方案详情
文
The ethanolic extract of Aloe barbadensis Miller leaf skin showed inhibitory activity against phosphodiesterase-4D (PDE4D), which is a therapeutic target of inflammatory disease. Subsequent bioassay-guided fractionation led to the isolation of two new anthrones, 6′-Oacetyl- aloin B (9) and 6′-O-acetyl-aloin A (11), one new chromone, aloeresin K (8), together with thirteen known compounds. Their chemical structureswere elucidated by spectroscopic methods including UV, IR, 1D and 2DNMR, and HRMS. All of the isolateswere screened for their inhibitory activity against PDE4D using tritium-labeled adenosine 3′,5′-cyclic monophosphate (3H cAMP) as substrate. Compounds 13 and 14were identified as PDE4D inhibitors,with their IC50 values of 9.25 and 4.42μM, respectively. These achievements can provide evidences for the use of A. barbadensis leaf skin as functional feed additives for anti-inflammatory purpose.
方案详情

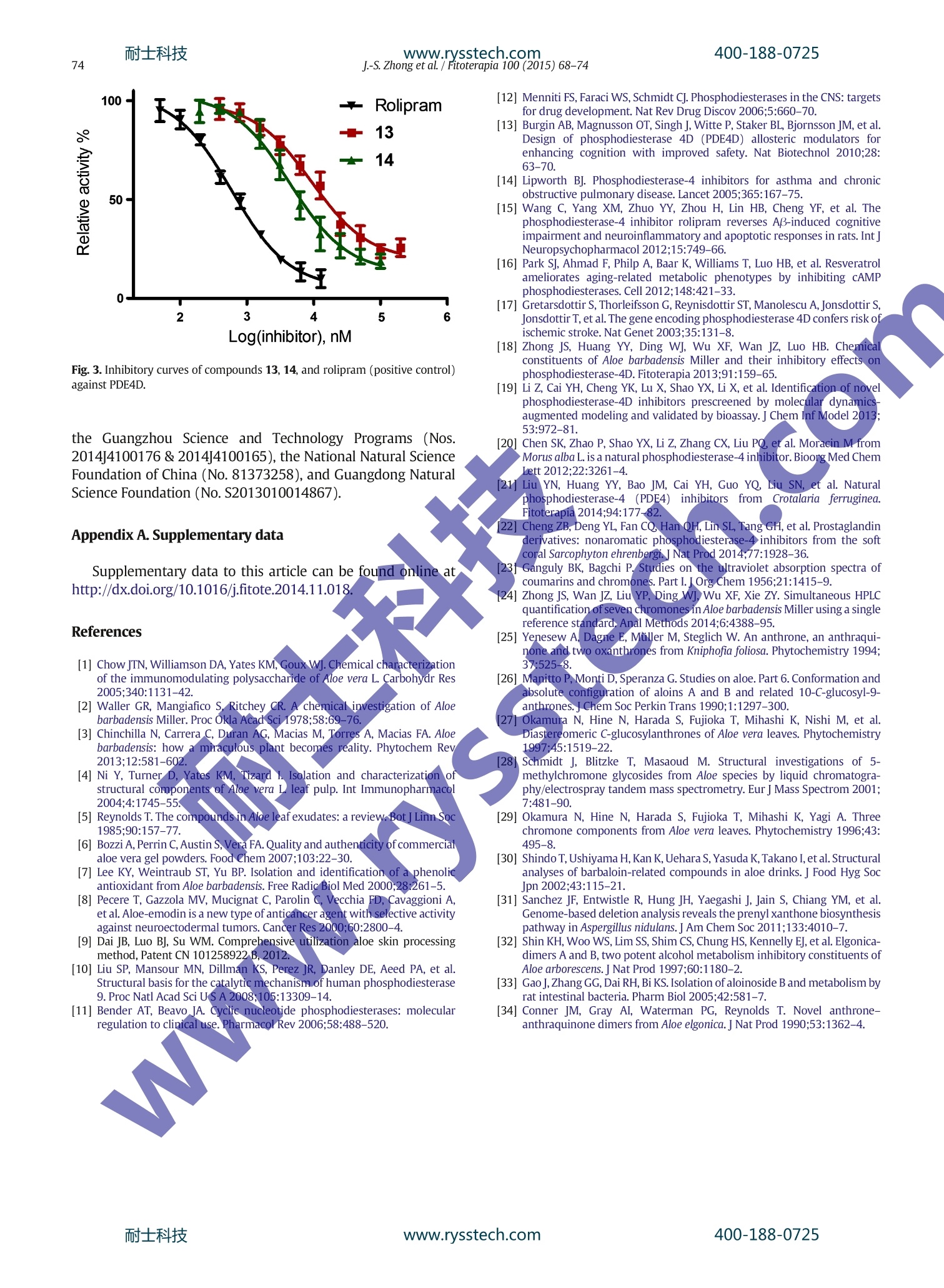
耐士科技400-188-0725 耐士科技400-188-0725www.rysstech.comJ.-S. Zhong et al. / Fitoterapia 100 (2015)68-7469 Contents lists available at ScienceDirect Fitoterapia ELSEVIER journalhomepage: www.elsevier.com/locate/fitote Natural phosphodiesterase-4 inhibitors from the leaf skin of Aloebarbadensis Miller Jia-Sheng Zhong,Yi-You Huang , Tian-Hua Zhang , Yu-Peng Liu, Wen-Jing Ding ,Xiao-Fang Wub.c, Zhi-Yong Xie, Hai-Bin Luoa*, Jin-Zhi Wan a.* School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, PR China DAnalysis and Testing Center, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, PR China ‘ Hainan Provincial Key Laboratory of Quality and Safety for Tropical Fruits and Vegetables, Haikou 571101, PR China Article history: Received 14October 2014 Accepted in revised form 19 November 2014Available online 28 November 2014 Chemical compounds studied in this article:Isoaloeresin D (PubChem CID: 76332505)Aloin B (PubChem CID: 5458894)Aloin A (PubChem CID:5458893)Aloe-emodin (PubChem CID: 10207)Aloinoside B (PubChem CID:46173998)Aloinoside A (PubChem CID: 46173997) Keywords: Aloe barbadensis Miller Anthrone The ethanolic extract of Aloe barbadensis Miller leaf skin showed inhibitory activity againstphosphodiesterase-4D(PDE4D), which is a therapeutic target of inflammatory disease.Subsequent bioassay-guided fractionation led to the isolation of two new anthrones, 6'-0-acetyl-aloin B (9) and 6'-0-acetyl-aloinA (11), one new chromone, aloeresin K (8), together withthirteen known compounds. Their chemical structures were elucidated by spectroscopic methodsincluding UV, IR, 1D and 2DNMR, and HRMS. All of the isolates were screened for their inhibitoryactivity against PDE4D using tritium-labeled adenosine3',5'-cyclic monophosphate (3H-cAMP) assubstrate.Compounds 13 and 14 were identified as PDE4D inhibitors, with their ICso values of 9.25and 4.42 uM, respectively. These achievements can provide evidences for the use of A. barbadensisleaf skin as functional feed additives for anti-inflammatory purpose. 1.Introduction Aloe barbadensis Miller, also known as “trueelaaloe”, is amedicinal and edible plant widely distributed in Europe, Asia andsouthern parts of North America[1,2]. In general, its leaf can bedivided into three major parts, the outer green leaf skin, the innerclear pulp and the bitter yellow exudate secreted by vascularbundles [3]. The pulp (aloe gel)is widely used for cosmetics,beverage and nutraceutical[4], and the exudate composed ofphenolic compounds [5]has a long history of medical use such asbeing a laxative [6], anti-oxidant [7] and anti-cancer agents [8]. ( *Corresponding aut h o r s . Tel.: +86 20 39943031; fax: + 86 20 39943040. ) ( E-mai l a ddresses : l u o h b77@mail.sy su. e d u. c n (H.-B. L uo), ) ( j in zhi wan 2 004@aliyun. c om (J.