnesium [16], though a convenient reagent, provedineffective. However, exposure of 7b to Raney nickel underhydrogen atmosphere (balloon) offered clean excision of thesulfone with concomitant alkene hydrogenation (Scheme 5) [17]. We next sought to further broaden the substrate scope byvarying the substituents on the a-carbon; however, this proved tobe highly challenging. Preparing secondary alkyllithium reagentsusing lithium di-tert-butylbiphenylide [18,19] followed by expo-sure to PhNTfz afforded triflones in low yields and presentedpurification challenges. Secondary Grignard reagents are not a Scheme 3. Palladium-catalyzed decarboxylative allylation of allyl a,a-dialkylated a-triflylacetate 3a. Scheme 4. Palladium-catalyzed decarboxylative allylation of allylic a,a-dialkylated a-triflylacetate 3b. Table 1Screening of solvents and catalyst loading Scheme 5. Desulfonylation of 7b with Raney nickel. suitable substitute [20,21]. A sequential alkylation/acylation ofbenzyl triflone [1,13,20] was also not fruitful [22]. At this point we began considering preparing compounds withother oxidation states of sulfur (i.e. sulfide and sulfoxide) andperforming subsequent oxidation. Secondary trifluoromethylsulfides have been accessed in modest yield via reaction ofdisulfides and thiocyanates with trifluoromethyltrimethylsilaneand TBAF [3]. However, in our hands these approaches did notafford sufficient yield of product when evaluated with dicyclohex-yldisulfide and cyclohexylthiocyanate. Entry Solvent Temperature Mol% Pd Time Yield (%) 1 Et2O rt 10 20 min 89 2 PhMe rt 10 20 min 86 3 PhMe rt 2 17h 7 DCM rt 10 20min 84 DCM rt 2 6h 11 THF rt 10 20 min 92 THF rt 5 30 min 79 8 THF rt 2 30 min 78 9 THF 0C 30 min 61 We were hopeful that our desired system could be accessedin an alternative fashion via the classicalAndersen-typenucleophilic attack on a sulfinate to afford the desired sulfoxide[23], which could then be oxidized to the sulfone. Moreover, asthis oxidation would erase any chirality at sulfur, we werecontent with producing racemicsulfoxide. We preparedcyclohexyl trifluoromethanesulfinate 10 via in situ conversionof sodium trifluoromethanesulfinate to trifluoromethanesulfinylchloride[1,13] and addition of cyclohexanol. When this com-pound was exposed tocyclohexylmagnesium bromide, thedesired product 11 was not obtained. Instead, to our surprise,the CF3 group had Ebeendisplaced,affording cyclohexylcyclohexanesulfinate 12 (Scheme t6)..Examination of the litera-ture offered some hints at this result. For example, Bertrand andco-workers reported thepreparation of 4-phenyl-3-trifluoro-methanesulfinyloxazolidin-2-one demonstrated that mildnucleophiles, such as alcohols, amines, and pyrroles could betriflinated [24]. However, there is no comment on reaction withharsher nucleophiles, such as Grignard reagents, despite the factthat the Evans group has reported the utility of oxazolidinoneauxiliaries for the preparation of enantiomerically enrichedsulfoxides [25]. In addition, Prakash, Hu, and Olah have reportedthe displacement of CF3- fromPhSO2CFupon exposure topotassium tert-butoxide in DMF[26]. At this stage we elected to revisit the alkylation of allylictriflylacetates[27]. Alkylationof this class of compounds isactually non-trivial.l.Triflylacetate esters are exceptionally acidicC-H acids: ethyl triflylacetate has a pKa of 6.4 in DMSO [28]; by acidhas a pka of 12.6 in DMSO comparison, acetic 1 1 29].Examination of anarray of alkylation conditions led only torecovery of starting material and/or decomposition by nucleo-philic attack of the halide byproduct on the ester alkyl group. Thisin turn leads to decarboxylation and a myriad of other side-products, as eleegantly elucidated by Langlois and co-workers(Scheme 7)[2a].Given the highly acidic nature of a-triflyl esters,we next considered the viability of diazoalkanes as alkylatingagents. The regioselectivity of such an alkylation was notimmediately