
Exploitation of the RNA interference (RNAi) pathway offers the promise of new and effective therapies for a wide variety of diseases. Clinical development of new drugs based on this platform technology is still limited, however, by a lack of safe and efficient delivery systems. Here we report the development of a class of structurally versatile cationic lipopolyamines designed specifically for delivery of siRNA which show high levels of target transcript knockdown in a range of cell types in vitro. A primary benefit of these lipids is the ease with which they may be covalently modified by the addition of functional molecules. For in vivo applications one of the core lipids (Staramine) was modified with methoxypolyethylene glycols (mPEGs) of varying lengths. Upon systemic administration, PEGylated Staramine nanoparticles containing siRNA targeting the caveolin-1 (Cav-1) transcript caused a reduction of the Cav-1 transcript of up to 60%, depending on the mPEG length, specifically in lung tissue after 48 h compared to treatment with non-silencing siRNA.In addition, modification with mPEG reduced toxicity associated with intravenous administration. The ability to produce a high level of target gene knockdown in the lung with minimal toxicity demonstrates the potential of these lipopolyamines for use in developing RNAi the rapeutics for pulmonary disease.
方案详情

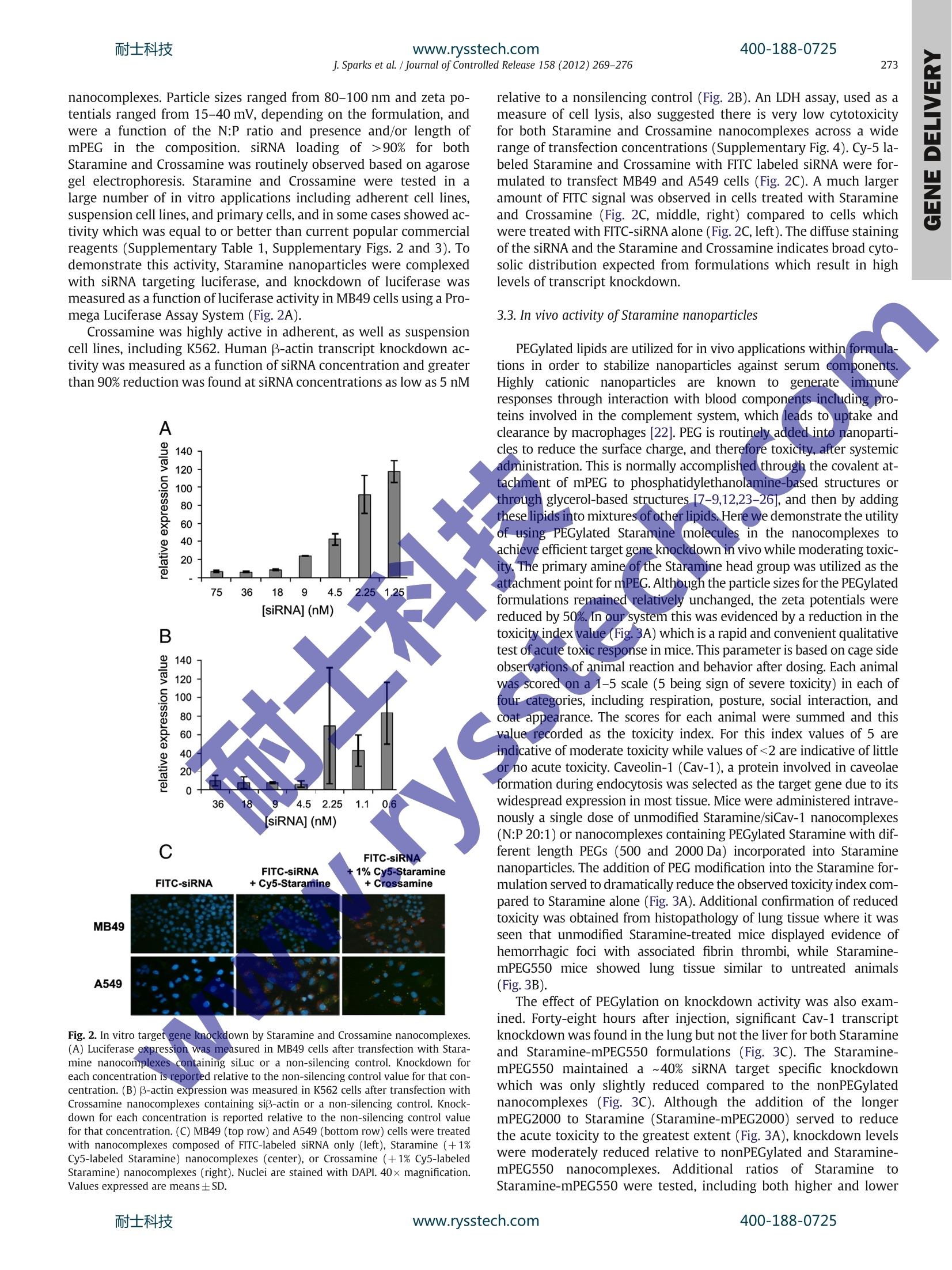
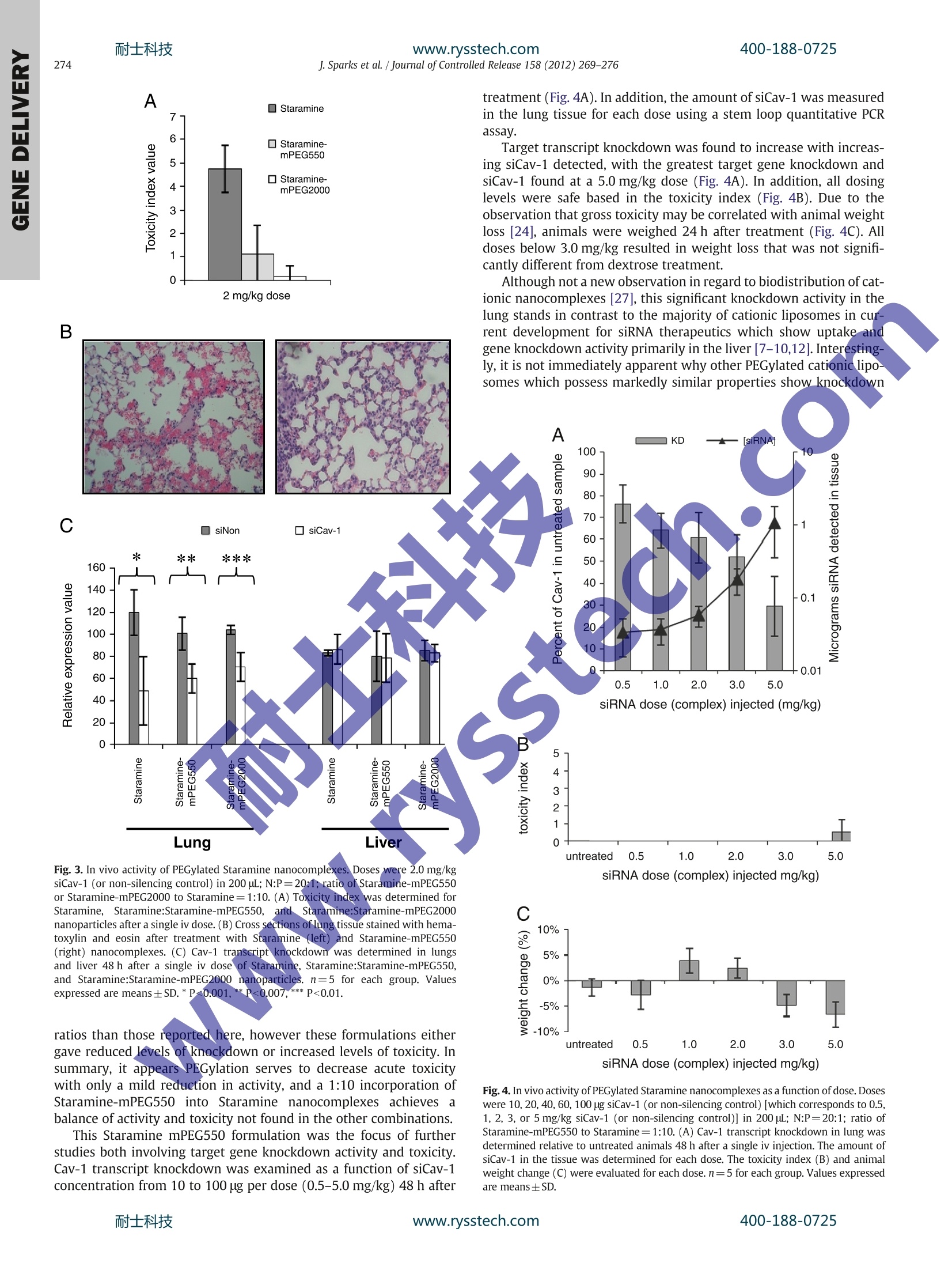
耐士科技400-188-0725www.rysstech.comJournal of Controlled Release 158 (2012)269-276 Contents lists available at SciVerse ScienceDirect Journal of Controlled Release RRI ELSEVIER journal hom epage: www.elsevier.com/locate/jconrel Versatile cationic lipids for siRNA delivery Jeff Sparks *, Gregory Slobodkin, Majed Matar, Richard Congo, David Ulkoski, Angela Rea-Ramsey,Casey Pence, Jennifer Rice, Diane McClure, Kevin J. Polach, Elaine Brunhoeber, Leslie Wilkinson,Kirby Wallace, Khursheed Anwer, Jason G. Fewell EGEN Inc., 601 Genome Way, Huntsville AL 35806, United States ARTICLE INFO A BSTRACT Article history: Received 12 September 2011 Accepted 5 November 2011 Available online 12 November 2011 Keywords:siRNA deliveryLipid nanoparticleLung deliveryPEGylationFunctionalization Exploitation of the RNA interference (RNAi) pathway offers the promise of new and effective therapies for awide variety of diseases. Clinical development of new drugs based on this platform technology is still limited,however, by a lack of safe and efficient delivery systems. Here we report the development of a class ofstructurally versatile cationic lipopolyamines designed specifically for delivery of siRNA which show highlevels of target transcript knockdown in a range of cell types in vitro. A primary benefit of these lipids isthe ease with which they may be covalently modified by the addition of functional molecules. For in vivoapplications one of the core lipids (Staramine) was modified with methoxypolyethylene glycols (mPEGs)of varying lengths. Upon systemic administration, PEGylated Staramine nanoparticles containing siRNA tar-geting the caveolin-1 (Cav-1) transcript caused a reduction of the Cav-1 transcript of up to 60%, dependingon the mPEG length, specifically in lung tissue after 48 h compared to treatment with non-silencing siRNA.In addition, modification with mPEG reduced toxicity associated with intravenous administration. The abilityto produce a high level of target gene knockdown in the lung with minimal toxicity demonstrates the poten-tial of these lipopolyamines for use in developing RNAi therapeutics for pulmonary disease. 1. Introduction The primary obstacle for nucleic acid-based therapeutics is the ef-ficient delivery to target cells.RNA-basedtherapeutics, which can re-sult in the sequence-specific reduction of gene expression, must beable to enter cells and become available to the RNA-induced silencingcomplex (RISC) in the cytosol [1,2]. Many different types of systemshave been utilized to deliver small inhibitory RNA (siRNA) includingsynthetic cationic polymers, natural polysaccharides, micelles, RNA,peptide and antibody conjugates,and microparticles, however liposo-mal systems have proven to be the most effective in vivo to date[3-6]. Yet obstacles remain for in vivo applications including toxicity,restricted tissue targets, and lack of active targeting. The developmentof siRNA delivery systems which can overcome these limitations hasbeen the subject of intense recent focus [7-11]. Cationic lipids have three general structural components: thecationic head group, the lipid tail(s), and the linkers that connectthe two. Although these features are common to many differentcationic lipids, a dear relationship between lipid structure andsiRNA delivery activity remains a goal in the field. When designingnew molecules, one way to separate the active variants from the ( * Corresponding author. Tel.: +1 256 327 0524; fax: + 1 256 327 0988. E-mail address: j sp arks@egeninc. c o m ( J.Sparks). ) inactive variants is to synthesize a large group of related moleculesand screen them [9,12]. Another technique is to use amino acids ortheir side chain components as the basis for head group design,thereby employing the unique chemical entities utilized by naturein proteins and peptides to achieve biological function [13-16].Alter-natively, once an active molecule is identified, its constituents may beincrementally varied in order to optimize its properties [10]. Ourapproach has been to utilize structural motifs from highly activepolyethylenimine (PEI)-based delivery systems which are beingused for plasmid DNA and siRNA delivery and adapting them towardconventional lipid structures. PEI has a higher charge