-Z. Wan). ) Instead, it seems that the leaf skin part of A. barbadensis isregarded as solid waste generated during the processing. Ex-citingly,recent studies and our preliminary experiments havedemonstrated that A. barbadensis leaf skin extract could serve asfunctional feed additives for immunity enhancement and anti-inflammatory purposes [9], which makes it possible to turn trashinto treasure. However, the compositions of A. barbadensis leafskin have rarely been reported. For further studies, it is urgent toclarify its chemical constituents and corresponding bioactivity. The phosphodiesterases (PDEs) are a superfamily of en-zymes that catalyze the hydrolysis of the intracellular secondmessengers cyclic adenosine monophosphate (cAMP) andcyclic guanosine monophosphate (cGMP) [10]. Among theeleven PDE families categorized by the human genomeencoding, the cAMP-specific PDE4, which is mainly distributed in immune and inflammatory cells, has gained increasingattention [11]. PDE4 is involved in inflammatory responsesand is proven as targets for the treatments of chronic obstructivepulmonary disease (COPD), asthma and central nervous system(CNS) disease [12-14]. Recent research also demonstrated thatPDE4 is bound up with anti-aging, reducing stroke risk, andtreating memory loss associated with Alzheimer’s disease[15-17], which make it a research hotspot. Our previous report indicated that A. barbadensis extractand several compounds possessed anti-inflammatory proper-ties through inhibiting the activity of PDE4D [18]. In continu-ation, it was found that the ethanolic extract of A. barbadensisleaf skin showed similar effects as well. Subsequent phyto-chemical investigation led to the isolation of two newanthrones (9 and 11), one new chromone (8), together withthirteen known compounds (Fig. 1). All of the isolates werescreened for their inhibitory activity against PDE4D. As a result,compounds 13 and 14 were identified as PDE4 inhibitors, withtheir IC5o values less than 10 uM. Herein, the details of theisolation, structural elucidation and the PDE4 inhibitory activityassay of these compounds are described. 2.Experimental Hackettstown,NJ, USA) with MeOH as solvent. UV spectrawere recorded on a Shimadzu UV2457 spectrophotometer(Kyoto, Japan) and IR spectra were obtained with a BrukerTensor 37 FT-IR spectrophotometer (Bruker Optics Inc., Biller-ica, MA, USA) with KBr pellets. NMR spectra were acquiredusing a Bruker AVANCE 400 spectrometer (Bruker BiospinGmbH, Rheinstetten, Germany) and chemical shifts (8) weregiven in ppm with TMS as internal standard. ESIMS data weredetermined on a TSQ Quantum mass spectrometer (ThermcFinnigan LLC, San Jose, CA, USA) and high-resolution massspectra (HRMS) were obtained on a Shimadzu LCMS-IT-TOFhybrid mass spectrometer (Kyoto,Japan). Semi-preparative RP-HPLC was performed on a Shimadzu LC-20AT liquid chroma-tography system (Kyoto, Japan) equipped with two LC-20ATpumps and a dual wavelength UV-VIS detector monitoring at300 and 355 nm. A semi-preparative ODS-A column (250 ×10 mm i.d., 5 um, YMC Co., Ltd., Kyoto,Japan) was employed forthe separation at a flow rate of 3.0 mL min. Reversed-phaseflash chromatography (RP-FC) was achieved oni a BiotageIsolera flashpurification system ((BiotageAB,. Uppsala,Sweden) coupled with an Eyela glass column (3000x:20 mmi.d.,Tokyo,Japan) packed with RP-C18 gel (20-40 um,Fuji SilisieChemical Ltd., Nagoya,Japan). Silica gel (300-400 mesh) usecfor column chromatography was produced by Qingdao MarineChemical Co., Ltd. (Qingdao, China). Thin layer chromatography(TLC) was carried out on silica gel Gprecoated plates (QingdaoMarine Chemical Co., Ltd., Qingdao, China) and spots werevisualized under UV 254 and/or 365 nm or by spraying with Fig. 1. Chemical structures of compounds 1-16. 10% H2SO4 in EtOH (v/v) followed by heating. Methanol usedfor HPLC analysis was of HPLC grade and purchased from SKChemicals (Ulsan, Korea). Ultrapure water was obtained from aMilli-Q laboratory water purification system (Millipore, Bed-ford, MA, USA). Other solvents were of analytical grade andmanufactured by Tianjin Zhiyuan Chemical Reagent Co., Ltd.