目标:本研究的目的是测量人类颌骨中钛的含量,并证明钛是从植入颌骨的牙齿中释放出来的。方法:以4例种植体患者7例为试验组,6例未种植体患者6例相似地形区骨标本为对照。采用电感耦合等离子体发射光谱法测定了人颌骨中各种元素的含量。用激光烧蚀-电感耦合等离子体质谱法(LA-ICP-MS)测定了人下颌骨不同同位素的分布。应用光镜对未脱钙、Giemsa-Eosin染色的下颌骨切片进行组织学分析,扫描电镜-能量色散x线对人骨髓颗粒进行鉴别。结果:在测试组相比,钛含量明显高于对照组。试验组Ti平均含量为400μg/kg,对照组为634μg/kg。人体下颌骨Ti含量*,为37,700μg/kg。在样本4-7(人体受试者II-IV)中,在距种植体556-1587μm的下颌组织中,LA-ICP-MS检测到Ti强度增加,且强度随着距种植体距离的减小而增大。在4-7个样本中,距种植体60-700μm距离的人类颌骨骨髓组织中发现了0.5-40μm大小的颗粒。
方案详情

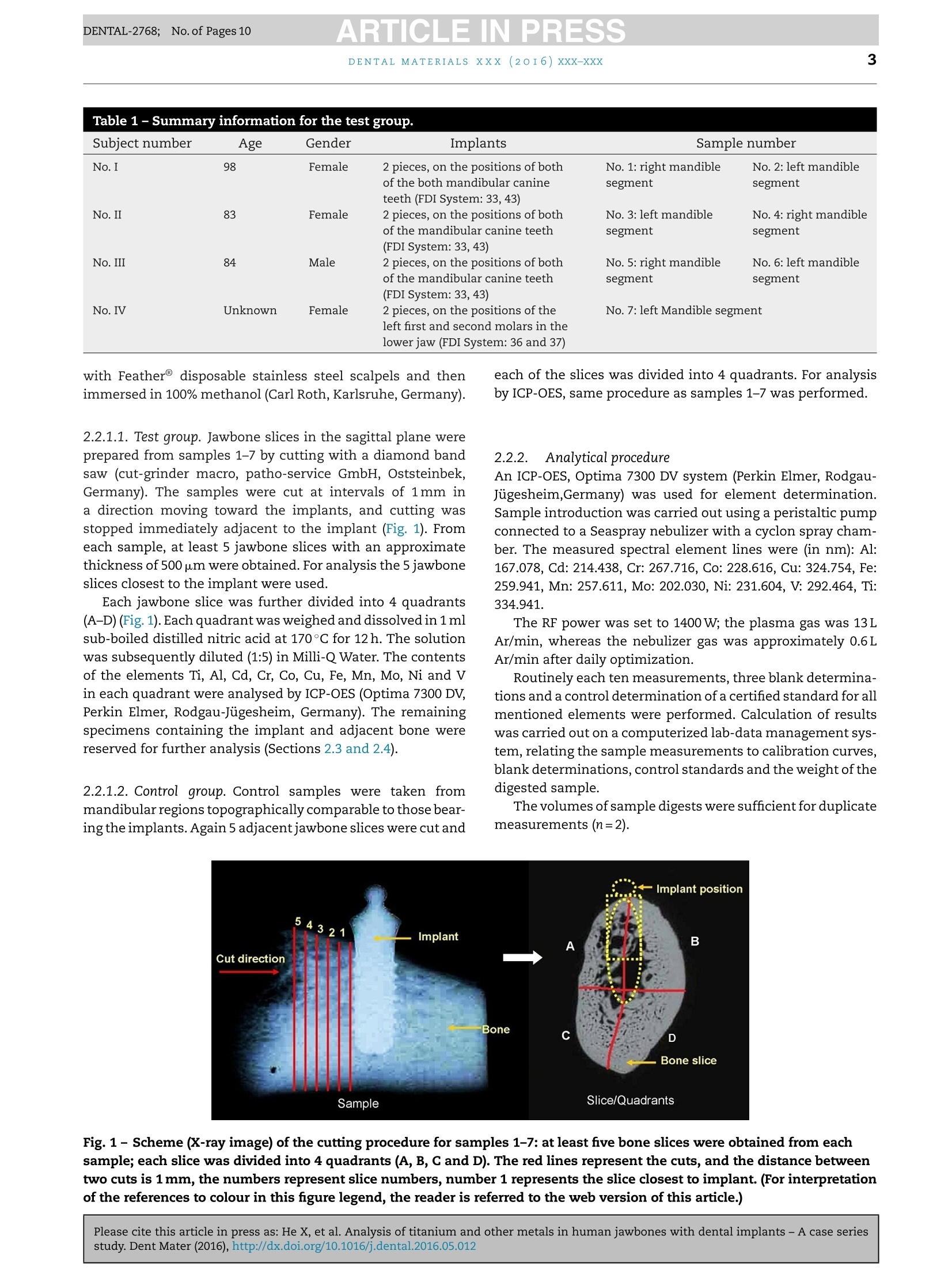
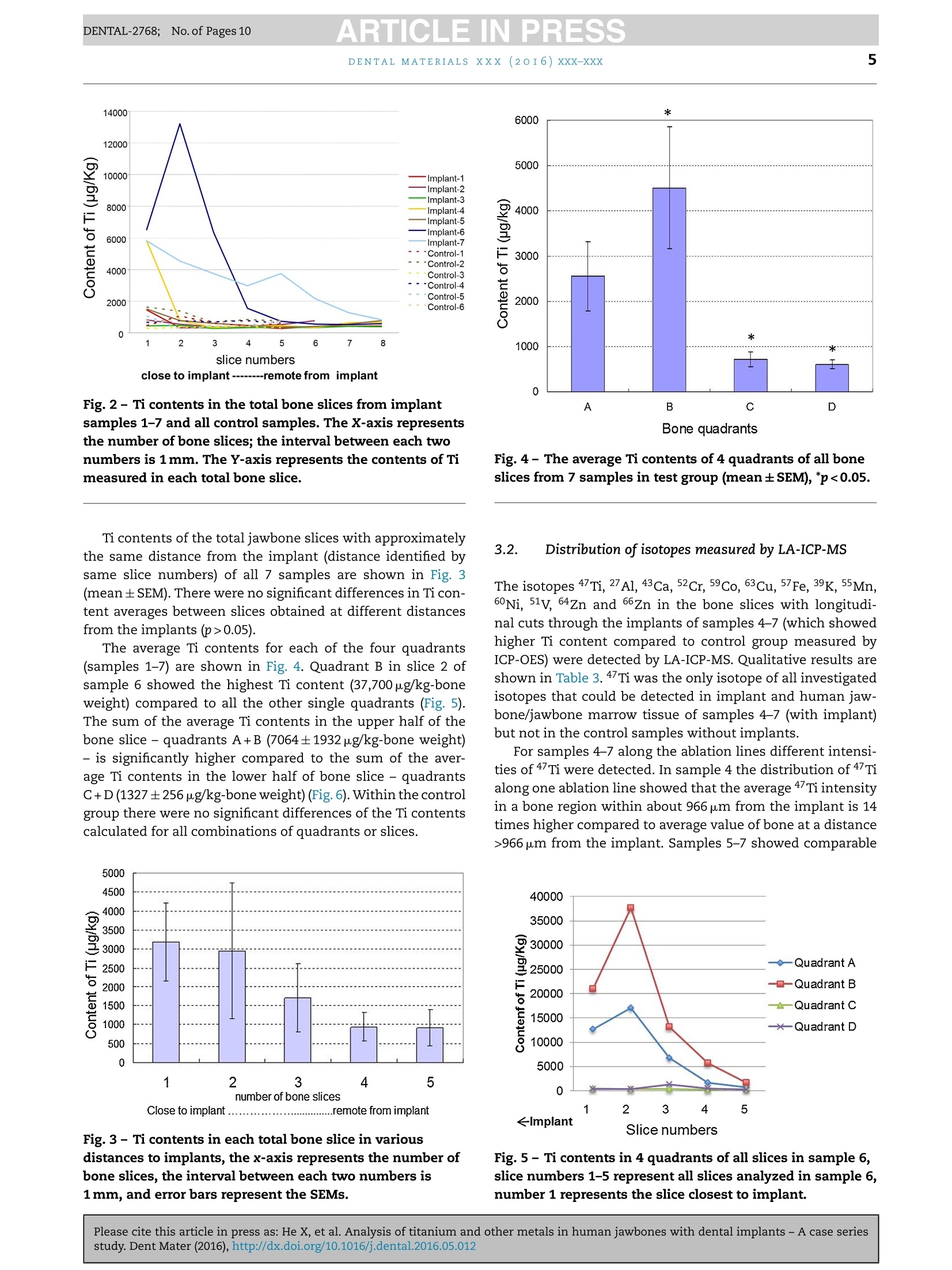
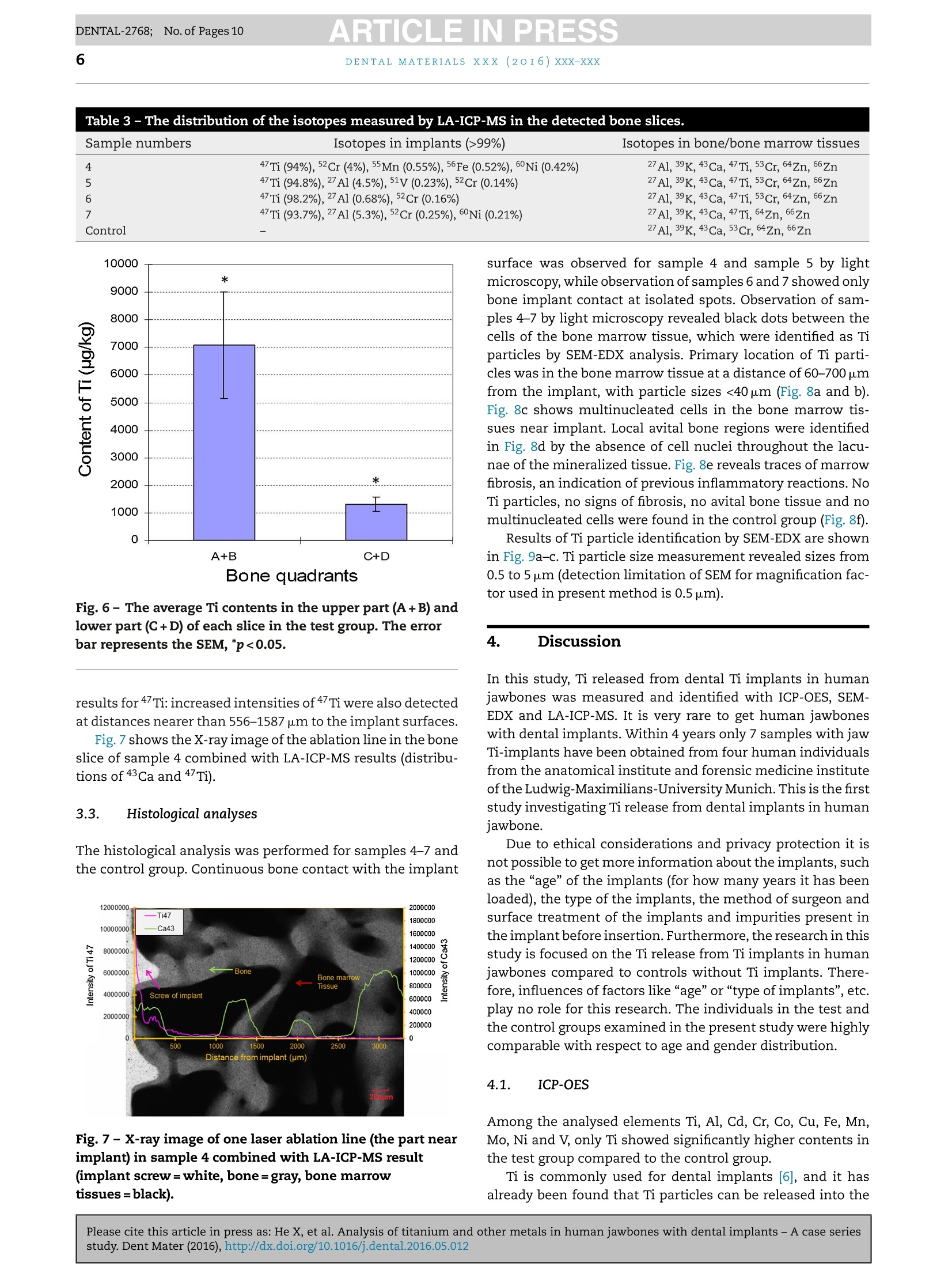
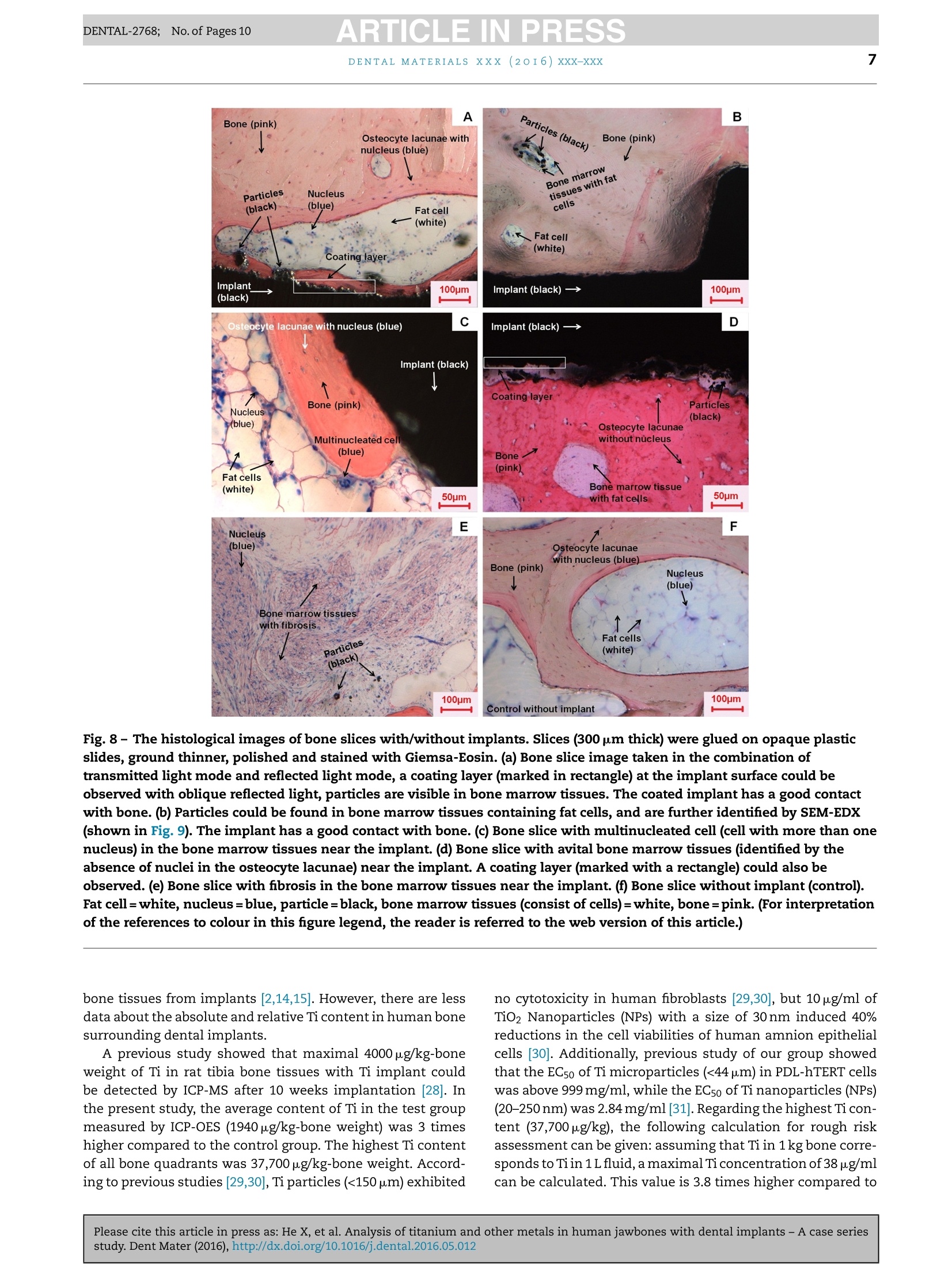
ARTICLE IN PRESSDENTAL-2768; No. of Pages 10DENTAL MATERIALSxxx (20I6) xxx-xxxAvailable online at www.sciencedirect.comScienceDirect ARTICLE IN PRESSDENTAL-2768; No. of Pages 102DENTAL MATERIALS XXX (2OI6)xxx-xxx journal homepage: www.intl.elsevierhealth.com/journals/dema Analysis of titanium and other metals in humanjawbones with dental implants - A case seriesstudy Xiuli Hea,b, Franz-Xaver Reichla,b, Yan Wanga,b, Bernhard Michalke,Stefan Milzd, Yang Yanga,b, Philipp Stolper, Gabriele Lindemaierf,Matthias Grawi, Reinhard Hickelb, Christof Hogg a,b,* a Department ofOperative/Restorative Dentistry, Periodontology and Pedodontics, Ludwig-Maximilians-Universityof Munich, Goethestr. 70, 80336 Munich, Germany Walther-Straub-Institute of Pharmacology and Toxicology, Ludwig-Maximilians-University of Munich,Nussbaumstr. 26, 80336 Munich, Germany C Research Unit Analytical Biogeochemistry, Helmholtz Zentrum Munich- German Research Center forEnuironmental Health (GmbH), Ingolstaedter Landstrae 1, 85764 Neuherberg, Germany ° Department of Anatomy II -Neuroanatomy, Ludwig-Maximilians-University of Munich, Pettenkoferstr. 11,80336 Munich, Germany e Fogra Forschungsgesellschaft Druck e.V., Streitfeldstr 1, 81673 Munich, Germany Institute of Forensic Medicine,Ludwig-Maximilian-University of Munich, Nussbaumstr. 26, 80336 Munich,Germany Article history: Received 27 October 2015 Received in revised form 23 December 2015 Accepted 31 May 2016 Available online xxx Keywords: Titanium content Human jawbones Dental implants Ti particles Objective. The aim of this study was to measure titanium (Ti) content in human jawbones and to show that Ti was released from dental implants inserted into these jawbones.Methods. Seven samples from four human subjects with dental implants were analysed astest group and six bone samples of similar topographical regions from six human sub-jects without implants served as control. The contents of various elements in humanjawbones were detected by inductively coupled plasma optical emission spectrometry. Thedistributions of various isotopes in human mandibular bone were measured with laserablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). Histological analy-ses of undecalcified, Giemsa-Eosin stained mandible sections were performed by lightmicroscopy and particles were identified in human bone marrow by scanning electronmicroscope-energy dispersive X-ray analysis. Results. In test group only Ti content was significantly higher compared to control group.Themean contents of Ti were 1940 ug/kg in test group and 634 ug/kg in control group. The highestTi content detected in human mandibular bone was 37,700 pg/kg-bone weight. In samples4-7 (human subjects II-IV), increased Ti intensity was also detected by LA-ICP-MS in humanmandibular tissues at a distance of 556-1587 um from implants, and the intensity increasedwith decreasing distance from implants. Particles with sizes of 0.5-40 um were found inhuman jawbone marrow tissues at distances of 60-700 um from implants in samples 4-7. * Corresponding author at: Department of Operative/Restorative Dentistry, Periodontology and Pedodontics, LMU Munich, Goethestr. 70,80336 Munich, Germany. Tel.: +49 89 2180 73842; fax:+49 89 2180 73841. E-mail address: christof.hoegg@lrz.uni-muenchen.de (C. Hogg). http://dx.doi.org/10.1016/j.dental.2016.05.012 0109-5641/ 2016 The Academy of Dental Materials. Published by Elsevier Ltd. All rights reserved. Significance. Ti released from dental implants can be detected in human mandibular bone and bone marrow tissues,and the distribution of Ti in human bone was related to the distance to the implant. C 2016 The Academy of Dental Materials. Published by Elsevier Ltd. All rights reserved. 