
方案详情
文
Previously it has been shown that glycerol can be regioselectively glucosylated by sucrose phosphorylase from Leuconostoc mesenteroides to form 2-O--d-glucopyranosyl glycerol.A series of compounds related to glycerol were investigated by us to determine the scope of the -glucosylation reaction of sucrose phosphorylase. Both sucrose
and glucose 1-phosphate (G1P) were applied as glucosyl donor. Mono-alcohols were not accepted as substrates but several 1,2-diols were readily glucosylated, proving that the vicinal diol unit is crucial for activity. The smallest substrate that was accepted for glucosylation appeared to be ethylene glycol,which was converted to the monoglucoside for 69%. Using high acceptor and donor concentrations (up to 2.5 M), sucrose or G1P hydrolysis (with H2O being the ‘acceptor’) can be minimised. In the study cited above, a preference for glucosylation of glycerol on the 2-position has been observed. For 1,2-propanediol however, the regiochemistry appeared to be dependent on the configuration of the substrate. The (R)-enantiomer was preferentialy glucosylated on its 1-position (ratio 2.5:1), whereas the 2-glucoside is the major product for (S)-1,2-propanediol (1:4.1). d.e.ps of 71–83% were observed with a preference for the (S)-enantiomer of the glucosides of 1,2-propanediol and 1,2-butanediol and the (R)-enantiomer of the glucoside of 3-methoxy-1,2-propanediol. This is the first example of stereoselective glucosylation of a non-natural substrate by sucrose phosphorylase. 3-Amino-1,2-propanediol, 3-chloro-1,2-propanediol, 1-thioglycerol and glyceraldehyde were not accepted as substrates.
方案详情

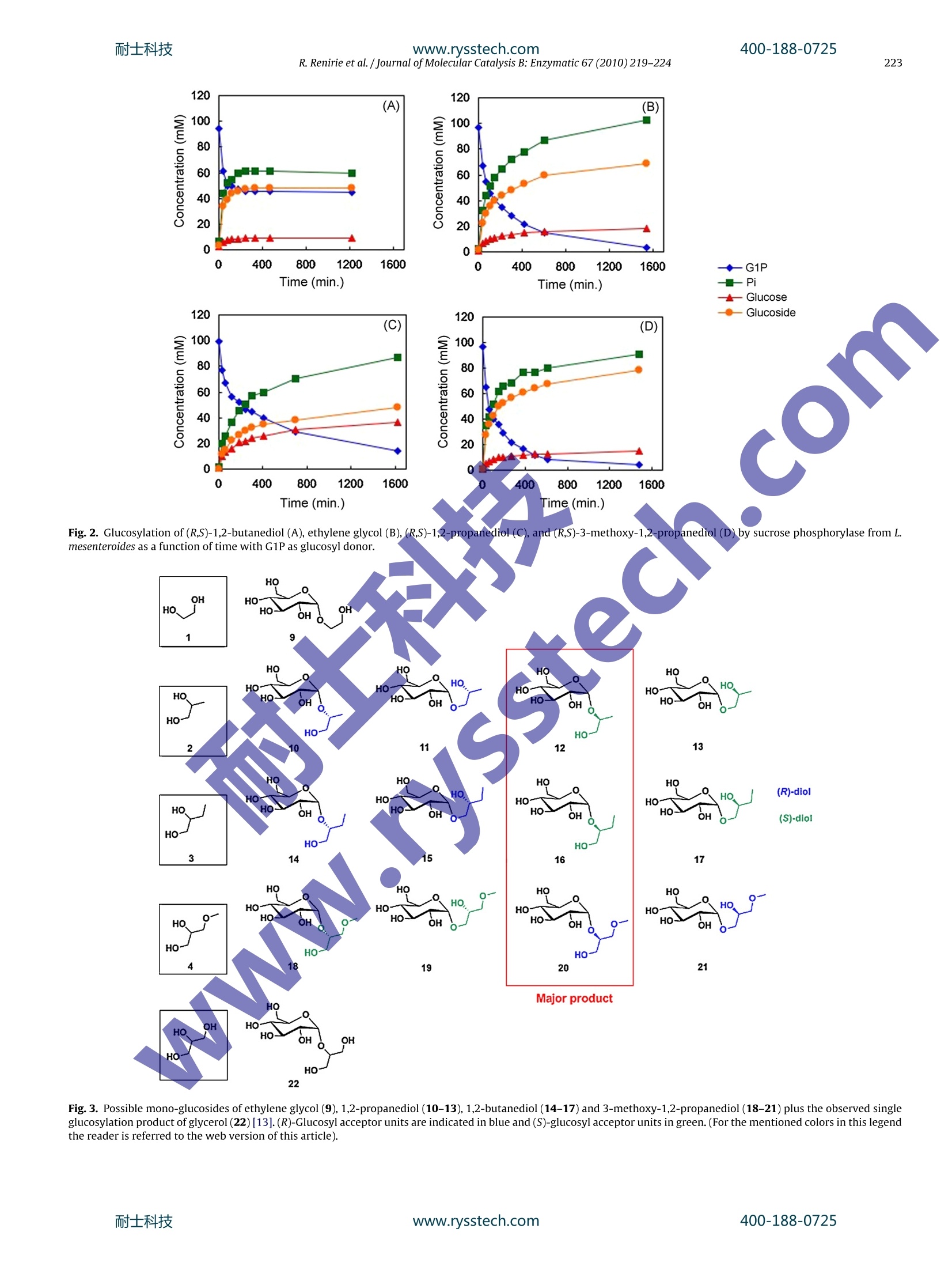
耐士科技400-188-0725www.rysstech.comJournal of Molecular Catalysis B: Enzymatic 67 (2010)219-224Contents lists available at ScienceDirect 耐士科技400-188-0725220R.Renirie et al. /Journal of Molecular Catalysis B: Enzymatic 67 (2010)219-224 Journal of Molecular Catalysis B: Enzymatic ELSEVIER journal homepage:www.elsevi er.com/locate/molcatb Regio- and stereoselective glucosylation of diols by sucrose phosphorylaseusing sucrose or glucose 1-phosphate as glucosyldonor Rokus Renirie, Aliaksei Pukin,Barend van Lagen, Maurice C.R. Franssen*Wageningen University, Lab of Organic Chemistry, Dreijenplein 8, 6703 HB Wageningen, The Netherlands ARTICLE INFO Article history: Received 27 April 2010Received in revised form 6 August 2010Accepted 12 August 2010 Available online 18 August 2010 Keywords:GlucosylationSucrose phosphorylaseRegioselectivityStereoselectivitySucroseGlucose 1-phosphate ABSTRACT Previously it has been shown that glycerol can be regioselectively glucosylated by sucrose phospho-rylase from Leuconostoc mesenteroides to form 2-0-a-D-glucopyranosyl-glycerol (Goedl et al., Angew.Chem. Int. Ed. 47 (2008)10086-10089). A series of compounds related to glycerol were investigatedby us to determine the scope of the a-glucosylation reaction of sucrose phosphorylase. Both sucroseand glucose 1-phosphate (G1P) were applied as glucosyl donor. Mono-alcohols were not accepted assubstrates but several 1,2-diols were readily glucosylated, proving that the vicinal diol unit is crucialfor activity. The smallest substrate that was accepted for glucosylation appeared to be ethylene glycol,which was converted to the monoglucoside for 69%. Using high acceptor and donor concentrations (upto 2.5 M), sucrose or G1P hydrolysis (with H2O being the'acceptor) can be minimised. In the study citedabove, a preference for glucosylation of glycerol on the 2-position has been observed. For 