
方案详情
文
Mechanotransduction in the regulation of cellular responses has been previously studied using elastic hydrogels. Because cells interact only with the surface of biomaterials, we are focusing on the molecular mobility at the outermost surface of biomaterials. In this study, surfaces with the mobile Arg-Gly-Asp-Ser (RGDS) peptide have been constructed. Cell culture substrates were coated with ABA-type block copolymers composed of poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate) segments (A) and a polyrotaxane (PRX) unit with RGDS bound to a-cyclodextrin (B). Adhesion, morphological changes and actin filament formation of human umbilical vein endothelial cells were reduced on the surfaces containing mobile PRX-RGDS in comparison to the immobile RGDS surfaces constructed from random copolymers with RGDS side groups (Prop-andom-RGDS). In the neurite outgrowth assay using rat adrenal pheochromocytoma cells (PC12), only 20% of adherent PC12 cells had neurites on PRX-RGDS surfaces, but more than 50% did on the Random-RGDS surface. The beating colony of dimethyl-sulfoxide-treated mouse embryonic carcinoma cells (P19CL6) were found 10 and 14 days after induction on
PRX-RGDS and Random-RGDS surfaces, respectively. After 22 days, the beating colony disappeared on PRX-RGDS surfaces, but many colonies remained on Random-RGDS surfaces. These data suggest that the molecular mobility of the cell-binding ligand on the outermost surface of materials effectively suppresses the actin filament formation and differentiation of these functional cell lines, and may be used as a culture substrate for immature stem cells or progenitor cells.
方案详情

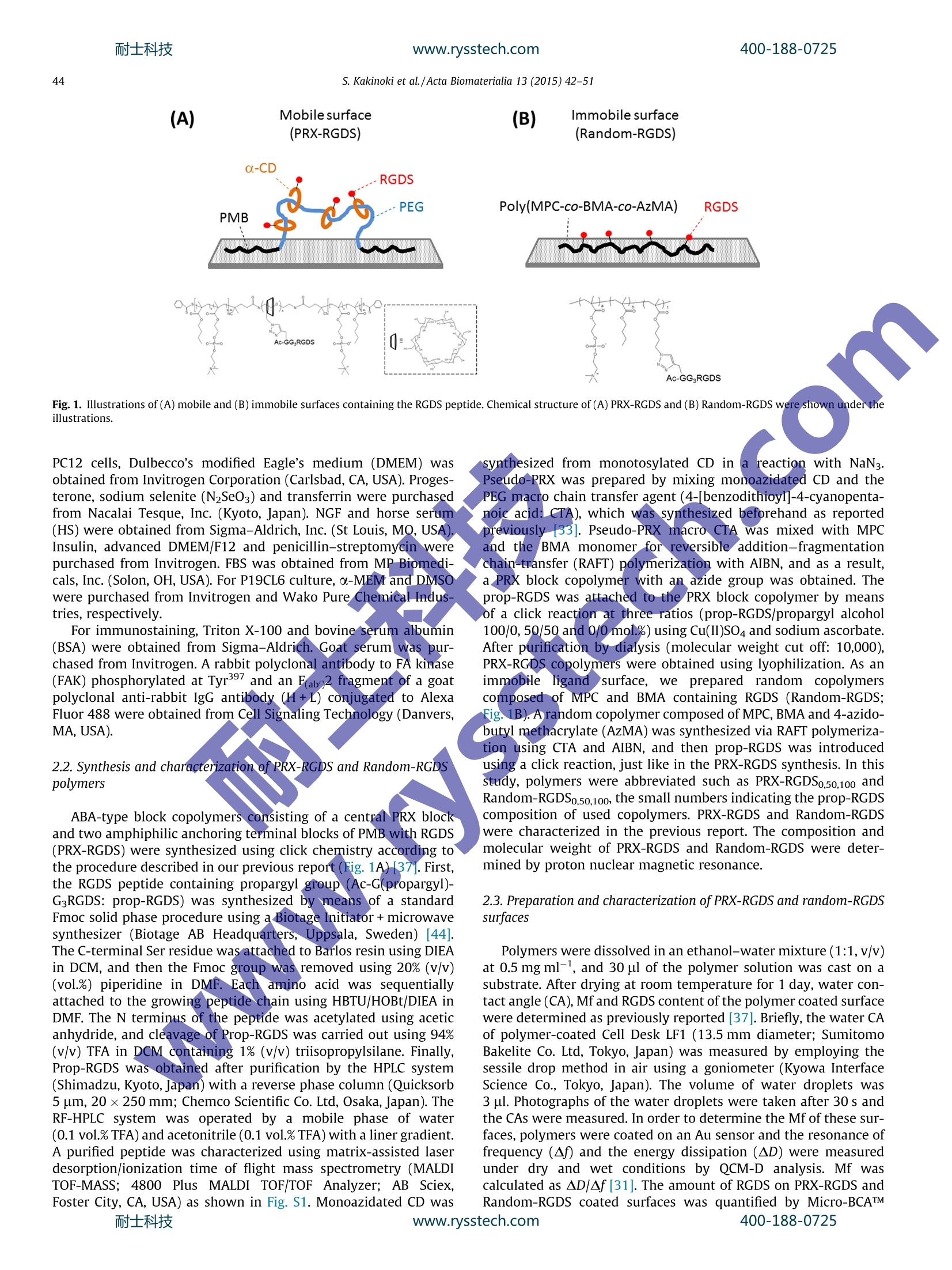
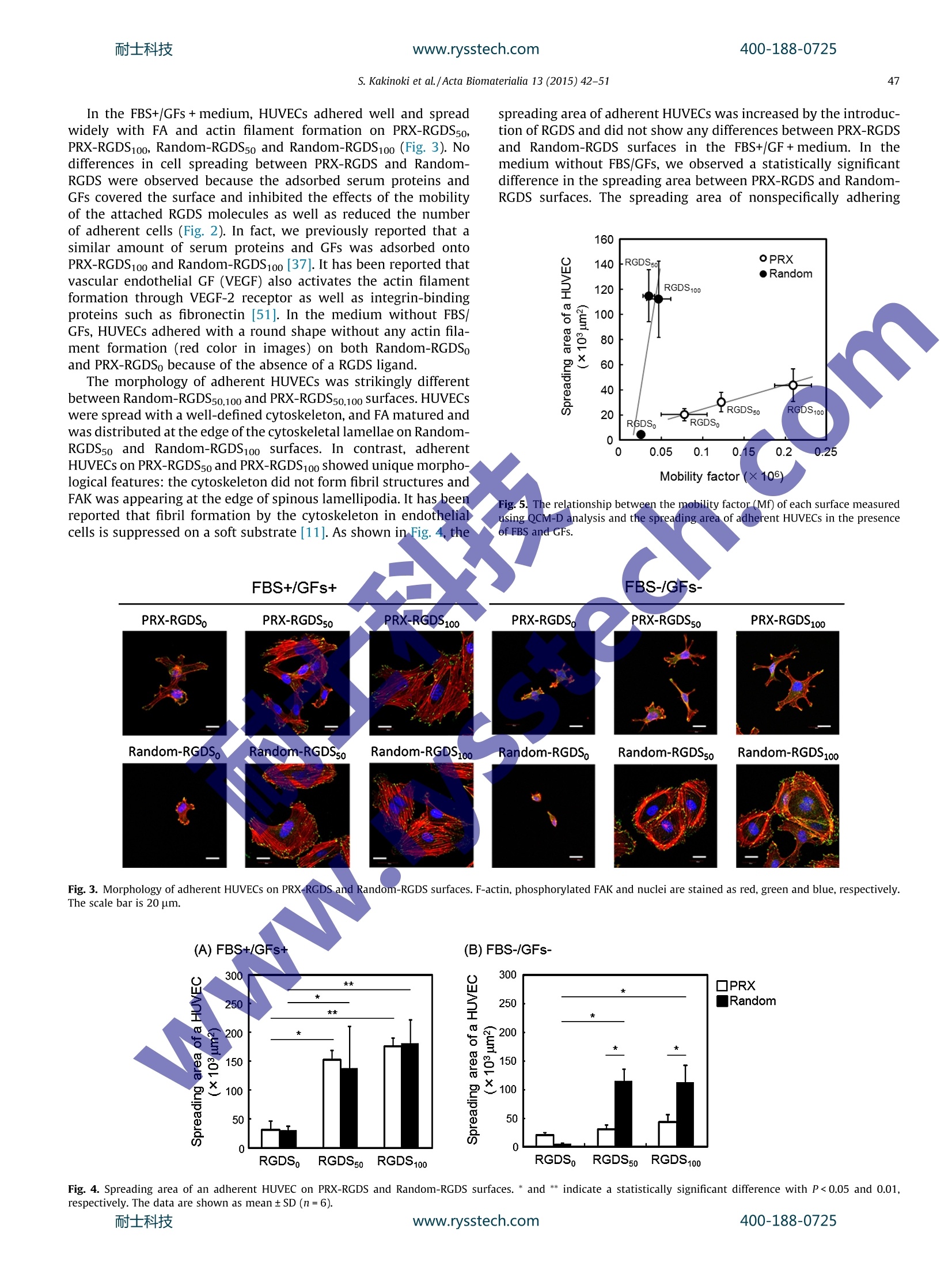
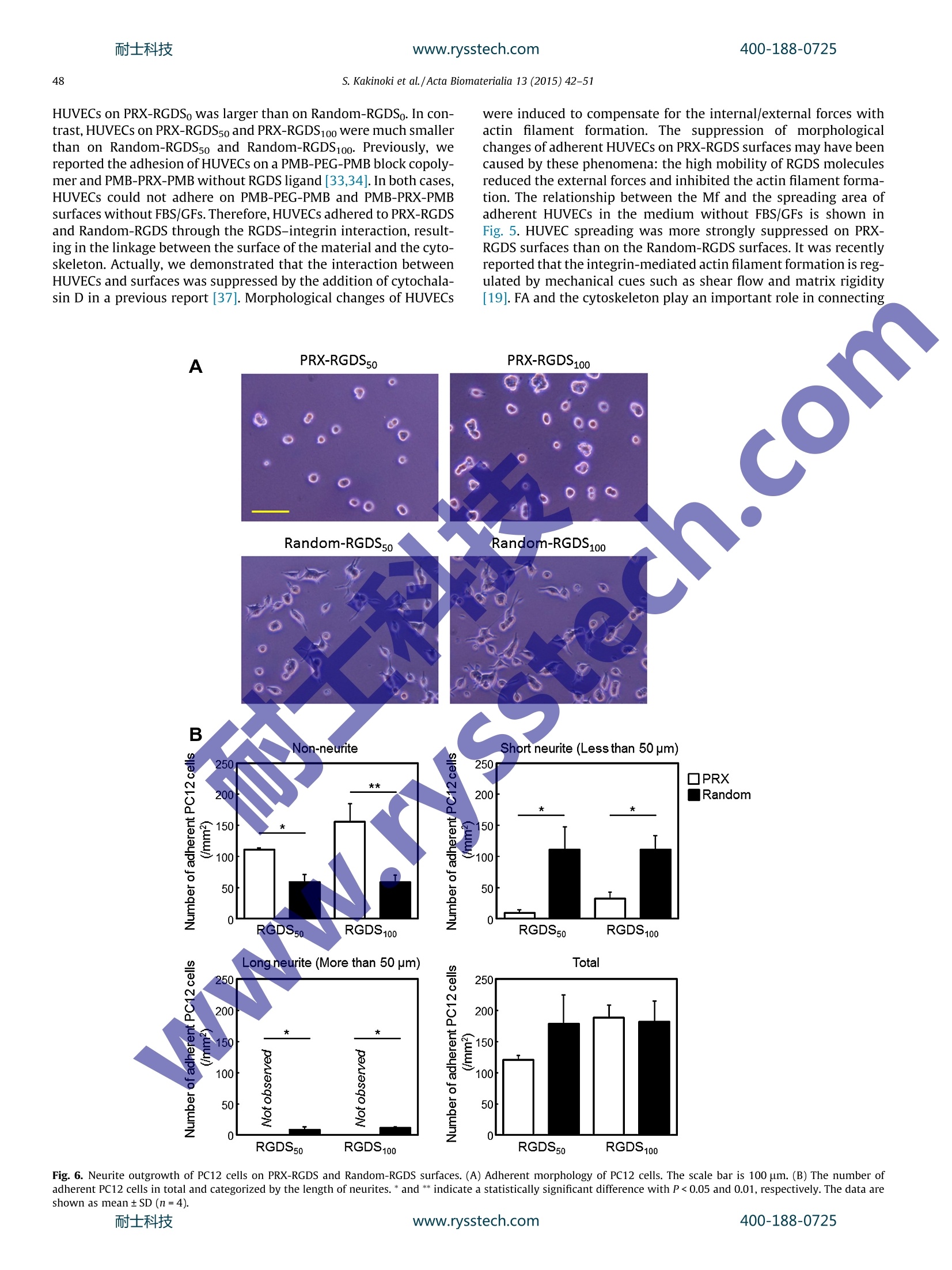
耐士科技400-188-0725www.rysstech.comActa Biomaterialia 13 (2015) 42-51 耐士科技400-188-0725www.rysstech.com43S. Kakinoki et al./Acta Biomaterialia 13 (2015) 42-51 Contents lists available at ScienceDirect Acta Biomaterialia ELSEVIER journal homepage: www.elsevier.com/locate /actabiomat Mobility of the Arg-Gly-Asp ligand on the outermost surface ofbiomaterials suppresses integrin-mediated mechanotransductionand subsequent cell functions Sachiro Kakinokiad, ji-Hun Seo b.d, Yuuki Inouecd, Kazuhiko Ishihara cd, Nobuhiko Yuib.d,Tetsuji Yamaoka a.d,* aDepartment of Biomedical Engineering, National Cerebral and Cardiovascular Center Research Institute, 5-7-1Fujishirodai, Suita, Osaka 565-8565, JapanDInstitute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, 2-3-10, Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan‘Department of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656,Japan ‘JST-CREST, 7 Gobancho, Chiyoda-ku, Tokyo 102-0076, Japan ABSTRACT 1. Introduction Highly functional scaffolds have been increasingly acceleratingprogress in the field of regenerative medicine[1,2]. These scaffoldsare expected to function as a temporary extracellular matrix (ECM)for controlling cell adhesion and for defining the shape and size ofregenerated tissues. The recent integrated understanding of cell-biomaterial interactions has given rise to the idea that well-designed scaffolds would lead to more effective tissue regenerationby modulating cell adhesion, migration,proliferation and stem celldifferentiation. Cells sense and respond to a wide variety of ( * C orresponding a uthor a t: Department of Biomedical E ngineering, National Cerebral a nd C a rdiovascular Ce n ter Res e arch Inst i tute, 5-7 - 1 Fuj i s hirodai, Sui t a, Osaka 565-8565,Japan. Tel.: +81 6 6833 5012; fax: +81 6 6835 5476. ) chemical, physical and biological features of the scaffolds such aswettability, electric charge, topology and biological activity of var-ious ligands; this phenomenon allows for regulation of cellularfunctions [3-5]. Recently, it was shown that matrix elasticity plays an importantrole in regulating numerous cell functions [6-9]. For instance,endothelial cells on the stiff hydrogel become stiffer, and theirproduction of podosomes is enhanced, as is the maturation of theactin cytoskeleton [10,11]. Stem cell fate is also affected by thematrix elasticity[8,9]. Human mesenchymal stem cells (hMSCs)were reported to be directed specific lineages when cultured onhydrogels of varying elasticity [8,12]. The hMSCs differentiate intoneurogenic, myogenic and osteogenic cells on the hydrogels withelasticity similar to that of brain, muscle and bone tissues. Theproliferation and differentiation of rat neural stem/progenitor cells ( h t tp: / /dx.d o i.org/ 1 0.101 6 /j . ac t bio.2014. 1 1 .020 1 742-7061/O 2 0 14 Acta Mat e rialia Inc. Published by E lsevier Ltd. All rights reserved. ) are stimulated better on the surfaces with moduli of 3.5 kPa than1 kPa [13]. Furthermore, rat embryonic cardiomyocytes functionlonger on surfaces with moduli of 11-17 kPa, which is similar tothe elastic modulus of normal myocardial tissue [14]. On the other hand, maintaining the undifferentiated state ofvarious stem cells or progenitor cells is also necessary for someresearch and clinical applications. Bone-marrow-derived hMSCsmaintain an ability for self-renewal and remain multipotent whencultured on collagen-coated polyacrylamide (PAAm) soft sub-strates (0.25 kPa)[15]. Gilbert and coworkers reported that skele-tal muscle stem cells also keep the undifferentiated state with aself-renewal ability on the laminin-immobilized soft polyethyleneglycol (PEG) hydrogel (12 kPa)[16]. In addition, Chowdhury et al.successfully cultured mouse embryonic stem cells as homoge-neous undifferentiated colonies with high expression of Oct3/4and alkaline phosphatase on the collagen-coated PAAm soft sub-strate (0.6 kPa) [17]. Cells adhere to the hydrogels coated with an ECM protein suchas collagen, fibronectin or laminin through focal adhesion (FA).Previous researchers reported that cells also sense the surface stiff-ness via FA points, and then integrin-mediated mechanotransduc-tion induces morphological changes, adhesion and differentiation[18-22]. The integrin-mediated mechanotransduction is closelyrelated to the dynamic integrin clustering at FAs [23,24]. In fact,an intercellular traction force increases in proportion to the elastic-ity of microspot substrates via reorganization of an actin filamentnetwork through integrin clustering:[25-27]. Trappmann et alevaluated the human epidermal and mesenchymal stem cellbehaviors on the collagen coated substrate with the elastic modu-lus of 0.1 kPa-2.3 MPa through the well-defined anchoring