1. Introduction In the last 50 years titanium (Ti) and its alloys have beenwidely used for dental and medical applications [1,2]. Clini-cally, pure Ti (Ti>99%) and the alloy Ti-6Al-4V are mainly usedfor endosseous implants [2]. Ti alloys with other metals (e.g.nickel (Ni), chromium (Cr), iron (Fe), and molybdenum (Mo))are applied for implant devices such as bone plates or screws[2,3]. Ti is the main component in those implants/implantdevices so that a stable TiO2 layer [4,5] on the surface canprovide Ti based implants/implant devices with a high bio-compatibilityand resistance to corrosion [6-9]. Although Ti based implants have been considered to bebiological inert, it has been found that implants in the bodycan undergo corrosion and release particulate debris overtime[10,11]. It has been reported that the metallic debrisfrom Ti based implants might exist in several forms includ-ing particles (micrometer to nanometre size), colloidal andionic forms (e.g. specific/unspecific protein binding) [12,13],organic storage forms (e.g. hemosiderin, as an iron-storagecomplex), inorganic metal oxides and salts [12]. Accordingto a previous study [14], the degradation of implants in thehuman body is primarily induced by wear and corrosion:wear is the mechanical/physical form of implant degrada-tion which produces particles; while corrosion refers to thechemical/electrochemical form of degradation that mainlyproduces soluble metal ions [14]. Ti particles released fromimplants have been found in the regenerated bone and peri-implant tissues in animals [7,15]. It has been shown thatalso Ti ions can be released from embedded implants inanimals [16,17]. Ti particles/ions are able to enter the circu-lation of blood and lymph [12,18,19]. Ti particles detachedfrom hip, knee and mandible implants have been detectedin organs such as liver, spleen, lung and lymph nodes [5,19].Increased levels of elementary Ti have also been detectedin the blood of patients with poorly functioning implants[12]. Increased concentrations of metals (e.g. Ti, Cr, Co andAl)derived from implants in body fluids might induce acute orchronic toxicological effects [12]. The long-term effects of Tiderived from implants are still not fully understood, but asso-ciated hypersensitivity and allergic reactions in patients havebeen reported [20,21]. In a clinical study, 0.6% of 1500 patientswere found to exhibit Ti allergic reactions [20]. Additionally,it has been found that detached metal debris from implantsmight cause marrow fibrosis, necrosis and granulomatosis[22-24]. The aim of our study was to measure the release of Tiand other metallic elements from dental implants throughdetailed post-mortem studies of human subjects with den-tal implants. In the present study, Ti released from implantsinserted into human jawbones was identified and quantified, and the spatial distribution of Ti in human jawbones nearimplants was also investigated. 2. Materials and methods 2.1. Materials The test group contained 7 samples from four human subjectswith dental implants (Table 1). The control group contained6 bone samples from similar topographical regions from sixhuman subjects without dental implants. The bodies were individually donated for medical research.Subjects I-III in the test group and all six of the subjectsfrom the control group were obtained during the dissectioncourse of the anatomical institute of the Ludwig-Maximilian-University Munich. Bone implant sample from subject ⅣVwas supplied by the institute of forensic medicine of theLudwig-Maximilian-University Munich. Subjects I-III carriedtwo implants (one on each side of the mandible)replacing themandibular canine teeth (FDI System: 33,43). Subject IV car-ried two implants that replaced the left first and second molarsin the mandible (FDI System: 36 and 37). All the lower jaw-bones in the test group contained no additional natural teeth.Four out of the six control subjects were toothless as well. Theother two control subjects were partiallyedentulous. The aver-age