1,2-propanediolhowever, the regiochemistry appeared to be dependent on the configuration of the substrate. The (R)-enantiomer was preferentialy glucosylated on its 1-position (ratio 2.5:1), whereas the 2-glucoside is themajor product for (S)-1,2-propanediol(1:4.1). d.e.ps of 71-83% were observed with a preference for the(S)-enantiomer of the glucosides of 1,2-propanediol and 1,2-butanediol and the (R)-enantiomer of theglucoside of 3-methoxy-1,2-propanediol.This is the first example of stereoselective glucosylation of anon-natural substrate by sucrose phosphorylase.3-Amino-1,2-propanediol,3-chloro-1,2-propanediol,1-thioglycerol and glyceraldehyde were not accepted as substrates. Generally, the glucoside yield is higherwhen sucrose is used as a donor rather than G1P, due to the fact that the released phosphate is a strongerinhibitor of the enzyme (in case of G1P) than the released fructose (in case of sucrose). Essentially thesame results are obtained with sucrose phosphorylase from Bifidobacterium adolescentis. 1. Introduction Glucosylation of alcohols and phenols is an important reactionin nature and industry. Glucosides, oligo- and polysaccharides areused by nature e.g. for energy storage, for strengthening cells andtissues, and for molecular recognition. In pharmaceutical indus-try, glucosylation is used to increase the solubility of drugs. Lastbut not least, regio- and stereoselectively formed glucosides areused as intermediates for other (bio)chemicals. Enzymatic glucosy-lation naturally occurs mostly by tailor-made glucosyltransferases,with a different glucosyltransferase for each glucosyl acceptor [1].These transferases usually use UDP-glucose as glucosyl donor andtherefore the equilibria of the reactions catalysed are far on the glu-coside side. However, the expensive nature of this donor is one ofthe major bottlenecks in industrial application of these enzymes. ( A bbreviations: G 1P, Glucose a-1-phosphate; SUC, Sucrose; Fru, Fructose;a·P Glc, Glucose; P , Phosphate; d.e.p,Diastereomeric excess of the product. ) * Corresponding author. Tel.:+31 317 482976; fax: +31 317 484914. ( E-mail address: maurice.franssen@wur.nl (M.C.R. Franssen). ) Aclass ofenzymes that has been intensely studied in their abilityto catalyseglucosylation with glucose as the donor are glucosidases[1-4]. The equilibrium of the reaction catalysed by these enzymes ishowever on the side of hydrolysis of the glucoside,leading to oftenlow yields with these enzymes. The equilibrium can be influencedby the use of glucose derivatives with good leaving groups, like4-nitrophenol [4], but these substrates are rather expensive. Alter-native approaches involve media with minimal water content [5-9]or continuous removal of the product [10]and they sometimes givereasonable yields, but still they have inherent limitations. More recently, phosphorylases have been extensively studiedwith respect to glucosylation, one of these being sucrose phos-phorylase. The enzyme from Bifidobacterium adolescentis has beenshown to catalyse the regioselective glucosylation of several sug-ars such as L-arabinose [11]. The natural reaction catalysed by thisenzyme is the phosphorolysis of sucrose, with the concomitantformation of a-glucose 1-phosphate (G1P) and fructose: The reaction proceeds through a B-glucosyl intermediate,whichis formed upon reaction of the enzyme with sucrose, as shown by www.rysstech.com Fig. 1. Potential glucosyl acceptors similar to glycerol used in this study. This reaction occurs with net retention of the a-glucosidic bond.The equilibrium of this reaction is between the extreme situationsof the glucosyl transferases and glucosidases; the exact equilibriumdepends on the nature of the glucosyl acceptor. Both sucrose andG1P can be used to form the glucosyl-enzyme intermediate. In thelatter case phosphate is released rather than fructose: Recently, Nidetzky and co-workers [13] have shown that glyc-erol can be regioselectively converted with high yield usingsucrose phosphorylase from Leuconostoc mesenteroides. 