
方案详情
文
Abstract—The reactions of 3-substituted 5-aminopyrazoles with arylidenepyruvic acids and their synthetic precursors, pyruvic acid and aromatic aldehydes, were studied. Several different reaction pathways for these cyclocondensation reactions were established depending on the reaction conditions and building block selection. The formation of pyrazolo[3,4-b]pyridine-6-carboxylic acids as major products and related compounds was discussed from the mechanistic point of view.
方案详情

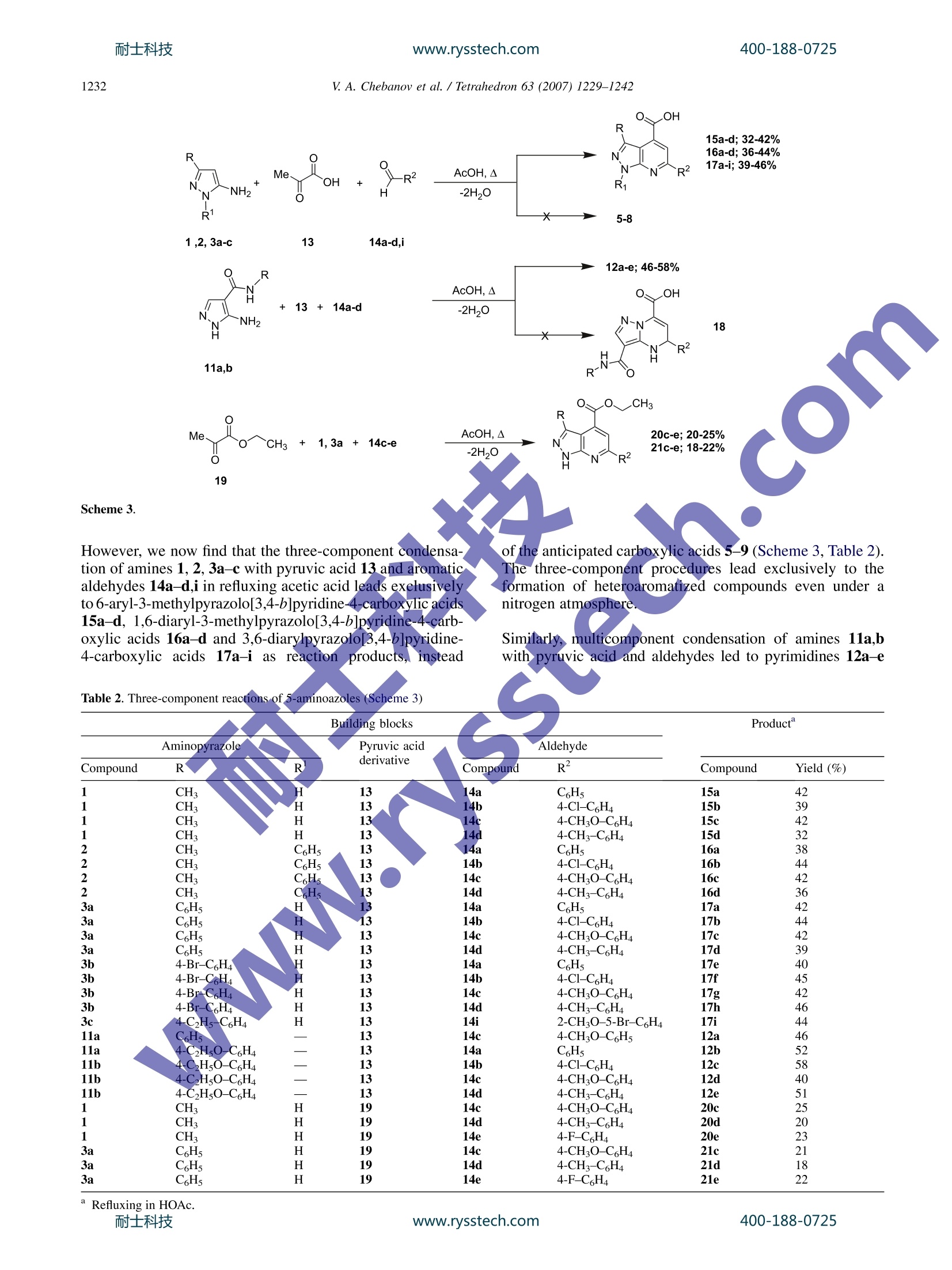
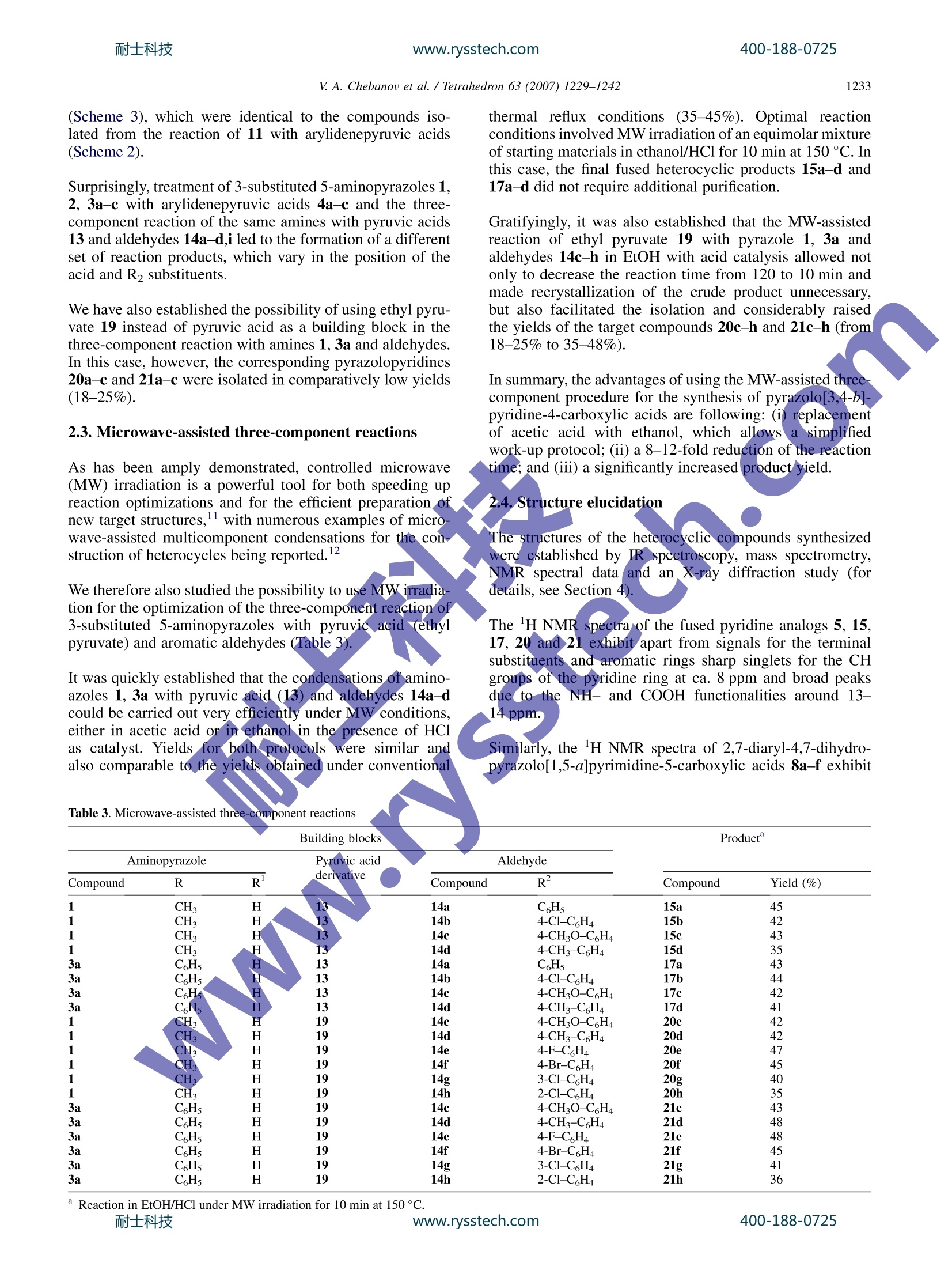
耐士科技400-188-0725www.rysstech.comAvailable online at www.sciencedirect.comTetrahedronTetrahedron 63 (2007)1229-1242 400-188-0725耐士科技www.rysstech.com1230 Cyclocondensation reactions of 5-aminopyrazoles, pyruvicacids and aldehydes. Multicomponent approaches topyrazolopyridines and related products Valentin A. Chebanov,a,b,* Yana I. Sakhno, Sergey M. Desenko,Vitaliy N. Chernenko,Vladimir I. Musatov, Svetlana V. Shishkina,Oleg V. Shishkin and C. Oliver Kappe’ ‘Department of Heterocyclic Compounds Chemistry, State Scientific Institution Institute for Single Crystals of National Academy of Sciences of Ukraine, Lenin Avenue 60, 61001 Kharkiv, Ukraine Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, A-8010 Graz, Austria mo Received 23 August 2006; revised 1 November 2006; accepted 16 November 2006Available online 11 December 2006 1. Introduction Cyclocondensations of aminoazoles and aminoazines witha,B-unsaturated ketoness ortheir synthetic precursors-aldehydes and ketonescontaining atLIleast twoactivehydrogen atoms-are the most widespread and investigatedpathways to fused dihydroazaheterocycles. In most cases,both types of reaction pathways,i.e., a sequential protocolinvolving the initialsynthesisof the (a,B-unsaturatedcompounds and the three-component reaction,n, yield thesame products. However, in rare cases the direct multi-component procedure may lead to the formation of differentproducts,connected to the complex reaction mechanisms ofthese transformations.3b,c Reactions of pyruvic acid derivatives with dinucleophileshave been applied for the synthesis of various types ofheterocycles since the beginning of the last century. Among号those, reactions involving acetyl-4 and arylidenepyruvic2b,5acids have been studied intensively. On the other hand, ( Keywords: H e terocycles; C y clocondensation; Mu l ticomponent reac t ions; Microwave-assisted o r ganic s y nthesis; Pyruvic a c id derivatives; 5-Amino-pyrazoles. ) ( * C orresponding author. Tel.: +380 57 341 01 91;fax: + 3 80 57 34 0 93 43 ; e-mail: c h e banov@isc.k h a r kov.com ) multicomponent reactions of pyruvic acid leading to hetero-cycles have rarely been investigated. Among those arepublications devoted to the treatment of pyruvic acidwith aromatic amines and aldehydes yielding either quino-linecarboxylic acids or pyrrolidines. Multicomponentreactions of pyruvic acid with aminoazoles have so far notbeen described. Previouslyywereported2b,c two alternative syntheticpathways to fused pyrimidinecarboxylic acids: by treatmentof 3-amino-1,2,4-triazole, 5-aminotetrazole and 2-amino-benzimidazole with either arylidenepyruvic acids or withpyruvic acid and aldehydes (Scheme 1). At that timewe noted that these three-component reactions carried outin refluxing DMF were not regioselective and yielded twoiSOmers. Our interest in pyridine and pyrimidine heterocycles con-taining fused pyrazole rings is mainly caused by the knownbiological activity of these systems reported in the litera-ture. On the other hand, reactions of the 5-aminopyrazolenucleus, having at least three nucleophilic centres, withpyruvic acid derivatives, which also possess several reactivecentres, can lead in several directions and are thereforeof interest from the point of view of their chemo- andregioselectivity. V. A. Chebanov et al./ Tetrahedron 63 (2007) 1229-1242 Ar Scheme 1. 2. Results and discussion procedures.10a,b Azomethine 22 was obtained as reportedin the literature.10c In this article, we disclose our results on the condensationreactions of 1-, 3- or 4-substituted 5-aminopyrazoles withboth (i) arylidenepyruvic acids and (ii) with their syntheticprecursors—pyruvic acids and aromatic aldehydes-leadingto fused pyridine or pyrimidine carboxylic acidsusingconventional thermal or microwave heating conditions. The starting a,B-unsaturated acids 4a-c were synthesizedaccording to the known literature procedures by reactionof aromatic aldehydes and pyruvic acid in an aqueousmethanolic solution of potassium hydroxide,9and weresubsequently used directly without additional purification.3-Substituted 5-aminopyrazoles 1, 2, 3a,b and 5-amino-N-phenylpyrazole-4-carboxamides 10a,b were either commercially available (amine 1) or synthesized using published 2.1. Reactions involving arylidenepyruvic acids Inthe course of our investigations we found that refluxing onan oil bath of equimolar mixtures of 5-amino-3-methylpyr-azole 1 with unsaturated acids 4a,c in DMF or acetic acidled to the formation of a pyridine ring. Addition of EtOHto the reaction mixture allowed to isolate 4-aryl-3-methyl-pyrazolo[3,4-b]pyridine-6-carboxylic acids 5a,b in satisfac-tory yields (Table 1). It should be noted that heterocyclicproducts in these reactions were not isolable as dihydro-derivatives, which indicate the high propensity of the ex-pected 4-aryl-3-methyl-4,7-dihydropyrazolo[3,4-b]pyridineintermediates to oxidation. Aminopyrazole Acid Compound R Compound R2 Compound Yield (%) 1 CH, H 4a C6H5 5a 38 1 CH H 4c 4-CHO-C,H4 5b 42 2 CH: C6H5 4a C6H5 6a 39 2 CH3 C,H5 4c 4-CH3O-C6H4 6b 48 2 CH3 C6H5 4a C6H5 7a 68° 2 CH: C6H5 4c 4-CHO-CH4 7b 72 3a H 4a C6H5 8a 75 3a H 4b 4-C1-C6H4 8b 78° 3a H 4c 4-CH3O-C6H4 8c+9c 52° and 26 3a H 4b 4-C1-C6H4 10b 31 3b 4-Br-C6H4 H 4a C6H5 8d 65° 3b 4-Br-C6H4 H 4b 4-CI-C6H4 8e 76° 3b 4-Br-C6H4 H 4c 4-CHO-C6H4 8f+10f 48° and 22° 3b 4-Br-C,H4 H 4c 4-CH,O-C,H4 9f 56° 11a C6H5 4c 4-CH;O-C6H4 12a 68 11b 4-C2H5O-C6H4 4a C6H5 12b 68 11b 4-C2H5O-C6H4 4b 4-C1-C6H4 12c 72° 11b 4-C2H5O-C6H4 4c 4-CHO-C6H4 12d 65° 11b 4-C2H5O-C6H4 4d 4-CH3-C6H4 12e 68 Refluxing in HOAc. Refluxing in DMF. Refluxing of 5-amino-3-methyl-1-phenylpyrazole 2 witharylidenepyruvic acids 4a,c in DMF also yielded hetero-aromatized 4-aryl-3-methyl-1-phenylpyrazolo[3,4-b]pyr-idine-6-carboxylic acids 6a,b, however, treatment: inboiling acetic acid allowed us to isolate the correspondingdihydro analogs 7a,b accompanied by only small amountsof the aromatized heterocycles 6a,b. Upon exposure toatmospheric conditions