
方案详情
文
A series of Arg-Phe-NH2 peptidomimetics containing an Arg mimetic were synthesized and tested as agonists of human MrgX1, rat MrgC, and mouse MrgC11 receptors. As predicted from the previously established species specificity, these peptidomimetics were found to be devoid of MrgX1 agonist activity. In contrast, these compounds acted as agonists of MrgC and/or MrgC11 with varying degrees of potency. These new peptidomimetics should complement the existing small molecule human MrgX1 agonists and enhance our ability to assess the therapeutic utility of targeting Mrg receptors in rodent models.
方案详情

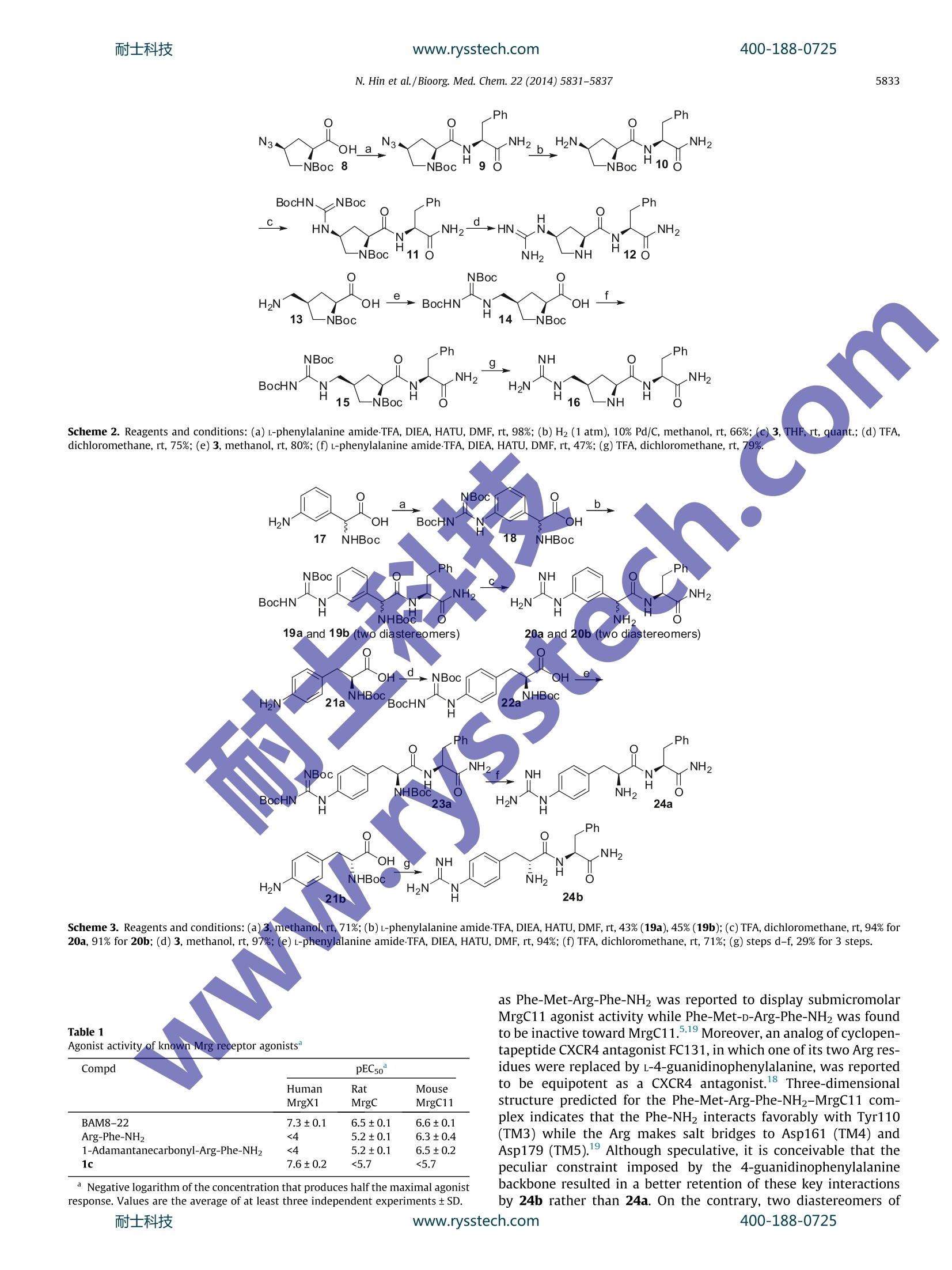
www.rysstech.comBioorganic & Medicinal Chemistry 22 (2014) 5831-5837耐士科技400-188-0725 耐士科技400-188-0725www.rysstech.comN. Hin et al./Bioorg. Med. Chem. 22 (2014) 5831-58375832 Bioorganic & Medicinal Chemistry ELSEVIER journal homepage: www.elsevier.com/locate/bmc Peptidomimetics of Arg-Phe-NH2 as small molecule agonistsof Mas-related gene C (MrgC) receptors CrossMark Niyada Hin, Jesse Alt, Sarah C. Zimmermann', Greg Delahanty, Dana V. Ferraris, Camilo RojasFengxian Li, Qin Liu, Xinzhong Dong, Barbara S. Slushera, Takashi Tsukamotoa,b,* Brain Science Institute, Johns Hopkins University,Baltimore, MD 21205,United States Department of Neurology, Johns Hopkins University, Baltimore, MD 21205, United States Center for the Study of Itch and Departments of Anesthesiology and Anatomy & Neurobiology, Washington University School of Medicine, St Louis, MO 63110, United States“The Solomon H. Snyder Department of Neuroscience, Johns Hopkins University, Baltimore, MD 21205, United States ARTICLE INFO ABSTRACT Article history: A series of Arg-Phe-NH2 peptidomimetics containing an Arg mimetic were synthesized and tested as ago- Received 27 July 2014 nists of human MrgX1, rat MrgC, and mouse MrgC11 receptors. As predicted from the previously estab- Revised 5 September 2014 lished species specificity, these peptidomimetics were found to be devoid of MrgX1 agonist activity. In Accepted 11 September 2014contrast, these compounds acted as agonists of MrgC and/or MrgC11 with varying degrees of potency. Available online 19 September 2014These new peptidomimetics should complement the existing small molecule human MrgX1 agonists and enhance our ability to assess the therapeutic utility of targeting Mrg receptors in rodent models. Keywords: ◎ 2014 Elsevier Ltd. All rights reserved. Mas-related gene (Mrg) receptors Agonist Peptidomimetic Arginine mimetic Arginine mimetic Mas-related gene (Mrg) receptors, also known as sensory-neu-ron specific receptors(SNSRs),, represent a family of orphanGPCRs. A subset of these receptors are expressed specifically inthe sensory neurons of the dorsal root ganglion (DRG), implicatinga role in modulating nociceptive signaling. Among them, humanSNSR4 (also known as MrgX1) has gained increased interest as atherapeutic target partly owing to the known peptide-based ago-nists as well as evidence for its role in nociception and pruritus. Of the rodent Mrg receptors, mouse MrgC115 and rat MrgCreceptors are believed to be the closest orthologs of human MrgX1because of their similar pattern of tissue expression in the DRG,substantial sequence homology,and existence of common pep-tide-based ligands including BAM8-22 (Fig.1). BAM8-22 is a pro-teolytic product (15-mer peptide) of proenkephalin A and is devoidof affinity for opioid receptors.Because of its high potency for Mrgreceptors and selectivity over opioid receptors, BAM8-22 hasserved as a valuable pharmacological tool for studying the physio-logical role of Mrg receptors. For example, intrathecal injection ofBAM8-22 was found to attenuate both mechanical and thermalhyperalgesia in miceand rats. Skin administration of BAM8-22 * Corresponding author. Tel.: +1 410 614 0982. ( E-mail address: ttsuk a moto@jhmi.edu (T.Tsukamoto). ) was also reported to induce itch in mice and humans’ in a hista-mine-independent manner. Val-Gly-Arg-Pro-Glu-Trp-Trp-Met-Asp-Tyr-GIn-Lys-Arg-Tyr-GlyBAM8-22 Figure 1. Representative Mrg receptor agonists. While BAM8-22 acts as a potent agonist for all three Mrg recep-tors, its truncated peptides appear to lose affinity to human MrgX1while retaining activity at the rodent forms, mouse MrgC11 and ratMrgC. In a systematic in vitro study using HEK293 cells expressinghuman MrgX1, BAM8-22 exhibited agonist activity with an EC5ovalue of 14 nM while neither N-truncated (BAM15-22) nor C-trun-cated (BAM8-18) analogs showed activity. In contrast, mouseMrgC11 was found to be activated by substantially smaller pep-tides containing a C-terminal-Arg-Phe(Tyr)-Gly or -Arg-Phe(Tyr)-NH2 motif.’ Even dipeptide Arg-Phe and its amide derivativeArg-Phe-NH2 exhibited submicromolar ECso values for activatingMrgC11. Although only limited number of peptides were testedin rat MrgC assay, several Arg-Phe containing peptides substan-tially shorter than BAM8-22 displayed potency comparable to thatof BAM8-22.10 This raises the possibility of cross-species variation in potencyfor small molecule-based agonists particularly between humanand rodent forms of Mrg receptors. Indeed, potent non-peptidehuman MrgX1 agonists 1a-c(Fig. 1) discovered by GSK were foundto have no detectable agonist activity against rat MrgC,thus hin-dering their utility in rat preclinical models. A non-peptidergicagonist 2 reported by ACADIA1 is selective to MrgX1 over MrgX2receptors in both humans and rhesus monkeys though its activityin rodent MrgC receptors is unknown. These findings have prompted us to explore the possibilityV ofidentifying a small molecule agonist with activity in rodent MrgCdSreceptors. It is evident from the prior work that the key residues inthese peptides are either Arg-Phe or Arg-Tyr,which can serve as acore template for the design of peptidomimetics with potent agonistactivity for Mrgreceptors. The presence of an arginine residue can beof particular advantage given the fact that a variety of argininemimetics have been explored in efforts to design peptidomimeticsderived from biologically active natural peptides containing an argi-nine residue.3In particular, development of fibrinogen receptorantagonists based on Arg-Gly-Asp motifrepresents one of the mostrelevant cases in which arginine mimetics were effectively uti-lized.14,15 Herein we report synthesis of short Arg-Phe-NH2 deriva-tives in which the arginine residue is substituted by an argininemimetic. The resulting short peptide derivatives were tested inMrg receptors of human, rat, and mouse for their agonist activityto examine the cross-species reactivity as well as their potentialutility as pharmacological tools in rodent studies. 2. Results and discussion 2.1. Chemistrv Preparation of Arg-Phe-NH2 analog 7containing 1-guanyl-4-piperidineglycine as an arginine mimetic is illustrated in Scheme 1.N-Boc-4-piperidineglycine 4 was reacted with N,N'-di-Boc-1H-pyr-azole-1-carboxamidine 3 to form tihee protectedd1-guanyl-4- Scheme 1. Reagents and conditions: (a) methanol, rt; (b) L-phenylalanineamideTFA, DIEA, HATU, DMF,rt, 5% from 4; (c) TFA, dichloromethane, rt, 94%. piperidineglycine 5. Coupling with L-phenylalanine amide pro-vided 6, which was subsequently deprotected with TFA to givethe desired product 7. Synthesis of proline-containing derivatives 12 and 16 is shownin Scheme 2.N-Boc-4-azido-L-proline 816 was coupled with l-phen-ylalanine amide to obtain 9. Subsequent catalytic hydrogenationfollowed by reaction with N,N'-di-Boc-1H-pyrazole-1-carboxami-dine 3 and deprotection with TFA afforded Arg-Phe-NH2 analog12 containing guanidino-L-proline as arginine mimic. Reaction of(4R)-N-Boc-4-(aminomethyl)-L-proline 13 with 3 provided fullyBoc-protected derivative 14. Coupling with L-phenylalanine amidefollowed by deprotection with TFA gave Arg-Phe-NH2 analog 16containing 4-guanidinomethyl-L-proline as an arginine mimic. Preparation of Arg-Phe-NH2 analogs 20 and 24 consisting ofarginine mimics with a benzene ring backbone is illustrated inScheme 3. N-Boc-DL-3-amino-phenylglycine 171’ was reacted with3 to form 18, which was subsequently coupled to L-phenylalanineamide to give 19. Chromatographic purification of the crude mate-rial resulted in the separation of the two diastereomers 19a and19b, which were deprotected with TFA to afford 20a and 20b (ste-reochemistry not assigned), respectively. A similar approach wastaken for the synthesis of 24a except that enantiomerically pureN-Boc-4-amino-phenylalanine 21a (L-form) and 21b(D-form) wereused as starting materials to obtain 24a and 24b, respectively. 