
Novel benzofuran-2-carboxamide ligands, which are selective for sigma receptors, have been synthesized via a microwave-assisted Perkin rearrangement reaction and a modified Finkelstein halogen-exchange used to facilitate Nalkylation. The ligands synthesized are the 3-methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamides (KSCM-1, KSCM-5 and KSCM-11). The benzofuran-2-carboxamide structure was N-arylated and N-alkylated to include both N-phenyl and N-(3-(piperidin-1-yl)propyl substituents, respectively. These new carboxamides exhibit high affinity at the sigma-1 receptor with Ki values ranging from 7.8 to 34 nM. Ligand KSCM-1 with two methoxy substituents at C-5 and C-6 of the benzofuran ring, and Ki = 27.5 nM at sigma-1 was found to be more selective for sigma-1 over sigma-2.
方案详情

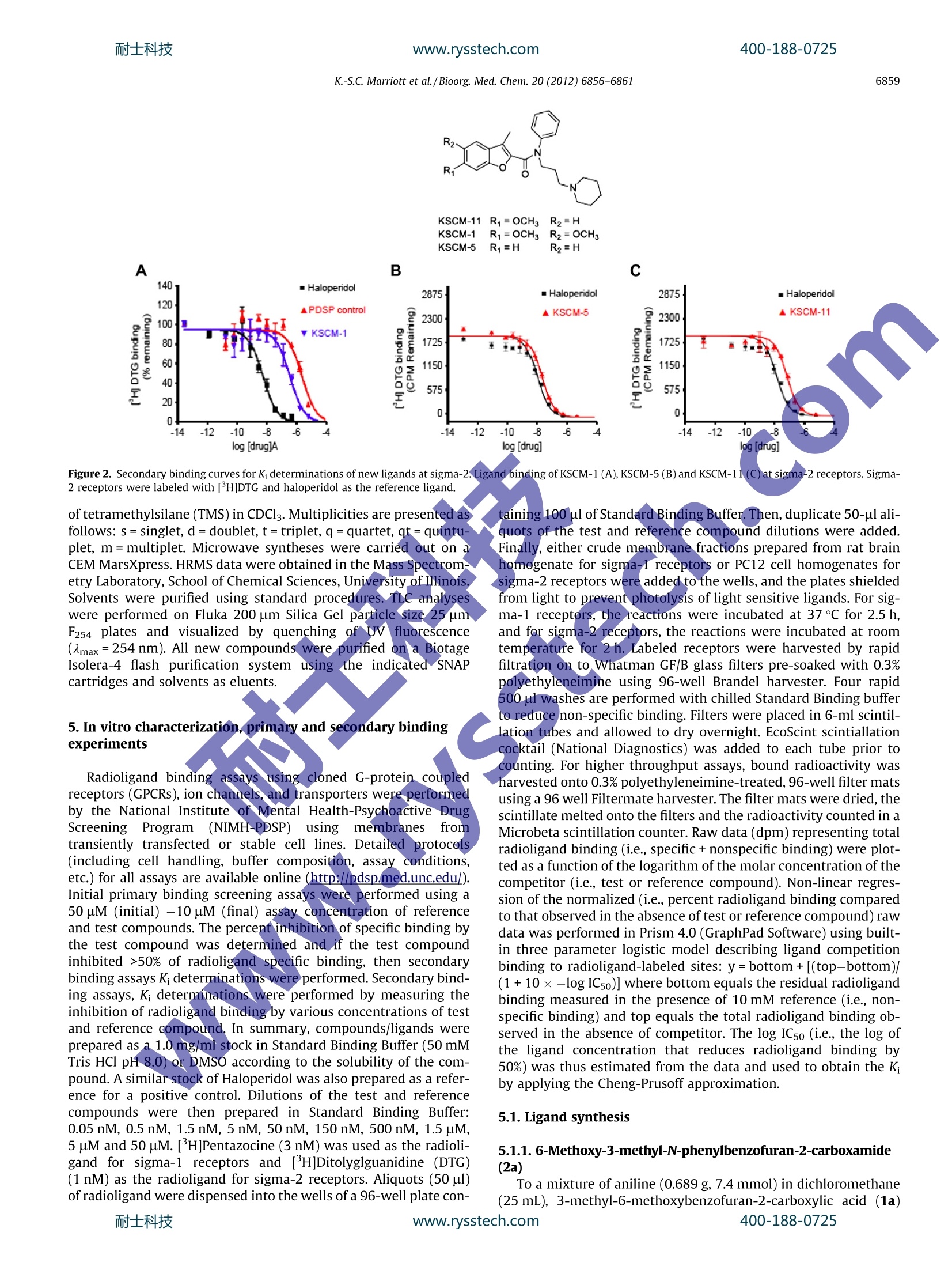
耐士科技400-188-0725www.rysstech.comBioorganic & Medicinal Chemistry 20 (2012)6856-6861 耐士科技400-188-0725www.rysstech.comK.-S.C. Marriott et al./Bioorg. Med. Chem. 20 (2012) 6856-68616857 Contents lists available at SciVerse ScienceDirect Bioorganic & Medicinal Chemistry ELSEVIER journal homepage:www.elsevier.com/locate/bmc Synthesis of N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamidesas new selective ligands for sigma receptors Karla-Sue C. Marriott*, Andrew Z. Morrison, Misty Moore, Olarongbe Olubajo, Leonard E. StewartDepartment of Natural Sciences, Savannah State University, College of Sciences and Technology, PO Box 20600, 3219 College Street, Savannah 31404, Georgia ARTICLE INFO ABSTRACT Article history:Received 9 July 2012Revised 12 September 2012Accepted 19 September 2012Available online 29 September 2012 Novel benzofuran-2-carboxamide ligands, which are selective for sigma receptors, have been synthesizedvia a microwave-assisted Perkin rearrangement reaction and a modified Finkelstein halogen-exchangeused to facilitate N-alkylation. The ligands synthesized are the 3-methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamides (KSCM-1, KSCM-5 and KSCM-11). The benzofuran-2-carboxamidestructure was N-arylated and N-alkylated to include both N-phenyl and N-(3-(piperidin-1-yl)propylsubstituents, respectively. These new carboxamides exhibit high affinity at the sigma-1 receptor withK values ranging from 7.8 to 34 nM. Ligand KSCM-1 with two methoxy substituents at C-5 and C-6 ofthe benzofuran ring, and K=27.5nM at sigma-1 was found to be more selective for sigma-1 over Keywords:SigmaReceptorLigandBenzofuranCarboxamideAlzheimer'sNeurodegenerative In the mid-70s sigma receptors were mistakenly characterizedas a new subtype of opioid receptors.However, cloning of theguinea pig sigma-1 binding site in 1996 revealed this protein tobe unniique sharing no homology with any other mammalian pro-ptein including all types of molecular chaperones. Sigma receptorsare classified into two subtypes, sigma-1 and sigma-2. To date,thesigma-2 receptor has not been cloned. Sigma-1 receptors are endo-plasmic reticular (ER) proteins consisting of 223-amino acids witha molecular mass of 24-kDa, and two-transmembrane-spanningregions.34Sigma-1 receptors are widely distributed throughoutthe body, centrally and peripherally, primarily functioning in amodulatory role on dopamine, acetylcholine, NMDA and opioidreceptors. They are known to translocate during signal transduc-tion and have been linked to the modulation or production of var-ious intracellular secondary messengers 3.6 Sigma-2 receptors areunderstood to be slightly smaller in size than the sigma-1 receptor.Pharmacological evaluations reveal that sigma-2 receptors may belipid raft proteins that affect calcium signaling via sphingolipidproducts and unlike sigma-1 receptors, sigma-2 receptors do nottranslocate. Both sigma receptors are highly expressed on tumorcell lines from human and rat cancer tissues. The overexpressionof sigma-2 receptors in human and murine tumors suggests thatthese receptors may be a biomarker of tumor cell proliferation. ( * C orresponding author. Tel.: +995 912 358 4454. ) ( E-mail address: marr i ottk@savannah st at e .edu ( K .