density[28]. Interestingly, they showed that the cell spreading and differ-entiation were affected by the collagen anchoring density, but notby the substrate elastic modulus. That is, stem cells decide theirown fate by sensing the integrin-ligand mobility of collagen fibers.Tsai and Kam reported that the lateral mobility of E-cadherinenhances the Rac1 recruitment of Madin-Darby canine kidney epi-thelial cells [29]. Moreover, Andreasson-Ochsner et al. also demon-strated that the lateral mobility of E-cadherin influences cellularresponses [30]. They evaluated the adhesion behavior of Chinesehamster ovary (CHO) cells on the supported lipid bilayer platformhaving laterally mobile or immobile E-cadherin ligands. Thespreading and the actin filament formation of CHO cells were sup-pressed by enhancing E-cadherin mobility. Although the molecularmobility of the outermost surface of substrates is one of the candi-dates for their key factors, these hydrogels and microspot substratefeatures affecting cell adhesion continue to be studied. Recently, we have succeeded at controlling the molecularmobility of biomaterial surfaces by meanss of polyrotaxane(PRX)-based supramolecules andi evaluated their function in vitro[31-37]. PRX has highly mobile structure, in which ring-shapedo-cyclodextrins (0-CDs) are threaded by a PEG chain, and thea-CDs are able to slide and rotate on the PEG chain. We foundthat the mobility of the PRX structure facilitates specific ligand-receptor interactions [38]. ABA-type block copolymers consistingof amphiphilic anchoring segments (A)that bind on various hydro-phobic substrates via hydrophobic interactions and a central PRXsegment (B) have been successfully designed and used for coatingthe surface of materials [33-37]. These triblock copolymers wereuseful for controlling the molecular mobility of the outermost sur-face irrespective of the substrate elastic modulus. We figured outthat the mobility factor(Mf) measured using quartz crystal micro-balance (QCM-D) analysis is useful for describing the mobility of asurface and strongly correlates with behavior the adherent cells[31]. The surface mobility is an influential feature because it causesconformational changes of adsorbed proteins and the morphologyof adherent platelets and other types of cells. In a previous study, we prepared the surfaces with mobile Arg-Gly-Asp-Ser (RGDS)ligands by means of RGDS bearing PRX (PRX-RGDS) [37]. The inte-grin-RGDS interaction was accelerated on PRX-RGDS surfaces atthe very early stage (within the first ~20 min). In the present study, the effects of the mobility of RGDS ligandson several features of cell behavior were evaluated for a muchlonger period of time in order to assess the downstream mecha-noresponse. Three types of cell functions - adhesion of humanumbilical vessel endothelial cells (HUVECs), neuronal differentia-tion of rat adrenal pheochromocytoma cells (PC12) and differenti-ation of mouse embryonic carcinoma cells (P19CL6) into beatingcolonies - were evaluated. The HUVEC is known to dynamicallyalter its morphology in response to the mechanical environmentssuch as the shear stress of blood flow and the tensile stress ofthe vascular wall [39]. PC12 cells which discontinue proliferatingand begin the neurite outgrowth by the treatment of nerve growthfactor (NGF) are widely used as a model of neural stem cells [40].The neurite outgrowth of PC12 cells is greatly affected by the celladhesion and morphology in regulating integrin activation [41].Furthermore, P19Cl6 cells are differentiated into beating cardio-myocytes with dimethyl sulfoxide (DMSO) treatment. The cardiacdifferentiation and maturation of P19CL6 is also affected by cell-material interaction [42]. ABA-type block copolymers consistingof a PRX block with RGDS-bearing a-CDs (A segment) and amphi-philic anchoring terminal blocks of poly(2-methacryloyloxyethylphosphorylcholine-co-n-butyl methacrylate) (PMB; B block) weresynthesized and used for constructing mobile surfaces (Fig. 1A).To prepare immobile RGDS surfaces, random copolymers com-posed of 2-methacryloyloxyethyl phosphorylcholine (MPC) andn-butyl methacrylate(BMA) (containing RGDS (Random-RGDS)were synthesized (Fig. 1B). The morphology of adherent HUVECswas examined after staining for F-actin and FA points. Neuriteoutgrowth of NGF-stimulated PC12 cells and differentiation ofP19CL6 cells into beating colonies after DMSO induction were alsoanalyzed. 2. Materials and methods 2.1. Materials Monotosylated &-CD, BMA,2,2'-azobis(isobutyronitrile)(AIBN),methacryloyl chloride, 4-azidobutanol and organic solvents werepurchased from Tokyo Kasei Co. (Tokyo, Japan). The organic sol-vents were of high purity chemical grade and were used withoutfurther purification. MPC was obtained from NOF Co. (Tokyo,Japan), which was prepared according to a previously publishedmethod [43]. Forirpeptidee synthesis,Fmoc-Gly-OH (whereFmocis9-fluorenylmethyloxycarbonyl group), Fmoc-Arg(Pbf)-OH, Fmoc-Asp(OtBu)-OH,1,Fmoc-Ser(tBu)-OH, Fmoc-Gly(propargyl)-OH,Barlos resin (1.57 mmolg-, 100-200 mesh), N,N-diisopropylethylamine (DIEA), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate (HBTU), 1-hydroxy-1H-benzotria-zole hydrate (HOBt), triisopropylsilane(TIS),piperidine,trifluoroacetic acid (TFA), N,N-dimethylformamide (DMF) anddichloromethane (DCM) were obtained from Watanabe ChemicalIndustries (Hiroshima, Japan). Acetic anhydride was purchasedfrom Wako Pure Chemical Industries, Ltd (Osaka,Japan). HUVECs were obtained from Cell Application, Inc. (San Diego,CA, USA). Rat adrenal pheochromocytoma cells (PC12) and mouseembryonic carcinoma cells (P19CL6) were purchased from theRiken BioResource Center (Ibaraki, Japan). For the culture ofHUVECs, the EBM-2 basal medium and EGM-2 SingleQuots kitincluding growth factors (GFs) and fetal bovine serum (FBS) wereobtained from Lonza (Walkersville, MD, USA). For the culture of400-188-0725 (A) Mobilesurface Fig. 1. Illustrations of (A) mobile and (B) immobile surfaces containing the RGDS peptide. Chemical structure of (A) PRX-RGDS and (B) Random-RGDS were shown under theillustrations. PC12 cells, Dulbecco's modified Eagle's medium (DMEM) wasobtained from Invitrogen Corporation (Carlsbad, CA, USA). Proges-terone, sodium selenite (N2SeO3) and transferrin were purchasedfrom Nacalai Tesque, Inc. (Kyoto, Japan). NGF and horse serum(HS) were obtained from Sigma-Aldrich, Inc. (St Louis, MQ, USA)Insulin, advanced DMEM/F12 and penicillin-streptomycin werepurchased from Invitrogen. FBS was obtained from MP Biomedi-cals, Inc. (Solon, OH, USA). For P19CL6 culture, a-MEM and DMSOwere purchased from Invitrogen and Wako Pure Chemical Indus-tries, respectively. For immunostaining, Triton X-100 and bovine serum albumin(BSA) were obtained from Sigma-Aldrich. Goat serum was pur-chased from Invitrogen. A rabbit polyclonal antibody to FA kinase(FAK) phosphorylated at Tyr397 and an Fab)2 fragment of a goatpolyclonal anti-rabbit IgG antibody (H+L) conjugated to AlexaFluor 488 were obtained from Cell Signaling Technology (Danvers,MA, USA). 