2.2. Biological evaluation In vitro experiments measuring agonist activityagainst Mrgreceptors were performed in a FLIPR assay using HEK293 cells sta-bly transfected with human MrgX1. For rat MrgC and mouseMrgC11, transientlytransfected HEK293 or KNRK cells were used.As shown in Table 1, BAM8-22 exhibited agonist activity againstMrg receptors of all three species in our assays. Arg-Phe-NH2 andadamantanecarbonyl-Arg-Phe-NH2 were only active in rat MrgCand mouse MrgC11.In contrast, non-peptide agonist 1c selectivelyactivated human MrgX1. Compound 1c had no measurable agonistactivity at concentrations up to 2 uM (the highest concentrationtested due to the limited solubility of 1c). Our in vitro assay resultsclearly differentiate ligand specificity of human MrgX1 from rat andmouse Mrg receptors. These findings are in a good agreement withthe cross-species variation known for Mrg receptors. Agonist activity of Arg-Phe-NH2 derivatives containing Argmimetics is summarized in Table 2. None of the peptidomimeticsdisplayed agonist activity in human MrgX1. This is not surprisinggiven th1ee n1egligible agonist activity of Arg-Phe-NH2 at humanMrgX1. Several Arg-Phe-NH2 peptidomimetics (compounds 12, 16,20a, and 20b) failed to show agonist activity at rat MrgC while allof them showed full agonist activity (relative to BAM8-22) at mouseMrgC11 with varying degrees of potency. For instance, despite thelack of activity toward MrgC, compound 16 was the most potentMrgC11 agonist with submicromolar potency (pEC50=6.3). Thesefindings highlight the significant species difference in ligand speci-ficity that exists not only between human and rodents but alsobetween rats and mice. Compounds 7, 24a, and 24b exhibited agonist activity at bothMrgC and MrgC11. It is worth noting that 1-guanyl-4-piperidine-glycine incorporated in 7 was also successfully used as an Argmimetic in HLA-DRRbinding peptidomimetic ligandss withimproved plasma stability and slightly increased binding affinity.Compound 24a containing L-4-guanidinophenylalanine as an Argmimetic showed weak yet appreciable agonist activity in both spe-cies. To our surprise, its diastereomer 24b derived from D-4-guani-dinophenylalanine was nearly 100- and 10-fold more potent than24a at rat MrgC and mouse MrgC11, respectively. In fact, com-pound 24b represents the most potent rat MrgC agonist amongthe tested Arg-Phe-NH2 peptidomimetics. This was unexpected 15 -NBoc 16 —NH o Scheme 2. Reagents and conditions:(a) L-phenylalanine amide-TFA, DIEA, HATU,DMF,rt, 98%;(b) H2 (1 atm), 10% Pd/C, methanol,rt, 66%; (c) 3, THF, rt, quant.;(d) TFA,dichloromethane, rt, 75%; (e) 3, methanol,rt, 80%; (f) l-phenylalanine amide-TFA, DIEA, HATU, DMF, rt, 47%;(g)TFA,dichloromethane, rt, 79%. Scheme 3. Reagents and conditions: (a)3, methanol, rt, 71%; (b)L-phenylalanine amide TFA, DIEA, HATU, DMF, rt, 43%(19a),45%(19b); (c) TFA, dichloromethane, rt, 94% for20a, 91% for 20b;(d) 3, methanol, rt, 97%; (e) L-phenylalanine amide.TFA,DIEA, HATU, DMF, rt, 94%;(f) TFA, dichloromethane, rt, 71%; (g) steps d-f, 29% for 3 steps. Table 1Agonist activity of known Mrg receptor agonists" Compd pEC5o° Human Rat Mouse MrgX1 MrgC MrgC11 BAM8-22 7.3±0.1 6.5±0.1 6.6±0.1 Arg-Phe-NH2 4 5.2±0.1 6.3±0.4 1-Adamantanecarbonyl-Arg-Phe-NH2 5.2±0.1 6.5±0.2 1c 7.6±0.2 <5.7 <5.7 Negative logarithm of the concentration that produces half the maximal agonistresponse. Values are the average of at least three independent experiments ±SD. as Phe-Met-Arg-Phe-NH2 was reported to display submicromolarMrgC11 agonist activity while Phe-Met-D-Arg-Phe-NH2 was foundto be inactive toward MrgC11.5.19 Moreover, an analog of cyclopen-tapeptide CXCR4 antagonist FC131, in which one of its two Arg res-idues were replaced by L-4-guanidinophenylalanine, was reportedto be equipotent as a CXCR4 antagonist. Three-dimensionalstructure predicted for the Phe-Met-Arg-Phe-NH2-MrgC11 com-plex indicates that the Phe-NH, interacts favorably with Tyr110(TM3) while the Arg makes salt bridges to Asp161 (TM4) andAsp179 (TM5).1 Although speculative, it is conceivable that thepeculiar constraint imposed by the 4-guanidinophenylalaninebackbone resulted in a better retention of these key interactionsby 24b rather than 24a. On the contrary, two diastereomers of 400-188-0725 Table 2Agonist activity of Arg-Phe-NH2 derivatives containing arginine mimetics" Human Rat Mouse MrgX1 MrgC MrgC11 NH H2N 7 <4 5.4±0.2 6.1±0.2 NH2 12 HN. NH2 -NH <4 <4 5.1±0.2 NH 16 H2N <4 <4 一NH NH 6.3±0.1 20a 4.5±0.1 and H2N 4.5±0.1 20b NH, 24a NH NH2 <4 4.3±0.3 4.8±0.3 H2N H 24b NH NH, <4 5 6.2±0.3 5.9±0.1 H2N H Negative logarithm of the concentration that produces half the maximal agonistresponse. Values are the average of at least three independent experiments ±SD. 20 (20a and 20b) containing 4-guanidinophenylglycine