
方案详情
文
Homopolymers and block copolymers of higher epoxides (butene oxide and hexene oxide) are synthesized using 1-alkanols and polyethylene glycol monomethyl ether (PEG-MME) 1100 as initiators by anionic ring opening polymerization in bulk.Most of thesampleswere synthesized with controlled microwave heating
in sealed vessels. Tri- and tetrablock copolymers with different repeat units in the individual blocks are synthesized by living polymerization with addition of the next monomer after complete consumption of the previous one. The products thus obtained are characterized using size exclusion chromatography (SEC), liquid chromatography under critical conditions (LCCC) and liquid adsorption chromatography (LAC).
方案详情

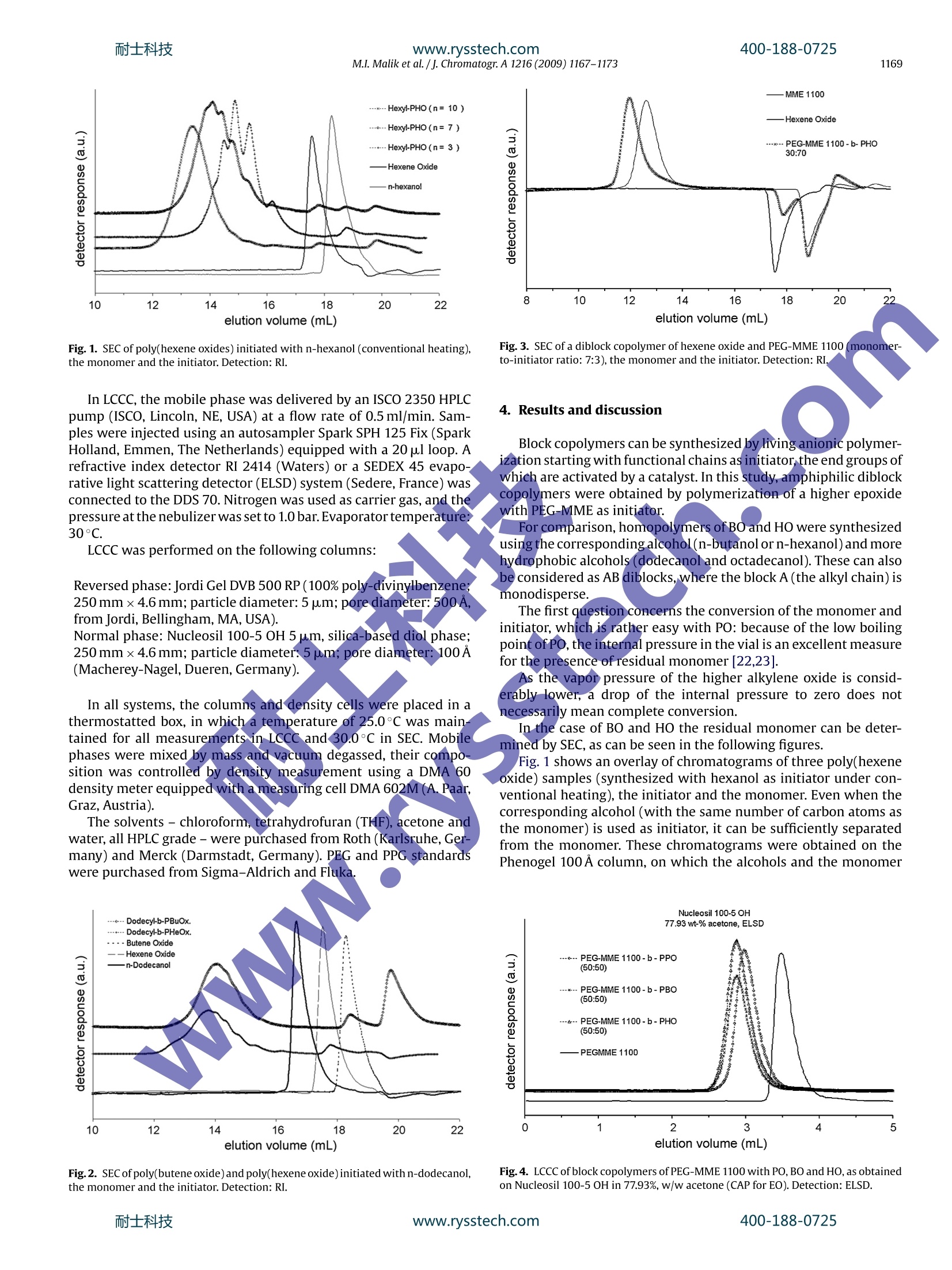
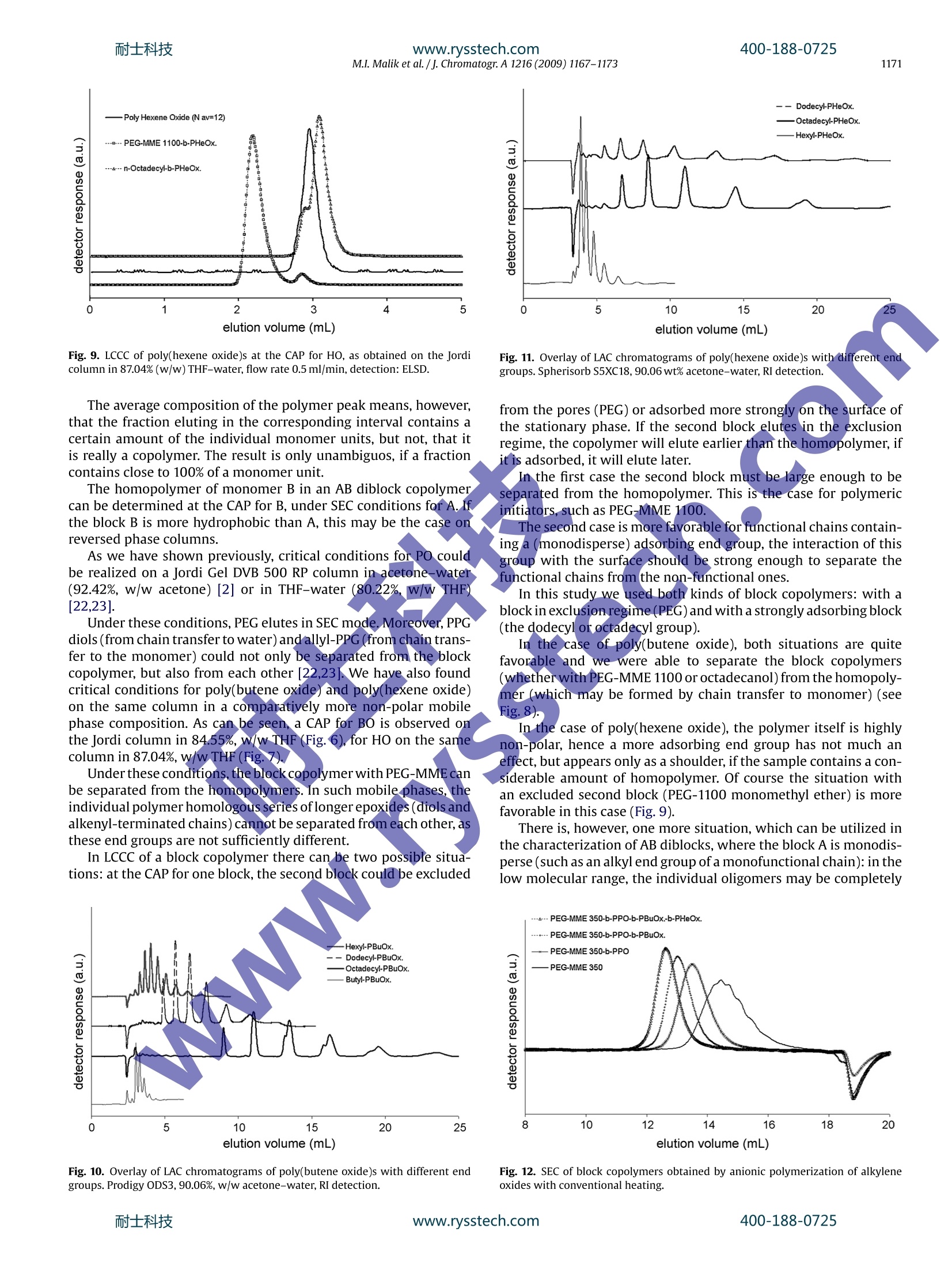
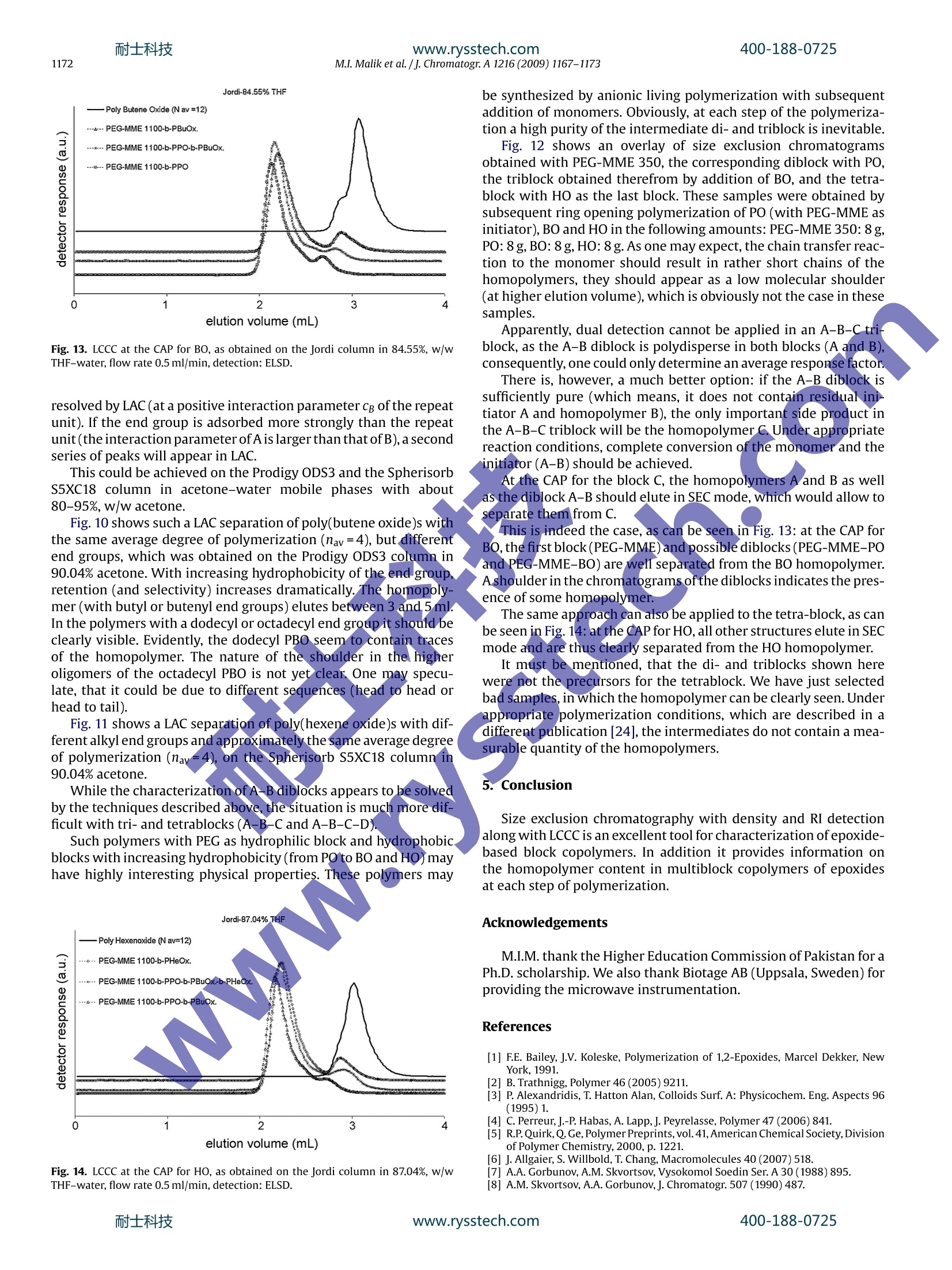
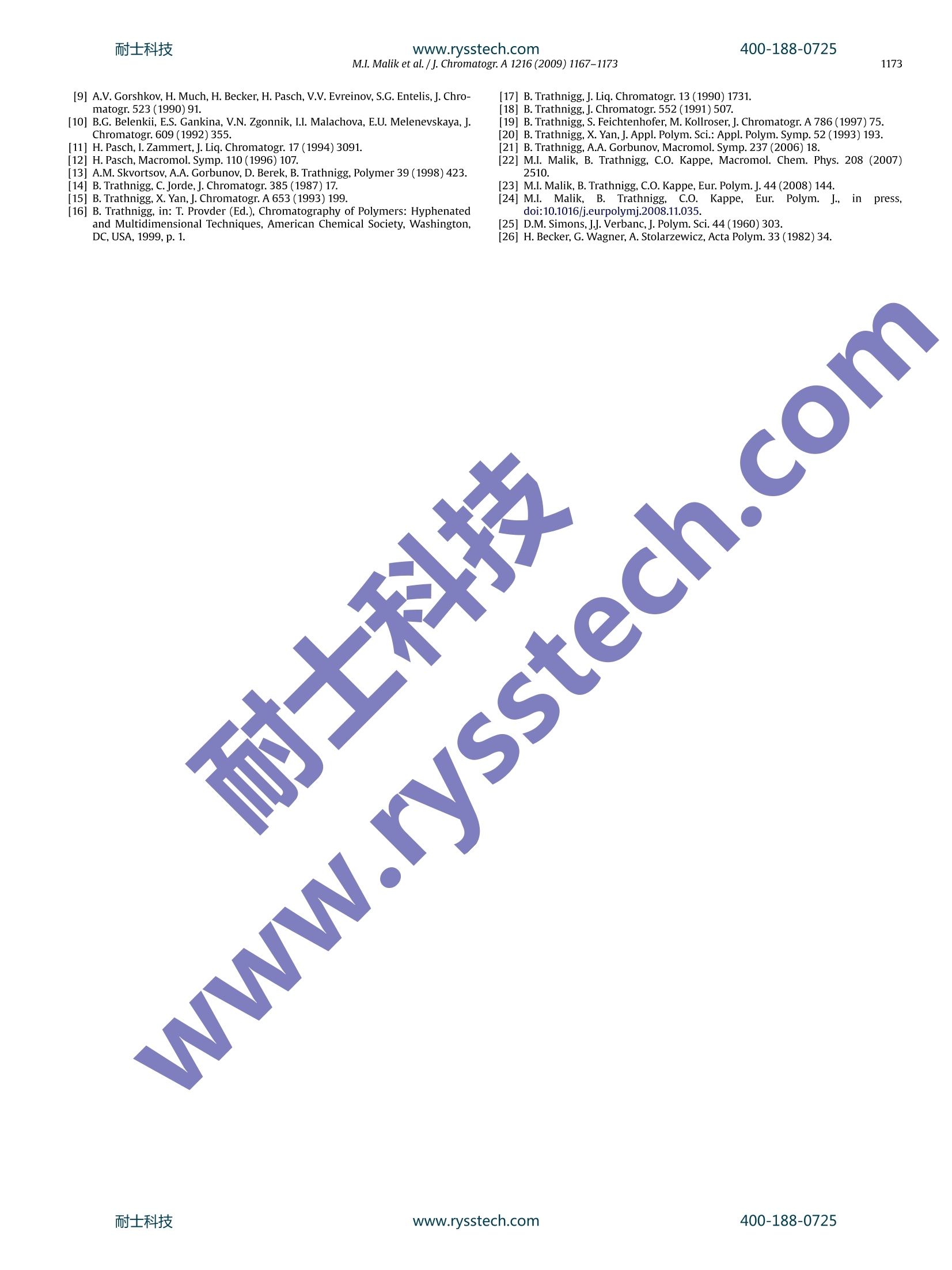
耐士科技400-188-0725www.rysstech.comJournal of Chromatography A, 1216 (2009) 1167-1173 耐士科技400-188-0725www.rysstech.com1168M.I. Malik et al./j. Chromatogr A 1216 (2009) 1167-1173 Contents lists available at ScienceDirect Journal of Chromatography A ELSEVIER journal homepage:www.els evier.com/locate/chroma Amphiphilic polymers based on higher alkylene oxidesSynthesis and characterization by different chromatographic techniques Muhammad Imran Malika,b, Bernd Trathnigg a,b,*, C. Oliver Kappe a,c a Institute of Chemistry, Karl-Franzens-University, Heinrichstrasse 28, A-8010 Graz, Austria Central Polymer Lab-Molecular Characterization (CePOL/MC), Heinrichstrasse 28, A-8010 Graz,Austria C Christian Doppler Laboratory for Microwave Chemistry (CDLMC),Heinrichstrasse 28, A-8010 Graz, Austria ARTICL E INFO ABSTRACT Article history:Received 1 August 2008Received in revised form24November 2008Accepted 22 December 2008Available online 27 December 2008 Keywords:Alkylene oxidesBlock copolymersCharacterizationChromatographyMicrowave-assisted polymerization X Poly(alkylene oxide)s are in widespread use for various applica-tions [1].Their properties depend on the substituents of the oxiranering: while poly(ethylene oxide)s (PEO) are soluble in water, onlythe lower poly(propylene oxide)s are water-soluble, and the poly-mers of higher epoxides -such as butene oxide(BO), hexene oxide(HO), etc. - are soluble only in non-polar organic solvents. Con-sequently, block copolymers containing ethylene oxide (EO) in thehydrophilic block and a higher epoxide in the hydrophobic block areamphiphilic materials, which can be used as surfactant or emulsi-fier. Up to now, only block copolymers of EO and propylene oxide(PO) are materials of commercial interest, which are produced inlarge amounts [2-4], while block copolymers containing higheralkylene oxides are still not in widespread use [5,6]. They may,however, have very special physical properties, as a block contain-ing longer alkyl side groups is much more hydrophobic than a POblock. One can imagine, that even more interesting materials maybe obtained by adding a third block with a different side chain: suchA-B-C triblocks and A-B-C-D tetrablocks (where A is EO, B is PO, ( * C o rresponding au t hor at: Ins t itute of Ch e mistry, Karl-Franzens-University, Heinrichstrasse 28, A -8010 Graz, Austria. Tel.:+43 31 63805328; ) fax: +43 31 63809840. ( E-mail a ddress: b ernd.trat hni gg@uni- g raz.at ( B. Trathnigg). ) ( 0 021-9673/$ - see front matter O 2 0 08 Elsevier B.V. All r ights reserved. ) C is BO, and D is HO) could be tailor-made surfactants for specialapplications. In principle, one may synthesize tri- and tetrablocks by livinganionic polymerization starting with the hydrophilic block (suchas a