(Tianjin, China). UP200S ultrasonic cell disruption processor(Hielscher, Teltow, Germany), 6K15 centrifugal machine(Sigma, Santa Clara, CA, USA), BioPhotomer spectrophotometer(Eppendorf, Hamburg, Germany) and nickel-nitriloacetic acid(Ni-NTA) column (Qiagen, Hilden, Germany) were employedfor the expression and purification of PDE4D. The radioactivity ofthe samples was measured on a Tricarb 2910 liquid scintillationcounter (PerkinElmer, Waltham, MA, USA). LB medium wasprepared using yeast extract and tryptone purchased fromOxoid Ltd. (Basingstoke, England). The isotope labeledsubstrate H-cAMP was manufactured by GE Healthcare(Waukesha, WI, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained fromAmresco Inc. (Solon, OH, USA). Other reagents such aspositive control rolipram were purchased from Sigma (SantaClara, CA, USA). The dried and powdered leaf skin of A. barbadensis (500.0g)was extracted three times with 95% EtOH (3x 1 L) underultrasonic. The extract was filtered and concentrated underreduced pressure to yield a dark residue (34.3 g). The crudeethanolic extract was suspended in distilled water (500 mL)and fractionated successively with 3x 500 mL volumes ofpetroleum ether, EtOAc and n-butanol. The EtOAc fraction(8.1 g) was then subjected to RP-FC and eluted with a gradientofMeOH-H20 (30:70 to 80:20,v/v) at a flow rate of 20 ml minto afford 10 fractions (E1-E10). Fraction E3 was furtherchromatographed by RP-FC using MeOH-Hz0 (26:74,v/v) asmobile phase to yield compounds 1 (321mg), 2 (362 mg) and3(738 mg). Fraction E5 was purified by semi-preparative HPLCusing a MeOH-H20 (56:44,v/v) system to obtain compounds 4(3 mg, tr 11.5 min),5 (2 mg, tr 12.3 min),6(3 mg, tr 13.2 min)and 7 (4mg, tr 13.9 min). Fraction E6 was subjected to semi-preparative HPLC eluted with an isocratic elution system ofMeOH-H20 (60:40, v/v) to give compounds 8 (45 mg, t8.9 min) and 9 (63 mg, tr 13.5 min). Using the same procedure,compounds 10(23 mg, tr 13.4 min) and 11 (82 mg, tR15.7 min) were obtained from fraction E7. Fraction E9 waschromatographed on silica gel column (100 ×10 mm i.d.)eluted with a step gradient of CH2Cl2-MeOH (10:1to 7:1, v/v)to afford compounds 12 (8 mg), 13 (3mg) and 14 (2 mg). Then-butanol extract (2.3g) was purified by RP-FC with MeOH-H20 (35:65 to 60:40, v/v) as mobile phase, followed by semi- 2.3.1. Aloeresin K (8) Yellowish amorphous powder; [a]20 D=-130.2°(c 0.66,MeOH); UV (MeOH) 入max: 226, 252 and 300 nm; IR (KBr)Vmax:3376 (br), 2934, 1706,1650,1600,1379,1246,1164 and1103 cm; positive ESIMS m/z 599.19 [M+H]+; HRESIMS m/z621.1973 [M + Na]+ (calcd. for C31H34012Na, 621.1942); lH(CD30D, 400 MHz) and ’c NMR (CD30D, 100 MHz) spectraldata see Table 1. 2.3.2.6'-O-acetyl-aloin B (9) Yellow amorphous powder;[α]20 D = -53.8°(c 0.66,MeOH); UV (MeOH) 入max: 269,297 and 356 nm; IR (KBr) Vmax:3403 (br), 2876,1721,1612,1286,1233 and 1069 cmpositive ESIMS m/z 461.05[M + H]+; HRESIMS m/z 483.1279[M + Na]t(calcd. for C23H24010Na, 483.1262); 1H (CD30D,400 MHz) and 13c NMR (CD3OD, 100 MHz) spectral data seeTable 1. 2.3.3. Aloeresin J (10) Yellowish amorphous powder; [a]20 D=-147.8°(c0.69,MeOH); UV (MeOH) 入max:225, 251 and 297 nm; IR (KBr) V'max:3385 (br), 2971,2926,1701,1647,1599,1382,1259,1166 and1078 cm-;positive ESIMS m/z 571.21[M+H]+; HRESIMS m/z593.1998 [M + Na]+(calcd. for C30H34011Na, 593.1993); H(CD30D, 400 MHz) and c NMR (CD30D, 100 MHz) spectraldata see Tabl:e1. 2.3.4.6-O-acetyl-aloin A (11) Yellow amorphous powder;[a]20 D= +5.2°(c 0.63,MeQH); UV(MeQH)入max: 269,297 and 356 nm; IR (KBr)Vmax:3423 (br), 2920,2872,1728,1622,1236,1101 and 1032 cm-;positive ESIMS m/z 461.08 [M + H]+; HRESIMS m/z 483.1285[M +Nat (calcd. for C23H24010Na, 483.1262); H(CD3OD,400 MHz) and c NMR (CD30D, 100 MHz) spectral data seeTable 1. 