-S.C. Marriott). ) Various drugs, including antipsychotics (morphine analogs),neuroleptics (haloperidol) and neuroactive steroids (progesterone,DHEA), bind to sigma-1 receptors. Ligands that bind to and mod-ulate the sigma-1 receptor have been proposed to exhibit effects inseveral therapeutic areas such as neurodegenerative disorders,pain, depression, schizophrenia, amnesia, Alzheimer’s disease,Parkinson’s disease and stroke. Sigma-1 receptors have also beendocumented as a target for cocaine and associated with the toxicand stimulant actions of cocaine. In general sigma-1 antagonistsmay have potential use in the treatment of addiction. The bindingsite of sigma-1 receptors is reported to consist of an amine bindingsite flanked on either side by hydrophobic binding pockets thatdisplay bulk tolerance and as such, typical pharmacophoricfeatures of sigma-1 receptors include an alkylamine moiety withinthe general molecular structure. Hence, a common pharmaco-phoric feature of sigma-1 ligands is an N-alkyl, N,N-dialkyl orN-arylalkyl amine moiety1 which poses a challenge in the synthe-sis of these ligands. Interestingly, progesterone which lacks a basicnitrogen, is considered one of the putative endogenous ligands forthe sigma-1 receptor.N,N-Dimethyltryptamine (DMT) has beenidentified as a potential endogenous sigma-1 receptor ligand, but,the role of DMT as a sigma-1 receptor modulator is unclear duetolow physiological concentrations in brain tissues.2 Our synthe-sized ligands have a benzofuran-2-carboxamide structural moietywhich has been N-arylated and N-alkylated to include both N-phe-nyl and N-(3-(piperidin-1-yl)propyl substituents, respectively, assummarized structurally in lFigure 1. These new carboxamideligands structurally consist of a basic alkylamine moiety the Figure 1. General ligand structure. N-(3-(piperidin-1-yl)propyl with a protonatable nitrogen and anaromatic phenyl ring and benzofuran moiety as two hydrophobicresidues (Fig. 1). Additionally, we introduced structural variationsat the benzofuran moiety in the form of methyl ether substituents(Ri and R2, Fig.1) to investigate the effect these substituents haveon receptor binding affinity. 2. Results and discussion 2.1. Chemistry Our microwave-assisted expedited synthetic pathwaystraightforward, involving the preparation of 3-methylbenzofu.ran-2-carboxylic acids (1a and 1b) in quantitative yields via aPerkin rearrangement reaction of mono- and di-methoxy-3-bro-mocoumarins as previously described. Mono-and di-hydrox-ycoumarins were methylated and subsequently brominated atposition-3 via a microwave-assisted regioselective brominationwith N-bromosuccinimide (NBS)to yield mono- and di-methoxy-3-bromocoumarins in 85-89% yields,13 3-Bromocoumarins traditionally undergo base-catalyzed Perkinrearrangement, which requires 3 hours reflux quantitatively yield-ing benzofuran-2-carboxylic acids.14However under microwavereaction conditions these reactions were completed in 5 min.Mono- and di-methoxy-3-methyl-N-phenylbenzofuran-2-carbox-amides (2a and 2b) were produced by reacting aniline with thecorresponding 3-methylbenzofuran-2-carboxylic acids (1a and1b) in the presence of DCC and DMAP at room temperature, asoutlined in Scheme 1.3-Methyl-N-phenylbenzofuran-2-carboxam-ide (2c) was produced by condensing commercially available3-methylbenzofuran-2-carboxylic acid (1c) with aniline. Syntheses of mono- and di-methoxy-3-methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamides (KSCM-11 andKSCM-1) were achieved bytreating carboxamides (2a and 2b) withNaH followed by N-alkylation with 