Understanding how the hydrodynamics of feeding, nutrient and gas exchange is affected by the morphology and orientation of jellyfish is important to distinguish their individual mechanisms of foraging. The differences between the currents generated by oblate medusa that rest on the ocean floor and the more commonly observed free-swimming medusa have previously not been explored. The pulsing kinematics and fluid flow around the upside-down jellyfish, Cassiopea spp., contained in laboratory aquaria is experimentally investigated using a combination of videography and digital particle image velocimetry measurements. The phase-averaged flow generated by bell pulsations is similar to a vertical jet, with induced flow velocities on the order of 1-10 mm/s. The bell margin is pushed downward during full contraction in a manner not previously observed in free-swimming medusae kinematics, due to the fact that the Cassiopea bell rests against a solid surface. This introduces a strong near-horizontal entrainment of the fluid toward the oral arms unlike the mostly upstream entrainment observed in free-swimming medusae. There is no evidence of the formation of a train of vortex rings as observed in oblate medusae exhibiting rowing propulsion. This is primarily due to the difference in the morphology of Cassiopea in which the oral arms extend to radial lengths greater than the maximum bell diameter. The resulting net fluid motion is not dominated by flow-reversal regions, suggesting that Cassiopea brings in new fluid with each bell pulse for the purposes of filter feeding, oxygen exchange and excretion.
方案详情

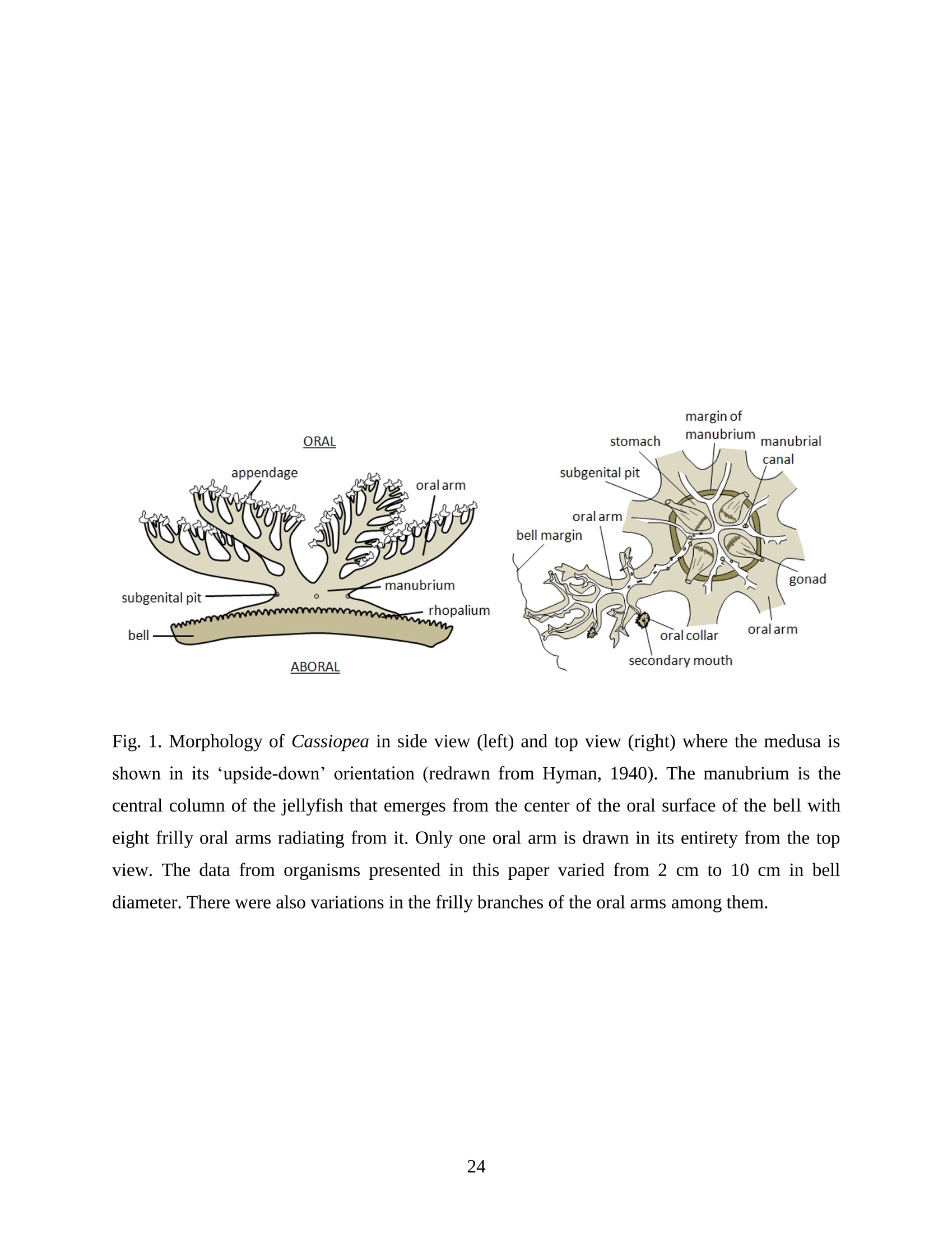
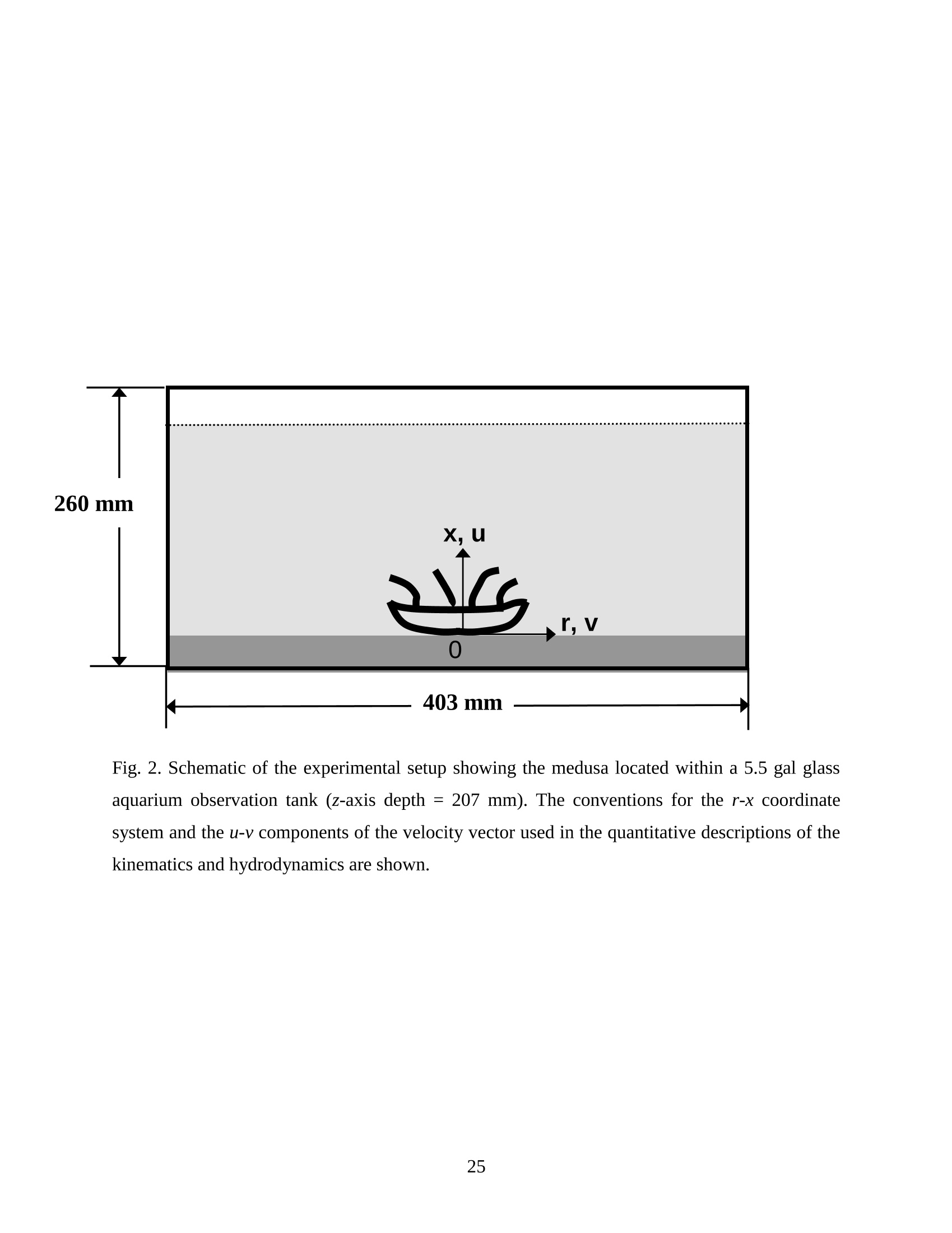
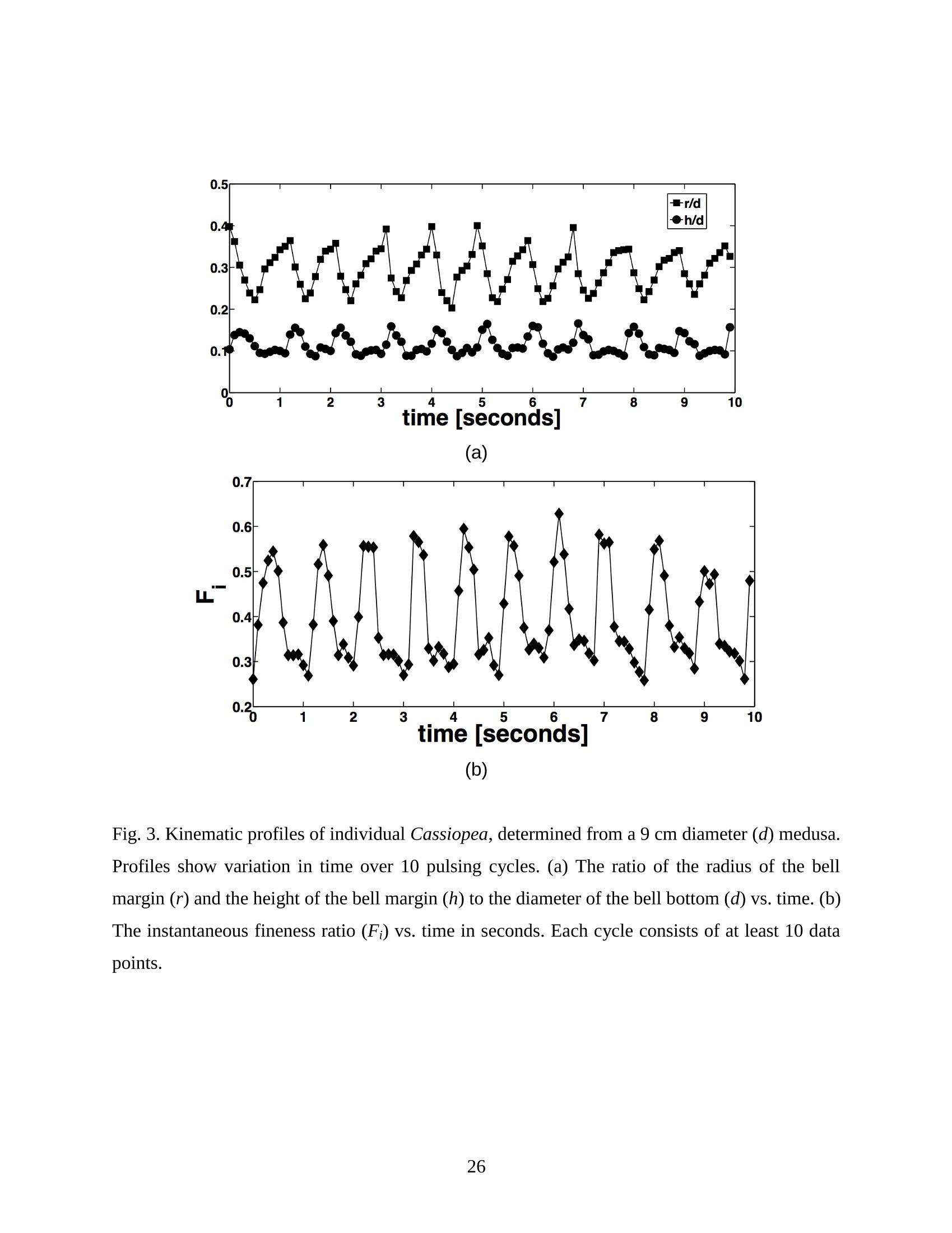
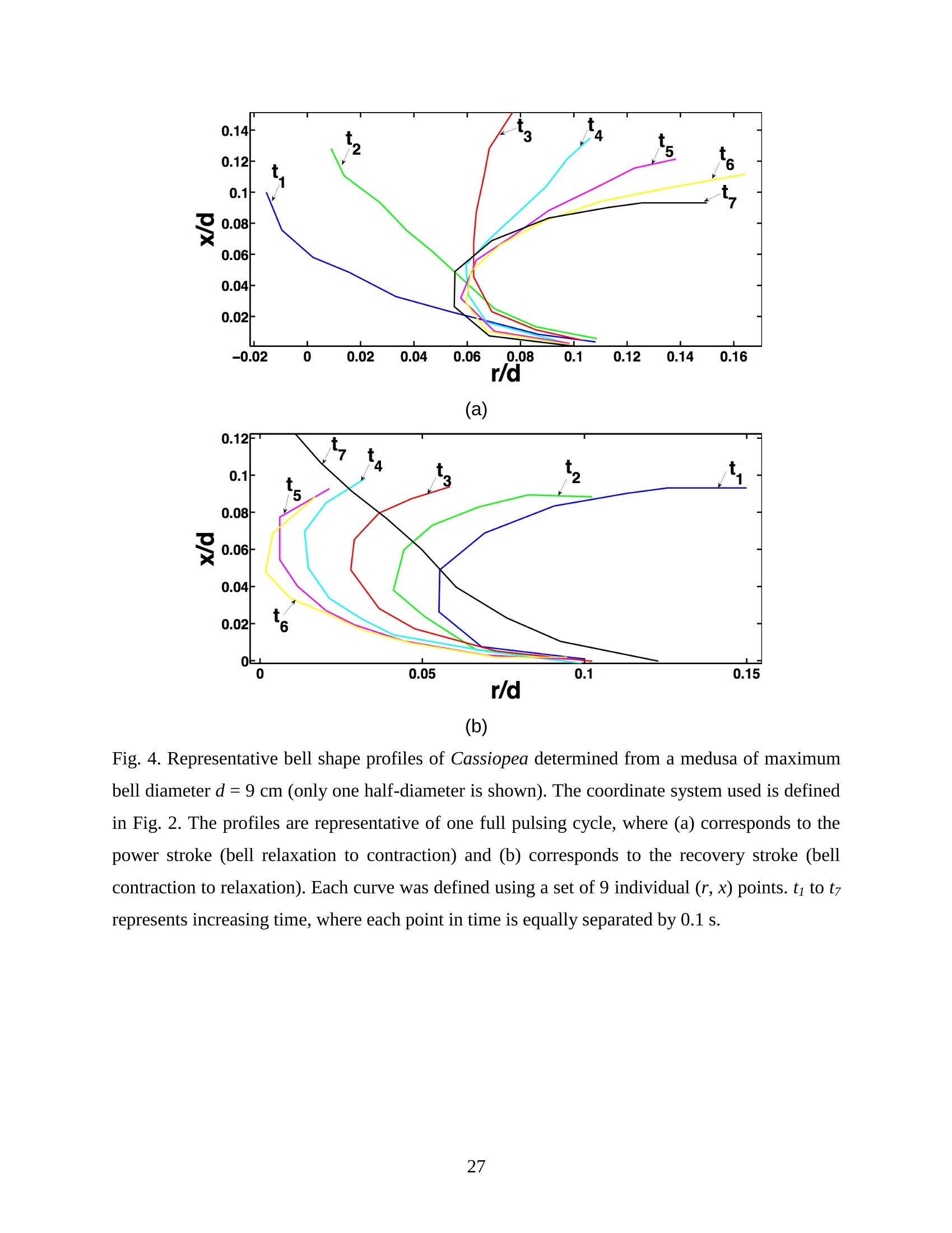
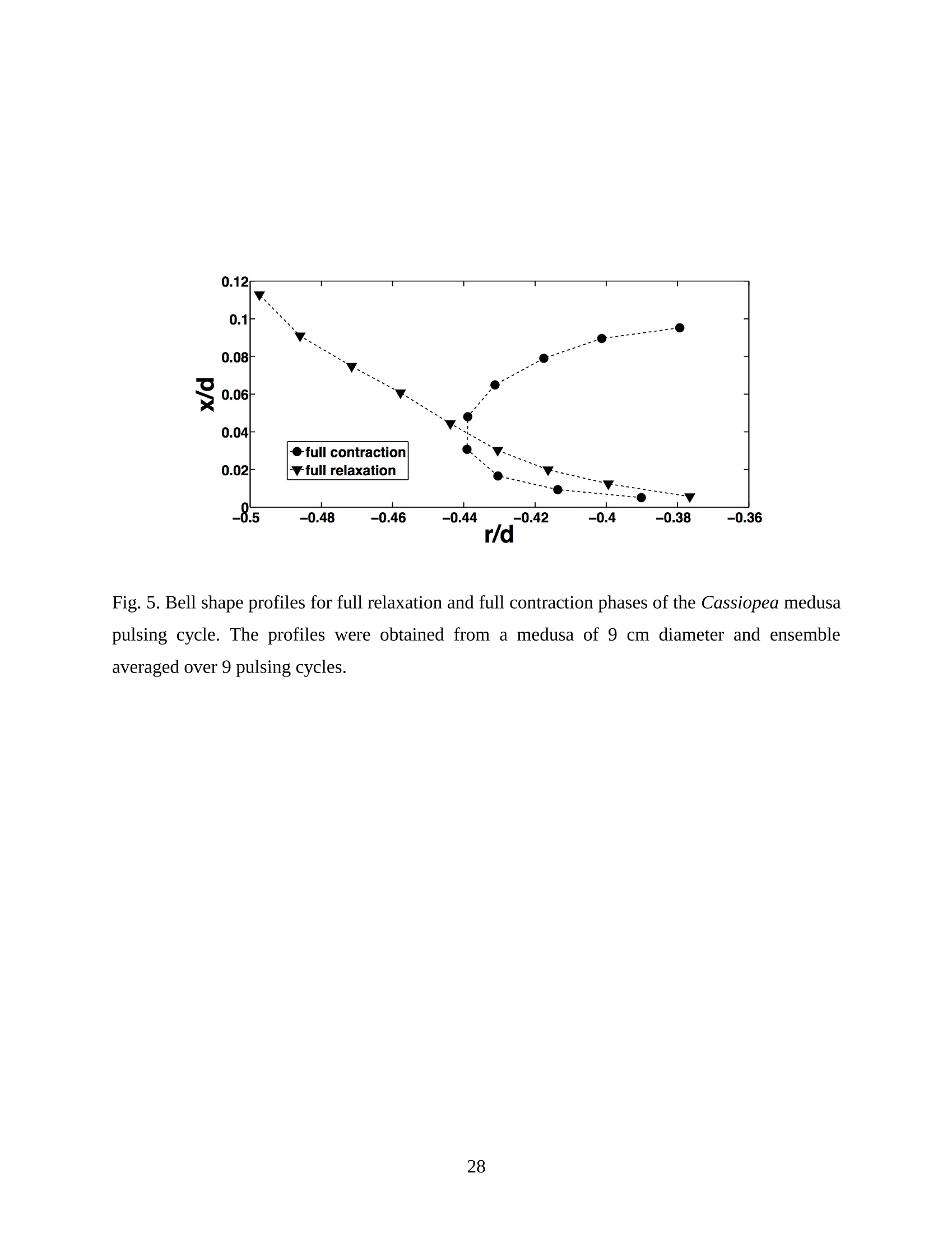
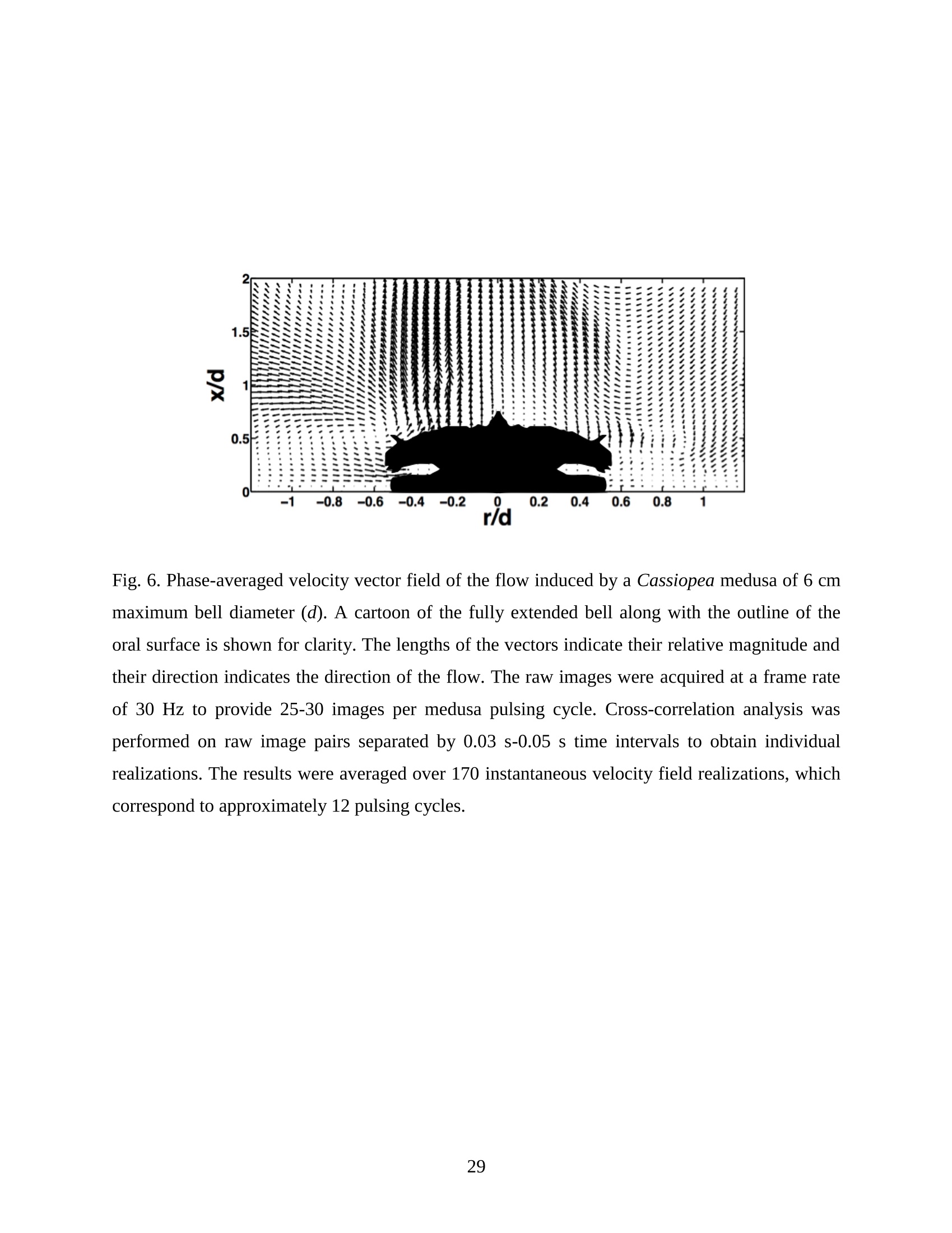
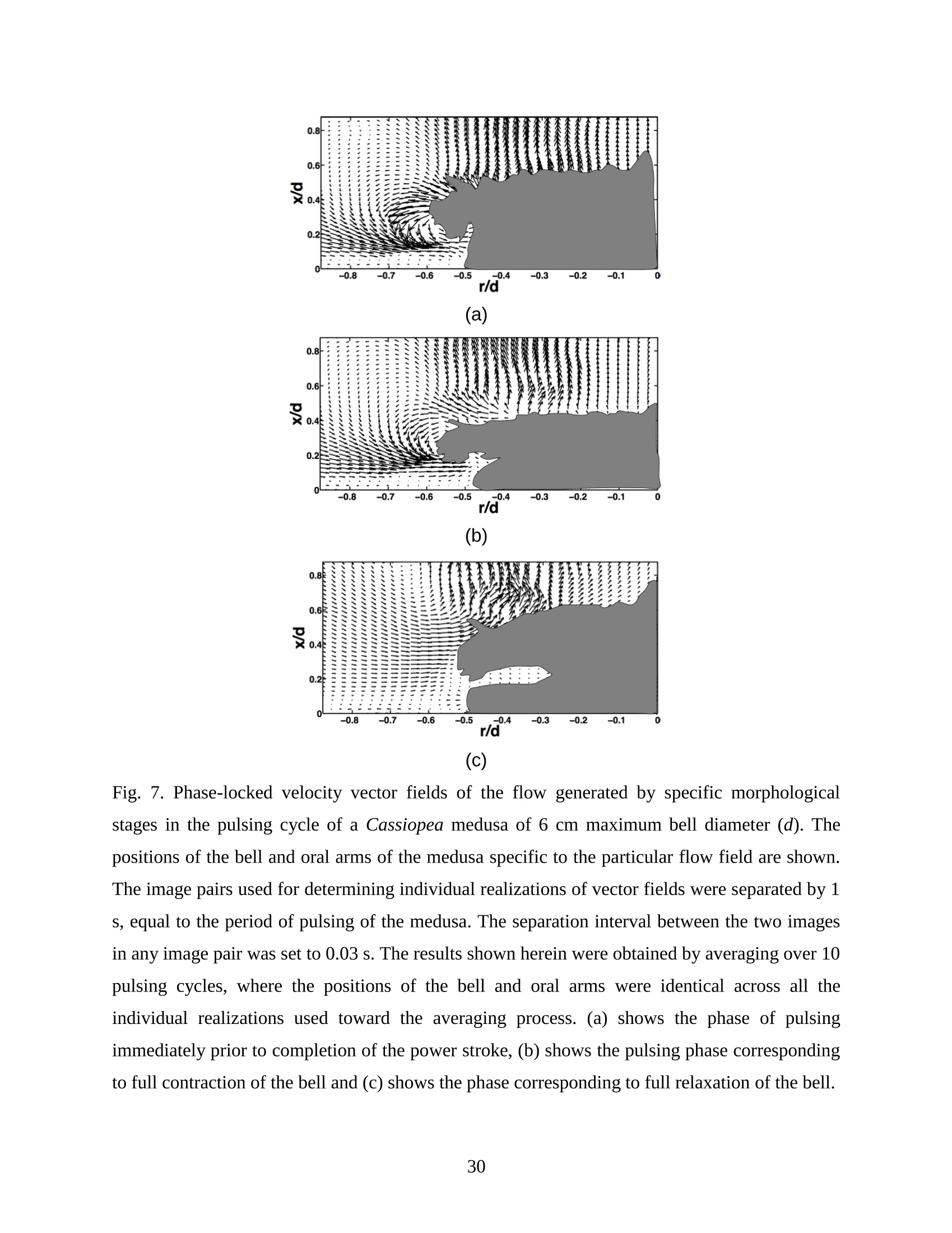
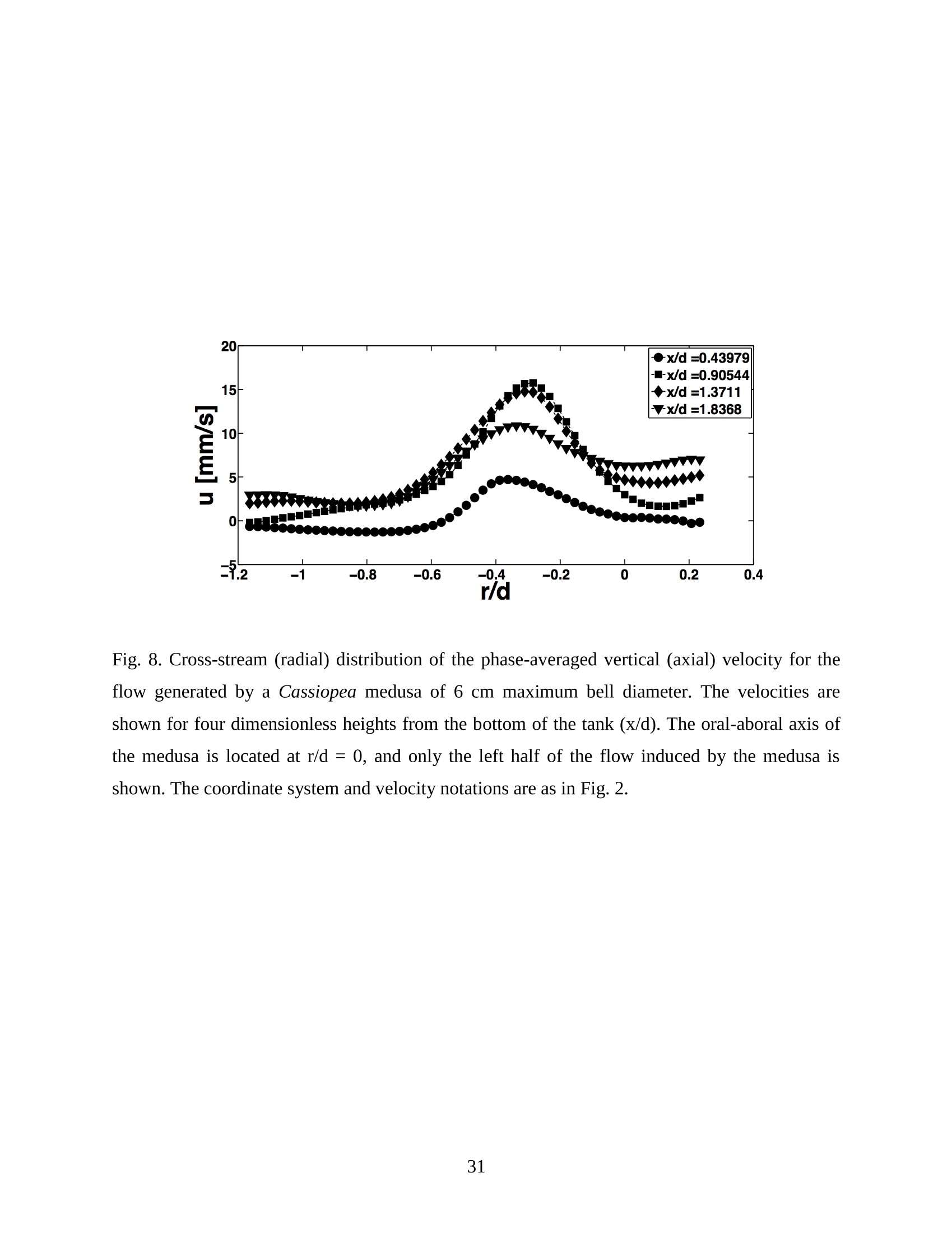
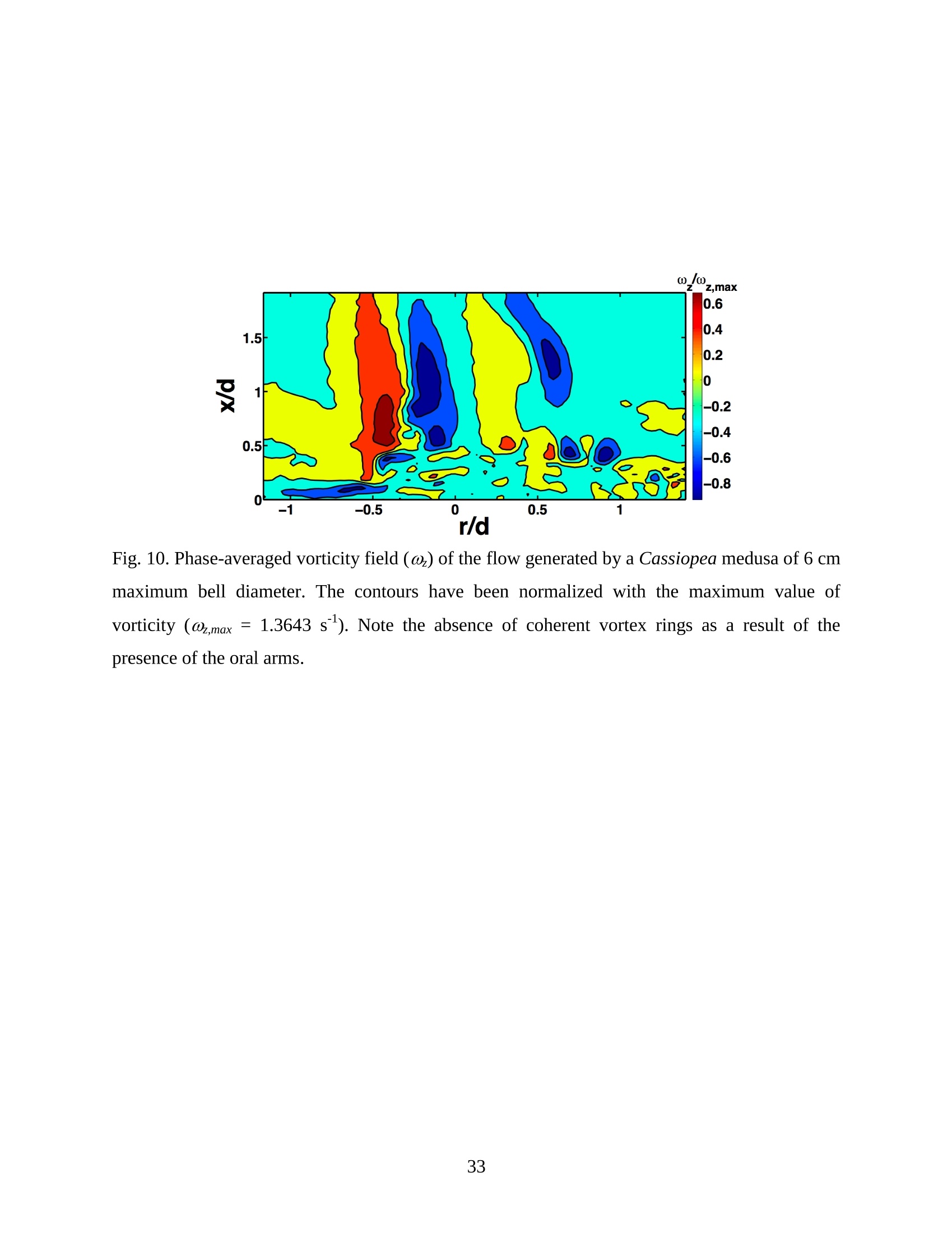
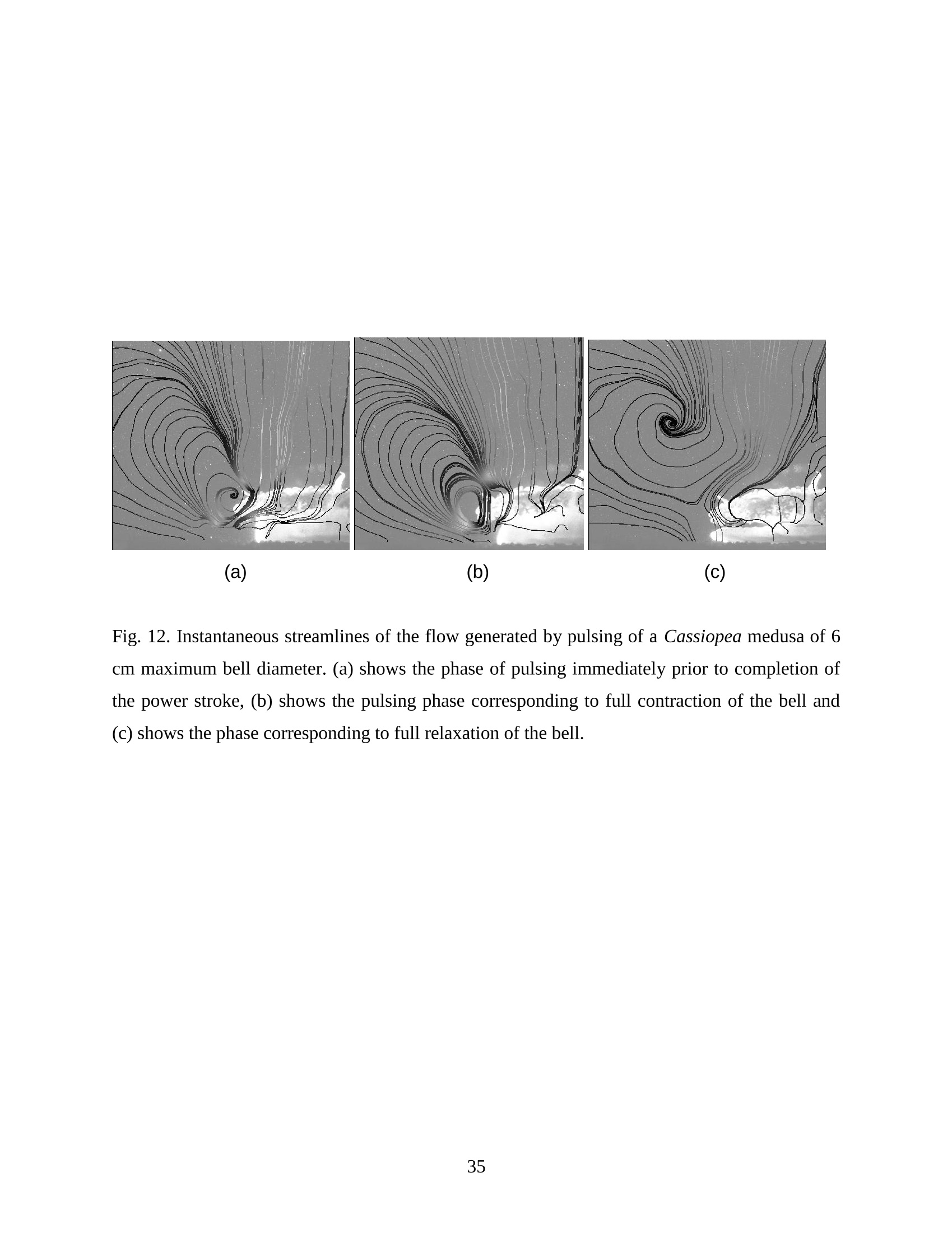
Feeding and Exchange Currents Generated by Upside-Down JellyfishCassiopea Arvind Santhanakrishnan , Makani Dollinger and Laura A. Miller Department of Mathematics, University of North Carolina, Chapel Hill, NC 27599, USA Author for correspondence (e-mail: asant0@email.unc.edu) Summary Understanding how the hydrodynamics of feeding, nutrient and gas exchange is affected by themorphology and orientation of jellyfish is important to distinguish their individual mechanismsof foraging. The differences between the currents generated by oblate medusa that rest on theocean floor and the more commonly observed free-swimming medusa have previously not beenexplored. The pulsing kinematics and fluid flow around the upside-down jellyfish, Cassiopeaspp., contained in laboratory aquaria is experimentally investigated using a combination ofvideography and digital particle image velocimetry measurements. The phase-averaged flowgenerated by bell pulsations is similar to a vertical jet, with induced flow velocities on the orderof 1-10 mm/s. The bell margin is pushed downward during full contraction in a manner notpreviously observed in free-swimming medusae kinematics, due to the fact that the Cassiopeabell rests against a solid surface. This introduces a strong near-horizontal entrainment of the fluidtoward the oral arms unlike the mostly upstream entrainment observed in free-swimmingmedusae. There is no evidence of the formation of a train ofvortex rings as observed in oblatemedusae exhibiting rowing propulsion. This is primarily due to the difference in the morphologyof Cassiopea in which the oral arms extend to radial lengths greater than the maximum belldiameter. The resulting net fluid motion is not dominated by flow-reversal regions, suggestingthat Cassiopea brings in new fluid with each bell pulse for the purposes of filter feeding, oxygenexchange and excretion. Keywords: upside-down jellyfish, Cassiopea, flow field, feeding, currents, swimming Introduction There has been a renewed interest in the medusan swimmers, from both the perspective ofunderstanding the flow patterns generated in relation to their morphology and foraging schemes,as well as their value as a case study of the biological fluid dynamics of locomotion usingunsteady propulsion. The specific details on the relationship between unsteady fluid dynamicsand swimming efficiency have been investigated using mathematical modeling (Daniel, 1983;Daniel, 1984; Dabiri et al., 2007), experiments (Costello and Colin, 1994; McHenry and Jed,2003; Dabiri et al., 2005) and numerical simulations (Lipinski and Mohseni, 2009). Thedifferences in the animal-fluid interaction as affected by the morphological differences betweenjellyfish medusa that are cruising swimmers for most of their life cycle to those that are mostlynon-swimming have not been examined. A thorough study of the flow generated by oblateupside-down scyphozoan medusae is important in understanding how the hydrodynamics of thecurrents generated by these organisms compare to better studied medusa that swim for most oftheir life cycle. Differences in the resulting fluid dynamics should have implications forstrategies of prey capture, oxygen exchange, and transport of matter including inorganic nutrientsand organic excreta. The upside-down jellyfish, Cassiopea spp., is found in shallow, protected and quiet marineenvironments saturated with sunlight. Cassiopea is an oblate scyphozoan medusa that belongs tothe order Rhizostomeae. These medusae are seldom observed to swim and instead prefer to resttheir bell upside-down on a substrate directing their oral arms to the free surface. Cassiopeamedusae have been examined for their ecological roles in influencing benthic oxygen andinorganic nutrient fluxes (Welsh et al., 2009), ability to thrive in eutrophic environments (Arai,2001), potential to accumulate trace metals from urban environments (Rainbow and Phillips,1993;Templeman and Kingsford, 2010), :and ability to functionnaasabiomonitor forenvironmental phosphates in marine coastal systems (Todd et al., 2006). Through periodiccontractions and relaxations of the bell margin, Cassiopea medusae drive water into and awayfrom the subumbrellar cavity and over the oral arms. This volume of entrained fluid is used tosample for particulate prey such as copepods and other zooplankton. The fluid flow is also usedfor the exchange of oxygen, nutrients, and the excretion of organic fecal material. The exact nature of the fluid flow generated by a Cassiopea medusa in comparison to free-swimmingoblate medusae is still unclear. In contrast to upside-down jellyfish, recent work using digital particle image velocimetry (DPIV)and computational fluid dynamics has elucidated the mechanisms by which free swimmingjellyfish propel themselves and feed. Medusae represent one of the oldest organisms to employunsteady propulsion for locomotion and feeding (Costello et al.,2008). The rowing or paddlingmode of swimming is found in oblate medusae such as Aurelia aurita (Dabiri et al., 2005), whilesmaller prolate hydromedusae such as Nemopsis bachei (Dabiri et al., 2006) use jet propulsion.In the rowing mode of swimming, there is a formation of a starting vortex ring during the powerstroke (contraction) due to flow separation at the bell margin. This is accompanied by anoppositely signed stopping vortex ring formed within the subumbrellar cavity during therecovery stroke (Dabiri et al., 2005). This train of counter-rotating vortex rings founddownstream of oblate medusae