age of the subjects in the test group (excluding subject IV)and control group were 88 and 85 years, respectively. For subjects I-III, each of the two implants with the adja-cent jawbone was taken as a single sample; for subjects ⅣV,two implants with adjacent jawbone on each side were takenas a single sample. Therefore, there were seven implant sam-ples in our experiment (Table 1). Implants used in samples5-6 were produced by Astra Tech implant system (Mannheim,Germany), and the implants used for sample 4 and sample7 were produced by Straumann AG (Basel, Switzerland). Themanufacturers of the implants of samples 1-3 were unknown.Due to ethical consideration, it is not possible to get moreinformation about the implants, such as the“age”of theimplants (for how many years it has been loaded), the typeof the implants and the surface treatment on the implants.Ti dental implants have no identification number to providedetail information (e.g. for age determination). 2.2. Sample analysis by inductively coupled plasmaoptical emission spectrometry (ICP-OES) 2.2.1. Sample preparation All 7 samples of test group and all 6 samples of controlgroup had been fixed in 4% buffered formalin (Merck, Darm-stadt, Germany). The soft tissue was cautiously removed Subject number Age Gender Implants Sample number No. I 98 Female 2 pieces,on the positions of both No. 1: right mandible No. 2: left mandible No. II 83 Female of the both mandibular canine segment segment teeth (FDI System: 33, 43) 2pieces, on the positions of both No. 3: left mandible No. 4: right mandible of the mandibular canine teeth (FDI System: 33, 43) 2 pieces, on the positions of both segment segment No. III 84 Male No.5: right mandible No.6: left mandible of the mandibular canine teeth (FDI System: 33, 43) 2 pieces, on the positions of the left first and second molars in the lower jaw (FDI System: 36 and37) segment segment No. 7:left Mandible segment No. IV Unknown Female with Feather disposable stainless steel scalpels and thenimmersed in 100% methanol (Carl Roth,Karlsruhe, Germany). 2.2.1.1. Test group. Jawbone slices in the sagittal plane wereprepared from samples 1-7 by cutting with a diamond bandsaw (cut-grinder macro, patho-service GmbH, Oststeinbek,Germany). The samples were cut at intervals of 1mm ina direction moving toward the implants, and cutting wasstopped immediately adjacent to the implant (Fig. 1). Fromeach sample, at least 5 jawbone slices with an approximatethickness of 500 um were obtained. For analysis the 5 jawboneslices closest to the implant were used. Each jawbone slice was further divided into 4 quadrants(A-D) (Fig.1).Each quadrant was weighed and dissolved in 1 mlsub-boiled distilled nitric acid at 170°C for 12 h. The solutionwas subsequently diluted (1:5) in Milli-Q Water.The contentsof the elements Ti, Al, Cd, Cr, Co, Cu, Fe, Mn, Mo, Ni and Vin each quadrant were analysed by ICP-OES (Optima 7300 DV,Perkin Elmer, Rodgau-Jugesheim, Germany). The remainingspecimens containing the implant and adjacent bone werereserved for further analysis (Sections 2.3 and 2.4). 2.2.1.2. Control group. Control samples were taken frommandibular regions topographically comparable to those bear-ing the implants. Again 5 adjacent jawbone slices were cut and each of the slices was divided into 4 quadrants. For analysisby ICP-OES,same procedure as samples 1-7 was performed. 2.2.2..Analytical procedure An ICP-OES, Optima 7300 DV system (Perkin Elmer,Rodgau-Jugesheim,Germany) was used for element determination.Sample introduction was carried out using a peristaltic pumpconnected to a Seaspray nebulizer with a cyclon spray cham-ber. The measured spectral element lines were (in nm): Al:167.078, Cd: 214.438, Cr: 267.716, Co: 228.616, Cu: 324.754, Fe:259.941, Mn: 257.611, Mo: 202.030, Ni: 231.604, V: 292.464, Ti:334.941. The RF power was set to 1400 W; the plasma gas was 13LAr/min, whereas the nebulizer gas was approximately 0.6LAr/min after daily optimization. Routinely each ten measurements, three blank determina-tions anda control determination of a certified standard for allmentioned elements were performed.Calculation of resultswas carried out on a