1-(3-iodopropyl)piperidineoutlined in Scheme 1. A modified halogen exchange Finkelstein reaction was employed to convert commercially available 1-(3-chloropropyl)piperidine hydrogen chloride salt in the presence ofpotassium iodide, tetrabutylammonium bromide (TBAB) andpotassium carbonate into the more reactive iodopropylpiperidinein situ, which then reacted with anions obtained from treating car-boxamides (2a and 2b) with sodium hydride producing KSCM-11and KSCM-1.3-Methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamide (KSCM-5) was similarly synthesized byN-alkylation of carboxamide (2c) with 1-(3-iodopropyl)piperidine. 2.2. Binding assay Compounds were screened by the National Institute of MentalHealth-PsychoactiveDrugScreening Program(NIMH-PDSP)against a panel of G-protein coupled receptors (GPCRs) and molec-ular targets due to the fact that many ligands with high affinity atsigma receptors have proven to also exhibit significant binding atone or more other central nervous system (CNS) relevant receptorsites. This evaluation has led to the discovery of three new sigmareceptor selective ligands. Primary bindingassays were performedat serotonin 5-HTs, adrenergic (Alpha1-A,-B, -D, Alpha2-A,-B,-C,Beta-1, -2, -3), cannabinoid (CB1and CB2), Dopamine (D1-D5),histamine (H1 and H2), opioid (KOR, MOR and DOR), Muscarinic(M1-M5), N-methyl-D-aspartate(NMDA), Sigma-1, Sigma-2,metabotropic glutamate (mGluR5 Rat Brain), and benzodiazepine(BZP Rat Brain) receptor sites, as well as, dopamine transporter(DAT),y-aminobutyric acid type A(GABAA), norepinephrine trans-porter (NET) and serotonin transporter (SERT) molecular targets.Compounds showing >50% inhibition of radioligand specific bind-ing at the stated GPCRs and molecular targets (Tables 1 and 2)were forwardedforadditional screening (secondary bindingassays) to determine K; values at the respective GPCR binding sitesusing radioligand binding assays. It was determined that com-pounds KSCM-1,KSCM-5 and KSCM-11 have the desired selectivityfor sigma receptors (sigma-1 and sigma-2) over non-sigmareceptors. Secondary binding assays of these compounds showed greateraffinity at sigma receptors over non-sigma receptors such as5-HT2A, 5-HT2B,5HT3,Alpha2A, Alpha2C, D3, and M4 (Tables 3and 4). Consistent with documented sigma-1 receptor ligands,the molecular structures of KSCM-1, KSCM-5 and KSCM-11 includea basic alkyl amine group, flanked by two hydrophobic residues (anaromatic phenyl and benzofuran ring). Based upon the sigma-1 receptor selective ligand pharmaco-phore profile [10], a sigma-1 selective ligand usually possesses aprimary and secondary hydrophobic site separated by an amine.The sigma-1 receptor site displays some bulk tolerance and so thisprompted us to explore introducing a benzofuran moiety to pro-duce the general molecular structure summarized in Figure 1. Allthree compounds have an N-arylated benzofuran-2-carboxamidescaffold which was then N-alkylated yielding N-(3-(piperidin-1- Table 1Primary binding assay at sigma receptors Compound % Inhibition G-1 G-2 KSCM-1 99.8 81.4 KSCM-5 100.6 93.5 KSCM-11 97.7 88.5 Primary binding assays performed in sigma binding buffer (50 mM Tris-HCl, pH8.0). Sigma-1 receptors were labeled with [H](+)-pentazocine and sigma-2receptors were labeled with [’H]Ditolylguanidine (DTG) with haloperidol as thereference compound in both cases. Data represent mean % inhibition (N=4 deter-minations) for compound tested at receptor subtypes. Significant inhibition isconsidered>50%. In cases where negative inhibition (-) is seen, this represents astimulation of binding. Occasionally, compounds at high concentrations will non-specifically increase binding. The default concentration for primary bindingexperiments is 10 uM. Table 2Primary binding assay at nonsigma receptors Compound % Inhibition 5-HT2A 5-HT2B 5HT3 Alpha2A Alpha2C