exhibitedlittle difference in potency. Neither of the analogs showed agonistactivity in MrgC, and both of them were equally weak agonists(pEC50=4.5) of MrgC11. here is a possibility that Arg-Phe-NH2 binds to the BAM8-22binding site of human MrgX1 receptor without activating intracel1-lular signaling and rather acts as an antagonist. To this end, weassessed antagonist activity of Arg-Phe-NH, and 24b in humanMrgX1 using BAM8-22 (200 nM) as an agonist and known MrgX1antagonists 252 and 262 as positive controls (Fig.2). In our assay,both 25 and 26 exhibited potent antagonist activity with ICso val-ues of 800 and 50 nM, respectively. Neither Arg-Phe-NH2 nor 24b,however, showed any antagonist activity at concentrations up to200 uM. Thus, it appears that Arg-Phe-NHz motif alone is not suf-ficient to enable the binding to human MrgX1 as opposed to rodentMrg receptors. Figure 2. Known MrgX1 receptor antagonists. Mrg receptors have gained increasing interest in connectionwith the development of novel therapeutic agents for pain andpruritus. While BAM8-22 has served as a valuable pharmacologicaltool in preclinical studies, development of smaller moleculesshould further facilitate understanding of the therapeutic utilityof targeting Mrg receptors. The new peptidomimetics describedin this paper have a preference for rodent Mrg receptors andshould complement the existing human MrgX1 agonists such ascompound 1c. Indeed, it is encouraging that compound 24b(termed JHU-58) attenuated neuropathic pain in both rats andmice but not in KO mice lacking a cluster of Mrg genes includingMrgC11.Collectively, these small molecule-based agonists shouldenhance our ability to assess the therapeutic utility of targetingMrg receptors in rodent models. 4. Experimental 4.1. Chemistry NMR spectra were recorded on a Bruker 400 instrument. Chem-ical shifts are reported in parts per million relative to TMS. Pre-parativeHPLC was performed using aJasco HPLC systemequipped with a Jasco U-987 pump and a Jasco RI-2031 refractiveindex detector.The instrument was fitted with a Phenomenex Luna10 um Silica 100 A (250×21.2 mm). An isocratic flow (10 mL/min)of 100%EtOAc was used unless otherwise specified. HPLC analyseswere performed on a JASCO HPLC system fitted with a Phenome-nex-Luna 5 um C18 (250×4.6 mm) using a gradient of solventsA (0.1% TFA in waterand B (0.1% TFA in acetonitrile) at 1.0 mL/minflow rate. The gradient was 15% to 80% B over 40 min (detection at210 nm). Elemental analyses were performed by Atlantic Microl-abs, Norcross, GA. BAM8-22 was purchased from Tocris Bioscience(Bristol, UK). Arg-Phe-NH2 and 1-adamantanecarbonyl-Arg-Phe-NH2 were purchased from Bachem Biosciences (King of Prussia,PA). Compounds 1c,25,2 and 262 were prepared according tothe previously reported procedures. 4.1.1.2-(1-(N,N'-Bis(tert-butoxycarbonyl)carbamimidoyl)piperi-din-4-yl)-2-(tert-butoxycarbonylamino)acetic acid (5) A milky mixture of 2-(tert-butoxycarbonylamino)-2-(piperidin-4-yl)acetic acid 4 (0.5g, 1.94 mmol, 1 equiv) and tert-butyl(1H-pyrazol-1-yl)methanediylidenedicarbamate 3 (0.72 g, 2.32 mmol,1.2 equiv) in methanol (12 mL) was stirred at rt over 2 days. Asthe reaction was not complete, excess methanol was added untilthe solution became colorless. Stirring continued for another dayand the solvent was removed by rotary evaporation.The white res-idue was triturated in EtOAc and the resulting semi-solid 5 wasused in the next step without further purification. 1H NMR(CD30D)8 1.46 (s,27H), 1.4-2.07 (m, 5H), 2.95 (m, 2H), 3.80 (m,2H), 4.02(m, 1H). 4.1.2. 2-(1-(N,N'-Bis(tert-butoxycarbonyl)carbamimidoyl)piperi-din-4-yl)-2-(tert-butoxycarbonylamino)acetyl-l-phenylglycineamide (6) To a solution of 5 (from the above experiment) and phenylala-nine amide.TFA salt (0.58g, 2.09 mmol)in DMF (10 mL) diisopro-pylethylamine (1 mL, 5.70 mmol) and HATU (0.79 g, 2.08 mmol)were added at 0C. The bright yellow solution was stirred at 0℃and gradually warmed to rt overnight. DMF was removed in vacuoand the residue was dissolved in EtOAc(50 mL). The organic solu-tion was subsequently washed with 10% KHSO4, satd NaHCO3,brine, dried over NazSO4, and concentrated in vacuo. The resultingresidue was purified by preparative HPLC followed by triturationwith EtOAc to afford 6 (60 mg, 5% yield from 4) as a white solid.400-188-0725 'HNMR (CD30D) 8 0.63-0.80 (m,2H), 1.12 (m, 1H), 1.42-1.50(m,27H), 1.58(m,2H),2.59(t,J=12.1Hz, 1H), 2.73 (t,J=13.0 Hz, 2H),3.42 (dd,J=3.8, 13.9 Hz, 1H), 3.61 (m,1H), 3.74(br s, 1H), 3.98 (brs, 1H), 4.65 (dd,J=3.8, 11.9 Hz, 1H), 7.22-7.29(m,5H). 