dihydropyridines 7a,b very easilyoxidized to pyridines 6a,b. Treatment of arylidenepyruvic acids 4a-c with 5-amino-3-arylpyrazoles 3a,b in most cases was not regioselective andyielded mixtures of several regioisomers and products oftheir heteroaromatization (Scheme 2). Thus, in reactions ofunsaturated acid 4a with amines 3a,b, and of acid 4b withamine 3b,carboxylic acids 8a,d,e were obtained as pure sin-gle isomers whereas reaction of acid 4b with amine 3ayielded pyrazolopyridine 10b. It is interesting that under ni-trogen atmosphere the reaction of acid 4b and amine 3a pro-vided dihydropyrimidine 8b. On the other hand, treatment ofpyrazolesS3a,bbwith unsaturated acid 4c containinga methoxyaryl group was less selective and led to the forma-tion of several products: in reaction of amine 3a with acid 4ca mixture of 2-phenyl-7-(4-methoxyphenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acids 8c (majorisomer) and 3-phenyl-4-(4-methoxyphenyl)-4,7-dihydropyr-azolo[3,4-b]pyridine-6-carboxylic acids 9c (minor isomer) with small traces of pyridine derivative 10c was isolated(compound 10c was not separated from mixture). At thattime, treatment of 3b with unsaturated acid 4c led to a mixtureof 8f (major isomer) and 10f (minor isomer), whereas dihy-dropyridine derivative 9f was obtained as the single productin the same reaction under nitrogen atmosphere (Table 1). When the nucleophilic position 4 on the pyrazole ring wasblocked by an N-arylcarboxamide group as in structures11a,b treatment with acids 4a-d in boiling acetic acid ledto the clean formation of pyrimidine derivatives 12a-e asthe only isolable product (Scheme 2). It should be noted that condensation of the unsaturated acids4a-d with 5-aminopyrazoles 1, 2, 3a,b never yielded iso-mers with opposite location of the aryl and carboxyl groupsSat the pyridine or pyrimidine rings, respectively. 2.2. Three-component reactions involving pyruvic acidand aldehydes As it was pointed out in Section 1, both the sequential andthe one-pot multicomponent approach can yield the sameor. different reaction products. In the case of 3-amino-11.,2,4-triazole and 5-aminotetrazol, both types of protocolsled to the formation of the same compounds, i.e., 5-aryl-5,8-dihydroazolo[1,5-alpyrimidine-7-carboxylic acids.2b,c Scheme 2.耐士科技 www.rysstech.com V. A. Chebanov et al./Tetrahedron 63 (2007) 1229-1242 Scheme 3. However, we now find that the three-component condensa-tion of amines 1, 2,3a-c with pyruvic acid 13 and aromaticaldehydes 14a-d,i in refluxing acetic acid leads exclusivelyto 6-aryl-3-methylpyrazolo[3,4-b]pyridine-4-carboxylic acids15a-d, 1,6-diaryl-3-methylpyrazolo[3,4-b]pyridine-4-carb-D-oxylic acids 16a-d and 3,6-diarylpyrazolo[3,4-b]pyridine-4-carboxylic acids 17a-i as reaction hi products, instead Similarly, multicomponent condensation of amines 11a,bwith pyruvic acid and aldehydes led to pyrimidines 12a-e Table 2. Three-component reactions of 5-aminoazoles (Scheme 3) Compound Aminopyrazole R Pyruvic acid Aldehyde R derivative Compound R² Compound Yield (%) CH 14a C6H5 15a CH 14b 4-C1-C6H4 15b CH 4-CHO-C,H4 15c CH: 148 4-CH3-C6H4 15d CH: C6H5 14a CH5 16a CH: C6H5 14b 4-C1-C6H4 16b CH: C6H5 14c 4-CHO-C6H4 16c CH, C,H 14d 4-CH3-C6H4 16d C,H 14a C6H5 17a C6H5 14b 4-C1-C,H4 17b C6H5 14c 4-CHO-C,H4 17c C6H5 14d 4-CH3-C6H4 17d 4-Br-C6H4 14a C6H5 17e 4-Br-C,H4 14b 4-C1-C6H4 17f 4-Br-C,H 14c 4-CHO-C,H4 17g 4-Br-C6H 14d 4-CH3-C6H4 17h 4-C2H5-C6H4 14i 2-CHO-5-Br-C6H4 17i 11a 16Hs 14c 4-CH,O-CH5 12a 12b 12c 12d 12e5 20c 20d 20e 11a C2H5O-C6H4 14a C6H5 11b C2H50-C6H4 14b 4-C1-C6H4 11b 4-C,H5O-C6H4 14c 4-CH,O-C,H4 11b 4-C2H5O-C,H4 14d 4-CH3-C6H4 CH3 14c 4-CH;O-C,H4 CH3 14d 4-CH3-C6H4 CH; 14e 4-F-CH4 C6H5 14c 4-CH,O-C,H4 21c CH5 14d 4-CH3-C6H4 21d c,Hs 14e 4-F-C6H4 21e (Scheme 3), which were identical to the compounds iso-lated from the reaction of 11 with arylidenepyruvic acids(Scheme 2). Surprisingly, treatment of 3-substituted 5-aminopyrazoles 1,2, 3a-c with arylidenepyruvic acids 4a-c and the three-component reaction of the same amines with pyruvic acids13 and aldehydes 14a-d,i led to the formation of a differentset of reaction products, which vary in the position of theacid and R2 substituents. We have also established the possibility of using ethyl pyru-vate 19 instead of pyruvic acid as a building block in thethree-component reaction with amines 1, 3a and aldehydes.In this case, however, the corresponding pyrazolopyridines20a-c and 21a-c were isolated in comparatively low yields(18-25%). 2.3. Microwave-assisted three-component reactions thermal reflux conditions (35-45%). Optimal reactionconditions involved MW irradiation of an equimolar mixtureof starting materials in ethanol/HCl for 10 min at 150°C. Inthis case, the final fused heterocyclic products 15a-d and17a-d did not require additional purification. Gratifyingly, it was also established that the MW-assistedreaction of ethyl pyruvate 19 with pyrazole 1, 3a andaldehydes 14c-h in EtOH with acid catalysis allowed notonly to decrease the reaction time from 120 to 10 min andmade recrystallization of the crude product unnecessary,but also facilitated the isolation and considerably raisedthe yields of the target compounds 20c-h and 21c-h (from18-25% to 35-48%). In summary, the advantages of using the MW-assisted threecomponent procedure for the synthesis of pyrazolo[3,4-b]-pyridine-4-carboxylic acids are following: (i) replacementof acetic acid with ethanol, which allows: a simplifiedwork-up protocol; (ii) a 8-12-fold reduction of the reactiontime; and (iii) a significantly increased product yield. The structures of the heterocyclic compounds synthesizedwere established by IR sjspectroscopy, mass spectrometry,NMR spectral data.a nadn dan X-ray diffraction study (fordetails,see Section 4). The 'H NMR spectra of the fused pyridine analogs 5, 15,17, 20 and 21 exhibit apart from signals for the terminalsubstituents and aromatic rings sharp singlets for the CHgroups of the pyridine ring at ca. 8 ppm and broad peaksdue to the NH- and COOH functionalities around 13-14 ppm. Similarly, the 'H NMR spectra of 2,7-diaryl-4,7-dihydro-pyrazolo[1,5-a]pyrimidine-5-carboxylic acids 8a-f exhibit Aminopyrazole Pyruvic acid Aldehyde Compound R R derivative Compound R² Compound Yield (%) 1 CH: H 13 14a C Hs 15a 45 1 CH3 H 13 14b 4-C1-C6H4 15b 42 1 CH3 H 13 14c 4-CH;O-C6H4 15c 43 1 CH3 H 13 14d 4-CH3-C6H4 15d 35 3a C6H5 H 13 14a C6H5 17a 43 3a C6H5 H 13 14b 4-C1-C6H4 17b 44 3a C6H5 H H 13 14c 4-CHO-C,H4 17c 42 3a C6H5 H 13 14d 4-CH3-C6H4 17d 41 1 SH H 19 14c 4-CHO-C6H4 20c 42 1 CH H 19 14d 4-CH3-C6H4 