D3 M4 KSCM-1 76.6 43.8 64.2 55.2 78.9 38.8 54.4 KSCM-5 18.1 69.2 2.9 77.7 61.9 60.6 43.1 KSCM-11 61.2 93.9 7.3 81.1 49.0 40.6 68.4 Assays were performed using transiently or stably transfected cell lines (e.g.,HEK293, COS, CHO, NIH3T3). Refer to Table 5 for radioligands and reference comn-pounds listing. Data represent mean % inhibition (N=4 determinations) for com-pound tested at receptor subtypes. Significant inhibition is considered >50% Incases where negative inhibition (-) is seen, this represents a stimulation of binding.0ccasionally, compounds at high concentrations will non-specifically increasebinding. The default concentration for primary binding experiments is 10uM. Table 3Secondary binding assay, K, determination at sigma receptors Compound o-1(K, nM) o-2(K,nM) 6-2/o-1 KSCM-1 27.5 528 19 KSCM-5 7.8 16 2 KSCM-11 34 41 1.2 Haloperidol 1.7 13 8 Affinities K(nM) were determined in rat brain homogenate (sigma-1), PC12 cells(sigma-2), sigma binding buffer (50 mM Tris-HCl, pH 8.0). Sigma-1 receptors werelabeled with [H](+)-pentazocine and sigma-2 receptors were labeled with [H]DTGwith haloperidol as the reference compound in both cases. yl)propyl introducing a basic protonatable nitrogen as well as athree-carbon alkyl linker as summarized structurally1 in |Figure 1.Additionally, the benzofuran molecular structure was varied bysubstitutions at C-5 and C-6 with methyl ether (Ri and R2, Fig. 1)to explore the impact that introduction of additional hydrogenbond acceptor (HBA) centers would have on receptor binding affin-ity and selectivity. We observed that the inclusion of methoxy sub-stituents at both C-5 and C-6 of the benzofuran moiety resulted inboth high affinity and selectivity at the sigma-1 receptor over thesigma-2 receptor as observed for KSCM-1 with K=27.5nM atsigma-1 and 528nM at sigma-2 (19-fold selectivity for sigma-1 Table 4 over sigma-2, Table 3). KSCM-1 showed no significant affinity atnon-sigma receptors selected for secondary binding assay, K, deter-mination (Table 4), with K, values ranging from 945 nM at Alpha2Ato 7,612 nM at 5-HT3. The exclusion of a methoxy substituent atC-5 of the benzofuran moiety to produce KSCM-11 resulted in aslightly decreased affinity at sigma-1 (K;=34nM, Table 3), how-ever,a significant increase in affinity at sigma-2 (K;=41 nM, Table3) in comparison to KSCM-1. The mono-methoxy compoundKSCM-11 has similar affinity at both sigma receptor binding sitesand thus no significant selectivity of sigma-1 over sigma-2 wasobserved (K=34nM at sigma-1 and 41 nM at sigma-2,Table 3).KSCM-11 showed no significant affinity at non-sigma receptors se-lected for secondary binding assay, K, determination(Table 4), withK values ranging from 204 nM at 5-HT2B to>10,000 nM at M4. Theexclusion of both methoxy substituents at C-5 and C-6of thebenzofuran moiety to produce KSCM-5 resulted in a significantlyincreased affinity at both sigma-1(K=7.8nM, Table 3) and sig-ma-2(K=16 nM, Table 3) in comparison to KSCM-1 and KSCM-11. However, selectivity of KSCM-5 for sigma-1 over the sigma-2was only twofold. KSCM-5 showed no significant affinity atnon-sigma receptors selected for secondary binding assay, K deter-mination (Table 4), with K values ranging from 249 nM at Alpha2Cto 6,739 nM at D3. The sigma-2 receptor secondary binding curvesfor compounds KSCM-1, KSCM-5, KSCM-11, PDSP control, and hal-operidolaresshown in Figure 2.The sigma-2 receptor bindingcurves illustrate the readily noticeable link between affinity atthe sigma-2 receptor and the loss of methoxy substituents at C-5and C-6 of the benzofuran ring. Compound KSCM-5 without meth-oxy substituents wasfound to bea potent sigma-2 ligand withcomparable potency to haloperidol at sigma-2 as is readily discern-ible from the binding curve (Fig. 2). 