方案详情
文
浮萍作为未来食物:代谢物、营养和微生物分析的证据Duckweed as a future food Evidence from metabolite profile, nutritional and microbial analyses
方案详情

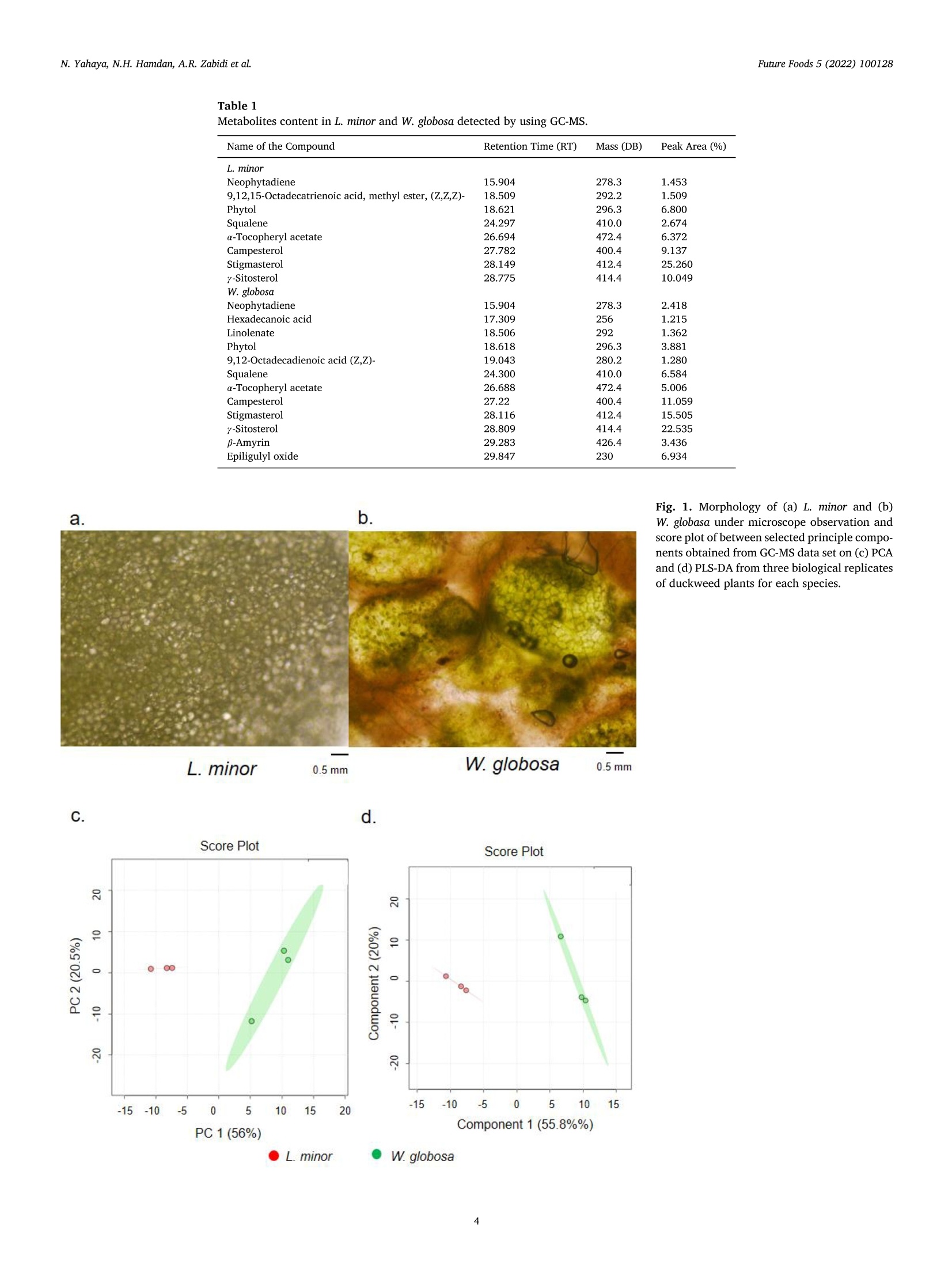
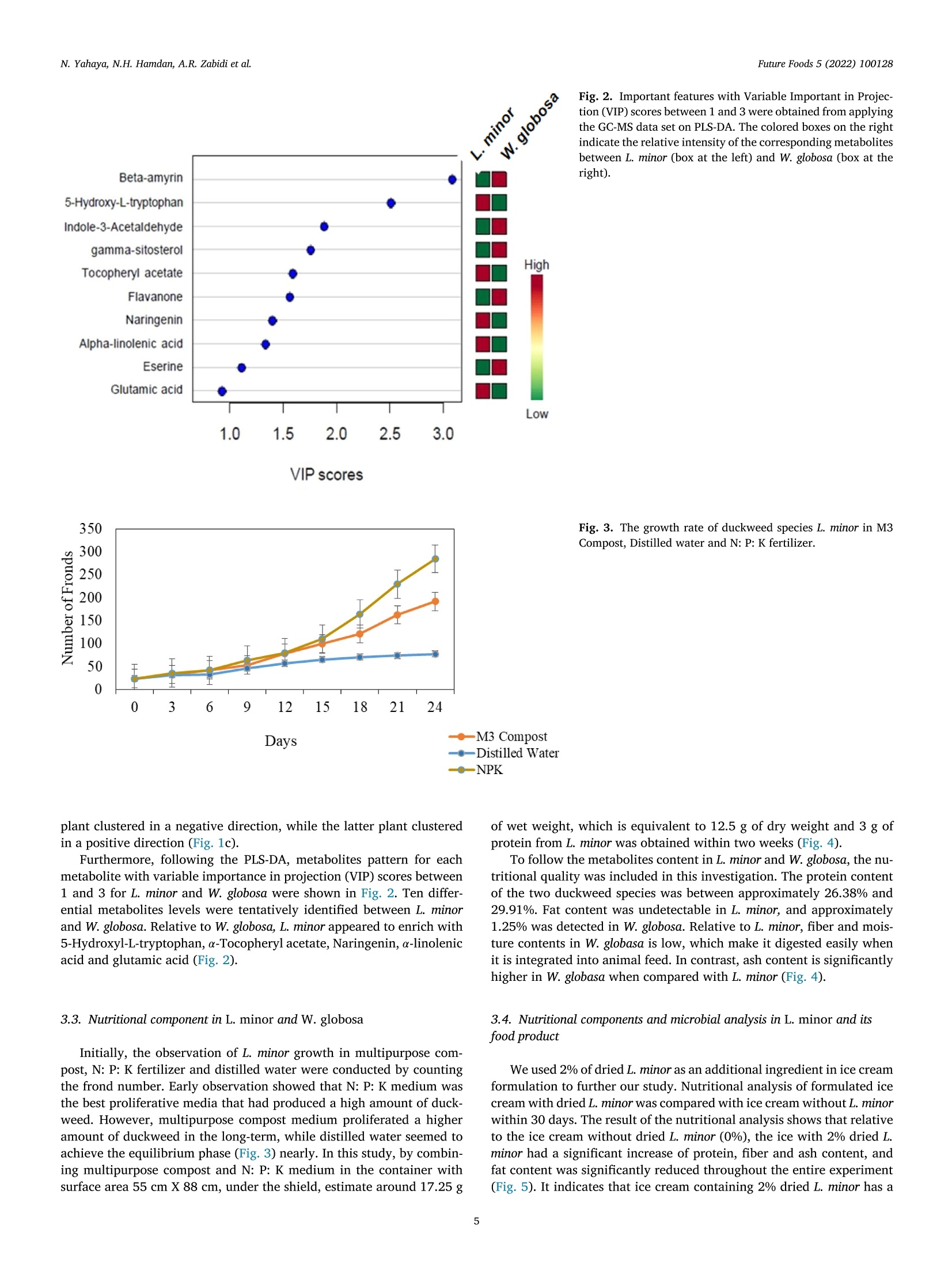
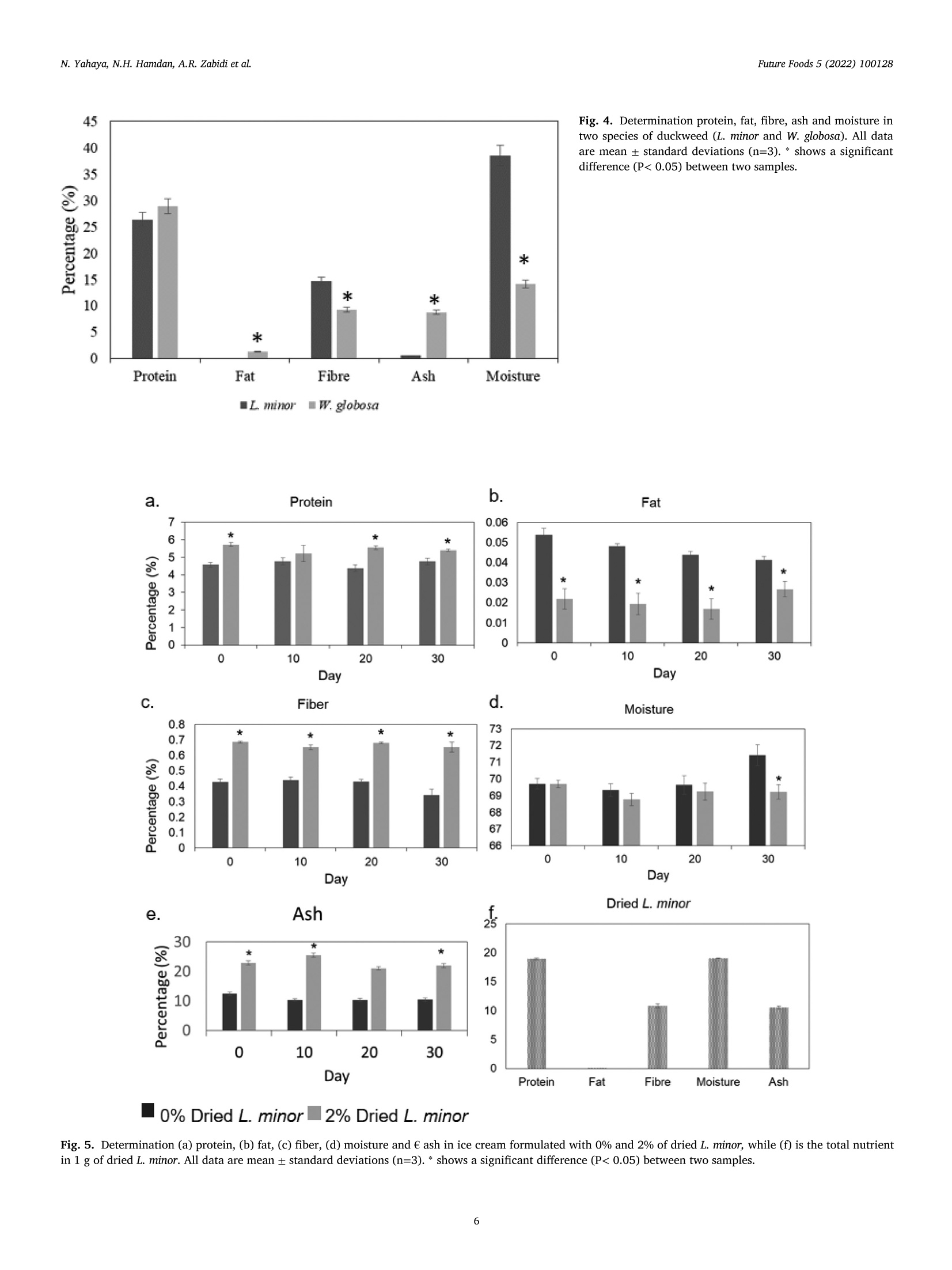
浮萍作为未来食物:代谢物、营养和微生物分析的证据Duckweed as a future food Evidence from metabolite profile, nutritional and microbial analysesFuture Foods 5 (2022) 100128 Future Foods 5 (2022) 100128N. Yahaya, N.H. Hamdan, A.R. Zabidi et al. Contents lists available at ScienceDirect Future Foods ELSEVIER journa l homepage: www.elsevier.com/locate/fu fo 浮萍作为未来食物:代谢物、营养和微生物分析的证据 Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses Nazariyah Yahaya*, Nabila Huda Hamdan, Atiqah Ruqayyah Zabidi, Ammar Mirza Mohamad,Mohammad Luqman Hakim Suhaimi , Muhammad Azhan Azfar Md Johari, Hanis Nadia Yahya,Hafiza Yahya Food Biotechnology Programme, Faculty of Science and Technology, Universiti Sains Islam Malaysia (USIM), 71800 Bandar Baru Nilai, Negeri Sembilan, Malaysia ARTICLE INFO ABSTRACT Keywords:Duckweed GC-MS -based metabolomics Lemna minor Wolffia globosa Duckweed species are nutritionally meaningless plant which grow wildly in unattended areas. Therefore, un-derstanding the metabol i tes content in duckweed species is essential for designing a future food products. Here,we report an untargeted Gas Chromatography-Mass Spectrometry (GC-MS)-based metabolomics approach for comprehensively discr i minating between Lemna minor and Wolffia globosa of duckweeds species using principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA). Ten differential metabol i tes levels were tentatively identified between L. minor and W. globosa. Relative to W. globosa, L. minor appeared to enrich with 5-Hydroxyl-L-tryptophan, Tocopheryl acetate, Naringenin, a-linolenic acid and glutamic acid. Fur-thermore, the nutritional and microbial analyses of ice cream formulated with dried L. minor were investigated.The nutritional analysis results show that relative to control , the i ce cream with 2% dried L. minor had signif-icantly increased protein, fiber and ash content. In addition, total plate count (TPC) for microbial analysis of duckweed ice cream was performed. The result suggested that the smal l amount of bacteria (3.82 cfu/g) was traced in formulated i ce cream with 2% dried L. minor. Overall, the metabolites profile, nutritional and microbial analyses of food used L. minor plant indicate that duckweed is a good candidate for future food. 1. Introduction Duckweed can be considered as a plant-based ingredient for future food products and a sustainable alternative source of protein that has the potential of replacing animal meat, healthier and more affordable protein (Ap penroth et al ., 2018). Duckweed i s an aquatic green plant,t i ny and known as floating freely plants that can commonly be found in slowly-moving bodies of water l ike lakes and ponds (Van Hoeck et al. 2015; Sree et al. 2016). I t is the smallest flowering plant glob.ally and consists of less than three leaves with a single root hanging in the water (Sree et al. 2016). However, leaves of duckweed can be mul-tiplied by vegetative method to result in a 50% increase of the yield per day. Duckweed nutrient content and metabol i te composi t ion have gained extensive attention, particularly in the animal feed industry, aquacul-ture, health supplement, biofertilizer, biofuel and emerging food prod-ucts for humans (Anthonius et al. 2018; de B eukelaar et al . 2019;Naseem et al. 2020). It has been reported that duckweed contains 20% to 30% of protein, which is higher than cereal (de Beukelaar et al. 2019). The main protein in duckweed i s ribulose-1,5-bisphosphate carboxylase (RuBisCO), which is a good source of essential amino acids.Moreover, RuBisCO protein is a good candidate as a functional food due to its nutritional value, in vitro digestibility and non-allergenic (Chakrabarti et al . 2018). In addition, RuBisCO protein has good gelling and emulsification properties, high foaming capacity, and high solu-bility (Di Stefano et al. 2018). Duckweed also contains 4% to 7% of fat, 4% to 10% of starch, carotenoids and polyphenols like flavonoids and anthocyanins (Ap penroth et al ., 2018). The fat content in duck-weed consists of polyunsaturated fatty acids, 60% to 63% of tota l fatty acids and 41% to 47% of a-linolenic acid (Chakrabart i et al. 2018). The starch of duckweed comprises 35.7% amylose and 26.5% amylopectin (Lee et al . 2016). Lemna minor, one of the duckweeds species in the family Lemnaceae,shows elliptica l form, regular species size with 1.7 mm diameter on av-erage, 2-4 fronds colonies and developed roots system, which was de-scribed extensively by Save r i and Fornasiero (1983). L. minor uses both roots and fronds for taking up nutr i ents (Cederg re en and Madsen, 2002),while Wolffia globosa, which is a rootless plant, uses the fronds surface to E-mail address: naza r iyah@usi m .edu.my (N. Yahaya). https://doi .org/10.1016/j.fufo.2022.100128 Received 22 December 2021; Received in revised form 3 February 2022; Accepted 7 February 2022 2666-8335/C 2022 The Author(s). Publ i shed by Elsevier B.V. This is an open access article under the CC BY-NC-ND l icense take up the nutrient from the growth medium. L. minor and Lemna gibba are commonly used for research (A pp enroth et al., 2018; Xie et al. 2019;Alkimin et al. 2019), whereas Wolffiella and Wolffia, which grow fast and has high starch content, are more useful in renewable biorefinery feedstock (An et al. 2018). Additionally, Wolffia has been recognized as a rich source of protein and is tradi t ionally employed as a natural food source in Southeast Asia. There are many subspecies in the Wolf-fia genus, and the most common one is known as Wolffia globosa. W.globosa is familiar as watermeal or Khai Nam in Thailand. Raw Wolffia is utilized to make certain meals such as salads, omelettes or vegetable curries. Duckweed fully depends on optimum temperature range between 17.5 and 30℃ and sunlight to grow. It also requires nitrogen,phosphorus and potassium as supplemental nutrients (Hasan and Chakrabart i , 2009). However, since duckweed is a phytoremediation plant that extracts metals, radionuclides and other pollutants from wastewater and accumulates those in their tissues, the safety of con-suming this aquatic plant has become a concern (Landesman et al. 2005;K a my ab et al. 2017; Radulov i c et al. 2019). In addition , a high amount of pathogens such as Escherichia coli, Clostridium botulinum, Salmonella spp., fungi and microscopic invertebrate could contaminate duckweed SPP.,collected from wastewater or open greenhouse pools (Moyo et al. 2003;Islam et al. 2004; Ansa et al . 2012; Markou et al . 2018). Therefore, to produce duckweed as a food source for humans, it must be grown in a sustainable environment such as axenic culture, which could produce an abundant amount of duckweed under control environment with low bacteria abundance and less contaminant. However, to mass-produce duckweed for food production, the laboratory set is extensively expen sive. Thus, an open-air system with direct sunlight and minimum nu-trient usage is required to commercialize duckweed as a future food to low-income communities. In this study, we initially i nvestigated metabolite contents in L. minor and W. globosa by using GC-MS. The data was integrated with principle component analysis (PCA) and partia l least squares-discriminant anal-ysis (PLS-DA). We also investigated protein, fat, f iber, ash and moisture content in both duckweed species and their minimum requirement for growth. As a part of the food product development, we focused on L.minor by investigating the nut r i t ional composition and microbial anal-ysis of duckweed ice cream, which were relevant for human consump-tion and safety. Hence, our study was to reveal duckweed as a potential future food in the aspects of metabol i te profile, nutritional values and microbial analysis in duckweed developed food product. 2. Materials and methods 2.1. Biological materials and growth condition Two species of duckweed, L. minor and W. globosa, were used in this experiment. Initia l material of L. minor and W. globosa were purchased from Megauppy Aquatic Supplies, Malaysia and Yuren Aquatic Sdn. Bhd.Store, Malaysia, respectively. Both duckweed species were maintained in 300 mL of water with 500g multipurpose compost and 300 mL Nitrogen: Phosphate: Potassium (N: P: K) solution by mixing 1.52 g N: P: K (12:12; 17, Baja Taiping)pellet into 1 litre of distilled water in the outside environment under shield with temperature around 29℃ day/12 hours and 24℃ at night/12hours. For large scale preparation of duckweed growth to develop food products, duckweed was grown in a 70 L container with surface area 55 cm X 88 cm, under shield with 1 kg of multipurpose compost and 5 L of N: P: K ferti l izer (per week) in similar environment mentioned above.Duckweed was harvested every two weeks. Metabolite extraction of L. minor and W. globosa was conducted ac-cording to the method described by Gonzalez-Pena et al. (2017) with modi fi cation. Two duckweed species were dr i ed in t he oven dryer (65℃for 24 hours) and ground into fine powder. In this experiment, 100 mg of dried duckweed powder was mixed with 1 mL of methanol for each replicate. The mixture was vortexed for 10 s, left on ice for 10 min and later centrifuged at 17000 g for 2 min at room temperature. The super-natant was collected in a 1.5 mL centrifuged tube and stored at 80℃ for GC-MS analysis. In GC-MS analysis, t hree replicates were performed for each duckweed species. Following metabolite extraction, samples were analyzed using Agi-lent JandW Gas Chromatography Column with Triple Quadrupole mass spectrometry operated at 70 eV. An aliquot of a 1.0 ul sample was in-jected into the DB-35MS (30 m length x0.250 mm diameter x 0.25 um f ilm thickness) column. Helium was used as a carrier gas, and the scan range was set to 40 to 500 Da. The initial oven temperature was set to 60℃ for 10 min and was increased by 40℃/min to 280℃, then held for 10 min and increased by 40℃/min unt il 310℃. Both injector and transfer temperatures were set to 250℃ while the source temperature was adjusted to 300℃. The full scan range was acquired after 30 min with a spl i t ratio of 10:1 (Carry et al. 2018). Each recorded spectrum was compared with the standard mass spectra l ibrary of the National Institute of Standards and Technology (NIST). 2.3. Metabolite profiling by multivariate analysis The raw data from GC-MS was converted to Microsoft Excel in .csv format and processed by the MetaboAnalyst 4.0 website (h ttps://www.metaboana ly st .ca ). A data integrity check and normal-ization by sum, log transformation and data scaling and mean centring were performed before being subjected to principal component analysis (PCA) and partia l least square-discriminant analysis (PLS-DA). Finally,predictive abil i ty (Q2) and goodness-of-fit (R2) parameters assessed the quality of the resultant PLS-DA models. 