PEG-monomethyl ether) or the hydrophobic block (such as afatty alcohol) and add the next monomers after consumption of theprevious one. The problem in the evaluation of such materials is their charac-terization: as traces ofthe homopolymers(A,B,orC)and the diblockcopolymers (A-B, B-C) may influence the physical properties verystrongly, one must be sure to keep the concentration of these impu-rities as low as possible. The situation is even more complex withA-B-C-D tetrablocks. In this study, analytical techniques have been developed, whichallow the identification and determination of side products in thesynthesis of A-B-C and A-B-C-D block copolymers. 2. Characterization of block copolymers A-B diblock copolymers and triblocks containing just two dif-ferent repeat units (A-B-A and B-A-B) can be characterized usingdifferent chromatographic techniques, as has already been shownin several publications [2,7-12,13]. The overall molar mass can be obtained by size exclusion chro-matography (SECft), which separates according to the hydrodynamicvolume.SEC can be performed with two different concentration Column Mobile phase polarity Hydrophilic (EO) Hydrophobic (PO,BO, HO) RP High LCCC LAC RP LOW SEC LCCC NP High LCCC SEC NP LOW LAC LCCC detectors (such as refractive index and density [14-16]), the sen-sitivity of which for the repeat units is sufficiently different. Usingjust one concentration detector may result in serious errors, if thecomposition of the copolymer changes along the molar mass dis-tribution (MMD). SEC with dual detection yields information on the (average)chemical composition along the MMD [16-20], it is, however,not capable of discriminating block copolymers and a mixture ofhomopolymers. A separation according to functionality or to the length of oneblock can be achieved by liquid chromatography under critical con-ditions (LCCC), which is performed in mixed mobile phases with aspecial composition and at a special temperature. At the so-calledcritical adsorption point (CAP) entropic and enthalpic effects com-pensate each other, and retention becomes independent on molarmass, which means, that the corresponding block becomes“chro-matographically invisible”,and a separation according to otherblocks or the end groups becomes possible. Depending on thechromatographic system, there are different options, as has beendiscussed previously [2,21] (See Table 1). On reversed phase columns, there may be critical conditions foithe hydrophilic block in a mobile phase with rather high polarity. Insuch a mobile phase the hydrophobic block will elute in LAC mode.On the other hand, critical conditions may also be found for thehydrophobic block in a rather non-polar mobile phase. In this case,the hydrophilic block will elute in SEC mode. On normal phase columns, a CAP for the hydrophilic block maybe found in a polar mobile phase, in which the hydrophobic blockwill elute in SEC mode. In a mobile phase with low polarity a CAPmay exist for the hydrophobic block: in this case, the hydrophilicblock will elute in LAC mode. For the purpose of this study, the situation with an excludedsecond block (at the CAP for the first one) is more favorable, as itworks almost for any length of the non-critical block. Such conditions have already been realized previously forEO-PO block copolymers [22,23].Obviously, at the CAP for PEO ona normal phase column higher polyalkylene oxides will also elutein SEC mode (as PPO). On the other hand, the more polar block (PEOor PPO) will elute in SEC mode on a reversed phase column at theCAP for a higher polyalkylene oxide. Hence the next step was to findcritical conditions for PBO and PHO on a reversed phase column. 