computerized lab-data management sys-tem,relating the sample measurements to calibration curves,blank determinations, control standards and the weight of thedigested sample. The volumes of sample digests were sufficient for duplicatemeasurements (n=2). Fig. 1- Scheme (X-ray image) of the cutting procedure for samples 1-7: at least five bone slices were obtained from eachsample; each slice was divided into 4 quadrants (A, B,C and D). The red lines represent the cuts, and the distance betweentwo cuts is 1 mm, the numbers represent slice numbers, number 1 represents the slice closest to implant. (For interpretationof the references to colour in this figure legend, the reader is referred to the web version of this article.) 2.2.3.CCalculations and statistics For a total jaw bone slice the content (ug/kg)of each inves-tigated element was calculated by dividing the summedconcentrations of the 4 quadrants with their summed masses. The results are presented as mean±standard error of themean (SEM). Independent two-sample t-test and a one-wayANOVA analysis followed by post hoc Bonferroni adjustmentwere performed for statistical analysis. Differences were con-sidered statistically significant only when the p-value was lessthan 0.05 (p<0.05) [25]. 2.3. Content distribution measured by LaserAblation-Inductively Coupled Plasma-Mass Spectrometry(LA-ICP-MS) 2.3.1. Sample preparation The remaining parts of samples 4-7 (the bone surroundingthe implants) and bone samples from the control group weredehydrated in ascending ethanol fractions (70%,80%, 90% and100%), defatted in xylene (Merck, Darmstadt, Germany), andembedded in methylmethacrylate (Fluka, Switzerland). Sam-ples 4-7 were analysed because in these samples significantlyhigher Ti contents (compared to controls) were detected withICP-OES measurement in Section 2.2. Details of the embedding and cutting process can be foundin a previous study [26]. The polymerized methacrylate blockswere cut at a thickness of approximately 300 um parallel tothe long axis of the implants in the mesio-distal plane usinga Leica SP 1600 saw-microtome (Leica, Wetzlar, Germany).The sections were used for spectroscopy and for histologicalanalysis. The distribution of the isotopes 47Ti, 27Al, 43Ca, 52Cr,59Co,63Cu. 57Fe, 39K, 55Mn, 60Ni, 51v, 64Zn and 66Zn in each sec-tion were analysed by Laser Ablation-Inductively CoupledPlasma-Mass Spectrometry (LA-ICP-MS)- NWR-213 (NewWave Research Co. Ltd.) coupled to a NexION 300 ICP-MS(PerkinElmer). 2.3.2. Analytical procedure The laser ablations started from the bone region at a dis-tance of 5 mm from the implant and ended in the implantregion. In this manner, sample-specific backgrounds couldbe measured by moving from low to high contents [27], andmemory effects were avoided. Two to three parallel ablationlines with each time 6 measurements into the deep (10 pmdepth ablation per measurement) were performed for eachjawbone slice. The average value of 2-6 measurements wascalculated for each line (the first measurement was discardedin order to eliminate possible contamination on the surface ofthe bone sections which could have occurred during the cut-ting process). Instrument settings and parameters are shownin Table 2. After the laser ablations, contact radiographs (Faxitron X-ray Corporation, Lincolnshire, IL, USA) for each section weretaken on Agfa Strukturix X-ray sensitive film (Agfa-Gevaert,Mortsel, Belgium). The laser lines were clearly visible onthe X-ray film and were photographed with an Axiophotmicroscope (Zeiss, Goettingen, Germany),equipped with ZeissPlan-Neofluar objectives (5x and 10x) in transmitted lightmode. Table 2 -Instrument settings for LA-ICP-MS. Laser Energy: 0.930 mJ Power: 100% Pulse repetition rate: 10Hz Scan speed: 35 p.m/s ICP Spot size: 50um Ablation pattern: line Depth: approx. 10 pm Plasma power: 1200 W Transport gas: 1.2 L/min Ar Auxiliary gas: 0.8L/min Ar Cool gas:17 L/min Ar Registered isotopes: 47Ti, 27Al, 43 Ca,Cr,Co,63Cu, 57Fe,39K,55Mn,60Ni, 51v,64Zn and 65Zn MS Dwell time/isotope: 25 ms 2.4. Histological analysis 2.4.1. Sample preparation From each of the samples 4-7, one of the sections (cuttingprocedure described in Section 2.3.1) was glued (Cyanolit201, Panacol LTD., Ziirich, Switzerland) on opaque plasticslides,ground thinner, polished (EXAKT 400CS grinding sys-tem, EXAKT Vertriebs GmbH, Norderstedt, Germany) andstained with Giemsa-Eosin stain (Sigma Aldrich, Steinheim,Germany). The same procedure was applied for the control samples. 