2.4. Nutritional analysis of raw materials and food product The nutritional analysis was determined by measuring the proximate content of both duckweed species according to the Association of Of f i-cial Analytical Chemists (AOAC, 1985) methods. Among the parameters measured were protein content, fat content, fiber content, ash content and moisture content. All analysis of proximate composition was per-formed with three samples for each type of analysis. Protein analysis was carried out using the Kjeldahl method, which i s based on the determination of nitrogen content . The procedure can be divided into three steps, digestion, dist i llation and titration. At the end of the process, the percentage of Nitrogen present in the sample was calculated using the following e quation 1. Where c: Concentration of the standard-acid solution: Hydrochloric acid 0.1N or c =0.1 mol/L; Alternative: sulfuric acid 0.1N or c=0.05mol/L, which N=Normality of standard acid. V: Consumption of the standard acid in ml (Sample) Vb: Consumption of the standard acid in ml (Blank Sample) Equivalent weight of NH is 17g=14 g Nitrogen. Factor 1.4 is cal-culated from the 14 g of Nitrogen divided by 1000 ml of 1 N acid and multiply with 100. The percentage of protein (% raw protein) in the sample need to calculate by mult i ply percentage of N which calculated from e quation 1 with 6.25 (%N*6.25). Conversion factor 6.25 is equiva-lent to 0.16 g Ni t rogen per gram of protein. Following protein analysis, fat content was determined using the au-tomated Soxtherm method. Initially, 2 g of dried sample was weighed on crude fi l ter paper and placed into extraction thimble in the beaker with 3-5 boi l ing stones. Next, 130 mL of petroleum ether was added i nto the beaker ahead of the extraction of the sample in automated Soxtherm.After the extraction, the beakers containing the exudate are dried in the drying oven for an hour at 105℃. Then, the extraction beakers were transferred to a desiccator to cool down. Finally, the extracted samples were weighed. The sample was l eft to dry for another 30 minutes before weighed again to determine the consistency of the weight. At the end of the process, the total fat content in the sample was calculated using the following e quation 2. Where mj: Mass of the empty extraction beaker with boi l ing stones in g m: Mass of the extraction beaker with fat after drying in g mg: Weight of the dried duckweed sample at the start of the analysis in g The crude fiber was extracted and analyzed using the Fiber Bag method. In this analysis, 1 g of sample was put into the Fiber Bag, and the mass was weighed with 1 mg preciseness. Afterwards, the glass spencer was inserted into Fiber Bag, and then the bag was added to the carousel.Next, the analysis was continued by de-fatting the sample by immersing the carousel three times in a row into 100 ml of 40/60 petroleum ether.Shortly after, the drained Fiber Bag was taken out and transferred into the crucible. Next, the crucible was placed in the drying oven overnight at 105℃. Finally, the crucible and residue left were transferred into a desiccator to let i t cool before ascertaining the final weight by using equation 3. Blank Value E=D-F A = Mass Fiber Bag in g B = Mass Sample weight in g (has to be adjusted according to dry content) C = Mass Crucible and dried FiberBag after digestion in g D = Mass Crucible and Ash in g E = Blank Value of the empty FiberBag in g F = Mass Crucible in g Ash content was measured by taking 3 g of sample and burned on the electric hot plate unti l the residue had ceased smoking. Later, the cru-cible was ignited at 550℃ overnight in the muf f le furnace. The sample was transferred into the desiccator before measuring the final weight and recorded using equation 4. To determine moisture content, 1 g of sample was placed on the pan surface in the moisture analyzer and let run for a few minutes before it shut off automatically, which signalled that the measurement was com-pleted. Then, the data was taken and recorded from the reading of the moisture analyzer. 2.5. Duckweed ice cream formulation and development The duckweed was dried using oven drying at 65℃ for 24 hours and ground using a dry blender. The powder then was sieved using a strainer to obtain a finer powder. Control ice cream and duckweed ice cream with specific formulations were prepared. For control, the formu-lation was 75% of full cream milk, 9% non-fat milk solids, 5% butter,10% Castor sugar, 1% ovalette and 0.4% vanilla essence. In duckweed ice cream, 2% of duckweed powder was added into the control formula tion. First, full cream milk was heated at 55-56℃ for 5 min. After that,butter and ovalette were melted together in the pot. Then, all ingredi-ents were combined together into a stainless-steel container and mixed using a hand mixer at a speed of 3 for 2 min. The mixture was stirred for 15 min under a freezing temperature turning into soft ice cream. All the ice creams were stored in a freezer at -20°C. The nutritional anal-ysis of the ice cream was conducted using the method mentioned in section 2.4. The protein content, fat content, fiber content, ash content and moisture content were analyzed every 10 days within 30 days with three replicates. 