3. Experimental 3.1. Polymerizations PO,BO,HO and n-alcohols(butanol,hexanol,dodecanol, octade-canol) were purchased from Fluka (Buchs, Switzerland). The lower alcohols (butanol and hexanol) were dried over anhy-drous sodium sulfate,while epoxides were refluxed over calciumhydride prior to use. Dodecanol and octadecanol were used as received. Polyethyleneglycol monomethyl ether (PEG-MME) with an average molar massof 350 and 1100(specification by the producer) was purchased fromFluka (Buchs, Switzerland). PEG-MME was dried by azeotropic dis-tillation with toluene. Sodium hydride (60% dispersion in mineraloil) from Aldrich was used as received. The polymerization of PO, BO and HO was performed by anionicring opening using the different initiators (different alcohols andPEG-MME 1100) and sodium hydride as co-initiator. The details ofthe synthesis will be discussed in another publication [24]. All polymerizations were performed in 2-5 ml (filling volume)mirowave process vials (Biotage AB), which were oven dried undervacuum prior to use. The initiator (alcohol or PEG-MME 1100) andsodium hydride were added to the vials which were subsequentlysealed with an aluminum crimp top and Teflon septum. The vialswere then evacuated and purged with argon (three cycles). The cal-culated volume of epoxide was added with a syringe through theseptum. The quantity of sodium hydride was 0.1% of total productand quantities of initiator (alcohol or PEG-MME) and epoxide in dif-ferent experiments depend upon the intended average number ofmonomer units in the chain (calculated by molar ratios). The sealed microwave process vials were subsequently intro-duced into the cavity of the single-mode microwave reactor(Biotage Initiator Eight EXP 2.0, absorbance level: high).The reac-tion temperatures ranged from 160 to 200°C. The total reaction timedepended on the monomer and the reaction temperature. Afterthe polymerization the active end was neutralized by adding anequimolar quantity of acetic acid. The time required for completeconversion was determined by measuring the monomer content,as will be described later on. The same procedure was followed for the synthesis ofamphiphilic multiblocks. After complete conversion of the firstmonomer the reaction vial was cooled down to approximately100°C and the next monomerwas added by a syringe through theTeflon septum without unsealing the vial, which was again intro-duced into the cavity of the microwave reactor. After polymerization of the last monomer the active end wasneutralized by adding an equimolar quantity of acetic acid. Detailsof the procedure will be given elsewhere [24]. Tetrablock copolymer with PEG-MME 350 as hydrophilic blockand PPO, PBO and PHO as second, third and fourth blocks was pre-pared by slow addition of monomer with conventional heating.8.0 gof PEG-MME 350and 0.08 gof Na metal was added in a reactionflask equipped with a reflux condenser and a dropping funnel. Afterazeotropic distillation with toluene, slow addition of PO (8.0g) wasstarted under reflux at 160°C. After complete conversion of PO (which took about 5h), slowaddition of BO (8.0g) (under reflux) took about 6 h at 160°C. Aftercomplete conversion of BO, 8.0g of HO was added and heatedovernight (12h) at 160°C. Finally, the active chain end was neu-tralized by an equimolar quantity of acetic acid. 3.2. Chromatography These investigations were performed using a density detec-tor (according to the mechanical oscillator principle) DDS70(Chromtech, Graz, Austria) in all chromatographic systems. Dataacquisition and processing was performed using the softwareCHROMA, which has