2.4.Expression and purification of PDE4D The protocols for expression and purification of PDE4Dwere referred to our previous reports [19-22]. In brief, therecombinant plasmid pET15b subcloned with PDE4D2 (cata-lytic domain, residues 86-413) was introduced into the E. colistrains BL21 (codonplus) and the transformed cells werecultivated in LB medium containing 100 ug mL-ampicillinand 0.4% glucose at 37℃ until OD600 = 0.7. Then 0.1 mMisopropyl B-D-1-thiogalactopyranoside (IPTG) was applied toinduce PDE4D protein expression for another 20 h at 15°C.PDE4D protein was purified by Ni-NTA column eluted withimidazole buffer and the effluent was monitored throughOD280.Typically 40-60 mg of PDE4D was obtained from 1 L cellculture, with a purity of greater than 90% as determined bySDS-PAGE. 2.5. Enzymatic assays Table 1 1H(400 MHz) and 13c (100 MHz) NMR data of compounds 8, 9, 10 and 11 in CD3OD (6 in ppm, J in Hz). (pH7.5),10 mM MgCle and 0.5 mMDTTto 2×104-3×104cpmper assay. The reaction was carried out in a reaction mixturecontaining PDE4D enzyme, substrate and different concentra-tions of inhibitor dissolved in DMSO at room temperature for15 min, and terminated by adding 0.2 M of ZnS04. Then thereaction product H-AMP was precipitated out by 0.2 N ofBa(OH)2, whereas unreacted H-cAMP remained in the super-natant. The radioactivity in the supernatant was measured in2.5 mL of Ultima Gold liquid scintillation cocktails by a liquidscintillation counter. All of isolates were preliminary screened ata concentration of 10 pM and the lC5o values of activecompounds were further measured by nonlinear regression.Each test was measured in triplicate. Rolipram, a representativePDE4 inhibitor, was selected as positive control. 2.6. Cytotoxic assays The cytotoxicity of compounds 13 and 14 on the murinemacrophage RAW 264.7 cells (obtained from ATCC, Manassas,VA, USA) was evaluated by MTT assay. RAW 264.7 cells werecultured in DMEM (Gibco-BRL, Grand Island, NY, USA) supple-mented with 10% fetal bovine serum (Zhejiang TianhangBiological Technology Co., Ltd., Hangzhou, China) in 5% CO2 at37 C. Cells were seeded into 96-well culture plate with a density of 1 ×104 cells per well and allowed to adhere for 24 hand then treated with various concentrations of each compoundfor another 24 h. After treatment, cells were incubated with0.5 mg mL- MTT solution for 4 h at 37℃ and thenthe supernatant was discarded and the formazan crystal wasdissolved in DMSO. The optical density at 570 nm was measuredon a FlexStation 3 microplate reader (Molecular Devices,Sunnyvale, CA, USA) with SoftMax Pro 5 software onboard. 3. Results and discussion 3.1. Structural elucidation of the isolated compounds Compound 8 was obtained as a yellowish amorphouspowder. Its molecular formula was established as C31H34012from a pseudomolecular ion peak at m/z 621.1973 [M + Na](calcd. for C31H34012Na, 621.1942) in the HRESIMS. The IRspectrum of 8 indicated the presence of hydroxyl group(3376 cm-, br), conjugated ester carbonyl (1706 cm-),chelated carbonyl group (1650 cm-1) and aromatic moiety(1600 cm). The UV spectrum gave maximum absorptions at226, 252 and 300 nm, which were the characteristics ofchromones[23]. The H-NMR spectroscopic dataaof 8(Table 1) combined with its H-1H COSY and HSQC spectra exhibited an AA'BB’ system for two pairs of ortho-coupledaromatic proton signals at 6 7.31 (2H, d,J=8.6Hz) and 8 6.74(2H,d,J=8.6Hz), a trans-olefinic bond proton signals at 67.35(1H,d,J=16.1 Hz) and 8 6.03 (1H, d,J=15.9 Hz), twoisolatedaromatic proton signals on chromone skeleton at 8 6.77 (1H,s) and 6 6.12 (1H,s), a B-linked glucosyl moiety at 6 3.57-5.75(anomeric proton at 8 5.20 (1H, d,J= 10.1 Hz)), a 2-hydroxypropyl group at 6 2.83 (1H, dd,J=14.2, 5.0 Hz), 82.76 (1H, d,J=7.8 Hz), 8 4.45 (1H,m) and 8 1.37 (3H,d,J=6.2 Hz), a methoxyl group at 8 3.87 (3H, s), as well as twomethyl groups at 6 2.71 (3H, s) and 2.03 (3H,s). The 13C-NMRspectrum, in combination with HSQC experiments and afore-said analysis, indicated 31 carbons attributable to threecarbonyl groups (6c 182.2,173.0 and 168.0), eight spquaternary carbons (8c 167.2 (bearing oxygen atoms), 162.0,161.3,159.6,144.8,127.0, 117.2 and 111.4), eight spmethines(146.6,131.1,116.9,114.6 and 112.6), six glucosyl carbons (8c80.1,77.7,73.7,72.8,72.4 and 64.4), a methoxy group (8c57.1),a sp’ methylene (6c 44.9), a sp’ methine (8c 66.3), andthree methyl groups (8c 23.7 and 20.9). The aforementioneddata preliminary indicated that compound 8 was a 2,5,7,8-tetrasubstituted chromone. The chromone skeleton was con-firmed by the key HMBC correlations (Fig.2) ofH-6 to C-4a andC-8, and H-3 to C-2 and C-4a. The 2-hydroxypropyl group,methoxyl group and methyl group were respectively attachedto C-2, C-7 and C-5 of the chromone ring, as indicated by the HMBCcorrelations of H-9 to C-2, the protons of methoxy to C-7,and the protons of methyl to C-4a, C-5 and C-6, respectively.B-glucosyl moiety was detected as being connected to C-8 due tothe HMBC correlations of H-1' to C-8 and C-1a. The HMBCcorrelations from H-5" to C-3", H-2" to C-1" and H-3" to C-1"implied the presence of a p-(E)-coumaroyl. The above-mentioned information was quite similar to that of isoaloeresinD (1), except for the presence of an additional acetyl group (8H2.03, 3H, s; 8c20.9 and 173.0). The location of the acetyl groupwas assigned at 6'-OH by an HMBC correlation from H-6' to thecarbonyl group at 6c 173.0. The absolute configuration of thehydroxy-bearing carbon (C-10) of the 2-hydroxypropyl chainin 8 was assigned by acid-catalyzed hydrolysis method,Approximate1 mg of compound 8 was refluxed with 1 mL of1 M HCl(MeOH-H20,3:1,v/v) for 1 h to convert 8 to 8a. Afterremoval of solvent and HCl under vacuum, the residue wasdissolved in MeOH and analyzed by HPLC using the previouslyreported procedures [24]. The S absolute configuration of C-10was confirmed by comparison of the retention time of 8a (t11.3 min) with reference standard. Thus, compound 8 wasidentified as 6'-O-acetyl-isoaloeresin D, and was given thetrivial name aloeresin K. Compound 9, a yellow amorphous powder, possessed amolecular formula of C23H2401o, as established by HRESIMS(m/z 483.1279 [M +Na]+, calcd. for C23H24010Na, 483.1262).The IR spectrum of 9 showed absorption bands for hydroxyl group (3403 cm-, br), ester carbonyl (1721 cm-) andchelated carbonyl group (1612 cm-). The UV absorptionbands at 入max 269,297 and 356 nm implied the presence of ananthrone chromophore [25]. The H-NMR (Table 1) and HSQCspectra of 9 exhibited signals for three vicinal aromatic protonsat 86.98 (1H,d,J=7.4Hz),87.44(1H,t,J=7.9 Hz) and 6 6.84(1H,d,J=8.1 Hz), as well as two isolated aromatic protons at 86.96 (1H,s) and 66.85 (1H, s) belonging to the anthrone ring.Combined with the signals of two strongly chelated phenolprotons at 8 11.88 (1H,s) and 6 11.87 (1H,s) and typical H-10signals at 64.47(1H,d,J=1.6Hz), compound 9 was deducedas a 1,8-dihydroxyl-3,10-disubstituted anthrone. The spectraalso showed a -linked glucosyl group at 62.81-4.06(anomericproton at 63.28(1H,dd,J=9.1,2.5 Hz)), a hydroxymethyl at 84.65(2H, s), and a methyl group at 8 1.88(3H,s). The 13C-NMRspectrum together with the HSQC experiment revealed 23carbon signals including those of two carbonyl groups (8c195.4 and 172.6), seven sp quaternary carbons (8c 163.0,162.9,152.3,147.2,142.3,119.1 and 117.8, two of thembearing oxygen atoms), five spmethines (8c 136.0,121.3,117.4,117.2 and 114.1), six glucosyl carbons (8c 85.9,79.7,78.7,71.7,71.5 and 64.7), an oxygenated methylene (8c64.5), a sp’ methine (8c 64.5), together with two methylgroups (8c 45.4 and 20.5). The HMBC correlations (Fig. 2)from hydroxymethyl protons (8H 4.65, H-11) to C-2, C-3 andC-4 indicated that a hydroxymethyl was attached to C-3 ofthe anthrone ring. The location of glucosyl moiety wasassigned at C-10 by an HMBC correlation from the anomericproton (8H 3.28, H-1') to C-10. An acetyl group was locatedat6'-OH as indicated by the HMBC correlations of H-6' to thecarbonyl group at 8c 172.6. The NOESY interactions of H-4and H-1'(Fig.2) further assigned the R configuration of C-10[26]. Hence, compound 9 was assigned as an aloin-likecompound named 6'-0-acetyl-aloin B. Compound 11, a yellow amorphous powder, possessed thesame molecular formula as that of 9, implying that they wereisomers. The lHand 13cNMR spectra