2-0-(a-D-glucopyranosyl)-sn-glycerol, a natural osmolyte, is formed andis currently marketed under the trade name Glycoin@ [14]. Todetermine the scope and selectivity of sucrose phosphorylase, aseries of compounds related to glycerol were investigated by us(Fig.1), using the enzymes from L. mesenteroides and B. adolescentis.The formation of glucosylated ethylene glycol (1), 1,2-propanediol(2), 1,2-butanediol (3) and 3-methoxy-1,2-propanediol (4) isdescribed, using sucrose or G1P as the glucosyl donor. The gluco-sylation products of 1,2-propanediol, 3-methoxy-1,2-propanedioland 1,2-butanediol were isolated and analysed. The glucosylationreactions of 1,2-propanediol, 1,2-butanediol and 3-methoxy-1,2-propanediol show interesting regio- and stereoselectivity. 2. Experimental Sucrose phosphorylase from L. mesenteroides, sucrose, glucose1-phosphate, glycerol, ethylene glycol, (R,S)-1,2-propanediol,(R)-1,2-propanediol,(S)-1,2-propanediol, (R,S)-1,2-butanediol,(R,S)-3-methoxy-1,2-propanediol, 3-chloro-1,2-propanediol,1-thioglycerol, (R,S)-3-amino-1,2-propanediol, (R)-3-amino-1,2-propanediol, (S)-3-amino-1,2-propanediol,glyceraldehyde,magnesium chloride, MES buffer,disodium hydrogenphos-phate, hydrogen chloride were obtained from Sigma-Aldrich(Zwijndrecht, The Netherlands). Sucrose phosphorylase from B.adolescentis was produced and isolated as described before [11].Methanol (Biosolve, Valkenswaard, The Netherlands) and ethy-lacetate (Fischer Scientific, Loughborough, UK) were HPLC grade.NANOpure water (18.3M9cm) was used during all experiments(Barnstead, Thermo Scientific). Pre-packed flash purification silicacartridges (SNAP 10g KP-SIL) were obtained from Biotage. 2.2. Enzymatic glucosylation Typical enzymatic reactions (250 uL) were done using 100 mMG1P or 100 mM sucrose. A stock solution of G1P was broughtto pH 6.6 using HCl without adding additional buffer, whereasin the case of sucrose (100mM) MES buffer pH 6.6 (50mM)was added. Incubations at T=30°C further contained glucosyl donor (see below), 10mM MgCl2 and 25U/mL sucrose phospho-rylase. The highest practical glucosyl acceptor concentration wasused, namely: 2.5 M ethylene glycol, 2.5 M(R,S)-1,2-propanediol,2.5M(R)-1,2-propanediol, 2.5M (S)-1,2-propanediol, 2.5M 1,2-butanediol, 2.5M 3-methoxy-1,2-propanediol, 0.25M 3-chloro-1,2-propanediol,2.5M1-thioglycerol, 0.25M glyceraldehyde,1.7 M 3-amino-1,2-propanediol. The 3-amino-1,2-propanediolstock solution was brought to pH 7 with 37% HCl prior to use. Con-versions were followed in time by HPLC. The pH ofthe mixtures waschecked after completion. In the case of G1P a maximum increaseto pH 7.0 was observed. Two standard controls for all reactionswere performed: (i) same conditions without enzyme; (ii) sameconditions without glucosyl acceptor. For product isolation with acharcoal column, 1.0M sucrose and 1.0M1,2-butanediol were used(1 mL scale); this reaction was followed using HPLC. 2.3. HPLC analysis An Alltech OA-1000 column plus a wide pore eC 4C 4S SecurityGuard (Phenomenex, Utrecht, The Netherlands) were used with25mM H2SO4 as eluent at a