density thanother conventional polymers used in transfection such as polylysine[17] and chitosan [18], and it is this property which is thought tocause the“proton sponge effect”[19], a key component of the endo-somal escape process of internalized particles. Under physiologicalconditions, the pKa values for PEI amines can vary and drop substan-tially depending on the protonation state of neighboring aminegroups due to charge repulsion [20]. This close-proximity theory hasalso been advanced for small pseudo-linear PEI molecules such asspermine in the context of DNA delivery [21]. Branched PEI containsroughly 25% tertiary amine groups which have pKas of approximately7, and it is thought that the majority remains unprotonated andbecome protonated only when the pH drops within the endosome.The presence of a tertiary amine within the lipid head group isthought to be a necessary structural component for some cationic lipids [10]. The general alkyl amine structural motif of PEI was used togenerate symmetric cationic lipid analogs which form liposomalassemblies upon formulation and which retain the beneficialtransfective properties of PEI. Liposomes composed entirely of thesecore structures can produce nanocomplexes that have very hightransfection activity. In addition, a distinct design feature of theselipids is the presence of at least one primary amine group that is avail-able for covalent attachment of modifiers such as small molecules,serum stabilizers, and targeting ligands. This inherent versatilityallows the generation of formulations with a wide range of potentialproperties and does not require the co-formulation of commercialhelper or PEG-lipids. Through rational design, we have developed aseries of lipopolyamines that are distinguished by the number of reac-tive groups available for modification, having either one (Staramine)or two (Crossamine) such groups. Here we describe the synthesis ofthese systems and the evaluation of their ability to deliver siRNA invitro and in vivo. 2. Materials and methods Unless otherwise indicated, all solvents were HPLC-grade andpurchased from ThermoFisher Scientific (Waltham, MA) and allcompounds purchased from Sigma-Aldrich (St. Louis, MO). 2.1. Lipopolyamine synthesis 2.1.1. Staramine Oleoyl chloride (250 mmol) was heated at reflux with trifluoroetha-nol (600 mmol) for 16 h. After the evolution of HCl ceased, excesstrifluoroethanol was removed under vacuum. Vacuum distillation ofthe residue afforded trifluoroethyl oleate (241 mmol) as colorlessliquid. Yield=96%. Boiling point 148-152℃ (at 0.3 mm Hg); NMR(CDCl3): 6 5.37 (m, 2H), 4.5 (m, 2H), 2.15 (t, 2H), 2.01(m, 4H), 1.68(m,2H), 1.35 (m,24H), 0.9 (t, 3H) (Fig.1). Tris-(2-aminoethyl)amine (10 mmol) was dissolved in 20 mL ofethanol. To this solution trifluoroethyl oleate (15 mmol)was added,and the reaction mixture was refluxed for 24 h. The mixture wasconcentrated under vacuum, the residue dissolved in 90% acetonitrile/10% water mixture and then acidified to pH 2-3 with trifluoroaceticacid (TFA). The crude mixture was split into seven aliquots and purifiedusing an Biotage Isolera One system with a reverse phase Cg silica(Fluka, 400 g) column cartridge (eluent consisted of a linear gradientfrom 49.9% acetonitrile/50% water/0.1% TFA to 89.9% acetonitrile/10%water/0.1% TFA over 15 column volumes). The purification afforded N,N'-dioleoyl tris(2-aminoethyl)amine (Staramine) (3 mmol) as its TFAsalt. Yield=23%.HPLC: single peak eluted at 5.5 min (SupplementaryFig.1). MS (TFA salt): 675 [M + 1]; NMR (CDCl3): 8 5.37 (m, 4H), 3.55,3.37 and 3.05 (poorly resolved, 12H total), 2.15 (t,4H), 2.01 (m,8H),1.68(m,4H), 1.35 (m,48H),0.9 (t, 6H). Detection and quantification of Staramine samples were carried outby analytical HPLC (Agilent, Santa Clara, CA). Separation was performedat 25 Con a Zorbax SB-CN 5 um particle, 4.6×250 mm column (Agilent).The mobile phase consisted of 69.9% acetonitrile/30% water/0.1%TFA.Chromatographic separation was performed at 1 mL/min and monitoredat 205 nm (Agilent-1100 UV detector) and a PL-ELS-2100 detector. Thenebulizer was set at 50℃, the evaporator at 70°℃, and the ultra-highpurity nitrogen gas flow rate at 1.6 slpm. Data collection and analysiswere performed using ChemStation software (Agilent). 2.1.2. Staramine derivatives Methoxypolyethyleneglycol(mPEG)(polydisperse,averageMW=550 Da, approximately 12 ethylene glycol repeating units;1.30 mmol) was dissolved in 8 mL of dry toluene. To this solutionwas added phosgene solution in toluene (4 mL of a 2 M solution,8 mmol). The reaction mixture was stirred at room temperature for3 h, then concentrated in vacuum at room temperature to afford 1.30 mmol of crude mPEG550 chloroformate. mPEG550 chlorofor-mate (1.30 mmol) was dissolved in 6.313 g of dry methylene chlorideand 5.66g of this solution (1.04 mmol mPEG550 chloroformate) wasadded to a rapidly stirred solution of Staramine free base (1.04mmolin 6 mL methylene chloride). The mixture was stirred for 3 h at roomtemperature, then concentrated and purified chromatographicallyusing normal phase silica (50 g) with 3, 5, 10, and 15% methanol inmethylene chloride (200 mL each) elutions. Reaction progress andpurification was monitored by HPLC. The purification affordedStaramine-mPEG550 (0.664 mmol). Yield=51%. HPLC: multiplepeaks corresponding to Staramine-mPEG conjugates derived fromeach individual mPEG species eluted between 6 and 8 min, centeredaround 7 min (Supplementary Fig. 1). MS (TFA salt): multiple molec-ular weights observed in increments of 44 centered around 1192[M+2].NMR (CDCl3): 6 5.37 (m, 4H), 4.15 (t, 2H), 3.6 (m,~48H3.5 (t, 2H), 3.3 (s, 3H), 3.55, 3.37 and 3.05 (poorly resolved, 12Htotal), 2.15 (t, 4H), 2.01 (m, 8H), 1.68 (m, 4H), 1.35 (m, 48H), 0.9(t, 6H) (Fig. 1). Staramine-mPEG2000 was synthesized inananalogous manner. Staramine-Cy5 was generated likewise usingiCyy-5-NHS (Lumiprobe, Hallandale Beach, FL). 