3. Conclusion In this study, we have identified three new sigma receptorselective ligands based on features of the sigma-1 receptor bindingsite which consists of an amine binding site flanked on either sideby hydrophobic binding pockets. All three ligands have high affin-ity at sigma-1 with K; values ranging from 7.8 to 34 nM. KSCM-1was the most selective with K=27.5nM at sigma-1 and a 19-foldselectivity for sigma-1 over sigma-2. Ligands KSCM-1, KSCM-5and KSCM-11 all possess basic nitrogen atoms and are structurallycomposed of benzofuran-2-carboxamide moieties which havebeen N-arylated and N-alkylated to include both N-phenyl andN-(3-(piperidin-1-yl)propyl substituents. The expedited syntheticprocedures employed include regioselective microwave-assistedNBS bromination as well as microwave-assisted Perkin rearrange-ment reactions to prepare benzofuran-2-carboxylic acids (1a and1b) from the corresponding 3-bromocoumarins in very high yields. 4. Experimental section lH and 13c NMR spectra were recorded on a JEOL 300 MHz spec-trometer. Chemical shifts for lH and 13c NMR spectra are reportedin 8 values (parts per million, ppm) relative to an internal standard Secondary binding assay, K, determination at nonsigma receptors K(nM) Compound 5-HT2A 5-HT2B 5HT3 Alpha2A Alpha2C D3 M4 KSCM-1 2564 NT 7612 945 1542 NT 2871 KSCM-5 NT 936 NT 1232 249 6739 NT KSCM-11 2766 204 NT 653 NT NT >10,000 Assays were performed using transiently or stably transfected cell lines (e.g., HEK293,COS, CHO, NIH3T3). Refer to Table 5 for radioligands and reference compounds listing.NT= not tested. B C log [drug] Figure 2. Secondary binding curves for K, determinations of new ligands at sigma-2. Ligand binding of KSCM-1(A), KSCM-5(B) and KSCM-11 (C) at sigma-2 receptors. Sigma-2 receptors were labeled with[H]DTG and haloperidol as the reference ligand. of tetramethylsilane (TMS) in CDCl3. Multiplicities are presented asfollows: s=singlet, d=doublet, t=triplet,q=quartet, qt=quintu-plet, m=multiplet. Microwave syntheses were carried out on aCEM MarsXpress.HRMS data were obtained in the Mass Spectrom-etry Laboratory,School of Chemical Sciences, University of Illinois.solvents were purified using standard procedures. TLC analyseswere performed on Fluka 200 um Silica Gel particle size 25 umF254 plates and visualized by quenching of UVfluorescence(八max=254nm). All new compounds were purified on a BiotageIsolera-4 flash purification system usingthe indicated SNAPcartridges and solvents as eluents. 5. In vitro characterization, primary and secondary bindingexperiments Radioligand binding assays using cloned G-protein coupledreceptors (GPCRs), ion channels, and transporters were performedby the National Institutee of Mental Health-Psychoactive DrugScreeningProgram(NIMH-PDSP))using membranes fromtransiently transfected or stable cell lines. Detailed protocols(including cell handling, buffer composition, assay conditions,etc.) for all assays are available online (http:/pdsp.med.unc.edu).Initial primary binding screening assays were performed using a50 uM (initial) -10 uM (final) assay concentration of referenceand test compounds. The percent inhibition of specific binding bythe test compound was determined and if the test compoundinhibited >50% of radioligand specific binding, then secondarybinding assays K, determinations were performed. Secondary bind-ing assays, K determinations were performed by measuring theinhibition of radioligand binding by various concentrations of testand reference compound. In summary, compounds/ligands wereprepared as a 1.0 mg/ml stock in Standard Binding Buffer (50 mMTris HCl pH8.0) or DMSO according to the solubility of the com-pound. A similar stock of Haloperidol was also prepared as a refer-ence for a positive control. Dilutions of the test and referencecompounds were then prepared in Standard Binding Buffer:0.05 nM, 0.5 nM, 1.5 nM, 5 nM, 50 nM, 150 nM, 500 nM, 1.5 uM,5uM and 50 uM.