上市前三周的热应激对商业肉鸡、本地鸡和杂交鸡中脂肪浸润和炎症相关基因的组织学和基因表达的影响Influences of Thermal Stress During Three Weeks Before Market Age on Histology and Expression of Genes Associated With Adipose Infiltration and Inflammation in Commercial Broilers, Native Chickens, and Crossbreeds
方案详情

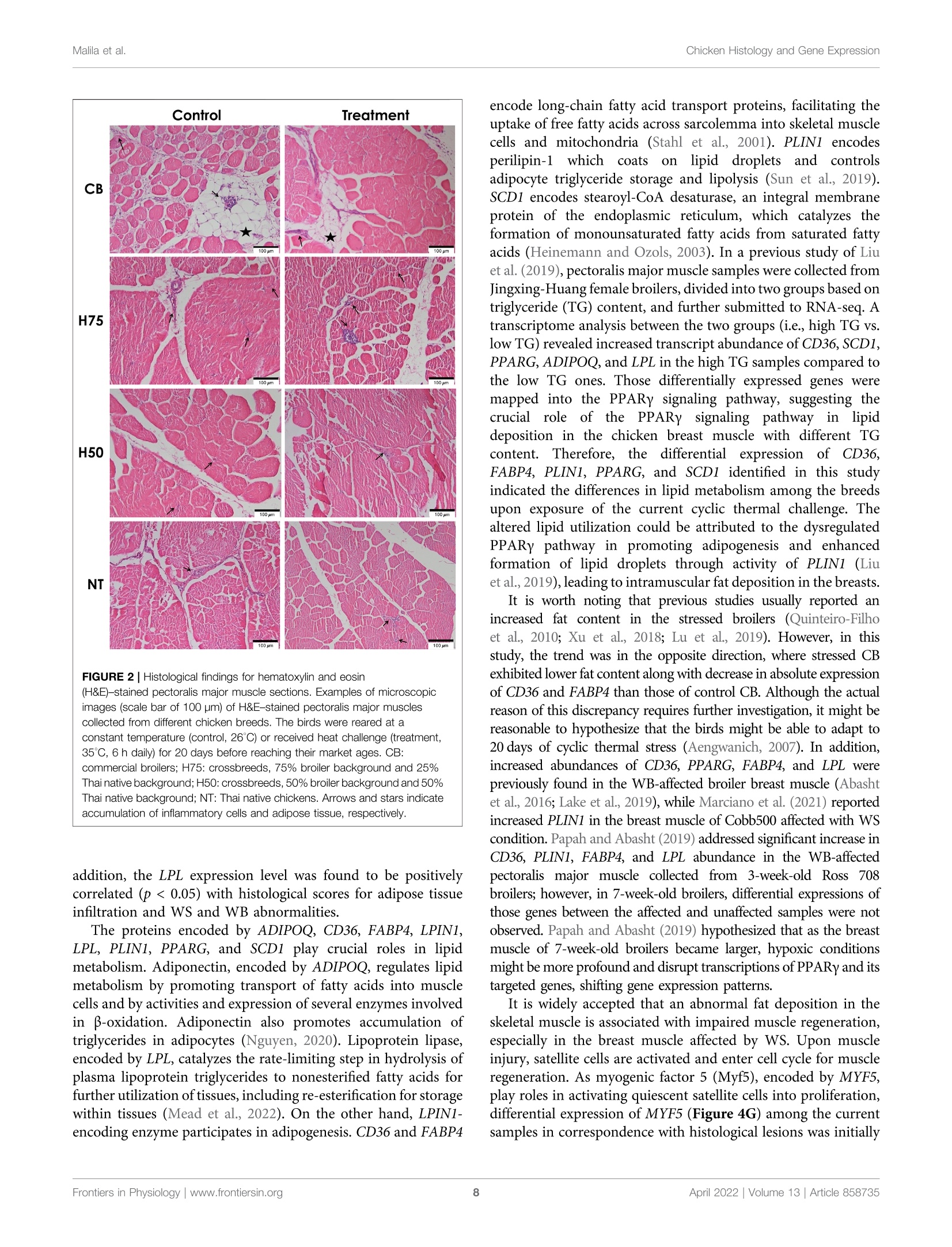
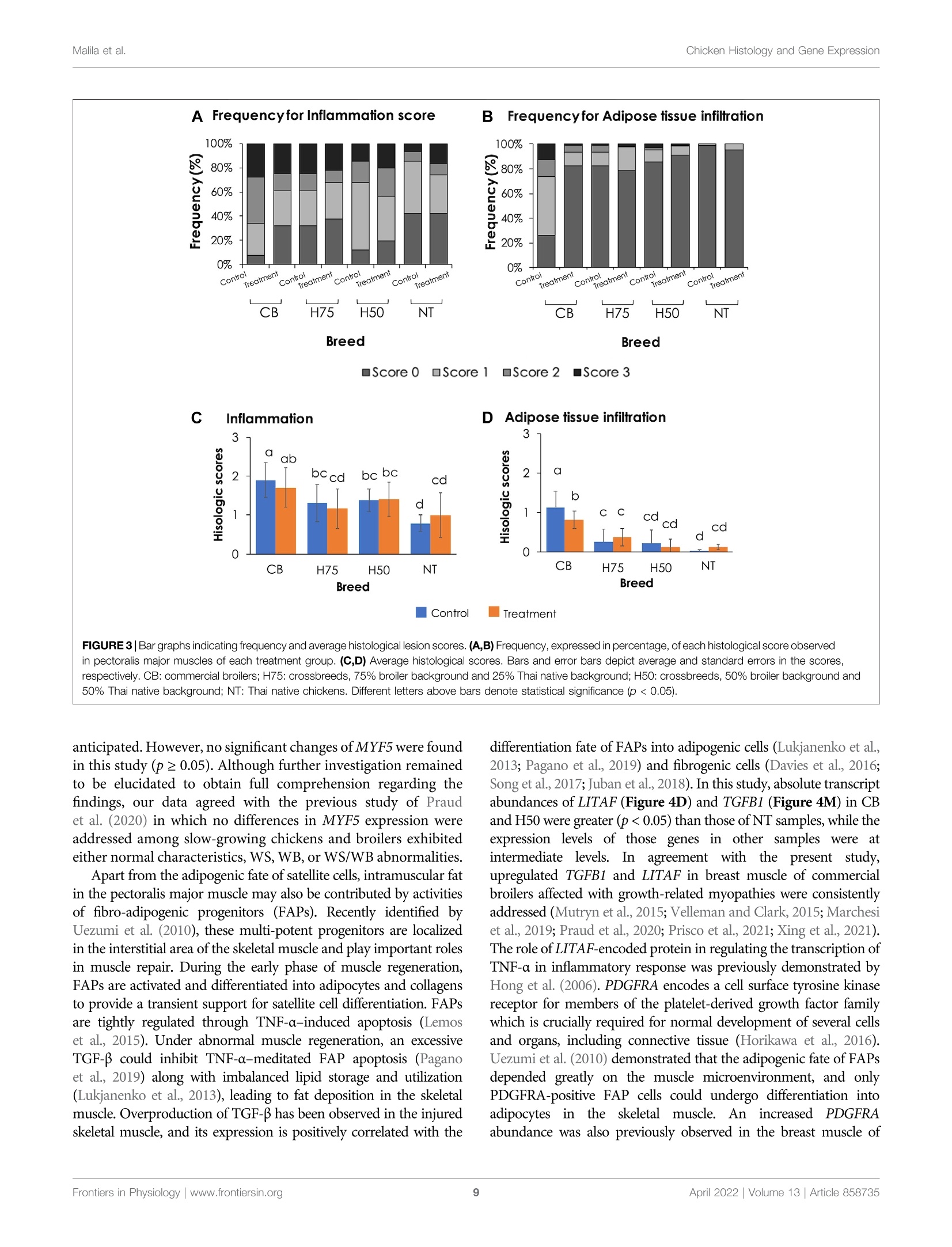
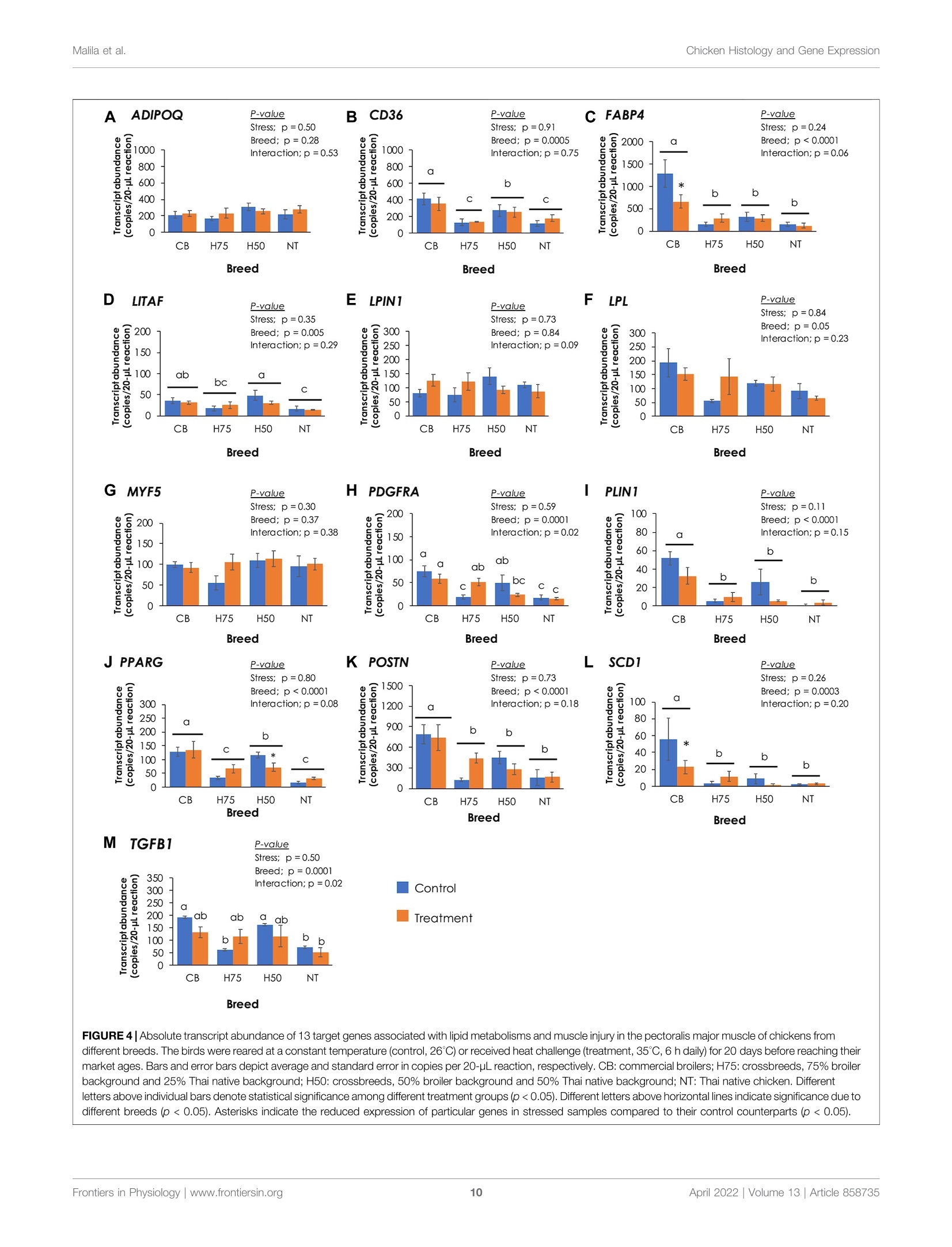
上市前三周的热应激对商业肉鸡、本地鸡和杂交鸡中脂肪浸润和炎症相关基因的组织学和基因表达的影响Influences of Thermal Stress During Three Weeks Before Market Age on Histology and Expression of Genes Associated With Adipose Infiltration and Inflammation in Commercial Broilers, Native Chickens, and CrossbreedsORIGINAL RESEARCHFrontiers in Physiologypublished: 12 April 2022doi: 10.3389/fphys.2022.858735 Chicken Histology and Gene ExpressionMalila et al. O PEN ACCESS Edited by: Vincent M. Cassone,University of Kentucky, United States Reviewed by:Mauri z io Ma z zoni,University of Bologna, Italy E l i z abeth Ruth Gilber t ,Virginia Tech, United States *Correspondence:Yuwares Malila yuwares.ma/@biotec.or.th Specialty section: This article was submitted to Avian Physi o logy,a section of the j ournal Fronti e rs in Physiology Received: 20 January 2022 Accepted: 15 March 2022 Published: 12 April 2022 Citation: Malila Y, Sanpinit P, Thongda W,Jandamook A, Srimarut Y, Phasuk Y a nd Kunhareang S (2022) Influences of Thermal Stress During Three Weeks Before Market Age on Histology and Expression of Genes Associated With Adipose I nfiltration and Inflammation in Commercial Broilers. Native Chickens,and Crossbreeds Front. Physiol. 13:858735. doi: 10.3389/fphys.2022.858735 上市前三周的热应激对商业肉鸡、本地土鸡和杂交鸡中脂肪浸润和炎症相关基因的组织学和基因表达的影响 Influences of Thermal Stress During Three Weeks Before Market Age on Histology and Expression of Genes Associated With Adipose Infiltration and Inflammation in Commercial Broilers, Native Chickens, and Crossbreeds Yuwares Malila’*, Pornnicha Sanpinit ', Wilawan Thongda12, Anuwat Jandamook,Yanee Srimarut ’, Yupin Phasuk’ and Sajee Kunhareang3 1National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand Science Park, Pathum Thani, Thailand,center of Excellence for Shrimp Molecular Biology and Biotechnology (CENTEX Shrimp), Faculty of Science, Mahidol University,Bangkok, Thailand, Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand The object i ves of this study were to examine the effects of cyclic thermal stress on histological characteristics of breast muscle and gene expression regarding adipose infiltration and inflammation in breast muscles collected from different breeds of chickens. The birds, from commercial broilers (CB, Ross 308, 3 weeks), native (NT,100%Thai native Chee, 9weeks), H75 (crossbred;775%broiler and 25%NT.5 weeks), and H50 (crossbred; 50% broiler and 50% NT, 7 weeks), were equally assigned into control or treatment groups. The control samples were reared under a constant temperature of 26±1℃, while the treatment groups were exposed to 35±1℃(6 h per day ). After a 20-day thermal challenge, 12 male birds per t reatment group were randomly collected for determination of live body weight, breast weight, numbers of growth-related myopathies, and breast meat chemical composition. Histological lesions were evaluated in the pectoralis major muscle immediately collected within 20 min postmortem based on hematoxylin and eosin staining. The results indicated that despite interaction between thermal stress and breed effects, thermal challenge significantly reduced feed intake, live body weight, and breast weight of the birds and increased moisture content in breast meat (p<0.05). An interaction between the two main factors was found for protein content (p < 0.05) for which control CB showed less protein than the other groups. Heat stress decreased histological scores for adipose infiltrat i on in CB (p <0.05), but it did not significantly i nfluence such scores i n the other groups. CB received histological scores for adipose tissue at greater extent than those for the other groups. Differential absolute abundance of CD36, FABP4, LITAF, PDGFRA, PLIN1,PPARG, POSTN, SCD1, and TGFB1 in t he muscle samples well-agreed with the trend of histological scores, suggest i ng potential involvement of dysregulated f ibro-adipogenic progenitors together with imbalanced lipid storage and utilization in the breast muscle. The findings demonstrated that the cyclic thermal challenge restricted growth performance and breast mass of the birds, but such effects attenuated infiltration of adipose tissue and inflammatory cells in the CB breast muscle. Keywords: chicken, droplet digital polymerase chain reaction, fat accumulation, immune cell infiltration, heat stress An increase in average environmental temperatures has urgently raised global attention as i t inevitably i mpacts the well-being of lives on Earth. For chickens, their physiological response to thermal challenge depends on several factors, including breed, size, and age (Lu et al, 2007; Awad et al., 2020; Malila et al, 2021a).Commercial broiler S s,, meat-type e chickens S ,, exhibit more susceptibility to thermal stress than their ancestors (Tabler et al.,2020) and other slow-growing strains (Washburn et al., 1980;Aengwanich, 2007; Malila et al ., 2021a ). This has been considered a negative consequence of an artificial breeding selection program focused on production performance and massive mass to meet high consumer demand. Development of thermoregulatory systems of broilers does not complement rapid growth of the birds (Havenstein et al., 2003). An ambient temperature above 30℃ is considered suf f icient to induce stress among the birds (Azad et al., 2010; Adu-Asiamah et al., 2021). The negative impacts of thermal challenge include diminished growth performance (Azad et al, 2010;Quinteiro-Filho et al., 2010; He et al ., 2018; Aslam et al .,2021),increased mortality (Al-Fataftah and Abdelqader, 2014),compromised gut health, (Quinteiro-Filho et al., 2010; Awad e t al., 2020), and deviated meat quality (Sandercock et al., 2001;Lu et al, 2017; Mal i la et al, 2021a). A recent meta-analysis performed by Andretta et al. (2021) highlighted that broilers with increasing age and weight become more sensitive to heat as their heat dissipation area becomes smaller. Their findings also i ndicated that heat stress negatively affects broilers over 21 days of age at a greater extent than the birds at an initial growing phase. In regard to altered meat quality, increased fat along with decreased protein content was frequently reported in the meat yielded from chickens exposed to heat stress (Aksit et al., 2006; Lu et al., 2007; Zhang et al., 2012). The actual molecular etiology of the changes remained not fully comprehended. An increased level of serum corticosterone was observed in heat-stressed chickens and further linked with induced insulin resistance, stimulated lipid synthesis, and fat accumulation in abdominal, cervical, and thigh adipose tissues (Quinteiro-Filho et al., 2010; Xu et al., 2018).Serum corticosterone might also activate protein breakdown in the skeletal muscle of stressed animals (Scanes, 2016). Lu et al.(2019) reported widespread deposition of lipid droplets inside the liver of Arbor Acres broilers exposed to 14-day constant heat stress (32℃). They found that in the stressed animals, both apolipoprotein B gene and protein levels in the chicken liver and plasma corticosterone concentration remained unchanged,suggesting the disrupteddt transportation of the excessive triglycerides from the liver in the broilers exposed to chronic heat stress. Recently, Adu-Asiamah et al..