2.1.3.Crossamine Tetrakis(aminomethyl)methane tetrahydrochloride was preparedby a known procedure [12]. A 50 mL flask was charged with tetraki-++s(aminomethyl)methane tetrahydrochloride (2.88 mmol), methanol10mL)andsodium methoxide/methanols)solution (2.10g of5.457 Molal, 11.50 mmol). The mixture was stirred and refluxed for16h then cooled. The methanol solution was decanted from theinorganic salts and the salts resuspended in 15 mL of absoluteethanol. The suspension was centrifuged and the combined superna-tant solutions concentrated under vacuum. The residue was dissolvedin 15 mL of methylenechloride and filtered from the remaininginorganic salts using a syringe filter (0.45 um). Concentration of thefiltrates afforded free tetrakis(aminomethyl)methane in a quantita-tive yield as a white semi-solid material (the residual solvents wereestimated by NMR). NMR (D20): 6 2.9 (s, CH2). Tetrakis(aminomethyl)methane (2.86 mmol) was dissolved in15 mL of absolute ethanol to which TFA (3.50 mmol) and 2,2,2-tri-fluoroethyloleate (5.72 mmol) were added (see Staramine prepara-tion). The homogeneous mixture was allowed to react for 72 h atroom temperature, and then concentrated under vacuum. The residuewas dissolved in methanol/water (10%)/TFA (0.1%) (40 mL) and thepH adjusted to 2 with TFA. The residue was purified by preparativeHPLC on Cg silica, using 89.6% methanol/10% water/0.4% TFA aseluent. N,N'-dioleoyl tetrakis(aminomethyl)methane (Crossamine)was isolated as the bis-TFA salt (1.01 mmol). Yield=35%. HPLC: sin-gle peak eluted at 17.5 min (Supplementary Fig. 1). MS: (bis-TFAsalt) 661 [M + 1]; NMR (CDCl3): 65.37 (m, 4H), 3.07 and 2.95 (poorlyresolved, 4H each); 2.15 (t, 4H), 2.01 (m, 8H), 1.35 (m, 48H), 0.9(t,6H) (Fig.1). Detection and quantification of Crossamine samples were carriedout by analytical HPLC (Agilent). Separation was performed at 25℃on a Zorbax XDB-Cg 5 um particle, 4.6×150 mm column (Agilent).The mobile phase consisted of 89.6% methanol/10% water/0.4% TFA.Chromatographic separation and data collection remained the sameas in the Staramine assay. 2.1.4. Formulation of Staramine liposomes and siRNA cargo siRNAs targeting luciferase and the non-silencing controls werepurchased from ThermoFisher Scientific (siSTABLE). The sequencesare as follows: siNon sense: 3'-UGGUUUACAUGUCGACUAAUU-5';siNon antisense: 5'-UUAGUCGACAUGUAAACCAUU-3'; siLuc sense: 3'-CGUACGCGGAAUACUUCGAUU-5'; siLuc antisense: 5'-UCGAAGUAUUCCGCGUACGUU-3'. siRNA targeting B-actin were purchased fromThermoFisher Scientific (siGENOME SMARTpool) and were an equalmixture of four siRNAs (sense): 3'-GAAAUCGUGCGUGACAUUA-5';3'- Fig. 1. Lipid structures and H NMR peak assignments.(A) Staramine (B) Staramine-mPEG (C) Crossamine. Based on the manufacturer’s specified molecular weight for each PEGspecies, for mPEG550, n=~12; for mPEG2000,n= 5. GGGCAUGGGUCAGAAGGAU-5;3-AAACCUAACUUGCGCAGAA-5';3'-ACUUGAGACCAGUUGAAUA-5'.Chloroform solutions of Staramine orCrossamine alone or 10:1 mixtures of Staramine and Star-mPEG550 orStaramine-mPEG2000 were rotary-evaporated to a film. The flaskcontaining this film was held under high vacuum overnight. Water forinjection (B. Braun Medical Inc., Irvine,CA) was then added to the filmto give the desired Staramine concentration. This suspension was soni-cated in a bath for approximately 30 min followed by a probe sonicationfor 5 min using a continuous pulse sonication with 5-10 watt output(ThermoFisher Scientific, Sonic Dismembrator, Model 100). The lipo-some solution was then filtered through a 0.2 um filter. The liposomesolution was diluted with 5% dextrose and mixed with the desiredamount of siRNA. Particle size and zeta potential measurements were conducted using Brookhaven 90 Plus Particle Size and Zeta PotentialAnalyzer (Brookhaven Instruments, Holtsville,NY). 2.1.5. Serum studies Staramine, Staramine:Staramine-mPEG550, and Staramine:Stara-mine-mPEG2000 (10:1) nanocomplexes were prepared at an siRNAconcentration of 0.1 mg/mL (N:P=20:1) in 5%dextrose. For turbidityassessment, samples were diluted 1:1 in serum and incubated at37℃. Each reading was blanked against 50% serum in 5% dextroseand absorbance measured at 600 nm at each time point. For particlesize assessment, readings of freshly prepared samples were takenwithout serum (-5 min), then dilutions were made into 10% serum 2.1.6. In vitro transfection assays Twenty four hours prior to transfection, MB49 cells (murine blad-der carcinomas, kindly provided by Dr. Yi Luo,University of Iowa),which stably express firefly luciferase, were plated in a 24-wellplate at an approximate density of 8×10 cells per well. On the dayof transfection a Staramine nanocomplex solution was prepared bymixing the siRNA (siLuc or non-silencing control) solution (1 uL of a1 mg/mL solution of siLuc or siNon in 99 uL of 5% dextrose) with theStaramine solution (25pL of a 1mg/mL solution of Staramineliposomes prepared as above in 75 pL of 5% dextrose). Dilutions(50 uL, 25, 12.5, 6.25, 3) of the nanocomplex solution were made in5% dextrose (total volume 50 pL). Fifty microliters of the Staraminenanocomplex solutions were added to 200 uL of 12.5% FBS in RPMImedia to give a total volume of 250 pL, which were then added toeach previously aspirated well of a 24 well plate. After the plate wasincubated at 37℃ (5%CO2) for 4 h, 250 uL of additional 10% FBSwere added to each well. The plate was then incubated for an addi-tional 48 h, at which time the luciferase signal was measured usinga microplate luminometer (Berthold Detection Systems, Huntsville,AL). Cell lysates (20 uL per sample) were analyzed by BCA proteinassay (ThermoFisher Scientific) to determine total protein accordingto the manufacturer's instructions. K562 (human bone-marrow-derived lymphoma cells, ATCC) cells were plated to a density ofapproximately 200,000 cells/well in 24-well culture plate.siB-actinor a non-silencing control (0.25 ug) were complexed with Crossamineat (20:1) N:P ratio in 5% dextrose. The transfection complex wasadded to cells in presence of 10% FBS. Cells were incubated for 48 hbefore the gene knockdown assay was performed. Fluorescenceassays were performed with Staramine or Crossamine nanocom-plexes containing 1% of Cy5-labeled Staramine, complexed withFITC-siRNA. MB49 and A549 (human lung carcinoma cells, ATCC)cells (1 mL of cell suspension) were plated into each chamber of achamber slide at a concentration of 7,5×10 cells/mL in growthmedia. After 24 h, the cells were transfected as above. Four hoursafter transfection cells were washed and fixed/stained, then viewedat 40x magnification using a Zeiss Axiovert 40 CFL microscope. 2.1.7.LDH assay An LDH assay (CytoTox-ONE, Promega, Madison, WI) was pei.一formed to assess the relative cytotoxicity of Staramine and Crossa-mine. Transfections were performed as above with the addition of2% (v/v) Triton-X 100 as a positive control (maxLDH). After the cellswere treated with Staramine or Crossamine nanocomplexes, or withTriton-X 100, cells were incubated at 37 ℃ at 5% CO2 for 4h. The su-pernatant from each well (250uL) was collected and centrifuged at10,000 rpm for 5 min. To a black 96-well plate was added 100 uL ofeach supernatant in duplicate. To each well containing supernatantwas added 100 uL of substrate solution (11.25 mL assay buffer inone vial of Substrate Mix). The plate was incubated in the dark atroom temperature for 10 min and was then read in a fluorescenceplate reader at an excitation wavelength of 560 nm and an emissionwavelength of 590 nm.Percent cytotoxicity was calculated accordingto the formula::[(sample-background)/(maxLDH-background)x100].The amount of LDH detected is reported for decreasing amountsof siLuc relative to Triton X-100. 2.1.8. Animals All procedures used in animal studies were performed in accor-dance with local, state and federal regulations and approved by theInstitutional Animal Care and Use Committee. Female ICR mice wereobtained from Harlan Laboratories (Houston, TX) and ranged from8 to 10 weeks of age (17-22 g) at the time of each study. siRNAs targeting Cav-1 and a non-silencing control sequence werepurchased from Dharamcon (siSTABLE, in vivo purified) and containedthe following sequences: siCav-1 sense: 3'-GUCCAUACCUUCUGCGAU-CUU-5'; siCav-1 antisense: 5'-GAUCGCAGAAGG UAUGGACUU-3';siRNA nanocomplexes were diluted into sterile water containing 5%dextrose and injected into the tail vein of female ICR mice. Injectiondoses were adjusted by altering the concentration of particles in eachinjection; injection volumes were held constant at 10 pL/g. At varioustimes post-injection, organs were collected and immediately frozen inliquid nitrogen for storage at -80℃. Total RNA was collected from tis-sues using Tri-Reagent (Molecular Research Center, Cincinnati, OH)according to the manufacturer's protocol. These RNA stocks were usedas templates for synthesis of complementary DNAs (cDNAs) using ran-dom hexamer primers and reverse transcriptase (Multiscribe, AppliedBiosystems, Carlsbad,CA). The reverse transcription reaction contained:10 mM Tris-HCl (pH8.3), 50 mM KCl, 5.5 mM MgCl2, 50 ng total RNA,2.5 uM random hexamer primer, 500 uM each dNTP, 0.4 unit/uLRNAseinhibitor, 1.25 U/uL Reverse Transcriptase. TaqMan primers and probesets (Applied Biosystems) were used in qPCR to amplify the transcriptof interest from the corresponding cDNA template. The qPCR reactioncontained: TaqMan Gene Expression Master Mix (Applied Biosystems),50ngcDNA,900 nM forward and reverse primers, 250 nM FAM-labeledMGB probe. All changes were measured against an internal controlusing primers specific for housekeeping genes (GAPDH or Ppib). 3.Results and discussion3.1. Synthesis of Staramine, Crossamine, and their derivatives The core Staramine and Crossamine lipopolyamines were designed toprovide high levels of target geneknockdown while possessing structureswhich are readily modifiable. We chose head groups which had such mo-lecular geometry as to potentially possess primary amine groups either inclose proximity to tertiary amine groups or to one another (Fig.1). The synthetic linker was selected in order to maximize synthesissimplicity. The choice of acylating agent, a mildly activatedtrifluoroethyl1leester, was dictated by the considerations of reactivity.The degree of its reactivity should be high enough for the reaction toproceed in a reasonably short time, but at the same time low enoughto allow for the acylating agent to be dispersed in the reaction mixture.This precluded the use of functionalities such as acyl chlorides andchloroformates.Ultimately, the ease of the byproduct trifluoroethanolremoval was a benefit of using trifluoroethyl activation. Although thenature of the lipid tail was not optimized, oleoyl-based lipids were cho-sen based on the large number of reports of their utility as componentsof nucleic acid delivery systems. Structural versatility is a distinguishing design feature of theselipids. A primary goal was to reduce the total number of unique com-ponents required to generate active formulations. The majority ofcurrent therapeutic liposomal formulations are composed of a cation-ic lipid, a fusogenic lipid, a PEG-lipid and cholesterol. We felt that bydesigning a single highly active cationic lipid which has endosomoly-tic properties as well as the ability to be readily modified, we mightgain utility in the simplicity of the formulation. Through covalent at-tachment of small molecule drugs, serum stabilizers, and targeting li-gands, the properties of the resulting nanoparticles may theoreticallybe tuned to fit a specific delivery application or target a specific tissueor organ. As a validation of this structural advantage, we synthesizedPEGylated derivatives of Staramine in order to control the in vivoproperties of the lipopolyamine formulations. 