2.2. Synthesis and characterization of PRX-RGDS and Random-RGDSpolymers ABA-type block copolymers consisting of a central PRX blockand two amphiphilic anchoring terminal blocks of PMB with RGDS(PRX-RGDS) were synthesized using click chemistry according tothe procedure described in our previous report (Fig. 1A) [37]. First,the RGDS peptide containing propargyl group (Ac-G(propargyl)-G3RGDS: prop-RGDS) was synthesized by means of a standardFmoc solid phase procedure using a Biotage Initiator + microwavesynthesizer (Biotage AB Headquarters,, Uppsala, Sweden) [44].The C-terminal Ser residue was attached to Barlos resin using DIEAin DCM, and then the Fmoc group was removed using 20% (v/v)(vol.%) piperidine in DMF. Each amino acid was sequentiallyattached to the growing peptide chain using HBTU/HOBt/DIEA inDMF. The N terminus of the peptide was acetylated using aceticanhydride, and cleavage of Prop-RGDS was carried out using 94%(v/v) TFA in DCM containing 1% (v/v) triisopropylsilane. Finally,Prop-RGDS was obtained after purification by the HPLC system(Shimadzu, Kyoto,Japan) with a reverse phase column (Quicksorb5 um, 20×250 mm; Chemco Scientific Co. Ltd, Osaka, Japan).TheRF-HPLC system was operated by a mobile phase of water(0.1 vol.% TFA) and acetonitrile (0.1 vol.%TFA) with a liner gradient.A purified peptide was characterized using matrix-assisted laserdesorption/ionization time of flight mass spectrometry (MALDITOF-MASS; 4800 Plus MALDI TOF/TOF Analyzer; AB Sciex,Foster City, CA,USA) as shown in Fig. S1. Monoazidated CD was synthesized from monotosylated CD ina reaction with NaN3.Pseudo-PRX was prepared by mixing monoazidated CD and thePEG macro chain transfer agent (4-[benzodithioy1]-4-cyanopenta-noic acid: CTA), which was synthesized beforehand as reportedpreviously [33]. Pseudo-PRX macro CTA was mixed with MPCand the BMA monomer for reversible addition-fragmentationchain-transfer (RAFT) polymerization with AIBN, and as a result,a PRX block copolymer with an azide group was obtained. Theprop-RGDS was attached to the PRX block copolymer by meansof a click reaction at three ratios (prop-RGDS/propargyl alcohol100/0,50/50 and 0/0 mol.%) using Cu(II)SO4 and sodium ascorbate.After purification by dialysis (molecular weight cut off: 10,000),PRX-RGDS copolymers were obtained using lyophilization. As animmobileliganddsurface, we prepared random copolymerscomposed of MPC and BMA containing RGDS (Random-RGDS;Fig.1B). A random copolymer composed of MPC, BMA and 4-azido-butyl methacrylate(AzMA) was synthesized via RAFT polymeriza-tion using CTA and AIBN, and then prop-RGDS was introducedusing a click reaction, just like in the PRX-RGDS synthesis. In thisstudy, polymers were abbreviated such as PRX-RGDSo,50,100 andRandom-RGDSo,50,100, the small numbers indicating the prop-RGDScomposition of used copolymers. PRX-RGDS and Random-RGDSwere characterized in the previous report. The composition andmolecular weight of PRX-RGDS and Random-RGDS were deter-mined by proton nuclear magnetic resonance. 2.3. Preparation and characterization of PRX-RGDS and random-RGDSsurfaces Polymers were dissolved in an ethanol-water mixture (1:1,v/v)at 0.5 mg ml-1, and 30 ul of the polymer solution was cast on asubstrate. After drying at room temperature for 1 day, water con-tact angle (CA), Mf and RGDS content of the polymer coated surfacewere determined as previously reported [37]. Briefly, the water CAof polymer-coated Cell Desk LF1 (13.5 mm diameter; SumitomoBakelite Co. Ltd, Tokyo, Japan) was measured by employing thesessile drop method in air using a goniometer (Kyowa InterfaceScience Co., Tokyo, Japan). The volume of water droplets was3 ul. Photographs of the water droplets were taken after 30 s andthe CAs were measured. In order to determine the Mf of these sur-faces, polymers were coated on an Au sensor and the resonance offrequency (Af) and the energy dissipation (AD) were measuredunder dry and wet conditions by QCM-D analysis. Mf wascalculated as AD/Af[31]. The amount of RGDS on PRX-RGDS andRandom-RGDS coated surfaces was quantified by Micro-BCATM400-188-0725 assay (Pierce Chemical, Rockford, IL, USA). The polymer-coated CellDesk LF1 was immersed in 1 ml of sodium dodecyl sulfate solution(10 mg ml-) containing 0.5 ml of micro-BCATM reagent solution.After sonication for 20 min and reaction for 40 min, the concentra-tion of the liberated RGDS in the solution was determined from theabsorbance at 570 nm. For the evaluations using cells, PRX-RGDS and Random-RGDSwere dissolved in1 an ethanol-water mixture (1:1,v/v) at0.5 mg ml-1, and 30 ul of the polymer solution was cast on a CellDesk LF1. After drying at room temperature for 1 day, we placedthe polymer-coated substrates in 24-well plates (Iwaki Co., Tokyo,Japan). When evaluating P19CL6 cells, we cast polymer solutions in24-well plates directly. Prior to in vitro assessment, polymer-coated substrates were stabilized for 24 h in phosphate-bufferedsaline (PBS), pH 7.2, ionic strength 0.167 M (GIBCO Invitrogen Cor-poration,CA, USA). The elastic modulus of PRX-RGDS and Random-RGDS surfaceswas measured using atomic force microscopy (AFM) as shown inFig. S2. All surfaces (n=3) were hydrated in Milli-Q water for24h before AFM. A force-separation curve was built using aDimension 3000 AFM (Bruker AXS, CA, USA) with a spherical sili-con oxide cantilever (CP-PNP; sQube, Wetzlar, Germany) in water.The elastic modulus was calculated using the Hertz model to fit thefirst 10 nm of slope in the force-separation curve. 