((2021) analyzed histopathological characteristics of 56-day-old Chinese broiler chickens exposed to acute heat stress (35℃) for 8 h and reported the extent of tissue damage in the liver, spleen, breast, and leg muscles. In addition, experiments conducted using turkey pectoralis major muscle satellite cells showed that temperature extremes altered the adipocyte-like properties of the satellite cells isolated from f ast-growing and non-selec t ed random-bred control lines (Clark et al ., 2017). The cyclic thermal stress, resembling the daily rise of temperature in the tropical region or in the summer of the temperate zone,exerted adverse impacts on growth performance of commercial broilers, although the impact was at a lesser extent than that of the constant heat challenge (Souza et al ., 2016; Aslam et al., 2021).Under cyclic s tress, commercial broilers are partly able to adjust to the stressed environment and have compensatory gain during t he non-stressed period (Aen gw anich, 2007; Andretta et al., 2021). Still,reduced growth performance and less meat yield, attributed to an exposure to cyclic heat stress, were consistently reported among previous studies (Quinteiro-Filho et al., 2010; Shao et al., 2019;El-Tarabany et al., 2021). Heat stress may exert a negative impact on meat quality through oxidative damages (Lu et al, 2017). This could impair the muscle regeneration process and lead to muscle abnormalities, including emerging myopathies known as white striping (WS) and wooden breast (WB), among the chickens exposed to thermal challenge (Aslam et al., 2021). Several studies indicated chemical changes , that is, reduced protein and increased fat content in chicken 11meat affected with tthose abnormalities (Kuttappan et al., 2012; Petracci et al., 2014; Malila et al, 2018;Thanatsa n g et al ., 2020). Overall, t he incidence could not only affect economic returns of broilers but also potentially dilute nutritional properties of chicken breast meat, which is generally recognized as an inexpensive source of food protein in several regions. The objectives of this study were to examine the effects of cyclic thermal stress, mimicking the rise of temperature during the day in tropical regions, on histological characteristics of the breast (pectoralis major) muscle of chickens from different breeds. Absolute transcript abundance of associated genes was also evaluated to provide i nsights at molecular levels. The chickens included commercial broiler (CB,Ross 308), native (NT , 100% Thai native Chee), H75 (crossbred; 75%broiler and 25%NT), and H50 (crossbred; 50% broiler and 50% NT).We focused on the exposure of the challenge during the l ast 3 weeks before the specific market ages of each breed. MATERIALS AND METHODS Experimental Design, Animals, and Management Al l chickens , inc l uding commercial broiler (CB, Ross 308), native (NT, 100% Thai native Chee), H75 (crossbred; 75% broiler and 25% NT), and H50 (crossbred; 50% broiler and 50% NT), were raised and maintained under an environmentally controlled Nutrient Starting phase (1 day-21 days) Growing phase (22 daysto Market age) Metabolizable energy (kcal/kg) 3,100 3.200 Moisture (%) Crude protein (%) Crude fiber (%) 54 Fat (%) poultry facility of t he Department of Animal Science, Faculty of Agriculture, Khon Kaen University (Khon Kaen, Thailand)following the standard practice for commercial broilers. The birds were housed in f loor pens (1.3 m x2.0 m) with rice hulls provided as bedding materials and received standard commercial broiler diets (Table 1). Feed and water were provided ad libitum.A routine vaccination program against coccidiosis, infectious bronchitis virus, and Newcastle disease was applied. Three weeks prior to their specific market ages, the chickens of each strain were randomly divided i nto two groups: control and stressed treatment . The control (4 replications/group, 12-13 birds/replication, 5 birds/m) group was kept at a constant temperature of 26 ± 1℃, while the treatment groups (4 replications/group, 12-13birds/replication, 5 birds/m ) were exposed to 35 ± 1℃ during 10:00a.m. to 4:00 p.m., accounting for 6h daily. The thermal challenge period was carried on for 20 days. Upon completion of the thermal challenge, a total of 96male chickens (n = 12 per group, 3 birds per pen replicate)were proceeded to the slaughtering process with 12-h fasting prior to the slaughter. The right side of the breast was immediately removed from the carcass and monitored for occurrences (presence or absence) of white striping (WS)and wooden breast (WB)abnormalities based onthe criteria previously described by Malila et al..((2018)) and Ti jare et al . (2016), respectively. The evaluation was carried out by one fully trained staff to minimize the variation. The muscle samples were excised, oriented along the muscle fiber,from the cranial portion (approximately 1 cm deep from the ventral surface) of the muscle, and cut into 0.5 cm’cubes. Half of the muscle specimen was then snap-frozen in liquid nitrogen within 20 min postmortem and stored at -80℃until RNA isolation, whereas the other half was fixed within 10% buffered formalin fixative (pH 7.0) and stored at 4℃ unt i l histological evaluation. The left side of the breast was collected, weighed, packed i n a plastic bag, kept on ice during samples collection, and subsequently stored at refrigerated temperature until it reached 24 h postmortem.The samples were then ground and stored at -20℃ until the analyses of chemical composition were performed. Proximate Composition Chemical compositions, including moisture, protein, fat , ash,and carbohydrate, of the samples were determined following the standard methods of AOAC (2016). In brief, moisture content was analyzed based on weight loss upon drying the samples at 105℃. Crude protein was determined following a B Cases of meat abnormalities Dumas combustion principle, and the conversion factor of 6.25 was used for calculation (AOAC 990.03). Crude fat was extracted from the breast samples with petroleum ether by using a Soxtherm (mode l SOX416, C. Gerhardt GmbH & Co.KG, Konigswinter, Germany) following a Soxhlet method. Ash content was examined based on an incineration of the samples at 600℃. T he analyses were performed in two technical replicates. FIGURE 1|Live body we i ght and cases o f meat abnormalities i n the chickens f r om different breeds. The bi r ds were reared at a constant temperature (control, 26℃) or received heat challenge (treatment, 35℃, 6h daily) for 20 days before reac h ing their market ages. (A) Markers and error bars depict average and standard deviation of li ve body weight (n=12per group), respectively. (B) Venn diagrams illust r ate occurrence of g r owth-related myopathies, including white st r ip in g (WS) and wooden breast (WB), i n breast muscles (n = 12 per group). CB: commercial broil e rs; H75:crossbreeds, 75% broiler background and 25% Thai native background; H50:crossbreeds, 50% broiler background and 50% Thai nat i ve backg r ound; NT:Thai nat i ve ch i cken. Histological Evaluation and Scoring The muscle specimen stored in 10% buffered formalin was dehydrated i n ethanol solutions with a seria l concentration of 70, 80, 95% (repeated a t t his step three times ), and 100%. The specimen was then embedded in paraffin and cross-sectioned into 5-um sections. The tissue section sl i des were stained with hematoxylin and eosin (H&E staining). The slide section was examined under a bright-field microscope (Olympus, Tokyo,Japan) equipped with a digital camera (Olympus D D P73Microscope Digital Camera, Tokyo, Japan) and visualized using Olympus cellSens software (Olympus, Tokyo, Japan).The histopathological characteristics, including inflammation and adipose infiltration, were scored based on the criteria described by Prisco et al. (2021). In brief, inflammation was assessed based on the number of inflammatory cells as follows:score 0= no infiltrated cells; score 1 = 5 to 25 cells per field; score 2 = 26 to 50 cells per field; and score 3 = more than 50 cells per field. As for adipose t issue infiltration: score 0= no appearance of adipose tissue; score 1 =less than 10% of the skeletal muscle area was infiltrated by adipose tissue; score 2=10 to 20% i nfiltration;and score 3= more than 20% infiltration. A total of ten fields at x200 magnification (x20 for objective lens and x10 for eyepiece lens) for each sample were selected for the evaluation. The scores for each muscle sample were an average score calculated from the ten fields. Total RNA Isolation and cDNA Synthesis Total RNA was isolated from the frozen breast muscle tissues using TriReagent (Molecular Research Center, Inc., Cincinnati, OH,United States ) following the manufacturer's protocol.Contaminated DNA was removed by incubating the isolated total RNA with RNase-free DNase I (Thermo Fisher Scientific, Rockford,IL, United States), according to the company's i nstruction. The total RNA samples were t hen purified using a column-based ReliaPrep RNA clean-up and concentration system (Promega Corporation,Madison, WI, United States). The quantity and quality of total RNA were determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific) and a fragment analyzer system (model 5300,Agilent), respectively. Only RNA samples exhibiting RNA i ntegrity number greater than 7..00 were u used for cDNA synthesis.Subsequently, total RNA (1.5 ug)w was reverse-transcribed into cDNA using oligo(dT) as a primer and an ImProm-II reverse transcription system (Promega Corporation). The