3.2. In vitro activity of Staramine and Crossamine nanoparticles Staramine and Crossamine nanoparticles were formed using thethin film hydration method, and mixed with siRNA to form J. Sparks et al. / Journal ofControlled Release 158 (2012) 269-276 nanocomplexes. Particle sizes ranged from 80-100 nm and zeta po-tentials ranged from 15-40 mV, depending on the formulation, andwere a function of the N:P ratio and presence and/or length ofmPEG in the composition. siRNA loading of >90% for bothStaramine and Crossamine was routinely observed based on agarosegel electrophoresis. Staramine and Crossamine were tested in alarge number of in vitro applications including adherent cell lines,suspension cell lines, and primary cells, and in some cases showed ac-tivity which was equal to or better than current popular commercialreagents (Supplementary Table 1, Supplementary Figs. 2 and 3). Todemonstrate this activity, Staramine nanoparticles were complexedwith siRNA targeting luciferase, and knockdown of luciferase wasmeasured as a function of luciferase activity in MB49 cells using a Pro-mega Luciferase Assay System (Fig.2A). Crossamine was highly active in adherent, as well as suspensioncell lines, including K562. Human B-actin transcript knockdown ac-tivity was measured as a function of siRNA concentration and greaterthan 90% reduction was found at siRNA concentrations as low as 5 nM MB49 A549 Fig. 2. In vitro target gene knockdown by Staramine and Crossamine nanocomplexes.(A) Luciferase expression was measured in MB49 cells after transfection with Stara-mine nanocomplexes containing siLuc or a non-silencing control. Knockdown foreach concentration is reported relative to the non-silencing control value for that con-centration. (B) B-actin expression was measured in K562 cells after transfection withCrossamine nanocomplexes containing sip-actin or a non-silencing control. Knock-down for each concentration is reported relative to the non-silencing control valuefor that concentration. (C) MB49 (top row) and A549 (bottom row) cells were treatedwith nanocomplexes composed of FITC-labeled siRNA only (left), Staramine (+1%Cy5-labeled Staramine) nanocomplexes (center), or Crossamine (+1% Cy5-labeledStaramine) nanocomplexes (right). Nuclei are stained with DAPI. 40× magnification.Values expressed are means±SD. relative to a nonsilencing control (Fig. 2B). An LDH assay, used as ameasure of cell lysis, also suggested there is very low cytotoxicityfor both Staramine and Crossamine nanocomplexes across a widerange of transfection concentrations (Supplementary Fig. 4). Cy-5 la-beled Staramine and Crossamine with FITC labeled siRNA were for-mulated to transfect MB49 and A549 cells (Fig.2C). A much largeramount of FITC signal was observed in cells treated with Staramineand Crossamine (Fig. 2C, middle, right) compared to cells whichwere treated with FITC-siRNA alone (Fig. 2C,left). The diffuse stainingof the siRNA and the Staramine and Crossamine indicates broad cyto-solic distribution expected from formulations which result in highlevels oftranscript knockdown. 3.3. In vivo activity of Staramine nanoparticles PEGylated lipids are utilized for in vivo applications within formula-tions in order to stabilize nanoparticles against serum components.Highly cationic nanoparticles are known togenerateimmuneresponses through interaction with blood components including pro-teins involved in the complement system, which leads to uptake andclearance by macrophages [22]. PEG is routinely added into nanoparti-cles to reduce the surface charge, and therefore toxicity, after systemicadministration. This is normally accomplished through the covalent at-tachment of mPEG to phosphatidylethanolamine-based structures orthrough glycerol-based structures [7-9,12,23-26], and then by addingthese lipids into mixtures of other lipids. Here we demonstrate the utilityof using PEGylated Staramine molecules in the nanocomplexes toachieve efficient target gene knockdown in vivo while moderating toxic-ity. The primary amine of the Staramine head group was utilized as theattachment point for mPEG. Athough the particle sizes for the PEGylatedformulations remained relatively unchanged, the zeta potentials werereduced by 50%. In our system this was evidenced by a reduction in thetoxicity index value (Fig.3A) which is a rapid and convenient qualitativetest of acute toxic response in mice. This parameter is based on cage sideobservations of animal reaction and behavior after dosing. Each animalwas scored on a 1-5 scale (5 being sign of severe toxicity) in each offour categories, including respiration, posture, social interaction, andcoat appearance. The scores for each animal were summed and thisvalue recorded as the toxicity index. For this index values of 5 areindicative of moderate toxicity while values of<2 are indicative of littleor no acute toxicity. Caveolin-1 (Cav-1), a protein involved in caveolaeformation during endocytosis was selected as the target gene due to itswidespread expression in most tissue. Mice were administered intrave-nously a single dose of unmodified Staramine/siCav-1 