increases the volume of fluid entrained within the subumbrellarcavity where water sampling occurs. The most important benefit to having a vortex-laden wakeis toward the cruising mode of predation observed in these medusae. The tentacles in Aurelia arepositioned within the starting (during power stroke) and stopping (recovery stroke) vortices so asto maximize the possibility of capturing prey throughout the entire pulsing cycle. Furthermore,the prey captured in the strongly rotational regions of the fluid are less likely to escape. Prolateforms rely primarily on a jet type flow downstream of their bell. This has implications on theirecological role as ambush predators, where there is an increased demand for short-time impulsesof large thrust generation and not necessarily efficient swimming (Colin and Costello,2002).During the contraction of the bell, a starting vortex ring forms in the wake that pinches off with ajet advecting downstream (Dabiri et al., 2005; Sahin et al., 2009). For both oblate and prolateforms, these unsteady fluid interactions have to be considered when modeling the wake in bothmodes of swimming. Prey capture in scyphozoan medusae is accomplished via injecting nematocysts from thetentacles as in Aurelia or oral arms as in Cassiopea. These nematocysts are capable of paralyzingsmaller organisms such as copepods and brine shrimp. Another mechanism of prey captureobserved in scyphozoans is through secretion of a layer of protein-rich mucus (Ducklow and Mitchell, 1979). In both cases, the trapped prey is transferred to the primary mouth of themedusa (or secondary mouths in Cassiopea) by either the motion of ciliary or the contraction ofthe tentacles or oral arms. The choice of mechanism of prey capture is dictated by the size of theprey. Microzooplankton are typically captured by secretion of mucus, while mesozooplanktonare captured by the action of discharging nematocysts.The diet of many species ofSemaeostomeae consists of a wide variety including copepod nauplii, veligers, larvaceans,nematodes, rotifers and fish larvae (Larson, 1991; Arai,1997). In Cassiopea specifically, prey capture is accomplished by pulsatile contractions of the bellmargin. The idea is that the contraction of the bell moves the surrounding fluid over the oralarms. Since Cassiopea belong to the Order Rhizostomeae, they do not possess tentacles incontrast to the commonly studied Aurelia spp. that are of the Order Semaeostomeae. Ascompared to adult Aurelia, the bell of Cassiopea is thicker with substantial mesoglea. The oralsurface of Cassiopea (Fig. 1) consists of eight adradial, fleshy, pinnately or irregularly branchedappendages that are fused together to the thick and short manubrium. Common to members ofthe Order Rhizostomeae is the absence of a central primary mouth; contained within each ofthese frilly oral arm branched vesicles are multiple secondary mouths. Depending upon thespecies, these oral arms can extend to lengths larger than the maximum bell radius. More detailson the anatomy and development of Cassiopea xamachana can be found elsewhere (Bigelow,1900). Although they are capable of ingesting particulate, Cassiopea obtains most of its carbonnutrition by harboring symbiotic photosynthetic zooxanthellae within its tissues (Drew, 1972;Verde and McCloskey, 1998). The focus of this paper is to experimentally characterize the pulsing kinematics and the resultantfluid dynamics of the currents generated by a Cassiopea medusa. Digital particle imagevelocimetry is used to calculate the phase averaged and phase locked large scale flow fieldsaround the bell. The results will be compared to flow patterns measured in the commonly studiedfree-swimming oblate scyphozoan medusa Aurelia spp. Specifically, the dependence of the fluiddynamics upon the morphology of the two oblate medusae (upside-down and free-swimming) aswell as the implications of the results toward transport processes in Cassiopea medusae arediscussed. Since this is the first investigation of the fluid dynamics of currents generated by Cassiopea, the focus of the study is to characterize the large scale flow fields generated by thepulsing bell. Small scale flows through the opaque oral arms and around the secondary mouthswill be the focus of future work. Materials and Methods Specimen collection and handling Two species ofmedusae, Cassiopea frondosa and Cassiopea xamachana, were obtained fromGulf Specimen Marine Lab, Florida, USA during July 2009, and Carolina Biological Supply,North Carolina, USA during September 2009. The organisms were transported overnight to thelaboratory and placed in a 29 L glass aquarium maintained at a temperature of around 20 to 22℃. Individuals ranged from 2 to 10 cm in bell diameter. The medusae were typically allowed atleast 4daysto adjustt to thelaboratory conditionsbefore conducting experimentalmeasurements. For the purposes of observation, an individual medusa was transferred from the holding tank to asmaller 10 L glass aquarium that was 20.7 x 40.3 cm at the base and 26 cm in height (Fig. 2).Prior to transferring the medusa, the saltwater from the larger holding tank was used to fill thevolume of the observation tank. The transfer of individuals was accomplished over a period of atleast 2 to 3 hours to reduce the potential of environmental shock, especially due to any relativedifferences in the salinity and water temperatures of the two tanks. Marine sand was added to thebottom of the storage/holding tank, while black sand was used in the observation tank to reduceglare in the optical measurements. Individuals placed in the observation tank took between 5 to20 minutes to settle to the sandy bottom in their natural ‘upside-down’orientation. Kinematics The medusa within the observation tank was filmed using a Canon XH A1 camcorder at a rate of30 frames s. Standard room lighting was used for most video captures and an additionalbacklight was employed in certain cases to obtain better resolution. A centimeter grid was usedbehind the aquarium for scale reference. The focal planes of the videos were adjusted to align with the plane passing through the centre of the bell and parallel to the front and back walls ofthe aquarium. The videos were digitized using the DLTdv3 program (Hedrick, 2008) in MATLAB. From eachframe, the instantaneous bell profile was extracted. The instantaneous fineness ratio Fi, definedas the ratio of the bell height (h) to the diameter (d) at any particular time, was estimated fromthe digitized images. For quantifying the bell profiles, radial symmetry about the oral-aboral axiswas assumed and only one half of the bell motion was digitized. It must be noted that since themedusae were resting on the bottom, only the portions of the bell that noticeably moved during apulsing cycle were digitized. To describe the bell curvature, 9 points were selected per framestarting from the bell margin to the resting point of the bell on the sand bottom. These 9 pointswere tracked across 14-15 frames per pulsing cycle. Digital particle image velocimetry (DPIV) To quantify the flow generated by the bell pulsations of Cassiopea in an initially quiescentmedium, measurementsi wVere performed on these medusae using two-dimensional phase-averaged and phase-locked DPIV. For illuminating the flow field, a laser sheet approximately 2mm thick was generated from a 50 mJ double-pulsed neodymium-doped yttrium aluminumgarnet (Nd:YAG) laser manufactured by Continuum Inc., which emitted light at a wavelength of532 nm with a maximum repetition rate of 15 Hz. The