2.4.2.Light microscopy obseruation and scanning electronmicroscope-energy dispersive X-ray (SEM-EDX) measurements The stained sections were examined with an Axiophot micro-scope (Zeiss, Goettingen, Germany) that was equipped withZeiss Plan-Neofluar objectives (5x and 10x) in transmittedlight mode. Images were recorded with an Axciocam HRc dig-ital camera (Zeiss, Goettingen, Germany). The sections that contained visible dark particles werecoated with gold and investigated with a SEM (EVD MA 15)equipped with secondary electron (SE) and back-scatteredelectron (BSE) detectors. The energy dispersive X-ray (EDX)analyses were performed using an Oxford INCA Penta Fetx 3. The samples of the control group were also histologicallyinvestigated, coated with gold and analysed by SEM-EDX. 3. Results 3.1. Sample analysis by ICP-OES Among all the investigated elements (Ti, Al, Cd, Cr, Co, Cu,Fe, Mn, Mo, Ni and V), only the element Ti in the test group(samples 1-7) showed significantly higher content comparedto the control group (p<0.05). Ti contents of each total jawbone slice from samples 1-7and control group are shown in Fig. 2. The highest Ti con-tent (13,205 pg/kg-bone weight) of all slices was detected insample 6. The average Ti content across all 35 bone slices(n=35) of samples 1-7 (1940±469pg/kg-bone weight) was sig-nificantly higher compared to the average content across the30 bone slices (n=30) from the control group (634±58pg/kg-bone weight). Fig. 2- Ti contents in the total bone slices from implantsamples 1-7 and all control samples. The X-axis representsthe number of bone slices; the interval between each twonumbers is 1 mm. The Y-axis represents the contents of Timeasured in each total bone slice. Ti contents of the total jawbone slices with approximatelythe same distance from the implant (distance identified bysame slice numbers) of all 7 samples are shown in Fig. 3(mean±SEM). There were no significant differences in Ti con-tent averages between slices obtained at different distancesfrom the implants (p>0.05). The average Ti contents for each of the four quadrants(samples 1-7) are shown in Fig. 4. Quadrant B in slice 2 ofsample 6 showed the highest Ti content (37,700 pg/kg-boneweight) compared to all the other single quadrants (Fig. 5).The sum of the average Ti contents in the upper half of thebone slice - quadrants A+B (7064±1932pug/kg-bone weight)- is significantly higher compared to the sum of the aver-age Ti contents in the lower half of bone slice - quadrantsC+D(1327±256pg/kg-bone weight) (Fig.6). Within the controlgroup there were no significant differences of the Ti contentscalculated for all combinations of quadrants or slices. Fig.3- Ticontents in each total bone slice in variousdistances to implants, the x-axis represents the number ofbone slices, the interval between each two numbers is1 mm, and error bars represent the SEMs. .Fig.4- The average Ti contents of 4 quadrants of all boneslices from 7 samples in test group (mean±SEM),*p<0.05 3.2. Distribution of isotopes measured by LA-ICP-MS The isotopes 47Ti, 27 Al, 43 Ca, 52Cr, 59Co, 63Cu, 57Fe, 39K, 55Mn,60Ni, 51v, 64Zn and 66Zn in the bone slices with longitudi-nal cuts through the implants of samples 4-7 (which showedhigher Ti content compared to control group measured byICP-OES) were detected by LA-ICP-MS. Qualitative results areshown in Table 3. 