clear. Diazoalkanes are certainly “hard”reagents[30]; however, given the triflyl group's extraordinary electronwithdrawing character and ability to stabilize negative charge(which is comparable in strength to that of the nitro group [28],though the two groups operate via different mechanisms), it is notunambiguous whether the hard site in a-triflyl esters is theenolate oxygen or carbon. This results in six potential reactionproducts, arising from mono- or bis-C-methylation, E- or Z-selective O-methylation, and C-methylation followed by O-methylation. In order to evaluate the viability of this hypothesis, we preparedcyclohexyl triflylacetate 13 from the corresponding bromide and1[1]. Exposure of 13 to trimethylsilyldiazomethane (10 equiv.) inMeOH at room temperature for 1 h resulted in a mixture of threemajor methylation products: a 1.5:1 ratio of C-methylation (14) toO-methylation (15) (Scheme 8). Alkene 15 was isolated as a 1.8:1mixture of olefin isomers. The bis-c,o- and bis-C,c-methylationproducts were not observed. Scheme 6. Sulfinate approach to trifluoromethylsulfoxides. Scheme 7. Decarboxylation of a-triflyl acetates. Scheme 8. Methylation of ester 13 with trimethylsilyldiazomethane. Scheme 10. Rationale for regio- and stereoselectivity in methylation of a-triflylcarbonyls. Several a-triflyl amides were prepared from two equivalents ofthe corresponding amine and bromoacetyl bromide or 2-bromo-propionyl bromide, followed by Sn2 reaction with sodiumtrifluoromethanesulfinate in DMA (Table 2). The preparation ofa-triflyl esters and ketones has been reported previously [2a];however, to the best of our knowledge this is the first work [27] onthe synthesis of a-triflyl tertiary acetamides. Table 2Synthesis of a-triflyl amides Entry R, R R Equiv. 1 Step2 temp. Product Yield (℃), time (%,2 steps) 1 (CH2)4 H 1.5 60, 2d 22 90 2 (CH2)20 H 2.0 70,3 d 23 52 3 i-Pr2 H 1.5 70,3 d 24 54 4 Et2 H 1.5 70, 3 d 25 86 5 n-Bu, H H 1.2+0.5 70,4d 26 72 after 2 d 6 (CH2)4 CH3 1.5+0.85 after 4 d 70,7d 27 41 We: also synthesizedda selection of(a-triflyl ketones(Scheme 11). The requisite a-halo ketones 28 and 30 werecommercially available, while a-bromination of the correspond-ing ketone afforded 34 and 36 [33]. Since cyclohexyl methylketone is not commercially available, bromomethyl ketone 32was accessed via reactionoff cyclohexanecarbonyl chloridewith trimethylsilyldiazomethane, followed by treatment withHBr [34]. Upon examination of their H NMR spectra, we discoveredthat several of the ketones exhibited significant enol content, aphenomenon previously observed anecdotally in a-perfluoro-sulfonyl ketones [35]. We elected to carry out a survey of thisphenomenon by varying the solvent and structure of the ketoneand observed several general trends (Table 3). Conjugatedketones exhibit less enol tautomer thannon-conjugated ketones.In CDCls and DMSO-d6 the cyclic ketone 29 displayed a higherenol content than the acyclic examples. In all cases but two (31and 37 in DMSO-d6), the keto/enol tautomerization was slowerthan the NMR timescale, thus affording distinct peaks for theketo and enol tautomers. Perhaps the most intriguing resultsarise from 35, which offered no detectable enol content in CDCl,while affording 92% enol content in acetone-d6.We surmise thatthis latter result is due to the ability of acetone to act as ahydrogen bond acceptor for the enol or 35 and acetone's planarcharacter provides a less sterically crowded environment thanthe trigonal pyramidal DMSO. Moreover, the acyclic nature of 35could allow it to adopt conformations more favorable tostabilization of the enol form, including the E configuration ofthe enol. This is in contrast to the cyclic 29. We presume stericsalso play a role, as the ethyl group of 29 is less bulky than thephenyl and cyclohexyl groups of 31 and 33, respectively. With an assortment of a-triflylketones and amides in hand, weselected amide 22 as our model. Reaction with 