20d 42 1 CH3 H 19 14e 4-F-C6H4 20e 47 1 H H 19 14f 4-Br-C6H4 20f 45 CH H 19 14g 3-C1-C6H4 20g 40 1 CH, H 19 14h 2-C1-C6H4 20h 35 3a C6H5 H 19 14c 4-CH,O-C,H4 21c 43 3a C6H5 H 19 14d 4-CH3-C6H4 21d 48 3a C6H5 H 19 14e 4-F-C6H4 21e 48 3a C6H5 H 19 14f 4-Br-C6H4 21f 45 3a C6H5 H 19 14g 3-C1-C6H4 21g 41 3a C,H5 H 19 14h 2-C1-C6H4 21h 36 doublet of doublets for protons at positions 6 (~5.8 and6.2 ppm, 3J~4.2 Hz, 4J~1.5 Hz with NH of pyrimidinePring) and a doublet of the CH proton at position 7(~5.8 ppm, 3J~4.2 Hz). The presence in spectra of a sharpsinglet for the pyrazole CH at ~6 ppm shows that treatmentdid not affect this nucleophilic centre and confirms the for-mation of the pyrimidine ring. The resonances for the pyrim-idine NH and COOH groups are present in the spectra atapproximately 9.3 and 12.5 ppm, respectively. The signals for the C=CH-CH fragment of the pyridinenucleus in dihydroderivatives 7a,b and 9a-c,e,f are shiftedupfield as compared to compounds 8 and 12, and the ethyl-ene proton (doublet) does not show coupling with the aminogroup. The presence of a NH resonance signal for the pyr-azole nucleus (for compound 9) and the absence of a singletfor the pyrazole CH group confirmed the formation of thepyridine but not pyrimidine ring. The structures of compounds 17a-i were finally proven byan X-ray analysis (Fig. 1). Here it was also established thatthe bicyclic fragment is a planar within 0.02 A. The bondalternation is observed within the pyridine ring (the C(3)-C(4) 1.419(6) A and C(5)-C(6)1.415(6) A bonds are longer Figure 1. Molecular structure (X-ray diffraction data) of 3-(4-ethylphenyl)6-(5-bromo-2-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine-4-carboxylicacid (17i). * NOE NOE Figure 2. NOE experiments for structure determinations.耐士科技 www.rysstech.com and the C(4)-C(5) 1.366(6) A bond is shortened as com-pared to their mean value for a pyridine ring 1.380 A).The carboxylic group at the C(4) atom and the aromaticring of the substituent at the C(2) atom are not coplanarwith respect to the bicyclic fragment (the C(3)-C(4)-C(14)-O(1) and the C(3)-C(2)-C(15)-C(20) torsion anglesare-31.9(7)° and -40.3(8)°, respectively). Such anarrangement of these substituents is caused, probably, bythe repulsion between them (the shortened intramolecularcontacts C(14)…C(20) 3.34 A (the sum of the correspond-ing van der Waals radii 4 is 3.42 A), O(1)…C(20) 2.94 A(3.00A)). The benzene ring of the substituent at the C(6) atom is turnedrelatively the mean plane of the bicyclic fragment (the C(5)C(6)-C(7)-C(8) torsion angle is 34.9(7)). It can be assumedthat such orientation of the substituent is stabilized bythe attractive interactions C(5)-H(5)…O(3)2.28 A, and theC(12)-H(12)…N(1) 2.57 A. The methoxygroup and thebenzene ring are almost coplanar (the C(13)-O(3)-C(8)-C(9) torsion angle is 6.2(7)°) despite of the repulsionbetween the methyl group and atoms of the aromatic ring(the shortened intramolecular contacts H(9)…C(13) 2.51 A(2.87A). H(9)…H(13)2.28 (2.34 A), H(13b)…C(9)2.71 A (2.87 A),H(13c)C(9) 2.79 A (2.87 A)). To establish the relative location of substituents in the otherfused pyridine or pyrimidine derivatives, several additionalexperiments were carried out. Thus, compound 9f was hetero-aromatized in NMR tubes in DMSO-d6, which confirmedthat the products of oxidation have 'HNMR spectra differentfrom structures 17f and equivalent to 10f. On this basis, andwith regard to the additional spectral data, the structure of3,4-diaryl-4,7-dihydropyrazolo[3,4-b]pyridine-6-carboxylicacids were assigned to 9c,f. The locations of the substituents in pyrazolopyridines andpyrazolopyrimidines 5-9, 16, 17 were also derived on thebasis of NOE experiments (Fig. 2). Thus, the presence orabsence of an NOE between specific protons allowed toestablish the structures for these compounds unequivocally. V. A. Chebanov et al./ Tetrahedron 63 (2007)1229-1242 The similarity of the H NMR spectra between dihydro-pyrimidines 12a-d and compounds 8a-f allowed to assignthem the structure of 7-aryl-3-(arylcarbamoyl)-4,7-dihydro-pyrazolo[1,5-a]pyrimidine-5-carboxylic acids. The different reaction pathways experienced in the trans-formation studies in the present investigation can be ratio-nalized from the mechanistic point of view. It is most likely that the three-component condensation of3-substituted 5-aminopyrazoles 1, 2, 3a,b with pyruvicacid (or ethyl pyruvate) and aromatic aldehydes proceedsthrough initial formation of the corresponding azomethine(intermediate A) with subsequent heterocyclization withpyruvic acid yielding the observed pyridine carboxylic acids15-17 as products (Scheme 4). The condensation of inde-pendently synthesized azomethine 22 with pyruvic acidconfirmed this assumption. As it was pointed out above, three-component condensationsinvolving aminopyrazoles 11a,b leading to the formation ofdihydropyrimidine derivatives 12a-e do not proceed throughazomethine formation. This is most likely the result of theinfluence of the carboxamide fragment on the nucleophilic-ity of the amino group via iu-electron withdrawing effect andthe formation of an intramolecular hydrogen bond (shownby the H NMR spectrum of amines 11a,b, having non-equivalent NH2 protons). Here, the reaction is likely toproceed via a different pathway, for example, through initial nucleophilic attack of the endocyclic NH of thealdehyde carbonyl group (intermediate B, Scheme 5). For the condensation of 3-methyl-5-aminopyrazoles 1, 2with arylidenepyruvic acids 4a-c the initial reaction steppossibly involves a nucleophilic attack of the CH group ofthe aminopyrazole to the C=C double bond of the unsatu-rated acid with the formation of Michael adduct C and itssubsequent cyclization to pyridines 5-7 (Scheme 6). Onthe (other hand, in the case of 3-aryl-5-aminopyrazoles3a-c the C=C double bond of the unsaturated acid maybe attacked by both the endocyclic nitrogen atom (interme-diate D) and(less likely) by the sterically hindered CH group(intermediate E), leading to the formation of pyrazolopyr-imidines or mixtures with pyrimidine derivatives (structures8 and 9,respectively). As it was pointed out above, the yields of pyridine deriva-tives 15-17, 20,21 were always lower than 50%, both underatmospheric and inert conditions. Participation of one of theintermediates in the mechanistic pathway of the oxidation Scheme 6. 耐士科技 www.rysstech.com process may be a reason for this fact. Unfortunately, ourattempts to establish the composition of the mother liquorafter isolation of the target compounds by preparativeHPLC were unsuccessful. 