2.4. HUVEC adhesion HUVECs were cultivated in the EBM-2 medium supplementedwith 2.0%(v/v) FBS and GFs in a humidified incubator at 37 °C with5% CO2 and were used at passage 6. Polymer-coated substrates thatwere previously stabilized in PBS were washed with the EBM-2medium once. Cultured HUVECs were trypsinized, washed withthe EBM-2 medium and manually counted using a hemocytometer(Watson Co. Ltd, Tokyo, Japan). The concentration of HUVECs wasadjusted to 2×104ml-1, and then 1.0 ml of HUVEC suspensionwas seeded on the samples using the EMB-2 medium with or with-out 2.0%(v/v) FBS and GFs. After 3 h of incubation, the cell sampleswere gently rinsed three times with the EBM-2 medium to removeunattached HUVECs. The number of adherent HUVECs in the sam-ples was quantitated using the WST-1 assay (Takara Bio Inc., Shiga,Japan). Washed samples were moved to new 24-well plates, andthen 500 ul of a mixture of the EBM-2 medium and WST-1(10:1,v/v) was added. After 1 h of incubation in a humidified incubatorat 37℃ with 5% CO2, absorbance of the solution at 460 nm wasmeasured on a microplate reader (Multiskan FC, Thermo Fisher Sci-entific, St Herblain, France). F-actin and phosphorylated FAK of adherent HUVECs wereimmunostained to examine cellularrmorphology. AdherentHUVECs in the samples were fixed with 4%(v/v) formaldehyde atroom temperature for 15 min. Fixed samples were incubated withPBS containing 1.0% (v/v) of Triton X-100 for 5 min at room tem-perature and were then immersed in a blocking solution consistingof PBS with 5.0% (v/v) goat serum and 0.30%(v/v) Triton X-100 for1 h at room temperature. The samples were incubated with theprimary antibody, rabbit polyclonal antibody to FAK phosphory-lated at Tyr397 for 12 h at 4C. After washing with PBS three times,we incubated the samples with the secondary antibody, the F(ab)2fragment of a goat polyclonal antibody to rabbit IgG (H+L) conju-gated to Alexa Fluor 488, for 1 h at room temperature in the dark.After rinsing with PBS three times, we stained F-actin of adherentHUVECs with 100 nM of rhodamine-phalloidin (Cytoskeleton Inc.,Denver, CO, USA) at room temperature for 30 min. The stainedsamples were mounted on ProLong Gold with 4,6-diamidino-2-phenylindole (Molecular Probes, Invitrogen), and images wereobtained using confocal laser-scanning microscopy (FV1000-D;Olympus, Tokyo, Japan) and the viewer software (Fluoview; Olympus). The spreading area of adherent HUVECs was determinedfrom the images of the immunostaining experiment using ImageJsoftware (National Institute of Mental Health, Maryland, USA)[45]. 2.5. The neurite growth assay of PC12 cells PC12 cells were cultivated on poly-D-Lys-coated cell culturedishes (Becton Dickinson, NJ, USA) in a humidified incubator at37C with 5% CO2 in DMEM supplemented with 100U ml-peni-cillin, 100 pg ml-1 streptomycin, 10% (v/v) FBS and 7.5%(v/v)HS.For the neurite outgrowth assay, PC12 cells were primed with100 ng ml-1 NGF for 24 h at 37C in tissue culture polystyrenedishes. The cells were collected by agitation, placed in the culturemedium for 30 min at 37 C and resuspended with the serum-freeadvanced DMEM/F12 containing 5 pg ml- insulin, 100 ng ml-1NGF, 20 nM progesterone, 30 nM Na2SeO3 and 100 mg mllttrans-ferrin. Then, the cells were seeded at the density of 2.0 ×104 persample on PRX-RGDS50, PRX-RGDS100, Random-RGDS5o and Ran-dom-RGDS100, which were stabilized beforehand in PBS in 24-wellcell culture plates. After incubation for 24 h at 37C, the sampleswere gently rinsed with DMEM/F12 with NGF to remove nonad-herent PC12 cells. After that, the samples were moved to new24-well cell culture plates containing 500 ul per well of DMEM/F12 with NGF. The morphological features of adherent PC12 cellswere examined undera microscope and the number of cells withor without neurites was determined from images using the Imagejsoftware in order to evaluate the neurite outgrowth activity. 2.6. Differentiation of P19CL6 cells into beating colonies P19CL6 cells were cultivated on regular tissue culture polysty-rene dishes (Iwaki Co., Tokyo, Japan) in a humidified incubator at37℃ with 5% CO using a-MEM supplemented with 100 U ml-penicillin, 100 ug ml- streptomycin and 10% (v/v) FBS. To assessthee ddifferentiation of P19CL6 cells into beating colonies, the cellswere seeded at the density of 3.8×104 per sample on PRX-RGDS5oand PRX-RGDS100 and Random-RGDS5o and Random-RGDS100 sur-faces in o-MEM containing 1%(v/v) of DMSO. P19CL6 cells wereincubated with this concentration of DMSO for 4 days [46,47],and then maintained using the culture medium without DMSO.Beating colonies were visually identified under a microscope andcounted in 12.0 cm² (6wells of a 24-well plate) (n=4). 2.7. Statistics and data analysis In order to investigate the relationship among PRX-RGDS andRandom-RGDS surfaces, statistical analysis was performed bytwo-way analysis of variance followed by Tukey's post hoc test. 3. Results and discussion 3.1. Characterization of PRX-RGDS and Random-RGDS Molecular profiles of the PRX and a random copolymer thatwere used for the preparation of PRX-RGDS and Random-RGDSare summarized in Table 1. The MPC content in the flanking seg-ment of PRX and in random copolymers was similar: 15.4% and18.9%, respectively. It was previously reported that the MPC con-tent of 20 to 30% is optimal for coating hydrophobic substratesand for reducing nonspecific protein adsorption [48-50].The azidegroup content of PRX and random copolymers was 2.3% and 2.4%(by weight), respectively. Prop-RGDS was mixed with propargylalcohol (PA) at three ratios (Prop-RGDS/PA=0/100, 50/50 and100/0 mol.%), and it reacted with PRX and random copolymers,yielding PRX-RGDSo, PRX-RGDS5o, PRX-RGDS100, Random-RGDSo,400-188-0725 Table 1Molecular composition of random and PRX copolymers [37]. Copolymer Molecular composition (mol.%) Azide group (wt.%) Number of a-CDs (/polymer) MW(DP) BMA MPC AzMA PEG PRX 38.4 15.4 N/A 46.2 2.3 25 144000 (530) Random 71.1 18.9 10.0 N/A 2.4 N/A 25400 (145) Calculated from lH-NMR spectrum. Random-RGDS5o and Random-RGDS100 with different RGD con-tents. These copolymers were cast on Cell Desk LF1 to constructthe mobile and immobile RGDS surfaces. Surface characteristicsof PRX-RGDS and Random-RGDS are summarized in Table 2[37].Water CA of PRX-RGDS surfaces decreased from 75 to 25°withan increase in RGDS content and was slightly lower compared toRandom-RGDS surfaces. Mf, which expresses the hydrated visco-elasticity determined using QCM-D analysis, in PRX-RGDS surfaceswas different from that of Random-RGDS surfaces. Althoughhydrophilicity of PRX-RGDS was similar to that of Random-RGDSin each RGDS composition, Mf of PRX-RGDS surfaces was greaterthan threefold higher than that of Random-RGDS surfaces. Theelasticity of the hydrogel-type substrate for cell behavior was eval-uated using AFM [8]. The elastic modulus (E) of Random-RGDS andPRX-RGDS surfaces, as measured by AFM, was too high forhydrated polymer substrates of 25 to 100 MPa, irrespective of theRGDS incorporation ratio (Fig. S2). This effect may have beencaused by the extreme thinness of the coating layers, which isresponsible for the properties of the base cell desk materials (E900 MPa: the data were provided by Sumitomo Bakelite Co..LLtd(Tokyo,Japan)). These results indicate that we succeededin con-trolling the mobility of only the outermost surface with similarbulk properties. The amount of surface RGDSWas~0.4 and 0.7 ug cm-2 for RGDSso and RGDS100, respectively, for both ofPRX and random copolymers. Here we prepared PRX-RGDS andRandom-RGDS surfaces with well-defined molecular mobility andRGDS density. 