方案详情
文
采用LaVision公司的DaVis软件,FASTCAM SA3 120K 单色高速相机(Photron,Tokyo, Japan),1WLED光源,构成一套显微粒子成像测速系统。测量了砂眼蛤蜊悬浮喂养时4mmX4mm视场区域的流动速度矢量场分布。
方案详情

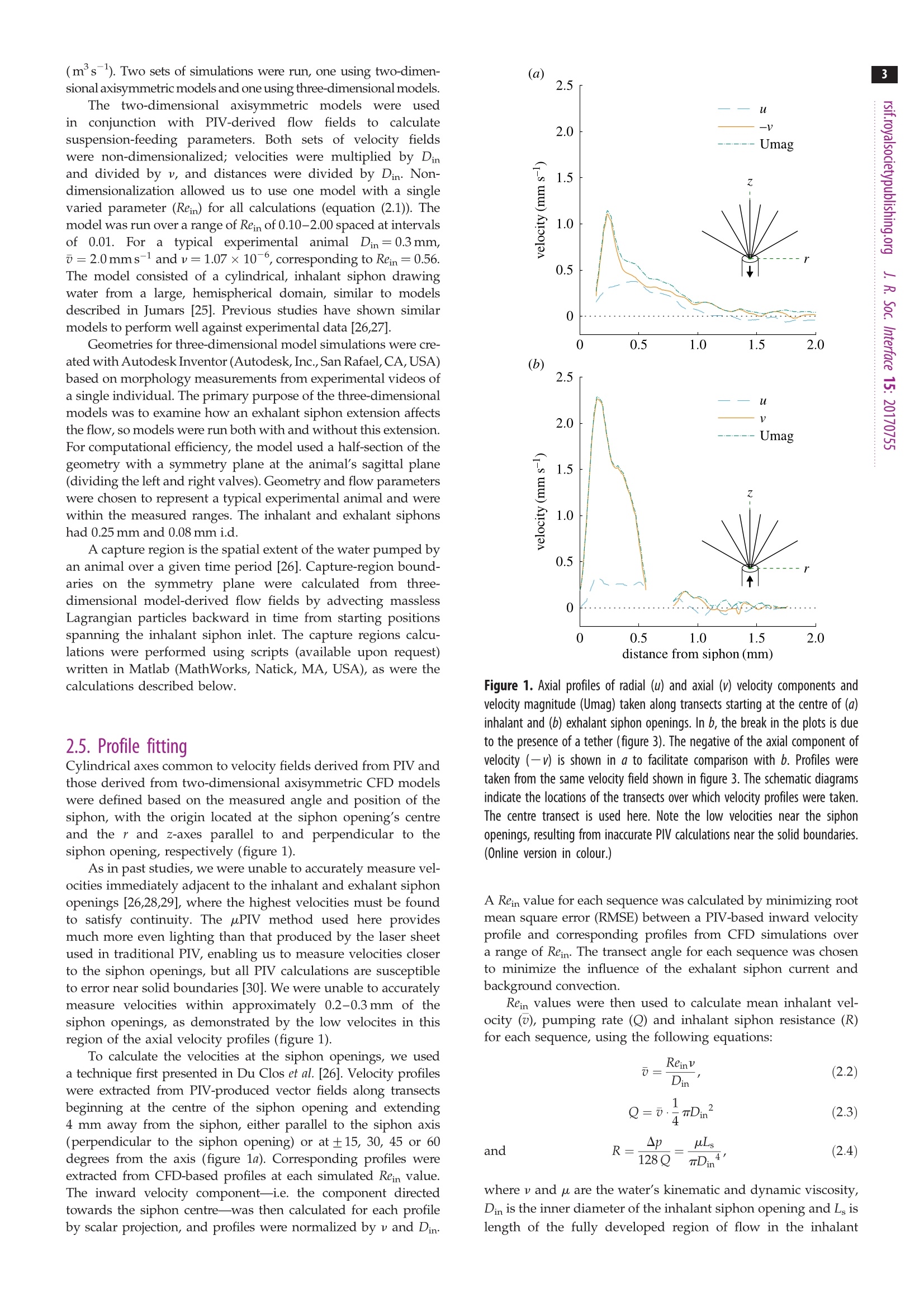
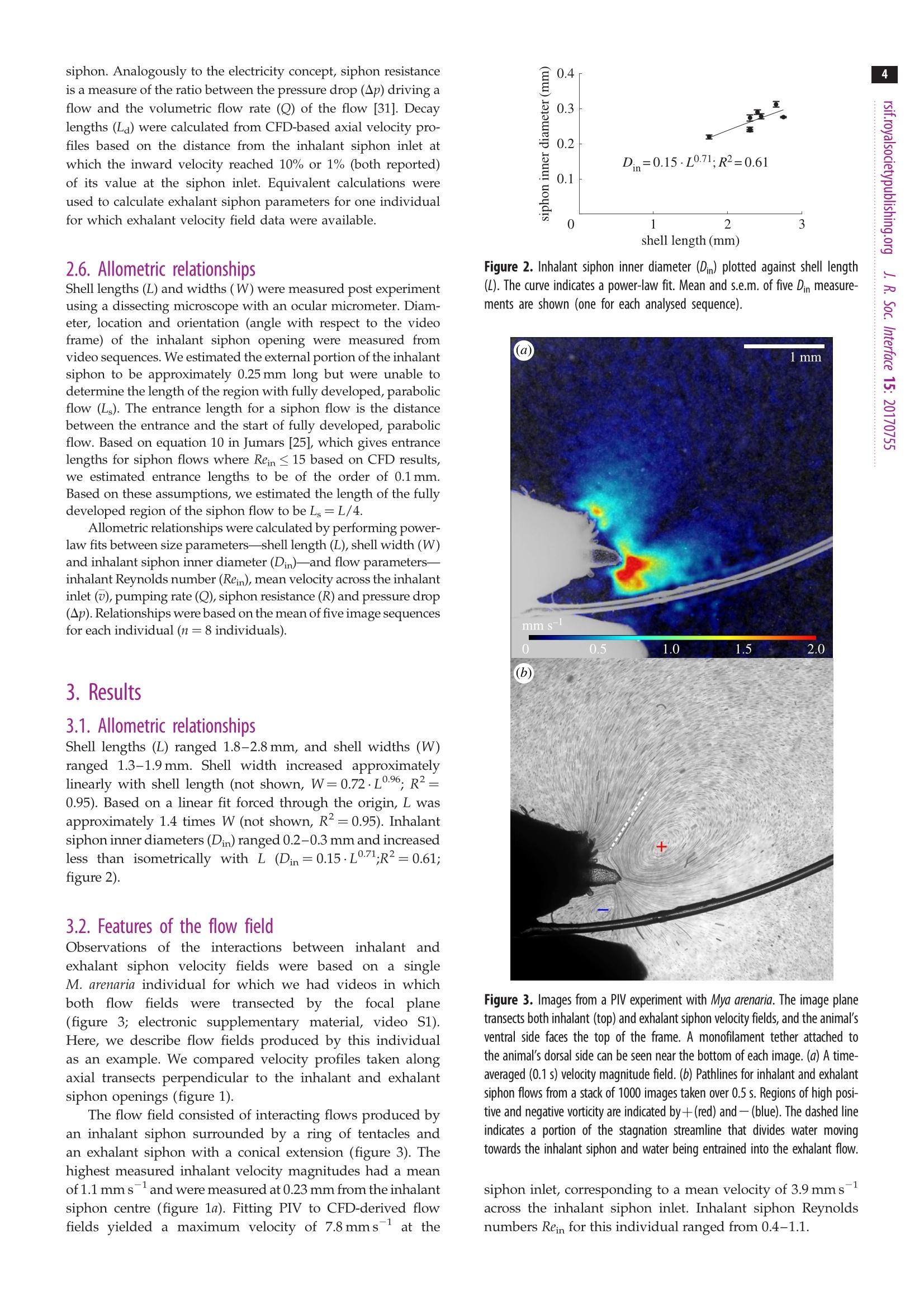
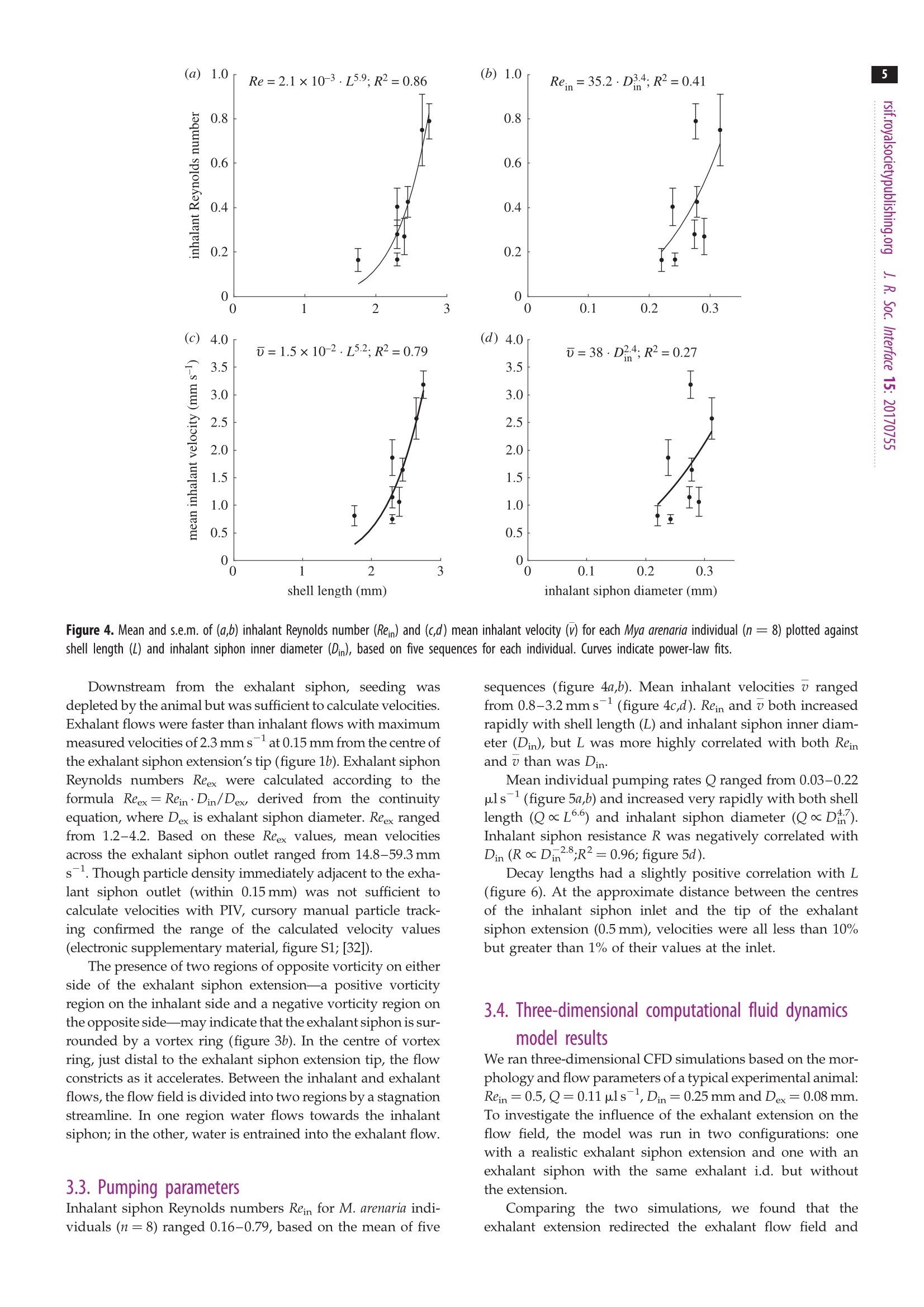
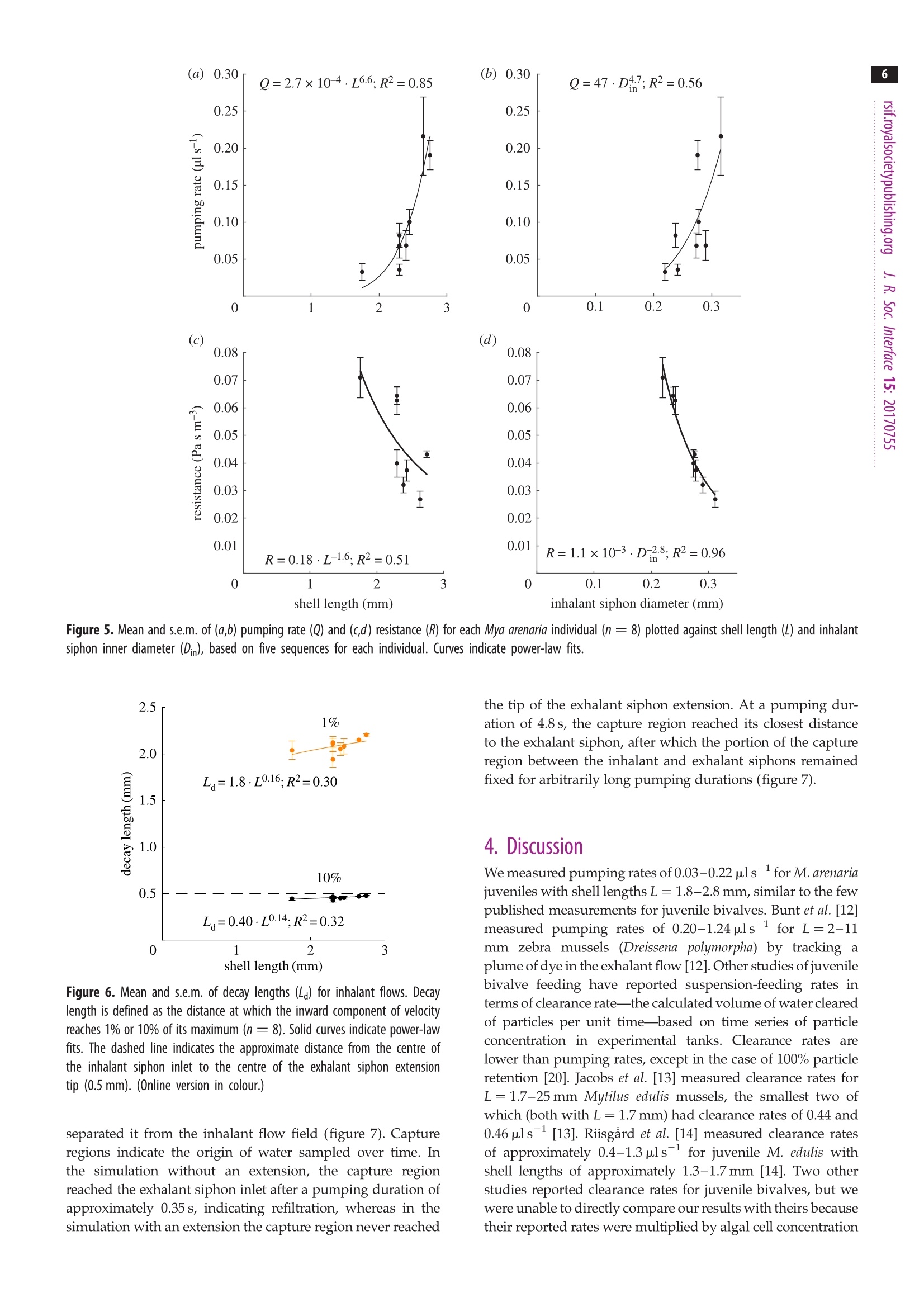
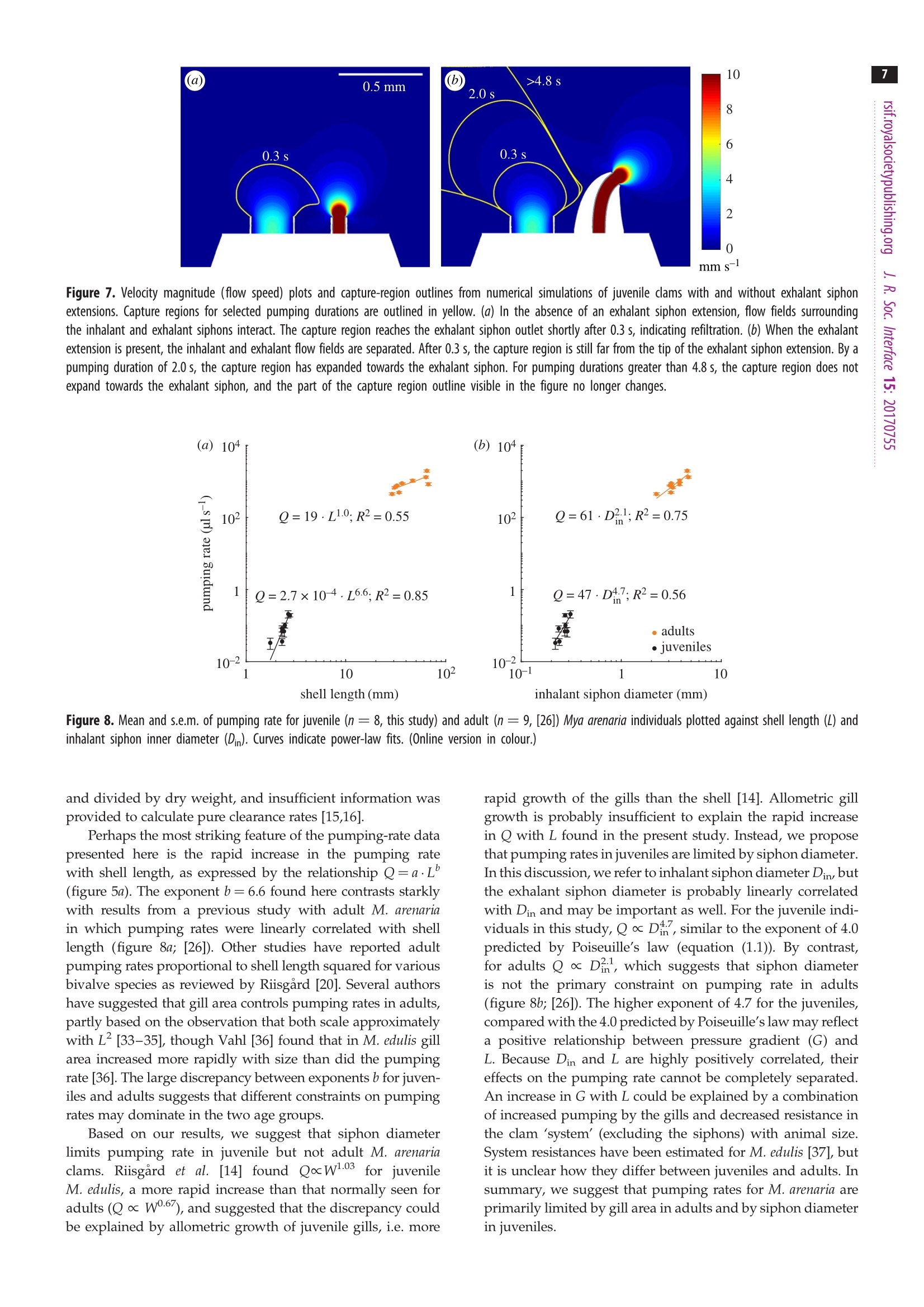
3 4 C 2018 The Author(s) Published by the Royal Society. All rights reserved. INTERFACE rsif.royalsocietypublishing.org ResearchCheck forupdates Cite this article: Du Clos KT, Jiang H. 2018 Overcoming hydrodynamic challengesin suspension feeding by juvenile Mya arenariaclams.J. R. Soc. Interface 15: 20170755.http://dx.doi.org/10.1098/rsif.2017.0755 Keywords: Present address: Integrative BiologyDepartment, University of South Florida, 4202E. Fowler Avenue, Tampa, FL 33620-9951, USA . Electronic supplementary material is availableonline at http://dx.doi.org/10.6084/m9.figshare.c.3969024. THE ROYAL SOCIETYPUBLISHING Overcoming hydrodynamic challengesin suspension feeding by juvenileMya arenaria clams Kevin T. Du Closltand Houshuo Jiang? -Darling Marine Center, School of Marine Sciences, University of Maine, 193 Clarks Cove Road, Walpole,ME 04573-3307, USA -Applied Ocean Physics and Engineering Department, Woods Hole Oceanographic Institution,266 Woods Hole Road, Woods Hole, MA 02543-1535, USA KTD, 0000-0002-3017-7777 We present some of the few suspension-feeding measurements and to ourknowledge the first velocity-field measurements for early post-settlementjuvenile bivalve clams. We verify and extend our experimental results withnumerical simulations. For 1.8-2.8 mm shell length Mya arenaria clams,pump-ing rates ranged 0.03-0.22 uls-, inhalant siphon Reynolds numbers (Re)ranged 0.16-0.79 and mean inhalant velocities ranged 0.8-3.2mms.Owing to the low Re at which they pump and the small diameters of theirsiphons, juvenile clams are subject to unique hydrodynamic challenges,including high siphon resistance and susceptibility to refiltration. At leastthree features of juvenile clam siphons differentiate them from those ofadults-shorter inhalant siphon length, a more rapid increase in inhalantsiphon diameter with shell length, and the presence of a prominent exhalantsiphon extension. These features are probably adaptations to the challengesof suspension feeding at low Re. 1. Introduction Mya arenaria clams are infaunal marine suspension feeders. Owing to their highsuspension-feeding rates and often high population densities, M. arenaria pro-vide an important link between benthic and pelagic ecosystems. In areas ofthe Baltic Sea, for example, M. arenaria have been shown to process the entirewater column in less than 1 day [1]. They also support valuable fisheries [2]. The juvenile stage of bivalve development has received less research attentionthan either the adult or larval stages. The early post-settlement juvenile stage ofM. arenaria development, which has been called the 'swimming-crawling'phase, lasts two to five weeks and precedes eventual burrowing into sediments[2]. Juvenile M. arenaria initially settle onto rocks and macroalgae where theymay remain attached by byssal threads until they have reached a shell length ofapproximately 7 mm before detaching, sinking and burrowing into the sedimentbelow [3]. The early post-settlement stage is a crucial one for M. arenaria. Post-settlementprocesses, including predation and hydrodynamic transport, may be as impor-tant as larval transport and settlement in determining distributions of adultpopulations [4]. Juvenile M. arenaria grow quickly relative to size. Under optimalconditions, an M. arenaria individual with a shell length of 2.6 mm clam growsapproximately 4 mm per month [5]. Results from a series of growth experimentswith juvenile M. arenaria from a single spawning event suggest that initial rapidgrowth by some individuals allows them to outcompete smaller individuals, apositive feedback that leads to a skewed size distribution [5]. Rapid growthmay also help juvenile M. arenaria avoid size-specific predation [6,7]. For example,the shrimp Crangon crangon feeds only on M. arenaria up to a shell length of 3 mm[8]. Predation is often the primary cause of mortality for juvenile M. arenaria [9,10],and mortality rates as high as 89% per year [9] and 77% per month [11] have been reported. Because of the energy required for their rapidgrowth, suspension feeding rates are particularly importantfor juvenile clams. These rates are difficult to measure, how-ever, so despite their importance they have rarely beenreported (but see [12-16]) and to our knowledge only oncefor M. arenaria [16]. Suspension feeding poses distinct hydrodynamic chal-lenges for juvenile M. arenaria and other small, siphonatesuspension feeders. One challenge results from the smalldiameters of a juvenile M. arenaria's siphons. According toPoiseuille’s equation for well-developed, laminar flow througha pipe or siphon, where Q is the volumetric flow rate through the siphon in cubicmetres per second, u is the dynamic viscosity of the fluid (Pa s),D is the inner diameter of the inhalant or exhalant siphon (Din orDex) in metres and G is the pressure gradient along the length ofthe siphon(Pa m-)-i.e. the pressure drop (Ap) divided by thesiphon length (Ls) [17,18]. Volumetric flow rate (Q) through asiphon is a product of the fourth power of siphon inner diameter(D), so suspension feeding rates may be constrained in suspen-sion feeders with small siphon diameters. At steady state, Apis equal to the viscous