
An efficient route to dipyridone acid HIV integrase inhibitors is developed. The key steps include a onepot three-step formation of the core template (containing one point of structural diversity) followed by a regioselective benzylation and in situ deprotection to afford the title compounds.
方案详情

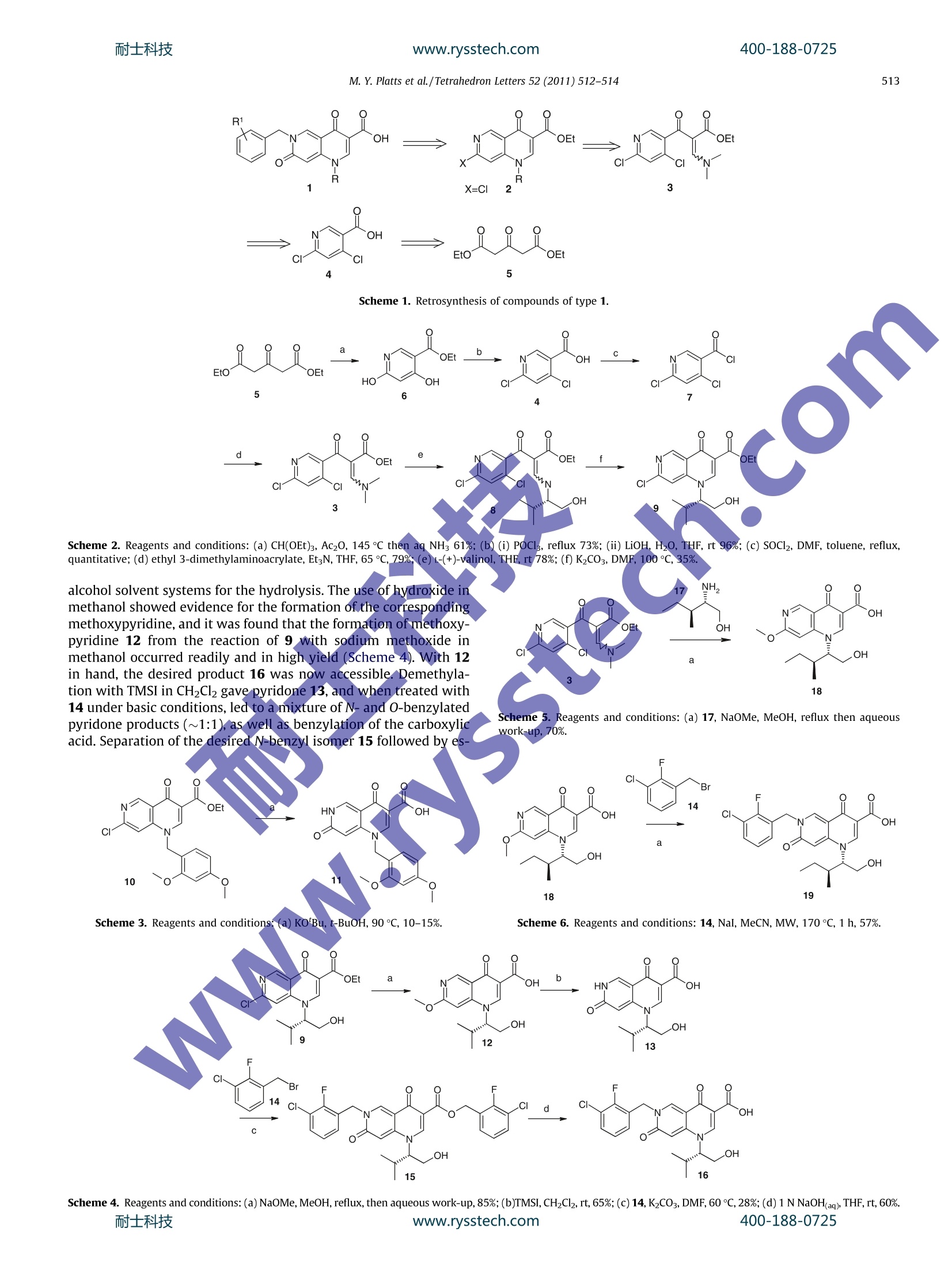
耐士科技400-188-0725www.rysstech.comTetrahedron Letters 52 (2011) 512-514 耐士科技400-188-0725www.rysstech.com513 Contents lists available at ScienceDirect Tetrahedron Letters ELSEVIER journal homepage:www.elsevier.com/locate/tetlet A concise synthesis of HIV integrase inhibitors bearing the dipyridone acid motif Michelle Y. Platts *, Christopher G. Barber, Jean-Yves Chiva, Rachel L. Eastwood, David R. Fenwick,Kerry A. Paradowski, David C. Blakemore Pfizer Global Research and Development, Sandwich Laboratories, Ramsgate Road, Sandwich, Kent CT13 9NJ, UK ARTICLE:INFO ABSTRACT An efficient route to dipyridone acid HIV integrase inhibitors is developed. The key steps include a one-pot three-step formation of the core template (containing one point of structural diversity) followed by aregioselective benzylation and in situ deprotection to afford the title compounds. Article history:Received 15 July 2010Revised 9 November 2010Accepted 19 November 2010 1Available online 24 November 2010 Keywords:Dipyridone acidIntegraseHeterocyclesAntiviral agentsRing-closure In connection with a programme to discover novel HIV integr-ase inhibitors, we report our successful exploits in the preparationof 6-benzyl-1-alkyl-4,7-dioxo-1,4,6,7-tetrahydro-1,6-naphthyri-dine-3-carboxylic acids (dipyridone acids) 1, Figure 1. From theoutset, we desired a facile and reliable method for the preparationof dipyridone acids 1, which would allow rapid access to a range ofanalogues in order to studythe structure-activity relationships(SARs) in this series. Our retrosynthetic analysis (Scheme 1) reveals that 1 may bederived from the hydrolysis and alkylation of pyridine ester 2.The bromo analogue of 2 (X=Br, R=CH2CH3) has been previously,reported by Strehlke and was synthesised in nine steps from 2-amino-5-methylpyridine. We required a more concise route to2, which we believed could be formed from the addition of a suit-able amine to ethyl (2E/Z)-2-[(4,6-dichloropyridin-3-yl)carbonyl]-3-(dimethylamino)prop-2-enoate (3), followed by ring-closureonto the chloropyridine moiety. Compound 3 should be accessiblefrom 4,6-dichloronicotinic-3-carboxylic acid (4), which can be pre-pared from diethyl 1,3-acetonedicarboxylate (5). As outlined in Scheme 2, acid 4 was synthesised in three-stepsfrom commercially available 1,3-acetonedicarboxylate 5. Reactionof 5 with triethyl orthoformate in acetic anhydride, followed bycyclisation with aqueous ammonia, gave 6. Treatment of 6 withphosphorus oxychloride under reflux conditions followed by esterhydrolysis under basic conditions gave the desired