4.1.3.(2-Amino-2-(1-carbamimidoylpiperidin-4-yl)acetyl-L-phenylglycine amide bis(trifluoroacetate) (7) A solution of 6 (0.045 g, 0.07 mmol) in dichloromethane(1 mL)was treated with TFA (1 mL) for 1 h at rt. The solvents wereremoved and the excess of TFA was co-evaporated 3 times withdichloromethane. The residue was dried in vacuo, dissolved inwater, and freeze-dried to give 7 (40 mg, 94%) as a white fluffysolid (bis-TFA salt). H NMR (D20)8 0.50-0.69 (m,2H), 1.33 (d,J=11.9 Hz, 2H), 1.79 (m, 1H), 2.78 (m, 3H), 3.34 (dd, J=4.0,14.4 Hz, 1H), 3.55 (t,J=15.9 Hz, 2H), 3.75 (d,J=4.6Hz, 1H), 4.82(dd, J=4.0, 12.4 Hz, 1H), 7.28 (m, 5H); 13C NMR (100MHz, D20); 26.7,27.9,37.7,38.1,46.1,46.3,55.6,57.9,128.6,130.0, 130.2,137.9,157.0,169.2, 176.8. Anal. Calcd for C17H26N6022TFA-2H2O: C, 41.45; H, 4.97; N, 13.81. Found: C, 41.57; H, 5.08; N,13.44.HPLC purity: 98%. 4.1.4.(2S,4S)-tert-Butyl 2-((S)-1-amino-1-ox0-3-phenylpropan-2-ylcarbamoyl)-4-azidopyrrolidine-1-carboxylate (9) To a solution of 8 (0.30 g,1.17 mmol,1.0 equiv) and phenylalanine amide.TFA salt (0.39 g, 1.40 mmol, 1.2 equiv) in DMF(10mL),diisopropylethylamine (0.82 mL, 4.64 mmol, 4.0 equiv) and HATU(0.54 g, 1.42 mmol, 1.2 equiv) were added at 0℃.The bright yel-low mixture was stirred at 0℃ and gradually warmed to rt over-night. DMF was removed in vacuo and the residue was dissolvedin EtOAc (30mL). The organic solution was subsequently washedwith 10% KHSO4, satd NaHCO3, brine, dried over Na2SO4, and con-centrated in vacuo. The resulting residue was purified by prepara-tive HPLC to afford 9 (0.46 g, 98% yield) as a white solid. HNMR(CD30D) 8 1.34-1.43 (m, 9H), 1.99 (m, 2H),),2.46(m, 1H), 2.96-3.17(m,2H), 3.64(m, 1H), 4.42 (m,2H), 4.65(m,1H), 7.27(m, 5H). 4.1.5.(2S,4S)-tert-Butyl 4-amino-2-((S)-1-amino-1-0x0-3- phenylpropan-2-ylcarbamoyl)pyrrolidine-1-carboxylate (10) A solution of 9 (0.42 g, 1.04 mmol) in methanol (10mL) washydrogenated overnight at balloon atmosphere in the presence ofa catalytic amount of 10% Pd/C. The reaction was filtered througha pad of Celite and the filtrate was concentrated to afford 10(0.26g, 66%) as an off-white foam. HNMR (CD30D)61.28-1.44(m, 9H), 1.56-1.68 (m,1H), 2.0(m, 1H), 2.32-2.44(m,1H), 2.80-3.20 (m, 2H), 3.42 (m, 1H), 3.66 (m, 1H), 4.10 (m, 1H), 4.57 (m,1H), 7.28 (m,5H). 4.1.6.(2S,4S)-tert-Butyl2-((S)-1-amino-1-0x0-3-phenylpropan-2-ylcarbamoyl)-4-(2,3-bis(tert-butoxycarbonyl)guanidino)pyrrolidine-1-carboxylate(11) A mixture of 10 (0.24g, 0.64 mmol, 1.0 equiv) and 3 (0.30 g,0.97 mmol, 1.5 equiv) in THF (7mL) was stirred at rt over 3 days.The reaction mixture was concentrated and the resulting residuewas filtered through a short pad of silica before being purified bypreparative HPLC to afford 11(H NMR yield: 97%) as colorlesscrystals containing 0.5 equiv (by NMR) of inseparable pyrazole.'H NMR (CDCl;) 81.36-1.50 (m, 27H), 2.46 (m, 1H), 3.15 (m,2H), 3.27 (m, 1H), 3.80 (m, 1H), 4.26 (m, 1H), 4.72 (m,2H), 5.37(m, 1H), 6.60 (br s, 1H), 6.82(m, 1H), 7.21-7.31 (m, 6H), 8.60 (brs, 1H). 4.1.7.(2S,4S)-N-((S)-1-Amino-1-ox0-3-phenylpropan-2-yl)-4-guanidinopyrrolidine-2-carboxamide bis(trifluoroacetate) (12) A solution of 11 (0.48 g, 0.78 mmol) in dichloromethane (5 mL)was treated with TFA (5mL) for 2 h at rt. The solvents wereremoved and the excess of TFA was co-evaporated 3 times with dichloromethane. The residue was dried in vacuo, dissolved inwater, and freeze-dried to give a fluffy solid foam which was puri-fied by HPLC (isocratic 5% acetonitrile/water, 0.1% TFA) to give0.22 g of 12 (containing 0.15 equiv of pyrazole) as a white fluffysolid (75% yield): HNMR (D20)82.16 (m, 1H), 2.73 (m, 1H),2.98(dd,J=8.6, 13.9 Hz, 1H), 3.05 (dd,J=6.8, 13.4 Hz, 1H), 3.45(dd,J=3.8, 12.6 Hz, 1H), 3.60 (dd,J=6.3, 12.6 Hz, 1H), 4.34 (m,1H), 4.41 (dd,J=5.6, 8.8 Hz, 1H), 4.54 (t, J=7.7 Hz, 1H), 7.23-7.30 (m, 5H). 1c NMR (100 MHz, D20)8 35.6, 37.2, 50.4, 50.6,55.6, 58.7,127.6,129.1,129.5,136.4,156.5,168.8, 175.5. Anal.Calcd for C15H22N602·2.5TFA-1.2 H20-0.15pyrazole: C, 38.51; H,4.11; N, 13.22; F, 22.34. Found: C, 38.24; H, 3.93; N, 13.42; F,22.08. HPLC purity: 93%. 4.1.8.(2S,4R)-4-((2,3-Bis(tert-butoxycarbonyl)guanidino)methyl).0)- 1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid (14) A mixture of 13 (0.45 g, 1.84 mmol, 1.0 equiv) and 3 (0.86 g,2.77 mmol, 1.5 equiv) in methanol (8mL) was stirred at rt over5 days. The reaction mixture was concentrated and the resultingresidue was purified by flash chromatographyy((eluent: hexanes/EtOAc/AcOH, 1:1:0.1%) to afford 14 (0.78g,80% H NMR yield) asa white solid containing 1.3 equiv of inseparable pyrazole. 