laser beam was converted into a planarsheet approximately 3 mm thick using a set of focusing optics. The laser sheet was adjusted so asto be located in the plane passing through the oral-aboral axis and parallel to the front and backwalls of the aquarium. There was a shadow formed after the laser sheet intersected the opaqueCassiopea medusa. By adjusting the laser and focusing optics, this region was limited in heightto not exceed bell dimensions.The accuracy of the DPIV method in tracking the displacementsof particle groups is lowered in shadow regions with low light intensity and particle scattering.As a result, only half the flow field on one side of the oral-aboral axis will be presented. A 14-bitcharge-coupled device (CCD) camera (Imager Intense, LaVision Inc.) with a 1376 x 1040 pixelarray was used for capturing images. To seed the water for the purpose of tracking the flow,small volume concentrations of Artemia spp. nauplii (1-2 day old, around 0.5 mm in size) wereinserted in the tank (on the order of 3-5 mL) and mixed in the water prior to each experiment. Phase-averaged data (in the form of image pairs) was acquired at a camera frame rate of 15 Hz.For phase-locked DPIV measurements, the frame rate of the camera was adjusted to beapproximately equal to the bell pulsing frequency of the medusa (1-2 Hz). The time interval ofseparation between two images in an image pair was adjusted to the range of 0.01-0.03 sthroughout all experiments. The choice of value depended upon the desired field of view, pulsingfrequency and size of the medusa. The maximum displacement of the seed particles was alwaysmaintained to be below 4 pixels. For processing the raw images, a single-pass FFT-based cross-correlation algorithm was used (Davis 7.0, LaVision Inc.).The interrogation window size used inthe cross-correlation analysis was 32 x 32 pixels with 50% overlap. The velocity gradients in theflow field were sufficiently low and the seed particle displacements were consistently wellwithin the interrogation window to justify this choice. No pre-processing of the raw data wasperformed prior to this step. For each PIV run, 340 individual images were recorded forprocessing resulting in a minimum of 170 velocity vector fields from which to generate flowinformation (two-dimensional velocity and velocity gradients) and statistics. Each instantaneousDPIV realization consisted of an array of 100 x 75 vectors, with each vector being separated by awidth of 12 pixels. Postprocessing was performed in MATLAB. No smoothing algorithms orother post-processing techniques was used on the data. Results Kinematic observations The pulsing cycle of Cassiopea consists of a power stroke where the bell undergoes fullcontraction, followed by a recovery stroke that is a phase of full bell relaxation. In general, theaverage frequency at which the pulsing occurred was in the range of 0.9 ± 0.1 Hz. Variationsfrom this cycle duration were observed during target feeding of Artemia spp. nauplii to amedusa, where the pulsing activity was more frequent and reached values as high as 3-4 Hz. Inaddition, certain environmental conditions appeared to marginally affect the pulsing cycle aswell, including the presence or absence of baseline currents in the aquarium, proximity toneighboring medusae, and ambient light intensity. While Cassiopea typically prefer to rest on thefloor in an upside-down orientation, they would swim if exposed to significant mechanicaldisturbances in the fluid medium. All measurements reported herein refer to non-swimming medusae in conditions of no baseline fluid flow, i.e. the experiments were conducted on amedusa placed in an observation tank that was initially quiescent and pulsing in upside-downorientation. Both the relative durations and spatial profiles of bell motion between the power and recoveryphases of the pulsing cycle were different (Figs. 3 and 4). Such asymmetries have been observedin other schyphomedusae (Costello and Colin, 1994). Due to the preference of Cassiopeamedusae to rest their bell on a flat surface, the resulting bell motion was expected to be differentfrom free-swimming medusae. Furthermore, the bell motion was chiefly restricted to the bellmargin of the Cassiopea due to both the presence of higher mesoglea content and their upside-down orientation. This is different from upward cruising oblate swimmers such as Aurelia wherelarge deformations have been observed across the bell (Bajcar et al., 2009). From the kinematicanalyses, the position of the bell margin was tracked as a function of both the spatial location andtime. The center of the bell was used as the reference point for tracking the position of the bellmargin, and only one half of the bell diameter was analyzed assuming radial symmetry. The power stroke was approximately 40% of the period of the pulsing cycle (0.4±0.07 secondscompared to a 1.1.1±(0.1second period, averaged over 10 cycles). The change ininstantaneous bell height at any point in time was out of phase with that of the bell diameter (Fig.3(a)). The maximum bell height occurred during contraction, while the maximum bell diameteroccurred at the end of the relaxation phase of pulsing. During the contraction phase, the belldiameter decreased resulting in an increase in the fineness ratio (Fig. 3(b)). This movement wasobserved to draw in water toward the oral arms that enveloped the bell closely. Duringrelaxation, the bell diameter decreased and thus the fineness ratio decreased, such that the waterentrained near the subumbrellar cavity was gradually ejected out by a decrease in the pressurewithin the bell surface.The bell height remained constant for most of the recovery stroke,followed by a sharp increase during the power stroke. This finding is consistent with thekinematics of the free-swimming oblate medusae Aurelia aurita reported elsewhere (Costelloand Colin, 1994). In addition to temporal variation, the spatial bell profiles also changed differentially across bothpulsing phases as shown in Fig. 4. The bell height varied by a larger extent during the powerstroke as compared to the recovery stroke, while the variations in bell diameter were morepronounced during the recovery stroke. Compared to Aurelia, the bell margin in the fullycontracted state of Cassiopea was moved further inward and closer to the axis of radialsymmetry. It is important to note that during the end of the power stroke, the bell was pusheddownward against the floor of the tank (Fig.5). This is not seen in oblate medusan swimmerssuch as Aurelia and primarily arises from the difference in the preferred orientation of Cassiopeaand the presence of the floor. The oral appendages of Cassiopea medusae used in this studytypically extended to more than the bell radius. The extent of this structure significantly differentfrom medusae that swim. Consequently, the currents generated by Cassiopea can also beexpected to be different due to the unfused oral arms functioning as obstacles to the path of thefluid flow. Near the end of the power stroke, the oral arms were oriented slightly in an upwarddirection to possibly increase the encounter rate of particulate matter within the local fluidentrained by the medusae. Induced flow measurements The phase-averaged flow generated by the pulsations in Cassiopea resembles a vertical blowingjet (Fig. 6), with mean velocities on the order of 1-10 mm/s for a medusa with a maximum belldiameter of 9 cm. Fluid is entrained near the bell margin and ejected above the oral surface of themedusa. A starting vortex is