energy dissipation per unit volume[19, pp. 118,119]. Thus, for a juvenile M. arenaria individualwith shell length and inhalant siphon diameter one tenth thoseof an adult to pump at a flow rate one hundredth that of anadult (as predicted by a scaling of Q=L, where L is shelllength [20]), it would need to produce a pressure gradient 100times as strong to balance viscous dissipation. Entrance-regioneffects further reduce flow rates [21]. Another challenge for juvenile M. arenaria is avoiding refil-tration, pumping particle-depleted exhalant water in throughthe inhalant siphon. Because of its fused siphons, M. arenaria’sinhalant and exhalant siphon openings are adjacent. M. arenariaretains particles 4 um and larger with nearly 100% efficiency[22], so pumping exhaled water in through inhalant siphonwastes energy, regardless of the particle content of the waterabove the animal. In well-mixed water, refiltration at the popu-lation scale is much less important (i.e. bivalves will be lessaffected by neighbours upstream), but refiltration at the indi-vidual scale still occurs if exhaled water enters the inhalantsiphon, regardless of the initial particle concentration of thewater. Adult M. arenaria produce strong, coherent exhalantjets that carry depleted water away from the inhalant siphoninlet. Juvenile M. arenaria, however, produce exhalant jetswith much lower inertia (i.e. lower Re), so they must rely onother mechanisms to separate inhalant and exhalant flows. Inthis study, we measure the flow fields produced by suspensionfeeding juvenile M. arenaria and examine how these flow fieldsdiffer from those produced by adults to better understand howjuvenile M. arenaria overcome the hydrodynamic challengesresulting from their size. 2. Material and methods 2.1. Animals Juvenile Mya arenaria clams (n =8; shell length L=1.8-2.8mm),approximately 2 months post-settlement, were obtained from theDowneast Institute (Beals, ME, USA) and transferred to WoodsHole Oceanographic Institution (Woods Hole, MA, USA) where they were maintained in a recirculating seawater tank through-out the course of the experiments, which were conducted over3 days. Actively pumping individuals were haphazardly selectedfor experiments andgently separated from aggregations ifnecessary. 2.2. Video capture Experiments were performed in a glass tank with inner dimen-sions 44×34.5×97 mm (length x width × depth). The tank sizewas large enough to have negligible wall effects. The tank wasfilled to approximately 100ml with filtered seawater (25 umfilter,17-19C, practical salinity 33) seeded with 3 um polystyrenebeads (Polysciences, Inc., Warrington,PA,USA). Seawater densityand viscosity were calculated for each individual based on thewater temperature during the experiments with that individual.Individual clams were positioned on a circular stage 20 ×4 mm(heightxdiameter) centred in the tank. Videos of suspension feeding were captured at 2000 fps using aFASTCAM SA3 120K monochrome high-speed camera (Photron,Tokyo, Japan) fitted with an infinity-corrected, long-working-distance microscope objective (4×18.5 mm working distance)plus a 200 mm focal length objective lens. The lens combinationprovided a4×4mm (1024×1024px) field of view. This opticalset-up was advantageous for achieving sharp imaging under lowillumination. The illumination used was a collimated 1 W LEDwhite light source, with part of the collimated beam blocked toform a beam cross-sectional area only slightly larger than thefield of view. A similar optical set-up has been used to observe pro-tist swimming behaviours at even higher magnification under lowillumination [23]. The focal depth of the present high-magnifi-cation optical set-up was thin (less than 100 um), and thereforethe brightfield imaging of particles along the thin focal planewas used for micro particle image velocimetry (uPIV) analysis.This uPIV approach has previously been implemented by a differ-ent optical set-up that used direct projection with only a finitemicroscope objective but required much higher illumination [24]. 2.3. Vector field calculation For each M. arenaria individual, five 200-frame video sequences(0.1 s) were selected and imported into DaVis (LaVision, Goettingen,Germany) for PIV processing. Only videos in which the individualwas feeding for the entire 0.1s were used. For each sequence,frames were intensity inverted, and a two-pixel sliding backgroundfilter was applied. Then, an intensity threshold-based mask wasapplied to exclude the animal and the stage from vector calcu-lations. Vector fields were calculated in single-frame mode usingone pass with 64×64px interrogation windows (50%overlap) fol-lowed by four passes with 32×32 px windows (75% overlap).Calculated vector fields were time averaged and exported forfurther processing. Inhalant siphon position and diameter wererecorded for each video sequence. 