amount of the synthesized dcDNA Was determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific). Primers and Droplet Digital Polymerase Chain Reaction The absolute expressions of 13 target genes (Table 2) associated with l ipid metabolism and musc l e i njury were evaluated using an EVAGREEN droplet digital polymerase chain reaction (ddPCR)assay. The primers were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). To confirm primer specificity, PCR mixture, containing 1X EvaGreen supermix (Bio-Rad Laboratories , Inc., Hercules, CA, United States), 0.25 uM of each forward and reverse primer, and the cDNA template (Table 2), was prepared (Table 2) and performed using a thermocycler with conditions set as 95℃ for 5 min; 40 cycles of 95℃ for 30 s, 60℃ for 1 min, 4℃ for 5 min, and 90℃ for 5 min.Primer specificity was then confirmed by the presence of a single band of amplicon products that corresponded to the correct molecular weight on a 2% agarose gel . To perform ddPCR, the ddPCR reaction mixture consisting of 1X EvaGreen supermix (Bio-Rad Laboratories, Inc., Hercules, CA,United States), 0.25 uM of each forward and reverse primer, and the cDNA template (Table 2), was set up in a sterile microcentrifuge tube. The template was replaced by an equal volume of nuclease-free water for no template control. The ddPCR reaction mixture (20 uL)was then mixed with Evagreen generator oil (Bio-Rad Laboratories,Inc., Hercules, CA, United States) using a QX100droplet generator (Bio-Rad Laboratories, Inc.), according to the company’s protocol .The generated droplets (40uL) were subsequently transferred into a 96-well plate. The plate was then heat-sealed with an aluminum foil cover. The target genes in the samples were amplified in a thermocycler (model T100M, Bio-Rad Laboratories, Inc.) with a condition set as follows: 95℃ for 5 min; 40 cycles at 95℃ for 30 s,60℃ for 1 min, and 4℃ for 5 min; and 90℃ for 5 min. Afterward,the fluorescent signal intensity of the droplets was measured using a QX200droplet reader (Bio-Rad Laboratories, Inc.). The number of positive and negative droplets was automatically counted by QuantaSoft software (Bio-Rad Laboratories, Inc.), and transcript abundance was expressed as copies per 20-uL reaction. Statistical Analysis A statistical analysis was performed using the R package version 3.4.3.The significant level for all statistical analyses was set at α =0.05. The effects of thermal challenge and chicken breeds, as the main effects,were determined according to a 2 × 4 factorial analysis of variance (ANOVA). The mean differences were assessed using Duncan’s new multiple range test. Prior to ANOVA analysis, the assumptions of normality and homogeneity of variance were examined using the Shapiro-Wilk normality test and Bartlett test , respectively. The data set that did not follow the assumptions was transformed using a function varIdent f rom the l ibrary nlme of the R package. Pearson's correlation coefficient was calculated to define the correlation among histological scores and absolute expression of the tested genes. In t his case, average histological scores from 10observed microscopic fields and absolute transcript abundance (in the unit of copy number per 20 uL reaction) were included in the test. As for the relationship between growth-related myopathies and gene expression l evel , this categor i cal variable was labeled as 1 (presence) or 0 (absence) before the data were submitted to Spearman's rank correlation test. RESULTS AND DISCUSSION Feed Intake, Live Body Weight, Breast Weight, Chemical Composition, and Cases of Growth-Related Myopathies Table 3 shows feed i ntake (during the 20-day thermal stress), live body weight, breast weight, and breast yield of the tested chickens as affected by the t hermal challenge. Focusing on the main effects, daily) for 7 days compared to the birds reared under thermal the heat condition reduced feed i ntake, l ive body weight, and neutral condition (23℃). Using similar thermal challenge breast weight (p<0.05) but not breast yield (p≥0.05). Live body conditions (cyclic temperature of 35℃, 8h daily vs. control weight of the stressed CB, stressed H50, and stressed NT was condition of 23 ℃), Emami et a l. (2021) observed a significant approximately 13, 11, and 12%, respectively, less than their decrease in body weight of broilers exposed to the stress during control counterparts. The current stress condition on reduced the age of 29-42 days . The decreased production performance has breast weight was more pronounced in CB as the breast weight been shown to be associated with restricted feed i ntake upon was reduced to 28, 8, and 13% in stressed CB, stressed H50,and exposure to thermal challenge (Souza et al., 2016). In this study,stressed NT, respectively, in comparison to their control the difference in feed intake during the 20-day challenge was counterparts. The reduced body weight and breast weight due observed only between the control and stressed H50 (p<0.05).to the thermal challenge in those breeds well agreed with that The previous meta-analysis conducted by Andretta et al. (2021)observed in previous studies (Sandercock et al., 2001; Duangjinda found that under cyclic thermal stress, commercial broilers at t he et al., 2017; Shao et al., 2019; Emami et al., 2021). Duangjinda age of 21 days or older could sometime compensate their i ntake et al. (2017) compared growth performance of commercial during the cooling period. The lack of t hermal effects on growth broilers and Thai indigenous chickens under either and meat yield of H75 and NT suggested better heat tolerance and thermoneutral condition (26℃) or heat challenge (36-38℃, better adapta t ion of both strains over CB and H50 (Aengwanich,6 h daily) for 3 weeks prior to market age and reported 2007).decreased growth performance for broilers but not in the indigenous birds. Shao et al. (2019) reported reduced body weight, average daily gain, and average feed i ntake in yellow-feathered broilers exposed to cyclic temperature of 35℃ (8h NCBI accession Gene ID Gene annotation Sequence (5' Amplicon Template Annealing number →3) length amount temperature (bp) (ng) (C) NM 206,991.1 ADIPOQ Adiponectin F: AGCAGAACCACTACGACAGC R: ACGTTGTTCTCCTGG 166 10 60 AACTGG NM_001,030,731 CD36 CD36 molecule F: TACCAGACCAGTAAGACC GTGAAGG 156 25 60 R: AATGTCTAGGACTCCAGC CAGTGT NM_204,290.1 FABP4 Fatty acid-binding protein 4 F: TATGAAAGAGCTGGGTGTGG R: GCTGTGGTCTCATCAAACTC F: ACTATCCTCACCCCTACC 168 NM 204.267.1 LITAF Lipopolysaccharide-induced tumor necrosis factor-alpha factor CTGTC R: TGTTGGCATAGGCTGTCCTG XM 040,666,999.1 LPIN1 Lipin 1 F: CCTTTCACTGTAATGCTGGT 173 R: TGGAGTGGTATGGTCATCAG NM_205,282.1 LPL Lipoprotein lipase F: AGGAGAAGAGGCAGCAATA 222 一 R: AAAGCCAGCAGCAGATAAG NM_001,030,363.1 MYF5 Myogenic factor 5 F: TGAACCAAGCATTCGAGACC R: AGTAGTTCTCCACCTGTT 141 CCCT NM 204,749.2 PDGFRA Platelet-derived growth factor receptor, alpha F: AAGAGAGTGCCATTG AAACCG 155 10 60 R: GCAGTTAGAAGGTGTCTG GGAT NM_001,127,439.1 PLIN1 Perilipin 1 F: GCCAAGGAGAACGTGCT R: TCACTCCCTGCTCATAGACC F: CCAGCGACATCGACCAGTTA 142 NM_001,001,460.1 PPARG Peroxisome proliferator activated 182 100 Receptor, gamma R: TCCCATCCTTAAAGAGTTCA NM 001,030,541.1 POSTN Periostin F: GCCTGGTGTGACAAACATCC R: TGGTTGCCATGAGAT 119 10 CAGGTT NM 204,890.1 SCD1 Stearoyl-CoA desaturase 1 F: GGCTGACAAAGTGGTGATG R: GGATGGCTGGAATGAAGA F: GACGATGAGTGGCTC 137 NM_001,318,456.1 TGFB1 Transforming growth factor beta 1 195 TCCTTC R: GTGCTTCTTGGCAATGCTCT The H50 birds exhibited the greatest live body weight (p <0.05), followed by CB and H75, whereas NT showed the lowest (p <0.05) body weight. In this regard, the different market age of each chicken strain must be emphasized. The CB, H75, H50, and TABLE 3| Live body weight at slaughter age, breast weight, and breast yield (%) of the chicken samples. CB (42d) Control 98.3±11.6 1.962.5±188.8 164.8±43.6 8.3±1.5 一 Ireatment 85.6±3.0 1,695.0±106.3 119.3±13.5 7.0±0.4 H75 (56d) Control 85.0±3.8 1,575.0±125.8 82.3±19.1 5.2±1.0 一 Treatment 88.2±13.2 1.576.3±115.9 88.8±15.9 5.6±0.8 H50 (70d) Control 94.4±8.6 2.150.0±122.5 132.8±15.7 6.2±0.7 二 Treatment 79.8±6.3 1,905.0±85.4 121.5±10.1 6.4±0.4 NT (84d) Control 64.4±3.8 1,345.0±68.1 60.5±8.2 4.5±0.4 一 Treatment 63.9±8.2 1,185.0±54.5 52.5±4.4 4.4±0.3 Main effects — Stress Control 86.9±15.05 1,758.1±347.5 110.1±48.1 6.8±1.9 一 Treatment 78.8±11.54 1,590.3±283.2 95.5±30.8 6.5±1.2 Breed CB 92.0±10.4 1,828.8±201.4 142.0°±38.5 8.4±1.4 一 H75 86.4±8.3 1,575.6°±112.0 85.5°±16.7 6.1±1.0 一 H50 87.1±10.5 2,027.5±163.4 127.1±13.6 