2.6. Microbial analysis on formulated ice cream For the microbial analysis of the formulated ice cream (without duck-weed and 2% dried duckweed), the total plate count (TPC) method was conducted by mixing the sample i nto peptone water in a ratio of 1:7using a stomacher bag. The diluted mixture with a factor 10-1 to 10-6was prepared by adding 1 ml of the mixture from the stomacher bag into 9 ml of peptone water in the f irst tube, vortex and labeled it as the solution with di l ution factor 10-1. Then, 1 ml of the mixture f rom dilu -tion factor 10-1 was t ransferred i nto the second tube (10-2 dilution fac-tor) and mixed homogeneously using a vortex. The steps were repeated until 10-6 dilution factor. After all of the samples were mixed homoge-neously using a vortex, 0.1 mL of sample culture from dilution factor 10-1 to 10-6 were dispensed onto the surface of Total Plate Count agar (TPC) and spread the sample evenly using the sterile spreader. The agar plates were inverted and incubated at 35℃ for 24 hours. The number of the bacteria growth on the plate was calculated using e quation 5. The calculated number of colonies was normalized by logio transformation. 3. Results 3.1. Overview of metabolites content i n L. minor and W. globosa Two genera of duckweeds (Lemna minor and Wolffia globosa) were investigated concerning the content of their metabolites by using GC-MS. GC-MS prof il ing of the L. minor and W. globosa revealed a total of 67 and 74 identified peaks, respectively. Nine chemical compounds in L. minor and 13 chemical compounds in W. globosa with a peak area of more than 1% are presented in T able 1. The major compounds present in L. minor and W. globosa with the highest percentage of peak area included y-Sitosterol, Stigmasterol and Campesterol. 3.2. Metabolites differences between L. minor and W. globosa The morphological level difference between L. minor (Fig.1a) and W.globosa (F ig . 1b) could reflect the metabolites profile in both duckweed species. The morphology of two duckweed genus was recorded using microscopic analysis. Microscopic image of L. minor showed suborbicu-lar to elliptic-obovate in the outline of frond which nearly symmetrical,whereas W. globosa microscopic image showed oval i n outline (Fig. 1a).Compared to L. minor, W. globosa i s a tiny f loat i ng rootless plant whose size is less than 1 mm in any dimension. Next, the variation within the metabolic profiles corresponds to two duckweed genus using principa l component analysis (PCA) (Fig.1c) and partial least squares-discriminant analysis (PLS-DA) (F i g . 1d). T he anal-ysis was performed on the metabolites dataset of L. minor and W. globosa from methanolic extracts of their fronds. The outcomes of PCA clearly discriminate the L. minor and W. globosa plant s which indicate that these two genera have different component profiles (Fig. 1c). The profile of L. minor clustered in a PC1-negative direction, while W. globosa clus-tered in a PC1-positive direction. PCA analysis showed both PC1 and PC2 accounted for 77.4% of the total accumulative variance. Then the metabolites data set was subjected to PLS-DA. PLS-DA is a supervised method, which used to build models that discriminate be-tween data. F ig . 1d shows PLS-DA models (Component 2 plotted against Component 1) on the recorded data of GC-MS analysis. In PLS-DA, Com-ponent 1 discriminates L. minor and W. globosa; the profile of the former Table 1 Metabolites content in L . minor and W. globosa detected by using GC-MS. Name of the Compound Retention Time (RT) Mass (DB) Peak Area (%) L. minor Neophytadiene 15.904 278.3 1.453 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- 18.509 292.2 1.509 Phytol 18.621 296.3 6.800 Squalene 24.297 410.0 2.674 α-Tocopheryl acetate 26.694 472.4 6.372 Campesterol 27.782 400.4 9.137 Stigmasterol 28.149 412.4 25.260 y-Sitosterol 28.775 414.4 10.049 W. globosa Neophytadiene 15.904 278.3 2.418 Hexadecanoic acid 17.309 256 1.215 Linolenate 18.506 292 1.362 Phytol 18.618 296.3 3.881 9,12-Octadecadienoic acid (Z,Z)- 19.043 280.2 1.280 Squalene 24.300 410.0 6.584 α-Tocopheryl acetate 26.688 472.4 5.006 Campesterol 27.22 400.4 11.059 Stigmasterol 28.116 412.4 15.505 y-Sitosterol 28.809 414.4 22.535 B-Amyrin 29.283 426.4 3.436 Epiligulyl oxide 29.847 230 6.934 Fig. 1. Morphology of (a) L. minor and (b)W. globasa under microscope observation and score plot of between selected principle compo-nents obtained from GC-MS data set on (c) PCA and (d) PLS-DA from three biological replicates of duckweed plants for each species. L.minor 0.5m m W. globosa 0.5m m C. d. Fig. 2. I mportant features with Variable Important in Projec-tion (VIP) scores between 1 and 3 were obtained from applying the GC-MS data set on PLS-DA. The colored boxes on the right indicate the relative i ntensity of the corresponding metabolites between L. minor (box at the left) and W. globosa (box at the right ). V IP scores Fig. 3. The growth rate of duckweed species L. minor in M3Compost, Distilled water and N: P: K fertilizer. Days -M3 Compost Distilled Water 一 N PK plant clustered in a negative direction, while the latter plant clustered in a positive direction (F ig. 1c). Furthermore, following the PLS-DA, metabolites pattern for each metabol i te with variable importance in projection (VIP) scores between 1 and 3 for L. minor and W. globosa were shown in Fi g. 2. Ten di f fer-ential metabolites levels were tentatively ident i fied between L. minor and W. globosa. Relative to W. globosa, L. minor appeared to enrich with 5-Hydroxyl-L-tryptophan,a-Tocopheryl acetate, Naringenin, a-l i nolenic acid and glutamic acid (Fig. 2). 