[H]Pentazocine (3 nM) was used as the radioli-gand for sigma-1 receptors and [H]Ditolyglguanidine (DTG)(1 nM) as the radioligand for sigma-2 receptors. Aliquots (50 ul)of radioligand were dispensed into the wells of a 96-well plate con- taining 100 ul of Standard Binding Buffer.Then,duplicate 50-ul ali-quots of the test and reference compound dilutions were added.Finally, either crude membrane fractions prepared from rat brainhomogenate for sigma-1 receptors or PC12 cell homogenates forsigma-2 receptors were added to the wells, and the plates shieldedfrom light to prevent photolysis of light sensitive ligands. For sig-ma-1 receptors, the reactions were incubated at 37C for 2.5 h,and for sigma-2 receptors, the reactions were incubated at roomtemperature for 2 h. Labeled receptors were harvested by rapidfiltration on to Whatman GF/B glass filters pre-soaked with 0.3%polyethyleneimine using 96-well Brandel harvester. Four rapid500 pl washes are performed with chilled Standard Binding bufferto reduce non-specific binding. Filters were placed in 6-ml scintil-lation tubes and allowed to dry overnight. EcoScint scintiallationcocktail (National Diagnostics) was added to each tube prior tocounting. For higher throughput assays, bound radioactivity washarvested onto 0.3% polyethyleneimine-treated,96-well filter matsusing a 96 well Filtermate harvester. The filter mats were dried, thescintillate melted onto the filters and the radioactivity counted in aMicrobeta scintillation counter. Raw data (dpm) representing totalradioligand binding (i.e.,specific + nonspecific binding) were plot-ted as a function of the logarithm of the molar concentration of thecompetitor (i.e., test or reference compound). Non-linear regres-sion of the normalized (i.e., percent radioligand binding comparedto that observed in the absence of test or reference compound) rawdata was performed in Prism 4.0 (GraphPad Software) using built-in three parameter logistic model describing ligand competitionbinding to radioligand-labeled sites: y=bottom+[(top-bottom)/(1+10×-log ICso)] where bottom equals the residual radioligandbinding measured in the presence of 10 mM reference (i.e., non-specific binding) and top equals the total radioligand binding ob-served in the absence of competitor. The log ICso (i.e., the log ofthe ligand concentration that reduces radioligand binding by50%) was thus estimated from the data and used to obtain the Kby applying the Cheng-Prusoff approximation. 5.1. Ligand synthesis 5.1.1. 6-Methoxy-3-methyl-N-phenylbenzofuran-2-carboxamide(2a) To a mixture of aniline (0.689 g, 7.4 mmol) in dichloromethane(25 mL), 3-methyl-6-methoxybenzofuran-2-carboxylic acid (1a) (1.501 g, 7.3 mmol) was added with stirring. Then DMAP (0.094 g,0.77 mmol) was added, followed by DCC (1.583 g, 7.7 mmol). Thereaction was left to stir at room temperature for 23 h. The reactionmixture was then filtered and the filtrate washed with water(20 mL×2), then 5% acetic acid (20 mL×2)and again with water(20 mL×2). The crude product was recrystallized from methanolto yield 2a as white crystals (1.37g, 67%), mp 176-178℃. 1HNMR (300 MHz, CDCl3) 8 2.64 (s,3H), 3.88 (s, 3H), 6.93-7.00(m,2H), 7.12-7.16 (t,J=7.41 Hz, 1H) 7.35-7.40 (t,J=7.65 Hz, 2H),7.48-7.50(d,J=8.6 Hz, 1H), 7.68-7.71 (d,J=8.5 Hz, 2H), 8.26 (s,1H). 13C NMR (300 MHz, CDCl3) 8 9.2, 55.8,95.6,112.9, 119.9,121.4, 123.3,124.4,129.1,137.7,141.8,154.5,158.1,160.5. MS(E-SI)* calcd for C17H16NO3[M+H]*:282.1130, found: 282.1124. 