observed in the phase-averaged flow field close to the bell margin. Itis formed when the unsteady entrained flow separates at the bell margin due to insufficientmomentum (and hence kinetic energy) to navigate the steep pressure gradient imposed during thecontraction phase. The formation of counter-rotating starting and stopping vortex rings does notoccur unlike what has been observed for Aurelia aurita (Dabiri et al., 2005). The velocity fieldgenerated by the medusa was not centerline symmetric, as evidenced by the shorter length vectorfield arrows (vector length indicative of relative flow speed) about the oral-aboral axis (r/d=0).Interestingly, a similarity between the flow fields of the upside-down jellyfish and the oblatefree-swimmers such as Aurelia is in the nature of the spreading of the jet itself. The startingvortex induced by Cassiopea is directed away from the centerline of the medusa and the induced flow is toward the centerline axis. This same pattern was also reported in the case of Aureliaaurita (Dabiri et al.,2005). The principal reasons behind the spreading of the jets in Cassiopea and Aurelia are not identical.In Aurelia aurita, the flow field induced by the bell pulsations for any particular cycle consists ofcounter-rotating starting and stopping vortex rings that interact with equivalent shed vortex ringsfrom the previous cycle. Such interactions include the point when the stopping vortex formedduring the recovery stroke contributes to formation of starting vortex in the power stroke of thenext cycle. These interactions proceed in a manner such that a portion of the previous cyclestopping vortex ring gets pinched off with a portion of the newly formed starting vortex. At theend of every pulsing cycle,the added mass contributed by these vortices increase the momentumof the jet. Viscous dissipation in the flow causes the spreading to move away from the centerline.It must be noted that this process is heavily dependent upon the spacing between the vortexrings, which is effectively controlled by the pulsatile undulations in the system. In Cassiopea,trains of coherent vortex rings are broken up by the oral arms, and the mechanism behind the jetspreading and resulting flow field is not as straightforward. To describe the flow generated by the different morphological positions of the medusa bell in acycle, DPIV measurements were acquired at an image pair capture rate phase-locked to themedusa pulsing frequency of 1 Hz (Fig. 7). Matching the data acquisition rate to the pulsingfrequency of the medusa permitted the assessment of the relative effects of the bell positions onthe resultant ensemble-averaged flow field. Specifically, three discrete bell positional phases inthe pulsing cycle were closely examined: near the end of the power stroke (close to fullcontraction, Fig. 7(a)), at the end of the power stroke (Fig. 7(b)), and the end of the recoverystroke (Fig. 7(c)). Both the positions of the medusa bell as well as the oral arms vary duringpulsing, and these play an important role in how the resulting currents are generated. During thephase when the bell is almost fully contracted (Fig.7(a)), the flow adjacent to the bell marginshows the formation of a starting vortex. This arises from entrainment of the fluid horizontallyfrom the sides of the bell margin and consequent separation of the flow at the edges of the bell.Prior to passing through the edges of the bell, the flow is partially sampled through the oralsurface of the medusa at the distal end. On progressing to the next stage of full bell contraction (power stroke), the horizontal entrainment from the sides of the bell becomes more pronouncedand the vertical flow directly above the oral surface becomes weaker than the previous phase(Fig. 7(b)). Additionally, the bell moves further inward toward the center and downward duringfull contraction (as also discussed above in kinematics). This motion allows a slightly largerpassage for water to enter the subumbrellar cavity as compared to the phase of near full bellcontraction where only the edges of the oral arm cylinders were exposed to the flow. Eventhough these two stages appear to be almost similar, the bell position in relation to the oralsurface arrangement brings about different interactions with the flow. Upon full relaxation(recovery stroke) of the bell, the starting vortex moves outward and away from the centerline asexpected (Fig. 7(c)). Most of the flow is from the vertically directed jet above the oral surface,and the magnitude of the horizontal flow adjacent to the bell margin is drastically reduced duringrecovery. In contrast to the free-swimming oblate medusa Aurelia, there is no formation of two counter-rotating vortical structures per pulsing cycle in Cassiopea, This is most ostensibly due to thepresence of the elaborate cylindrical oral arms that extends to nearly the bell radius. Such amorphological difference prevents flow from filling the entire subumbrellar space (as observedin Aurelia aurita) and thus the formation of a stopping vortex. During the recovery stroke inCassiopea, the bell expands in diameter, and this increase in bell cavity volume is accompaniedby advection of the starting vortex outward from the centerline oral-aboral axis. The direction ofinduced motion, however, is toward the centerline axis. This is evidenced by an examination ofthe radial distribution of phase-averaged axial velocity (Fig. 8). The axial velocity reaches themaximum value at a distance of about 1 diameter downstream from the sand bottom, and theprofile peaks are centered at x/d=-0.5, which is the location of the medusa bell margin. Withinthe field of observation in these experiments, the axial velocity is reduced in magnitude uponmoving from the second (x/d=1)to last (x/d=1.8) downstream station. This is indicative of jetspreading about an axis that is centered at bell margin. The direction of induced motion is madeclear by observing the centerline velocities about the oral-aboral axis (radial symmetry line). Themagnitude of centerline velocity increases as we move downstream from the medusa, and thepeak value (about the oral-aboral axis) occurs at the farthest axial location at x/d=1.8. Thespreading of the jet about the bell margin axis is accompanied by a loss in the momentum of the flow. This is compensated by an increase in the centerline velocity with increasing distance fromthe medusa, in order to account for mass conservation. Thus the direction of the induced motionin the flow field is toward the centerline, the direction of starting vortex advection is away fromthe centerline, and the peak value of axial velocity is near the bell margin where the entrainmentoccurs upon bell contraction. Such a directed vertical motion of fluid is necessary in Cassiopeato provide vertical force (in the form of a reaction force) to effectively press their bell to theocean floor. While the direction of motion is similar to what is observed in Aurelia, it must bedistinguished that the mechanisms of counter-rotating vortex shedding and interaction are notpresent in the case of Cassiopea. This marks an important difference in the hydrodynamics of theflow between oblate non-swimming to free-swimming medusae, driven by differences in theirmorphology. The entrainment patterns in the flow field can be examined by looking at the axial (downstream)distribution of the radial component of phase-averaged velocity (Fig.9). The velocity changes insign at a distance of 0.8 diameters downstream from the sand floor, which is approximately