3.3. Nutritional component in L. minor and W. globosa Initially, the observation of L. minor growth in multipurpose com-post, N: P: K fertilizer and distilled water were conducted by counting the frond number. Early observation showed t hat N: P: K medium was the best prol i ferative media that had produced a high amount of duck-weed. However, multipurpose compost medium proliferated a higher amount of duckweed in the long-term, while distilled water seemed to achieve the equi l ibrium phase (F ig. 3) nearly. In this study, by combin -ing multipurpose compost and N: P: K medium in the container with surface area 55 cm X 88 cm, under the shield, estimate around 17.25g of wet weight, which is equivalent to 12.5 g of dry weight and 3 g of protein from L. minor was obtained wi t hin two weeks (F ig.4). To follow the metabolites content in L. minor and W. globosa, the nu-tritional quality was included in this investigation. The protein content of the two duckweed species was between approximately 26.38% and 29.91%. Fat content was undetectable in L. minor, and approximately 1.25% was detected in W. globosa. Relative to L. minor, fiber and mois-ture contents in W. globasa is low, which make it digested easily when it is i ntegrated i nto animal feed. In contrast, ash content i s significantly higher in W. globasa when compared with L. minor (Fig. 4). 3.4. Nutritional components and microbial analysis in L. minor and its food product We used 2% of dried L. minor as an addi t ional ingredient in ice cream formulation to further our study. Nutritional analysis of formulated ice cream with dried L. minor was compared with ice cream without L. minor within 30 days. The result of the nutritional analysi s shows that relative to the ice cream without dried L. minor (0%), the ice with 2% dried L.minor had a significant increase of protein, fiber and ash content, and fat content was signi f icantly reduced throughout the entire experiment (Fig. 5). It indicates that ice cream containing 2% dried L. minor has a 45 40 Fig. 4. Determination protein, fat , fibre, ash and moisture i n two species of duckweed (L. minor and W. globosa). Al l data are mean ± standard deviations (n=3). * shows a significant difference (P< 0.05) between two samples. 35 30 10 5 0 0% Dried L. minor2% Dried L. minor Fig. 5. Determination (a) protein, (b) fat, (c) fiber, (d) moisture and e ash in ice cream formulated with 0% and 2% of dried L. minor, while (f) is the total nutrient in 1 g of dr i ed L. minor. Al l data are mean ± standard deviations (n=3). * shows a significant difference (P< 0.05) between two samples. Total plate count (TPC) (cfu/g) in ice cream formulated without dried duckweed (Control) and ice cream formulated with 2% of dried duckweed. The data shows mean of colonies was normalized by logio transformation. Time Point Week 1 Week 2 Week 3 Week 4 Samples Mean Logio (cfu/g) Ice cream (0% dried duckweed) <0 <0 <0 <0 Ice cream (2% dried duckweed) 3.82 3.70 3.82 3.82 Note: Descriptive statistics of TPC-Mean Log1o·<0 shows no colonies was detected at cfu g-as <1 t imes at dilution factor 10-5. The TPC was conducted every seven days within four weeks with a dilution factor of 10-5, and the total volume of t he plate was 10 mL. high protein content with essential amino acids and low fat, which are suitable for human nutritional needs. The total plate count (TPC) is a t radi t ional method used to assess the quality and l evel of hygiene of formulated ice cream with the presence of dried L. minor. A high level of bacteria indicates a generally poor condition in the growing and cleaning of duckweed. In this study, the smal l amount of bacteria was traced in formulated ice cream with 2%of dried L. minor (Table 2). 4. Discussion In this study, the detected metabolites are mostly related to the secondary metabolism pathway and created by the enzymatic process in plant systems. For instance, y-sitosterol, stigmasterol and campes-terol, which were detected in GC-MS in both duckweeds species, are types of phytosterol that naturally occurred in plant leaves. y-Sitosterol is believed to have antidiabetic properties (Balamurug an et al. 2011;Tripathi et al . 2013), whereas stigmasterol acts as a precursor in the synthesis of progesterone and is also known as an intermediate prod-uct in biosynthesis of vitamin D3, estrogen, androgen and corticoids (Chaudhary et al.2011; Gabay et al. 2010). Estimate around 200-400 mg of sitosterol and campesterol have been consumed in the daily western diet, and the intestine absorption of campesterol is higher than sitosterol (Behrman and Gopalan , 2005). However, the differences in metabo-lite profile between L. minor and W. globosa are not wel l understood.I t has only been reported that dif f erent duckweed species are involved in the application of wastewater treatment systems and toxicology tests (Zhao et al., 2014; Alkimin et al. 2019). In terms of duckweed growth conditions, it has been reported that with a small trace of N and P and high temperature, duckweed can grow rapidly. However, the plant will die if the water temperature rises to 35℃ (Hasan and Chakrabarti, 2009). In addition, recent research showed that N: P: K fertilizer enriched with molasses and trace elements was the most promising medium of stimulating duckweed growth and,in the meantime, suppressing microalgae growth (Saty a et al . 