of 11 and 9 (Table 1) werealmost identical. The NOESYYassociations of H-5 with H-1'(Fig. 2) suggested thes configuration of C-10 in 11. Theirabsolute configuration was confirmed by comparison of corre-sponding optical rotation ([a]20D+5.2°and-53.8° for 11 and9, respectively) with aloin-like isomers as well [27]. Therefore,compound 11, the diastereoisomer of 9, was elucidated as 6'-0-acetyl-aloin A. Compound 10 had previously been identified from Aloerubroviolacea Schweinf. by LC-MS method [28]. The presentstudy gave a detailed description of its NMR data (Table1) andabsolute configuration for the first time. The methoxyl groupwas confirmed being connected to C-7" by HMBC correlationsof methoxyl protons (8n3.79) to C-7"(8c 163.2). Using thesame procedure as compound 8, 10 was converted to 8a byacid-catalyzed hydrolysis.Hence, the absolute configuration ofC-10 in 10 was assigned as S. Compound 10 was given thetrivial name aloeresin J. The other twelve known compounds were identified asisoaloeresin D (1)[29], aloin B (2) [26], aloin A (3)[26], aloin-dimer A (4)[30], aloin-dimer B (5)[30], aloin-dimer C (6)[30],aloin-dimer D (7)[30], aloe-emodin (12)[31], elgonica-dimerA (13) [32], elgonica-dimer B (14) [32], aloinoside B (15) [33]and aloinoside A (16) [33] by comparison of their spectroscopicdata with those reported in literatures. 3.2. In vitro inhibitory activity towards PDE4D All of the isolates were screened for their inhibitory activityagainst PED4D. The positive control, rolipram, herein gave anICso value of 0.59 uM, which was comparable to the literaturedata of 1.0 uM. The bioassay results indicated that compounds13 and 14 exhibited remarkable inhibitory activity towardsPDE4D (Table 2), with ICso values of 9.25 and 4.42 pMrespectively. The inhibitory curves of compounds 13, 14 androlipram were represented in Fig. 3. Interestingly, it was found that anthraquinones with 10-carbonyl group like compounds 12, 13 and 14 showedremarkable enzyme inhibitory activity, while anthrones in-cluding compounds 2-7, 9, 11, 15 and 16 were inactive. Theseresults verified our previous structure-activity relationship(SAR) observation that anthraquinones with glucosyl group onC-10 of the ring decreased inhibitory activity against PDE4D[18]. Compounds 13 and 14 are a pair of diastereoisomersdiffering in the configuration of C-10. They are anthrone-anthraquinone dimers composed of anthrone emodin-10'-C-B-D-glucopyranoside and anthraquinone aloe-emodin moieties[34]. Our previous study showed that their subunit aloe-emodin (12) exhibited moderate inhibitory activity with anIC5o of ~20 uM [18]. Taken together, it can be concluded thataanthraquinone ring may contribute to good activity. However,ffurther investigation should be carried out to explore theirinhibitory mechanism. For further study, RAW 264.7 cell, which is the mostcommon used cell line in in vitro anti-inflammatory experi-ment, was applied to the cytotoxicity test of active compoundsMTT assays (Table 2) indicated that compounds 13 and 14 didnot show significant cytotoxicity when the concentration wasup to 50 pM. In summary, two new anthrones (9 and 11) and one newchromone(8), as well as thirteen known compounds, wereisolated from the 95% EtOH extract of A. barbadensis leaf skin. Inthe bioassay ofPDE4D, compounds 13 and 14 were found to bePDE4D inhibitors, with their ICso values of 9.25 and 4.42 uMrespectively. These achievements can provide evidences for theuse of A. barbadensis leaf skin as functional feed additives foranti-inflammatory purpose. Conflict of interest The authors declare no conflict of interest. Acknowledgments This work is financially supported by the National KeyTechnology R&D Program during the Twelfth Five-Year PlanPeriod of People's Republic of China (No. 2013BAD10B04-2), Table 2ICso values of compounds 13 and 14 against PDE4D and their cytotoxicity. Compound IC50(uM) Cell viability (%) 13 9.25±0.64 98.3 ± 2.8 14 4.42±0.74 100.5±2.4 Rolipram 0.59±0.05 Determined on RAW 264.7 