3.2. Adhesion and morphological changes of HUVECs HUVEC adhesion on PRX-RGDS and Random-RGDS surfaces isshown in Fig. 2. The number of adherent HUVECs increased withRGDS contents in the presence (Fig. 2A) and absence (Fig.2B) ofFBS/GFs. There were no statistically significant differences in thenumber of adherent HUVECs between PRX-RGDSand Random-RGDS surfaces for any RGDS composition in the presence of FBSand GFs. Because the supplemented proteins adsorbed onto the sur-face first, RGDS of PRX-RGDS likely loses the mobility and its effectson HUVEC adhesion. Meanwhile,in the absence of FBS/GFs, thenumber of adherent HUVECs on PRX-RGDS5o and PRX-RGDS100was much smaller than on Random-RGDS5o and Random-RGDS100,respectively. The number of adherent HUVECs was similar on PRX-RGDSo and Random-RGDSo, suggesting that it is not necessary toconsider the effect of the backbone of polymers on the cellularresponses Table 2Characteristics of PRX-RGDS and Random-RGDS surfaces [37]. All measurements wereperformed with n=3. *Group effect(P)=9.02×107; **p<0.01. Surfaces C.A.() Mf (x10) RGDS(mg/cm) PRX-RGDS0 74.9±2.6 0.078±0.028 N.D. PRX-RGDS 45.3±1.9 0.122±0.000 0.4±0.32 PRX-RGDS100 25.3±4.9 0.210±0.022 0.6±0.25 Random-RGDS 77.7±1.8 0.025±0.004 N.D. * 64.8±1.6 0.035±0.007 0.34±0.15 Random-RGDS5o Random-RGDS100 34.7±4.9 0.047±0.015 0.7±0.3 (B) FBS-/GFs- ¥]PRX Random Fig. 2. Adhesion of HUVECs on PRX-RGDS and Random-RGDS surfaces (A) with or (B) without 2% FBS and GFs determined by WST-1 assay.*and ** indicate a statisticallysignificant difference with P<0.05 and 0.01, respectively. The data are shown as mean ± SD (n=3).耐士科技 www.rysstech.com 400-188-0725 In the FBS+/GFs +medium, HUVECs adhered well and spreadwidely with FA and actin filament formation on PRX-RGDS5o,PRX-RGDS100, Random-RGDS5o and Random-RGDS100(Fig. 3). Nodifferences in cell spreading between PRX-RGDS and Random-RGDS were observed because the adsorbed serum proteins andGFs covered the surface and inhibited the effects of the mobilityof the attached RGDS molecules as well as reduced the numberof adherent cells (Fig. 2). In fact, we previously reported that asimilar amount of serum proteins and GFs was adsorbed ontoPRX-RGDS100 and Random-RGDS100[37]. It has been reported thatv+;ascular endothelial GF (VEGF) also activates the actin filamentformation through VEGF-2 receptor as well as integrin-bindingproteins such as fibronectin [51]. In the medium without FBS/GFs, HUVECs adhered with a round shape without any actin fila-ment formation (red color in images) on both Random-RGDSoand PRX-RGDSo because of the absence of a RGDS ligand. The morphology of adherent HUVECs was strikingly differentbetween Random-RGDS50,100 and PRX-RGDS50,100 surfaces. HUVECswere spread with a well-defined cytoskeleton, and FA matured andwas distributed at the edge of the cytoskeletal lamellae on Random-RGDSso and Random-RGDS100 surfaces. In contrast, adherentHUVECs on PRX-RGDS5o and PRX-RGDS100 showed unique morpho-logical features: the cytoskeleton did not form fibril structures andFAK was appearing at the edge of spinous lamellipodia.It has beenreported that fibril formation by the cytoskeleton in endothelialcells is suppressed on a soft substrate [11]. As shown in Fig. 4, the spreading area of adherent HUVECs was increased by the introduc-tion of RGDS and did not show any differences between PRX-RGDSand Random-RGDS surfaces in the FBS+/GF+ medium. In themedium without FBS/GFs, we observed a statistically significantdifference in the spreading area between PRX-RGDS and Random-RGDS surfaces. The spreading area of nonspecifically adhering Fig.55.. The relationship between the mobility factor (Mf) of each surface measuredusing QCM-D analysis and the spreading area of adherent HUVECs in the presenceof FBS and GFs. Fig. 3. Morphology of adherent HUVECs on PRX-RGDS and Random-RGDS surfaces. F-actin, phosphorylated FAK and nuclei are stained as red, green and blue, respectively. The scale bar is 20 um. (B)FBS-/GFs- Random Fig. 4. Spreading area of an adherent HUVEC on PRX-RGDS and Random-RGDS surfaces. * and ** indicate a statistically significant difference with P<0.05 and 0.01,respectively. The data are shown as mean±SD (n=6). www.rysstech.com HUVECs on PRX-RGDSo was larger than on Random-RGDSo. In con-trast, HUVECs on PRX-RGDS5o and PRX-RGDS100 were much smallerthan on Random-RGDS5o and Random-RGDS10o. Previously, wereported the adhesion of HUVECs on a PMB-PEG-PMB block copoly-mer and PMB-PRX-PMB without RGDS ligand [33,34]. In both cases,HUVECs could not adhere on PMB-PEG-PMB and PMB-PRX-PMBsurfaces without FBS/GFs.Therefore, HUVECs adhered to PRX-RGDSand Random-RGDS through the RGDS-integrin interaction, result-ing in the linkage between the surface of the material and the cyto-skeleton. Actually, we demonstrated that the interaction betweenHUVECs and surfaces was suppressed by the addition of cytochala-sin D in a previous report [37]. Morphological changes of HUVECs were induced to compensate for the internal/external forces withactin filament formation. The suppression of morphologicalchanges of adherent HUVECs on PRX-RGDS surfaces may have beencaused by these phenomena: the high mobility of RGDS moleculesreduced the external forces and inhibited the actin filament forma-tion. The relationship between the Mf and the spreading area ofadherent HUVECs in the medium without FBS/GFs is shown inFig. 