1HNMR'HNMR (CDCl3) 8 1.40-1.50 (m, 27H), 1.96 (m, 1H), 2.39-2.53(m,2H), 3.15 (m, 1H), 3.33-3.48 (m, 2H), 3.69-3.80 (m,1H),4.26-4.36 (dt,J=7.6, 38.9 Hz, 1H). 4.1.9. (2S,4R)-tert-Butyl2-((S)-1-amino-1-0x0-3-phenylpropan-2-ylcarbamoyl)-4-((2,3-bis(tert-butoxycarbonyl)guanidino)methyl)pyrrolidine-1-carboxylate (15) To a solution of 14 (0.33 g, 0.57 mmol,1 equiv) and phenylala-nine.TFA salt (0.19 g, 0.68 mmol, 1.2 equiv) in DMF (7 mL), diiso-propylethylaminee(0.40 mL, 2.27 mmol, 4.0 equiv) and HATU(0.26 g,0.68 mmol, 1.2 equiv) were added at 0 C. The bright yel-low mixture was stirred at 0°℃ and gradually warmed to rt overa period of 3 h. DMF was removed in vacuo and the residue wasdissolved in EtOAc (20 mL). The organic solution was subsequentlywashed with 10% KHSO4, satd NaHCO3, brine, dried over Na2SO4,and concentrated in vacuo. The resulting residue was purified bypreparative HPLC to afford 15 (0.17 g, 47% yield) as a white solid.H NMR (CD3OD) 8 1.32-1.54 (m, 27H), 2.01 (m, 1H), 2.21-2.44(m, 2H), 2.93-3.31 (m, 4H), 3.65 (m, 1H), 4.08 (m, 2H), 4.65 (m, 1H), 7.25 (m, 5H). 4.1.10. (2S,4S)-N-((S)-1-Amino-1-0x0-3-phenylpropan-2-yl)-4-(guanidinomethyl)pyrrolidine-2-carboxamidebis(trifluoroacetate) (16) A solution of 15 (0.15g, 0.24 mmol) in dichloromethane (4mL)was treated with TFA (4mL) for 2.5 h at rt. The solvents wereremoved and the excess of TFA was co-evaporated 3 times withdichloromethane. The residue was dried in vacuo, dissolved inwater, and freeze-dried to give 16 (75 mg, 50%) as a fluffy solidfoam (bis-TFA salt). H NMR (D20) 8 1.68(m, 1H), 2.57 (m, 1H),2.70 (m, 1H), 3.00 (m, 1H), 3.08 (m, 2H), 3.21 (dd,J=5.3, 7.1 Hz,1H), 3.49 (dd,J=7.6, 11.9 Hz, 1H), 4.32 (t,J=8.3 Hz, 1H), 4.54 (d,J=7.3Hz, 1H), 4.54 (t, J=7.7 Hz, 1H), 7.25 (m, 5H), 13c NMR(100MHz, D20); 33.3,37.2,37.8,42.4,48.8,55.5,59.7,127.6,129.1,129.5,136.5,156.5,169.1, 175.5. Anal. Calcd for C16H24N6022.3TFA2H2O: C, 38.51; H, 4.11; N, 13.22; F, 22.34. Found:C, 38.24; H, 3.93; N, 13.42; F, 22.08. HPLC purity:>98%. 4.1.11.2-(3-(2,3-Bis(tert-butoxycarbonyl)guanidino)phenyl)-2-(tert-butoxycarbonylamino)acetic acid (18) A mixture of 17 (0.15 g, 0.56 mmol 1.0equiv) and 3 (0.18 g,0.58 mmol, 1.0 equiv) in methanol (5mL) was stirred at rt for1 day. The reaction mixture was concentrated and the resultingresidue was subjected to purification by using a Biotage Isolera400-188-0725 One with the eluent hexanes/EtOAc (1:1 containing 1% AcOH) togive 0.21 g of 18 as a yellow foam (73%). H NMR (CDCl3) 8 1.42(br s, 9H), 1.46 (br s, 9H), 1.50(s, 9H), 5.20 (d, J=7.1 Hz, 1H),5.69 (d,J=7.1 Hz, 1H), 7.12 (d,J=7.3Hz, 1H), 7.29(m, 2H), 7.65(d,J=8.1 Hz, 1H), 11.24(br s, 2H). 4.1.12.(2S)-2-(3-(2,3-Bis(tert-butoxycarbonyl)guanidino)phe-nyl)-2-(tert-butoxycarbonylamino)acetamido-3-phenylpro-panamide bis(trifluoroacetate)(19a and 19b) To a solution of 18 (0.10 g, 0.20 mmol, 1.0 equiv) and phenylal-anine amide.TFA salt (0.066g, 0.24 mmol, 1.2 equiv) in DMF(5 mL), diisopropylethylamine (0.14 mL, 0.80 mmol, 4.0 equiv)and HATU (0.090 g, 0.24 mmol, 1.2 equiv) were added at 0℃. Thebright yellow solution was stirred at 0 ℃ and gradually warmedto rt overnight. DMF was removed in vacuo and the residue was dis-solved in EtOAc (30 mL). The organic solution was subsequentlywashed with 10% KHSO4, satd NaHCO3, brine, dried over Na2SO4,and concentrated in vacuo. The resulting residue was purified bypreparative HPLC to afford 55 mg (42%) of product 19a as the lesspolar compound and 58 mg (44%) of product 19b as the more polarcompound. Compound 19a: 1HNMR (CDCl;) 8 1.39-1.55 (m, 27H),3.16(d,J=6.6 Hz, 2H), 4.72 (dd,J=6.6, 14.4Hz, 1H), 4.98 (d,J=4.3 Hz, 1H), 5.35 (s, 1H), 5.57 (d,J=4.6 Hz, 1H), 6.25 (s, 1H),6.49(d,J=8.6Hz, 1H), 6.92 (d,J=7.6 Hz, 1H), 7.19-7.21(m,2H),7.25-7.32(m, 4H), 7.44 (m, 1H), 7.50(m, 1H), 10.33(s, 1H), 10.63(s, 1H). Compound 19b: H NMR (CDCl3) 8 1.40-1.55 (m, 27H)3.03 (s, 2H), 4.67 (dd,J=6.8, 14.7Hz, 1H), 5.04 (d,J=5.8 Hz, 1H),5.49 (s, 1H), 5.73 (d,J=6.1Hz,1H), 6.28 (s,1H), 6.63 (d,J=8.1Hz,1H), 6.97-7.03 (m, 3H), 7.17 (m, 3H), 7.29 (m, 1H), 7.43(s,1H),7.64(d,J=7.8Hz, 1H), 10.35 (s, 1H), 11.65 (s, 1H). ( 4.1.14.(S)-3-(4-( 2 ,3 - Bis(tert - butoxycarbonyl)guanidino)phenyl)- 2-(tert-butoxycarbonylamino)propanoic acid (22a) ) ( A m ixture o f 21a (0.7 0 g, 2 .50mmol, 1. 