3. Conclusions The sequential and multicomponent reactions of 1-, 3- or4-substituted 5-aminopyrazoles with arylidenepyruvic acidsor their synthetic precursors were studied. Several differentreaction pathways for these cyclocondensation reactionsincluding the formation of pyrazolo[3,4-b]pyridine-6-carb-oxylic acids, pyrazolo[1,5-a]pyrimidine-5-carboxylic acidsand pyrazolo[3,4-b]pyridine-4-carboxylic acids were estab-lished depending on the specific reaction conditions andbuilding block selection. Facile and rapid microwave-assis-ted procedures for the synthesis of pyrazolo[3,4-b]pyridine-4-carboxylic acids were elaborated, which allowed a simplework-up protocol, reduction of the reaction time and a signif-icant increase in yields. The formation of different reactionproducts was discussed from the mechanistic point of view. C, 66.40; H, 4.38; N, 16.59. Found: C, 66.51; H, 4.25; N,16.61. 4.1.1.2. 4-(4-Methoxyphenyl)-3-methyl-1H-pyrazolo-[3,4-b]pyridine-6-carboxylic acid (5b). Yield: 130 mg(42%) of yellow crystals, mp>300°C. IH NMR (DMSO-d6) 2.24 (s, 3H, CH3), 3.83 (s, 3H, OCH3) 7.0-7.5 (m,4H, ArH), 7.62 (s,1H,5-H),13.60(s,1H, NH). Anal. Calcdfor C15H13N303: C, 63.60; H, 4.63; N, 14.83. Found: C,63.71; H, 4.50;N, 14.87. 4.1.2. General procedure for the synthesis of 1,4-diaryl-3-methyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acids6a,b. A mixture of 5-amino-3-methyl-1-phenylpyrazole 2(1.1 mmol) and the appr4opriate arylidenepyruvie acid(1.1 mmol) in 0.7 mL of DMF was refluxed for 30 min.After cooling, 5 mL of EtOH was added and the mixturewas allowed to stand overnight. The precipitate formed wasisolated by filtration, crystallized from EtOH and air dried. 4. Experimental 4.1. General All microwave experiments were performed using theEmrysTM Creator EXP and EmrysTM Initiator 8 synthesizersfrom Biotage AB (Uppsala, Sweden) possessing a singlee-mode microwave cavity producing controlled irradiation at2.45 GHz. Experiments were carried out in sealed micro-wave process vials utilizing the high absorbance level. Reac-tion time reflects irradiation times at theset reactiontemperature (fixed hold times), 4.1.1. General procedure for the synthesis of 4-aryl-3-methyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acids5a,b. A mixture of 5-amino-3-methylpyrazole 1 (1.1 mmol)and the appropriate arylidenepyruvic acid 4 (1.1 mmol) in1mL of acetic acidwas refluxed for 1 h. After cooling,5 mL of EtOH was added and the mixture was allowed tostand overnight. The precipitate formed was isolated by filtra-tion, crystallized from EtOH and air dried. 4.1.1.1. 3-Methyl-4-phenyl-1H-pyrazolo[3,4-b]pyr-idine-6-carboxylic acid (5a). Yield: 105 mg (38%) of yel-low crystals, mp>300°C. IH NMR (DMSO-d6) 8 2.19 (s,3H, CH3), 7.1-7.6 (m, 5H, ArH), 7.65 (s, 1H, 5-H), 12.0(br s, 1H, COOH), 13.68 (s, 1H, NH). 13C NMR (DMSO-d6)6 12.4, 110.3,119.0,124.6,125.8,128.3,140.1, 143.3,144.0, 146.5,148.2,166.3. Anal. Calcd for C14H11N3O2: 4.1.2.2.4-(4-Methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acid (6b). Yield:190 mg(48%) of yellow crystals, mp 262-264°C. HNMR (DMSO-d6)8 2.32 (s, 3H, CH3), 3.85 (s, 3H,OCH3)7.10-8.33 (m, 10H, ArH), 7.75 (s, 1H, 5-H), 13.5 (br s,1H, COOH). Anal. Calcd for C21H17N3O3: C,70.18; H,4.77;N, 11.99. Found: C, 70.21; H,4.68; N, 12.02. 4.1.3. General procedure for the synthesis of 1,4-diaryl-3-methyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-6-carb-oxylic acids 7a,b. A mixture of 5-amino-3-methyl-1-phenylpyrazole 2 (1.1 mmol) and the appropriate arylidene-pyruvic acid 4 (1.1 mmol) in 3 mL of acetic acid wasrefluxed for 30 min. The mixture was allowed to stand over-night and the precipitate formed was isolated by filtration.The by-products 6 were removed by crystallization fromEtOH. 4.1.3.1. 3-Methyl-1,4-diphenyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acid (7a). Yield: 250 mg (68%)of yellow crystals, mp 222-224°C. IR (KBr): 3264, 1708,1596,1252.IH NMR (DMSO-d6) 6 1.75 (s, 3H,CH), 4.91(d, J=4Hz, 1H, 4-H), 5.73 (d, J=4Hz, 1H, 5-H), 7.1-7.7(m, 10H, ArH), 13.5 (br s, 2H, NH+COOH). 13C NMR(DMSO-d6) 6 15.1, 44.2,99.1,115.6,123.7,124.0,125.1,128.1,129.2,129.1,131.4,138.6,141.8,145.15, 145.7,169.3. Anal. Calcd for C20H17N302: C, 72.49; H, 5.17; N,12.68. Found: C, 72.52; H, 5.06; N, 12.71. 4.1.3.2. 4-(4-Methoxyphenyl)-3-methyl-1-phenyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acid (7b).Yield: 290mg (72%) of yellow crystals, mp 238-240 °C.400-188-0725 H NMR (DMSO-d6) 8 1.75 (s, 3H, CH3), 3.71 (s, 3H,OCH3),,4.85 (d, J=4.3Hz,, 1H, 4-H),5.70 (d, J=4.3 Hz, 1H, 5-H), 6.8-7.6 (m, 9H, ArH), 13.2 (br s, 2H,NH+COOH). Anal. Calcd for C21H19N303: C, 69.79;H,5.30;N, 11.63.Found: C, 69.84; H, 5.20; N, 11.64. 4.1.4. General procedure for the synthesis of 2,7-diaryl-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylicacids 8a-c,d-f, 3,4-diaryl-4,7-dihydro-1H-pyrazolo[3,4-blpyridine-6-carboxylic acids 9c,f and 10b,f. A mixtureof the appropriate 5-amino-3-arylpyrazole 3 (1.1 mmol)andd of thecorresponding arylidenepyruvic acid4(1.1 mmol) in 3 mL of acetic acid was refluxed for 30 min.After cooling, the precipitate formed was filtered andif needed recrystallized from EtOH. The acids 8c,f wereisolated from 9c and 10f by crystallization from EtOH.Compounds 8b and 9f were obtained using the same proce-dure under a nitrogen atmosphere. 4.1.4.1.2,7-Diphenyl-4,7-dihydropyrazolo[1,5-a]pyr-imidine-5-carboxylic acid (8a). Yield: 260 mg (75%) of yel-lowish crystals, mp 215-217°C. IR (KBr): 3350,1710, 1597.'H NMR (DMSO-d) 6 5.69 (dd, 3J=4.5, 1.7Hz, 1H, 6-H),6.01 (s, 1H,3-H), 6.20 (d,J=4.5 Hz, 1H,7-H), 7.1-7.7 (m,10H, ArH), 9.28 (d, J=1.7Hz, NH), 12.53 (br 1H.COOH). 13C NMR (DMSO-d6) 6 61.1, 83.0,117.1,125.4,127.7,128.5,130.9,132.1,133.8,134.7,138.3,139.刀141.1,147.9, 166.3. Anal. Calcd for C19H15N3O2:C,71.9H, 4.76;N, 13.24. Found: C,72.02; H, 4.65; N, 13.27 4.1.4.3. 7-(4-Methoxyphenyl)-2-phenyl-4,7-dihydro-pyrazolo[1,5-a]pyrimidine-5-carboxylic acid (8c). Yield:200 mg (52%) of yellowish crystals, mp 214-216°℃. lHNMR (DMSO-d6) 8 3.69 (s, 3H, OCH3),;5.67(dd,J=4.2, 1.8 Hz, 1H, 6-H), 5.98 (s, 1H, 3-H), 6.13 (d,J=4.2 Hz, 1H, 7-H), 6.82-7.68 (m, 9H, ArH)9.22 (d,J=1.8 Hz, NH), 12.56 (br s, 1H, COOH). Anal. Calcd forC20H17N303: C, 69.15; H, 4.93;, N, 12.10. Found: C,69.16;H, 4.85; N,12.13. 4.1.4.4. 2-(4-Bromophenyl)-7-phenyl-4,7-dihydropyr-azolo[1,5-a]pyrimidine-5-carboxylic acid (8d). Yield:280 mg (65%) of yellowish crystals, mp 222-225C.IHNMR (DMSO-d6) 6 5.69 (dd, 3J=4.2 Hz,4J=1.8 Hz, 1H,6-H), 6.02 (s, 1H,3-H), 6.19 (d, J=4.2 Hz, 1H,7-H), 7.1-7.6 (m, 9H, ArH),9.32 (s, 1H, NH), 12.58 (br s, 1H,COOH). Anal. Calcd for C19H14BrN3O2: C,57.59; H,3.56;N,10.60. Found: C, 57.62; H, 3.42; N,10.62. 