cell line at 50 uM.Positive control. Fig. 3. Inhibitory curves of compounds 13, 14, and rolipram (positive control)against PDE4D. the Guangzhou Science and Technology Programss (Nos.2014J4100176 &2014J4100165), the National Natural ScienceFoundation of China (No.81373258), and Guangdong NaturalScience Foundation (No.S2013010014867). Appendix A. Supplementary data Supplementary data to this article can be found online athttp://dx.doi.org/10.1016/j.fitote.2014.11.018. References ( [1 ] Cho w JT N , Wi l l ia mso n D A , Y a tes K M, Goux WJ.Chem i cal c haracterization o f the i mmun omo dulat in g po l ysac charid e of Aloe v e ra L. Car b ohy d r R es 2 005;3 40 : 1 131- 4 2. ) ( [2 ] W all e r G R, M a ngi a f i c o S . R it c he y CR . A c hemi ca l investigation o f A lo e b arbadensi s M ille r. Pr oc Ok l a A c ad Sc i 197 8 :5 8 : 6 9-76. ) ( [3] Ch i n ch il l a N, Car rer a C, D uran AG, Ma ci a s M , T o rres A. M a ci as F A . A lo e b ar ba de nsi s: h o w a m i raculo u s p l a nt b e co m es reality. Phy to che m R ev 2 01 3;12 : 5 81 - 6 0 2 2 . ) ( ] L N i Y , T u rne r D , Y ates K M KM , , T T izard I . Isol a tion an d char a cte rization of s tructural components o f A l oe vera L . leaf p u lp. In t Immunophar ma col 20 04 ;4: 1 7 45-55. ) ( [5] 1 R eyn ol ds T . The co mpounds i n A loe lea f e x ud ate s: a r e v ie w. Bo t J L in n Soc 19 85;90: 1 5 7 -7 7 . ) ( [6] B ozz i A , Pe rr i n C , A u s t i n S, V era F A. Q ua l it y an d a u th e n t ic i t y of comm er ci a l a l oe v era ge l pow d er s. Fo o d Che m 2 0 07 ; 1 03:2 2-3 0 . ) ( [7] L e e K Y, W eint r aub S T, Yu BP . Is o l ati on an d i de n t ifi cat ion o f a phenol i c a n t io x idant from A loe barbadensis. F r ee R a d ic Bio l M ed 2 0 00;28:261-5. ) ( [8] Pe c e r e T, G azzola MV, M u cignat C , Pa r o lin C , V e cchia FD , C a vaggi o ni A, e t a l. A loe - emodin is a new type o f ant ican cer agen t with sele c tive ac tiv ity a ga i n st ne ur oectoder ma l tumo r s . C a n c er Res 20 0 0;60:280 0 - 4. ) ( [9] Dai JB, Luo BJ , Su WM. C omprehen s ive u t il i z a tion a loe s kin p rocessing method, Patent CN 101258922 B,2012. ) ( [10] L i u SP, M anso u r M N , D i llma n KS , Per ez JR, D a nle y D E, A ee d PA , e t a l. S tr uc tu ra l bas i s f o r th e ca t alyt i c m ec ha ni sm of h u m an p ho s ph o di est e ra s e 9 . Pr oc N atl Ac a d S ci U S A 2008; 1 05:1 330 9- 14 . ) ( [11] Ben d er A T , B ea vo J A . C yclic n ucl e otide p h o sphodiesterases: m ole c ular r eg ulation t o clin i cal use . Phar m ac o l Rev 2 00 6;58 :4 88-52 0 . ) ( [12] M e n ni t i FS , Faraci WS, Schmidt C J . P h osp h odi e sterases in the C N S: t a r ge t s f or d r ug d e vel op ment. Nat Re v Drug D is c o v 200 6;5:66 0- 7 0 . ) ( [13] B u rg i n A B, M a g nus s on O T, S in g h J , Witte P , S t ak e r BL, Bj or nsso n JM , e t a l . D es ig n o f p h os ph odies te r as e 4 D ( P D E 4D ) a l l os t e ri c m o d ul ator s f o r enhan c in g c ogniti o n w i t h i m p r oved s a f e t y. N a t Bi o t e ch no l 20 1 0 ;28 : 6 3- 70. ) ( [ 14] L i p wor t h B J. P h osp h odies t er a se -4 i nhi b itors f or as thma a nd c h ro ni cobstr u cti v e p u lmon a ry disease. L ancet 2 0 0 5 ;365:16 7 -75 . ) ( [15] Wang C, Y ang XM, Z h uo YY, Zho u H, L in HB , C h e ng YF , e t a l . T he p ho s ph od i es te ra se- 4 i n hib itor r o l i pram rev e r ses A B- i n du c e d c o gn i t i v e imp air me nt a n d neu ro infl am m a tory an d ap o p t o t i c r esp o n ses in r a t s . I nt ] Neurops ycho ph armacol 2 0 1 2 ;15 :7 4 9-6 6. ) ( [ 16] P a rk S J, Ahmad F, Ph ilp A , Ba ar K , W il l i am s T, L u o H B , et a l . Res ve ra trolam e lio rates a gi n g-rel a te d m et a bo l ic phen o typ e s b y in h i b i t in g c AMP p h osp h od i estera se s . C el l 20 1 2 ;1 48 :4 21 -33 . ) ( [17] G ret ar sdott i r S , Tho rl ei fsso n G, R e yn i sdot ti r ST , M a no lesc u A , J on sd ott ir S, J onsd ot tir T , et a l . T h e g