been developed for the DDS70. In SEC, we used a modular system consisting of a Gynkotek300C pump, a VICI injector equipped with a 100 pl sample loop,two column selection valves Rheodyne 7060 (Rheodyne, Cotati, CA,USA), a density detection system DDS 70(Chromtech)coupled witha Bischoff 8110 refractive index detector (Bischoff, Leonberg, Ger-many). SEC measurements were performed on a 600 mmx7.8mmPhenogel 100A column (Phenomenex, Torrance, CA, USA) or a300mmx7.8 mm PLgel MIXED E column (Polymer Laboratories,Church Stretton, UK) in chloroform at a flow rate of 1.00 ml/min.Sample concentrations were 3.0-10.0g/l. Thecolumns were calibrated with PPGs from Sigma-Aldrich andFluka (Buchs, Switzerland). Molar mass distributions were calcu-lated using CHROMA. Fig. 1. SEC of poly(hexene oxides) initiated with n-hexanol (conventional heating),the monomer and the initiator. Detection: RI. Fig. 3. SEC of a diblock copolymer of hexene oxide and PEG-MME 1100 (monomer-to-initiator ratio: 7:3), the monomer and the initiator. Detection: RI. 4. Results and discussion In LCCC, the mobile phase was delivered by an ISCO 2350 HPLCpump (ISCO, Lincoln, NE, USA) at a flow rate of 0.5 ml/min. Sam-ples were injected using an autosampler Spark SPH 125 Fix (SparkHolland, Emmen, The Netherlands) equipped with a 20 ul loop. Arefractive index detector RI 2414 (Waters) or a SEDEX 45 evapo-rative light scattering detector (ELSD) system (Sedere, France) wasconnected to the DDS 70. Nitrogen was used as carrier gas, and thepressure at the nebulizer was set to 1.0 bar. Evaporator temperature?30°C. LCCC was performed on the following columns: The solvents - chloroform, tetrahydrofuran (THF), acetone andwater, all HPLC grade-were purchased from Roth (Karlsruhe, Ger-many) and Merck (Darmstadt, Germany). PEG and PPG standardswere purchased from Sigma-Aldrich and Fluka. Fig.2. SEC of poly(butene oxide) and poly(hexene oxide)initiated with n-dodecanol,the monomer and the initiator. Detection: RI. Block copolymers can be synthesized by living anionic polymer-ization starting with functional chains as initiator, the end groups ofwhich are activated by a catalyst. In this study, amphiphilic diblockcopolymers were obtained by polymerization of a higher epoxidewith PEG-MME as initiator. For comparison, homopolymers of BO and HO were synthesizedusing the corresponding alcohol(n-butanolorn-hexanol) and morehydrophobic alcohols (dodecanol and octadecanol). These can alsobe considered as AB diblocks, where the block A (the alkyl chain) ismonodisperse. The first question concerns the conversion of the monomer andinitiator, which is rather easy with PO: because of the low boilingpoint of PO, the internal pressure in the vial is an excellent measurefor the presence of residual monomer [22,23]. As the vapor pressure of the higher alkylene oxide is consid-erably lower, a drop of the internal pressure to zero does notnecessarily mean complete conversion. In the case of BO and HO the residual monomer can be deter-mirined by SEC, as can be seen in the following figures. Fig. 1 shows an overlay of chromatograms of three poly(hexeneoxide) samples (synthesized with hexanol as initiator under con-ventional heating), the initiator and the monomer. Even when thecorresponding alcohol (with the same number of carbon atoms asthe monomer) is used as initiator, it can be sufficiently separatedfrom the monomer. These chromatograms were obtained on thePhenogel 100 A column, on which the alcohols and the monomer coawo Fig. 4. LCCC ofblock copolymers of PEG-MME 1100 with PO, BO and HO,as obtainedon Nucleosil 100-5 OH in 77.93%, w/w acetone (CAP for EO). Detection: ELSD. Fig. 5. MMD and chemical composition of the sample shown in Fig.3, as obtainedby SEC with coupled density and RI detection. elute as quite unsymmetric peaks, which may be due to a mixedretention mode for these small molecules. In one of the samples shown here the conversion of themonomer was almost complete, while the other one still containssome monomer and initiator. Both peaks can be reasonably inte-grated. Fig. 2 shows chromatograms of a poly(butene oxide) (PBO)and a poly(hexene oxide) (PHO), the initiator (dodecanol), andthe monomers. Dodecanol elutes clearly later than the lowestoligomers of BO and HO, but earlier than the monomers. Evidently,both polymers shown here still contain some residual monomer,but no initiator. The peak eluting at 20 ml is a solvent peak result-ing from preferential solvation (the chloroform used contained 1%ethanol as a stabilizer). In the case of a polymeric initiator (PEG-MME) the situation iseven more favorable for the determination of residual monomer. Fig. 3 shows an overlay of the chromatograms obtained by SECwith a diblock copolymer of hexene oxide initiated with PEG-MME 1100 (monomer-to-initiator ratio: 7:3), the initiator and themonomer. The monomer (hexene oxide) elutes before the solvent peak (inthis chromatogram THF was used as internal standard for flow ratecorrection) and can thus be quantified very well. As can be seen,thissample still contains considerable amounts of the monomer. Thepeaks of the initiator (PEG-MME 1100) and the block copolymer,however, overlap in SEC. The information in the presence of residual initiator can be pro-'vided by LCCC, as has been shown previously [2]: at the CAP for Fig. 6. LCCC:molar mass vs. Ve, as obtained for poly(butene oxide) on the Jordi DVB500A RP column in THF-water, flow rate 0.5 ml/min, detection: ELSD. Fig.7. LCCC: molar mass vs. Ve, as obtained for poly(hexene oxide) on the Jordi DVB500ARP column in THF-water, flow rate 0.5 ml/min, detection: ELSD. block A (in this case EO) on a normal phase column (NucleoSil 100-5OH) in an acetone-water mobile phase (78%,w/wacetone) the ini-tiator (PEG-MME) elutes close to the void volume, while the blockcopolymer as well as the homopolymer of the hydrophobic unit(PO)elute in SEC mode. The same system works also with the higherepoxides. Under the :same conditions,,Lboth poly(butene oxide) andpoly(hexene oxide) elute in SEC mode and can thus be clearly sep-arated from PEG (and PEG-MME). As can be seen in Fig. 4, all threeblock copolymers (containing different poly(alkylene oxides) ashydrophobic block) do not contain residual initiator. The next question concerns the presence of the homopolymerof the second monomer (butene oxide or hexene oxide), which maybe formed by a chain transfer reaction [1,6,22,25,26]. The diblock copolymer of hexene oxide with PEG-MME 1100shown in Fig. 3(monomer-to-initiator ratio: 7:3), contains not onlyconsiderable amounts of the monomer: the copolymer peak has alow molecular end, which may be due to residual initiator or theside product poly(hexene oxide). As has already been discussed above, SEC with dual detectionallows the determination of the chemical composition along theMMD[16,19]. The result is shown in Fig. 5: the low molecularfraction contains indeed approximately 100% poly(hexene oxide).While the overall composition of the sample agrees with the the-oretical value (70%), the main fraction contains considerably lessthan the intended 70% hexene oxide, which is reasonable, as a con-siderable part of the monomer had not been incorporated in thecopolymer. Fig. 8. LCCC of poly(butene oxide)s at the CAP for BO, as obtained on the Jordi