4.1.4.5. 2-(4-Bromophenyl)-7-(4-chlorophenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acid (8e).Yield: 360 mg (76%) of yellowish crystals, mp 220-222 °C. IR (KBr): 3413,1710,1595. H NMR (DMSO-d6); 5.68 (dd, J=4.3, 1.7 Hz, 1H, 6-H), 6.03 (s, 1H, 3-H), 6.22 (d, J=4.3 Hz, 1H, 7-H), 7.14-7.65 (m, 8H, ArH), 9.38 (s,NH),12.70(br s,1H, COOH). Anal. Calcd forC19H13BrClN3O2: C, 52.99; H, 3.04; N, 9.76. Found: C,53.01;H, 2.98;N, 9.80. 4.1.4.6. 2-(4-Bromophenyl)-7-(4-methoxyphenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acid(8f). Yield: 220 mg (48%) of yellowish crystals, mp214-216 °C. H NMR (DMSO-d6) 8 3.7 (s, 3H, OCH),8 5.66 (dd, J=4.3, 1.9 Hz, 1H, 6-H), 6.0 (s, 1H, 3-H),6.13 (d, J=4.3 Hz, 1H, 7-H), 6.8-7.6 (m, 8H, ArH),9.27 (d, J=1.9 Hz, NH). Anal. Calcd for C20H16BrN3O3:C,56.35; H, 3.78; N, 9.86. Found: C, 56.38; H, 3.65; N,9.82. 4.1.4.7. 4-(4-Methoxyphenyl)-3-phenyl-4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acid (9c). Yield:100 mg (26%) of yellow crystals, mp 265-267C. IH NMR(DMSO-d6) 3.63 (s, 3H, OCH3), 5.11 (d,J=4.9 Hz, 1H,4-H), 5.54(d,J=4.9 Hz, 1H,5-H), 6.68-7.47 (m, 9H,ArH),7.78(s, NH), 14.22 (br s, NH), 12.78 (br s, 1H, COOH).Anal. Calcd for C20H17N303: C, 69.15;H, 4.93; N, 12.10.Found: C,69.18;H,4.82;N, 12.12. 4.1.4.8.3-(4-Bromophenyl)-4-(4-methoxyphenyl)-4,7-di-hydro-1H-pyrazolo[3,4-blpyridine-6-carboxylic acid (9f).Yield: 260 mg (56%) of yellow crystals, mp 276-278 °C. IR(KBr): 3480, 1711, 1666,1605,1509.1H NMR (DMSO-d)8 3.64 (s, 3H, OCH3), 5.12 (d, J=4.7Hz, 1H, 4-H), 5.53 (d,J=4.7 Hz, 1H, 5-H), 6.7-7.5 (m, 8H, ArH), 7.86 (s, NH),14.3 (br s, NH), 12.85 (br s, 1H, COOH). Anal. Calcd forC20H16BrN3O3: C, 56.35; H, 3.78; N,9.86. Found: C, 56.39;H, 3.65;N,9.75 4.1.4.9.4-(4-Chlorophenyl)-3-phenyl-1H-pyrazolo[3,4-b]-pyridine-6-carboxylic acid (10b). Yield: 120 mg (31%) ofyellowishcrystals,S,mp>300℃. H NMR(DMSO-d)6 7.80 (s, 1H, 5-H), 7.0-7.4 (m, 9H, ArH), 14.3 (br s, 1H,NH), 13.9 (br s, 1H,COOH). Anal. Calcd for C19H12CIN302:C, 65.24; H, 3.46; N, 12.01. Found: C, 65.31; H, 3.36; N,12.05. 4.1.4.10. 3-(4-Bromophenyl)-4-(4-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine-6-carboxylic acid (10f). Yield:100 mg (22%) of yellow crystals, mp>300°C. H NMR(DMSO-d6) 8 3.75 (s, 3H, OCH3), 7.78 (s, 1H, 5-H), 6.7-7.4 (m, 8H, ArH), 14.31 (br s, 1H, NH), 13.95 (br s, 1H,COOH). Anal. Calcd for C20H14BrN3O3: C, 56.62;H, 3.33;N, 9.90. Found: C, 56.69; H, 3.22; N, 9.87. 4.1.5. General procedures for the synthesis of 7-aryl-3-(arylcarbamoyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acid 12a-e. Method A: A mixture of theappropriate 5-amino-N-aryl-1H-pyrazole-4-carboxamide 11(1 mmol) and the corresponding arylidenepyruvic acid 4(1 mmol) was refluxed in 5 mL of acetic acid for 10-20 min until a solid started to precipitate. After cooling,the crystals formed were removed by filtration, washedwith EtOH and air dried. If required,products were crystal-lized from EtOH. ( Method B: A m i xture of the a p propriate 5-amino-N-aryl- 1H-pyrazole-4-carboxamide 11 (1 mmol), py r uvic acid 13400-188-0725 ) (1 mmol) and the corresponding aldehyde 14 (1 mmol) wasrefluxed in 3 mL of acetic acid for 2 h. After cooling, thecrystals precipitated were removed by filtrw2ation, washedwith EtOH and air dried. If required, products were crystal-lized from EtOH. 4.1.5.1. 7-(4-Methoxyphenyl)-3-(phenylcarbamoyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acid(12a). Yields:265 mg (68%, method A) and 180 mg (46%,method B) of yellowish crystals, mp 254-256°C.IH NMR(DMSO-d6) 8 3.71 (s, 3H, OCH3), 5.81 (dd,J=4.1, 1.8 Hz,1H, 6-H), 6.17 (d, J=4.1 Hz, 1H, 7-H), 6.8-7.7 (m, 9H,ArH), 8.06 (br s, 1H, 2-H), 8.39 (d, J=1.8 Hz, 1H,NH), 9.76 (s, 1H, CONH). Anal. Calcd for C21H18N4O4:C, 64.61; H, 4.65; N, 14.35. Found: C, 64.63; H, 4.57; N,14.37. 4.1.5.2. 3-[(4-Ethoxyphenyl)carbamoyl]-7-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxylic acid (12b).Yields: 275 mg (68%, method A) and 210 mg (52%, methodB) of yellowish crystals, mp 266-268°C. IR (KBr): 3400,1746,1722,1600,1509.'H NMR (DMSO-d6) 6 1.3 (t, 3H,OCH2CH3), 3.98 (q, 2H, OCH2CH3), 5.83 (dd, J=4.1.1.8 Hz, 1H, 6-H), 6.23 (d, J=4.1 Hz, 1H, 7-H), 6.8-7.6 (m9H, ArH), 8.04 (s, 2-H), 8.42 (d, J=1.8 Hz, 1H, NH), 9.68(s, 1H, CONH). Anal. Calcd for C22H20N4O4: C, 65.34; H,4.98;N, 13.85. Found: C, 65.38; H, 4.87;N, 13.86. 4.1.5.3. 5-(4-Chlorophenyl)-3-[(4-ethoxyphenyl)carb-amoyl]-4,5-dihydropyrazolo[1,5-a]pyrimidine-7-carboxy-lic acid (12c). Yields: 310 mg (72%, method A) and 250 mg(58%,method B) of yellowish crystals, mp 267-269C. IR(KBr):3393,3361,1755,1721,1603, 11510.H NMR(DMSO-d6) 8 1.3 (t, 3H, OCHCH), 3.98 (q,2H,OCH2CH3), 5.82 (d,J=4.2 Hz, 1H, 6-H),6.27 (dd, J=4.1,1.7Hz, 1H,7-H), 6.8-3-7.6(m,8H, ArH), 8.05 (s, 1H, 2-H)8.43 (d, J=1.7 Hz, 1H,NH), 9.68 (S, 1H, CONH). AnalCalcd for C22H9CIN4Q4: 60.21; H, 4.36; N, 12.777.Found: C, 60.25; H, 4.24,N, 1.80. 4.1.5.4. 3-[(4-Ethoxyphenyl)carbamoyl]-7-(4-methoxy-V-phenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-5-carboxy-lic acid (12d). Yields: 290 mg (68%, method A) and 170 mg(40%, method B) of yellowish crystals, mp 267-269°C. IR(KBr): 3388,1720,1599,1509. H NMR (DMSO-d6) 6 1.3(t, 3H, OCH2CH3), 3.98 (q, 2H, OCHCH3), 3.72 (s, 3H,OCH3), 5.81 (dd, J=4.1, 1.8 Hz, 1H, 6-H), 6.17 (d, J=4.1 Hz, 1H, 7-H), 6.8-7.6 (m,8H, ArH), 8.02 (s, 1H, 2-H),8.4(d,J=1.8 Hz, 1H, NH),9.66 (s, 1H, CONH). Anal. Calcdfor C23H22N405: C, 63.59; H, 5.10; N, 12.90. Found: C,63.61;H, 5.00; N,12.92. 4.1.5.5. 3-[(4-Ethoxyphenyl)carbamoyl)-5-p-tolyl-4,5-dihydropyrazolo[1,5-a]pyrimidine-7-carboxylic acid (12e).Yields: 270 mg (65%, method A) and 210 mg (51%, methodB) of yellowish crystals, mp 265-267°C. IR (KBr): 3416,1717, 1602, 1510. H NMR (DMSO-d6) 6 1.3 (t, 3H,OCH,CH3), 3.98 (q, 2H, OCH2CH3), 2.26 (s, 3H, CH),5.80 (dd, J=4.1, 1.7 Hz, 1H, 6-H), 6.18(d,J=4.1 Hz, 1H, 7-H), 6.8-7.6 (m, 8H, ArH), 8.02 (s, 1H, 2-H), 8.39 (d,J=1.7 Hz, 1H, NH), 9.67 (s, 1H, CONH). Anal. Calcd forC23H22N4O4: C, 66.02; H, 5.30; N, 13.39. Found: C,66.07;H, 5.19;N, 13.42. 