6.9±0.6 一 NT 64.2±5.7 1,265°±102.8 56.5°±7.5 5.0°±0.4 p-value 一 一 一 Stress 一 0.03 0.0004 0.04 0.34 Breed 一 <0.0001 <0.0001 <0.0001 <0.0001 Interaction 0.12 0.11 0.09 0.15 NT chickens were slaughtered a t the age of 42, 56,70, and 84 days,respectively , following their specific market ages. The superior yield of H50 over H75 might be mainly due to the later age of slaughter as both strains exhibited a similar growth rate (Figure 1A). Although the live body weight of H50 birds was greater than that of CB, the average weight of the breast portion collected from CB, particularly the control group, was greater than the others. The results wel l-agreed with those of previous studies comparing growth performance among the chickens with different growing rates and with the fact that modern broilers have been selected for their growth rate and pectoralis major yield (Zuidhof et al., 2014). Concerning WS and WB cases (Figure 1B) of 12 control CB samples,only one breast S samplee exhibited 1unaffected characteristics, while four showed WS, and the other seven were classified as WS+WB 3.. As for stressed CB, four unaffected, two WS, and six WS+WB breasts were observed.On the other hand, three of 12 birds from the control H75 and control H50 exhibited the abnormalities, whereas no WS or WB cases were observed among the NT chickens. It is worth mentioning that all WS-af f ected samples were classified as mild lesions (1-40 white lines clearly observed with line thickness < 1 mm), whereas t he majority of t he WB-affected breasts fell into the category of Grade 1 in which the breasts were hard mainly i n the cranial region but flexible otherwise (Tijare et al ., 2016). Of seven WS+WB breasts among the control CB samples, two samples were classified as moderate WB lesion (hardness throughout but slightly flexible in the middle) and one sample fell into severe WB severity (extreme hardness throughout the meat). The results strongly supported the influences of genetic selection for growth rate and meat yield on the development of abnormalities (Kutt app an et al., 2012; Pampoui ll e et al., 2018; Lake et al., 2021). The lower number of cases for WS and WB abnormalities in the stressed CB group might be related with restricted growth among the stressed CB birds. This hypothesis was in agreement with previous reports of Kutta ppa n et al.(2013a) and Malila et al . (2018) that the high degree of WS and WB was significantly related with heavier birds and pectoral muscle yield. In accordance with our current findings, a recent study of Aslam et al. (2021) showed that Arbor Acres broilers received cycle heat stress (32℃, 12 h daily, 21-42 days) exhibited reduced body weight gain with less cases of WS in comparison to the birds raised under control condition (22-24℃). Focusing on the chemical composition of the breast samples (Table 4), the cyclic heat stress significantly reduced the moisture content in breast samples (p <0.05), whereas breed difference signi f icantly influenced moisture, protein, fat , and ash content (p <0.05). The interaction (p<0.05) between the main effects (i.e., heat stress and breed) was observed for crude protein content. The control CB comprised lower protein than other samples (p<0.05). Focusing on CB breeds, average values of moisture and fat content of the control CB were about 1.5 and 30% greater than those of their stressed counterparts. The results for CB breast were consistent with the characteristics of the breasts affected with growth-related myopathies (Sogl i a et al.,2016; Cai et al ., 2018; Mal i la et al., 2018; Dalle Zotte et al., 2020;Maharjan et al., 2020; Thanatsan g e t al ., 2020). It is worth mentioning here that the temperature for control samples in t his study was out of the optimum range (21-22℃)recommended for growing commercial broilers. However, as global ambient temperature continues to rise, it is difficult,particularly in tropical and subtropical regions, even for the houses equipped withh an evaporative coolingg facility, to maintain an optimum range during the day. The average Sample Moisture Crude protein Crude fat Carbohydrate Ash CB Control 75.87±0.55 20.87±0.84 1.09±0.19 1.01±0.50 1.16±0.12 一 I reatment 74.74±0.22 22.51°±0.27 0.76±0.12 0.59±0.27 1.40±0.07 H75 Control 73.85±0.29 23.03C±0.43 0.72±0.30 0.70±0.26 1.69±0.16 一 Treatment 73.96±1.03 23.05℃±1.08 0.60±0.17 0.82±0.37 1.57±0.30 H50 Control 74.12±0.41 23.27abc±0.34 0.63±0.27 0.47±0.49 1.51±0.13 一 I reatment 73.25±0.87 24.12±0.57 0.45±0.13 0.45±0.20 1.74±0.20 NT Control 73.31±0.40 23.91ab±0.60 0.28±0.08 0.57±0.21 1.94±0.08 一 Treatment 73.39±0.21 23.63±0.34 0.42±0.13 0.71±0.32 1.85±0.02 Main effects 一 一 — Stress Control 74.29±1.06 22.77±1.29 0.68±0.36 0.69±0.41 1.57±0.32 一 Treatment 73.89±0.87 23.33±0.85 0.56±0.18 0.64±0.30 1.64±0.24 Breed CB 75.31±0.72 21.69°±1.05 0.93±0.23 0.80±0.43 1.28°±0.16 一 H75 73.91±0.70 23.04±0.76 0.66°±0.24 0.76±0.30 1.63°±0.23 一 H50 73.69°±0.78 23.69±0.63 0.54 bc±0.22 0.46±0.35 1.62±0.20 一 NT 73.35°±0.30 23.77°±0.48 0.35°±0.12 0.64±0.26 1.89±0.07 p-value 一 一 一 一 Stress 一 0.03 0.02 0.08 0.71 0.25 Breed 一 <0.0001 <0.0001 <0.0001 0.23 <0.0001 Interaction 一 0.08 0.02 0.10 0.38 0.07 temperature of 24-26℃ or even higher was frequently observed in the rearing houses (Tirawattanawanich et al, 2011). We observed that in 1some e recent studies, the thermoneutral condition approximately at 24-26℃ was also used (Sohail et al., 2012; Dua ngj inda et al., 2017; Xu et al., 2018). Histological Findings The examples of histological findings of the pectoralis major muscles of each sample group are il l ustrated in Figure 2. Based on histological scores (Figure 3), the control CB samples f requently received score 2 for inflammation, with less samples receiving score 0 (Figure 3A). Hence, the average score for i nflammation (Figure 3C) of the control CB was greater (p<0.05) than that of the samples from the other strains, but the average scores for such histological characteristics were not significantly different from that of the stressed CB (p ≥0.05). The average scores for inflamma t ion were least pronounced in the control NT, of which the average scores did not significantly d i ffer from stressed NT and stressed H75. Concerning adipose t issue infiltration (Figure 3B), the majority of the control CB received score 1, while the majority of the other g S, Wa roups, particular l y NT samples, was scored 0 for adipose t issue infiltration. The average score (Figure 3D) was t he most pronounced in control CB, followed by the stressed CB. The results indicated the significant inflammation and adipose infiltration in the pectoral muscle of the control CB at a greater extenttin comparison 1to the other strains.The histopathological appearance was i n correspondence with the abnormal histological characteristics of WS and WB consistently reported in commercial broilers (K utta ppa n et al., 2013b; R usso et al ., 2015; Sihvo et al., 2017; Prisco et a l ., 2021). A previous study of 上 Adu-Asiamah et al. (2021)demonstrated that local Chinese broilers exposed to an acute temperature rise from 30 to 35℃ for 8 h at the age of 56 days exhibited infiltrations of fat tissues and inflammatory cells along with mild fibrosis and degenerated muscle fibers in the breast muscle. In this study, although the average histological scores of H75, H50, and NT followed the trend reported in the previous studies, no significant effects were observed, which again supported the ability of those strains to adapt under t he current challenge. The discrepancy between our results and the findings of Adu-Asiamah et al. (2021)might be explained by t he different thermal intensity between the studies. On the contrary, the CB chickens exposed to the thermal stress were affected with adipose infiltration at a lesser extent than control CB. The current results were in congruence with the report of Aslam et al. (2021) in which less incidence and severity of myodegeneration, inflammatory and lipid infiltration, and fibrosis V were observed in the pectoralis major muscle collected from Arbor Acres Plus broilers exposed to cyclic heat stress compared to the birds reared under thermoneutral condition. Absolute Gene Expression To further elucidate the molecular events associated with histological characteristics, absolute expressions of 13 target genes were examined (Figure 4). Concerning the main ef f ects (heat stress and breed), no significant effects of the cyclic heat stress on t ranscript abundance of the tested genes were detected (p>0.05). The significan t impacts