nanocomplexes(N:P20:1) or nanocomplexes containing PEGylated Staramine with dif-ferent length PEGs (500 and 2000 Da) incorporated into Staraminenanoparticles. The addition of PEG modification into the Staramine for-mulation served to dramatically reduce the observed toxicity index com-pared to Staramine alone (Fig.3A). Additional confirmation of reducedtoxicity was obtained from histopathology of lung tissue where it wasseen that unmodified Staramine-treated mice displayed evidence ofhemorrhagic foci with associated fibrin thrombi, while Staramine-mPEG550 mice showed lung tissue similar to untreated animals(Fig.3B). The effect of PEGylation on knockdown activity was also exam-ined. Forty-eight hours after injection, significant Cav-1 transcriptknockdown was found in the lung but not the liver for both Staramineand Staramine-mPEG550 formulations (Fig. 3C). The Staramine-mPEG550 maintained a ~40% siRNA target specific knockdownwhich was only slightly reduced compared to the nonPEGylatednanocomplexes (Fig. 3C). Although the addition of the longermPEG2000 to Staramine (Staramine-mPEG2000) served to reducethe acute toxicity to the greatest extent (Fig. 3A), knockdown levelswere moderately reduced relative to nonPEGylated and Staramine-mPEG550iInanocomplexes. Additional ratios of Staraminet0Staramine-mPEG550 were tested, including both higher and lower treatment (Fig. 4A). In addition, the amount of siCav-1 was measuredin the lung tissue for each dose using a stem loop quantitative PCRassay. C 口 siNon 口 siCav-1 Fig. 3. In vivo activity of PEGylated Staramine nanocomplexes. Doses were 2.0 mg/kgsiCav-1 (or non-silencing control) in 200 uL; N:P=20:1; ratio of Staramine-mPEG550or Staramine-mPEG2000 to Staramine=1:10. (A) Toxicity index was determined forStaramine, Staramine:Staramine-mPEG550, andStaramine:Staramine-mPEG2000nanoparticles after a single iv dose. (B) Cross sections of lung tissue stained with hema-toxylin and eosin after treatment with Staramine (left) and Staramine-mPEG550(right) nanocomplexes. (C) Cav-1 transcript knockdown was determined in lungsand liver 48h after a single iv dose of Staramine, Staramine:Staramine-mPEG550,and Staramine:Staramine-mPEG2000 nanoparticles. n=5 for each group. Valuesexpressed are means±SD.*P-0.001,**P<0.007,*** P<0.01. ratios than those reported here, however these formulations eithergave reduced levels of knockdown or increased levels of toxicity. Insummary, it appears PEGylation serves to decrease acute toxicitywith only a mild reduction in activity, and a 1:10 incorporation ofStaramine-mPEG550 into Staramine nanocomplexes achieves abalance of activity and toxicity not found in the other combinations. This Staramine mPEG550 formulation was the focus of furtherstudies both involving target gene knockdown activity and toxicity.Cav-1 transcript knockdown was examined as a function of siCav-1concentration from 10 to 100 pg per dose (0.5-5.0 mg/kg) 48 h after Target transcript knockdown was found to increase with increas-ing siCav-1 detected, with the greatest target gene knockdown andsiCav-1 found at a 5.0 mg/kg dose (Fig. 4A). In addition, all dosinglevels were safe based in the toxicity index (Fig. 4B). Due to theobservation that gross toxicity may be correlated with animal weightloss [24], animals were weighed 24 h after treatment (Fig. 4C). Alldoses below 3.0 mg/kg resulted in weight loss that was not signifi-cantly different from dextrose treatment. Although not a new observation in regard to biodistribution of cat-ionic nanocomplexes [27], this significant knockdown activity in thelung stands in contrast to the majority of cationic liposomes in cur-rent development for siRNA therapeutics which show uptake andgene knockdown activity primarily in the liver [7-10,12]. Interesting-ly, it is not immediately apparent why other PEGylated cationic lipo-somes which possess markedly similar properties show knockdown siRNA dose (complex) injected (mg/kg) B5xx 4 3 21 0 untreated 0.5 1.0 2.0 3.0 5.0 siRNA dose (complex) injected mg/kg) siRNA dose (complex) injected mg/kg) Fig. 4. In vivo activity of PEGylated Staramine nanocomplexes as a function of dose. Doseswere 10, 20, 40,60, 100 ug siCav-1 (or non-silencing control) [which corresponds to 0.5,1, 2, 3, or 5 mg/kg siCav-1 (or non-silencing control)] in 200uL; N:P=20:1; ratio ofStaramine-mPEG550 to Staramine=1:10.(A) Cav-1 transcript knockdown in lung wasdetermined relative to untreated animals 48 h after a single iv injection. The amount ofsiCav-1 in the tissue was determined for each dose. The toxicity index (B) and animalweight change (C) were evaluated for each dose. n=5 for each group. Values expressedare means±SD. activity in the liver rather than the lung [24]. This effect could beexplained, in part, by variable particle aggregation induced byserum components, which may lead to uptake in lung fenestrationdue to alteration of the particle size and surface properties [28].How-ever, PEGylation of the liposomes routinely serves as a modificationto reduce such an effect [29-31], one which we have observed bymeasuring both turbidity and particle sizes in the presence of serumfor Staramine-based formulations (Supplementary Figs. 5 and 6). Inthese assays, particle sizes observed for non-PEGylated Staramine in-creased significantly, which was reflected in the increase in turbidity.Although Staramine-mPEG550 nanocomplexes showed turbidity inserum that was similar to nonPEGylated Staramine, the particlesizes of the Staramine-mPEG550 nanocomplexes were impactedmuch less dramatically and were similar to Staramine-mPEG2000nanocomplexes, which showed very little turbidity.The effect of theincreased stability of the Staramine-mPEG550 