2.4. Numerical models Computational fluid dynamics (CFD) model simulations wereperformed to further characterize the relevant flow fields. All simu-lations were carried out in COMSOL Multiphysics (COMSOL Inc.,Burlington, MA, USA), using the PARDISO solver and the BDFtime-stepping method. Initial simulations were performed toensure mesh independence. The key dimensionless parameter forthe model is inhalant siphon Reynolds number: wherev is the mean velocity across a cross section of the inhalantsiphon in metres per second,Din is the inhalant siphon inner diam-eter (i.d.) in metres and v = u/pis the water's kinematic viscosity The two-dimensional axisymmetric modelswere usedincconjunction withPIV-derived1 flow fieldsto calculatesuspension-feeding parameters. Both sets of velocity fieldswere non-dimensionalized; velocities were multiplied by Dinand divided by v, and distances were divided by Din. Non-dimensionalization allowed us to use one model with a singlevaried parameter (Rein) for all calculations (equation (2.1)). Themodel was run over a range of Rein of 0.10-2.00 spaced at intervalsof 0.01. For a ttypical experimental animalDin=0.3mm,o=2.0mms-l and v=1.07×10-, corresponding to Rein= 0.56.The model consisted of a cylindrical, inhalant siphon drawingwater from a large, hemispherical domain, similar to modelsdescribed in Jumars [25]. Previous studies have shown similarmodels to perform well against experimental data [26,27]. Geometries for three-dimensional model simulations were cre-ated with Autodesk Inventor (Autodesk, Inc.,San Rafael, CA, USA)based on morphology measurements from experimental videos ofa single individual. The primary purpose of the three-dimensionalmodels was to examine how an exhalant siphon extension affectsthe flow, so models were run both with and without this extension.For computational efficiency, the model used a half-section of thegeometry with a symmetry plane at the animal’s sagittal plane(dividing the left and right valves). Geometry and flow parameterswere chosen to represent a typical experimental animal and werewithin the measured ranges. The inhalant and exhalant siphonshad 0.25 mm and 0.08 mm i.d. A capture region is the spatial extent of the water pumped byan animal over a given time period [26]. Capture-region bound-aries on the symmetry plane were calculated from three-dimensional model-derived flow fields by advecting masslessLagrangian particles backward in time from starting positionsspanning the inhalant siphon inlet. The capture regions calcu-lations were performed using scripts (available upon request)written in Matlab (MathWorks, Natick,MA, USA), as were thecalculations described below. 2.5. Profile fitting Cylindrical axes common to velocity fields derived from PIV andthose derived from two-dimensional axisymmetric CFD modelswere defined based on the measured angle and position of thesiphon, with the origin located at the siphon opening’s centreand the r and z-axes parallel to and perpendicular to thesiphon opening, respectively (figure 1). As in past studies, we were unable to accurately measure vel-ocities immediately adjacent to the inhalant and exhalant siphonopenings [26,28,29], where the highest velocities must be foundto satisfy continuity. The uPIV method used here providesmuch more even lighting than that produced by the laser sheetused in traditional PIV, enabling us to measure velocities closerto the siphon openings, but all PIV calculations are susceptibleto error near solid boundaries [30]. We were unable to accuratelymeasure velocities within approximately 0.2-0.3mm of thesiphon openings, as demonstrated by the low velocites in thisregion of the axial velocity profiles (figure 1). To calculate the velocities at the siphon openings, we useda technique first presented in Du Clos et al. [26]. Velocity profileswere extracted from PIV-produced vector fields along transectsbeginning at the centre of the siphon opening and extending4 mm away from the siphon, either parallel to the siphon axis(perpendicular to the siphon opening) or at ±15, 30, 45 or 60degrees from the axis (figure 1a).Corresponding profiles wereextracted from CFD-based profiles at each simulated Rein value.The inward velocity component-i.e. the component directedtowards the siphon centre-was then calculated for each profileby scalar projection, and profiles were normalized by v and Din distance from siphon (mm) Figure 1. Axial profiles of radial (u) and axial(v) velocity components andvelocity magnitude (Umag) taken along transects starting at the centre of(a)inhalant and (b) exhalant siphon openings. In b, the break in the plots is dueto the presence of a tether (figure 3). The negative of the axial component ofvelocity (-V) is shown in a to facilitate comparison with b. Profiles weretaken from the same velocity field shown in figure 3. The schematic diagramsindicate the locations of the transects over which velocity profiles were taken.The centre transect is used here. Note the low velocities near the siphonopenings, resulting from inaccurate PIV calculations near the solid boundaries.(Online version in colour.) A Rein value for each sequence was calculated by minimizing rootmean square error (RMSE) between a PIV-based inward velocityprofile and corresponding profiles from CFD simulations overa range of Rein. The transect angle for each sequence was chosento minimize the influence of the exhalant siphon current andbackground convection. Rein values were then used to calculate mean inhalant vel-ocity (v), pumping rate (Q) and inhalant siphon resistance (R)for each sequence, using the following equations: and where v and uare the water’s kinematic and dynamic viscosity,Din is the inner diameter of the inhalant siphon opening and L islength of the fully developed region of flow in the inhalant siphon. Analogously to the electricity concept, siphon resistanceis a measure of the ratio between the pressure drop (Ap) driving aflow and the volumetric flow rate (Q) of the flow [31]. Decaylengths (La) were calculated from CFD-based axial velocity pro-files based on the distance from the inhalant siphon inlet atwhich the inward velocity reached 10% or 1% (both reported)of its value at the siphon inlet. Equivalent calculations wereused to calculate exhalant siphon parameters for one individualfor which exhalant velocity field data were available. 