4.1.6. General procedure for the synthesis of 6-aryl-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids15a-d and 3,6-diaryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids 17a-i.Conventional method C: A mixtureof the appropriate 5-aminopyrazole 1 or 3a (2 mmol),pyruvic acid 13 (2 mmol) and the corresponding aromaticaldehyde 14 (2 mmol) in 3 mL of acetic acid was refluxedfor 10-40 min until a solid started to precipitate. After cool-ing, the crystals formed were removed by filtration, crystal-lized from EtOH and air dried. Microwave-assisted method D: A mixture of the appropriate5-aminopyrazole 1 or 3a (1.3 mmol), pyruvic acid13(1.3 mmol) and the corresponding aromatic aldehyde 14(1.3 mmol) in 1 mL of EtOH with two drops of HCl was ir-radiated under sealed vessel microwave conditions at 150°Cfor 10 min in a 5 mL microwave process vial. After cooling,the crystals formed were removed by filtration, washed withEtOH and air dried. 13.50 (br s, 1H, COOH), 14.13 (s, 1H, NH). 13C NMR(DMSO-d6) 114.92, 118.3,128.7,128.9,129.3,130.1,130.3, 131.0,134.1,135.7,142.1,143.6,147.3,156.1,167.5. Anal. Calcd for C19H13N3O2: C, 72.37; H, 4.16; N,13.33. Found: C, 72.25; H, 4.22; N, 13.36. 4.1.6.6. 6-(4-Chlorophenyl)-3-phenyl-1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid (17b). Yields: 310 mg(44%, method C) and 200 mg (44%, method D) of yellowcrystals, mp>300°C. IR (KBr): 3264, 1712, 1596. HNMR (DMSO-d6) 8 7.37-8.33 (m, 9H, ArH), 8.00 (s, 1H,5-H), 13.49 (br s, 1H, COOH), 14.16 (br s, 1H, NH). 13CNMR (DMSO-d6)8107.8,113.7,128.6,128.61,129.1,129.2,129.66,129.69,134.51,135.44,137.45,137.62,154.37, 155.19, 168.04. Anal.Calcd for C19H12CIN302: C,65.24;H, 3.46;N, 12.01. Found:C, 65.18;H,3.58;N, 12.05. 4.1.6.7. 6-(4-Methoxyphenyl)-3-phenyl-1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid (17c). Yields: 290 mg(42%, method C) and 190 mg (42%, method D) of yellowcrystals, mp>300℃. IR (KBr): 3260, 1708, 1596. HNMR (DMSO-d6) ; 7.0-8.30 (m, 9H, ArH), 7.91(s, 1H,5-H), 13.58 (br s, 1H, COOH), 14.02 (br s, 1H, NH), 3.83(s,3H, OCH3). Anal. Calcd for C20H15N303: C, 69.56; H,4.38; N, 12.76. Found: C, 69.51; H, 4.25; N, 12.68. 4.1.6.8. 3-Phenyl-6-p-tolyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid (17d). Yields: 260 mg(39%method C) and 180 mg (42%, method D) of yellow crystals,mp>300C.1HNMR (DMSO-d6)6 7.3-8.2 (m,9H, ArH),7.94 (s, 1H, 5-H), 13.65 (br s, 1H, COOH), 14.08 (br s, 1H,NH), 2.38 (S, 3H, CH3). Anal. Calcd for C20H15N302:C,72.94;H, 4.59;N, 12.76. Found: C, 72.90;H, 4.67;N, 12.79. 4.1.6.9. 3-(4-Bromophenyl)-6-phenyl-1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid (17e). Yield: 310 mg(40%, method C)) ofyellowcrystals, mp>300°C. HNMR (DMSO-d6) .2(m,, 9H, ArH), 8.00(s, 1H,5-H), 14.20 (br s, 1H, NH). Anal. Caled for C19H12BrN302C, 57.89; H, 3.07; N, 10.66. Found:C, 57.84; H, 3.15; N,10.61. 4.1.6.10.3-(4-Bromophenyl)-6-(4-chlorophenyl)-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid (17f), Yield:380 mg (45%, method C) of yellow crystals, mp>300°C.H NMR (DMSO-d6)8 7.5-8.3 (m, 8H, ArH), 8.02 (s, 1H,5-H), 13.4 (br s, 1H, COOH), 14.24 (br s, 1H, NH). Anal.Calcd for C19H11BrClN302: 53.24; H, 2.59; N, 9.80.Found: C, 53.20; H, 2.50; N, 9.84. 4.1.6.11. 3-(4-Bromophenyl)-6-(4-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid (17g). Yield:350 mg (42%, method C) of yellow crystals, mp>300°C.H NMR (DMSO-d ) 0 7.0-8.30 (m, 8H, ArH), 7.95 (s, 1H,5-H), 14.12 (br s, 1H, NH), 3.83 (s,3H,OCH3). Anal. Calcdfor C20H14BrN3O3: C, 56.62; H, 3.33; N,9.90. Found: C,56.58;H, 3.23; N, 9.94. 4.1.6.12. 3-(4-Bromophenyl)-6-p-tolyl-1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid (17h). Yield: 370 mg(46%, method C) of yellow crystals, mp>300C. 1H NMR(DMSO-d6) 8 7.3-8.2 (m,8H, ArH), 7.96 (s, 1H, 5-H), 13.6(br s, 1H, COOH), 14.12 (br s, 1H, NH), 2.38 (s, 3H, CH3). Anal. Calcd for C20H14BrN302: C, 58.84; H, 3.46; N,10.29. Found: C, 58.87; H, 3.33; N, 10.34. 4.1.6.13. 6-(5-Bromo-2-methoxyphenyl)-3-(4-ethyl-phenyl)-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid(17i). Yield: 395 mg (44%, method C) of yellow crystals,mp>300℃. IH NMR.(DMSO-d6)) 1.22(t,3H,CH2CH3), 2.66 (q, 2H, CH2CH3), 3.86 (s, 3H, OCH3),7.3-8.2 (m, 8H, ArH), 7.86 (s, 1H, 5-H), 13.5 (br s, 1H,COOH),14.09 (br s,1H, NH). Anal. CalcdfforC22H18BrN303: C, 58.42; H, 4.01; N, 9.29. Found: C,58.46; H, 4.11;N,9.26. 4.1.7. General procedure for the synthesis of 1,6-diaryl-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids16a-d. A mixture of 5-amino-3-methyl-1-phenylpyrazole2 (1.1 mmol), pyruvic acid 13 (1.1 mmol) and the appropri-ate aromatic aldehyde 14 (1.1 mmol) in 0.5 mL of DMF wasrefluxed for 20-25 min. Then 5 mL of MeOH was added andthe reaction mixture was allowed to stand overnight. Thesolid precipitated was removed by filtration, washed withMeOH and air dried. If required, the crude compoundswere recrystallized from EtOH. 4.1.7.1..3-Methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]-pyridine-4-carboxylic acid (16a). Yield: 140 mg (38%) ofyellow crystals, mp) 285-287°C. IH NMR (DMSO-d6)8 2.70 (s, 3H, CH3),, 7.3-8.4 (m, 10H, ArH), 8.15 (s, 1H,5-H), 13.70 (brS,1H, COOH). 13C NMR (DMSO-d6);13.5,114.3,118.3,121.1, 126.6,127.1,129.0,129.1,130.3.},133.3,134.7,1138.5,142.7,150.7,155.4,.1170.1.Anal. Calcd for C20H15N302: C, 72.94; H, 4.59; N, 12.76.Found: C, 73.03;H, 4.41;N, 12.79. ( 4.1. 7. 2. 6-(4-Chlorophenyl)-3-methyl-1-phenyl-1H-pyrazol o [3,4-b]pyridine-4-carboxylic acid (1 6 b). Yi e ld: P 180 mg (44%) of yellow crystals, mp 280-282 °C.H NMR(DMSO-d6)8 2 .68 (s , 3H, CH3), 7.3-8.4 (m, 9H, ArH), 8 . 1 4 (s, 1H,5-H). Anal. Calcd for C20H14CIN302: C,66.03;H, 3 .88; N, 11.55. Found: C, 66.07;H, 3.72; N, 1 1.57. ) 4.1.7.3. 6-(4-Methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid (16c). Yield:170mg (42%) of yellow crystals, mp 278-280°C. HNMR (DMSO-d6) 8 2.68 (s, 3H, CH3), 3.84 (s, 3H,OCH3), 7.1-8.35 (m, 9H, ArH), 8.09 (s, 1H, 5-H), 14.1 (brs, 1H, COOH). Anal. Calcd for C21H17N3O3: C, 70.18; H,4.77;N, 11.69. Found: C,70.22; H,4.58;N,11.72. 4.1.7.4.3-Methyl-1-phenyl-6-p-tolyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid (16d). Yield: 140 mg (36%)of yellow crystals, mp 275-277°C. IH NMR (DMSO-d6); 2.68 (s, 3H, CH3), 2.38 (s, 3H, CH3), 7.25-8.25 (m, 9H,ArH), 8.11 (s, 1H, 5-H). Anal. Calcd for C21H17N302: C,73.45;H,4.99;N,12.24. Found: C, 73.48;H,4.82;N,12.21. 4.1.8. General procedures for the synthesis of ethyl6-aryl-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxyl-ates 20c-h and ethyl 3,6-diaryl-1H-pyrazolo[3,4-b]-pyridine-4-carboxylates 221c-h. Conventional methodE: A mixture of corresponding 5-aminopyrazol 1 or 3a(1.3 mmol), ethyl pyruvate 19 (1.3 mmol) and the appropri-ate aromatic aldehyde 14 (1.3 mmol) in 5 mL of acetic acid400-188-0725 was refluxed for 100-120 min (TLC control). Then 10 mL ofEtOH/H2O(1:1) was added to the reaction mixture, the crys-tals formed were removed by filtration, washed with EtOH/H2O (1:1) and air dried. The crude products were recrystal-lized from EtOH. MW-assisted method F: A mixture of the corresponding5-aminopyrazole 1 or 3a (1.3 mmol), ethyl pyruvate 19(1.3 mmol) and the appropriate aromatic aldehyde 14(1.3mmol) in 1 mL of EtOH containing one drop of concdHCl was irradiated under sealed vessel microwave condi-tions at 150°C for 10 min in a 5 mL microwave processvial. Then 4 mL of EtOH/H2O (1:1) was added to the reac-tion mixture, the crystals formed were removed by filtration,washed with EtOH/H2O (1:1) and air dried. 