
方案详情
文
The discovery of potent benzimidazole stearoyl-CoA desaturase (SCD1) inhibitors by ligand-based virtual screening is described. ROCS 3D-searching gave a favorable chemical motif that was subsequently optimized to arrive at a chemical series of potent and promising SCD1 inhibitors. In particular, compound SAR224 was selected for further pharmacological profiling based on favorable in vitro data. After oral administration to male ZDF rats, this compound significantly decreased the serum fatty acid desaturation
index, thus providing conclusive evidence for SCD1 inhibition in vivo by SAR224.
方案详情

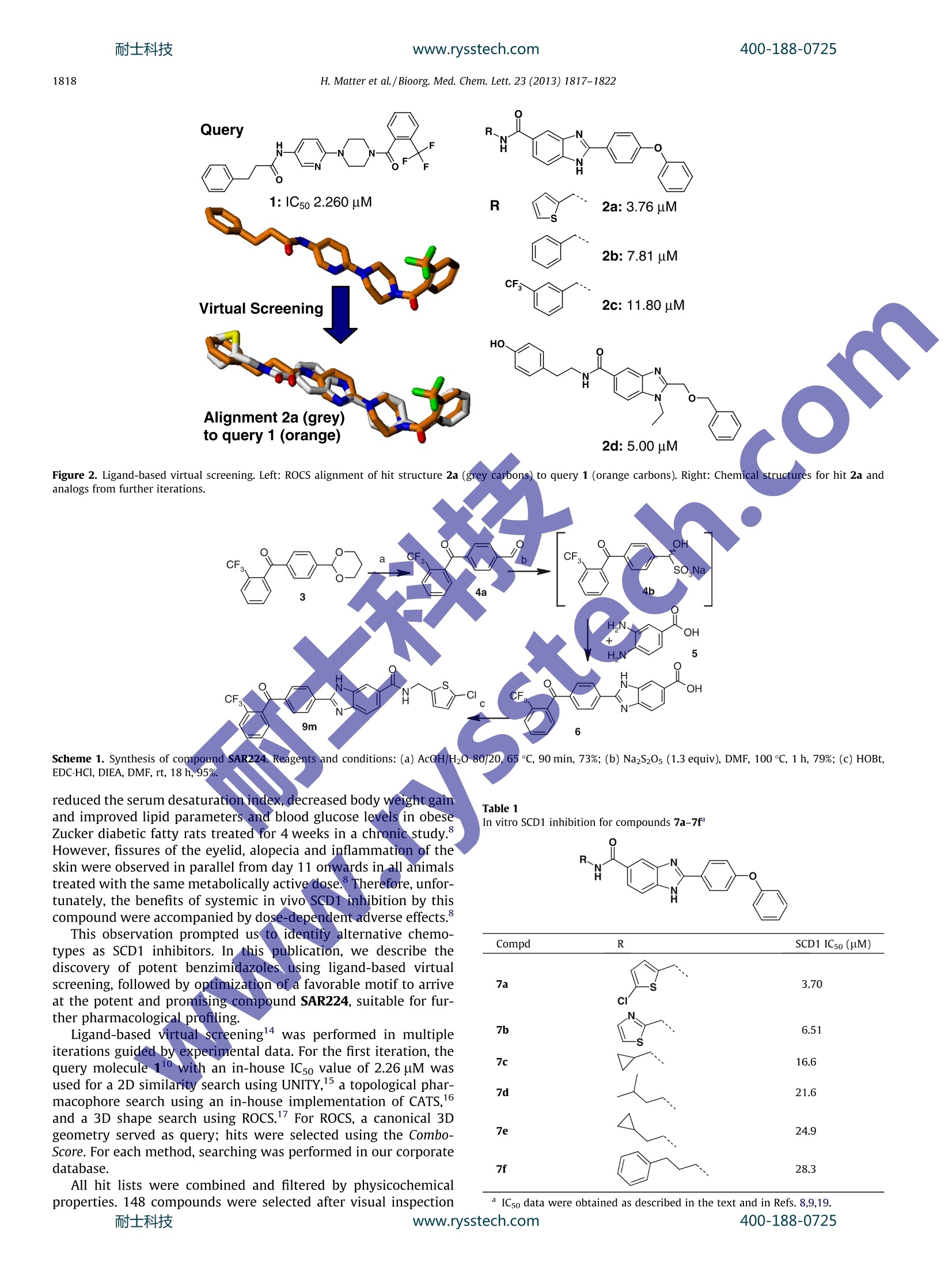
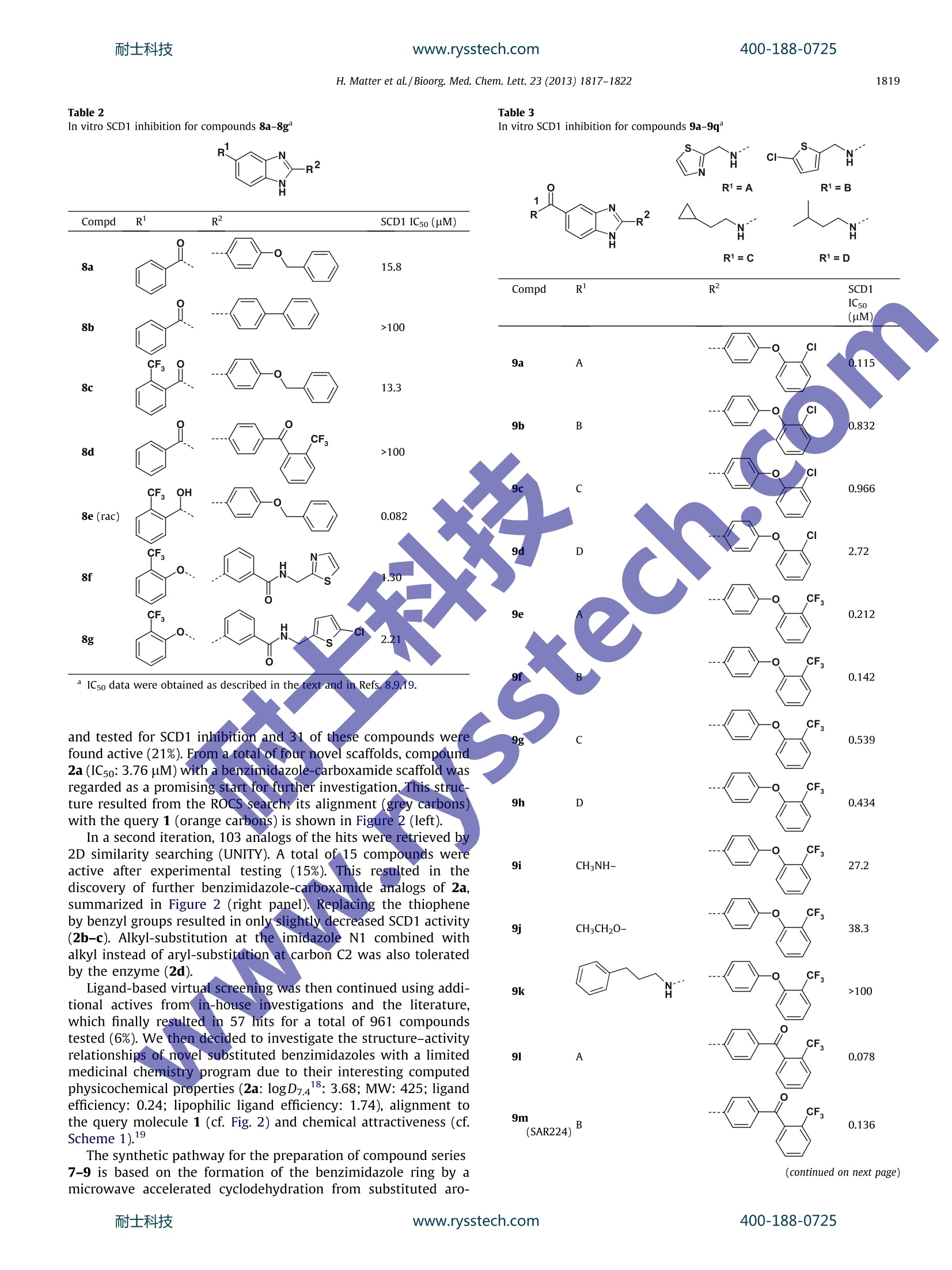
www.rysstech.comBioorganic & Medicinal Chemistry Letters 23 (2013) 1817-1822耐士科技400-188-0725 400-188-0725耐士科技www.rysstech.comH. Matter et al./Bioorg. Med. Chem. Lett. 23 (2013) 1817-18221818 Bioorganic & Medicinal Chemistry Letters ELSEVIER journal homepage:www.elsevier.com/locate/bmcl Benzimidazole-carboxamides as potent and bioavailablestearoyl-CoA desaturase (SCD1) inhibitors from ligand-based virtualscreening and chemical optimization Hans Matter *, Gerhard Zoller, Andreas W. Herling,Juan-Antonio Sanchez-Ariasb,tChristophe Philippo, Claudie Namane, Markus Kohlmann, Anja Pfenninger, Marc D. Voss Sanofi-Aventis Deutschland GmbH, Research and Development,D-65926 Frankfurt am Main, Germany "Sanofi-Aventis, Research and Development, Avenida de Leganes, 62, 28925 Alcorcon, Madrid, SpainSanofi-Aventis, Research and Development, 1 Avenue Pierre Brossolette, F-91385 Chilly-Mazarin, France O ARTICL E INFO ABSTRAC Article history: Received 19 November 2012 Revised 7 January 2013 Accepted 9 January 2013 Available online 22 January 2013 Keywords: The increasing prevalence of obesity and associated metabolicdisorders such as type 2 diabetes in societies following a Westernlifestyle highlights the necessity to discover new pharmaceuticalsolutions for this area. Recently, elevated activity of stearoyl-CoA desaturase (SCD1), one of the essential enzymes in lipogenesis,has been linked to the pathogenesis of obesity,metabolic disorders,dyslipidemia and type 2 diabetes.34 SCD1 is an iron-containingmicrosomal enzyme, which catalyzes the formation of a cis-doublebond at the carbon-9 position of saturated fatty acyl-Coenzyme-Aesters, the rate-limiting step in the synthesis of mono-unsaturated16:1 n-7 and 18:1 n-9 fatty acyl-CoAs.’ SCD1 expression is regu-lated by different nutritional and pharmacological stimuli..SCD1-deficient mice were reported to exhibit reduced body weight,body fat mass, increased oxygen consumption and improvedinsulin sensitivity in a glucose tolerance test. Therefore substantialefforts in the pharmaceutical industry have been undertaken todiscover small-molecule SCD1 inhibitors for the treatment ofmetabolic disorders. * Corresponding author. Tel.: +49 69305 84329. E-mail address: Hans.Matter@sanofi.com (H. Matter). ( f Current address: Small Molecule Discovery Platform, Center for Applied MedicalResearch, University Of Navarra, P amplona, Spain. ) In a previous publication we reported the discovery andpharmacological profiling of SAR707 as a promising member of aclass of potent hexahydro-pyrrolopyrrole based SCD1 inhibitors(Fig. 1).8.9 Our starting point was patent applications from XenonPharmaceuticals1 and Merck Frosst Canada, describing chemicalseries reported to modulate SCD1 activity, thereby regulatingplasma-lipid levels. Further structure-activity relationship datafor SCD1 inhibitors was also available in reports from AbbottLaboratories.12,13 Rescaffolding the central piperidine substructure resulted in thediscovery of the hexahydro-pyrrolopyrrole scaffold, which subse-quently was optimized to result in SAR707 with an IC5o value of0.00848 uM in a rat liver microsome SCD1 assay. In particular,thiscompound is characterized by high potency,selectivity and favor-able properties in enzymatic and cellular assays. In vivo, SAR707 Figure 1. Chemical structure of SAR707. Figure 2. Ligand-based virtual screening. Left: ROCS alignment of hit structure 2a (grey carbons) to query 1 (orange carbons). Right: Chemical structures for hit 2a andanalogs from further iterations. Scheme 1. Synthesis of compound SAR224. Reagents and conditions: (a) AcOH/H20 80/20,(, 65C, 90 min, 73%; (b) Na2S20s (1.3 equiv), DMF, 100℃, 1 h, 79%;(c) HOBt,EDC·HCl, DIEA, DMF, rt, 18 h, 95% reduced the serum desaturation index, decreased body weight gainand improved lipid parameters and blood glucose levels in obeseZucker diabetic fatty rats treated for 4 weeks in a chronic study.However, fissures of the eyelid, alopecia and inflammation of theskin were observed in parallel from day 11 onwards in all animalstreated with the same metabolically active dose. Therefore, unfor-tunately, the benefits of systemic in vivo SCD1 inhibition by thiscompound were accompanied by dose-dependent adverse effects.° This observation prompted us to identify alternative chemo-types as SCD1 inhibitors. In this publication, we describe thediscovery of potent benzimidazoles using ligand-based virtualscreening, followed by optimization of a favorable motif to arriveat the potent and promising compound SAR224, suitable for fur-ther pharmacological profiling. Ligand-based virtual screening14 was performed in multipleiterations guided by experimental data. For the first iteration, thequery molecule 11 with an in-house IC5o value of 2.26 uM wasused for a 2D similarity search using UNITY,15 a topological phar-macophore search using an in-house implementation of CATS,and a 3D shape search using ROCS.’ For ROCS, a canonical 3Dgeometry served as query; hits were selected using the Combo-Score. For each method, searching was performed in our corporatedatabase. All hit lists were combined and filtered by physicochemicalproperties. 148 compounds were selected after visual inspection Table 1In vitro SCD1 inhibition for compounds 7a-7f SCD1 IC50 (uM) Compd 7a S 3.70 7b 6.51 7c 16.6 7d 21.6 7e 24.9 7f 28.3 SCD1 IC5o (uM) and tested for SCD1 inhibition and 31 of these compounds werefound active (21%). From a total of four novel scaffolds, compound2a (IC5o: 3.76 uM) with a benzimidazole-carboxamide scaffold wasregarded as a promising start for further investigation, This struc-ture resulted from the ROCS search; its alignment (grey carbons)with the query 1 (orange carbons) is shown in Figure 2 (left). In a second iteration, 103 analogs of the hits were retrieved by2D similarity searching (UNITY). A total of 15 compounds wereactive after experimental testing (15%). This resulted in thediscovery of further benzimidazole-carboxamide analogs of 2a,summarized in Figure 2 (right panel). Replacing the thiopheneby benzyl groups resulted in only slightly decreased SCD1 activity(2b-c). Alkyl-substitution at the imidazole N1 combined withalkyl instead of aryl-substitution at carbon C2 was also toleratedby the enzyme (2d). Ligand-based virtual screening was then continued using addi-tional actives from in-house investigations and the literature,which finally resulted in 57 hits for a total of 961 compoundstested (6%). We then decided to investigate the structure-activityrelationships of novel substituted benzimidazoles with a limitedmedicinal chemistry program due to their interesting computedphysicochemical properties (2a: logD7.418: 3.68; MW: 425; ligandefficiency: 0.24; lipophilic ligand efficiency: 1.74), alignment tothe query molecule 1 (cf. Fig. 2) and chemical attractiveness (cf.Scheme 1).19 The synthetic pathway for the preparation of compound series7-9 is based on the formation of the benzimidazole ring by amicrowave accelerated cyclodehydration from substituted aro- (continued on next page) Compd SCD1 IC5o data were obtained as described in the text and in Refs. 8,9,19. Next, we studied the influence of the carboxamide linker onSCD1 activity with a small set of compounds,which are summa-rized in Table 2. Replacing the carboxamide by a keto-linker in-cluded in a benzophenone moiety does not improve activity,asshown for derivatives 8a-8d. Adding a CF3-group at the ortho-position does not significantly improve activity, as can be seenby comparing compounds 8a and 8c with IC5o values of 15.8 