thecenter of the starting vortex formed during bell contraction (Fig. 7 (a)). The peaks in the radialvelocity values occur at distances close to the medusa (x/d=0.2), and the profiles are directedtoward the centerline in a nearly horizontal manner. This suggests that the entrainment in theprimarily non-swimming medusae Cassiopea occurs from the sides of the bell margin, which isdifferent than the animal-fluid interaction of free-swimming oblate medusae Aurelia auritawhere the fluid entrained is primarily from the upstream direction. While such horizontalentrainment is not necessarily advantageous from the standpoint of propulsive swimming, itprovides a highly directed flow to the oral arms of Cassiopea. As a result, the volume of sampledwater undergoes recirculation for only short periods of time before being advected into thevertical jet. Since the advection of sampled water is toward the centerline, the volume of waterentrained horizontally during the next power stroke is least contaminated from the previouscycle. This may allow the non-swimming medusa to effectively feed, exchange gas excretewastes. Figure 10 shows the phase-averaged vorticity field in the currents generated by the medusa,which are indicative of the rotational characteristic of the fluid flow. An examination of this data reveals the presence of two opposite-signed regions of shear flow centered about the oral-aboralaxis. The flow near the bell margin is symmetric about the oral-aboral axis (r/d=0). Given thelow momentum of the induced flow at any particular instant, the starting vortex ring formedduring the power stroke is subject to viscous forces in the fluid. Although the flow through theoral arms has not been resolved here, one could consider the structure as an array of porouscircular cylinders that move with the bell pulsations. When the fluid separates at the bell marginduring the power stroke, the reversed flow from the starting vortex ring next encounters the oralappendages of the medusa. While the numerous mouths contained in the frilly oral arms functionas porous openings, the bulk fluid flow is nevertheless subject to resistance from the entire oralassembly as a whole. Subsequently, the identity of the starting vortex ring is lost from suchviscous interactions with the oral arms. This is in sharp contrast to the flow around the highlyflexible and slender tentacles observed in Aurelia, where the identity of the starting vortexformed at a particular pulsing cycle is preserved at least until the next pulsing cycle. Cancellationof the starting vortex vorticity in such free-swimming oblate medusae has been attributed to theinteraction between the counter-rotating starting and stopping vortices. It must be noted in thecase of Cassiopea that there is no formation of the latter structure in the flow field. This suggeststhat the morphology of the oral appendages of Cassiopea is responsible for this starting vortexvorticity cancellation action, which results in a diffuse vorticity distribution. Discussion From experimental measurements of the bell kinematics and the flow induced by a Cassiopeamedusa, the fluid-structure interactions in this system could be described as a cyclic alternationbetween two distinct patterns. One pattern is generated by the power stroke and the second bythe recovery stroke (Fig. 11). During the phase of bell contraction, fluid is entrained almostentirely from the sides of the bell margin toward the oral-aboral axis. Similar to what is observedin mechanically generated vortex rings (Glezer, 1988), the flow entrained by the medusaseparates at the edges of the bell margin and subsequently forms a cylindrical vortex sheet thatrolls up into a vortex ring. This starting vortex ring is formed at the phase of pulsing where thebell is near full contraction. The vortex ring is centered roughly on the distal ends of the oral arms that typically extend to lengths greater than the bell radius. The circulation of the startingvortex sustains the fluid entrainment process, and the added mass of this structure aids inincreasing the momentum of the wake. The flow induced by the entrainment and vortexformation processes was chiefly directed vertically upward and toward the oral-aboral axis. In addition to the pulsatile motion of the bell, there was an asymmetry between the durations ofthe power and recovery strokes. Contraction times were observed to be roughly 40% of thepulsing period, and this asymmetry in pulsing introduced another component of unsteadiness inthe induced flow field such that the starting vortex was not entirely dissipated at the end of thepower stroke. During the longer duration recovery stroke, the starting vortex ring formationprocess stopped, and this structure began to advect with the induced vertical jet flow. Comparedto the contraction phase, the vertical extent of the jet was greater in the relaxation phase. Theaxial component of jet velocity reached its maximum value at approximately the core of thestarting vortex during both phases of the pulsing cycle. This suggests that the increased verticalextent of the jet during recovery was mostly due to vortex advection. The fluid entrained duringcontraction was expelled during the recovery stroke. As a result, the flow induced in the recoverystroke mostly consisted of the vertical jet with minimal horizontal components. No coherentcounter-rotating vortex pairs were formed during the pulsing cycle. In addition, the oral armsmoved with the bell contractions and served as an obstacle to the flow. Comparison to oblate free-swimming medusa In this section, the morphology, bell kinematics and resulting flow fields around Cassiopea willbe compared to that of oblate free-swimming scyphozoan medusa, such as the most commonlystudied Aurelia aurita. It is useful to first discuss the comparative morphologies of the bell andthe oral surface of these two species. A detailed description of the anatomy of the bell rim ofAurelia aurita can be found elsewhere (Chapman, 1999). In the case of Aurelia, the oral orsubumbrellar surface consists of a central mouth opening below the manubrium. Four unfusedoral arms with frilly edges surround this orifice. Numerous tentacles are present from the sides ofthe umbrella above the margin. The bell is convex-shaped, and a layer of circumferential tissueflange (pseudovelarium) is present below the subumbrella. In Cassiopea, the bell is concave- shaped everywhere other than at the margin where it is convex. There is the absence of a centralmouth. In its place, eight elaborately branched oral arms with numerous secondary mouths arepresent. The extent of the oral arms in Cassiopea typically exceeds the bell radius (Bigelow,1900). As a result, the arms act as an obstacle to the flow expelled vertically during the powerstroke and move with the contractions of the bell. In contrast, the oral arms of Aurelia do notextend beyond the bell radius. Additionally, the tentacles of Aurelia are thinner than the oralarms in Cassiopea and typically trail along in the flow during the power stroke (Dabiri et al.,2005). The structure and distribution of the mesoglea in each organism also play a role in the kinematicsof bell contraction and the resulting fluid motion. The mesoglea in medusae is primarilyresponsible for providing the shape and the force required to expand the bell during the recoverystroke. Compared to the even distribution of exumbrellar mesoglea in Aurelia, the exumbrellarmesoglea in Cassiopea is joined to the subumbrellar surface through large elastic fibers (Arai,1997). Furthermore, there is a greater distribution of mesoglea on the concave part of theCassiopea bell that is in contact with the floor. This uneven distribution of exumbrellar mesogleain Cassiopea permits a larger range of motion at the bell margin. The connectivity of thesubumbrellar surface restricts motion elsewhere. This pattern of motion has been described in thekinematic measurements presented here,particularly during the downward motion of the bellduring full contraction. In Aurelia, the motion of the bell is not restricted to the bell margin. Theentire convex bell contracts and expands evenly throughout its surface (Bajcar,2009). Thesedifferences in the extent and areas of pulsation of the medusa bell affect the entrained flowpatterns between Aurelia and Cassiopea. While both Aurelia and Cassiopea medusae rely on generating localized unsteady flows fortransport, the flow field in Aurelia medusae is heavily dominated by vortical interactions in thewake. A detailed description of the fluid dynamics of Aurelia swimming can be found elsewhere(see Dabiri et al., 2005, Ichikawa et al.,2006). During the power stroke, the flow is entrainedfrom upstream of the exumbrellar surface in Aurelia as compared to along the sides of the bellmargin as observed in Cassiopea. The flow expelled from the subumbrellar cavity and theentrained flow that separates at the bell margin generate the starting vortex ring in both medusae. The fluid dynamics during the recovery stroke in the two species are noticeably different due tothe presence of oral arm cylinders in Cassiopea. In Aurelia, the bell expands during the recoverystroke and the subumbrellar space is filled with entrained fluid. The flow directed inwards to thebell separates at the bell margin and results in the formation of a stopping vortex ring. Thisstopping vortex spins opposite to the starting vortex ring. The stopping vortex is pinched offfrom the bell margin at the onset of the next power stroke along with the formation of a newstarting vortex, and the events repeat in this manner. The asymmetry in the power and recoverystrokes leads to interactions between the starting vortex of the current cycle and the counter-rotating stopping vortex from the previous cycle. Such a vorticity cancellation mechanismdecreases the loss of kinetic energy spent in generating the wake vortices and reduces the overallrotational motion in the flow field (Dabiri et al., 2007). The spacing between the vorticesthemselves can be tuned for increasing swimming efficiency. In contrast, there is no stoppingvortex formation in the recovery stroke in Cassiopea, and this can be attributed to themorphology of the oral arms that disturb the fluid entrained during bell relaxation. Moreover,there is minimal horizontal flow in the recovery stroke of Cassiopea. The fluid dynamic eventsin this phase primarily consist of the starting vortex advecting with its self-induced velocityfollowed by a vertical jet in its wake that serves to remove the fluid entrained in the powerstroke.The vorticity cancellation mechanism in Cassiopea is due to the obstruction of the flowby the oral arms that extend to lengths beyond the bell radius. Implications toward biological transport processes in CassiopeaThe structure of the large scale flow fields in Cassiopea likely has implications for themechanisms by which the organism captures prey, transports organic excreta away, andexchanges inorganic nutrients and oxygen. The medusa obtains its carbon nutrition via symbioticphotosynthetic zooxanthellae contained in the mesoglea (Drew, 1972; Verde and McCloskey,1998). Only large medusae are able to obtain all the carbon necessary for respiratory metabolismthis way, and most other Cassiopea medusae have to obtain other nutrients through feeding andabsorption of dissolved nutrients (Fitt and Costley, 1998). Due to their preference to not swim,Cassiopea medusae spend most of their time in the same environment. Therefore, the transportprocesses would be efficient if relatively unsampled volumes of water were brought to the bellduring each pulsing cycle. This can be accomplished by minimal recirculation in the nearby flow field. The expansion of the bell draws new fluid to the organism that is then driven upward andaway during the power stroke. The oral arms of Cassiopea provide a means for mixing this fluidduring the power stroke such that the overall circulation in the flow is minimized. The movement of the fluid through and around the oral arms may also have implications for theefficiency of prey capture. Cassiopea's eight oral arms are individually further divided into many(7-8 pairs) smaller branches. Contained on the distal ends of these oral arms are numerousdigitata that bear nematocysts at the tips. In addition, there are club-shaped vesicles in betweenthe arms that also bear nematocysts at the distal ends. These two regions are responsible for preycapture. Upon trapping prey via injecting nematocysts, the digitata bend inwards, allowing thecilia present within the oral arms to move the prey to the brachial canal that leads into thestomach for digestion. During the power stroke, the oral arms of Cassiopea move upward andthe bellis contractedinwardand downwardsuchthatttwotasksaire accomplishedsimultaneously: (i) the distal ends of the oral arms are brought outward and closer together in amanner that exposes the nematocyst bearing digitata to the incoming entrained flow, and (ii) thepassage in between the oral arms and the vesicles that are present underneath them is widenedallowing for some fluid to collect in this subumbrellar space. The peripheral coronal musclescontract first resulting in raising the bell margin so as to drive flow to the oral arms (Arai, 1997).Contrasting the morphology with the flow streamlines at the instants where the power strokeoccurs (Figs. 12 (a) and 12 (b)) shows that the flow is primarily entrained along the sides of thebell margin. The core of the starting vortex ring is centered on the distal ends of the oral arms.The rotational nature of the starting vortex ring prevents the escape of prey contained in theentrained fluid, and the inertial forces that are predominant in the core of the vortex provide ameans for the prey to impact the oral arm cylinders. During bell relaxation when the entrainedfluid is expelled, the force of the vertical jet produced in the wake of the advecting startingvortex ring forces the water trapped within the passage between the vesicles and oral arms topass through the nematocyst bearing surfaces once again (Fig. 12(c)). The oral arms expandoutward during recovery in a manner that increases their surface area, and the arms also movedownward so as to prevent any possible roll-up of the entrained flow in the form of a stoppingvortex. Prey capture during the recovery stroke is therefore accomplished by passing the fluidthrough the vesicles and oral arms that are acting together in the form of a sieve or a biological ‘filter’(Rubenstein and Koehl, 1977). These observations are in agreement with the prey captureprocess observed in other oblate swimming rhizostome medusae such as Stomolophus meleagris(Larson, 1991). It is important to note that the prey capture process is made possible inCassiopea during both the power and recovery strokes through these fluid-structure interactions,so as to maximize the chance for prey to encounter the oral arms during both phases of thepulsing cycle. Apart from feeding, the fluid flow generated by bell pulsations in Cassiopea serves to bring