4.1.8.1. Ethyl 6-(4-methoxyphenyl)-3-methyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (20c). Yields: 100 mg(25%, method E) and 170 mg (42%, method F) of yellowishcrystals, mp 177-180℃. IH NMR (DMSO-d6) 6 1.41 (t,J=7.0Hz, 3H, CH3CH2), 2.58 (s, 3H, CH3), 3.83 (s, 3H,CH;O), 4.45 (q, J=7.0 Hz, 2H, CH,CH2), 7.07 (d, J=9 Hz, 2H, ArH), 7.97 (s, 1H, 5-H), 8.12 (d, J=9 Hz, 2H,ArH), 13.54 (s, 1H, NH). 13C NMR (DMSO-d) 8 14.5,16.2,55.8, 62.2,108.8,113.5,114.8,129.0,130.8,134.0.141.0,154.4,155.6,161.2,165.9. MS (El, 70eV): m/z(%)=312(100%) [M*]. Anal. Calcd for C17H17N303:(C65.58;H, 5.50; N,13.50. Found: C,65.40;H, 5.59;N, 1133.48 4.1.8.2. Ethyl 3-methyl-6-p-tolyl-1H-pyrazolo[3,4-blpyridine-4-carboxylate (20d).).Yields::80 mg(20%.method E) and 140 mg (42%, method F) of yellowish crystals,mp188-191°C. H NMRL(DMSO-d6) ;1.38(t,J=7.0 Hz, 3H, CH3CH2), 2.34 (S, 3H, CH3), 2.57 (s, 3H,CH;O), 4.43 (q, J=7.0Hz, 2H, CH,CH2), 7.30 (d, J=8.3 Hz, 2H, ArH), 7.96(s, 1H, 5-H), 8.02 (d,J=8.3 Hz, 2HArH 13.58 (s, 1H, NH). 1133CNMR (DMSO-d6) 814.5,21.2,62.21,109.1,11.13.8,127.4,130.0,134.0,135.6,140.0,141.0,154.4,155.8,,I165.8.MS (El, 70eV): m/z (%)=296(100%) [M*]. Anal. Calcd for C17H17N3O2: C, 69.14; H,5.80; N, 14.23. Found: C, 69.25;H, 5.71; N, 14.26. 4.1.8.3. Ethyl 6-(4-fluorophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylate (20e). Yields: 90 mg(23%, method E) and 180 mg (47%, method F) of yellowishcrystals, mp 160-163℃. H NMR (DMSO-d6) 6 1.39 (t.J=7.0 Hz, 3H, CH3CH2), 2.57 (s,3H.CH3), 4.43 (q, J=7.0 Hz, 2H, CH3CH2), 7.33 (m, 2H, ArH), 7.97 (s, 1H,5-H), 8.17 (m, 2H, ArH), 13.61 (s, 1H, NH). 13C NMR(DMSO-d6)8 14.5, 16.2,62.3,109.2,113.9,116.1,116.4,129.7,129.9,134.2,134.8,1141.0,145.3,154.7,162.3,165.1, 165.7. Anal. Calcd for C16H14FN3O2: C, 64.21; H,4.71; N, 14.04. Found: C, 64.14;H, 4.87; N, 14.02. 4.1.8.4.Ethyl(6-(4-bromophenyl)-3-methyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (20f). Yield: 210 mg(45%, method F) of yellowish crystals, mp 131-135°℃.HNMR (DMSO-d6) 8 1.39 (t, J=7.0 Hz, 3H, CH3CH2), 2.58(s, 3H, CH3), 4.44 (q, J=7.0 Hz, 2H, CH3CH2), 7.70 (d,J=8.8 Hz, 2H, ArH), 7.99 (s, 1H, 5-H), 8.08 (d, J=8.8 Hz, 2H, ArH), 13.66 (s, 1H, NH). 13C NMR (DMSO-d6); 14.5, 16.2,62.3,109.5,113.9,123.9,129.6,132.4,134.3,137.5,141.0,154.3,154.5, 166.0. MS (El, 70eV): mlz (%)=362 (100%) [M*]. Anal. Calcd for C16H14BrN302: C,53.35; H, 3.92; N, 11.67. Found: C, 53.21;H, 4.06; N, 11.63. 4.1.8.5. Ethyl 6-(3-chlorophenyl)-3-methyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (20g). Yield: 160 mg(40%, method F) of yellowish crystals, mp 112-114°C.H NMR (DMSO-d6) 8 1.39 (t, J=7.0 Hz, 3H, CH3CH2),2.57 (s, 3H, CH3), 4.44 (q, J=7.0 Hz, 2H, CH3CH2), 7.53(d, J=4.6 Hz, 2H, ArH), 8.00 (s, 1H, 5-H), 8.07 (dd,J=4.6,1.5 Hz, 1H, ArH), 8.15 (d, J=1.5 Hz, 1H, ArH), 13.66(s, 1H, NH). 13C NMR (DMSO-d6) 6 14.5, 16.1, 62.4,109.7, 114.1,126.2,127.2,130.0,131.3,134.3,134.4,140.4, 154.1,165.7.MS (El, 70 eV): m/z(%)=316(100%)[M*]. Anal. Calcd for C16H14CIN302: C, 60.86; H, 4.47;N, 13.31.Found: C, 60.71; H, 4.54;N, 13.28. m/z(%)=362(100%)[M*]. Anal. Calcd for C22H19N3O2: C,73.93; H, 5.36;N,11.76. Found: C, 74.09;H,5.22;N, 11.79. 4.1.8.10. Ethyl 6-(4-bromophenyl)-3-phenyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (21f). Yield: 240 mg(45%, method F) of yellowish crystals, mp 131-135°C.'H NMR (DMSO-d6) 8 0.75 (t, J=7.0 Hz, 3H, CHCH2),3.94 (q, J=7.0 Hz, 2H, CH3CH2), 7.4-8.3 (m, 9H, ArH),8.04 (s, 1H, 5-H), 14.20 (s, 1H, NH). MS (El, 70 eV): m/z(%)=422 (100%) [M*]. Anal. Calcd for C21H16BrN302: C,59.73; H, 3.82; N, 9.95. Found: C, 59.60; H, 3.99;N, 10.00. 4.1.8.11. Ethyl 6-(3-chlorophenyl)-3-phenyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (21g). Yield: 200 mg(41%, method F) of yellowish crystals, mp 173-175C.H NMR (DMSO-d6) 8 0.75 (t, J=7.0 Hz, 3H, CH3CH2),3.94 (q, J=7.0 Hz, 2H, CH,CH2), 7.4-8.4 (m, 9H, ArH),8.09 (s, 1H, 5-H), 14.18 (s, 1H, NH). MS (El, 70 eV): m/z(%)=378(100%)[M*].Anal. Calcd for C21H16ClN3O2: C,66.76;H, 4.27;N,11.12. Found: C, 66.89;H,4.15;N, 11.14. 4.1.8.12. Ethyl 6-(2-chlorophenyl)-3-phenyl-1H-pyr-azolo[3,4-b]pyridine-4-carboxylate (21h). Yield: 180 mg(36%, method F) of yellowish crystals, mp 120-123℃H NMR (DMSO-d6) 8 0.73 (t, J=7.0 Hz, 3H, CH3CH2)3.91 (q, J=7.0Hz, 2H, CH3CH2), 7.4-7.8 (m, 9H, ArH),7.69 (s, 1H, 5-H), 14.15 (s, 1H, NH). Anal..CaledC21H16ClN3O2: C, 66.76; H, 4.27; N, 11.12.Found:66.60; H, 4.37; N, 11.09. 4.2. X-ray diffraction data The crystals of C22H18N303Br are monoclinic.c. AAt293Ka=7.622(2), b=16.991(6),c=15.354(6)A, β=95.92(3)°,V=1978(1) A’, space group P21/c,Z=4, dcalc=1.519 g/cm’,u=2.108mm-,F(000)))=920. Intensity of 3657 reflections(3392 independent, Rint=0.055) was measured on an auto-matic four circles Siemens P3/PC diffractometer (graphitemonochromated Mo K. radiation,/2 scanning,20max=50°). The absorption correction was performed analytically(Tmin=0.365,Tmax=0.678). The structure was solved by di-rect method using SHELX97 package.15 The positions ofhydrogen atoms were located from the electron densitydifference maps and refined by the riding’model withUiso=nUeq of non-hydrogen atoms bonded to given hydro-gen atoms (n=1.5 for methyl and hydroxyllggroups andn=1.2 for other hydrogen atoms). Full-matrix least-squaresrefinement against Fin the anisotropic approximation using3355 reflections converged to R1=0.061 (for 1865 reflec-tions with F>4o(F)),wR2=0.130,S=0.991. Atomic coordi-nates and crystallographic parameters have been depositedto the Cambridge Crystallographic Data Centre, depositionnumber CCDC 617647. Copies of the data can be obtainedfree of charge on application to CCDC, 12 Union Road,Cambridge CB2 1EZ, UK (fax: +44 1223 336 033, e-mail:deposit@ccdc.cam.ac.uk). Acknowledgements The present work was supported by an INTAS grant (Ref.no.04-83-2844). We thank Biotage AG for the use of EmrysCreator EXP and Initiator 8 microwave reactors. ( 1. (a) Chebanov, V. A.; Desenko, S. M. Curr. Org. Chem. 2 006,10, 297; (b) Desenko, S. M. C hem. H e terocycl. Compd.(Engl. Transl.) 