10 equiv. of Keto/enol ratios of several a-triflyl ketones in different deuterated solvents. Ketone CDCl: DMSO-ds Acetone-ds 65:35 13:87 91:9 .SO2CF3 29 94:6 100:0 65:35 Ph' SO2CF3 31 91:9 Broad peaks 74:26 SO2CF3 33 0 .SO2CF3 100:0 85:15 8:92 35 94:6 Broad peaks 80:20 Ph SO2CF3 a All samples were 1 mM in the noted solvent and all spectra (H, 1’c and 19FNMR) were acquired at ambient temperature. Scheme 12. Optimization of methylation of a-triflyl ketone 33. Table 4Optimization of methylation of a-triflyl amide 22. Entry Me3SiCHN2 Solvent Conc. Time Conv. (equiv.) (M) (h) (%) 1 10 MeOH 0.5 3 100 2 10 EtOH 0.5 3 100 3 10 i-PrOH 0.5 3 68 4 5 MeOH 0.5 24 100 5 5 EtOH 0.5 24 100 6 5 i-PrOH 0.5 24 86 7 2.5 MeOH 0.5 12 50 8 2.5 EtOH 0.5 12 100 9 2.5 EtOH 1 12 100 10 1.5 EtOH 12 47 11 2.5 EtOH/THF 12 90 12 2.5 EtOH/Et2O 12 94 13 2.5 EtOH/EtOAc 1 12 91 aDetermined by integration of the 'H NMR spectrum of the crude reactionmixture. trimethylsilyldiazomethane at 0.5 M in MeOH for 3 h resulted in100% conversion (Table 4). Gratifyingly, the Z-alkene 38 was theonly observed product; no C-methylation or bis-methylationproducts were found. We then set out to optimize the reactionconditions. In order to ensure complete desilylation, we initiallylimited our solvents to simple alcohols. Though methanol iscommonly used in conjunction with trimethylsilyldiazomethane[36], ethanol proved to be the superior solvent for the formation of38, as it enabled the reduction of the number of equivalents of Scheme 14. Competitive methylation of a-triflyl ketone 31 and a-triflyl amide 22. www.rysstech.com Table 5 Methylation of a-triflyl amides and ketones S.M. Product Conv. (%) Yield (%) Z/E Ratio" www.rysstech.com Table 5 (Continued) aDetermined by integration of the lH NMR spectrum of the crude reaction mixture. Assigned via 2D NOESY experiment. Under standard conditions. ° 5 equiv. MegSiCHN2, 1M in EtOH, rt, 5 days. and, conversely, amides enolate are more reactive than theirketone analogues, we opted to perform a competition experimentto determine whether the rate-limiting step of the reaction isdiazoalkane protonation or enolate methylation. When equimolaramounts of ketone 31 and amide 22 were combined with 1equivalent of trimethylsilyldiazomethane we observed 46 as thesole methylation product; amide methylation product 38 was notobtained (Scheme 14). Accordingly, we concluded that theprotonation of diazomethane is likely the rate-limiting step inthe reaction mechanism; however, given the apparent termole-cular pathway of carboxylic acid esterification with trimethylsi-lyldiazomethane [38], we cannot rule out more complexbehaviour. It is also notable that these conditions offered only50% conversion of 31, consistent with our previous observationthat excess trimethylsilyldiazomethane is required toeffectcomplete reaction. 3. Conclusion In conclusion, we have demonstrated the rapid Pd(0)-catalyzeddecarboxylation of allylic a-triflyl esters, despite significantchallenges in substrate preparation. However, these challengesled us to utilize the exceptional acidity of a-triflyI carbonylcompounds to explore their methylation with trimethylsilyldia-zomethane. We have also prepared an array of a-triflyl amides andketones and the latter exhibit varyingdegrees of keto/enoltautomerism. We have also demonstrated a facile method forthe stereoselective synthesis of a-triflyl enol ethers and a-triflylketene aminals, both of which can be classified as ambiphilicalkenes. We are currently exploring the chemistry of thesecompounds as electrophiles and substrates for cycloadditions,including cyclopropanation to afford a new class of donor-acceptorcyclopropanes (Scheme 15) [39] and results will be reported in duecourse. 