inwater for the diffusion of dissolved oxygen (Welsh et al., 2009), the expulsion of organic matterin the form of mucus or fecal material (Niggl et al., 2010), and the intake or expulsion ofinorganic nutrients through the orifices in the oral arms. At the shallow benthic waters in whichthese medusae are found, the levels of dissolved oxygen are low. Depending upon the intensityof exposed light, the metabolism in these medusae changes. Cassiopea are autotrophic duringdaylight where they function as oxygen sources and sinks for inorganic nutrients. They areheterotrophic during the night where they function as oxygen sinks and inorganic nutrientsources (Welsh et al.,2009). It seems likely that the process of oxygen and nutrient diffusion inand out of Cassiopea would be the most efficient if a fresh volume of water were to be sampledduring each pulsing cycle. As compared to cruising pelagic medusae such as Aurelia where thevortex-laden wake has been shown to be beneficial for locomotion, the non-swimmingCassiopea would derive the most energy efficient means for transport of oxygen and inorganicnutrients by avoiding the possibility of recirculation in the flow between each pulsing cycle.Thisis directly confirmed with what was observed in the induced fluid flow. No counter-rotatingvortical structures were generated. Similarly, the excretion process would be not be as effectiveif the organic matter were recirculated after release. This again suggests that the absence ofinteracting vortex rings in the flow is more efficient for the transport of fecal material away fromthe non-swimming medusa. Given that the flow expelled from the medusa during recovery strokeis slower than the entrained flow during the power stroke, the directionality of the entrained flowalso plays an important role in the efficiency of nutrient exchange and excretory transfer. Thechiefly horizontal direction of entrainment prevents the sampled water during a particular pulsingcycle from being contaminated with organic and inorganic material exchanged in the previouscycle. Implications toward the modeling ofnon-swimming medusae The results of this study suggest that both the morphology and the orientation of a medusa withrespect to a surface significantly affect the overall hydrodynamics of currents generated bypulsatile undulations of the bell. Most mathematical models are focused on free-swimmingmedusae and describe the fluid flow induced by unsteady pulsing. Such models treat the flow asan output characteristic that is exclusively dependent upon the bell kinematics as an input.However, the morphology and the dynamics of the oral surface also affect the flow andassociated processes such as the capture of prey. In organism with elaborate oral arm structures,the true nature of the fluid-structure interactions occurs at multiple levels of structuralcomplexity. These interactions are dictated by the morphology of the particular medusa ofinterest. Despite the simplifications, these ‘black-box’approaches do provide reasonableapproximations of the flow in the case of free-swimming medusa with reduced oral armstructures (Daniel, 1984; Dabiri et al., 2007; Lipinski and Mohseni, 2009). For medusa withpronounced oral arms, such simplified approaches may not capture some of the relevant fluiddynamics in the wake of the organism. For Cassiopea, the motion of the bell against a surfaceand the structure of the oral arms heavily influence vortex generation and the entrainmentpatterns around the organism. This research was supported by a Burroughs Wellcome Fund CASI award ID #1005782.01 (to L.A. M.). The authors would like to thank the following people: Sean Colin and John Dabiri foradvice on maintaining the medusae and flow visualization; Christina Hamlet, Terry Campbelland Walt Douglas for their assistance in setting up and maintaining the medusae in the laboratoryaquaria;William Kier for insightful discussionson invertebrate swimming and musclemechanics, and Tyson Hedrick for advice on the use of the digitizing code for quantifyingmedusa kinematics. References Arai, M.N. (1997). A Functional Biology of Scyphozoa. London: Chapman and Hall. Arai, M. N. (2001). Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451,69-87. Bajcar, T., Malacic, V., Malej, A. and Sirok, B. (2009). Kinematic properties of the jellyfishAurelia sp. Hydrobiologia 616, 279-289. Bigelow, R. P. (1900). The anatomy and development of Cassiopeia xamachana. Boston Soc.Nat. Hist. Mem.5, 191-236. Brusca,R. C. and Brusca, G. J. (1990). Invertebrates. Massachusetts: Sinaur Associates Inc. Chapman,D. M. (1999).Microanatomy of the bell rim of Aurelia aurita (Cnidaria: Scyphozoa).Can.J.Zool.77,34-46. Colin, S. P. and Costello, J. H. (2002). Morphology, swimming performance, and propulsivemode of six co-occurring hydromedusae.J. Exp. Biol. 205, 427-437. Costello, J. H. and Colin, S. P. (1994). Morphology, fluid motion and predation by thescyphomedusa Aurelia aurita. Mar. Biol. 121, 327-334. Costello, J. H. and Colin, S. P. (1995). Flow and feeding by swimming scyphomedusae. Mar.Biol. 124,399-406. Costello, J. H., Colin, S. P. and Dabiri, J. O. (2008). Medusan morphospace: phylogeneticconstraints, biomechanical solutions, and ecological consequences. Invertebrate Biol. 127, 265-290. Dabiri,J. O., Colin, S. P., Costello,J. H. and Gharib, M. (2005). Flow patterns generated byoblate medusan jellyfish: field measurements and laboratory analyses. J. Exp. Biol. 208, 1257-1265. Dabiri, J.O., Colin, S. P. and Costello, J. H. (2006). Fast-swimming jellyfish exploit velarkinematics to form an optimal vortex wake. J. Exp. Biol. 209,2025-2033. Dabiri, J. O., Colin, S. P. and Costello, J. H. (2007). Morphological diversity of medusanlineages is constrained by animal-fluid interactions. J. Exp. Biol. 210, 1868-1873. Daniel, T. L. (1983). Mechanics and energetics of medusan jet propulsion. Can. J. Zool. 61,1406-1420. Daniel, T. L. (1984). Unsteady aspects of aquatic locomotion. Am. Zool. 24, 121-134. Drew, E. A. (1972). The biology and physiology of alga-invertebrate symbioses. I. Carbonfixation in Cassiopea sp. at Aldabra atoll. J. Exp. Mar. Biol. Ecol. 9,65-69. Ducklow, H. W. and Mitchell, R. (1979). Composition of mucus released by coral reefcoelenterates. Limnol. Oceangr.24,706-714. Fitt, W. K. and Costley, K. (1998). The role of temperature in survival of the polyp stage of thetropical rhizostome jellyfish Cassiopea xamachana. J. Exp. Mar. Biol. Ecol. 222,79-91. Glezer, A. (1988). The formation of vortex rings. Phys. Fluids. 31, 3532-3542. Hedrick., T. L. (2008). Software techniques for two- and three-dimensional kinematicmeasurements of biological and biomimetic systems. Bioinspiration & Biomimetics 3, 034001. Ichikawa, S., Yazaki, Y. and Mochizuki, O. (2006). Flow induced by jellyfish. Phys. Fluids18,091104. Larson, R. J. (1991). Diet, prey selection and daily ration of Stomolophus meleagris, a filter-feeding scyphomedusa from the NE Gulf ofMexico.Estuarine, Coastal and Shelf Science 32,511-525. Lipinski,D. and Mohseni, K. (2009) Flow structures and fluid transport for the hydromedusaeSarsia tubulosa and Aequorea victoria. J. Exp. Biol. 212, 2436-2447. McHenry, M. J. and Jed, J. (2003). The ontogenetic scaling of hydrodynamics and swimmingperformance in jellyfish (Aurelia aurita). J. Exp. Biol. 206, 4125-4137. Niggl, W. and Wild, C. (2009). Spatial distribution of the upside-down jellyfish Cassiopea sp.within fringing coral reef environments of the Northern Red Sea: implications for its lifecycle.Helgol. Mar. Res. Niggl, W., Naumann, M. S.,Struck, U., Manasrah, R. and Wild, C. (2010). Organic matterrelease by the benthic upside-down jellyfish Cassiopea sp. fuels pelagic food webs in coral reefs.Journal of Experimental Marine Biology and Ecology 384,99-106. Rainbow, P. S. and Phillips, D. J. H. (1993). Cosmopolitan biomonitors of trace metals. Mar.Poll. Bull. 26,593-601. Rubenstein, D. I. and Koehl, M. A. R. (1977). The mechanisms of filter feeding: sometheoretical considerations. The American Naturalist 111, 981-994. Sahin, M., Mohseni, K. and Colin, S. P. (2009). The numerical comparison of flow patternsand propulsive performances for the hydromedusae Sarsia tubulosa and Aequorea victoria, J.Exp. Biol. 212, 2656-2667. Templeman, M. A. and Kingsford, M. J. (2010). Trace element accumulation in Cassiopea sp.