acid 4.4 Com-pound4 was readily converted into the acid chloride 7 and wassubsequently treated with ethyl 3-dimethylaminoacrylate, using ( * C orresponding author. Tel.: +44 130 464 9141; fax: +44 130 4651821. E-mail address: michelle.plat t s@pfizer.com (M.Y. Platts). ) 、c the conditions developed by Shinkai and co-workers, to provideenaminoate 3. Enaminoate 3 reacted readily with a series ofamines. In this example it reacts with L-(+)-valinol to provide 8,and after cyclisation under basic conditions, afforded ketoester 9in moderate yield. This step was unoptimised, but allowed accessto sufficient material to provide key analogues for our SAR analysis.We then attempted chloropyridine-ketoester hydrolysis to thecorresponding pyridone acids directly. We anticipated that hydro-lysis of intermediate 10, for example (prepared from 2,4-dime-thoxybenzylamine using the route outlined in Scheme 2), usingsodium hydroxide would be straightforward. However, initial worksuggested that this reaction was far from trivial and a range of bothacidic and basic hydrolysis conditions met with little success; ourmost successful attempt to form a pyridone acid utilised potassiumtert-butoxide in t-butanol, which converted ketoester 10 into thedesired compound 11, albeit in poor isolated yield (Scheme 3). As expected, in the presence of sodium hydroxide it appearedthat the initial ester hydrolysis was facile (by LCMS) while dis-placement of the chloro substituent was hampered due to the poorsolubility of the intermediate chlorocarboxylates in the reactionsolvents used (water, DMSO and DMF). This led us to investigate Figure 1. ( 0040-4039/$ - s ee front ma t ter @ 201 0 Else v ier Ltd. All rights reserved.doi:10.1 0 16/j.te t let . 2010.11 . 112 ) M. Y. Platts et al./Tetrahedron Letters 52 (2011)512-514 Scheme 1. Retrosynthesis of compounds of type 1. Scheme 2. Reagents and conditions: (a) CH(OEt)3, Ac20, 145 ℃ then aq NH 61%; (b)0(i) POClg, reflux 73%; (ii) LiOH, H20, THF, rt 96%; (c) SOCl2, DMF, toluene, reflux,quantitative; (d) ethyl 3-dimethylaminoacrylate, Et3N, THF, 65 ℃, 79%;(e)L-(+)-valinol, THE, rt 78%; (f) K2CO3, DMF, 100 C, 35%. alcohol solvent systems for the hydrolysis. The use of hydroxide inmethanol showed evidence for the formation of the correspondingmethoxypyridine, and it was found that the formation of methoxy-pyridine 12 from the reaction of 9 with sodium methoxide inmethanol occurred readily and in high yield (Scheme 4). With 12in hand, the desired product 16 was now accessible. Demethyla-tion with TMSI in CH2Cl2 gave pyridone 13, and when treated with14 under basic conditions, led to a mixture of N- and O-benzylatedpyridone products (~1:1), as well as benzylation of the carboxylicacid. Separation of the desired N-benzyl isomer 15 followed by es- Scheme 5. Reagents and conditions: (a) 17, NaOMe, MeOH, reflux then aqueouswork-up, 70%. Scheme 6. Reagents and conditions: 14, NaI, MeCN, MW, 170℃, 1 h, 57%. Scheme 4. Reagents and conditions:(a)NaOMe,MeOH, reflux, then aqueous work-up, 85%;(b)TMSI, CHzClz,rt, 65%;(c)14,K2CO,,DMF,60℃, 28%;(d)1N NaOH(aq), THF,rt, 60%.