en e e nco di n g phosphodi e s t erase 4D c o nf e r s risk o f i sc he mi c s t roke. Nat Gen e t 200 3 ; 3 5: 1 3 1 - 8 . ) ( [18] Zho n g JS , H uang Y Y, D ing WJ , Wu XF, Wan JZ , L uo H B . Ch e micalco n st itu ent s of A l oe b a rb ad ensi s M i l ler an d th e i r i nh i b it o ry ef f e c t s on p h os ph od i es ter ase - 4D. F i t otera pi a 20 1 3;91: 1 5 9 -6 5 . ) ( [ 1 9] L i L Z , C ai Y H, C h eng YK, L u X , S h a o YX, L i X , e t al . I de ntif i c a ti o n o f n o vel p h osp h od i es t er a s e -4D i n h ib i t ors p r e sc re ene d b y m ol ec ula r d y na mi cs- aug men te d m ode l i n g an d v alidate d by b io as say . J C h em I n f M od e l 2 0 1 3; 53:9 7 2 - 8 1. ) ( [20] C h en S K , Z h a o P , Sh a o Y X , L i Z, Zha ng C X , Liu P O, et al. Moracin M fro m M o rus alba L . is a natura l phos p h o d ie ster as e-4 inhi b ito r . Bioor g Me d Chem Let t 2 0 1 2 ; 22 : 32 6 1 - 4. ) [21]11Liu YN, Huang YY, Bao JM, Cai YH, Guo YQ, Liu SN, et al. Naturalphosphodiesterase-4 (PDE4)inhibitors fromCrotalaria ferruginea.Fitoterapia 2014;94:177-82. [22]] Cheng ZB, Deng YL, Fan CQ. Han QH, Lin SL, Tang GH, et al. Prostaglandinderivatives: nonaromatic phosphodiesterase-4 inhibitors from the softcoral Sarcophyton ehrenbergi. J Nat Prod 2014;77:1928-36. [23] Ganguly BK, Bagchi P. Studies on the ultraviolet absorption spectra ofcoumarins and chromones. Part I. J Org Chem 1956;21:1415-9. [24] Zhong JS, Wan Jz, Liu YP, Ding WJ,Wu XF. Xie ZY. Simultaneous HPLCquantification of seven chromones in Aloe barbadensis Miller using a singlereference standard. Anal Methods 2014;6:4388-95. [25] Yenesew A, Dagne E, Muller M, Steglich W. An anthrone, an anthraqui-one and two oxanthrones from Kniphofia foliosa. Phytochemistry 1994;37:525-8. ( [26] M a N nitto P, M o n ti D, S p e r an z a G. Stu die s o n al o e. Pa r t 6. Co nfo r m ati o n a nd absolu t e c on f i g u r ati o n o f a lo i n s A and B a nd r elated 10 -C- glucosy l- 9- a n t h ron es . j C he m Soc Pe rkin Trans 1 990; 1:1 2 97- 3 00. ) ( [ 27]O k amura N, H i n e N , H ar a da S , Fuj i ok a T, M iha sh i K, N is h i M, e t a l. D i ast ereo me ri c C - gluc o syl an t h r o n es o f A l o e ver a l e a ve s . P h yt o c he m ist ry 1 997; 4 5 :15 19 -22. ) ( [28 1 S c hmid t J , B litzk e T , M a sa oud M . S t r uc tural i n v e st i gati o ns o f 5 -m e t h ylch r omon e glyc o side s fr o m Aloe spe c i es b y l iq u id ch r omato gra - p hy/el e ct ros pray t ande m m a ss s p ectromet ry. E u r J Mass Sp e ctr om 2 0 0 1 ; 7 :4 8 1 - 9 0 . ) ( [29] O k am u r a N , H i ne N , H a rad a S, Fuj i o ka T , M i ha sh i K , Yagi A. T h ree c hrom on e c o mpo nent s fr o m Al o e ve ra l e a ves. P hytoc hemist ry 199 6 ; 4 3: 49 5 -8 . ) [30] Shindo T,Ushiyama H, Kan K, Uehara S,Yasuda K, Takano I, et al. Structuralanalyses of barbaloin-related compounds in aloe drinks. J Food Hyg SocJpn 2002;43:115-21. [31]Sanchez JF, Entwistle R, Hung JH, Yaegashi J, Jain S, Chiang YM, et al.Genome-based deletion analysis reveals the prenyl xanthone biosynthesispathway in Aspergillus nidulans. J Am Chem Soc 2011;133:4010-7. ( [32] S hin K H, W o o WS, Li m S S, Shi m CS , C h u n g H S , Ke n n e l ly E , et a l. Elgoni c a- d im e rs A and B, t w o po te n t a lc o h o l m eta bo l ism i nh i b it o ry c onst itu en t s o f A loe a rb ore s c ens. J Na t Pro d 1 997;6 0 : 1180 - 2 . ) [33] GaoJ,Zhang GG, Dai RH, Bi KS. Isolation of aloinoside B and metabolism byrat intestinal bacteria. Pharm Biol 2005;42:581-7. ( [34] C o n ner J M, G ray Al , Wat e rman P G, R e yno l ds T . N ove l a nt h rone - a nthraqui n one di m ers f rom Alo e elgo n ic a . J Nat Prod 1 9 9 0 ;53 : 1 36 2 - 4. ) http://dx.doi.org/j.fitote./O Elsevier B.V. All rights reserved.士科技www.rysstech.com 耐士科技ww.rysstech.com
确定






还剩5页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《库拉索芦荟叶皮中磷酸二酯酶检测方案(制备液相色谱)》,该方案主要用于中药材和饮片中含量测定检测,参考标准--,《库拉索芦荟叶皮中磷酸二酯酶检测方案(制备液相色谱)》用到的仪器有
相关方案
更多
该厂商其他方案
更多