4. Experimental 4.1. General methods All reagents were purchased from commercial sources and wereused as received, without further purification. Methylene chloridewas distilled from CaH2 prior to use. THF was distilled from lithiumaluminum hydride prior to use. Crude products were analyzed by TLC using glass-backed Extra Hard Layer (60 A) TLC plates fromSilicycle and visualized by fluorescence quenching under UV lightand/or staining using potassium permanganate. Flashchromatographic purification of products was performed either onSilia-P Flash silica gel from Silicycle using a forced flow of eluent bythe method of Still [18] or by automated chromatography on aBiotage Isolera One equipped with a UV detector. Concentration invacuo refers to rotary evaporation at 40°C at the appropriatepressure. Yields refer to purified and1spectroscopically purecompounds unless explicitly indicated as crude. NMR spectrawere recorded on a Bruker Avance III 300 or Bruker AMX 400 MHzspectrometer. Chemical shifts are reported in ppm. Tetramethyl-silane was used as the internal standard for lH and 13c NMRspectra. 19F NMR spectraare referenced to trifluorotoluene(--163.7ppm).IR spectra were recorded on a Varian 1000 ScimitarSeries or an ABB Bomem MB Series spectrometer. Absorptions aregiven in wavenumbers (cm ). 1High-resolution electron impactmass spectrometry (HR-EI-MS) was performed on a KratosConcept-11A mass spectrometer with an electron beam of 70 eVat the Ottawa-Carleton Mass Spectrometry Center. High-resolutionelectrospray mass spectrometry (HR-ESI-MS) was conducted usingan ABI SciEx QSTAR XL electrospray quadrupole time-of-flightspectrometer with cesium iodide as aninternal reference.Compounds 22-27 [27],29[27], and 31-48 [27] were preparedas previously reported. 4.2.EExperimental procedures 4.2.1. Allyl trifluoromethanesulfonylacetate (2) A mixture of sodium trifluoromethanesulfinate (1,672 mg, 4.3imol, 1.0 equiv.), allyl chloroacetate (0.50 mL, 4.3 mmol,1.0 equiv.), and sodium iodide (155 mg, 6.5 mmol, 1.5 equiv.)were sequentially added to MeCN (5 mL) and the solution washeated to reflux for 40 h. After cooling to room temperature water(10 mL) was added and the mixture was washed with EtOAc(3×5 mL). The combined organic layers were dried over magne-sium sulfate, filtered, and concentrated in vacuo. The residue waspurified by column chromatography (7% EtOAc/hexanes) to afford2 as a colourless oil (589 mg, 59%). Rr=0.16 (10%EtOAc/hexanes); 1H NMR (CDCl3, 400 MHz): 85.94 (m, 1H), 5.31-5.45 (m, 2H), 4.77 (d,J=5.9Hz, 2H), 4.28 (s,2H); 13C NMR (CDCl, 75 MHz): 8 159.2,130.2, 120.3, 119.1 (q,J=327 Hz),67.9,54.6;19FNMR(377MHz): 8-77.8; IR (thin film): Scheme 15. Potential applications of ambiphilic alkenes. 3093,3000,2946,1750,1651,1379,1283,1211,1120;HR-ESI-MS:m|z calcd for C6H6F304S (M-H*) 230.9944; found:230.9945. 4.2.2. Allyl 2-methyl-2-(trifluoromethanesulfonyl)butanoate (3a) sec-Butyllithium (1.4M in cyclohexane, 1.0mL, 1.4 mmol,2.5 equiv.) was added dropwise to solution of N-phenyl-bis(tri-fluoromethanesulfonimide) (200 mg, 0.56 mmol, 1.0 equiv.) inTHF (2.8mL, 0.2M) at -78℃ in a Schlenk flask. The reactionmixture was allowed to warm over 1 h and was then re-cooled to-78℃. Allyl chloroformate (0.056 mL, 0.53 mmol, 0.95 equiv.)was added dropwise and the reaction mixture was stirred for 8 hwithout further addition of dry ice to the cooling bath. The reactionwas quenched with saturated aqueous ammonium sulfate.Mixture was extracted with 2x 10 mL diethyl ether, and thecombined organic extracts were washed with brine and then driedover magnesium sulfate. Filtration, followed by concentrationyielded aipale yellow oil, which was purified by columnchromatography on silica gel (2% EtOAc/hexanes) to afford 3a(94 mg, 62% yield) as a colourless oil. solution. PPhs (6.6mg, 0.025 mmol, 10 mol%) was added tothe reaction mixture, and then left to stir for 30 min. The solutionprogressively changed from the original deep red colour to aclear dark green. A solution of ester 3a (68.0mg, 0.25 mmol,1.0 equiv.) in THF (200 uL) was then added to the Pd mixturedropwise over a 2-min period. The reaction was monitored byTLC and was complete in less than 30 min. The solvent wasremoved in vacuo, then the crude mixture was purified via flashchromatography (2.5% EtOAc/hexane) to give a colorless oil(53mg, 92%). Rr=0.477(10%ethylacetate/hexanes);1HNMRR(CDCl3,400 MHz): 8 5.80 (ddt, J=16.8, 10.0, 7.6 Hz, 1H), 5.22 (dd,J=16.8,1.6 Hz, 1H), 5.24-5.26 (m, 1H), 2.76 (dd,J=14.4, 7.2 Hz,1H), 2.57 (dd,J=14.0, 7.2 Hz, 1H), 2.03 (dq,J=14.8, 6.8 Hz, 1H),1.88 (dq,J=14.8, 7.2Hz, 1H), 1.48 (d,J=1.2 Hz, 3H), 1.09(t,J=7.6Hz,3H). 