versus13.3 uM, respectively. However, replacing the keto-linker with a hydroxyl-group as in8e (racemic mixture) results in a significant improvement of activ-ity (ICso: 0.082 uM). Due to the chirality of this motif and its highermetabolic lability, this motif was not followed further. Finally, weintroduced an ortho-trifluoromethyl-phenoxy-substituent on thisposition of the benzimidazole. The rational of derivatives 8f and8g was to check if a reversed superposition compared to thealignment in Figure 2 of the benzimidazole (grey) with thevirtual screening query 1 (orange) could be possible and whether 导88 88 891111 三 8858111导 o山aao> xN8M111g coNaaoa 2N 11O MN OO5c 6o寸品品品品E品 a CF3-group would improve binding affinity. While derivatives 8fand 8g exhibit a slightly increased activity with 1.30 and2.21 uM compared to the original hit, this option was not exploredfurther with a larger range of R'substituents, since alternative sub-stituents combined with the carboxamide linkers lead to signifi-cantly improved activity (cf. Table 3). Table 3 summarizes our efforts to investigate the ortho-positionon the distal phenoxy-moiety of the original hit 2a. The ROCS-de-rived alignment of 2a with the virtual screening query 1 suggeststhat inserting the lipophilic substituent of the query 1 at this posi-tion in our series should be tolerated by the enzyme. We exploredthis option combined with different carboxamide substituents (R'in Table 3). Introducing an ortho-chlorine substituent on the distalring with a thiazolyl-substituent attached to the carboxamide(R=A) results in potent derivatives, for example, 9a (ICso:0.115 pM). The nature of the amine substituent of R significantly influ-ences SCD1 activity. Replacing the thiazole-ring by a chloro-thio-phene (Rl=B) results in a lower activity (9b: 0.832 uM), whilealiphatic substituents do not improve activity, as demonstratedby 9c and 9d with ICso values of 0.966 and 2.72 uM, respectively. Interestingly this ranking of R'-substituents is not respected ifthe ortho-chlorine of substituent Ris replaced by an ortho-trifluo-romethyl group. Here the most active compound is obtained withthe chloro-thiophene moiety (R=B), 9f (ICso: 0.142 uM) ratherthan with the thiazolyl-moiety, 9e (IC5o: 0.212 uM). Combining ali-phatic R-substituents with ortho-CFs-substituted biarylethersdoes not improve activity, as seen by compounds 9g and 9h withICso values of 0.539 and 0.434 uM, respectively. Replacing the R-carboxamide substituent by a methyl-group significantly affectsactivity (9i: 27.2 pM), and even more so with a phenylpropyl-substituent (9k: >100 uM). A similar decrease inaactivity isobserved for the corresponding ethyl-ester substitution at positionR(9j: 38.3 uM). Finally we replaced