47Ti was the only isotope of all investigatedisotopes that could be detected in implant and human jaw-bone/jawbone marrow tissue of samples 4-7 (with implant)but not in the control samples without implants. For samples 4-7 along the ablation lines different intensi-ties of 4 Ti were detected. In sample 4 the distribution of 47Tialong one ablation line showed that the average 4Ti intensityin a bone region within about 966 pm from the implant is 14times higher compared to average value of bone at a distance>966 pm from the implant. Samples 5-7 showed comparable Fig.5- Ti contents in 4 quadrants of all slices in sample 6,slice numbers 1-5 represent all slices analyzed in sample 6,number 1 represents the slice closest to implant. Table 3 - The distribution of the isotopes measured by LA-ICP-MS in the detected bone slices. Sample numbers Isotopes in implants (>99%) Isotopes in bone/bone marrow tissues 4 47Ti(94%),52Cr (4%),55Mn (0.55%), 56Fe (0.52%), 60Ni (0.42%) 27Al, 39K, 43Ca,47Ti, 53Cr, 64Zn,65Zn 5 47Ti (94.8%), 27Al (4.5%), 51V (0.23%), 52Cr (0.14%) 27Al 39K, 43Ca, 47Ti,53Cr,64Zn, zn 6 47Ti i((998.2%), 27Al (0.68%), 52Cr (0.16%) 27Al, 39K, 43Ca, 47Ti, 53Cr, 64Zn, 6 Zn27A1. 39K, 43Ca, 47Ti, 64Zn, 66Zn 7 47Ti(93.7%), 27Al (5.3%), 52Cr (0.25%), 60Ni (0.21%) Control 27Al, 39K, 43 Ca, 53Cr, 64Zn,65Zn Fig.6-The average Ti contents in the upper part (A+B) andlower part (C+D) of each slice in the test group. The errorbar represents the SEM, *p<0.05. results for 47Ti: increased intensities of 47Ti were also detectedat distances nearer than 556-1587 pm to the implant surfaces. Fig.7 shows the X-ray image of the ablation line in the boneslice of sample 4 combined with LA-ICP-MS results (distribu-tions of 43 Ca and 47Ti). 3.3. Histological analyses The histological analysis was performed for samples 4-7 andthe control group. Continuous bone contact with the implant Fig.7-X-ray image of one laser ablation line (the part nearimplant) in sample 4 combined with LA-ICP-MS result(implant screw=white, bone=gray, bone marrowtissues=black). surface was observed for sample 4 and sample 5 by lightmicroscopy,while observation of samples 6 and7 showed onlybone implant contact at isolated spots. Observation of sam-ples 4-7 by light microscopy revealed black dots between thecells of the bone marrow tissue, which were identified as Tiparticles by SEM-EDX analysis. Primary location of Ti parti-cles was in the bone marrow tissue at a distance of 60-700 umfrom the implant, with particle sizes <40 pm (Fig.8a and b).Fig. 8c shows multinucleated cells in the bone marrow tis-sues near implant. Local avital bone regions were identifiedin Fig. 8d by the absence of cell nuclei throughout the lacu-nae of the mineralized tissue. Fig. 8e reveals traces of marrowfibrosis, an indication of previous inflammatory reactions. NoTi particles, no signs of fibrosis, no avital bone tissue and nomultinucleated cells were found in the control group (Fig. 8f). Results of Ti particle identification by SEM-EDX are shownin Fig.9a-c. Ti particle size measurement revealed sizes from0.5 to 5 pm (detection limitation of SEM for magnification fac-tor used in present method is 0.5pm). 4. Discussion In this study, Ti released from dental Ti implants in humanjawbones was measured and identified with ICP-OES, SEM-EDX and LA-ICP-MS. It is very rare to get human jawboneswith dental implants. Within 4 years only 7 samples with jawTi-implants have been obtained from four human individualsfrom the anatomical institute and forensic medicine instituteofthe Ludwig-Maximilians-University Munich. This is the firststudy investigating Ti release from dental implants in humanjawbone. Due to ethical considerations and privacy protection it isnot possible to get more information about the implants, suchas the “age” of the implants (for how many years it has beenloaded), the type of the implants, the method of surgeon andsurface treatment of the implants and impurities present inthe implant before insertion. Furthermore, the research in thisstudy is focused on the Ti release from Ti implants in humanjawbones compared to controls without Ti implants. There-fore, influences of factors like “age”or “type of implants”,etc.play no role for this research. The individuals in the test andthe control groups examined in the present study were highlycomparable with respect to age and gender distribution. 