flow rate of 0.4 mLmin1,Cou-pled to a refractive index detector (Gilson M 131, Gilson France,Villiers le Bel, France). Samples were diluted 10-fold in waterafter which 20 uL was injected via a fixed volume loop. Cal-ibration curves for phosphate, glucose, fructose, sucrose andglucosyl acceptors were linear in the concentration range mea-sured; the same holds for G1P after correction for the overlapwith the negative void peak. Concentrations of these com-pounds were calculated using these curves; for the glucosidesthe concentrations were calculated assuming that glucose produc-tion+glucoside production=glucoside-1-phosphate consumption(=phosphate production). 2.4. TLC analysis Prior to product isolation reactions were checked by TLC analy-sis to determine optimal eluent conditions, using various ratio'sof H20, methanol and ethyl acetate. Spots were colored with amolybdate-cerium based reagent (42gL-1 Mo7024(NH4)64H20,3.6gL-1Ce(NH4)(SO4)42H20,6.2%(v/v)H2SO4). 2.5. Product isolation 2.5.1. Silica column Glucoside producttss rom (R,S)-1,2-propanediol,(R)-1,2-propanediol,I, (S)-1,2-propanediol,:(R,S)-1,2-butanediol and(R,S)-3-methoxy-1,2-propanediol obtained with G1P as glu-cosyl donor were isolated using pre-packed silica cartridgesusing the Isolera One flash chromatography system from Bio-tage. This procedure led to the removal of most of the excessglucosyl acceptor, some remaining G1P, the formed phosphateand most of the formed glucose; glucoside regio- or stereoiso-mers could not be separated in this way. A mixture of ethylacetate:methanol:water=7:2:1 was used for elution. Fractionswere analysed by HPLC and pooled fractions were freeze dried andtaken up in D20 for NMR analysis. 2.5.2. Charcoal column Using equimolar concentrations of 1,2-butanediol and sucrose(1.0M) resulted in 87% sucrose consumption after 96h reactiontime. The product mixture consisted of 85%glucosides (correspond-ing to 750 mM) and 15% glucose (120mM). The glucoside productmix was isolated with a charcoal column based on the procedurethat Goedl et al. [13,14] used for the isolation of the glucoside ofglycerol. This method removed sucrose, formed fructose and glu-cose and remaining 1,2-butanediol, but could not separate product R. Renirie et al./Journal of Molecular Catalysis B: Enzymatic 67(2010)219-224 oooaa''号ooooscc5oooooooo .. 寸二o一卜Noa+++一oN……寸o9一No+M++寸N二1 11IN >co >oo >兰 日 >uoo18 888品斗。C09 寸仍Noa00oo'aaN''ccxoooooooE''''m >o二u>一'o regio-or stereoisomers. A pre-packed silica cartridge (SNAP 50gKP-SIL) was emptied and filled with a 1:1 mixture of activated char-coal Norit (type Norit SX Ultra, Sigma-Aldrich) and calcined Celite503 filter aid (Sigma-Aldrich). Elution was done in 4 steps: 250 mLH20,250 mL 2%EtOH, 140 mL 15%EtOH, 90 mL 25%EtOH. Fractionswere dried, taken up in water and analysed by HPLC as describedabove. On a 1 mL scale and using a charcoal column we obtainedan isolated yield of 124 mg glucoside mix (49%) with a purity>90%.The compounds eluted in the order: fructose (0-2%EtOH),glucose(2%EtOH), sucrose(15%EtOH), glucoside isomers (15-25% EtOH),1,2-butanediol (25%EtOH). 2.6. NMR analysis Pooled fractions from the silica column or charcoal column werecombined, freeze dried and taken up in 600 pL D20 for NMR anal-ysis.1H,13C(APT), COSY, HSQC and HMBC spectra were taken ona Bruker Avance III 400MHz NMR spectrometer.Typically about50 mg of product (purity 75-99%) was isolated for each conversionand analysed by NMR. For the purity analysis of the samples see theSupplementary file. 2.6.1.