the ortho-substituted biphenylether bysubstituted benzophenone-derivatives (91-9q). Our intention wasto explore the effect of altered hydrogen-bond accepting propertieson SCD1 inhibition by comparing the carbonyl oxygen to the aro-matic ether-oxygen which cannot play the role of H-bond acceptor.Once again the most significant influence on activity is observedwith a thiazole-substituentt(RA; 91: 0.078uM) and chloro-thiophene (R=B; 9m: 0.136 uM), while aliphatic substituents atR once more result in decreased SCD1 inhibition (9n: 3.86uM).These favorable motifs in 9l and 9m were further explored bychanging the distance between the aromatic thiazole ring andthe carboxamide linker. Removing the methylene-linker fromR=A reduces activity, ascan be seen by comparing 91 (IC5o:0.078pM) and 9o (IC5o:4.52 uM. Removal of the chlorine substituent in 9m has only aslightly favorable effect on SCD1 inhibition, leading to 9p with anIC50 value of 0.099 uM. Our final investigation in this series wasto reverse the order of the carboxamide linker, resulting in themuch less active compound 9q (3.20 pM). This systematic SAR investigation had lead to several moleculesthat were interesting as potent SCD1 inhibitors, and which werethen profiled in further assays. A summary of additional in vitrodata, giving cellular SCD1 activities in rat H4IIE and human HepG2liver cell lines, effect on ACC phosphorylation in human HepG2cells (at 10 uM concentration with in-cell Western immunolabel-ing), ADME and physicochemical properties is provided in Table4. In general, the entire chemical series is characterized by highCaco-2 permeability and acceptable to moderate metabolic labilityrates (e.g., column MLab Human reporting the percentage ofa com-pound metabolized). Addition of the CYP3A4/5 inhibitor ketocona-zole to the metabolic lability assay reveals an important influenceof CYP3A4/5 on the observed metabolic degradation of the com- pounds (column MLab Keto in Table 4). In contrast CYP2D6 appar-ently is much less likely to be involved in this metabolizationprocess, as the addition of the CYP2D6 inhibitor quinidine (columnMLab Quin in Table 4) does not significantly alter the experimen-tally observed metabolization rates. From the inspection of Table4, compound 9m exhibited interesting in vitro ADMET and physi-cochemical properties with moderate human metabolic labilityand still acceptable logD7.4. This compound is the most potentmember of this series in HepG2 and H4IIE cell-based SCD1 assayswith ICso values of 0.157 and 0.182 uM (see Table 4), respectively.It was also potent with respect to ACC phosphorylation. Therefore,this compound was selected for further investigation and renamedSAR224. An in-depth pharmacokinetic and pharmacological