formulation (as indi-cated by a maintained relatively small particle size) is most readilyseen in the amount of siRNA found in tissues shortly after intravenousinjection (Supplementary Fig. 7). The vast majority of material isfound in the liver rather than the lung after 5 min. The nanocom-plexes necessarily passed through the lung without entrapmentprior to being taken up by the liver. In addition, we have demonstrat-ed that Staramine-mPEG550 nanocomplexes achieve target geneknockdown in lung in part due to the significantly slower clearancerate of the siRNA from lung tissue relative to other tissues, which results in prolonged accumulation on the timescale of RNAi-mediatedtranscript depletion [32]. It is clear from these studies that PEGylationhas a physical impact on the interaction of the nanocomplexes withserum, but the shielding effect provided by PEGylation only minimal-ly reduces target gene knockdown. 4. Conclusion Two cationic lipids, Staramine and Crossamine,were synthesizedwith the goal of producing active and easily modifiable structureswhich could be formulated into nanoparticles for the delivery ofsiRNA. We have demonstrated the versatility of the compounds byfunctionalizing Staramine with mPEG. While both lipids exhibitedhigh degrees of in vitro activity, Staramine:was found to possess ahigh level of target gene knockdown in vivo. Its toxicity upon intrave-nous administration could be mitigated by the addition of PEGylatedStaramine molecules to the formulation without significantly reduc-ing knockdown activity. A thorough mechanistic investigation ofthis system is underway, including analysis of siRNA accumulationand elimination kinetics, as well as use in therapeutic applicationssuch as pulmonary arterial hypertension and lung cancer. Appendix A. Supplementary data Supplementary data to this article can be found online at doi:10.1016/j.jconrel.2011.11.006. References ( [ 1] R . Juliano,J. Bauman, H. Kan g , X. M i n g, Bio l ogical bar r iers to t he r apy with anti - s ense and siRNA olig on ucleotides, Mol. Pharm.6 (2009) 68 6 -695. ) ( [2] K.A. Whitehead, R . Lange r , D . G. Anderson, Knocking down b a rriers: advances in s iRNA deliv e ry , Nat. Rev . Drug Discov. 8 ( 2009) 12 9 -138. ) ( [3] M. F rank-K am enets ky , A. Grefhorst, N.N. A n derson, T . S. Racie, B. Bramlage, A . A kinc, D . B utler , K. Cha r isse, R. Dorkin, Y. Fan, C . Gamba-Vitalo, P. Hadwiger, M .Jayaraman, M. J o hn, K . N. J ayaprakash, M . Maier, L . Nechev, K .G. Rajeev, T. Read, I . Rohl, J.Soutschek, P. Tan, J . Wong, G . Wang, T. Zimmermann, A. de Fougerolles,H.P. Vornlocher, R . Langer, D.G. Anderson, M . Manoharan, V. Koteliansky, J . D. Horton, K . F itzgerald , Therapeutic RNAi targeting PCSK9 acutely lowers plasma c holesterol in rodents and L DL cholesterol i n n onhuman pr i mates, Pr o c.Nat l . A cad. S ci. U. S. A. 105 (2008 ) 11915-11920. ) ( [ 4] T . W. Geisbert, A.C. L ee, M . Robbins, J.B. Geisbert, A.N.Honko, V. S o od, J.C. Joh n son, S. d e Jong, I . Tavakoli, A . J udge, L .E. Hensley, I . Maclachlan, P ostexposure ) ( p rotection of non-human primates ag a inst a le t hal E b ola v i rus challenge with R NA i n terference: a proof-of-concept study, Lancet 375 (2010) 1896-1905. [5] T .S. Zimmermann, A.C. Lee, A. Ak i nc, B. Br a mlage, D. B um c rot, M.N. Fedo r u k, J. ) ( H arborth, J . A. Heyes, L . B. Jeffs, M. J ohn, A.D. Judge, K . L a m, K. Mc C lintock, L.V .N echev, L.R . P almer, T . Racie, I . Rohl , S. Seiffert, S . Shanmugam, V . Sood, J . Soutschek, I . T oudjarska, A.J. Wheat, E. Yaworski, W. Zed a lis, V. Koteliansky, M. M anoharan, H.P. V ornlocher, I . MacLachlan, RNAi-mediated g ene silencing in n on-human primates, N a ture 441 (2006) 1 11 -114 . ) ( [6] A. S chroeder, C .G. Levins, C . C ortez, R . L anger, D.G. A n derson, Li p id-based n anotherapeutics for siRNA d elivery, J Intern Med 267 (2010) 9-21. ) ( [z] M . T. Abrams, M.L. Koser, J. Seitzer, S.C . Williams , M.A. DiPietro, W. Wang, A.W. S haw, X. Mao, V. Jadhav, J.P . Da v ide, P.A . Bur k e,A.B. Sachs, S.M. Stirdivant, L. S epp-Lorenzino, Evaluation of efficacy, biodistribution, and in f lammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment, Mol. Ther. 18 (2010) 171-180. ) ( [8] A. Akinc, W. Q uerbes, S. D e,J. Q in, M. F rank-Kamenetsky, K.N. Jayaprakash, M. J ayaraman, K. G . Rajeev, W. L . Can tl ey, J.R. D orkin, J.S. B utl e r, L. Qin, T. Racie, A. S prague, E. F ava, A . Z eigerer,M.J. Hope, M. Zerial, D . W. Sah, K. Fitzgerald, M . A. T racy, M. M anoharan, V . K o teliansky, A . F o ugerolles, M .A. M aier, T a rg e t e d 1 1 delivery of RNAi therapeutics with endogenous and exogenous ligand- b a s ed m echanisms, Mol. Ther. 18 (2010)1357-1364. ) ( [9 | ] K.T. Love, K.P. Mahon, C . G. L e vins, K . A. Whitehead,W. Querbes, J.R. Dorki n , J. Qin , W. Cantley, L .L. Qin, T . Racie, M. Frank-Kamenetsky, K.N. Yip, R . Alvarez, D . W. Sah,A. de Fougerolles, K . Fitzgerald,V. Koteliansky, A . Akinc, R . La n ger, D .G. Ander s on, L ipid-like materials for low-dose, in vivo gene silencing, Proc. Natl. A cad.Sc i. U . S. A. 107 (2010) 1864-1869. ) ( [10 ] S .C. Semple, A. Akinc, J. Chen, A.P. Sandhu, B .L. Mui, C.K. C h o, D .W. S ah, D . Stebbing, E .J. Crosley, E. Yaworski, I.M. Hafez, J. R. Dork i n, J . Qin , K. Lam, K.G. R ajeev, K.F. W ong, L. B . J e ffs, L . N echev, M . L. E isenhardt, M . Ja y araman, M. ' Kazem, M.A. Maier, M. Srinivasulu, M.J. Weinstein, Q. Chen, R. A l varez, S.A. Barros, S . De, S.K. Klimuk, T. Borland, V. Kosovrasti, W.L. C ant l ey, Y .K. Tam , M.Manoharan,M.A. C iufolini, M.A. T racy, A. d e F ougerolles, I . M acL a ch l an, P . R. Cullis, T.D. M adden, M . J . Ho p e, Rational design of c a ti o nic lipi d s for siRNA delivery, Nat. Biote ch nol . 