1995, 31, 125; ( c) Desenko, S. M.; O r lov, V. D. A zaheterocycles Based o n Aromatic U n saturated Ketones; Folio: Kharkov, 1 998; pp 38-101. ) ( 2. (a) Lipson, V. V .; Desenko, S . M . ; Shirobokova, M . G. ; Borodina, V . V. Chem. H e terocycl. C o mpd. (En g l. Transl.)2003, 39,1213;(b)Chebanov, V . A.; D e senko, S. M.; Sakhno, Y. I.;Panchenko, E. S.; Saraev, V . E.; Musatov, V.I.;Konev, V . F. Fiziol. Akt. Rechovini 2002, 3 3, 10; ( c )Chebanov, V. A .; S akhno, Y. I .; Desenko, S. M.; Shishkina , S. V.; Musatov, V. I.; Shishkin, O.V.; Knyazeva, I. V. Synthesis2005, 2597; (d) Lipson, V. V .; Desenko, S. M.;Borodina, V .V. ; Shirobokova,M . G . ; M u satov, V. I. Russ. J. Org. Chem. 2005, 41, 114; ( e) Lipson, V. V.; Desenko, S. M .; Shishkina, S. V ; Shirobokova, M. G.; S hishkin, O . V.; Orlov, V . D . C he m .Heterocycl. Compd.(Engl.Transl.)2003,39, 10 4 1; ( f ) Li pson, V . V.;Borodina, V.V.; Shirobokova, M . G.; K arnoz hi tskaya, ) T. M. Fiziol. Akt. Rechovini 2002,2,10. ( 3 . ( a) Desenko, S. M.; O rlov, V.D . ; Getmanskii, N. V . ; Shishkin, o . V; Lindeman, S. y ; S truchk ov , Y u. T . Chem. H eterocycl. Co mpd. ( (Engl. T rans l .) 1 993, , 2 9 , 4 06; ( b) Wermann, K .; Hartm a n, M. S y nthesis 19 9 1, 1 8 9; (c) D esenko, S. M.; Orlov,V. D .; G etmanskii, N . V ; Shishkin, O .V . ; Lindeman, S. V ; Struchkov, Yu. T . Dokl . Aka d , Nauk 1992, 324, 801; (d) Desenko, S. M.: O rl o v, V .D .; Estrada, Kh. Chem. Heterocycl.Compd. (Engl. T ransl . ) 1990, 26, 839;(e) D esenko, S. M.;Orlov, V.D.; Estrad a, Kh.; Ivkov, S. M. Chem. Heterocycl.Compd. (Engl. T r an sl. ) 1991,27,5 5 5. ) ( 4. See, for example : ( a) Kozminykh, V . O.; Kozminykh, E. M. Selecte d Metho d s for S ynthesis and Modification o f Hetero- cy c l es , Kartsev, V. G. , Ed.; IBS: Moscow, 2002; pp 263-279; (b) S a loutin, V. I; Burgart, Ya. V.; Chupakhin, O. N . Usp. Khi m . 1 999,6 8 , 227;(c) Perevalov, S. G. ; Bu r gart, Ya . V. ; Saloutin, V . I.; Chupakhin , O. N . Usp. Khim . 2001,70,1039; ( d) Nosek, E. Collect. Czec h . Chem. Commun. 1950, 15,335;(e) Mueller, R. J. Prakt. Chem. 1959, 132;(f) Armand, J . ; Armand, Y.; Boulares, L.; Bellec, C.; Pinson, J. J . H eterocycl. Chem. 1 9 85, 22, 15 1 9; (g) Kr u glenko, V. P .; Kl y uev, N. A.;Povstyanoi, M. V; Timoshin, A. A . Chem. H eterocycl.Compd. 1998, 34,232; (h) Berezina, E . S.; K o z’minykh,V. O.; Igidov, N. M.; Shirinkina, S. S. ; Koz’minykh, E. N.; Makhmudov, R . R . ; B u kanova, E. V. Russ. J. O r g. Chem.2001,37,539; (i ) K i stanova, N. S . ; M a shevskaya, I. V.;Bozdyreva, K. S .; Maslivets, A . N. C h em. Heterocycl. Compd. 2 003, 39, 6 73. ) ( 5. (a) Abdel-Haby, S. A. L.; Badawy, M. A .; K adry, A. M .;Mosselhi, M. A. N. J. Heterocycl . Chem.1987,24,1 5 87;(b)Abdel-Megid, M. P harmazie 2000, 55, 263;(c) El-Maati, T. M. A. Boll . Chim . Farm 1999 , 138,272. ) ( 6. (a) Epling, G. A.; Provatas , A. A. Chem. C ommun. 2002, 1036; (b) Garzarolli-Thurnlackh, K. M onatsh. C h em. 18 9 9, 20, 487; (c) Jablonski, W. Ber. Dtsch. Chem. Ges. 1 900,33,2925. ) ( 7 . Borsche, W. Ber. Dtsch . Chem. Ges. 1908,41,3886. ) ( 8. See, for example: ( a) Alajarin, R.; Avarez-Buila, J.; Vaquero,J. J.; Sunkel, C.; Fau, J.; Statkov, P. ; Sanz, J . Tetrahedron:Asymmetry 1993,4, 617; (b ) Bossert, F ; V a ter, W . Med. Res. Rev. 1989, 9 , 2 91; (c) Trigle, D . J . ; L angs, D . A . ; J a nis, R. A . M ed. R e s. R e v.19 8 9, 9, 12 3 ; (d ) Tsuda, Y. ; Mi s hina, ) T.;Obata, M.; Araki, K.; Inui, J.; Nakamura, T., Patent8,504,172 PCT Int., 1985; Chem. Abstr. 104, 207298;(e)Tsuda, Y.; Mishina, T.; Obata, M.; Araki, K.; Inui, J.;Nakamura, T. Patent 61,227,584 Jpn., 1986; Chem. Abstr.109,120988; (f) Tsuda, Y.; Mishina, T.; Obata, M.; Araki,K.; Inui, J.; Nakamura, T.19,870,408 Eur. Pat., 1987; Chem.Abstr.106, 213976; (g) Atwal, K. S.; Vaccaro, W.;Lloyd, J.;Finlay, H.; Yan, L.; Bhandaru, R. S. 20,010,607 PCT Int.,2001; Chem. Abstr.135,19660; (h) Atwal, K. S.; Moreland,S. Bioorg. Med. Chem. Lett. 1991, 1, 291. 9. Annan, N.;Paris, R.; Jordan, F. J. Am. Chem. Soc. 1999, 111,8895. 10. (a) Hartmann, H.; Liebscher, J. Synthesis 1984, 276; (b)Nenaidenko, V. G.; Golubinskii, I. V.; LenkoD..va, O. N.;Shastin, A. V.; Balenkova, E. S. Russ. J. Org. Chem. 2004,40, 1518; (c) Hennig, L.; Hofmann, J.; Alva-Astudillo, M.;Mann, G. J. Prakt. Chem.1990,332,351. 11. (a) Kappe, C. O. Angew. Chem., Int. Ed. 2004, 43, 6250; (b)Hayes, B. L. Aldrichimica Acta 2004, 37, 66; (c) Larhed, M.;Hallberg, A. Drug Discov. Today 2001,6, 406; Wathey, B.;Tierney, J.; Lidstrom, P.; Westman, J. Drug Discov. Today2002, 7, 373; (d) Al-Obeidi, F; Austin, R. E.; Okonya, J. F;Bond, D. R. S. Mini-Rev. Med. Chem. 2003, 3,449; (eKappe, C. O.; Dallinger, D. Nat. Rev. Drug Discov. 2006.5D,51; (f) Shipe, W. D.; Wolkenberg, S. E.; Lindsley, C. WDrug Discov. Today Technol. 2005, 2, 155;(g) Kappe, C.O.;Stadler, A. Microwaves in Organic and Medicinal Chemistry;Wiley-VCH:Weinheim, 2005. 12. (a) Xu, Y.;Guo,Q.-X. Heterocycles 2004,63,903;(b) Hamelin,J.; Bazureau, J.-P.; Texier-Boullet, F. Microwaves in OrganicSynthesis; Loupy, A., Ed.; Wiley-VCH: Weinheim, 2002;pp 253-294; (c) Besson, T.; Brain, C. T. Microwave AssistedOrganic Synthesis; Lidsrom, P., Tieney, J. P., Eds.; Blackwell:Oxford, 2005;(d) Stadler, A.; Kappe, C. O. J. Comb. Chem.2001, 3,624;(e) Kappe, C. O.; Stadler, A. CombinatorialChemistry, Part B; Bunin, B. B., Morales, G., Eds.; ElsevierScience: 2003; pp 197-223; (f) Ohberg, L.; Westman, J.Synlett 2001, 1296; (g) Bagley, M. C.; Singh, N. Synlett 2002,1718; (h) Wilson,N. S.; Sarko, C. R.; Roth, G. P. TetrahedronLett. 2002,43, 581; (i) Devi, I; Bhuyan, P. J. TetrahedronLett. 2004, 45, 8625; (j) Mont, N.; Teixid6, J.; Borell, J. IKappe, C. O. Tetrahedron Lett. 2003,44, 5385; (k) Mont, N.;Teixid6, J.; Borell, J. I.; Kappe, C. O. Mol. Diversity 2003,,153; (1) Mont, N.; Fernandez_Megido, L.; Teixid6, J.; Kappe,C. o.; Borell, J. I.QSAR Comb. Sci. 2004, 23, 836; (m) TyeH.; Whittaker, M. Org. Biomol. Chem. 2004, 2, 813; (n)Molteni, V.; Hamilton, M. M.; Mao, L., Crane, C. M.;Termin, A. P.; Wilson, D. M. Synthesis 2002, 1669;(o)Ireland, S.M.; Tye, H.; Whittaker, M. Tetrahedron Lett. 2003,44,4369. 13. Burgi, H.-B.; Dunitz, J. D. StructureCorrelation; VCH:Weinheim, 1994; Vol. 2, p 741. 14. Zefirov, Yu. V.; Zorky, P. M. Usp. Khim. 1989, 58, 713. 15Sheldrick, G. M. SHELXTL PLUS. PC Version. A system ofcomputer programs for the determination of crystal structurefrom X-ray diffraction data. Rev. 5.1,1998. ww.rysstech.com耐士科技 ° Refluxing in HOAc under nitrogen atmosphere.ww.rysstech.com耐士科技
确定












还剩12页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