1CNMR (CDCl3, 75 MHz): 8 129.5,120.9,120.7(q,J=332 Hz),56.2,37.4,26.7,19.2,8.2.19FNMR(CDCl3,376.5 MHz):8 -71.1. IR (thin film): 1622, 1594,1439,1419,1341,1204,1121,948, 887. HR-EI-MS: (M*) not observed; m/z calcd for C7Hi3(M*-SO2CF3): 97.1012; found: 97.1039. 4.2.6. (E)-(6-Methyl-6-((trifluoromethyl)sulfonyl)oct-3-en-1-yl)benzene (7b) 4.2.4.(E)-5-Phenylpent-2-en-1-yl 2-methyl-2- ((trifluoromethyl)sulfonyl)butanoate (3b) Prepared in analogy to 3a to afford 3b as a colourless oil (40%).Rf=0.47’ (10% ethyl acetate/hexanes);H NMR (CDCl3,400 MHz): 8 7.26-7.31(m, 2H), 7.16-7.21 (m, 3H), 5.88 (m,1H),5.60 (m, 1H), 4.64(m,2H),4.59(d, minor isomer), 2.71 (t,J=7.2 Hz,2H),2.47 (m, unresolved due to overlap with q at 2.40), 2.40 (q,J=7.6 Hz, 2H), 2.02 (dq,J=15.2, 6.8 Hz, 1H), 1.72 (s, 3H), 0.98 (t,J=7.2Hz,3H).13CNMR(CDCl,100 MHz) (major isomer): 8165.5,141.3,137.3,128.4,126.0,124.0,122.9,120.4(q,J=330Hz), 74.7,67.7, 35.2, 33.9, 25.9,16.0,8.3. 19F NMR (CDCl3, 376.5 MHz): 8-70.9.IR (thin film): 3029,2944,2858,1742,1673,1604,1497,1456,1362,1316,1202,1155,1127,1096,1052,1030. HR-EI-MS:(M*) not observed; m/z calcd for C5HgF302S*[M*-C(O)OC11H13]:189.0175; Found: 189.0192; m/z calcd for C11H13*: 145.1012;Found:145.1026. 4.2.5. 4-Methyl-4-((trifluoromethyl)sulfonyl)hex-1-ene (7a) Pd2(dba)3 (5.8 mg, 6.3 umol, 2.5 mol%) was dissolved in THF(2.5 mL)under argon with constant stirring to produce a deep red Rf=0.40) (10%6 ethyl acetate/hexanes);1'H NMR (CDCl3,400 MHz): 8 5.92 (ddt, J=17.1,10.3, 6.0Hz, 1H), 5.42 (ddt,J=17.1,1.4,1.3 Hz, 1H), 5.32(m, 1H), 4.73 (m, 1H), 2.50 (m, 1H),2.04(m, 1H), 1.74 (s, 3H), 1.01 (t,J=7.6 Hz, 3H); 13C NMR(CDCls,100 MHz): 8165.5,130.4,122.6(q,J=330Hz), 120.1, 74.7, 67.6,25.9,16.0,8.3;19FNMR(CDCl3, 376.5 MHz):8-70.9; IR(thin film):3092,2987,2952,2918,2890,2849,1745,1462,1363,1316,1203,1156,1128,1097,1052; HR-EI-MS: (M*) not observed; m/z calcdfor C6HgF303S (M*-OC3H5): 217.0135; found: 217.0121. 4.2.3. (E)-5-Phenylpent-2-en-1-yl chloroformate Prepared in analogy to 7a as a colorless oil (77%). The E/Z ratio(determined by integration of19F NMR spectrum) of 7b was 88:12.Rr=0.56(10% EtOAc/hexanes); H NMR (CDCl3, 400 MHz): 87.15-7.30(m,5H),5.57-5.71(m, 1H,mixture of cis/trans isomers),5.32-5.43 (m, 1H, mixture of cis/trans isomers), 2.71 (t,J=7.2 Hz,2H), 2.60-2.67 (m,1H, mixture of cis/trans isomers), 2.44-2.52(m,1H,mixture of cis/trans isomers), 2.39 (td,J=7.2,7.2Hz,2H),1.81-1.98 (m, 1H, mixture of cis/trans isomers), 1.69-1.79 (m, 1H,mixture of cis/trans isomers), 1.40 (d, J=0.8 Hz, minor isomer),1.35 (d,J=1.2Hz, 3H), 1.03 (t, J=7.2 Hz, minor isomer, over-lapping with peak for major isomer at 1.02 ppm), 1.02(t,J=7.6 Hz,3H);13C NMR (CDCl3, 100 MHz): 8 141.4, 136.1,134.1,128.5(minor isomerr),128.5, 128.4 (minor isomer), 128.3,126.0 (minorisomer), 125.9,122.4, 122.3(minor isomer), 120.2(q,J=246 Hz),70.1 (minor isomer), 69.9, 36.1, 35.5 (minor isomer), 35.4, 34.1,30.4 (minor isomer), 29.4, 26.8 (minor isomer), 26.6, 19.0, 18.9(minor isomer), 8.4(minor isomer), 8.3.;.;19F NMR (CDCl3,376.5MHz): 8 -71.1 (minor isomer), -71.2 (major isomer). IR(thin film): 3028,2985,2945,2857,1604,1497,1456,1349,1199,1138,1123,1103,1030,974.HR-EI-MS: m/z calcd for C16H21F302S:334.1214;Found: 334.1218. 4.2.7. 6-Methyl-1-phenyloctane (9) Raney 2800 nickel, slurry in water, was washed five timeswith distilled water until the pH of the supernatant was neutral,then the Raney Ni was slurried in anhydrous ethanol.