耐士科技 www.rysstech.com 400-188-0725 M. Y. Platts et al./Tetrahedron Letters 52 (2011) 512-514 Figure 2. ter hydrolysis afforded dipyridone acid 16 in reasonable yield. Gi-ven the difficult isolation of intermediate 13 and the subsequentlow yielding unselective alkylation we decided to investigate amore direct and selective route to compounds of type 1, sincethe synthetic complexity of the existing route precluded an effi-cient elaboration of the SAR. The first improvement made to the route was to telescope thethree-steps of amine addition, ring-closure and chloro to methoxyconversion into a single step. This was achieved by reacting enami-noate 3 with the desired amine, in this case 17, in the presence ofsodium methoxide in methanol at reflux,to afford the correspond-ing product 18 in an impressive overall yield, (Scheme 5). The abil-ity to utilise the methoxide as base and nucleophile in such asequence is, to our knowledge, unprecedented. We then explored a further improvement to the route which involved direct benzylation of the methoxypyridine intermediate18. It was anticipated that benzylation would occur selectivelyon the pyridine nitrogen and that the resulting quaternary ammo-nium salt would be readily demethylated using an appropriatenucleophile. Under the conditions reported by Bowman and Bridge,who had shown a similar reaction with 2-methoxypyridine to besuccessful, we attempted the regioselective synthesis of N-benzylpyridone 19. Our initial results in refluxing MeCN showed no reac-tion, although pleasingly, at the higher temperature achieved un-der microwave conditions,,thedesiredreaction did occur.indicating that the pyridine nitrogen in our system was less nucle-ophilic than that of methoxypyridine (Scheme 6).Under theseconditions N-benzylation and demethylation occurred exclusivelyto afford dipyridone acid 19. Similar methodology was applied to prepare further analoguesof compound 19 (Fig.2). These derivatives possessed potent anti-HIV integrase activity and a further explanation of theSAR willbe described elsewhere. In summary, a novel and concise synthetic route to dipyridoneacids of type 1 has been achieved. Significant improvements madeto the initial route made for a highly efficient synthesis of cyclisedmethoxypyridine intermediatesandregioselective N-benzyldipyridone acid preparation. Late stage N-benzyl variation has al-lowed rapid access to a range of analogues. A key feature of this work has been the reduction of the route complexity as a meansto explore rapidly the SAR. References and notes 1. Semenova, E.A.; Marchand, C.; Pommier, Y. Adv. Pharmacol. 2008,56,199. 