of breed on the expression of CD36,FABP4, LITAF,PDGFRA, PLIN1, PPARG, POSTN,SCD1,and TGFB1 were observed (p<0.05). The interaction (p<0.05)between the two main effects was observed for TGFB1. The absolute expression levels of those genes, except for LITAF,were positively correlated (p <0.05) with histological scores and the occurrence of WS and WB abnormalities (Table 5).Positive correlation (p<0.05) between LITAF abundance was detected with adipose tissue i nfiltration and WS incidence. In FIGURE 2| Histological findings for h ematoxylin and eos i n (H&E)-stained pectoralis major muscle sections. Examples of microscopic images (scale bar o f 100 pm) o f H&E-stained pectoralis major muscles col l ected from di f ferent chicken breeds. T he birds were reared at a constant temperature (control, 26℃) or received heat challenge (treatment,35℃, 6 h daily) for 20 days before r eaching thei r market ages. CB:commercial broilers; H75: crossbreeds, 75% broi l er background and 25%Th ai native background; H50: crossbreeds, 50% broiler background and 50%Thai n ative backgrou n d; NT: Thai n ative chickens. Arrows and sta r s indicate accumulation of i nflammatory cells and adipose t iss u e, respect i vely. addition, the LPL expression level was found to be positively correlated (p<0.05) with histological scores for adipose tissue infiltration and WS and WB abnormalities. The proteins encoded by ADIPOQ, CD36, FABP4, LPIN1,LPL, PLIN1, PPARG, and SCD1 play crucial roles in lipid metabol i sm. Adiponectin, encoded by ADIPOQ, regulates lipid metabolism by promoting transport of fatty acids i nto muscle cells and by activities and expression of several enzymes involved in B-oxidation. Adiponectin also promotes accumulation of t r iglycerides i n adipocytes (Nguyen, 2020). Lipoprotein l ipase,encoded by LPL, catalyzes the rate-limiting step in hydrolysis of plasma lipoprotein triglycerides to nonesteri f ied f atty acids for further utilization oftissues, including re-esterification for storage within tissues (Mead et al., 2022). On the other hand, LPIN1-encoding enzyme participates i n adipogenesis. CD36 and FABP4 encode long-chain fatty acid transport proteins, facilitating the uptake of free fatty acids across sarcolemma into skeletal muscle cells and mitochondria (Stahl et al., 2001). PLIN1 encodes perilipin-1which 1coattss on 1lipid droplets S andcontrols adipocyte triglyceride storage and lipolysis (Sun et al., 2019).SCD1 encodes stearoyl-CoA desaturase, an integral membrane protein of the endoplasmic reticulum, which catalyzes the formation of monounsaturated fatty acids from saturated fatty acids (Heinemann and Ozols, 2003). In a previous study of Liu et al . (2019),pectoralis major muscle samples were collected f rom Jingxing-Huang female broilers, divided into two groups based on triglyceride (TG) content, and further submitted to RNA-seq. A transcriptome analysis between the two groups (i.e., high TG vs.low TG) revealed increased transcript abundance of CD36, SCD1,PPARG,ADIPOQ, and LPL in the high TG samples compared to the low TG ones. Those differentially expressed genes were mapped into the PPARy signaling pathway, suggesting the crucial role of the PPARy signalingpathwayinlipid deposition in the chicken breast muscle with different TG contentt..Therefore,the differential e expressionn of CD36,FABP4, PLIN1, PPARG, and SCD1 identified in this study indicated the differences in lipid metabolism among the breeds upon exposure of the current cyclic thermal challenge. The altered lipid utiliza t ion could be attributed to the dysregulated PPARy pathway in promoting adipogenesis and enhanced formation of lipid droplets through activity of PLIN1 (Liu et al ., 2019),leading to intramuscular fat deposition in the breasts. It is worth noting that previous studies usually reported an increased fat content in the stressed broilers (Quinteiro-Filho et al., 2010; Xu et al., 2018; Lu et al ., 2019). However, in this study, the trend was in the opposite direction, where stressed CB exhibited lower fat content along with decrease in absolute expression of CD36 and FABP4 than those of control CB. Although the actual reason of t his discrepancy requires f urther i nvestigation, i t might be reasonable to hypothesize that the birds might be able to adapt to 20 days of cyclic thermal stress (Aengwanich, 2007). In addition,increased abundances of CD36, PPARG, FABP4, and LPL were previously found in the WB-affected broiler breast muscle (Abasht et al., 2016; Lake et al ., 2019), while Marciano et al. (2021) reported increased PLIN1 in the breast muscle of Cobb500 affected with WS condition. Papah and Abasht (2019) addressed significant increase in CD36, PLINl, FABP4, and LPL abundance in the WB-affected pectoralis major muscle collected from 3-week-old Ross 708broilers; however, in 7-week-old broilers, differential expressions of those genes between the affected and unaffected samples were not observed. Papah and Abasht (2019) hypothesized t hat as the breast muscle of 7-week-old broilers became l arger, hypoxic conditions might be more profound and disrupt transcriptions of PPARy and its targeted genes, shifting gene expression patterns. It i s widely accepted that an abnormal fat deposition in t he skeletal muscle is associated with i mpaired muscle regeneration,especially in the breast muscle affected by WS. Upon muscle injury, satellite cells are activated and enter cell cycle for muscle regeneration. As myogenic factor 5 (Myf5), encoded by MYF5,play roles in activating quiescent satellite cells into proliferation,differential expression of MYF5 (Figure 4G) among the current samples i n correspondence with histological lesions was ini t ially FIGURE 3|Bar graphs indicating frequency and average hi stological l esion scores. (A,B) Frequency, expressed in percentage, of each histo l ogical score observed in pectoralis major muscles of each treatment group. (C,D) Average histological scores. Bars and error bars depict average and standard errors i n the scores,respect i vely. CB: commercial broi l ers; H75: crossbreeds,75% broiler background and 25% Thai native background; H50: crossbreeds, 50% broi l er background a n d 50% Thai native background; NT: T h ai native chick e ns. Differen t le t t e rs abov e bars denote statistical significance (p<0.05). ant i cipated. However, no significant changes of MYF5 were found in this study (p≥0.05). Although f urther investigation remained to be eluc i dated to obtain ful l comprehension regarding the findings, our data agreed with the previous study of Prau d et al. (2020) in which no dif f erences in MYF5 expression were addressed among slow-growing chickens and broilers exhibited either normal characteristics, WS, WB, or WS/WB abnormal i ties. Apart from the adipogenic f ate of satellite cells, i ntramuscular fat in t he pectoralis major muscle may also be contributed by activities of f ibro-adipogenic progenitors (FAPs). Recently identified by Uezumi et al. (2010), these multi-potent progenitors are l ocalized in the interstitial area of the skeletal muscle and play i mportant roles in muscle repair. During t he early phase of muscle regeneration,FAPs are activated and differentiated into adipocytes and collagens to provide a transient support for satellite cell differentiation. FAPs are tightly regulated t hrough TNF-a-i nduced apoptosis (Lemos et al, 2015). Under abnormal muscle regeneration, an excessive TGF-B could inhibit TNF-a-meditated FAP apoptosis (Pag ano et al ., 2019) along with imbalanced l ipid storage and ut i lization (Lukjanenko et al, 2013), l eading to fat deposition i n the skeletal muscle. Overproduction of TGF-B has been observed in the injured skeletal muscle, and i ts expression i s positive l y correlated with the differentiation fate of FAPs i nto adipogenic cells (Lukjanenko et al.,2013; Pagano et al., 2019) and fibrogenic cells (D avies et al., 2016;S on g et al., 2017; J uban et al., 2018). I n this study, absolute transcript abundances of LITAF (Figure 4D) and TGFB1 (Figure 4M) in CB and H50 were greater (p<0.05) than those of NT samples, while the expression levels of those genes in other samples were at intermediate levels. In agreement with the present study,upregulated TGFB1 and LITAF in breast muscle of commercial broilers affected with growth-related myopathies were consistently addressed (Mut ry n et al ., 2015; Velleman and Clark, 2015; Marchesi et al., 2019; Praud et al., 2020; Prisco et al ., 2021; Xing e t al ., 2021).The role of LITAF-encoded protein in regulat i ng