(6-methyl-6-(trifluoromethanesulfonyl)oct-3-en-1-yl)benzene(51.7mg, 0.15mmol, 1 equiv.) was dissolved in anhydrous ethanol in a Schlenkflask, then the washed slurry of Raney Ni was transferred to theflask. The flask headspace was briefly evacuated under vacuum,then backfilled with a balloon of hydrogen gas. This was repeatedthree times. The reaction flask was left under H2 pressure at roomtemperature for 8 h while monitoring by TLC. The mixture wasfiltered through Celite, then concentrated in vacuo to yield a lightyellow oil. Flash chromatography (100% hexane) gave 9 as acolorless oil (24 mg,78%). R=0.72(hexane); H NMR (400 MHz, CDCl;) 8 7.25-7.29(m,2H), 7.15-7.19 (m, 3H), 2.60 (t,J=8.0 Hz, 2H), 1.58-1.65 (m,2H),1.24-1.37(m,7H),1.07-1.17 (m,2H), 0.83-0.87(m,6H).13CNMR(100MHz, CDCl3)8143.0,128.4,128.2,125.5,36.5,36.0,34.4, 31.6,29.7,29.5,27.0,19.2, 11.4. IR (thin film): 3027, 2960,2928,2856,1605,1496,1463,1377,1030. HR-EI-MS: m/z calcd for C15H24:204.1878; Found: 204.1869. A solution of mesitylenesulfonyl chloride (3.32 g, 15.2 mmol,1.0 equiv.) and sodium1 trifluoromethanesulfinate(1,2.50g,16.0 mmol, 1.05 equiv.) in acetonitrile (20 mL) was stirred at roomtemperature for 1 h, then cooled in an ice bath. A solution ofcyclohexanol (1.61 mL, 15.2 mmol, 1.0 equiv.) and pyridine(1.23 mL, 15.2 mmol, 1.0 equiv.) in acetonitrile (7.0 mL, 2.2 M)was prepared and added dropwise over 5 min to the cooled solutionprepared previously. The reaction mixture was allowed to warmslowly to room temperature over 18 h. The milky reaction mixturewas then diluted with diethyl ether (100 mL) and washed withwater (5x 50 mL). The organic phase was washed with 3x 50 mLbrine, then dried over magnesium sulfate, filtered and concentratedin vacuo to produce a yellow oil (2.30 g, 70%) that was sufficientlypure to proceed to the next step. An analytical sample was purifiedby flash chromatography (10% EtOAc/hexanes). Rr=0.64(10%ethyl acetate/hexanes);: 1H NMRR(CDCl3,400 MHz): 8 4.54 (septet, J=4.0 Hz,1H), 1.19-2.02 (m, 10H);1c NMR (CDCl3,75 MHz): 8 122.7(q,J=334Hz), 82.0, 33.1, 33.0,24.8,23.5,23.4; 19FNMR(276 MHz):8-81.1; IR (thin film): 2942,2864, 1732, 1453,1373,1200,1132,1033, 1004; HR-EI-MS: m|zcalcd for C6H1102S(M*-CF3)147.0474; found: 147.0498; m/z calcdfor CF3OS: 116.9622; found:116.9594; m/z calcd for C6H11(M*-SO2CF3): 83.0855; found: 83.0877. 4.2.9. Cyclohexyl cyclohexanesulfinate (12) Cyclohexyl trifluoromethanesulfinate (10, 505 mg, 2.3 mmol,1.0 equiv.) was dissolved in toluene (12.5mL, 0.2M)) and thesolution was cooled in an ice bath. Cyclohexylmagnesium chloride(2.9 mL,2.0 M in diethyl ether, 5.8 mmol, 2.5 equiv.)was addeddropwise over 5 min. Cooling bath was maintained for 15 min, thenreaction mixture was allowed to warm to room temperature over45 min.Reaction was quenched with saturated aqueous ammoniumchloride and extracted with 3× 25 ml methylene chloride. Thecombined organic extracts were washed with brine(3×25 mL),thendried over sodium sulfate, filtered and concentrated in vacuo. Thecrude product was purified via flash chromatography (10% EtOAc/hexane) to yield 12 (213 mg, 40%) as a colourless oil. Rf=0.24(10%ethylacetate/hexanes);;H INMRR(CDCl3,400 MHz): 8 4.81 (tt,J=9.6,4.0 Hz, 1H), 2.52 (tt, J=11.2, 4.0 Hz,1H), 1.68-2.01 (m,10H),1.21-1.61(m,12H).13CNMR(100MHz,CDCl3)878.8,63.8,33.8,32.9,25.7,25.2,25.2,25.1,24.5,23.8,23.7(2C). IR (thin film): 2933,2856,1451,1370,1134,1035,1012.HR-EI-MS: m/z calcd for C12H2202S: 230.1341;Found: 230.1326. Acknowledgements J.M.M. thanks NSERC (Discovery and RTI programs), CanadaFoundation for Innovation (Leaders Opportunity Fund), OntarioResearch Fund, and Carleton University for financial support.M.A.G. acknowledges the Government of Ontario for an OntarioGraduate Scholarship.M.A.G. and H.I.K. thank Carleton Universityfor financial support. The authors also wish to thank Prof. JeffSmithand Karl Wasslen for performing high-resolution electrospraymass spectrometry experiments. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version, athttp://dx.doi.org/10.1016/j.jfluchem.2013.03.020. References [1](a) For reviews of the Hendrickson group’s work, see: J.B. Hendrickson, D.D.Sternbach, K.W. Bair, Acc. Chem. Res. 10 (1977) 306-312; (a) F.Eugene, B. Langlois, E. Laurent, J. Fluorine Chem. 66 (1994) 301-309; ( ( b ) M .Tor d eux , B. Langlois, C. W aksel m an , J. Org. Chem. 5 4 (1989) 2452-2453. ( a) T . B illard , B.R. Lang l ois, Tetrahed r on L et t . 37 (1996) 6 865-6868; ) ( ( b) T . B il l ard , S. Larg e, B.R. Langloi s, Tetrahedro n L ett . 3 8 (1997) 6 5-6 8 . ) ( ] J .B. He n dri c k son, K.W. Bai r , J. Or g . Chem . 4 2 (1977 ) 3875 - 3878. ) J.B. Hendrickson, D.A. Judelson, T. Chancellor, Synthesis (1984) 320-322. ( 456 ( a) P . E isen b erger, I . Kieltsch, R . Koller, K. Stanek, A . T o gni, Org. Synth.8 8 (2011 ) 1 68-180: ) ( ( b) I . Kielts c h , P. Eisenberger, A. T ogni,Ange w . Ch e m . Int. Ed. 46(200 7 )754-757; (c) S . Ca p one, I . Kielts c h ,O . F logel, G. Lelais, A. Togni, D.Seeb a ch, Helv.C h im. Acta ) 91(2008)2035-2056. ( [ 7] C . Chen, Y.Xie, L . Chu, R.-W. Wang,X. Zhang,F.- L . Qing, Angew. Chem. Int . Ed. 5 1 (201 2 ) 2492. ) ( G . Teverovs ki y, D .S. Surr y , S.L . Buchwald, Angew. Chem. Int. Ed. 50 (201 1 )7312. ) (a) For recent reviews on metal-catalyzed decarboxylative allylation, see: J.D. ( W eaver, A. Reci o I I, A.J. Grenning, J . A. Tunge, Che m .Re v . 1 1 1 (2 0 1 1)1846-191 3 ; nning,J.A . lu n g (b) J.F. Hartwig, L.M. Stan l ey, A cc . C hem. Res . 43 (2010) 1 461-14 75 . ) ( [10 ( ] a)J . D. Weaver, J.A. T un g e, O rg. L e tt. 10 (2008) 4657- 46 60; ) ( ( b) J .D. Weaver, B .J. Ka, D.K. Morris, W. T h ompson,J.A. T u nge,J. Am. Chem. So c . 132(2010 ) 12179- 1 2181. ) ( [11] M . C hilds, W. W ebber, J . Org. C h em . 41 (197 6 ) 3 4 8 6. [12 B ] . M . Tr o st, J . Xu, J. Org. C h em. 7 2 (2007) 9 3 72. ) ( [13] ] j J.B. Hen d rickso n , P.L . Skipper, Tetrah ed ron 32 (1 976) 1627- 1 6 3 5 . ) ( [14] F . G . Bordwel l , N.R. Van i er,W.S. M a tt he w s, J.B. H en d rick s on , P . L . Ski p pe r, J . A m. C hem. Soc. 97 (1975) 7160-7162. ) ( [ 1 5] SciFinder Scholar . Calculate d usin g Advanced Chemistry Develo p ment (ACD/ L abs) Software V 11 . 02. ) ( [1 6 ] S e e also Ref.[ 8 ]. Magnesium is typically limited to r eduction of a romatic sulfones. S ee: A .C. Brown, L .A. Carpino, J . Org. Chem. 50 (1985) 1 749-1 7 5 0. ) ( [17 ] C areful T LC monitoring of t h e r e action demonstrated t h a t sulfone cleavage and a lkene reduction proceeded at competitive rates. ) ( 1 8] L ithium 4, 4 '- d i -tert-butylbiphenylide. e - Ency cl opedia of Rea g ents for O rga n ic ) ( Synthesis [Onli n e]; W i ley, Posted 15. 4. 0 1 . ht t p : // on lin eli b r a r y.wiley.c o m/o/ero s / a rt i c le s /rl088/frame.htm l (accessed 1 5.10.12) ) ( [1 9 ] F ew secondary alkyllithiums are commercially avai l able and t heir prep a ration is c onsi d erably m ore tedious than their Grignard brethren. We explored the gener-ation o f the requisite alk y llithiums from t h e corresponding halides usi n g lithium t v d i-tert-butylbiphenylide but p roduct yields were quite low and contrary t o our i nitial expectati o ns, our t riflone s were actually quite non-polar and co-elutedwith the di-tert-butylbi p heny l byproduct. ) [20] X. Creary,J. Org. Chem. 45 (1980) 2727-2729. ( [21 ] Given t h a t secondary G r ignard r eagents a re f ar m ore r e adily a vailable and p repared, w e c o nsidered t h eir utility here. H owever, Creary ( s ee R e f. [ 2 0 ]) reported that organomagnesium bromides and iodides re a ct with Tf20 to form b IY rom i ne a n d i o d i ne , r espectively. Primary organomagnesium chlorides were shown t o prod u ce t r i flones in moderate to high yield b u t s e condary Grignards a fforded poor r e sults. I n addition, Grignard r eagents a r e u n reactive toward P hNT f2 ( s ee Ref. [4]) and thus we ruled out this route. ) ( [ 22] Benzy la tion of benzyl triflone (see Ref. [1 , 3 ,20]) with benzyl bromide and sodium h y d ri d e s u ccessfully a f forded 2-(benzyl)benzyl triflone (s e e Ref.