2.6. Allometric relationships Shell lengths (L) and widths (W) were measured post experimentusing a dissecting microscope with an ocular micrometer. Diam-eter, location and orientation (angle with respect to the videoframe) of the inhalant siphon opening were measured fromvideo sequences. We estimated the external portion of the inhalantsiphon to be approximately 0.25 mm long but were unable todetermine the length of the region with fully developed,parabolicflow (Ls). The entrance length for a siphon flow is the distancebetween the entrance and the start of fully developed, parabolicflow. Based on equation 10 in Jumars [25], which gives entrancelengths for siphon flows where Rein ≤ 15 based on CFD results,we estimated entrance lengths to be of the order of 0.1 mm.Based on these assumptions, we estimated the length of the fullydeveloped region of the siphon flow to be Ls=L/4. Allometric relationships were calculated by performing power-law fits between size parameters-shell length (L), shell width (W)and inhalant siphon inner diameter (Din)-and flow parameters-inhalant Reynolds number (Rein), mean velocity across the inhalantinlet (V),pumping rate (Q), siphon resistance (R) and pressure drop(Ap). Relationships were based on the mean of five image sequencesfor each individual (n=8individuals). 3. Results 3.1. Allometric relationships Shell lengths (L) ranged 1.8-2.8 mm, and shell widths (W)ranged 1.3-1.9mm. Shell width increased approximatelylinearly with shell length (not shown, W=0.72·L0.96, R2=0.95). Based on a linear fit forced through the origin, L wasapproximately 1.4 times W (not shown, R²=0.95). Inhalantsiphon inner diameters (Din) ranged 0.2-0.3 mm and increasedless than isometrically with L (Din=0.15·L;R²=0.61;figure 2). 3.2. Features of the flow field Observations of the interactions between inhalant andexhalant siphon velocity fields were based on a singleM. arenaria individual for which we had videos in whichboth flow 1fields weretransectedttby trthe focal plane(figure 3; electronic supplementary material, video S1).Here, we describe flow fields produced by this individualas an example. We compared velocity profiles taken alongaxial transects perpendicular to the inhalant and exhalantsiphon openings (figure 1). The flow field consisted of interacting flows produced byan inhalant siphon surrounded by a ring of tentacles andan exhalant siphon with a conical extension (figure 3). Thehighest measured inhalant velocity magnitudes had a meanof 1.1 mms-1 and were measured at 0.23 mm from the inhalantsiphon centre (figure 1a). Fitting PIV to CFD-derived flowfields yielded a maximum velocity of 7.8 mmsat the Figure 2. Inhalant siphon inner diameter (Din) plotted against shell length(L). The curve indicates a power-law fit. Mean and s.e.m. of five Din measure-ments are shown (one for each analysed sequence). 1mm Figure 3. Images from a PIV experiment with Mya arenaria. The image planetransects both inhalant (top) and exhalant siphon velocity fields, and the animal'sventral side faces the top of the frame. A monofilament tether attached tothe animal's dorsal side can be seen near the bottom of each image. (a) A time-averaged (0.1 s) velocity magnitude field. (b) Pathlines for inhalant and exhalantsiphon flows from a stack of 1000 images taken over 0.5 s. Regions of high posi-tive and negative vorticity are indicated by+(red) and-(blue). The dashed lineindicates a portion of the stagnation streamline that divides water movingtowards the inhalant siphon and water being entrained into the exhalant flow. ( siphon inlet, corresponding to a mean v elocity of 3.9 mmsacross the inhalant siphon inlet . Inhalan t siphon Reynoldsnumbers Rein f o r this individual ranged from 0.4-1.1. ) Downstream from the exhalant siphon, seeding wasdepleted by the animal but was sufficient to calculate velocities.Exhalant flows were faster than inhalant flows with maximummeasured velocities of 2.3 mmsat 0.15 mm from the centre ofthe exhalant siphon extension's tip (figure 1b). Exhalant siphonReynolds numbers Reex were calculated according to theformula Reex=Rein·Din/Dex, derived from the continuityequation, where Dex is exhalant siphon diameter. Reex rangedfrom 1.2-4.2. Based on these Reex values, mean velocitiesacross the exhalant siphon outlet ranged from 14.8-59.3 mms-. Though particle density immediately adjacent to the exha-lant siphon outlet (within 0.15mm) was not sufficient tocalculate velocities with PIV, cursory manual particle track-ing confirmed the range of the calculated velocity values(electronic supplementary material, figure S1; [32]). The presence of two regions of opposite vorticity on eitherside of the exhalant siphon extension-a positive vorticityregion on the inhalant side and a negative vorticity region onthe opposite side-may indicate that the exhalant siphon is sur-rounded by a vortex ring (figure 3b). In the centre of vortexring, just distal to the exhalant siphon extension tip, the flowconstricts as it accelerates. Between the inhalant and exhalantflows, the flow field is divided into two regions by a stagnationstreamline. In one region water flows towards the inhalantsiphon; in the other, water is entrained into the exhalant flow. sequences (figure 4a,b). Mean inhalant velocities v rangedfrom 0.8-3.2 mms-1(figure 4c,d). Rein and v both increasedrapidly with shell length (L) and inhalant siphon inner diam-eter (Din), but L was more highly correlated with both Reinand v than was Din. Mean individual pumping rates Q ranged from 0.03-0.22uls-(figure 5a,b) and increased very rapidly with both shelllength (QoL6.6) and inhalant siphon diameter (QcDh)Inhalant siphon resistance R was negatively correlated withDin (Ro D;R2= 0.96; figure 5d). Decay lengths had a slightly positive correlation with L(figure 6). At the approximate distance between the centresof the inhalant siphon inlet and the tip of the exhalantsiphon extension (0.5 mm), velocities were all less than 10%but greater than 1% of their values at the inlet. 