0 equiv) and 3 (0.64 g, 2.06 mmol, 0.83 equiv) in methanol (10 mL) wa s stirred at rt o v erweekend. The reaction mixture was concentrated a nd the r esidue was subjected to purification by B i otope Isolera O n e us i ng EtOAc/ hexanes (containing 2% AcOH) to give 1 .04g(97%) of product 22a. 1 H NMR (CDCl3) 8 1 . 4 4 (S, 9 H) , 1.50 (s, 9H) , 1.5 3 (s, 9H) , 3.06(m, 2H),4.58(m , 1H), 4. 9 9 (d , J=7.6Hz, 1H), 7. 1 3 (d, J=8.4Hz, 2 H ), 7.50 (d,J=8.6 Hz, 2H), 10.34 (br s, 1H). ) 4.1.15.(2S)-2-[(2S)-3-(4-(2,3-Bis(tert-butoxycarbonyl))guanidi-nophenyl)-2-(tert-butoxycarbonylamino)propanoyl]amino-3-phenylpropanamide (23a) To a solution of 22a (0.28 g, 0.54 mmol, 1.0 equiv) and phenyl-alanine amide.TFA salt (0.18g, 0.63 mmol, 1.2 equiv) in DMF(10mL), diisopropylethylamine (0.40 mL, 2.10 mmol, 4.0equiv)and HATU (0.24 g, 0.63 mmol, 1.2 equiv) were added at OC. Thebright yellow solution was stirred at 0℃ and gradually warmedto rt overnight. DMF was removed in vacuo and the residue wasdissolved in EtOAc (50 mL). The organic solution was subsequentlywashed with 10% KHSO4, satd NaHCO3, brine, dried over NazSO4,and concentrated in vacuo. The resulting residue was purified byBiotage Isolera One using EtOAc-hexanes to afford 0.33g(92%)of product 23a as a white solid cake. H NMR (CD30D) 8 1.39 (s,9H), 1.46 (br s, 9H), 1.65 (br s, 9H),2.81 (m, 1H), 2.91(m, 2H),3.11 (m,1H), 4.21 (m, 1H), 4.58 (m, 1H), 7.15 (m, 2H), 7.25 (m,5H), 7.37 (m,2H). 4.1.16.(2S)-2-Amino-N-((2S)-1-amino-1-0x0-3-phenylpropan-2-yl)-3-(4-guanidinophenyl)propanamide bis(trifluoroacetate) (24a) A solution of compound 23a (0.32 g, 0.48 mmol) in dichloro-methane (6 mL) was treated with TFA (6mL) for 4 h at rt. The sol-vents were removed and the excess of TFA was co-evaporated 3times with dichloromethane. The residue was dried in vacuo, dis-solved in water, and freeze-dried to give 24a (0.25 g, 71%) as awhite fluffy solid (bis-TFA salt). 1H NMR (D20) 8 2.91 (m, 2H),3.04 (dd,J=8.8, 13.6Hz, 1H), 3.18 (dd,J=6.3, 13.9 Hz, 1H), 4.13(dd,,J=6.6,8.6 Hz, 1H), 4.51 (t,J=7.6Hz, 1H), 7.18 (m, 7H), 7.25(m, 2H); 1’cNMR (100 MHz, D20) 8 36.3, 37.1,53.9,54.4,126.3,127.2,128.7,129.1,130.8,133.3,133.8,136.0,156.2,168.2,174.1. Anal. Calcd for C19H24N6022.65TFA4.1 H2O: C, 39.35; H,4.38; N, 11.33. Found: C,338.95; H, 3.98; N, 11.73. HPLC purity:>98%. 4.1.17.(R)-3-(4-(2,3-Bis(tert-butoxycarbonyl)guanidino)phenyl)-2-(tert-butoxycarbonylamino)propanoic acid (22b) A mixture of 21b (0.58 g, 2.07 mmol, 1.0 equiv) and 3 (0.53 g,1.71 mmol, 0.83 equiv) in methanol (20 mL) was stirred at rt over-night. The reaction mixture was concentrated and the residue wassubjected to purification by flash chromatography (eluent: 1:1:1%hexanes/EtOAc/AcOH) to give 0.64g (72%) of product 22b.1HNMR (CDCl3) 8 1.46 (s, 9H), 1.51 (s, 9H), 1.54 (s, 9H), 3.13 (m,2H), 4.56(m, 1H), 4.94 (m, 1H), 7.14 (d,J=8.6Hz, 2H),7.53 (d,J=8.6Hz,2H), 10.34 (br s, 1H). 4.1.18.(2S)-2-[(2R)-3-(4-(2,3-Bis(tert-butoxycarbonyl))guanidi-nophenyl)-2-(tert-butoxycarbonylamino)propanoyl]amino-3-phenylpropanamide (23b) To a solution of 22b (0.64 g, 1.22 mmol, 1.0 equiv) and phen-ylalanine amideTFA salt (0.41 g, 1.47 mmol, 1.2 equiv) in DMF(12 mL), diisopropylethylamine (0.85 mL, 4.90 mmol, 4.0 equiv)and HATU (0.56 g, 1.47 mmol, 1.2 equiv) were added at 0C.The bright yellow solution was stirred at 0℃ and graduallywarmed to rt overnight. DMF was removed in vacuo and the res-idue was dissolved in EtOAc (50 mL). The organic solution wassubsequently washed with 10% KHSO4, satd NaHCO3, brine, driedover Na2SO4, and concentrated in vacuo. The resulting residuewas purified by Biotage Isolera One using EtOAc as eluent toafford 0.37g(45%) of product 23b as a white solid. H NMR(DMSO-d6) 8 1.30 (s, 9H), 1.41 (s, 9H), 1.50 (s, 9H), 2.46 (m,1H), 2.58 (dd,J=3.3,13.1Hz, 1H), 2.75 (dd,J=10.1, 13.4 Hz,1H), 3.05 (dd,J=4.0, 13.9 Hz, 1H), 4.10 (m, 1H), 4.44 (m, 1H),6.80 (d, J=8.1Hz, 1H), 7.03 (d, J=8.3Hz, 2H), 7.17 (m, 2H),7.25 (m, 2H), 7.36-7.41 (m, 3H), 8.30 (d,J=8.8 Hz, 1H), 9.92 (s,1H), 11.43 (s,1H). 4.1.19. (2R)-2-Amino-N-((2S)-1-amino-1-0X0-3-phenylpropan-2-yl)-3-(4-guanidinophenyl)propanamide bis(trifluoroacetate)(24b) A solution of compound 23b (0.057 g, 0.085 mmol) in dichloro-methane (3mL) was treated with TFA (3 mL) for 30 min at rt. Thesolvents were removed and the excess of TFA was co-evaporated 3times with dichloromethane. The residue was dried in vacuo, dis-solved in water, and freeze-dried to give 24b (0.051 g, 88%) as awhite fluffy solid (bis-TFA salt). 