4.1. ICP-OES Among the analysed elements Ti, Al, Cd, Cr, Co, Cu, Fe, Mn,Mo, Ni and V, only Ti showed significantly higher contents inthe test group compared to the control group. Ti is commonly used for dental implants [6], and it hasalready been found that Ti particles can be released into the Fig.8- The histological images of bone slices with/without implants. Slices (300 um thick) were glued on opaque plasticslides, ground thinner,polished and stained with Giemsa-Eosin. (a) Bone slice image taken in the combination oftransmitted light mode and reflected light mode, a coating layer (marked in rectangle) at the implant surface could beobserved with oblique reflected light, particles are visible in bone marrow tissues. The coated implant has a good contactwith bone. (b) Particles could be found in bone marrow tissues containing fat cells, and are further identified by SEM-EDX(shown in Fig.9). The implant has a good contact with bone. (C) Bone slice with multinucleated cell (cell with more than onenucleus) in the bone marrow tissues near the implant. (d) Bone slice with avital bone marrow tissues (identified by theabsence of nuclei in the osteocyte lacunae) near the implant. A coating layer (marked with a rectangle) could also beobserved. (e) Bone slice with fibrosis in the bone marrow tissues near the implant. (f) Bone slice without implant (control).Fat cell=white, nucleus=blue, particle=black, bone marrow tissues (consist of cells)=white, bone=pink. (For interpretationof the references to colour in this figure legend, the reader is referred to the web version of this article.) bone tissues from implants [2,14,15]. However, there are lessdata about the absolute and relative Ti content in human bonesurrounding dental implants. A previous study showed that maximal 4000 pg/kg-boneweight of Ti in rat tibia bone tissues with Ti implant couldbe detected by ICP-MS after 10 weeks implantation [28]. Inthe present study, the average content of Ti in the test groupmeasured by ICP-OES (1940 pg/kg-bone weight) was 3 timeshigher compared to the control group. The highest Ti contentof all bone quadrants was 37,700 pg/kg-bone weight. Accord-ing to previous studies [29,30], Ti particles (<150 um) exhibited no cytotoxicity in human fibroblasts [29,30], but 10 ug/ml ofTiO2 Nanoparticles (NPs) with a size of 30 nm induced 40%reductions in the cell viabilities of human amnion epithelialcells [30]. Additionally, previous study of our group showedthat the EC5o of Ti microparticles (<44 um) in PDL-hTERT cellswas above 999 mg/ml, while the ECso of Ti nanoparticles (NPs)(20-250nm) was 2.84 mg/ml [31]. Regarding the highest Ti con-tent (37,700pg/kg), the following calculation for rough riskassessment can be given: assuming that Ti in 1kg bone corre-sponds to Tiin 1L fluid, a maximal Ti concentration of 38 ug/mlcan be calculated. This value is 3.8 times higher compared to Fig. 9- SEM image and EDX analysis of the section with particles in Fig. 8(b).(a) Backscattered (BSD) SEM image: Tiimplant=white gray, particles=white gray, bone marrow tissues=dark gray, bone=gray. (b) Magnification of the region withparticles shown in (a) by SEM. Particles (white gray) could be visualized inside the bone marrow tissues (gray). (c) EDXanalysis of the region with particles in (a). On the left is the SEM image of the detected region, in the middle is the intensityof Ca in detected region (bone=gray, bone marrow tissue=black), and on the right is the intensity of Ti in detected region (Tiparticles=white gray). The particles in the bone marrow tissues in Fig. 8(b) are identified as Ti particles. reported cytotoxic concentration (10ug/ml) of TiO2 NPs [30]and 74 times lower compared to the EC5o (2.84mg/ml) of TiNPs [31]. ICP-OES results revealed no distance-dependence of the4/Ti distributions in the total jawbone slices (slice 1-5) of eachsample, but the 47Ticontents in the upper quadrants A+B ofthe bone slices in test group were 5-time higher comparedto the lower quadrants C+D. The bone quadrants A+B areanatomically regarded as the alveolar bone, which is knownto have a remodeling behavior reacting very sensitive to thetransmittedload. Previous studies also reported that the max-imum stress (e.g. masticatory forces) is distributed around theimplant neck which is surrounded by alveolar bone [32,33].Obviously, this might explain the higher 47Ti content in theupper quadrants in the current study. Moreover, the main partof the implant is anchored in the upper part of the jawbone,so the upper quadrants A+B of the bone slices are closer tothe implant compared to the lower quadrants C+D, and thisshorter distance to implant may result in the higher 47Ti con-tent as well. Additionally, the upper quadrants might receive aconsiderable particle load during the insertion of the implant,which could also lead to higher 47Ti content in the upper quad-rants. 