5.1.2.5,6-Dimethoxy-3-methyl-N-phenylbenzofuran-2-carboxamide (2b) To a mixture of aniline (0.398 g, 4.27 mmol) in dichloromethane(15 mL), 3-methyl-5,6-dimethoxybenzofuran-2-carboxylic: acid(1b) (1.005 g, 4.25 mmol) was added with stirring. Then DMAP(0.090g, 0.44 mmol) was added, followed by DCC (0.934g,4.53 mmol). The reaction was left to stir at room temperature for23 h. The reaction mixture was then filtered and the filtratewashed with water (20 mL×2), then 5% acetic acid (20 mL×2)and again with water (20 mL×2). The crude product was recrys-tallized from methanol to yield 2b as white crystals (0.94g, 71%),mp 183-185℃.H NMR(300MHz, CDCl3) 8 2.64 (s,3H), 3.956H), 6.97 (s, 1H), 6.99 (s, 1H) 7.12-7.72(m, 5H), 8.26 (s, 1H) 13cNMR (300 MHz, CDCl3)8 9.2,56.3,56.4,94.9,101.2,119.8,121.7,124.3,129.1,137.7,141.8,147.2,148.3,150.8,158.0. MS(ESI)calcd for C18H18NO4 [M+H]*: 312.1236, found: 312.1235. 5.1.4.5,6-Dimethoxy-3-methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamide (KSCM-1) Compound 2b (0.100 g, 0.321 mmol)was added to dry dichloro-methane (15 mL) with stirring under nitrogen atmosphere. To thissolution NaH (0.130 g, 3.25 mmol) 60% dispersion in mineral oilwas added and the reaction heated at reflux for 1 h. The reactionmixture was then cooled in an ice-bath and 1-(3-chloropro-pyl)piperidine hydrogen chloride salt (0.110 g, 0.555 mmol),potas-sium carbonate (0.380g. 2.75 mmol), tetrabutylammoniumbr-omide (0.046g, 0.143 mmol) and potassium iodide (0.292g,1.76 mmol) added with stirring. The reaction mixture was thenheated at reflux for 24 h. The reaction mixture was then cooledand slowly quenched with ethanol. The reaction mixture waswashed with water (5 ml×2) and the organic layer dried overmagnesium sulfate. The crude product was purified by high perfor-mance flash purification using a Biotage Isolera 4 system, SNAP(SiO2) KP-NH column, solvent dichloromethane/methanol (9:1) aseluent to give 0.0841g(60%) of KSCM-1 as a light brown paste. 'H NMR (300 MHz, CDCl3)8 1.38-1.56 (m, 6H), 1.81-1.91 (qt,J=7.58 Hz, 2H), 2.31-2.36(m, 6H), 2.34 (s, 3H), 3.80 (s, 3H), 3.87(s,3H), 3.86-3.93(m,2H), 6.52 (s, 1H), 6.81 (s, 1H), 7.10-7.30 (m,5H).C NMR (300 MHz, CDCl3)8 9.9,10.1,24.5, 25.3, 26.0, 29.7,49.0,54.6, 56.2, 56.3,56.6 94.7,100.8,120.8,122.4,126.5,126.9,128.9, 143.2, 143.6,146.7,148.4,149.9, 161.4. MS(ESI)* calcd forC26H33N204[M+H]*:437.2440, found: 437.2440. 5.1.5.3-Methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl) benzofuran-2-carboxamide (KSCM-5) Compound 2c (0.200 g, 0.80 mmol) was added to dry dichloro-methane (25 ml) with stirring under nitrogen atmosphere. To thissolution NaH (290 mg, 7.25 mmol) 60% dispersion in mineral oilwas added and at reflux for 1 h. The reaction mixture was cooledin an ice-bath and 1-(3-chloropropyl)piperidine hydrogen chloridesalt (0.238 g, 1.2 mmol), potassium carbonate(0.660 g, 4.8 mmol),tetrabutylammoniumbromide (0.100g, 0.310 mmol) and potas-sium iodide (0.299 g, 1.8 mmol) added with stirring. The reactionmixture was reflux for 24 h. The reaction mixture was then cooledand slowly quenched with ethanol. The reaction mixture waswashed with water (10 ml×2) and the organic layer dried overmagnesium sulfate. The crude product was purified by high perfor-mance flash purification using a Biotage Isolera 4 system, SNAP(SiO2)KP-NH column, solvent dichloromethane/methanol (9:1) aseluent to give 0.189g (63%) of KSCM-5 as a light brown paste.1HNMR (300MHz, CDCl3)81.36-1.56 (m, 6H), 1.82-1.92 (qt,J==7.58Hz, 2H), 2.31-2.36 (m, 6H), 2.34 (s, 3H), 3.90-3.95 (t,j= 7.64Hz, 2H), 7.03-7.05 (d,J=8.03 Hz, 1H), 7.11-7.26(m,7H),7.43-7.45 (d,J=6.85Hz, 1H).C NMR (300 MHz, CDCl3) 8 9.1,24.5,25.3,26.0,49.0,54.6,56.6,111.4,120.3,121.2,122.6,126.2,126.7,127.0,128.9,129.0,142.8,144.4, 153.4, 161.5. MS(ESI)*cal-culated for C24H29N202[M+H]*: 377.2229,found: 377.2227. 