columnin 84.55%, w/w THF-water, flow rate 0.5 ml/min, detection: ELSD. Fig. 9. LCCC of poly(hexene oxide)s at the CAP for HO, as obtained on the Jordicolumn in 87.04%(w/w) THF-water, flow rate 0.5 ml/min, detection: ELSD. Fig. 11. Overlay of LAC chromatograms of poly(hexene oxide)s with different endgroups. Spherisorb S5XC18, 90.06 wt% acetone-water, RI detection. The average composition of the polymer peak means, however,that the fraction eluting in the corresponding interval contains acertain amount of the individual monomer units, but not, that itis really a copolymer. The result is only unambiguos, if a fractioncontains close to 100%of a monomer unit. The homopolymer of monomer B in an AB diblock copolymercan be determined at the CAP for B, under SEC conditions for A. Ifthe block B is more hydrophobic than A, this may be the case onreversed phase columns. As we have shown previously, critical conditions for PO couldbe realized on a Jordi Gel DVB 500 RP column in acetone-water(92.42%, w/w acetone) [2] or in THF-water (80.22%,\w/wTHF[22,23]. Under these conditions, PEG elutes in SEC mode.Moreover, PPGdiols (from chain transfer to water) and allyl-PPG(from chaintrans-fer to the monomer) could not only be separated from the blockcopolymer, but also from each other [22,23]. We have also foundcritical conditions for poly(butene oxide) and poly(hexene oxide)on the same column in a comparatively more non-polar mobilephase composition. As can be seen, a CAP for BO is observed onthe Jordi column in 84.55%, w/w THF (Fig..6), for HO on the samecolumn in 87.04%, w/w THF (Fig.7) Under these conditions,the block copolymer with PEG-MME canbe separated from the homopolymers. In such mobile phases, theindividual polymer homologous series oflonger epoxides (diols andalkenyl-terminated chains) cannot be separated from each other, asthese end groups are not sufficiently different. In LCCC of a block copolymer there can be two possible situa-tions: at the CAP for one block, the second block could be excluded Fig. 10. Overlay of LAC chromatograms of poly(butene oxide)s with different endgroups.Prodigy ODS3,90.06%, w/wacetone-water, RI detection. from the pores (PEG) or adsorbed more strongly on the surface ofthe stationary phase. If the second block elutes in the exclusionregime, the copolymer will elute earlier than the homopolymer, ifit is adsorbed, it will elute later. In the first case the second block must be large enough to beseparated from the homopolymer. This is the case for polymericinitiators. such as PEG-MME 1100. The second case is more favorable for functional chains contain-ing a (monodisperse) adsorbing end group, the interaction of thisgroup with the surface should be strong enough to separate thefunctional chains from the non-functional ones. In this study we used both kinds of block copolymers: with ablock in exclusion regime(PEG) and with a stronglyadsorbing block(the dodecyl or octadecyl group). In the case of poly(butene oxide), both situations are quitefavorable and we were able to separate the block copolymers(whether with PEG-MME 1100 or octadecanol) from the homopoly-mer (which may be formed by chain transfer to monomer) (seeFig.8). In the case of poly(hexene oxide), the polymer itself is highlynon-polar, hence a more adsorbing end group has not much aneffect, but appears only as a shoulder, if the sample contains a con-siderable amount of homopolymer. Of course the situation withan excluded second block (PEG-1100 monomethyl ether) is morefavorable in this case (Fig.9). There is, however, one more situation, which can be utilized inthe characterization of AB diblocks, where the block A is