5. HUVEC spreading was more strongly suppressed on PRX-RGDS surfaces than on the Random-RGDS surfaces. It was recentlyreported that the integrin-mediated actin filament formation is reg-ulated by mechanical cues such as shear flow and matrix rigidity[19]. FA and the cytoskeleton play an important role in connecting Fig. 6. Neurite outgrowth of PC12 cells on PRX-RGDS and Random-RGDS surfaces. (A) Adherent morphology of PC12 cells. The scale bar is 100 pm. (B) The number ofadherent PC12 cells in total and categorized by the length of neurites. * and ** indicate a statistically significant difference with P<0.05 and 0.01, respectively. The data areshown as mean±SD (n=4). the extracellular mechanical cues with intracellular signaling path-ways. In addition, it has been demonstrated that MSCs use tractionforces for the integrin clustering and reorganization with an RGDpeptide in nanoscale experiments [52]. We previously reported thatthe integrin B1-RGDS initial interaction is accelerated on PRX-RGDSsurfaces due to the high mobility of RGDS [37]. Nevertheless, here itdid not lead to the subsequent actin filament formation due to thehigh mobility of RGDS molecules resulting in the suppression ofadhesion and the immature morphology on PRX-RGDS5o and PRX-RGDS100 surfaces. 3.3. Neurite outgrowth of PC12 cells Fig. 7. Differentiation of P19CL6 cells into beating colonies on PRX-RGDS andRandom-RGDS surfaces.*and ** denote significant difference with P<0.05 and 0.01compared to both Random-RGDS5o and Random-RGDS100, respectively..andttdenote significant difference with P<0.05 and 0.01, respectively, compared to PRX-RGDSso. The data are shown as mean ±SD (n=4). Adherent morphology of PC12 cells on PRX-RGDS and Random-RGDS surfaces is shown in Fig. 6A, and the number of adherentPC12 cells with or without neurites is summarized in Fig. 6B. OnRandom-RGDS5o and Random-RGDS100, more than 65% of adherentPC12 cells were differentiated with neurites. In contrast, therewere no PC12 cells with a long neurite(s) and more than 80% ofadherent PC12 cells showed an undifferentiated state (roundshape) on PRX-RGDS50 and PRX-RGDS100. Differentiation of PC12cells after the addition of NGF was induced through the receptortyrosine kinase, TrkA, and the subsequent activation of the extra-cellular signal-regulated kinase(ERK) pathway,resulting in neuriteelongation [53,54]. This pathway of PC12 cells is activated by theintegrin-mediated signaling [55] and regulated by the cytoskele-ton, which senses the extracellular mechanical cues 56.It hasbeen reported that the neurite outgrowth of PC12 cells is stronglysuppressed on the softest substrate within the fibronectin-coatedPAAm hydrogels (7-19kPa) [57]. The behavior of PC12 cells onPRX-RGDS100 was remarkably similar to that on the soft substrate,suggesting that the mobility of RGDS molecules at the outermostsurface is primarily responsible for the suppressed differentiationof PC12 cells. 3.4. Differentiation of P19CL6 cells into beating colonies P19CL6 cells adhered both to PRX-RGDS and Random-RGDS sur-faces, and started to form embryoid body (EB)-like aggregates onPRX-RGDS100 after 48 h (Fig S3A, i, indicated with yellow arrows).After 10 days, the cell aggregates were found on Random-RGDS5oand Random-RGDS100, but not on PRX-RGDS5o (Fig. S3B). The beat-ing colonies were primarily observed on PRX-RGDS100 in 10 days,increased until 12 days and then gradually decreased (Figs. 3Band 7). In contrast, on Random-RGDS5o and Random-RGDS100,,beating colonies appeared in 14 days, increased rapidly until22 days and decreased thereafter. On the PRX-RGDS5o surface,the EB-like aggregates and beating colonies were not found duringthe observation period of 24 days. The cardiac differentiation of DMSO-treated P19CL6 cells pro-gresses through three phases: aggregation, differentiation into abeating colony and its maturation. It was reported that the forma-tion of EB-like aggregates is necessary for the cardiac differentia-tion of DMSO-treated P19CL6 cells through activation of theoxytocin pathway [58,59]. On the PRX-RGDS100 surface, P19CL6cells spread out poorly and cell-cell interactions were promoteddue to the high mobility of the RGDS motif; this result was similarto the cellular behavior on soft substrates [60]. Consequently, therapid formation of EB-like aggregates might enhance the cardiacdifferentiation of P19CL6 cells. P19CL6 cells did not form theEB-like aggregates, and hence did not differentiate into beating col-onies on the PRX-RGDS5o surface. The molecular mechanisms thatunderlie the aggregation of DMSO-treated P19CL6 cells in a mono-layer culture on different substrates are poorly understood [61].The different behavior of DMSO-treated P19CL6 cells on PRX-RGDS5o and PRX-RGDS100 meant that the slight differences in RGDS mobility affected the cell aggregation. Subsequent maturation ofbeating colonies was induced by the mechanotransduction withactin filament formation. Jacot et al. reported that beating cardiacmyocytes mature to adapt to the substrate elastic modulus(~10 kPa) with actin filament formation via the integrin-mediatedRho/ROCK pathway [62]. Moreover, integrin-mediated Rho/ROCKand Rac/PAK pathways control the contraction and relaxation cycleof beating cells [63].Many studies showed that the actin filamentformation as a result of Rho/ROCK signaling cannot be activatedbecause the cells do not sense a sufficient traction force on softsubstrates [12].According to the adhesion morphology, the actinfilament formation of adherent P19CL6 cells was not activated onPRX-RGDS100. On the other hand, P19CL6 cells confluently adheredand spread, suggesting that actin filament formation was activatedon the Random-RGDS surfaces. Accordingly, on PRX-RGDS100, thebeating colonies did not mature and stopped beating at the earlystage of differentiation, but they matured on Random-RGDS sur-faces, resulting in long-term beating. 3.5. Effect of RGDS mobility on different cells The adhesion and morphological changes of HUVECs, neuronaldifferentiation of PC12 cells and the maturation of beatingP19CL6 cells were remarkably suppressed on the highly mobilePRX-RGDS100 surfaces. These results indicate that the mobility ofRGDS ligands on the outermost surface can regulate the cellularfunctions related to actin filament formation in accordance withelastic modulus of the substrate's material. Stem cells such asembryonic stem cells and induced pluripotent stem cells hold greatpromise for regenerative medicine because of the strong ability todifferentiate into various types of cells. For clinical applications,the development of culture substrates for undifferentiated growthof stem cells is absolutely required. Actin filament formation alsomodulates the stem cell fate, including a lack of differentiation[15-17,64].It has been reported that an undifferentiated state ofstem cells could be maintained on soft substrates via reductionof the cell-matrix traction. However, soft substrates are not suit-able for the cultivation and self-renewal of undifferentiated stemcells due to the terribly low cell adhesion. The highly mobilePRX-RGDS10o surface that could suppress the integrin-mediatedmechanotransduction with good cell adhesion is expected to serveas an excellent substrate for maintenance of the undifferentiatedstate of stem cells. 