2020).On the contrary, when L. minor is grown in a high concentration of ammo-nium (NH4+), i t shows toxicity symptoms such as growth inhibition, cell death induction and the accumulation of reactive oxygen species (ROS)(W ang et al. 2016). In general, the plant requires N to create amino acids for growth and development , while P is important in energy stor-age and t ransfer , and K plays a role as an enzyme activator to promote metabolism (Uchida,2000). N can be obtained either in organics such as from animal manures like f ish and poultry or inorganic form. Inte-gration duckweed growth with fi shes in one pond or used wastewater from the agriculture industry could reduce the usage of the inorganic form of N (Landesman et al. 2005; Yao et al. 2017; Kamy a b et al. 2017;H ossain and Alam, 2020). For the rapid growth of duckweed, a small quantity of P is essential after N, and the sensitivity of duckweed to P or K is decreased after it reaches the adequate threshold. The small trace of minerals such as K, Calcium (Ca), Magnesium (Mg), Sodium (Na), Chlo-rine (Cl), Sulfate (S) and Bicarbonate (HCO) in water are essential for suppor t ing the survival of duckweed (Hasan and Chakrabar t i , 2009). In general, duckweed contains about 20-35% protein of its dry weight (Appenroth et a l ., 2018; Herawati et al . 2020) which aligned with our findings. Leucine, isoleucine and valine const i tuted of major-ity essential amino acids in duckweed species which are required in an-i mal feeds (Chakrabarti et al. 2018). This evidence also concluded that the potentiality of using duckweed as animal feeds was obvious due to the r i ch crude protein content and rapid accumulation of biomass of this genus in comparison to other terrestrial plants. It has been reported that duckweed contains approximately 1-5% fat of its dry weight and dom-inantly by polyunsaturated fats (PUFA), which a-l i nolenic acid mainly and followed by linoleic acid (Chakrabarti et al . 2018). Despite the fact that fat composition in duckweed is mainly low, the value of fatty acid profi l e in L. minor and W. globosa should be sufficient to meet the human diet ’s demand (Appenroth et al., 2018). In this study, squalene and a-Tocopheryl acetate (Table 1), which have antioxidant activity, were detected in L. minor and W. globosa metabol i tes profiles. However, the squalene compound is three times higher in W. globosa than L. minor. Squalene is a compound that has an antioxidant activity to protect the cel l from oxidative damage and inhibit tumor growth in human t issue. The human body could also be secreted 0.3-0.5 mg/g of squalene by the sebaceous glands for skin pro-tection (Lozano-Grande et al. 2018). It has been reported that fresh vir-gin olive oi l, which is a par t of the human diet, contains a high amount of squalene and are more stable after the first frying process when com-pared with other plant oil such as sunflower oil, cottonseed oil, corn oil,etc. (Kal o geropoulos and Andrikopoulos, 2004; T simidou et al ., 2010). Metabolite identified as a-Tocopheryl acetate, which was relatively higher i n L. minor than W. globosa, is known as a primary form of vitamin E, which is naturally present in the membrane of green photosynthetic leaves and is commonly present in the membrane used as a food addi-tive. In plant, a-Tocopheryl acetate, synthesized in the chloroplast, plays a role in stress tolerance and photo-protective and induces plant resis-tance against abiotic stresses such as metal toxicity (Sadiq et al. 2019).A previous study showed that o-Tocopheryl acetate was increased after duckweed was exposed to zinc and cadmium (Artetxe et al . 2002). In human, α-Tocopheryl acts as an antioxidant against free radicals and en-hance human immune function (Pekmezc i and Litwack, 2011). The pres-ence of chemicals properties that have antioxidant activity in duckweeds plants shows this plant can be beneficial to human health. Therefore, it can be a part of food supplement formulations and as a source of bioac-tive compounds. However, the food-friendly process of the duckweed plant needs to be established to produce high nutritional food with free food-borne microorganisms. According to microbiological standards in Food Act 1983 (Regulation 39), TPC in ice cream must be below 5×104cfu/g . I t shows that microbial analysis of ice cream formulated with 2%dried duckweed follows the Malaysian regulation of food-safe standards. In recent years, the published survey on duckweed acceptability as human food among consumers has allowed a better knowledge of human preferences on particular f i t t i ng meals t hat could be applied with duck-weed plants. The survey showed most participants prefer to eat duck-weed as a salad, and fewer participants accept duckweed plants added in their cookies, cream sauce , burrito or even chicken breast (de Beuke- la a r et al . 2019). However, the published study was conducted among western consumers only. More study on the human preference of duck-weed integrated food products should be conducted among a wide range of human ages, including children who prefer the taste and look rather than nutritional value. 