3.4. Three-dimensional computational fluid dynamicsmodel results We ran three-dimensional CFD simulations based on the mor-phology and flow parameters of a typical experimental animal:Rein=0.5, Q=0.11 p.ls-1, Din=0.25 mm and Dex=0.08 mm.To investigate the influence of the exhalant extension on theflow field, the model was run in two configurations: onewith a realistic exhalant siphon extension and one with anexhalant siphon with the same exhalant i.d. but withoutthe extension. Comparing the two simulations, we found that theexhalant extension redirected the exhalant flow field and inhalant siphon diameter (mm) Figure 6. Mean and s.e.m. of decay lengths (La) for inhalant flows. Decaylength is defined as the distance at which the inward component of velocityreaches 1% or 10% of its maximum (n=8). Solid curves indicate power-lawfits. The dashed line indicates the approximate distance from the centre ofthe inhalant siphon inlet to the centre of the exhalant siphon extensiontip (0.5 mm). (Online version in colour.) separated it from the inhalant flow field (figure 7). Captureregions indicate the origin of water sampled over time. Inthe simulation without an extension, the capture regionreached the exhalant siphon inlet after a pumping duration ofapproximately 0.35s, indicating refiltration, whereas in thesimulation with an extension the capture region never reached the tip of the exhalant siphon extension. At a pumping dur-ation of 4.8 s, the capture region reached its closest distanceto the exhalant siphon, after which the portion of the captureregion between the inhalant and exhalant siphons remainedfixed for arbitrarily long pumping durations (figure 7). 4. Discussion We measured pumping rates of 0.03-0.22 plsfor M. arenariajuveniles with shell lengths L=1.8-2.8 mm, similar to the fewpublished measurements for juvenile bivalves. Bunt et al.[12]measured pumping rates of 0.20-1.24 plsfor L=2-11mm zebra mussels (Dreissena polymorpha) by tracking aplume of dye in the exhalant flow [12]. Other studies of juvenilebivalve feeding have reported suspension-feeding rates interms of clearance rate-the calculated volume of water clearedof particles per unit time—based on time series of particleconcentration in experimental tanks. Clearance rates arelower than pumping rates, except in the case of 100% particleretention [20]. Jacobs et al. [13] measured clearance rates forL=1.7-25mm Mytilus edulis mussels, the smallest two ofwhich (both with L=1.7 mm) had clearance rates of 0.44 and0.46 uls-[13]. Riisgard et al. [14] measured clearance ratesof approximately 0.4-1.3uls-for juvenile M. edulis withshell lengths of approximately 1.3-1.7 mm[14]. Two otherstudies reported clearance rates for juvenile bivalves, but wewere unable to directly compare our results with theirs becausetheir reported rates were multiplied by algal cell concentration 7 Figure 7. Velocity magnitude (flow speed) plots and capture-region outlines from numerical simulations of juvenile dlams with and without exhalant siphonextensions. Capture regions for selected pumping durations are outlined in yellow.(a) In the absence of an exhalant siphon extension, flow fields surroundingthe inhalant and exhalant siphons interact. The capture region reaches the exhalant siphon outlet shortly after 0.3 s, indicating refiltration. (b) When the exhalantextension is present, the inhalant and exhalant flow fields are separated. After 0.3 s, the capture region is still far from the tip of the exhalant siphon extension. By apumping duration of 2.0 s, the capture region has expanded towards the exhalant siphon. For pumping durations greater than 4.8 s, the capture region does notexpand towards the exhalant siphon, and the part of the capture region outline visible in the figure no longer changes. Figure 8. Mean and s.e.m. of pumping rate for juvenile (n=8, this study) and adult (n= 9, [26]) Mya arenaria individuals plotted against shell length (L) andinhalant siphon inner diameter (Dn). Curves indicate power-law fits. (Online version in colour.) and divided by dry weight, and insufficient information wasprovided to calculate pure clearance rates [15,16]. Perhaps the most striking feature of the pumping-rate datapresented here is the rapid increase in the pumping ratewith shell length, as expressed by the relationship Q=a·L’(figure 5a). The exponent b=6.6 found here contrasts starklywith results from a previous study with adult M. arenariain which pumping rates were linearly correlated with shelllength (figure 8a;[26]). Other studies have reported adultpumping rates proportional to shell length squared for variousbivalve species as reviewed by Riisgard [20]. Several authorshave suggested that gill area controls pumping rates in adults,partly based on the observation that both scale approximatelywith L²[33-35], though Vahl [36] found that in