4. Conclusion In this work, adhesion and morphological changes of HUVECsand the differentiation or maturation of PC12 and P19CL6 cellswere assessed on surfaces containing either mobile or immobileRGDS ligands. PRX-RGDS and Random-RGDS surfaces possess thesame hydrophilicity and bulk rigidity (E≈ 25-100MPa), and dif-ferent hydrated viscoelasticity (Mf) when surfaces with similarRGDS composition are compared. We demonstrated that the cellu-lar behavior related to actin filament formation was remarkablysuppressed on PRX-RGDS surfaces. This finding suggests that theintegrin-mediated mechanotransduction and the corollary cellularfunctions are regulated by the molecular mobility of the cell-adhe-sive domain, i.e.,the outermost surface of biomaterials. For exam-ple, the highly mobile PRX-RGDS surfaces hold promise as a culturesubstrate for immature stem cells to support their self-renewal inthe long run. 5. List of abbreviations Appendix A. Figures with essential colour discrimination Certain figure in this article, particularly Figs. 1, 3 and 6 isdifficult to interpret in black and white. The full colour imagescan be found in the on-line version, at: http://dx.doi.org/10.1016/i.actbio.2014.11.020. Appendix A. Supplementary data Supplementary data associated with this article can be found, inthe online version, at http://dx.doi.org/10.1016/j.actbio.2014.11.020. ( References ) [1] Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellularmicroenvironments for morphogenesis in tissue engineering. Nat Biotechnol2005:23:47-55. [2] Place ES, Evans ND, Stevens MM. Complexity in biomaterials fortissueengineering. Nat Mater 2009;8:457-70. [3]11Lutolf MP, Gibert PM, Blau HM. Designing materials to direct stem-cell fate.Nature 2009;462:433-41. [4]] 0Cha C, Liechty WB, Khademhosseini A, Peppas NA. Designing biomaterials todilnirect stem cell fate. ACS Nano 2012:11:9353-8.y + [5]1Higuchi A, Ling Q-D, Chang Y, Hsu S-T, Umezawa A. Physical cue ofbiomaterialsguide stem celL ddiifferentiation fate. Chem Rev2013:113:3297-328. 61Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated bysubstrate flexibility. Proc Natl Acad Sci USA 1997;94:13661-5. [77IDischer DE, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness oftheir substrate. Science 2005:310:1139-43. [8]Engler AJ, Sen S, Sweeney HL, Disoher DE. Matrix elasticity directs stem celllineage specification. Cell 2006;126:677-89. [9] Levental I, George. Janmey PA. Soft biological materials and their impact oncell function. Soft Mattef 2006;2:1-9. [10]/Alexander NR, Branch KM, Parekh A. Clark ES, Iwueke IC, Guelcher SA, et al.ExtraceHlular matriixx rigidity promotes invadopodia activity. Curr Biol200818:1295-9. ( [1 1 ] Juin A , Planu s E G uille m ot F , H o rakova P , A lbiges-Rinzo C, G enot E, e t a l. Ext r r a cellul ar matr ix ri gid i t y control s podosome i nduction i n m icr o vascular e n n d o t h e l i al ce l l s. Biol Cell 2 0 13 ;1 05:46 - 5 7. ) [12] Nemir SS,, West JL. Synthetic materials in the study of cell response to substraterigidity.Ann Biomed Eng 2010;38:2-20. [I113]LLeipzigNND,Schoichet MS. The effect of substrate stiffness on adult neural stemcell behavior. Biomaterials 2009:30:6867-78. 1[114]Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY,Speicher DW, et al.Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity:scar-like rigidity inhibits beating. J Cell Sci 2008;121:3794-802. [15] Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived humanmesenchymal stem cells become quiescent on soft substrates remain.responsive to chemicalormechanicalstimuli.TissueEngPartA2009:15:147-54.4. [16]Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P,et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal inculture. Science 2010:329:1078-81. ( [17] ( C how d hu r y F, Li Y , Poh Y C, Yo k ohama-Ta m aki T , Wang N, T a naka TS . S o ft substrates promote h omogeneous se l f-renewal of e m bryo n ic st e m c e lls via downre gu lating cell - matrix tractions. PLoS ONE 2010;5:e15655 . ) [18]VogelV, Sheetz M. Local force and geometry sensing regulate cell functions.Nat Rev Mol Cell Biol 2006;7:265-75. 191Buxboim A, Lvanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of nucleus: how deeply do cells ‘feel' outside and in? j Cell Sci 2010;123:297-308. [20]IProvenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling.J Cell Sci 2011;124:1195-205. [21]1Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011:475:316-23. |22|Mammoto A. Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 2012;125:3061-73. [23]JJalali S, Pozo MA, Chen KD, Miao H, Li YS, Schwartz MA, et al. Integrin- mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 2001;98:1042-6. [24]Ivaska J. Unanchoringintegrinsinfocaladhesions.Nat CellBiol 2012;14:981-3. [25] IBalaban NQ, Schwarz US, Riveline D,Goichberg P, Tzur G,Sabanay I, et al. Force and focal adhesion assembly: a close relationship studies using elastic micropatterned substrates. Nat Cell Biol 2001;3:466-72. ( [26] Jianping F, Yang-Kao W, Mi c hael T Y , R a vi A D , Xi a ng Y, Z h i jun L, et al . Mechanical r egulation o f c ell f unction w i t h g eometrically m odula t ed ela s tom e r i c substr at es.Nat M eth o d 20 1 0;7:733- 6 . ) ( [27] N ikkhah M, Ed a l a t F , Manoucheri S, Khademhosseini A . Eng i n e ering microscale topogra p hies to c ontro l the cell-subs t rate i nterface. B ioma t erials 20 1 2:33:5230-46. ) ( [28] T rappmann B, Gautr o t JE, C o nnel l y JT , S trange D G , L i Y , Oyen ML , et al . E xtracellular-matri x t et h ering r egu l a t es ste m -cel l f f a te . Na t M at e r20 1 2:1 1 :642-9. ) ( [29] T sai J , Kam LC . L ateral mobility of E-cadherin enhances R ac1 r e sponse i n e pithelial cells. Cel l M ol Bioeng 201 0; 3:84-90. ) ( [30] Andreasson-Ochsner M, R omano G , Hakanson M , S m it h ML, Leckband ED, T ext o r M , e t a l . Sin g le cell 3 -D p l atform to st u dy lig a nd mobility in c e ll - cell contact. Lab Chip 20 1 1;11:2876-83. ) ( [31] I n ou e Y, Y e L , I s h ih a ra K, Y u i N . P r epa r ation a n d s u r face p r o pert ie s of polyrotaxane-containing t ri-bl o ck cop o l ym ers a s a de s ign f f or d y namic biomaterials surfaces. Colloids Surf B: Biointerf 2012;89:223-7. ) ( [32] Kaki n oki S , Yui N, Yam a oka T . P l atelet r esponses t o d ynamic b i omaterials surfaces wit h