monodis-perse (such as an alkyl end group of a monofunctional chain): in thelow molecular range, the individual oligomers may be completely Fig. 12. SEC of block copolymers obtained by anionic polymerization of alkyleneoxides with conventional heating. Fig. 13. LCCC at the CAP for BO, as obtained on the Jordi column in 84.55%, w/wTHF-water, flow rate 0.5 ml/min, detection: ELSD. resolved by LAC (at a positive interaction parameter Cg of the repeatunit). If the end group is adsorbed more strongly than the repeatunit(the interaction parameter of A is larger than that ofB), asecondseries of peaks will appear in LAC. This could be achieved on the Prodigy ODS3 and the SpherisorbS5XC18 column in acetone-water mobile phases with about80-95%,w/w acetone. Fig. 10 shows such a LAC separation of poly(butene oxide)s withthe same average degree of polymerization (nav=4), but differentend groups, which was obtained on the Prodigy ODS3 column in90.04% acetone. With increasing hydrophobicity of the end groupretention (and selectivity) increases dramatically.The homopoly-mer (with butyl or butenyl end groups) elutes between 3 and 5 ml.In the polymers with a dodecyl or octadecyl end group it should beclearly visible.Evidently, the dodecyl PBO seem to contain tracesof the homopolymer. The nature of the shoulder in the higheroligomers of the octadecyl PBO is not yet clear. One may specu-late, that it could be due to different sequences (head to head orhead to tail). Fig.11 shows a LAC separation of poly(hexene oxide)s with dif-ferent alkyl end groups and approximately the same average degreeof polymerization (nay=4), on the Spherisorb S5XC18 column in90.04% acetone. While thecharacterization of A-B diblocks appears to be solvedby the techniques described above, the situation is much more dif-ficult with tri- and tetrablocks (A-B-C and A-B-C-D). Such polymers with PEG as hydrophilic block and hydrophobic,blocks with increasing hydrophobicity (from PO to BO and HO) mayhave highly interesting physical properties. These polymers may Fig. 14. LCCC at the CAP for HO, as obtained on the Jordi column in 87.04%, w/wTHF-water, flow rate 0.5 ml/min, detection: ELSD. be synthesized by anionic living polymerization with subsequentaddition of monomers. Obviously, at each step of the polymeriza-tion a high purity of the intermediate di- and triblock is inevitable. Fig. 12 shows an overlay of size exclusion chromatogramsobtained with PEG-MME 350, the corresponding diblock with PO,the triblock obtained therefrom by addition of BO, and the tetra-block with HO as the last block. These samples were obtained bysubsequent ring opening polymerization of PO (with PEG-MME asinitiator), BO and HO in the following amounts: PEG-MME 350: 8g,PO: 8g,BO: 8g, HO: 8 g. As one may expect, the chain transfer reac-tion to the monomer should result in rather short chains of thehomopolymers, they should appear as a low molecular shoulder(at higher elution volume), which is obviously not the case in thesesamples. Apparently, dual detection cannot be applied in an A-B-C tri-block, as the A-B diblock is polydisperse in both blocks (A and B),consequently,one could only determine an average response factor. There is, however, a much better option: if the A-B diblock issufficiently pure (which means, it does not contain residual ini-tiator A and homopolymer B), the only important side product inthe A-B-C triblock will be the homopolymer C. Under appropriatereaction conditions, complete conversion of the monomer and theinitiator(A-B)should be achieved. At the CAP for the block C, the homopolymers A and B as wellas the diblock A-B should elute in SEC mode, which