M. edulis gillarea increased more rapidly with size than did the pumpingrate [36]. The large discrepancy between exponents b for juven-iles and adults suggests that different constraints on pumpingrates may dominate in the two age groups. Based on our results, we suggest that siphon diameterlimits pumping rate in juvenile but not adult M. arenariaclams. Riisgard et al. [14] found QoW103 for juvenileM. edulis, a more rapid increase than that normally seen foradults (W0.67),and suggested that the discrepancy couldbe explained by allometric growth of juvenile gills, i.e. more rapid growth of the gills than the shell [14]. Allometric gillgrowth is probably insufficient to explain the rapid increasein Q with L found in the present study. Instead,we proposethat pumping rates in juveniles are limited by siphon diameter.In this discussion, we refer to inhalant siphon diameter Din, butthe exhalant siphon diameter is probably linearly correlatedwith Din and may be important as well. For the juvenile indi-viduals in this study, Qo Din, similar to the exponent of 4.0predicted by Poiseuille's law (equation (1.1)). By contrast,for adults Qo Din, which suggests that siphon diameteris not the primary constraint on pumping rate in adults(figure 8b; [26]). The higher exponent of 4.7 for the juveniles,compared with the 4.0 predicted by Poiseuille’s law may reflecta positive relationship between pressure gradient (G) andL. Because Din and L are highly positively correlated, theireffects on the pumping rate cannot be completely separated.An increase in G with L could be explained by a combinationof increased pumping by the gills and decreased resistance inthe clam ‘system’ (excluding the siphons) with animal size.System resistances have been estimated for M. edulis [37], butit is unclear how they differ between juveniles and adults.Insummary, we suggest that pumping rates for M. arenaria areprimarily limited by gill area in adults and by siphon diameterin juvenilesS.. Whereas Kellogg ([3], fig. 2 therein) depicts the juvenileM. arenaria siphons as essentially scaled-down versions oftheir adult counterparts, our observations suggest that juvenilesiphons are specifically adapted to the particular hydrodynamicchallenges of suspension feeding at low Re. Because of theirsmall diameters, juvenile clam siphons have high resistances.Siphon resistance decreases as a function of Dand increaseslinearly with siphon length (equation (2.3)). Adult M. arenariahave long siphons that may account for 40-50% of their bodyweights [38]; selection pressure to avoid predation by burrow-ing deeper may outweigh the increased resistance associatedwith longer siphons [39]. In contrast with adults, juvenileshave short siphons relative to their size. Having short siphonsallows juvenile clams to partially compensate for the high resist-ance resulting from their small siphon diameters. We would alsoexpect juvenile clams to reduce resistance by increasing theirsiphon diameters rapidly as they grow. Indeed, Din increasesmore rapidly with L for juveniles (DincL9.71; figure 2b) thanfor adults (Din oL9.55 [26]). Interestingly, however, siphondiameter increases less than isometrically with shell length,suggesting that siphon diameter may be limited by factorsother than siphon resistance, such as shell gape. Avoiding refiltration appears to pose a particular challengefor juvenile clams, particularly in the case of M. arenaria, whichhas adjacent inhalant and exhalant siphons. The exhalant jetsof adult M. arenaria operate at relatively high Re (approx.300-1000) [26], making them highly directional and coherent.They are also directed away from the inhalant siphon by a foldof tissue surrounding the exhalant siphon outlet. JuvenileM.arenaria are unable to produce highly directional jet currentsbecause they operate at much lower Re than adults, with vis-cous effects dominating advection. The prominent exhalantsiphon extension, which is seen in juvenile but not adultM. arenaria, appears to be one adaptation to this challenge(figure 3b). This structure has been documented in juvenileMercenaria mercenaria [40], but its function has received littleattention in the literature. Based on our measured and calcu-lated flow fields, the exhalant extension appears to limitrefiltration in two ways. First, the extension increases the dis-tance between the inhalant siphon inlet and exhalant siphonoutlet by approximately 0.5 mm. Based on calculated decaylengths, this separation distance is enough to decrease the inha-lant velocity to less than 10% of its value at the siphon inlet(figure 6). Second, because of its shape, the extension redirectsthe exhalant flow away from the inhalant siphon. Both the pathlines (figure 3b) and the capture-region calculations(figure 7b) indicate a stagnation streamline between the inha-lant and exhalant siphon flows. The presence of thisstagnation streamline suggests limited refiltration. Bivalve suspension feeding flows cover a wide range offlow regimes. Over the course of an individual M. arenariaclam’s life it may produce inhalant siphon flows with Re ran-ging from of the order of 0.1 to 100 and exhalant flowsranging from of the order of 1 to 1000. This remarkable tran-sition has received very little attention. Juvenile M. arenariaclams appear to have several unique features compared withadults that enable them to feed at low Re, including the afore-mentioned shorter inhalant siphon lengths, more rapidincreases in inhalant siphon diameter with shell length andprominent exhalant siphon