'H NMR (D20) ; 2.79 (dd,J=10.1,14.2 Hz, 1H), 2.91 (m,2H), 3.09 (dd,J=5.3, 14.2 Hz, 1H),4.19 (t, J=6.6Hz, 1H),4.51 (dd,J=5.3, 9.9 Hz, 1H), 6.85 (d,J=8.3 Hz, 2H), 7.09(d,J=8.3 Hz, 2H), 7.22 (m,3H), 7.31 (m,2H);C NMR (100 MHz, D20) 36.3, 37.4,54.1,55.3,126.3,127.7,129.2,129.4,131.0,133.1,134.2,136.7,156.5,169.3,175.8. Anal.Calcd for C19H24N6022.5TFA-1.6 H2O: C, 42.28; H, 4.32; N, 12.33.Found: C, 42.09; H, 4.44; N, 12.23. HPLC purity:>98%. 4.2. In vitro Mrg receptor assays HEK293 cells stably transfected with human MrgX1, HEK293 orKNRK cells transiently transfected with Mouse MrgC or RatMrgC11 were p961lated in 96 well plates at 25,000 cell/well and incu-bated 2 days before imaging. On the day of imaging cells wereincubated in 100 uL HBSS with 2 uM Fluo 4AM and 1% TrypanRed for 50 min at 37°℃. The cells were then equilibrated for10 min at room temperature before imaging. Test compounds weredissolved in HBSS and diluted in a serial dilution. Test compounds,BAM8-22 (positive control) or HBSS (negative control) were added(50 uL into 100 uL) and cells were imaged on the FLIPR for 2 min.Data was exported as maximum-minimum fluorescent signal. Acknowledgments (to F.L.) from the Department of Anesthesiology, Zhujiang Hospital,Southern Medical University (Guangzhou, China). ( References and notes ) ( 1 . D ong, X . ; H an ,S.; Zylka, M. J ; Simon, M. I.; Anderson, D . J. C e l l 2001, 106, 619. ) ( 2. L 1 embo, P . M . ; G razzini, E .; Groblewski, T .; O'Donnell, D.; Roy , M . O. ; Zhang , J; Hof fert , C.; Cao, J . ; Schmidt, R .; Pel l e t i er , M .; L abar r e, M.; G o sselin, M . ; F o rti n , Y .; Banvil l e, D.; Shen, S. H. ; Str o m, P. ; Payza, K. ; D ray, A.; Walker , P.; Ahm a d, S. Nat. Neurosci . 2002, 5, 2 01. ) Chen, H.; Ikeda, S. R. J. Neurosci. 2004, 24, 5044. Liu,Q.; Tang, Z.; Surdenikova, L.; Kim, S.; Patel, K. N.; Kim, A.; Ru, F.; Guan,Y.;Weng, H. J.; Geng, Y.; Undem, B. J; Kollarik, M.; Chen, Z. F.; Anderson, D. J.;Dong, X. Cell 2009, 139, 1353. 5.Han, S. K.; Dong, X.; Hwang,J. I.; Zylka, M. J.; Anderson, D. J; Simon, M. I. Proc.Natl. Acad. Sci. U.S.A. 2002,99,14740. 6.Zylka, M.J; Dong, X.; Southwell, A. L.; Anderson,D. J. Proc. Natl. Acad. Sci. US.A.2003,100,10043. 7.Guan, Y.; Liu, Q; Tang, Z.; Raja, S. N.; Anderson, D. J.; Dong, X. Proc. Natl. Acad.1.Sci. U.S.A. 2010, 107,15933. 8. He,S. Q; Li, Z.; Chu, Y. X.; Han, L.; Xu, Q;Li, M.; Yang, F.; Liu, Q.; Tang. Z.; Wang,Y.; Hin,N.; Tsukamoto, T.; Slusher, B.; Tiwari, V.; Shechter, RR.;:VWei, F.; Raja, SN.; Dong, X.; Guan,Y. Pain 2014,155,534. ( 9. S ikan d , P.; Dong, X.; L aMotte, R . H . J. N eurosci. 2011,31, 756 3. ) 100.Grazzini, E.; Puma,C.; Roy, M. O.; Yu,X. H.; O'Donnell,D.; Schmidt, R.; Dautrey, ( S . ; Duc h arme,J; P e r kins, M.; Pa n etta, R.; L a ird , J. M.; Ah ma d, S. , L embo, P . M . P roc. Natl. Acad. Sci . U.S. A . 2004, 101,7175 . ) 11.Wroblowski, B.; Wigglesworth, M.J.; Szekeres, P. G.; Smith, G.D.; Rahman,S.Nicholson, N. H.; Muir, A. I.; Hall, A.; Heer, J. P; Garland,S. L.; Coates, W. J. J.Med.Chem. 2009.52.818. 12.Malik, L.; Kelly, N. M.;Ma,JN.;Currier, E. A.: Burstein, E. S.; Olsson, R. Bioorg.Med. Chem. Lett. 2009,19,9.17291 ( 13 . P e te rl in- Ma sic, L. ; Kikel j , D . Tet r ahedro n 200 1, 5 7, 7 073. ) ( 14. A n dr o nati, S. A . ; K arasev a , T . L L . ; K r y s ko, A . A . Curr . Med. Chem. 2004, 11 , 1 183. ) ( 1 5 . Ma s i c, L . P . C urr. Med, t . .C h em.2006 , 13 , 3627. ) ( 6. Webb, T. R . ;Eig e nb r ot, C. J . O . r g O . rg . Ch e m . 1991, 5 6, 3 00 9. ) 7.Atkinson,R. N.; Moore, L.: Tobin, j.; King, S. B. J. Org. Chem. 1999, 64, 3467. 18.RIosloniec, E. F.; Brandstetter,r, T.; Leyer, S.; Schwaiger, F. W.; Nagy, Z. A. J.Autoimmun. 2006, 182. 19.Heo,J;Hann,SS.K.;;Vaidehi,N.;Wendel,J.; Kekenes-Huskey, P.; Goddard,W.A.,.03rd ChemBioChem 2007/8,1527. 20.Kunapuli, P.;Lee 5.; Zheng, W.; Alberts,M.; Kornienko, O.; Mull, R.; Kreamer,IwangJ.I; Simon, M. I.; Strulovici, B. Anal. Biochem. 2006,351, 50. ( Ba yra k d arian, M. ; Butterworth, J; Hu, Y. J.;San t hakumar, V. ; Tomaszewski, M. | . B i oor g . Me d . Chem . Let t . 2011, 21,2102 . ) http://dx.doi.org/j.bmc.oElsevier Ltd. All rights reserved.士科技www.rysstech.com 耐士科技www.rysstech.com
确定






还剩5页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中主要物质含量分析检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中主要物质含量分析检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