28 (2010 ) 172-1 7 6. ) ( [11 1 1 S ] . Chono , S . D.L i , C.C. Conwell, L . H uang , An effi ci ent a n d low immunostimulatory nanoparticle formulation f or s yste m ic siRNA d e livery to the t u mor, J. Control. R elease 1 31 ( 2008) 6 4-69. ) ( [ 12] A . A kinc, A. Zumbuehl, M . G o ldberg, E.S. L e shchiner, V. Busini, N . Hossain, S.A. B acallado,D.N.Nguyen,J. Ful l er, R. A l var e z, A. Borodovsky, T. B o r land, R. Constien, A . d e F ougerolles, J.R . Do r kin , K . Narayanannair Jayaprakash, M. Jayaraman, M . J ohn,V. Koteli a nsky,M . Ma n oharan, L . N e c h ev, J. Qin , T.Rac i e, D. R aitcheva, K.G. R ajeev, D.W. Sa h, .J . So S utschek , I . Toudjarska, H.P . Vornlocher , T.S. Zimmermann, R . Langer, D . G. An derson, A combinatorial library of lipid-like materials for deliv- e ry o f R NAi t h erapeuti c s, N at. Biotechnol. 26 ( 2 008) 561-569. ) ( [13] M . D amen, J. Aarbiou, S . F. v an Dongen, R.M. Buijs-Offerman, P.P. Spijkers, M . vanden H eu vel, K . K v ashnina,R.J. No l te, B.J. Scholte, M.C. Feiters, Delivery of DNA ands i RNA by novel g e mini-like amphiphilic peptides, J. C ontrol. R elease 145 (2010)33 -39 ) ( 141 M . Mevel , N. Kamaly,S. C a rmona, M. H . Oliver, M.R. Jorgensen, C. Crowther, F.H. S alaz a r, P .L. Marion, M . Fujino, Y. Natori, M. Thanou, P. Arbuthnot, JJ. Yaouanc, P .A .A . J affres, A.D . Mille r , DODAG; a versatile new c ationic lipid tha t mediatesefficient delivery of pDNA a nd siRNA,J. Control. R elease 1 43 (2010) 2 2 2-232. ) [15 Y. Nakamura, K. Kogure, S. Futaki, H. Harashima, Octaarginine-modified multifunc-tional envelope-type nano device for siRNA, J. Control. Release119(2007)360-367. i[16]1M.S. Suh, G. Shim, H.Y. Lee, S.E. Han, Y.H. Yu, Y. Choi, K. Kim, I.C. Kwon, K.Y. Weon,Y.B. Kim, Y.K. Oh, Anionic amino acid-derived cationic lipid for siRNA delivery,J.Control. Release 140 (2009) 268-276. ( [17] U 1 .K. L aemmli, Characterization o f DNA c o ndensates i n duced by po l y(ethylene oxide) and p o lylysine, P r oc. N a tl. Acad. Sci. U . S. A. 72 ( 1 975)4288-4292. ) ( [ 18] F . C. MacLaughlin, R.J . M um per, J. Wang, J.M. Tagli a ferri,I. Gill, M. Hinc h c l iffe, A.P. R olland, Chitosan and d epolymerized chitosan oligomers as condensing carriers f or in vivo plasmid d elivery,J. Control. Release 5 6 (1998) 259-272. ) ( [19] | ( O. Boussif, F . Lezoualc'h, M.A. Zanta, M.D. Mergny, D. Scherman, B. Demeneix, J.P . B ehr, A versatile vector for gene an d oligonucleotide transfer into cells in cult u re a nd in vivo: p olyethylenimine, Proc. Natl. Acad. Sci. U. S.A. 92 (1995)7297-7301. ) ( [20] H .-j.P. Junghun S uh, Hwang Byung Keun, Ion i zation of Poly(ethylenimine) and P oly(allylamine) a t Various pH's, Bioorg. Chem. 22 (1994) 318-327. ) ( [21] J.P. Behr, B. D emeneix, J. P . L o effler, J. P erez-Mutul, E f ficient g e ne tr a nsfer into m ammalian primary end o crine cel l s wi t h lip o polyamine-coated DNA , Proc . N atl. Acad. Sci. U. S. A. 8 6 (1989) 6982-6986. ) [22] M.A. Dobrovolskaia, P. Aggarwal, J.B. Hall, S.E. McNeil, Preclinical studies to un-derstand nanoparticle interaction with the immune system and its potential ef-fects on nanoparticle biodistribution, Mol. Pharm.5 (2008) 487-495. [23] J.Heyes, L. Palmer, K. Bremner, I. MacLachlan, Cationic lipid saturation influencesintracellular delivery of encapsulated nucleic acids,J.Control.Release 107 (2005)276-287. [24A.] Santel, M. Aleku, O. Keil, J. Endruschat, V. Esche, G. Fisch, S. Dames, K. Loffler,M.Fechtner, W. Arnold, K. Giese, A. Klippel, J. Kaufmann, A novel siRNA-lipoplextechnology for RNA interference in the mouse vascular endothelium, Gene Ther.13 (2006) 1222-1234. [25] S.-D.L. Sumio Chono, Christine C. Conwell, Huang Leaf, An efficient and lowimmunostimulatory nanoparticle formulation for systemic siRNA delivery to thetumor,J. Control. Release 131 (2008) 64-69. [26] R.E. Vandenbroucke, I. Lentacker, J. Demeester, S.C.De Smedt, N.N. Sanders, Ultra-sound assisted siRNA delivery using PEG-siPlex loaded microbubbles,J. Control.Release 126 (2008)265-273. ( [ 27] B .M. Kinsey, C.L. Densmore, F.M. Orson, Non-viral gene delivery to the lungs, Curr. Gene Ther. 5 (2005) 1 81-194. ) ( [ 28] C . M archini, M . M o ntani, A. Am i ci, H. Am e nitsch, C. Mar i anecci, D. P ozzi, G. C aracciolo, S tructural s tability and i ncrease i n s ize r ationalize the e fficiency oflipoplexes in serum, Langmuir 25 (2009) 3013-3021. ) Nie A[29] T. Gjetting, N.S. Arildsen, C.L. Christensen, T.T. Poulsen, J.A. Roth, V.N. Handlos,H.S. Poulsen, In vitro and in vivo effects of polyethylene glycol (PEG)-modifiedlipid in DOTAP/cholesterol-mediated gene transfection, Int J Nanomedicine 5(2010)371-383. [30]J.K. Kim, S.H. Choi, C.O. Kim, J.S. Park, W.S. Ahn, C.K. Kim, Enhancement of poly-ethylene glycol (PEG)-modified cationic liposome-mediated gene deliveries: ( effects on serum stability and t ransfection efficiency, J . Pharm. Pharmacol. 55 (2003)453-460. ) [31] J. Heyes, K. Hall, V. Tailor,R. Lenz, I.MacLachlan,Synthesis and characterization ofnovel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery,J.Control.Release 112 (2006)280-290. ( [32] K 1 .J. Polach, M. M atar, J. Rice, G. Slobodkin, J.Sparks, R . Congo, A. Rea-Ramsey,D.McCl u re, E. Brunhoeber, M. Krampert, A. Schuster, K . Jahn-Hofmann, M. J o hn, H.P. Vornlocher, J . G. F ewell, K . Anwer, A . G e ick, Delivery of siRNA to the mou s e l ung v ia a f u nctionalized l i popolyamine, Mo l . Th e r . (2 0 11). doi : 10.1 038/ m t. 2 0 1 1 .2 1 0 . ) 耐士科技www.rysstech.com 士科技www.rysstech.com
确定






还剩6页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