extensions. The smallest, mostrecently settled juveniles may be unable to overcome thechallenges of suspension feeding at low Re. As recent settlers,many species of bivalves use a form of deposit feedingknown as pedal feeding before eventually switching to suspen-sion feeding [41,42]. Experiments with even more recentlysettled juvenile bivalves than those studied here may reveal aminimum size for siphonate suspension feeding. Additionalclearance-rate measurements for clams of intermediate sizemay clarify how suspension-feeding constraints change withanimal size. Data accessibility. Velocity field data from particle image velocimetry(PIV) experiments have been submitted to the Biological and Chemi-cal Oceanography Data Management Office (BCO-DMO) forarchiving (https://www.bco-dmo.org/dataset/713163). Processingcode is available from the corresponding author upon request. Authors’ contributions. K.T.D. conceived of the study, performed exper-iments, analysed data and drafted the manuscript. H.J. helped todesign experiments, provided expertise and equipment, and revisedthe manuscript. Both the authors gave their final approval forpublication. Competing interests. We declare we have no competing interests. Funding. K.T.D. was supported by a collaborative grant between thelaboratories of P. A. Jumars (NSF grant no. OCE-1260232) andJ. P. Crimaldi (NSF grant no. OCE-1260199). H.J. was supported byNSF grant nos. OCE-1433979 and IOS-1353937. Acknowledgements. Brian Beal, Kyle Pepperman and Cody Jourdet at theDowneast Institute in Beals, ME provided juvenile Mya arenaria clamsand care advice. Pascalle Jacobs provided clearance rate data for com-parison. Thanks are expressed to Peter Jumars and Damian Bradyand to the reviewers for helpful comments on the manuscript. ( .1. F orster S , Z ettler M L.2004 The capacity of the filter- f eeding b ivalve M ya ar e naria L. to af f ect w a tertransport in sandy b eds. M ar. B iol. 144,1183-1189. ( d oi:10.1007/s00227-003-1278-2) ) 2. Newell CR. 1991 The soft-shell dlam Mya arenaria(Linnaeus) in North America. In Estuarine andmarine bivalvemollusk culture (ed. W Menzel),pp. 1-10. Boca Raton, FL: CRC Press. 3. Kellogg JL. 1899 Observations on the life-history ofthe common clam, Mya arenaria. Bull. US FishComm. 19,193-202. 4. Bowen JE, Hunt HL. 2009 Settlement andrecruitment patterns of the soft-shell clam, Mya arenaria, on the northern shore of the Bay of Fundy,Canada. Estuaries. Coast 32, 758-772. (doi:10.1007/s12237-009-9151-2) ( 5. S tickney AP. 1964 Feeding and gro w th of juvenile soft- s hell dams, M ya a r enaria. Fish B ull. 63, 635-642. ) 6. Commito JA. 1982 Effects of Lunatia heros predationon the population dynamics of Mya arenaria andMacoma balthica in Maine, USA. Mar. Biol. 69,187-193.(doi:10.1007/BF00396898) 7. Gunther CP. 1992 Settlement and recruitment ofMya arenaria L. in the Wadden Sea. J. Exp. Mar.Biol. Ecol. 159, 203-215. (doi:10.1016/0022-0981(92)90037-B) 8. Moller P, Rosenberg R. 1983 Recruitment,abundance and production of Mya arenaria andCardium edule in marine shallow waters, westernSweden. Ophelia 22, 33-55. (doi:10.1080/00785326.1983.10427223) 9. Beal BF. 2006 Biotic and abiotic factors influencinggrowth and survival of wild and cultured individualsof the softshell dlam (Mya arenaria L.) in easternMaine. J. Shellfish Res. 25, 461-474.(doi:10.2983/0730-8000(2006)25[461:BAAFIG]2.0.C0;2) 10..FHunt HL, Mullineaux LS. 2002 The roles ofpredation and postlarval transport in recruitment ofthe soft shell dlam (Mya arenaria). Limnol. Oceanogr. 47, 151-164. (doi:10.4319/lo.2002.47.1.0151) ( 11. B B rousseau D J. 1 978 P opulation d ynamics o f t h esoft-shell clam Mya arenaria. Mar. Biol . 50 , 63-71. (doi:10.1007/BF00390542) ) ( 12 . B unt CM, Maclsaac H J, Sprules WG. 1993 Pumping r ates a nd projected fi l tering i m pacts of j uv e nilezebra m ussels (Dreissena polymorpha) i n w e stern L ake E r ie. C an.J . Fisheries A q uatic Sc i . 5 0 , 1 017-1022. ( d oi:10.1139/f93-117) ) ( 13 . Jacobs P, Troost K , Riegman R, Meer J. 2 015 Length- a n d weight-dependent clearance r ates of j uvenile m ussels (Mytilus e d ulis) onvarious p lanktonic p rey i t ems. H e lgolandMar. R es. 69, 1 01-112. ( d oi:10.1007/s10152- 014-0419-y) ) ( 14. Riisgard HU, R andlov A , K r istensen P S . 1 9 80 Ra t esof water processing, oxygen consumption a ndefficiency of particle retention in veligers and young p ost- m etamorphic My t ilus ed u lis. Op h elia 19 , 37-46. (doi:10.1080/00785326.1980.10425505) ) ( 15 . S humway S, C ucci T, Lesser M, Bo u rne N, B un t ing B. 1997 Particle clearance and selection in three species o f juvenile scallops. Aquaculture I nt. 5,89-99. (doi:10.1007/BF02764790) ) ( 16. L esser M P, S humway SE. 1993 Eff e cts of toxic dinoflagellates on clearance r ates and s urvival i n j uvenile bivalve molluscs. J . S h ellfish R es. 1 2, 377 - 381 . ) 17. Poiseuille JL. 1844 Recherches experimentales sur lemouvement des liquides dans les tubes de tres-petitsdiametres. Paris, France: Imprimerie Royale. 18.Sutera SP, Skalak R. 1993 The history of Poiseuille’slaw. Annu. Rev. Fluid Mech. 25, 1-20. (doi:10.1146/annurev.fl.25.010193.000245) 19. WiWlkes JO. 1999 Fluid mechanics for chemicalengineers. Englewood Cliffs, NJ: Prentice Hall. ( 20. R R iisqard HU. 2001 On m easurement of filtration r ates in bivalves: t he s t ony ro a d to reliable da t a: r eview and i n terpretation. Mar. Ecol. P r og. S e r. 211, 275-291. (doi:10.3354/meps211275) ) 21. LoLudon C, McCulloh K. 1999 Application of theHagen -Poiseuille equation to fluid feeding through ( short tubes. Ann . Entomol. Soc. A m .92 , 153-158. (doi:10.1093/aesa/92.1.153) ) ( 22 . Mo hlenberg F, Ri i sgard HU. 19 7 8 Eff i ciency o f p artidle r etention i n 1 3 s p ecies o f suspension f eeding b i valves. O phelia 17, 2 3 9-246. (do i :10. 