character-ization of SAR224 was performed. All experimental procedureswere conducted in accordance to the German Animal ProtectionLaw and international animal welfare legislation and rules and per-formed as previously described.8,9,19 After intravenous administra-tion of 5 mg/kg SAR224 in solution to male ZDF rats, a low meanplasma clearance (0.25 L/h/kg), a large volume of distribution(Vss=1.7 L/h/kg) and a long half-life (6.5 h) were observed. Aftera single oral dose of 30 mg/kg SAR224 in suspension to male ZDFrats, the mean plasma concentration profile showed a slowincrease from 0.25 to 6 h after administration and a mean Cmax va-lue of 1950 ng/mL after 4 h. SAR224 showed a long mean apparentplasma half-life (8.4 h) and a relative bioavailability of 25% (Fig. 3,Upper). To evaluate the in vivo pharmacological activity of SAR224,the compound was administered once orally at 30 mg/kg to8 week-old male ZDF rats, a well-known animal model of obesityand diabetes. After 6 h the compound significantly decreased theserum fatty acid desaturation index (-84.4±15.9%) compared to Figure 3. Upper: Pharmacokinetic parameters of SAR224 following an intravenousbolus administration of 5 mg/kg SAR224 and following a single oral dose of 30 mg/kg SAR224 to male obese ZDF rats. Results are means ±SD (n=3). Lower: Decreasedserum fatty acid desaturation indices (-84.4±15.9%) by treatment of male obeseZDF rats with 30 mg/kg SAR224. Results are means ±SEM (n=8) and *p<0.05versus the vehicle-treated, obese controls. vehicle in the obese animals (Fig. 3,lower), thus providing evidencefor SCD1 inhibition in vivo by SAR224. In conclusion, we have described the identification, design andstructure-activity relationships of a novel series of potent SCD1inhibitors based on a benzimidazole scaffold. This series was devel-oped following promising results from a ligand-based virtualscreen. Major parts of the scaffold were then appropriately substi-tuted to arrive at compounds having high affinity as SCD1 inhibi-tors and favorable physicochemical and ADMET properties. Finally SAR224 which showed the most promising in vitroproperties was characterized by in vivo PK and pharmacologystudies, which revealed strong evidence for in vivo SCD1 inhibitionby this molecule with suitable oral bioavailability and half-life.Hence SAR224 constitutes a promising starting point for furtherinvestigation of SCD1 inhibitors as potential treatments of diabetesand related diseases. Acknowledgments We would like to thank Petra Selle and Sascha Rauch for theirexcellent technical assistance. References and notes ( 1 . S myth, S . ; H eron, A. Nat. Med. 2 006, 1 2,75. ) ( 2. D obrzyn, P . ; D obrzyn, A. Expert Opin. T her. Pat. 2012,20, 8 49. ) ( 3 . E