5. Conclusion Overall, the presented outcomes of the study appear to suggest that L. minor and W. globosa contain nutritious metabol i tes that could en-hance the qual i ty of future food. However, the high fibre content i n L.minor relative to W. globosa suggests that L. minor was more suitable for human food, which is good for the human digestion system. However,further research should be performed in more depth to i nvestigate the capacity of duckweed as a sustainable and cheapest resource of health supplement with high antioxidant content. Conflict of interest Disclose any potential conflict of interest appropriately. The authors declare no conflict of interest. Acknowledgments Author contribution to this paper: Nazariyah Yahaya: Writing-original draf t preparation, edit i ng, supervision and project administra-tion, Nabila Huda Hamdan Atiqah Ruqayyah Zabidi, Ammar Mirza Mo-hamad, Mohammad Luqman Hakim Suhaimi, Muhammad Azhan Az-far Md Johari: Investigation, Hanis Nadia Yahya and Hafiza Yahya:Reviewing and editing. This research is supported by USIM Innova-tion Development Grant (grant code: PPP/GPI/FST/051014/60419)and a part of this research is supported by Ministry of Higher Edu-cation, Malaysia under Research Excellence Consortium (grant code:USIM/KKP-S03/IFFAH/FST/LUAR-K/44220). Supplementary materials Supplementary material associated with this article can be found,in the online version, at doi:10.1016/j.fufo.2022.100128. References Alkimin, G.D., Daniel, D., Frankenbach, S., SerA dio, J., Soares, A.M.V.M., Barata, C.,Nunes, B, 2019. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sc. Tota l Environ. 657, 926-937. doi:10.1016/j .scitote n v.2018.12.002. An, D., Li, C., Zhou, Y., Wu, Y., Wang, W., 2018. Genomes and transcriptomes of duck-weeds. Front . Chem. 6(230). doi:10.3389/fchem.2018.00230. Ansa , E.D.O., Lubberding, H.J., Ampofo, J., Amegbe, G.B., Gi j zen, H., 2012. Attach-ment of faecal col i form and macro-i nvertebrate activ i ty in the removal of faecal coliform in domestic wastewater treatment pond systems. Eco. Eng. 42, 35-41.doi:10.1016/j .ecol e ng .2012.01.018. Anthonius, C., Yong, A.S.K., Fui , C.F, 2018. Supplementation of duckweed diet and ci t ric acid on growth performance, f eed ut i l i zation, diges t ibil i ty and phosphorus ut i lization of TGGG hybrid grouper (Epinephelus fuscoguttatus x Epinephelus lanceolatus) juvenile.Songklanakar i n J . Sci. Technol. 40 (5), 1009-1016. doi:10.14456/sj st -psu.2018.123. Appenroth, K.-J., Sree, K.S., Bog, M., Ecker, J., Seeliger, C., Bohm, V., Lorkowski, S.,Jahreis, G., 2018. Nut r i ti onal value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front . Chem. 6 (483). doi:10.3389/fchem.2018.00483. Artetxe, U , Garcia-Plazaola, J.I, Hern ande, z A., Becerri l, J.M, 2002. Low l i ght -grown duckweed plants are more protected against the toxicity induced by Zn and Cd. Plant Physiol. Biochem 40 (10), 859-863. doi:10.1016/S0981-9428(02)01446-8. Balamurugan, R., Duraipandiyan, V., Ignacimuthu, S., 2011. Antidiabetic activity of y-sitosterol isolated from Lippia nodiflora L. in streptozotocin-induced diabetic rats. Eur.J. Pharmacol. 667 (1-3), 410-418. doi:10.1016/j.ejphar .2011.05.025. Behrman, E., Gopalan, V., 2005. Cholesterol and plants. J . Chem. Educ. 82 (12),1791.doi:10.1021/ed082p1791. Carry, E.,Zhao, D., Mogno, I ., Faith , J., Ho, L., Vi l lani, T., Wu, Q., 2018. Targeted analysis of microbial -generated phenolic acid metabolites derived f rom grape f l avanols by gas chromatography-triple quadrupole mass spectrometry. J. Pharm. Biomed . 159, 374-383. doi:10.1016/j.jpb a.2018.06.034. Cedergreen, N., Madsen , V.T, 2002. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol 155,285-292. doi:10.1046/j.1469-8137.2002.00463.x. Chakrabarti, R., Clark, W.D., Sharma , J.G., Goswami, R.K., Shrivastav, A.K., Tocher, D.R,2018. Mass production of Lemna minor and its amino acid and fatty acid prof i les.Front . Chem. 6 (479). doi:10.3389/fchem.2018.00479. Chaudhary, J., Jain,A., Kaur.Kishore, N., L.,2011.S Stigmasterol: A comprehensivee review.Int.J.Pharm. Sci . Res. 2 (9), 2259-2265.doi:10.13040/I J PSR.0975-8232.2(9).2259-65. de Beukelaar, M.F.A., Zeinstra, G.G., Mes, J .J., Fischer, A.R.H, 2019. Duckweed as human food. The influence of meal context and information on duckweed acceptability of Dutch consumers. Food Qual. Prefer. 71, 76-86. doi:10.1016/j .food q ual .2018.06.005. Di Stefano, E., Agyei , D., Njoku, E.N ., Udenigwe, C.C, 2018. Pl a nt RuBisCO: An under-utilized protein for food applications. J. Am. Oil Chem. Soc. 95 (8), 1063-1074.doi:10.1002/a oc s .12104. Gabay, O., Sanchez, C., Salvat, C., Chevy, F ., Breton, M., Nourissat, G., Berenbaum, F.,2010. Stigmaste r ol : A phytos t erol with potential anti -osteoarthr i tic properties. Os -teoarthr. Carti l . 18 (1), 106-116. doi:10.1016/j.joca.2009.08.019. Gonzalez-Pena , D., Dudzik, D., Garcia, A., de Ancos, B., Barbas, C., Sanchez-Moreno, C.,2017. Metabolomic fingerprinting in the comprehensive study of liver changes asso -ciated with onion supplementation i n Hypercholesterolemic Wistar r ats . Int . J. Mol.Sci. 18 (2), 267. doi:10.3390/i j ms18020267. Hasan, M.R., Chakrabar t i , R ., 2009. Floating a q uatic mac r ophytes-Duckwe e ds. In: Use of a lga e and aqua t ic macrophytes as feed -i n smal l-sca l e aquaculture -A review. FAO Fis h .T e ch. Pa p . 