1080/00785326.1978.10425487) ) ( 23. J J iang H , Johnson MD. 2 017 Jumping and o vercoming d if fusion l i mitation o f n u trient u p take i n the photosynthetic c iliate Mesodinium r ubrum.Limnol. Oceanogr. 62, 421-436.(doi:10.1002/ Ino.10432) ) ( 24. G emmell BJ, J i ang H, Buskey EJ. 2014 A n ew a pproach to m i cro-scale particle i m age ve l ocimetry (uPIV) for q uantifying f lows around free-swimming zooplankton.J. P lankton. R es. 3 6, 1 396-1401.(doi:10.1093/plankt/fbu067) ) ( 25. J umars P A . 2013 B o undary-trapped, i n halant siphon a nd d r ain flows: pipe e n try r e visited n umerically. Limnol. O ceanogr.: F luids Environ. 3, 1 -20 . (doi:10. 1215/21573689-2071927) ) ( 26. DuClos K T , Jones IT, Carrier TJ, B rady DC, J u mars PA . 2017 M odel-assisted measurements o f suspension- f eeding f l ow v e locities. J. Exp. B iol. 220, 2096-2107.(d o i:10.1242/jeb.147934) ) ( 27. T rue AC, C rimaldi JP. 2017 Hydrodynamics of viscous i nhalant f lows. P hys. Re v. E 9 5 , 0 53 107. (do i :10.1103/PhysRevE.95.053107) ) ( 28. 3 Frank DM, Ward JE, Shumway SE, Holohan BA, G ray C . 2008 Application o f particl e image v eloci m etry t o the s t udy o f suspension f eeding i n m a rine in v ertebrates. M ar . Fr e shw.Behav. Physiol. 41, 1-18. (doi:10.1080/ 10236240801896207) ) ( 29. Troost K, Stamhuis EJ, van Duren L A, Wolff WJ. 2009Feeding c u rrent characteristics o f t h reemorphologically different b ivalve suspension f eeders, C rassostrea g igas, Mytilus edulis and C e rastoderma e dule, i n relation t o f ood competition. Mar. Biol. 15 6 , 355-372.(d o i:10.1007/s00227-008-1088-7) ) ( 30. Kahler CJ, Scharnowski S, Cierpk a C . 2012 On theuncertainty o f d igital PIV and PTV n e ar wa l ls. E x p.Fluids 52, 1641-1656. (doi:10.1007/s00348-012- 1307-3) ) ( 31 . V V ogel S . 1994 Li f e in moving flu i ds: th e physical biology of flow, 2nd edn. Princeton, NJ: Princeton U niversity P ress. ) ( 32. . T T inevez J Y , P erry N, S c hindelin J , H oopes GM , R eynolds GD, L a plantine E, B e dnarek SY, Sh o rte SL, E liceiri KW. 20 1 7 TrackMate: a n open a n d extensible p lat f orm f or single-particle tracking . Methods 115,80-90.(d o i:10.1016/j.ymeth.2016.09.016) ) ( 33. M N eyhofer E. 1 985 Comparative pumping rates i n s uspension-feeding bivalves. M ar. B i ol. 8 5, 137-142. (doi:10.1007/BF00397432) ) ( 34. J ones HD, Richards OG, So u thern TA. 199 2 Gil l d imensions, w ater pumping rate and body s i ze i n the m ussel M y tilus ed u lis L. J.Exp. Ma r . Bio l . Eco l . 15 5 , 213-237. (doi: 1 0.1016/0022-0981(92)90064-H) ) ( 35. R R iisgard H U, S e erup DF. 20 0 3 Fil t ration ra t es in the s oft d lam Mya ar e naria: effects of t e mperature and b ody s i ze. S arsia 88,4 1 6-428. (doi:10.1080/ 00364820310003208) ) ( 36. Vahl 0. 1973 Pumping and oxygen cunsumption r ates o f M ytilus edulis L . o f different s izes. O phelia 12, 45-52. (doi: 1 0.1080/00785326.1973.1043 0 118) ) ( 37. J o rgensen CB. 1 986 The bivalve pump. Mar. E col.Prog. Ser. 34, 69-77. (doi:10.3354/meps034069) ) ( 38 . Zwarts L, W anink J. 1989 Siphon si z e an d bu r ying d epth in deposit- a nd s uspension-feeding b enthic bivalves. Mar. Biol . 100, 227-240. ( doi:10.1007/ BF00391963) ) ( 39 . Z Z aklan S D, Y denberg R. 1997 Th e bo d y size-burialdepth relationship i n the i nfaunal clam M ya a renaria.J. E xp. M ar. B iol. Ecol. 215, 1- 17. ( d oi:10.1016/S0022-0981(97)00021- X ) ) ( 40 . Carriker MR. 2 001 F unctional m orphology and b ehavior o f s h elled v e ligers and ea r ly juveniles. D e v.Aquaculture Fisheries Sci. 31 , 283-303. (doi:10. 1016/S0167-9309(01)80035-1) ) ( 41 . C C addy J. 1 969 Development o f mantle organs, feeding, and locomotion in postlarva l Macoma b althica ( L.) (Lamellibranchiata). Can. J. Z ool. 4 7, 609-617. ( d oi:10.1139/z69-105) ) ( 42. R R eid R , McMahon R, F o ighil D , F innigan R . 1992 A nterior i nhalant c urrents and pedal f eeding inbivalves. Velige r 35, 93-104. ) We present some of the few suspension-feeding measurements and to our knowledge the first velocity-field measurements for early post-settlement juvenile bivalve clams. We verify and extend our experimental results with numerical simulations. For 1.8–2.8mm shell length Mya arenaria clams, pumping rates ranged 0.03–0.22 ul/s, inhalant siphon Reynolds numbers (Re) ranged 0.16–0.79 and mean inhalant velocities ranged 0.8–3.2mm/s. Owing to the low Re at which they pump and the small diameters of their siphons, juvenile clams are subject to unique hydrodynamic challenges, including high siphon resistance and susceptibility to refiltration. At least three features of juvenile clam siphons differentiate them from those ofadults–shorter inhalant siphon length, a more rapid increase in inhalant siphon diameter with shell length, and the presence of a prominent exhalant siphon extension. These features are probably adaptations to the challenges of suspension feeding at low Re.
确定




还剩7页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《幼年砂眼蛤蜊中水流体速度矢量场检测方案(CCD相机)》,该方案主要用于其他中水流体速度矢量场检测,参考标准--,《幼年砂眼蛤蜊中水流体速度矢量场检测方案(CCD相机)》用到的仪器有LaVision HighSpeedStar 高帧频相机、显微粒子成像测速系统(Micro PIV)、LaVision DaVis 智能成像软件平台
推荐专场
CCD相机/影像CCD
更多
相关方案
更多
该厂商其他方案
更多