noch,H. G.; Catala,A.; Strittmatter, P. J. Biol. C hem. 1976,251,5095. ) ( 4. M 1 iller, C. W.; N t ambi, J. M. Pr o c. Natl. Acad. Sci. U . S.A. 1996, 93,9443. ) 7.NNtambi, J. M.; Miyazaki, M.; Stoehr, J. P.; Lan, H.; Kendziorski,C. I,M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J. M.; Attie, A. D. Proc. Natl. Acad. Sci. U.S.A.2002,17,11482. .8.Voss, M. D.; Zoller, G.; Matter,H.; Herling, A. W.; Biemer-Daub, G.; Pfenninger,A.; Haag-Diergarten, S.; Keil, S.; Kohlmann, M.; Schmidts, H.-L. Eur. J.Pharmacol., Submitted for publication. 9.Zoller, G.; Voss, M. D.; Keil, S.; Herling, A.; Matter, H. PCT Int. Appl. 2008,WO2008135141. ( 10. . (a)Abreo, M.; Chafeev, M.; Chakka, N . ; Chowdhury, S.; Fu, J.; Gschwend, H.W.; S un, S.; Sun, S.; Sviridov, S. ; Tu, C.; Winther, M. D.; Zhang, Z. PCT Int. Appl. 2005, WO2005011655.; (b) Fu, J.; Kodumuru, V.; Sun, S.; Winther, M.; Fine, R. M.; Harvey, D. F.; K lebansky,B.; Gray-Keller, M. P.; Gschwend, H. W.; Li, W. U.S. Pat. Appl. 2005, US20050119251. ) ( 11. L i, c. s. ; Ramtohul, Y. K.; Huang, Z. ; Lachance, N . In t . Pat. Appl 200 6 , WO 2006130986. ) ( 12. L iu, G.;L y nch, J. K . ; Freeman, J; Li u , B.; X i n,Z.; Zhao, H.; Serby, M. D .; Kym , P. R .; Suhar,T . S.; Smith, H. T.; C a o,N. ; Yang, R.; Janis, R . S.; K rauser,J. A.; C epa, P .; Beno, D. W . A . ; Sham, H. ; Co l lins, C. A.; S u rowy, T. K . ; Ca m p, H. S . J. M ed. Chem. 2007, 50 , 3086. ) ( 13. X in, Z.; Zhao, H.; S erby, M. D.; Liu, B .; Liu, M.; Szczepankiewicz, B. G . ; Nelso n , L. T .J . ; Smith, H. T.; S uhar, T. S . ; Janis, R . S.; Cao, N.; Camp, H . S.; C olli n s, C. A.;Sham, H. L.; Surowy, T. K.; L i u, G. Bioorg. M ed. Chem. Le t t. 2008, 18 , 42 9 8 . ) ( 14. O ( prea, T.; M atter, H. Curr. Opin. Chem. Biol. 2004, 8,34 9 . ) 15.【UNITY, implemented in Sybyl 8.2; Tripos International: St. Louis, MO, USA.http://www.tripos.com. ( 16. I nternal i mplementation: Ma t ter, H.; Giegerich, C. 2 0 08 fo H owing: Sch n eider, G . ; Neidhart, W.; Giller, T.Schmidt. G. Angew. Chem . 1999,1 1 1,3 0 68. ) ROCS version 3.1.1. OpenEye Scientific Software: Santa Fe, NM, USA. http://www.eyesopen.com. ACD/Labs version 12.0. ACD/Labs Inc., Toronto, CAN. http://www.acdlabs.com.Zoller, G.; Voss, M. D.; Matter, H.; Herling, A., Philippo,C.; Namane, C.;Sanchez-Arias, J.-A. PCT Int. Appl.2009, WO2009024287. 0..Ridley, H. F.; Spickett, R. G. W.; Timmis, G. M.J. Heterocycl. Chem. 1965,2, 453.Microwave assisted benzimidazole synthesis reactions were carried out insealed tubes in an Initiator 8 microwave oven (Biotage). /$ - see front matter @ Elsevier Ltd. All rights reserved.http://dx.doi.org/j.bmcl.ww.rysstech.com耐士科技 士科技www.rysstech.com
确定




还剩4页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