the transcript i on of TNF-a in inflammatory response was previously demonstrated by Hong et al . (2006). PDGFRA encodes a cell surface tyrosine kinase receptor for members of the platelet-derived growth factor family which is crucially required for normal development of several ce l ls and organs, including connective t issue (Horikawa et al., 2016).Uezumi et al . (2010) demonstrated that t he adipogenic f ate of FAPs depended greatly on the muscle microenvironment, and only PDGFRA-positive FAP cells could undergo differentiation into adipocytes inthe skeletal muscle. An n1increasea d PDGFRA abundance was also previously observed in the breast muscle of FIGURE 4|Absolute tra n scr i pt abundance of 13 target genes associated with lipid metabolisms and muscle injury in the pectoral i s major muscle of chickens fr om different breeds. The birds were reared at a constant temperature (control , 26℃) or rece i ved heat chall e nge (t reat m ent, 35℃,6 h daily) fo r 20 days before reaching their market ages. Ba r s and error bars depict average and standard error i n copies per 20-pL reaction, respectively. CB: commercial broilers; H75: crossbreeds, 75% broiler background and 25% Thai native background; H50: crossbreeds, 50% broiler background and 50% Thai native background; NT : Thai native ch i cken. Different letters above individ u al bars denote statistical signi f icance among different t r eatment groups (p <0.05). Different letters above hor i zontal l i nes indicate significance due to different breeds (p <0.05). Asterisks indicate the r educed expression of part i cu l ar genes in stressed samples compared to t heir cont r ol cou n terparts (p<0.05). TABLE 5| Correlation coefficient between histological scores, white str i ping (WS) and wooden breast (WB) abnormalities, and absolute transcript abundance.Pearson's correlation coefficient Spearman's rank correlation Inflammation Adipose tissue infiltration WS WB Inflammation na na 0.45 0.36 Adipose tissue infiltration na na 0.46 0.47** WS na 0.67*** WB mm mm 0.67*** na ADIPOQ -0.08 -0.12 0.03 0.01 CD36 0.43* 0.56** 0.66*** 0.51** FABP4 0.57** 0.76*** 0.61** 0.51** LITAF 0.29 0.37* 0.45* 0.31 LPIN1 -0.19 -0.17 0.14 0.03 LPL 0.27 0.40* 0.51 0.48** MYF5 -0.18 -0.03 0.37 0.30 PDGFRA 0.36* 0.73*** 0.51** 0.47** PLIN1 0.61** 0.81*** 0.57** 0.47** PPARG 0.49** 0.67*** 0.52** 0.43* POSTN 0.38* 0.66*** 0.44 0.48** SCD1 0.51** 0.62** 0.54** 0.47** TGFB1 0.47** 0.54** 0.54** 0.42* broilers affected with WB myopathy (Pampouille et al ., 2019; Praud et al, 2020). The difference i n the expression pattern of PDGFRA (Figure 4H) found in this study suggested a potential association between activities of FAPs and intensive histological lesions among the current chicken breast muscle samples. Although fibrosis was not the main focus in this study, we observed an increased POSTN absolute abundance (Figure 4K)in significant association with severity of histological lesions (Table 5) in thee current samples. POSTN-encoded protein,expressed in connective tissues rich in collagens, is recognized as a key player in regulation of organization of the extracellular matrix and shown to be induced by growth factors and cytokines,particularly TGF-B (Gonzalez-Gonzalez and Alonso, 2018). Our previous transcriptome analysis revealed an increased POSTN for approximately 2.5-fold in the WB-affected breast muscle of 7-week-old Ross 308 broilers compared to their normal counterparts (Malila et al , 2021b). The upregulated POSTN together with TGFB1identified in this study may also imply the fibronect i n fate of FAPs potentially through the regulation of TGF-B in t he control CB. CONCLUSION In summary, the results indicated that the current cyclic thermal condition significantly reduced live body weight and breast weight of the chickens (p <0.05). As per chemical composition, stressed CB samples exhibited i ncreased protein and ash (p<0.05) content compared with their control counterparts. In addition, infiltration of inflammatory cells and adipose tissues was prevalent in control CB, and the histological scores correlated with the incidence of white striping. Differential absolute transcript abundances of the target genes in the breast muscle sample F s B s A uggested potential involvement of dysregulated activit i es of FAPs together with perturbed lipid metabolisms i n fat deposition in the CB breast muscle. DATA AVAILABILITY STATEMENT The original contributions presented in the study are i ncluded in the article/Supplementary Material, further inquiries can be directed to the corresponding author. ETHICS STATEMENT The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at National Center for Genetic Engineering and Biotechnology (IACUC No. BT-Animal 13/2,564). AUTHOR CONTRIBUTIONS YM, YP, and SK conceived and designed the experiments . AJ , SK,and YP contributed to animal handling. YM, AJ, YS, PS, YP, and SK contributed to sample collec t ion. PS and WT performed histological evaluation. PS performed total RNA isolation,cDNA synthesis, and ddPCR. YM conducted data analysis and drafted the manuscript with revisions provided by WT, YS, and SK. All authors read and approved the f inal manuscript. FUNDING The research was f unded by the Agriculture Research Development Agency (Public Agency, Thailand) with project number 19597(PRP6405030420) and National Center for Genetic Engineering and Biotechnology (Thailand, Project number P21-50224). ACKNOWLEDGMENTS The authors thank T ThongsaBuasook,,Pattarawan Keela,Thi t iporn Kaisoda, and Thanawan Uopasai (KhonI Kaen University, Thailand)and d VWipakarn Songyou((BIOTEC,Tha i land) for their assistance during sample collection as well as Panadda Meeneam (CENTEX , Mahidol University, Thailand) REFERENCES Abasht, B., Mutryn, M. F., Michalek, R. D., and Lee, W. R. (2016). Oxidative Stress and Metabolic Perturbations in Wooden Breast Disorder in Chickens. PLoS One 11 (4), e0153750. doi:10.1371/journal .pone.0153750 Adu-Asiamah, P., Zhang, Y., Amoah, K., Leng, Q. Y., Zheng, J . H., Yang, H., et al.(2021). Evaluation of Physiological and Molecular Responses to Acute Heat Stress in Two Chicken Breeds. Animal 15 (2),100106. doi:10.1016/j .animal.2020.100106 Aengwanich, W. (2007). Comparative Ability to Tolerate Heat between Thai Indigenous Chickens, Thai Indigenous Chickens Crossbred and Broilers by Using Heterophil/lymphocyte Ratio. Pakistan J. Biol . Sci. 10 (11),1840-1844.doi:10.3923/pjbs .2007.1840.1844 Aksit, M., Yalcin, S., Ozkan, S., Metin, K., and Ozdemir , D. (2006). Effects of Temperature during Rearing and Crating on Stress Parameters and Meat Qual i ty of Broilers. Poult. Sci. 85 (11), 1867-1874. doi:10.1093/ps/85.11.1867 Al-Fataftah, A.-R.,and Abdelqader, A. (2014). Effects of Dietary Bacillus Subtilis on Heat-Stressed Broilers Performance, I ntest i nal Morphology and Microflora Composition. Anim. Feed Sci . Tech. 198, 279-285. doi:10.1016/j.anifeedsc i .2014.10.012 Andretta, I ., Kipper , M., Schirmann, G. D., Franceschina, C. S., and Ribeiro, A. M.L. (2021). Modeling the Performance of Broilers under Heat Stress. Poult. Sci.100(9), 101338. doi:10.1016/j.psj.2021.101338 AOAC (2016). Official Methods of Analysis. Rockville, MD: Association of Official Analytical Chemists. Aslam, M. A., I pek, E., Riaz, R., Ozsoy, S. Y., Shahzad, W., and Giiles, O.(2021).Exposure of Broiler Chickens to Chronic Heat Stress Increases the Severity of white Striping on the Pectoralis Major Muscle. Trop. Anim. Health Prod. 53 (5),502. doi :10.1007/s11250-021-02950-6 Awad, E. A., Najaa, M., Zulaikha, Z. A., Zulkifli, I., and Soleimani, A. F. (2020).Effects of Heat Stress on Growth Performance, Selected Physiological and Immunological Parameters, Caecal Microflora, and Meat Quality in Two Broiler Strains. Asian-australas. J. Anim. Sci. 33 (5), 778-787. doi:10.5713/ajas.19.0208 Azad, M. A. K., Kikusato, M., Maekawa, T., Shirakawa, H., and Toyomizu, M.(2010). Metabolic Character i stic s and Oxidative Damage to Ske l etal Muscle in Broiler Chickens Exposed to Chronic Heat Stress . Comp. Biochem. Physiol. A:Mol. Integr. Physiol. 155 (3), 401-406. doi :10.1016/j.cbpa.2009.12.011 Cai, K., Shao, W., Chen, X., Campbel l ,Y. L.,Nair, M. N., Suman, S. P., et al. (2018).Mea t Quality Traits and Proteome Profile of Woody Broiler Breast (Pectoralis Major) Meat. Poult. Sci . 