(R)-2-Hydroxy-1-propyl a-glucopyranoside (11, the majoregio-isomer that is formed) 11H1 NMR (400MHz, D20)8(ppm): 4.87(d,J=3.3Hz, 1H,H-1),4.00((1H, CH3CH(OH)CH20Glc), 3.80 (1H, H-6), 3.69 (1H, H-6),3.67(1H,H-3),3.65(1H,CH3CH(OH)CH2OGlc),3.62(1H,H-5), 3.49(1H,H-2),3.35(1H,H-4), 3.31 (1H,CH,CH(OH)CH2OGlc), 1.12(3H,CH3). 13C NMR(101MHz, D20) 8 (ppm): 98.6 (C-1), 73.1 (C-3),72.9 (CH3CH(OH)CH2OGlc),71.8(C-5), 71.5(C-2), 69.6 (C-4), 66.6(CH3CH(OH)CH2OGlc), 60.5 (C-6), 18.1 (CH3). 2.6.2. (R)-1-Hydroxy-2-propyl o-glucopyranoside (10, minorregio-isomer formed) H NMR (400MHz, D20) 8 (ppm): 5.02 (1H, H-1), 3.84 (1H,CH3CH(OGlc)CH2OH), 3.84-3.65 (2H, H-6, H-6'), 3.72 (1H, H-5) 3.65(1H, H-3), 3.59 (1H, CH3CH(OGlc)CH2OH), 3.51(1H,CHCH(OGlc)CH2OH), 3.47 (1H, H-2), 3.36 (1H, H-4), 1.17 (3H,CH3)13C NMR (101MHz, D20) 8 (ppm): 98.3 (C-1), 75.8(CH3CH(OGlc)CH2OH), 73.1(C-3),72.0(C-5),71.6(C-2),69.7(C-4),64.7 (CH3CH(OGlc)CH2OH), 60.6(C-6),16.9(CH3). 2.6.3. (S)-1-Hydroxy-2-propyl a-glucopyranoside (12, major regio-isomer formed) H NMR (400MHz, D20) 8 (ppm): 4.99 (1H, H-1), 3.84 (1H,CH3CH(OGlc)CH2OH), 3.79(1H,H-6), 3.74(1H,H-5), 3.70 (1H,H-6'),3.68(1H,H-3), 3.59 (2H, CH3CH(OGlc)CH2OH), 3.49 (1H,H-2),3.36 (1H, H-4), 1.08 (3H, CH3).13C NMR (101 MHz,D20)8 (ppm):95.6 (C-1), 73.2 (CH3CH(OGlc)CH2OH), 73.0 (C-3),71.8 (C-5), 71.3(C-2), 69.6(C-4),65.3(CH3CH(OGlc)CH2OH), 60.5(C-6),14.4(CH3). 2.6.4. (S)-2-Hydroxy-1-propyl a-glucopyranoside (13, minorregio-isomer formed) 'H NMR (400 MHz, D20) 8 (ppm): 4.86 (d,J=2.8Hz, 1H, H-1), 4.02 (1H, CH3CH(OH)CH2OGlc), 3.80-3.70 (2H,H-6, H-6'), 3.66(1H,H-3), 3.55(1H,CH;CH(OH)CH2OGlc),3.65(1H,H-5), 3.48 (1H,H-2), 3.34 (1H, H-4), 3.45(1H, CHCH(OH)CH2OGlc), 1.13 (3H,CH3). 13C NMR (101MHz, D20) 8 (ppm): 98.1 (C-1), 73.1 (C-3),72.4 (CH3CH(OH)CH2OGlc), 71.8 (C-5),71.5(C-2), 69.6(C-4), 66.2(CH3CH(OH)CH2OGlc), 60.6(C-6),18.1(CH3). 2.6.5. (S)-1-Hydroxy-2-butyla-glucopyranoside (16, majorregio-and stereoisomer formed) H NMR(400MHz, D20) 8 (ppm): 4.99 (1H, H-1), 3.77 (1H,H-6), 3.74 (1H, H-5), 3.68 (1H, H-6'), 3.67 (1H, H-3), 3.62 (2H,CH3CH2CH(OGlc)CH2OH),3.60(1H,CH3CH2CH(OGlc)CH2OH),3.48 (1H, H-2), 3.34 (1H, H-4), 1.55 (2H, CH3CH2CH(OGlc)CH2OH),0.86 (3H, CH3). 13C NMR (101 MHz, D20) 8 (ppm): 97.1 (C-1),79.5 (CH3CH2CH(OGlc)CH2OH), 73.0 (C-3), 71.7 (C-5), 71.6 (C-2),69.6(C-4), 63.2 (CH;CH2CH(OGlc)CH2OH), 60.6 (C-6), 22.4(CH3CH2CH(OGlc)CH2OH), 8.8 (CH3). 2.6.6. (R)-1-Hydroxy-3-methoxy-2-propyla-glucopyranoside(20, major regio- and stereoisomer formed) H NMR (400MHz, D20) 8 (ppm): 5.06(d,J=3.6Hz, 1H,H-1), 3.855(1H, CH3OCH2CH(OGlc)CH2OH),,33.78((1H, H-6),3.75 (1H, H-5), 3.69 (1H, H-6), 3.67 (1H, H-3), 3.66 (2H,CH3OCH2CH(OGlc)CH2OH), 3.58 (2H, CH3OCH2CH(OGlc)CH2OH),3.47 (1H,H-2), 3.36(1H, H-4), 3.33 (3H, CH3). 13C NMR (101 MHz,D20)8 (ppm): 97.6 (C-1), 76.4(CH3OCH2CH(OGlc)CH2OH), 72.9(C-3),72.1(C-5),71.6(C-2), 71.3 (CH3OCH2CH(OGlc)CH2OH), 69.6(C-4), 61.5 (CH3OCH2CH(OGlc)CH2OH), 60.6(C-6),58.5(CH3). 3. Results and discussion 3.1. Conversion of various alcohols with G1P as glucosyl donor First, sucrose phosphorylase was tested for its ability to glucosy-late mono-alcohols. Methanol and ethanol were not glucosylated.After this we tested various diols resembling glycerol. The enzy-matic glucosylation of (R,S)-1,2-butanediol with G1P as glucosyldonor was studied by HPLC. A clear formation of a glucoside prod-uct peak at tR=12.2 min is observed, which is absent in the controwithout enzyme (see Fig. S1, Supplementary material). In addition,phosphate release is observed, plus the formation of some glucose.As observed before for the ‘synthesis reaction'of phosphorylases[1], water