different poly(ethylene g l ycol) and polyro t axane mo lecular a rchitectures constr u cted o n gold substrates. J Biomat A ppl 2 012 ; 28:544- 5 1 . ) ( [33] S e o J H, Kakin o ki S, Inoue Y, Yam a oka T,I s hihara K, Y ui N . D esigning dy n amic s ur f aces f o r r egul a tion o f b iological r espon s es. S oft M at t er 2 0 12;8:5477-85. ) ( [34] K a kin o ki S, S eo J H, I no u e Y , Ishihara K, Yui N, Yamaoka T . A l a rge mobility of ,seojh, i n hy drophilic m olecules a t t h e o u tmost la y er co n tr o ls th e p r o tein adsorption and a dh e ring b e havior w ith the actin fiber ori e ntatio n o f human u m bilical vein e ndothe l ial c ells ( HUVEC). J Biomater S ci P olym Ed 2 013;24:13 20 -32. ) ( [35] S eo JH, Y ui N . T he e ffect of molecular mobility of supra m olecula r polym e r s urfaces on fib r oblast adhesi on . B i omat e rials 2 0 13;3 4 :55-63. ) [36] Seo JH, Kakinoki S, Inoue Y, Nam K, Yamaoka T, Ishihara K, et al. Thesignificance of hydrated surface molecular mobility in the control of themorphology of adhering fibroblasts. Biomaterials 2013;34:3206-14. [37]Seo JH, Kakinoki S, Inoue Y, Yamaoka T, Ishihara K, Yui N. Inducingg rabidcellular response on RGD-binding threaded macromolecular surfaces.j AnChem Soc 2013;135:5513-6. ( [38] O oya T, E guc h i M , Yu i N . Supramolecular desi g n for mul t i valen t int e racti on: m altose mobility alon g pol y rotaxane e n hanc ed bi n d ing with concan a v a lin A. A m Chem Soc 2003;125:1 3 016- 7 . ) ( [39] D a vies P F, T r ipathi SC. Mechanica l stres s mechanis m s s a a i nd t h e c e l l. An endotheli a l paradigm. C irc Res 1 993;72:239-45. ) ( [40] G reene L A , T i s chl e r A S . E s t ab lish m ent of a n o r adren e rgi c cl o n al l in e o f rat adrenal p he ochro m ocyto ma cell s w h ich r e spond t o nerve g rowth fa c t o f . ProcNatl Acad Sci USA 1 976:73:2424- 8 . ) ( [41] L ee JH, L ee HY, Kim H W . Adhesive p r oteins li nk ed w ith f oca l adh e s io n kinase r egulate n eur i te o utgrowth o f PC12 ce l ls. Act a Biomate r 2012;8:165 - 72. ) ( [42] M iskon A, Ehashi T, Mahar a A ,A , U yUaymaam Ha , H, Y am aoka T . Beati n g beh pri ma ry n e o natal card i o m yo c yt es an d cardiac -d iff e r entiate d P 1 9 .CL 6 cell s on d i f ferent extracellular m a trix c omp o n e n t s . J A r t i f O r gans 200 9 ; 1 2:11 1 -7. ) ( [43] I shihara K, Ueda T , N a k abayashi N . P r ep ar at ion o f p h osp h oli pi d po l ymers and ) ( thei r properties a s polym e r h ydr o gel membranes . Poly m j 1990;22:355-60 ) [44]Fields CG, Lloyd DH, Macdonald RL, Otteson KM, Noble RL. HBTU activation for ( automated F moc solid - pha syn t h es i s. Pe p t Res 1 991;4:95-101. [45] C o llins T J. Imagej f o r m ic r e DSSC y . Bi o Techni que s 2007;4 3 :S25-3 0 . ) ( [46] McBurney MW, Jo n es-Villeneuve EMB, Edwards MK S , Anderson PJ . Contro l o f mu s cle and n eur o nal d i f f erentiat i on i n a cultured embryonal c a rcinoma cell l in e . Nature 1982 ; 299:165- 7 . ) ( [47] H abara-Ohk ub o A. Di f ferentiation o f be a ting ca r diac mu s cle cel l s fr o m a d er ivative of P19 em b ryonal carcinoma cells. Cell S tru c t Fu n c 1996;21:10 1 - 1 0 . ) ( [48] I shihara K, Zia t s N P , Tierney BP, Nakabay a shi N, Anderson J M. P r otein adsorption from h u man p l asma is reduced on ph o spholipid po l ymers. j Biomed Mater R es 1 991:25:1 3 97-407. ) [49]Ishihara K, Oshida H, Endo Y, Ueda T, Watanabe A, Nakabayashi N.Hemocompatibility of human whole blood on polymers with a phospholipidpolar group and its mechanism. J Biomed Mater Res 1992;26:1543-52. [501Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why dophospholipid polymers reduce protein adsorption? J Biomed Mater Res1998;39:323-30. ( [51] I keda S,U s hio-Fukai M, Zuo L , Toj o T, D ik alov S, Pa t rushev NA, et al . N o v e l role of ARF6 i n 1 vascular endothelial g rowth f a ctor-induced s i gnaling g a n d angiogenesis. Circ Res 2005;96:4 6 7-75. ) ( [52] H uebsch N, A rany PR, Mao A S , Shvartsman D . A l i OA, B e ncher i f SA , e t Harn e ssin g t ractio n -m e di a ted m a n ipulation of t he cell-matrix i n terfac e e to control stem cell fate. Nat M a te r 2010:9:518 - 26. ) ( [53] j B B ene d itti M , L e vi A . Ch a o M V. D i fferenti a l exp r e s si o n o f n er v e g rowth f a cto r receptors l eads t o alte r ed binding a ffinity and neurotroph i n respo nsiven ess. P r oc Natl Acad Sc i USA 1993:90:7859-63. ) ( [54] V audry D, S t ork PJS, Lazarovic i P , E iden L E. Signaling pat h ways f o r PC1 2 cell d ifferentia ti on: Ma k in g t he r ig h t conn e cti o ns. Science 20 02;296: 1 64 8 - 9 . ) ( [55] W alowitz JL , Roth J A . Acti v ation of ERK1 and E RK 2 i s r e quir ed f o r m anganese- i nduced neurite out g rowth in r at pheochromoc yto m a (PC1 2 ) cells . J Ne uro sci R es 199 9 ;57: 8 47- 5 4. ) [56]Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth role of celladhesion molecules. Ann NY Acad Sci 2004;1014:140-54. [571Leach JB, Brown XQ. Jacot JG, DiMilla PA, Wong JY. Neurite outgrowth andbranching of PC12 cells on very soft substrates sharply decreases below athreshold of substrate rigidity.J Neural Eng 2007;4:26-34. [58] Paquin J, Danalache BA,,Jankowski M. McCann SM, Gutkowska J. Oxytocinindduced differentiation of P19 embryonic stem cells to cardiomyocytes. ProcNatl Acad Sci USA 2002;99:9550-5. [59]] Fathi F, Murasawa S, Hasegawa SS, Asahara T, Kermani AJ, Mowla SJ. Cardiacu1 F,differentiation of P19CL6 cells by oxytocin. Int J Cardiol 2009;134:75-81. [60]Guo W-H, Frey MT Burnham NA, Wang Y-L. Substrate rigidity regulates theformation and maintenance of tissues. Biophys J 2006;90:2213-20. [61] Hyden MAG, Defize LHK. Twenty one years of P19cells: what an embryonalcarncinoomat cell line taught us about cardiomyocyte differentiation. CardiovascRes 2003;58:292- [62]JJacot JG, iMcCulloch AD, Omens JH. Substrate stiffness affects the functionalmaaturatioon neonatal rat ventricular myocytes. Biophys2008:95:3479-87. ( [6 3] RRa ymond K, C a gne t S, Kreft M , Ja n ssen H , Sonnenb er g A . Glukhova MA. Contr o l ma m mar y myo e pithe l ial cel l co n tractile f u nction by a3b1 i n tegri n signaling.EMB O J 2011;30:1 8 96-906. ) ( 64] G u i la k F, Cohen DM, Estes BT, Gim b le J M , Liedtk e W, Chen CS. Co n trol of st e m c ell fate b y p hysical i nteraction s with t h e extracellul a r matrix. Ce l l Stem Cel l 2009:5:17-26. ) 士科技www.rysstech.com www.rysstech.com耐士科技
确定







还剩8页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