97 (1), 337-346. doi:10.3382/ps/pex284 Clark, D. L ., Strasburg, G. M., Reed, K. M., and Velleman, S. G. (2017). Influence of Temperature and Growth Selec t ion on turkey Pectorali s Major Musc l e Satellite Cell Adipogenic Gene Expression and Lipid Accumulation. Poult. Sci. 96 (4),1015-1027. doi:10.3382/ps/pew374 Dalle Zotte, A., Ricci , R., Cullere , M., Serva,L., Tent i , S., and Marchesini,G. (2020).Research Note: Effect of Chicken Genotype and white St r iping-Wooden Breast Condition on Breast Meat Proximate Composition and Amino Acid Profile.Poult. Sci . 99 (3), 1797-1803. doi:10.1016/j .psj.2019.10.066 Davies, M. R., Liu, X., Lee, L., Laron,D., Ning, A. Y., Kim, H. T.,et al. (2016).TGF-B Smal l Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration by Promoting Fibro/Adipogenic Progenitor Apoptosis . PLoS One 11 (5), e0155486. doi:10.1371/journal .pone.0155486 Duangjinda, M., Tunim, S., Duangdaen, C., and Boonkum, W. (2017). Hsp70Genotypes and Heat Tolerance of Commercial and Native Chickens Reared in Hot and Humid Conditions. Rev. Bras. Cienc. Avic . 19,7-18. doi:10.1590/1806-9061-2016-0245 El-Tarabany, M. S., Ahmed-Farid, O. A., Nassan, M. A., and Salah, A. S . (2021).Oxidative Stabil i ty, Carcass Traits, and Muscle Fatty Acid and Amino Acid Profiles in Heat -Stressed Broiler Chickens. Antioxidants 10 (11), 1725. doi:10.3390/antiox10111725 for her assistance in preparing H&E stained slides. Constructive advice of Natapong Jupatanakul (BIOTEC, Thailand) during histological evaluation is greatly appreciated. Emami, N. K., Greene, E. S., Kogut, M. H., and Dridi, S. (2021). Heat Stress and Feed Restriction Distinctly Affect Performance, Carcass and Meat Yield,Intestinal Integrity, and Inflammatory(Chemo)Cytokines inBroiler Chickens. Front. Physiol. 12 (1148), 707757. doi:10.3389/fphys.2021.707757 Gonzalez -Gonzalez, L., and Alonso, J . (2018). Per i ostin: a Matricel l ular Protein with Multiple Funct i ons in Cancer Development and Progression. Front. Oncol.8,225. doi:10.3389/fonc .2018.00225 Havenstein, G., Ferket, P., and Qureshi, M. (2003). Carcass Composition and Yield of 1957 versus 2001 Broilers when Fed Representative 1957 and 2001 Broi l er Diets. Poult . Sc i . 82 (10), 1509-1518. doi:10.1093/ps/82.10.1509 He, X., Lu, Z., Ma, B., Zhang, L., Li,J ., J iang, Y., et al . (2018). Effects of Chronic Heat Exposure on Growth Performance, Intestinal Epithelial Histology, Appeti t e -Re l ated Hormones and Genes Expression in Broilers. J. Sci. Food Agric. 98 (12),4471-4478. doi:10.1002/jsfa.8971 Heinemann, F. S., and Ozols, J. (2003). Stearoyl -CoA Desaturase, a Short -Lived Protein of Endoplasmic Re t iculum with Mult i ple Control Mechanisms.Prostaglandins, Leukot . Essent. Fatty Acids 68 (2), 123-133. doi:10.1016/S0952-3278(02)00262-4 Hong, Y., Li l lehoj, H., Hyenlee, S., Woonpark, D., and Lillehoj, E. (2006).Molecular Cloning and Characterization of Chicken Lipopolysaccharide-Induced TNF-a Factor (LITAF). Dev. Comp. Immunol. 30(10), 919-929.doi:10.1016/j.dci.2005.12.007 Horikawa, S., Ishii, Y., HamashimaYananoto, T. S., Yamamoto, S., Mori, H.,Fujimori, T ., et al . (2016). PDGFRa Plays a Crucial Role in Connective Tissue Remodeling. Sci . Rep. 5, 17948. doi :10.1038/srep17948 Juban, G., Saclier,M.,Yacoub-Youssef,H., Kernou, A., Arnold, L., Boisson, C., e t al.(2018). AMPK Activation Regulates LTBP4-dependent TGF-B1 Secretion by Pro-i nflammatory Macrophages and Controls Fibrosis in Duchenne Muscular Dystrophy . Cel Rep. 25 (8), 2163-2176. doi:10.1016/j .celrep.2018.10.077 Kuttappan, V. A., Brewer, V. B., Apple, J . K., Waldroup, P. W., and Owens, C. M.(2012). Influence of Growth Rate on the Occurrence of white Str i ping in Broi l er Breast Fi l lets. Poult . Sc i . 91 (10), 2677-2685. doi:10.3382/ps.2012-02259 Kuttappan, V. A., Brewer, V . B., Mauromoustakos, A., McKee, S. R., Emmer t , J. L.,Meullenet, J. F., et al. (2013a). Estimation of Factors Associated with the Occurrence of white Striping in Broiler Breast Fi l lets . Poult. Sci. 92 (3),811-819. doi :10.3382/ps.2012-02506 Kuttappan, V. A., Shivaprasad, H. L., Shaw, D. P., Valentine, B. A., Hargis, B. M.,Clark, F. D ., et al . (2013b). Pathologica l Changes Associated with white Striping in Broi l er Breast Muscles. Poult. Sci. 92 (2), 331-338. doi:10.3382/ps.2012-02646 Lake, J . A., Papah, M. B., and Abasht, B. (2019). Increased Expression of Lipid Metabolism Genes in Early Stages of Wooden Breast Links Myopathy of Broilers to Metabolic Syndrome in Humans. Genes 10 (10), 746. doi:10.3390/genes10100746 Lake, J . A., Dekkers, J . C. M., and Abasht, B. (2021). Genetic Basis and Identification of Candidate Genes for Wooden Breast and white St r iping in Commercial Broiler Chickens. Sc i . Rep. 11 (1),6785. doi :10.1038/s41598-021-86176-4 Lemos, D. R., Babaeijandaghi , F., Low, M., Chang, C.-K., Lee, S. T., Fiore, D., et al.(2015). Nilotinib Reduces Muscle Fibrosis in Chronic Muscle Injury by Promot i ng TNF-Mediated Apoptosis of Fibro/adipogenic Progenitors. Nat .Med. 21 (7), 786-794. doi:10.1038/nm.3869 Liu, L , Liu, X., Cui, H., Liu, R., Zhao, G., and Wen, J. (2019). Transcriptional Insights into Key Genes and Pathways Controlling Muscle Lipid Metabolism in Broiler Chickens. BMC Genomics 20 (1), 863. doi:10.1186/s12864-019-6221-0 Lu, Q., Wen, J., and Zhang, H. (2007). Effect of Chronic Heat Exposure on Fat Deposition and Meat Quality in Two Genetic Types of Chicken. Poult. Sci. 86(6), 1059-1064. doi:10.1093/ps/86.6.1059 Lu, Z., He, X., Ma, B., Zhang, L ., Li, J., J i ang, Y., et al. (2017). Chronic Heat Stress Impairs the Qual i ty of Breast-Muscle Meat in Broilers by Affecting Redox Status and Energy-Substance Me t abolism. J. Agric . Food Chem. 65 (51),11251-11258. doi:10.1021/acs.jafc.7b04428 Lu, Z., He, X. F., Ma, B. B., Zhang, L., Li , J . L., J iang, Y., et al . (2019). Increased Fat Synthesis and Limited Apolipoprotein B Cause Lipid Accumulation in the Liver of Broiler Chickens Exposed to Chronic Heat Stress. Poult. Sci. 98(9),3695-3704. doi:10.3382/ps/pez056 Lukjanenko, L., Brachat, S ., Pierrel, E., Lach-Trifilieff, E., and Feige, J . N. (2013).Genomic Profil i ng Reveals that Transient Adipogenic Activation I s a Hallmark of Mouse Model s of Skeletal Muscle Regeneration. PLoS One 8 (8), e71084.doi:10.1371/journal.pone.0071084 Maharjan, P., Hilton, K., Weil, J., Suesuttajit, N., Beitia, A., Owens, C. M., e t al.(2020). Characterizing Woody Breast Myopathy in a Meat Broiler Line by Heat Production, Microbiota, and Plasma Metabolites. Front . Vet. Sc i . 6,497. doi :10.3389/fvets.2019.00497 Malila, Y ., U-Chupaj, J., Srimarut, Y., Chaiwiwattrakul, P., Uengwetwanit, T .,Arayamethakorn, S., et al. (2018). Monitoring of white Striping and Wooden Breast Cases and Impacts on Qual i ty of Breast Meat Collected from Commercial Broi l ers (Gallus gallus ). Asian-australas. J. Anim. Sc i . 31 (11),1807-1817. doi:10.5713/ajas.18.0355 Malila, Y ., J andamuk, A., Uopasai, T., Buasook, T., Srimarut, Y., Sanpinit, P., et al.(2021a). Effects of Cyclic thermal Stress at Later Age on Product i on Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens. Animals 11 (12), 3532. doi:10.3390/ani11123532 Malila, Y ., Uengwetwanit , T., Thanatsang, K. V., Arayamethakorn, S., Srimarut, Y .,Petracci, M., et al . (2021b). Insights into Transcriptome Prof i les Associated with Wooden Breast Myopathy in Broilers Slaughtered at the Age of 6 or 7 Weeks.Front. Physiol. 12(996), 691194. doi:10.3389/fphys.2021.691194 Marchesi, J. A. P., Ibelli, A. M. G., Peixoto, J . O., Cantao, M. E., Pandolfi, J . R. C.,Marciano, C. M. M., et al . (2019). Whole Transcriptome Analysis of the Pectoralis Major Muscle Reveals Molecular Mechanisms Involved with white Striping in Broiler Chickens. Poult. Sci . 98 (2), 590-601. doi :10.3382/ps/pey429 Marciano, C. M. M., Ibelli, A. M. G., Marchesi, J. A. P., de Oliveira Peixoto, J .,Fernandes,L . T ., Savoldi , I . R., et al . (2021). Differential Expression of Myogenic and Calcium Signaling-Related Genes in Broi l ers Affected with white Striping.Front. Physiol. 12 (1172),712464. d o i :10.3389/fphys.2021.712464 Mead, J., Irvine, S., and Ramji, D. (2002). Lipoprotein Lipase: Structure, Function,Regulation, and Role i n Disease. J. Mol. Med. 80, 753-769. doi:10.1007/s00109-002-0384-9 Mutryn, M. F., Brannick, E. M., Fu, W., Lee, W. R., and Abasht, B. (2015).Characterization of a Novel Chicken Muscle Disorder through Differential Gene Expression and Pathway Analysis Using RNA-Sequencing.B BMC Genomics 16 (1), 399. doi:10.1186/s12864-015-1623-0 Nguyen, T . M. D. (2020). Adiponectin: Role i n Physiology and Pathophysiology.Int. J. Prev. Med. 11, 136. doi:10.4103/ijpvm.IJPVM _193_20 Pagano, A. F., Arc-Chagnaud, C., Br i oche, T., Chopard, A., and Py, G. (2019).Muscle Resting and TGF-B Inhibitor Treatment Prevent Fatty Infiltration Following Skeletal Muscle Injury. Cell Physiol. Biochem. 