competes as a ‘glycosyl acceptor', in this case formingglucose. Consequently, in the absence of (R,S)-1,2-butanediol, glu-cose formation is higher. While testing the mono-alcohols ethanoland methanol a similar amount of G1P hydrolysis was observed asin their absence, indication that the enzyme was not simply dena-tured or inhibited. At these time scales no glucose formation as aconsequence of non-catalytic hydrolysis of G1P is observed(see Fig.S1, Supplementary material). For (R,S)-1,2-butanediol, 55% of the G1P is consumed; an equi-librium is reached as can be seen in Fig. 2 (panel A), where theconversion in time is depicted. Fig. 2 also shows the conversionsof ethylene glycol,(R,S)-1,2-propanediol and(R,S)-3-methoxy-1,2-propanediol (Panels B-D). Table 1 summarises the amounts of glucosylateddprod-icts formed for all glycerol analogues that w re investi-gated. For (R,S)-1,2-propanediol, ethylene glycol andd(R,S)-3-methoxy-1,2-propanediol, >85% of G1Pisconsumed. Therate of product formation increases in the order (R,S)-1,2-propanediol→(R,S)-3-methoxy-1,2-propanediolethylene gly-col→(R,S)-1,2-butanediol, but differences are not large. With(R,S)-1,2-propanediol a relatively large amount of G1P hydrol-ysis is observed. For (R,S)-3-amino-1,2-propanediol (5), (R,S)-3-chloro-1,2-propanediol (6), (R.S)-1-thioglycerol (7) and (R,S)-glyceraldehyde (8) no glucoside product formation was observed.For (R,S)-1-thioglycerol a product was observed which wasalso formedin the:absence of enzyme, presumably dueto non-enzymatic disulfide formation. The presence of (R,S)-glyceraldehyde decreased glucose formation by 50%, indicatingbinding in the activesite without conversion. For 3-amino-1,2-propanediol both stereoisomers were also tested separately, bothshowing no conversion. During the conversions a decrease in rate of product formationin time is observed for all four products. To test if this is due toinhibition by released phosphate, the experiment with ethyleneglycol was repeated with an initial phosphate concentration of300 mM. The result was immediate inhibition for more than 95% (not shown). This could also be an explanation for the previouslyhigher observed yield of glucosylation of glycerol using sucrose asglucosyl donor compared to G1P [13]. We also tested glucosylation of all eight diols mentioned in thisstudy with sucrose phosphorylase from B. adolescentis [11,12], giv-ing essentially the same results during TLC and HPLC analysis. 3.2. Isolation and NMR analysis of product structure, regio- andstereoselectivity In Fig. 3 the possible products of all conversions in this studyare shown, plus product 22 that was found for the glucosylationof glycerol [13]. The product of ethylene glycol was not analysedby NMR; only one peak was observed during HPLC analysis so weassume it is the monoglucoside 9. For glucosylation of (R,S)-1,2-propanediol there is an overallpreference for the 2-position, as earlier observed for gluco-sylation of glycerol [13], but the ratio 2-glucoside:1-glucoside((10+12):(11+13)) is less marked (1.8:1). Interestingly, whenwe analysed glucosylation of the individual enantiomers weobserved the 1-regioisomer to be the major product for the (R)-1,2-propanediol (10:11=1:2.5), whereas the 2-regioisomer is themajor product for (S)-1,2-propanediol(12:13=4.1:1).Overall thismeans that the stereoselectivity R:S is 1:6 (d.e.p=71%), assumingthat in the racemate the preference of the enzyme for the 1-or2-pcosition of either of the enantiomers is the same as that for theindividual enantiomers. Also for