would allow toseparate them from C. This is indeed the case, as can be seen in Fig. 13: at the CAP forBO, the first block (PEG-MME)and possible diblocks (PEG-MME-POand PEG-MME-BO) are well separated from the BO homopolymer.Ashoulder in the chromatograms of the diblocks indicates the pres-ence of some homopolymer. The same approach can also be applied to the tetra-block, as canbe seen in Fig. 14: at the CAP for HO, all other structures elute in SECmode and are thus clearly separated from the HO homopolymer. It must be mentioned, that the di- and triblocks shown herewere not the precursors for the tetrablock. We have just selectedbad samples, in which the homopolymer can be clearly seen. Underappropriate polymerization conditions, which are described in adifferent publication [24], the intermediates do not contain a mea-surable quantity of the homopolymers. 5.Conclusion Size exclusion chromatography with density and RI detectionalong with LCCC is an excellent tool for characterization ofepoxide-based block copolymers. In addition it provides information onthe homopolymer content in multiblock copolymers of epoxidesat each step of polymerization. Acknowledgements M.I.M. thank the Higher Education Commission of Pakistan for aPh.D. scholarship. We also thank Biotage AB (Uppsala, Sweden) forproviding the microwave instrumentation. References ( [ 1 ] F.E. Bailey, J. V . Kolesk e , Polymerization of 1,2-Epoxides , Marcel Dekker, NewYork, 1991. ) ( B . Trathnigg, Polymer 46 (2005) 9211. ) P. Alexandridis, T. Hatton Alan, Colloids Surf. A: Physicochem. Eng.Aspects 96(1995)1. ( [4] C. Perreur,J.-P. Habas, A. Lapp,J . Peyrelasse, Polymer 47 (2006)841. ) [5] R.P. Quirk, Q.Ge,Polymer Preprints,vol. 41, American Chemical Society,Divisionof Polymer Chemistry, 2000,p. 1221. ( [6]J . Allgaier, S. Willbold, T. Chang, Macromolecules 40 (2007)518. ) ( [7] A.A. Gorbunov, A.M. Skvortsov, V ysokomol Soedin Se r . A 3 0 ( 1 988)895. ) ( [8] A.M.S k vortsov, A.A. G orbunov, J. C h romatogr.507 (1990) 487. ) ( [9] A .V. Gorshkov, H. Much, H. Bec k er, H. Pasch, V.V. Evreinov,S.G. Entel i s,J. Chro- m atogr.523 (1990)91. ) ( [10] B.G. Belenkii, E.S . Gankina,V.N. Zgonnik, I.I. Malachova, E.U. Melenevskaya,J. C hromatogr. 609 (1992)355. ) ( [ 1 1]H . Pasch, I. Zammert,J. L iq.Chromatogr. 1 7 (1994) 3091. [12] H. Pasch , Macromol. Symp. 110(1996)107. ) ( [13] A.M. Skvortsov, A.A. Gorbunov, D. Berek, B. Trathnigg, Polymer 39 (1998) 423.[14] B . Trathnigg, C . Jorde, J . Chromatogr. 3 8 5 (1987) 17 . ) ( [15] B . Trathnigg, X. Yan,J. Chromatogr. A 653 (1993)19 9 . ) ( [16] B. Trathnigg, in: T . Provder (Ed . ), Chromatography of Polymers: Hyphenatedand Multidimensional Techniques, American Chemical Society, Washington, DC,USA, 1999,p. 1 . ) ( [17] B . Trathnigg,J. Liq.Chromatogr. 13 (1990)1731. ) ( [18] B. Trathnigg,J. Chromatogr. 552 ( 1 991)507. ) ( [ 1 9] B . Trathnigg, S . Feichtenhofer , M . Kollroser,J. Chromatogr. A 786 (1997)75. ) [20] B. Trathnigg, X. Yan,J. Appl. Polym. Sci.: Appl. Polym. Symp. 52 (1993)193. ( [21] B. Trathnigg,A.A. Gorbunov, Macromol. Symp.237(2006)18. ) ( [22] M.I. Malik, B. Trathnigg, C.O. Kappe, Macromol. C h em. Ph y s. 20 8 (2007)2510. ) ( [ 23] M .I. Malik, B . Trathnigg,C.O. Kappe, Eur. Polym. J .44(2008)14 4 . ) ( [24] M.I . Malik, B. . Trathnigg, C.O. Kappe, E ur. Polym. J. J, in press, d oi:10.101 6 /j.e u rpolymj.20 0 8.11 . 035. ) ( [25] D .M. Simons, J.J. Verbanc,J. Polym. Sci. 44 (1960) 303. ) ( [26] H . Becker,G. Wagner, A. Stolarzewicz, Acta P o lym. 33 (1982) 34. ) 士科技www.rysstech.com 士科技www.rysstech.com
确定



还剩5页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