53 (1),62-75.doi:10.33594/000000121 Pampouil l e, E., Berri, C., Boitard, S., Hennequet-Antier, C., Beauclercq, S. A.,Godet, E., et al. (2018). Mapping QTL for white Striping in Relat i on to Breast Musc l e Yield and Meat Quality Traits in Broiler Chickens. BMC Genomics 19(1), 202. doi:10.1186/s12864-018-4598-9 Pampouille, E., Henneque t -Antier , C., Praud, C., J uanchich, A., Brionne, A., Godet, E.,et al . (2019). Different i al Expression and Co-expression Gene Network Analyses Reveal Molecular Mechanisms and Candidate Biomarkers Involved in Breast Muscle Myopathies in Chicken. Sci. Rep. 9 (1), 14905. doi:10.1038/s41598-019-51521-1 Papah, M. B., and Abasht, B. (2019). Dysregulation of Lipid Me t abolism and Appearance of Slow Myof i ber-specific Isoforms Accompany the Development of Wooden Breast Myopathy in Modern Broiler Chickens . Sci. Rep. 9(1),17170.doi:10.1038/s41598-019-53728-8 Petracci , M., Mudalal, S., Babini , E ., and Cavani, C. (2014). Effect of white Striping on Chemical Composition and Nutritiona l Value of Chicken Breast Meat. Ital.J. Anim. Sci. 13 (1), 3138. doi:10.4081/i j as.2014.3138 Praud, C., Jimenez, J., Pampouille , E., Courousse, N., Godet, E ., Le Bihan-Duval , E.,et al. (2020). Molecular Phenotyping of white Striping and Wooden Breast Myopathies in Chicken. Front. Physiol. 11, 633. doi:10.3389/fphys.2020.00633 Prisco, F., De Biase,D., Piegari , G., d Aquino, I., Lama, A., Comel l a, F., et al. (2021).Pathologic Character i zat i on of whi t e Striping Myopathy in Broi l er Chickens.Poult . Sci. 100 (7),101150. doi :10.1016/j.psj.2021.101150 Quinteiro-Filho, W. M., Ribeiro, A., Ferraz-de-Paula, V.,Pinheiro, M. L., Sakai,M.,Sá, L. R. M., et a l . (2010). Hea t Stress Impairs Performance Parameters, Induces Intestinal Injury, and Decreases Macrophage Ac t ivity in Broiler Chickens.Poult . Sci. 89 (9), 1905-1914. doi:10.3382/ps.2010-00812 Russo, E., Drigo, M., Longoni, C., Pezzotti , R., Fasoli, P., and Recordati, C. (2015).Evaluation of Whi t e Striping Prevalence and Predisposing Factors i n Broiler s at slaughter. Poult. Sc i . 94 (8), 1843-1848. doi:10.3382/ps/pev172 Sandercock, D. A., Hunter, R. R., Nute, G. R., Mitchell, M. A., and Hocking, P. M.(2001). Acute Heat Stress-Induced Alterations in Blood Acid-Base Status and Skeletal Muscle Membrane Integrity in Broiler Chickens at Two Ages:Implications for Meat Quality. Poult. Sci. 80 (4), 418-425. doi :10.1093/ps/80.4.418 Scanes,C. G. (2016). Bio l ogy of Stress i n Poultry with Emphasis on Glucocorticoids and the Heterophil to Lymphocyte Ratio. Poult. Sci . 95 (9), 2208-2215.doi:10.3382/ps/pew137 Shao, D., Wang, Q., Hu, Y., Shi, S., and Tong, H. (2019). Effects of Cyc l ic Heat Stress on the Phenotypic Response, Meat Quality and Musc l e Glycolysis of Breasts and Thighs of Yellow-Feather Broilers. Ital. J. Anim. Sci . 18 (1),301-308. doi :10.1080/1828051X.2018.1520051 Sihvo,H.-K., Linden, J ., Airas , N .,Immonen,K., Valaja,J ., and Puolanne,E.(2017).Wooden Breast Myodegeneration of Pectoralis Major Muscle over the Growth Period in Broilers. Vet. Pathol. 54(1),119-128. doi:10.1177/0300985816658099 Soglia, F.,Mudalal, S., Babini, E., Di Nunzio, M.,Mazzoni, M., Sirri, F., et al.(2016).Histology, Composition, and Quality Traits of Chicken Pectorali s Major Muscle Affected by Wooden Breast Abnormality. Poult. Sci. 95 (3), 651-659. doi:10.3382/ps/pev353 Sohai l , M. U ., Hume, M. E., Byrd, J. A., Nisbet, D. J ., Ijaz, A., Sohail, A., et al.(2012).Effect of Supplementation of Prebiotic Mannan-Oligosaccharides and Probiotic Mixture on Growth Performance of Broilers Subjected to Chronic Heat Stress.Poult. Sci. 91 (9), 2235-2240. doi:10.3382/ps.2012-02182 Song, Y ., Yao, S ., Liu, Y ., Long, L., Yang, H., Li , Q., et al . (2017). Expression Levels of TGF-B1 and CTGF Are Associated with the Severity of Duchenne Muscular Dystrophy. Exp. Ther. Med. 13 (4), 1209-1214. doi:10.3892/etm.2017.4105 Souza, L . F. A. d., Espinha, L. P., Almeida, E . A. d., Lunedo, R., Furlan, R. L., and Macar i , M. (2016). How Heat Stress (Continuous or Cyclical ) Interferes with Nut r ient Digestibility, Energy and Nitrogen Balances and Performance in Broilers. Livestock Sci . 192, 39-43. doi :10.1016/j.livsci .2016.08.014 Stahl , A., Gimeno, R. E., Tar t aglia, L. A., and Lodish, H. F. (2001). Fatty Acid Transport Proteins : a Current View of a Growing Fami l y. Trends Endocrinol.Metab. 12 (6), 266-273. doi:10.1016/S1043-2760(01)00427-1 Sun, Y., Li, R., Zhai, G., Zhang, X., and Wang, Y. (2019). DNA Methylation of the PLIN1 Promoter Downregulates Expression in Chicken Lines. Arch. Anim.Breed. 62 (2), 375-382. doi:10.5194/aab-62-375-2019 Tabler, T. W., Greene, E. S., Orlowski , s. K., Hiltz, J. Z., Anthony, N. B., and Dridi,S. (2020). Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild J ungle Fowl . Front. Vet. Sci. 7 (249), 249. doi:10.3389/fvets.2020.00249 Thanatsang,K V., Mal i la, Y .,, Arayamethakorn,S., Srimarut,Y .Tatiyaborworntham, N., Uengwetwani t , T., et al. (2020). Nut r i t ional Properties and Oxidative Indices of Broiler Breast Meat Affected by Wooden Breast Abnormality. Animals 10(12), 2272. doi:10.3390/ani10122272 T i jare, V. V, Yang, F . L., Kuttappan, V. A., Alvarado, C. Z., Coon, C. N., and Owens , C.M. (2016). Meat Quality of Broiler Breast Fillets with white Striping and Woody Breast Muscle Myopathies. Poult. Sc i . 95, 2167-2173. doi:10.3382/ps/pew129 Tirawattanawanich, C., Chantakru, S., Nimitsant i wong, W., and Tongyai, S.(2011). The Effects of Tropical Environmental Conditions on the Stress and Immune Responses of Commercial Broilers, Thai Indigenous Chickens , and Crossbred Chickens. J. Appl . Poult. Res. 20, 409-420. doi:10.3382/japr.2010-00190 Uezumi, A., Fukada, S.-i., Yamamoto, N., Takeda, S. i ., and Tsuchida, K. (2010).Mesenchymal Progenitors Distinct f rom Satellite Cells Cont ri bute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat. Cel Biol. 12 (2), 143-152. doi:10.1038/ncb2014 Velleman, S. G., and Clark, D. L . (2015). Histopathologic and Myogenic Gene Expression Changes Associated with Wooden Breast in Broiler Breast Muscles.Avian Dis. 59 (3), 410-418. doi:10.1637/11097-042015-Reg.1 Washburn, K. W., Peavey , R., and Renwick, G. M. (1980). Relationship of Strain Var i ation and Feed Restric t ion t o Variation in Blood Pressure and Response to Heat Stress. Poult. Sci . 59 (11), 2586-2588. doi:10.3382/ps.0592586 Xing, T ., Zhao, Z. R., zhao, X., Xu, X. L., Zhang, L., and Gao, F. (2021). Enhanced Transforming Growth Factor-Beta Signaling and Fibrosis in the Pectoralis Major Muscle of Broiler Chickens Affected by Wooden Breast Myopathy. Poult.Sci. 100 (3), 100804. doi:10.1016/j .psj.2020.10.058 Xu, Y., Lai, X., Li , Z.,Zhang, X., and Luo, Q. (2018). Effect of Chronic Heat Stress on Some Physiological and Immunological Parameters in Different Breed of Broilers. Poult. Sci. 97 (11), 4073-4082. doi:10.3382/ps/pey256 Zhang, Z. Y ., J ia, G. Q., Zuo, J. J ., Zhang, Y., Lei, J ., Ren, L., et al. (2012). Effects of Constant and Cyclic Heat Stress on Muscle Metabolism and Meat Quality of Broiler Breast Fi l let and Thigh Meat. Poult . Sci. 91 (11), 2931-2937. doi:10.3382/ps.2012-02255 Zuidhof, M. J., Schneider, B. L ., Carney, V. L ., Korver, D. R., and Robinson, F. E.(2014). Growth, Eff i ciency, and Yield of Commercial Broilers from 1957, 1978,and 2005. Poult. Sci . 93 (12), 2970-2982. doi:10.3382/ps.2014-04291 Conflict of Interest : The authors declare that the research was conducted in the absence of any commercial or financi a l relationships that could be construed as a potential conflict of interest. Publisher's Note: Al l claims expressed in this article are solely those of the authors and do not necessarily represent those of their affi l iated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by i ts manufacturer, is not guaranteed or endorsed by the publisher . Copyright @ 2022 Malila, Sanpinit , Thongda, Jandamook, Srimarut , Phasuk and Kunhareang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owne r (s ) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice . No use, distribution or reproduction is permitted which does not comply with these terms.
确定








还剩12页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《商业肉鸡、本地土鸡和杂交鸡鸡胸肉中脂肪和蛋白质》,该方案主要用于畜禽肉及副产品中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《商业肉鸡、本地土鸡和杂交鸡鸡胸肉中脂肪和蛋白质》用到的仪器有格哈特全自动超级总脂肪测定系统HT6+SOX416、格哈特杜马斯定氮仪DT N Pro、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多