531,29-51. Herawat i , V.E., Pinandoyo, Darmanto, Y.S., Rismaningsih, N., Windarto, S.,Radjasa, O.K,2020. The effect of fermented duckweed (Lemna minor ) i n feed on growth and nu -tritional quality of t ilapia (Oreochromis niloticus ). Biodiversitas 21 (7), 3350-3358.doi:10.13057/biodiv /d210759. Hoss a in, M.S., A lam, M.J ., 2020. Integration of fish cul t ure in hydroponic agriculture in f l ood-prone areas. Sain s Malays 49 (8), 1799-1808. Islam, M.S., Kabir , M.S., Khan, S.I ., Ekramullah, M., Nair, G.B., Sack, R.B., Sack, D.A.,2004. Wastewater-grown duckweed may be safely used as fish feed. Can . J. Microbiol.50 (1),51-56. doi:10.1139/w03-102. Kalogeropoulos, N., Andr i kopoulos, N.K., 2004. Squalene in oils and fats from domes-tic and commercial fryings of potatoes. Int. J. Food Sc i . Nutr. 55 (2), 125-129.doi:10.1080/09637480410001666531. Kamyab, H., Chelliapan, S., Din, M.F.M., Shahbazian-Yassar, R., Rezania, S., Khademi , T.,Az i mi , M., 2017. Evaluation of Lemna minor and Chlamydomonas to treat palm oil mill effluent and fertilizer production. J. Water Process. Eng. 17, 229-236.doi:10.1016/j .jw pe.2017.04.007. Landesman, L ., Parker , N.C ., F e dler , C., Konik o ff , M., 2005. Mode l ing duckwe e d growth in w a stewate r t r eatment systems . Livest . Res. Rura l . Dev. 17 (6). Lee, C.J ., Yangcheng, H., Cheng, J.J., Jane, J .L, 2016. Starch characterization and ethano l production of duckweed and corn kernel . Starch/Staerke 68 (3-4),348-354.doi:10.1002/st a r .201500126. Lozano-Grande, M.A., Gorinstein, S., Espit i a-Rangel, E., Davila-Ortiz, G., Martinez-Ayala, A.L., 2018. Plant sources, extraction methods, and uses of sq u alene. Int. J.Agron., 1829160 doi:10.1155/2018/1829160. Markou, G., Wang, L., Ye, J., Unc , A., 2018. Using agro-industria l wastes for the cultiva-tion of microalgae and duckweeds: Contamination risks and biomass safety concerns.Biotechnol . Adv . 36 (4), 1238-1254. doi:10.1016/j .biotechadv .2018.04.003. Moyo, S ., Dalu, J.M., Ndamba, J., 2003. The microbiological safety of duckweed fed chick-ens: a r i sk assessment of using duckweed reared on domestic wastewater as a pro-tein source in broiler chickens. Phys. Chem. Earth, Parts A/B/C 28 (20),1125-1129.doi:10.1016/j .pce.2003.08.021. Naseem, S., Bhat , S.U., Gani , A., Bhat, F.A, 2020. Perspectives on utilization of macro-phytes as a feed ingredient for f ish in future aquaculture. Rev. Aquac . 13 (1),282-300.doi:10.1111/raq .12475. Pekmezci, D., Lit wac k, G., 2011. Chapter eight -Vitam i n E and immunity. Vitamins and Hormones 86, 179-215 Ac a demic Press. Radulovic, O., Pet ri c, M., Raspor, M., Stan o jevic , O., J anaki e v, T ., Tadic, V.,Stankovic, S .A, 2019. Culture -depen d ent analysis of 16S rRNA sequences associated with the rhizosphere of Lemna minor and assessment o f bacteri al phen o l-resistance:Pl a n t /bacter i a sy s tem for potential bioremediation. Pol . J . Environ. Stud. 28 (2),811-822. Sadiq, M., Akram, N.A., Ashraf, M., Al-Qurainy, F ., Ahmad, P., 2019. Alpha-tocopherol-induced regulation of growth and metabolism i n plants under non-stress and stress conditions. J. Plant Growth Regul . 38 (4), 1325-1340.doi:10.1007/s00344-019-09936-7. Satya , A., Satya, I .A., Chrismadha, T., 2020. Nitrogen uptake compet i tion between minute duckweed (Lemna perpusillaTorr) and microalgae under various nutrient compositions.IOP Conf. Ser . Earth Environ. Sci. 535 (1). doi:10.1088/1755-1315/535/1/012027. Saveri, A., Fornasiero, B.R, 1983. Morphological variations in Lemna minor L.and possible relationships with abscisic acid.Caryologia a3366(1),57-64.doi:10.1080/00087114.1983.10797644. Sree, K.S., Bog, M., Appenroth, K.J ., 2016.T Taxonomy ofduckweeds (Lem-naceae), potential new crop plants. Emir. J. Food Agric. 28 (5), 291-302.doi:10.9755/ejfa.2016-01-038. Tripathi , N., Kumar,S., Singh, R ., Singh, P., Varshney, W.K, 2013. Isolation and i denti f ica10n 2tion of y -sitosterol by GC-MS from the l eaves of Girardinia heterophylla (Decne). The Open Bioactive Compounds Journal 4, 25-27. doi:10.2174/1874847301004010025. Tsimidou, M.Z., Preedy , V.R., Wat s on, R.R, 2010. Chapter 61-Squalene and t o copherols in oliv e oil: i mportance and method s of analysis. In: Oliv e s a nd Olive Oi l i n Hea l th a nd Disease Prevention. Ac a demic Press , San Diego, pp. 561-567. Uchida , R., 2000. Essential nutrients fo r pl a nt growth: nut r ient functions a nd deficienc y s ymptoms. Pl a nt Nutrient Management in Hawai i 's Soi l s , Approaches for Tropical and Subtropical Ag r i culture : Colleg e of Tropical Ag ri cul t ure and Human Resources.the University of Hawaii a t Manoa. Van Hoeck, A., Horemans, N., Monsieurs, P., Cao, H.X., Vandenhove, H., Blust, R., 2015.The first draf t genome of the aquatic model pl a nt Lemna minor opens the route for future stress physiology research and biotechnological applications. Biotechnol. Bio-fuels 8 (1),188. doi:10.1186/s13068-015-0381-1. Wang, W., Li, R., Zhu, Q., Tang, X., Zhao, Q., 2016. Transcriptomic and physiological analysis of common duckweed Lemna minor responses to NH(*) toxicity. BMC Plant Biol 16. doi:10.1186/s12870-016-0774-8,92-92. Xie, L., Solhaug, K.A.r., Song, Y., Brede, D.A., Lind, O.C., Salbu, B., Tollefsen, K.E, 2019.Modes of action and adverse effects of gamma radiation in an aquatic macrophyte Lemna minor. Sci . Tota l Environ. 680, 23-34. doi:10.1016/j .scitotenv .2019.05.016. Yao, Y., Zhang, M., Tian, Y., Zhao, M., Zhang, B., Zhao, M., Yin, B., 2017. Duckweed (Spirodela polyrhiza) as green manure for i ncreasing yield and reducing nitrogen loss in rice production. Field Crops Res 214, 273-282. doi:10.1016/j.fcr.2017.09.021. Zhao, Y., Fang, Y., J in, Y., Huang, J ., Bao, S., Fu, T., Zhao, H., 2014. Poten-tial of duckweed in the conversion of w CO astewater nutrients to valuable biomass:A pilot-scale comparison with water hyacinth. Bioresour. Technol . 163,82-91.doi:10.1016/j.bior t ech.2014.04.018.
确定






还剩7页未读,是否继续阅读?
中国格哈特为您提供《两种浮萍中蛋白质、脂肪和纤维含量的检测》,该方案主要用于水产品中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《两种浮萍中蛋白质、脂肪和纤维含量的检测》用到的仪器有格哈特全自动快速索氏提取SOXTHERM、格哈特凯氏消化系统KT8S、格哈特维克松废气实验室废物处理系统涤气VS、格哈特带自动进样器凯氏定氮仪VAP500C、格哈特全自动型纤维分析仪FT12、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多