5.1.6.6-Methoxy-3-methyl-N-phenyl-N-(3-(piperidin-1-yl)propyl)benzofuran-2-carboxamide (KSCM-11) Compound 2a (0.200 g, 0.71 mmol) was added to dry dichloro-methane (25mL) with stirring under nitrogen atmosphere. To thissolution NaH (0.290 g, 7.25 mmol) 60% dispersion in mineral oilwas added and the reaction heated at reflux for 1 h. The reactionmixture was then cooled in an ice-bath and 1-(3-chloropro-pyl)piperidine hydrogen chloridesalt((0.238g, 1.2mmol).potassium carbonate (0.660 g, 4.8 mmol), tetrabutylammoniumbr-omide (0.090g, 0.279mmol) and potassium iodide (0.357 g,2.15 mmol) added with stirring. The reaction mixture was thenheated at reflux for 24 h. The reaction mixture was then cooledand slowly quenched with ethanol. The reaction mixture waswashed with water (10ml×2) and the organic layer dried overmagnesium sulfate. The crude product was purified by high perfor-mance flash purification using a Biotage Isolera 4 system, SNAP(SiO2) KP-NH column, solvent dichloromethane/methanol (9:1) aseluent to give 0.168 g (58%) of KSCM-11 as a brown paste. lHNMR (300MHz, CDCl3)881.39-1.57 (m, 6H), 1.82-1.92 (qt,J=7.14Hz, 2H), 2.33-2.37 (m,6H), 2.35 (s, 3H), 3.74(s,3H), 3.90-3.95 (t,J=7.57 Hz,2H), 6.52 (s, 1H), 6.78-6.80 (d,J=8.61 Hz, 1H),7.12-7.33 (m, 6H). 1c NMR (300 MHz, CDCl3)8 9.2,24.5,25.3,26.0, 49.0, 54.6, 55.6,56.6,95.3,112.3,120.6,122.1,122.4,126.6,127.0, 129.0, 143.1,143.6,154.6,159.6,161.4. MS(ESI)* calcd forC25H31N203[M+H]*: 407.2335,found: 407.2339. Acknowledgments Special thanks and appreciation are extended to the NIMH Psy-choactive Drug Screening Program (PDSP). [K determinations,receptor binding profiles, agonist and/or antagonist functionaldata, HERG data, MDR1 data, etc. as appropriate] was generously provided by the National Institute of Mental Health’'s PsychoactiveDrug Screening Program, Contract # HHSN-271-2008-00025-C(NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD,PhD at the University of North Carolina at Chapel Hill and ProjectOfficer Jamie Driscol at NIMH, Bethesda MD, USA. For experimentaldetails please refer to the PDSP web site http://pdsp.med.unc.edu/and click on ‘Binding Assay’or 'Functional Assay’ on the menu bar(experimental details have been updated!). Thanks also extended to John C. Watts Jr. (external advisor),Savannah, GA. Karla-Sue C. Marriott received support for this work from theNational Institute of Health/National Institute on Drug Abuse(NIH/NIDA) (DA027086). Supplementary data Supplementary data associated with this article can be found, inthe online version, at http://dx.doi.org/10.1016/j.bmc.2012.09.044. 1. Martin, W. R.; Eades, C. G.; Thompson, J. A.; Huppler, R. E.; Gilbert, P. E. J.Pharmacol. Exp. Ther. 1976, 197,517. 2.H1anner, M.; Moebius, F. F.; Flandorfer, A.; Knaus, H.-G.; Striessnig,J.; Kempner,E.; Glossmann, H. Proc. Natl. Acad. Sci. U.S.A. 1996, 93,8072. ( 3. Aydar, E . ; P almer, C. P.; Klyachko, V. A.; Jackson, M . B. Neuron 2 0 02, 34, 399. ) ( 4 . jbilo, O.; Vidal, H.; Paul, R., et al J. B i ol. Ch e m. 1997,272,27107. ) 5.Bowen, W. D. Pharm. Acta Helv. 2000, 74,211. ( 6. Hayashi, T.; Su, T. P. P r oc. N a tl. Acad. Sci. U . S.A. 2001, 98,491. ) 17.Narayanan, S.; Mesangeau, C.; Poupaert,J; McCurdy,C. Curr. Top. Med. Chem.2011,9,1128. 8.Valade, A.; Cross, S. B.; Brown, C.;Detrait, E.; Ene, D.; Gillard, M.; Guyaux, M.;Lamberty, Y.; Maguire, M.; Namdev, N.; Provins, L.; Schwartz, E.;Vermeiren, C.Med. Chem. Commun. 2011,2,655. 9. Glennon, R. A. Mini-Rev. Med. Chem.2005,5,927. ( 10. Ablordeppey, S. Y.; Fischer,J. B.; Glennon,R. A. Bioorg. Med. Chem. 2000 , 8 , 21 0 5. ) 11. Hayashi, T.; Su, T. P. CNS Drugs 2004, 18,269. 12.F1ontanilla, D.; Johannessen, M.; Hajipour, A. R.; Cozzi, N. V13.; Jackson, M. B.;Ruoho, A. E. Science 2009, 323,934. 13.M1arriott, K.-S. C.; Bartee, R.; Morrison, A. Z.; Stewart, L.; Wesby,J. TetrahedronLett. 2012, 53,3319.http://dx.doi.org/10.1016/j.tetlet.2012.04.075. ( 14. Jackson,Y. A.;Marriott, K . -S. C. Molecules 2002, 7, 353. ) ww.rysstech.com耐士科技 Scheme . Reagents:(a) DCC, DMAP, aniline, CHCl; (b) NaH,-(-chloropropyl)piperidine·HCl, KCO, KI, TBAB,CHCl.www.rysstech.com耐士科技
确定





还剩4页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