[13 ] ) . We thenopted to revisit the acylation of triflone anions. Despite testing a thorough array of re action c onditions, w e, like H endrickson et al. (see Ref.[ 1 , 1 3 ] ) , w ere u n able to ) ( a chieve the desired outcome. ) ( [23] ( a) I . Fernan d ez, N. Khiar , J.M. Llera, F. Alcudia, J. Org. Ch e m. 57 (1992) 6789- 6 7 96: ) ( ( b) K .K. Andersen, Tet r ahedron L ett. 3 (196 2 ) 93-95; ) ( (c) K.K. Andersen, W. G a ffield, N.E. P ap a nikolaou, J . W. F o ley, R . I . Perkins, J. Am . Chem. Soc . 86 (196 4) 5637-5646. ) ( [ 24] V .D. Romanenko , C. T h oumazet, V. Lavallo, F . S. Tham, G . B er t rand, Chem. Com- m un. (2003) 1680-1681. ) ( [25] ( a) D .A.E v a n s, M.M. Fau l ,L. C o lombo, J.J. Bis a ha, J . C l ardy, D . Cherry, J. A m. Chem. Soc. 114 (1992 ) 5977 - 5985; ) (b) J.P. Marino, S. Bogdan, K. Kimura, J. Am. Chem.Soc. 114 (1992) 5566-5572. ( [26 G ] . K . S.P r akash, J. Hu, G.A. Olah, Org. L e tt . 5 (2003) 3 253-3256. ) ( [27] A prel i mi n ary a c count o f t h is work has been p u b li s h e d H.I . Kon g , J. Cricht o n, J.M . Manthorpe, Tetr a h e dron Lett. 52 (201 1 ) 3714-3717. ) ( [28] R . Goum o n t , E . Magnier , E . Kizilian, F . T er r ier,J . Org . Chem. 68 (2003) 6 566- 6570. ) ( [29] (a) I.M. Kolt h off , M.K . Chantooni, S . Bhowmik, J . Am. C hem. Soc. 90 ( 1 968) 23-28: ) ( ( b) F .G. Bordw e l l , D. Algrim, J.Org . Che m . 41 ( 1 976) 2 507-2 5 08. ) ( [30] F o r a r ela t ed exam p l e o f O-methylati o n of acetone dicarboxylic aci d anhydride, ) see: J.A. Ray, T.M. Harris, Tetrahedron Lett. 23(1982)1971-1974. ( [31]J . F. McGarrit y ,T. Smyt h , J. A m . Chem . Soc . 102 (1980) 7 303-7 3 08. ) [32] (a) D.A. Evans, J.M. Takacs, Tetrahedron Lett. 21 (1980) 4233-4236; ( ( b) D .A. Evans, J.M. Takacs, L . R. McGee, M. D . E n nis,D.J. M at h re, J. B a r troli, Pur e Appl . Chem. 53 (1981)1109-11 27 . ) ( [33]I . P ravst , M. Z u pan,S. S tavber, Tetrahedr o n Lett. 47 (2006) 4707-4710. ) ( [34] F or a related preparation o f ketone 22 , see: R.B. W a gner, J.A . Moore, J. Am. Chem. S oc. 72 (1950) 2884 -2 887. ) ( [35] ( a) F or t wo e x ample s of cy c lic a-perhalos ul fonyl ket o nes e xhibiting keto - enol ta utomeri s m, see Y. Kob a yashi , T. Y oshida, I . Kumadak i , T et r ahedron Lett. 2 0 ( 1979) 3 86 5 -386 6; ) ( ( b) X .-J . Wang, J.-T. Liu, Ch i n. J. Che m . 2 5 (2007)6 4 9 - 652. ) [36] Trimethylsilyldiazomethane.e-Encyclopedia of Reagents for Organic Synthesis[Online]; Wiley-InterScience, Posted 15.9.06. http://onlinelibrary.wiley.com/o/eros/articles/rt298/frame.html (accessed 20.10.12). [37]For an example of a similar decrease in acidity due to amide A13-interactions,see:D.A. Evans, J.S. Clark,R. Metternich, V.J. Novack,G.S. Sheppard,J. Am. Chem. Soc.112(1990)866-868. [38] The desilylation of trimethylsilyldiazomethane was already known not to be therate limiting step, as no trimethylsilylmethylated products were observed at anypoint in our work. However, it has been reported that the methylation of carboxylic acids with trimethylsilyldiazomethane proceeds via a termolecularpathway that involves concomitant desilylation see: E. Kuhnel, D.D.P. Laffan, G.C.Lloyd-Jones, T. Martinez del Campo, I.R. Shapperson, J.L. Slaughter, Angew. Chem.Int. Ed. 46 (2007)7075. [39](a) For recent reviews on donor-acceptor cyclopropanes, see T.P. Lebold, M.A.Kerr, Pure Appl. Chem. 82 (2010)1797-1812; ( (b) H. M.L. Da v ie s , J.R. De nton, Chem. Soc . Rev . 3 8 (2009) 306 1 - 307 1 . ) ( [40] S ee Sup p orti n g I n formation for Y . Kiyotsuka, H .P . Acharya,Y. Kat ay ama,T. Hyodo, Y . K obab y shi, O rg. Lett. 10 (2008) 1 719- 1 722. ) ww.rysstech.com耐士科技 耐士科技ww.rysstech.com
确定










还剩9页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