glucosylation of ((R,S)-1,2-butanediolpland (R,S)-3-methoxy-1,2-propanediol there is anoverall preferencefor the 2-position. The ratio 2-glucoside (14+16):1-glucoside(15+17) is 5.6:1 for (R,S)-1,2-butanediol (3). Likewise, the ratio(18+20):(19+21) is 7.4:1 for (R,S)-3-methoxy-1,2-propanedio(4). Both ratios are larger than for (R,S)-1,2-propanediol (2). For(R,S)-1,2-butanediol (3)the stereoselectivity is 1:6.6(d.e.p=74%) inbenefit of one of the enantiomers. If the analogy with propanediolcan beapplied, the preference would be for the S-enantiomeras well. For (R,S)-3-methoxy-1,2-propanediol (4) the stereose-lectivity is even higher (1:11, d.e.p=83%). If the analogy with(R,S)-1,2-propanediol can be applied, the preference would befor the R-enantiomer (which has the same three-dimensionalstructure as (S)-1,2-propanediol). Very recently it was shown by Schwartz et al. [15] that alsothe B-glucoside of glycerol can be formed enzymatically using athermostable cellobiose phosphorylase from Pyrococcus furiosusand cellobiose as the glucosyl donor. It is conceivable that alsointeresting enantioselectivity will be observed with this enzymein combination with the compounds studied by us. 3.3. Conversion of 1,2-butanediol: G1P vs. sucrose as glucosyldonor Having identified four new substrates for sucrose phosphory-lase, we subsequently compared the use of(cheap) sucrose and G1Pas glucosyl donors for this enzyme. As expected, all glucosides werealso formed using sucrose as a donor. Fig.4 shows the glucosylationof 1,2-butanediol (3) as a function of time. A higher conversion of aglycon is obtained with sucrose as adonor; in contrast to G1P (see Fig.2) sucrose is almost completelyconsumed, which is most likely due to a more favorable equilib-rium. However, the disadvantage of using sucrose is the releaseof fructose. This byproduct is more cumbersome to remove com-pared to phosphate in the case of G1P, which is also reflected inthe HPLC chromatograms (see Fig. S2, Supplementary material).Thus for product identification via NMR we used isolated glucosidesmade with G1P as a donor. Obviously, for industrial applicationsucrose is much cheaper, but it might be conceivable that when HO 22 Fig. 3. Possible mono-glucosides of ethylene glycol (9), 1,2-propanediol (10-13),1,2-butanediol (14-17)and 3-methoxy-1,2-propanediol (18-21) plus the observed singleglucosylation product of glycerol (22)[13].(R)-Glucosyl acceptor units are indicated in blue and (S)-glucosyl acceptor units in green.(For the mentioned colors in this legendthe reader is referred to the web version of this article). Fig. 4. Glucosylation of 1,2-butanediol(3) by sucrose phosphorylase from L.mesen-teroides as a function of time with sucrose as glucosyl donor. G1P is generated enzymatically this route become cost-effective aswell.We have preliminary results that by starting from glycogenand using a cascade of glycogen phosphorylase and sucrose phos-phorylase it is possible to form the glucoside of ethylene glycol (notshown). With both glucosyl donors no side product formation apart fromglucose was observed; we anticipated some formation of mal-tose, as a consequence of a-glucosylation of the formed glucose;however, at the retention time of maltose (8.40 min) no peak wasobserved in any of our experiments. 