(Scyphozoa) from urban marine environments in Australia. Marine Environmental Research 69,63-72. Todd, B. D., Thornhill, D. J. and Fitt, W.K. (2006). Patterns of inorganic phosphate uptake inCassiopea xamachana: a bioindicator species. Mar. Poll Bull. 52, 515-521. Welsh, D. T., Dunn, R. J. K. and Meziane, T. (2009). Oxygen and nutrient dynamics of theupside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges andprimary production.Hydrobiologia 635, 351-362. Verde, E. A. and McCloskey, L. R. (1998). Production, respiration, and photophysiology of themangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: effect of jellyfish sizeand season. Mar. Ecol. Prog. Ser. 168, 147-162. Fig.1. Morphology of Cassiopea in side view (left) and top view (right) where the medusa isshown in its ‘upside-down’orientation (redrawn from Hyman, 1940). The manubrium is thecentral column of the jellyfish that emerges from the center of the oral surface of the bell witheight frilly oral arms radiating from it. Only one oral arm is drawn in its entirety from the topview. The data from organisms presented in this paper varied from 2 cm to 10 cm in belldiameter. There were also variations in the frilly branches of the oral arms among them. Fig. 2. Schematic of the experimental setup showing the medusa located within a 5.5 gal glassaquarium observation tank (z-axis depth =207 mm). The conventions for the r-x coordinatesystem and the u-v components of the velocity vector used in the quantitative descriptions of thekinematics and hydrodynamics are shown. (a) (b) Fig. 3. Kinematic profiles of individual Cassiopea, determined from a 9 cm diameter (d) medusa.Profiles show variation in time over 10 pulsing cycles. (a) The ratio of the radius of the bellmargin (r) and the height of the bell margin (h) to the diameter of the bell bottom (d) vs. time. (b)The instantaneous fineness ratio (F;) vs. time in seconds. Each cycle consists of at least 10 datapoints. (b) Fig. 4. Representative bell shape profiles of Cassiopea determined from a medusa of maximumbell diameter d=9 cm (only one half-diameter is shown). The coordinate system used is definedin Fig. 2. The profiles are representative of one full pulsing cycle, where (a) corresponds to thepower stroke (bell relaxation to contraction) and (b) corresponds to the recovery stroke (bellcontraction to relaxation). Each curve was defined using a set of 9 individual (r, x) points. t to tzrepresents increasing time, where each point in time is equally separated by 0.1 s. Fig. 5. Bell shape profiles for full relaxation and full contraction phases of the Cassiopea medusapulsing cycle. The profiles were obtained from a medusa of 9 cm diameter and ensembleaveraged over 9 pulsing cycles. Fig. 6. Phase-averaged velocity vector field of the flow induced by a Cassiopea medusa of 6 cmmaximum bell diameter (d). A cartoon of the fully extended bell along with the outline of theoral surface is shown for clarity. The lengths of the vectors indicate their relative magnitude andtheir direction indicates the direction of the flow. The raw images were acquired at a frame rateof 30 Hz to provide 25-30 images per medusa pulsing cycle. Cross-correlation analysis wasperformed on raw image pairs separated by 0.03 s-0.05 s time intervals to obtain individualrealizations. The results were averaged over 170 instantaneous velocity field realizations, whichcorrespond to approximately 12 pulsing cycles. 0.881 0.6 0.22E -0.8 -0.7 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0 r/d (C) Fig. 7. Phase-locked velocity vector fields of the flow generated by specific morphologicalstages in the pulsing cycle of a Cassiopea medusa of 6 cm maximum bell diameter (d). Thepositions of the bell and oral arms of the medusa specific to the particular flow field are shown.The image pairs used for determining individual realizations of vector fields were separated by 1s, equal to the period of pulsing of the medusa. The separation interval between the two imagesin any image pair was set to 0.03 s. The results shown herein were obtained by averaging over 10pulsing cycles, where the positions of the bell and oral arms were identical across all theindividual realizations used toward the averaging process. (a) shows the phase of pulsingimmediately prior to completion of the power stroke, (b) shows the pulsing phase correspondingto full contraction of the bell and (c) shows the phase corresponding to full relaxation of the bell. Fig. 8. Cross-stream (radial) distribution of the phase-averaged vertical (axial) velocity for theflow generated by a Cassiopea medusa of 6 cm maximum bell diameter. The velocities areshown for four dimensionless heights from the bottom of the tank (x/d). The oral-aboral axis ofthe medusa is located at r/d=0, and only the left half of the flow induced by the medusa isshown. The coordinate system and velocity notations are as in Fig.2. Fig. 9. Axial distribution of the radial component of phase-averaged flow velocity generated by aCassiopea medusa of 6 cm maximum bell diameter. Velocities are shown for four positions ofvarying distance from the center of the bell (r/d). These radial locations correspond to the lefthalf of the flow field; r/d =0 indicates the oral-aboral axis, r/d= -0.5 indicates the edge of thebell margin and r/d=-1.0348 indicates the farthest location from the medusa. Fig. 10. Phase-averaged vorticity field (@) of the flow generated by a Cassiopea medusa of 6 cmmaximum bell diameter. The contours have been normalized with the maximum value ofvorticity (@,max = 1.3643 s). Note the absence of coherent vortex rings as a result of thepresence of the oral arms. Fig.11. Schematic of the flow induced by bell pulsations of a Cassiopea medusa in upside-downorientation. Note the presence of the starting vortex ring (SVR) about the oral-aboral axis ofradial symmetry (OA). The specific morphological configurations of the bell and oral arms arealso shown. (a) corresponds to the power stroke where the bell undergoes contraction, and (b)corresponds to the recovery stroke where the bell undergoes relaxation. (a) (b) (c) Fig.12. Instantaneous streamlines of the flow generated by pulsing of a Cassiopea medusa of 6cm maximum bell diameter. (a) shows the phase of pulsing immediately prior to completion ofthe power stroke, (b) shows the pulsing phase corresponding to full contraction of the bell and(c) shows the phase corresponding to full relaxation of the bell.
确定

























还剩33页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《朝天水母中生成的摄食和交换水流研究检测方案(粒子图像测速)》,该方案主要用于其他中生成的摄食和交换水流研究检测,参考标准--,《朝天水母中生成的摄食和交换水流研究检测方案(粒子图像测速)》用到的仪器有德国LaVision PIV/PLIF粒子成像测速场仪、Imager sCMOS PIV相机、PLIF平面激光诱导荧光火焰燃烧检测系统
推荐专场
相关方案
更多
该厂商其他方案
更多