2Strehlke, P. Eur. J. Med. Chem. 1977, 12, 541.34 .DDen Hertog Recl. Trav. Chim. Pays-Bas Belg. 1946, 65,129. Wallace, E.; Hurley, B.; Yang, H. W.; Lyssikatos, J.; Blake,J. U.S. Patent 049419,2005: Chem. Abstr. 2005,142,298094. 5 Fucini, R. V.; Hanan, E. J.; Romanowski, M. J; Elling, R. A.; Lew, W.; Barr, K. J.; Zhu,, J.; Yoburn,J. C.; Liu, Y.; Fahr, B. T; Fan,J.; Lu,Y.; Pham, P.; Choong, I. C.; VanderPorten, E. C.; Bui,M.; PurkeyA, H. E.; Evanchik,M. J.; Yang, W. Bioorg. Med.Chem. Lett.2008, 18,5648. 6.Sato, M.; Motomura, T.; Aramaki, H.;Matsuda, T.; Yamashita, M.; Ito, Y.; ( Kawakami, H.; Matsuzaki, Y.; Watanabe, W. ; Yamataka, K.; Ik e da, S.; Kodama, E. , Matsuoka, M.; Shinkai, H.J. M ed . C hem.2006,49,1506. ) 7.Preparation of 18. To a stirred suspension of3 (100 mg, 0.32 mmol) in MeOH (10 mL) was added17 (41 mg, 0.35 mmol) and the mixture was stirred at rt for 1 h. NaOMe(170 mg, 3.15 mmol) was added portionwise and the mixture was stirred atreflux for 4 h. The resulting mixture was concentrated under reduced pressureand diluted and acidified to pH 1 with 2 N HCl (aq). The aqueous was extractedwith 5% MeOHand CH2Cl2(15 mL) and the organics dried (Na2SO4), filtered andconcentrated under reduced pressure. The crude product was purified bychromatography (silica gel, eluting with 100% heptane-100% EtOAc) to provide18 as a white foam (71 mg, 70% yield). HNMR (400 MHz, CDCl3): 8=9.34(1H,S), 8.82 (1H,s), 6.79 (1H, s), 4.34(1H,m), 4.17-4.05 (5H, m), 2.19 (1H,m), 1.24(1H,m), 1.13(3H,d,J=6.6Hz), 1.03 (1H,m), 0.82 (3H,t, J=7.4Hz). LRMS (CI): 319[M-H]. Bowman, W. R.; Bridge, C. F. Synth. Commun. 1999,29, 4051. 8For an example of microwave-assisted 2-methoxypyridine quaternisation andMeO-group deprotection in one-pot, see: Tielmann, P.; Hoenke, C. TetrahedronLett. 2006, 47,261. 10. FPreparation of 19. ( T o a stirred suspension of 18 (7 1 mg,0.22 mmol) in M eCN (1 m L ) was added 14 (109 m g, 0 .49 mmol) and NaI (66 m g, 0.44 mmol) and the mixture was heated under m icrowave ir r adiation (Biotage, I n itiator 8 ) at 1 70C for 1 h . The r esulting mixture w as concentrated un d er re d uced pre s sure, dis s olved in CH2Cl2 ( 5 mL), washed with 1 0% aqueous N a 2S203 s o lution (5 mL), dried aq (Na2SO4), filtered and concentrated u nder reduced pressure. The crude product was p urified by reverse phase HPLC ( MeCN/H2O/TFA 5%/95%/0.1%, fl o w rate20 mL/min, column : Phenomenex C18 100A 150×15mm, 1 0 micron) toprovide 19 as a yellow solid (57 mg,57% yield). HNMR(400 MHz, DMSO-d6): 8=9.15 (1H,s), 8.67 (1H, s), 7.54( 1 H, dt,J=7.36, 2.32 Hz), 7.26-7.17(2H,m ) , 6 .78 ( 1H, s ), 5.43 (2H, s) , 5.1 5 (1H , t,J=5.13 Hz), 4.45 (1H, m), 3.84 (1H,m),3.65 (1H , m), 2.07 (1H, m), 1.24 (1H,m), 1 .04 (3H,d,J=6.50 Hz), 1.03 (1H,m ) , 0.77 (3H,t,J=7.40 Hz). L RMS (CI*): 449/451 [ M+H]t. ) ww.rysstech.com耐士科技 耐士科技www.rysstech.com
确定


还剩1页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案(微波合成仪)》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案(微波合成仪)》用到的仪器有
相关方案
更多
该厂商其他方案
更多