4. Conclusions Four new compounds resembling glycerol were found to beglucosylated by sucrose phosphorylase from L.imesenteroidesand B. adolescentis, namely ethylene glycol, 1,2-propanediol, 1,2-butanediol and 3-methoxy-1,2-propanediol. Since mono-alcoholsare not converted, the minimal unit necessary for these compoundsto be accepted as substrates appears to be ethylene glycol. Both a-glucose 1-phosphate and sucrose can be used as the glucosyl donor.If one terminal hydroxyl group of glycerol is substituted, regiose-lective a-glucosylation on the 2-position (overall) is observed forthe four compounds mentioned above, whereas for other com-pounds no glucosylation is observed. We show that glucosylationof the individual enantiomers of 1,2-propanediol results in a dif-ferent regioselectivity for each enantiomer; the 1-regioisomer(2-hydroxy-1-propyl a-glucopyranoside,11) is the major productfor the reaction of(R)-1,2-propanediol, whereas the 2-regioisomer(1-hydroxy-2-propyl a-glucopyranoside, 14) is the major productfor (S)-1,2-propanediol. For the compounds tested also enantiose-lectivity is observed with ee’s up to 83%. This is the first time thatthis stereoselectivity is observed for sucrose phosphorylase. It is conceivable that this type of stereoselectivity will also be found forother phosphorylases converting glycerol-like compounds. Note added in proof The Nidetzki group has also very recently published on thesucrose phosphorylase mediated glucosylation of 1,2-propanediol,see [16]. Acknowledgements This research was performed as part of the Program Integra-tion of Biosynthesis & Organic Synthesis (IBOS) of ACTS-NWO (TheNetherlands). Prof. Dr. Ron Wever is kindly thanked for the use of HPLC equipment. Mr. Louis Hartog, Dr. Teunie van Herk, Dr. Teris van Beekand Mr. Elbert van der Klift are acknowledged for their help duringHPLC measurements.Dr.Ben van den Broek and Dr. Gerrit Beldmanare kindly thanked for their generous gift of sucrose phosphorylasefrom B. adolescentis. Appendix A. Supplementary data Supplementary data associated with this article can be found, inthe online version, at doi:10.1016/j.molcatb.2010.08.009. References [1] C.A.G.M. Weijers, M.C.R. Franssen, G.M. Visser, Biotechnol. Adv. 26 (2008)436-456. [2] A. Millqvist-Fureby, I.S. Gill, E.NV.ulfson, Biotechnol. Bioeng. 60 (1998)190-196. [3] S. Takayama,G.J.McGarvey,C.H. Wong, Chem. Soc.Rev. 26(1997)407-415. [4] F. van Rantwijk, M. Woudenberg-vanOosterom, R.A. Sheldon, J.Mol. Catal.B:Enzyme 6(1999)511-532. Z. Chahid,D.Montet, M. Pina, J. Graille, Biotechnol. Lett. 14 (1992) 281-284. 5B.M. de Roode, S.W.P.G. Peters, M.C.R. Franssen, A. van der Padt, R.M. Boom,Biotechnol. Bioeng.72 (2001)568-572. [7]M.Gelo-Pujic, E. Guibe-Jampel,A. Loupy,A. Trincone,J. Chem. Soc.Perkin Trans.17(1997))1001-1002. [8]]G(. Ljunggeer,r, P. Adlercreutz, B. Mattiasson, Enzyme Microb. Technol. 16 (1994)751-7: [9] G. Vic, D. Thomas, D.H.G. Crout, Enzyme Microb. Technol. 20(1997)597-603. [10]B.M. de Roode, M.C.R. Franssen, A. Van der Padt, R.M. Boom, Biotechnol. Prog.19 (2003)1391-1402. [11] L.A.M. van den Broek, E.L. van Boxtel, R.P. Kievit, R. Verhoef, G. Beldman, A.J.G.Voragen, Appl.Microbiol. Biotechnol. 65(2004)219-227. [122]] O.Mirza,L.Skov,D.Sprogoe, L.A.M. Van den Broek, G. Beldman,J.S.Kastrup, M.Gajhede,J. Biol. Chem. 281 (2006)35576-35584. [13] C. Goedl, T. Sawangwan, M. Mueller, A. Schwartz, B. Nidetzky, Angew. Chem.Int. Ed. 47(2008)10086-10089. [14]C(. Goedl, T. Sawangwan, B. Nidetzky, M. Mueller, Patent WO 2008/034158(2008). [15] A. Schwartz, M.S. Thomson, B. Nidetzky, Biotechnol. Bioeng. 103 (2009)865-872. [16]C. Luley-Goedl,T. Sawangwan,L. Brecker, P. Wildberger, B. Nidetzki, Carbohyd.Res.345(2010)1736-1740. 士科技www.rysstech.com 耐士科技www.rysstech.com
确定





还剩4页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