4.2. LA-ICP-MS Due to its flexible spatial analysis capability and high sen-sitivity, LA-ICP-MS is applied to measure elements, suchas Ca, Mg and Sr in bones [27,34]. Moreover, this tech-nique is able to detect unique elemental distributions inmicro-spatial areas of dental tissuesSI[35]. In the present study, LA-ICP-MS was used to analyse the region immediatelyadjacent to the implant because bone slice samples in thisregion cannot be obtained by the cutting method used forICP-OES. The distributions of 13 isotopes 47Ti, 27 Al, 43 Ca, 52Cr, 59Co,63G 57Fe 39x 55Mn, 60Ni, 51v 64Zn and 66Zn in bone sam-ples 4-7 with implants were measured by LA-ICP-MS. In bonesamples without implant no increased 47Ti was detected.Increased intensity of 47Ti for samples 4-7 was detected inthe bone region adjacent to the implant (about 556-1587 umaway from the implant surface). In a previous study, Ti parti-cles from the implant were found in the peri-implant sheeptissue at a distance >200 pm from the implant [15].Obviously,Ti can migrate from the implant into the surrounding humanjawbone or jawbone marrow tissues. In addition, the intensityof 4/Ti increased with decreasing distance from the implants,which suggests that the distribution of 4 Ti in the jawbonedepends on the distance from the implant. This finding is con-sistent with the ICP-OES results showing that Ti content inupper quadrants A+B (closer to the implant) is significantlyhigher compared to the lower quadrants C+D. The lack ofobservations of distance-dependence across the total boneslices by ICP-OES might be attributable to the cutting methodnot being able to obtain jawbone slices that were sufficientlyclose to the implant. 43Ca can be taken as an indicator of bone [27]. In thisstudy, 43Ca distribution along the ablation line in the boneslices is shown. Interestingly, 47Ti is present not only in theperi-implant human jawbone marrow tissues but also in thecortical or trabecular bone matrix adjacent to the implant.This is in agreement with previously reported results showing that Ti particles are found not only in peri-implant animaltissues but also in the bone close to the implants [7,15]. 4.3. Histological analyses In the present study, the light microscopy and SEM-EDX resultsshow that Ti particles (0.5-40 um) are present in human jaw-bone marrow tissues at a distance of 60-700 um from theimplant. Nearly identical results have been shown by the fol-lowing studies: Ti particles (from the implant surface) wereable to be found in the peri-implant sheep tissue at a dis-tance >200pm from the implant [15]; Ti particles could notonly be observed in peri-implant tissues but also in newlyformed bone [7,15]. It was suggested that those Ti particlesmight be detached during the insertion of the implant [7,36]or released after insertion [14]. In the current study, SEM results showed Ti particles witha size of 0.5-5 um in human jawbone marrow tissues. Previ-ous studies reported that particles <1 pm could be taken upby non-phagocytic eukaryotic cells via endocytosis [37] andparticles with diameters exceeding 0.75 um can be taken upby macrophages, neutrophils and monocytes via phagocyto-sis or through macropinocytosis (>1 pm) by all cell types [38].Therefore,transportation of Ti particles with size of 0.5-5 umthat were observed in human bone marrow tissues of presentstudy into cells is possible. It should also be pointed out thata particle smaller than approximately 1 um can no longer beseen by transmitted light microscopy. In this study, bone marrow fibrosis, avital bone tissuesand multinucleated cells were observed near the implant.Similarly, a previous post-mortem study about metal particlesreleased from implants showed bone marrow fibrosis inlymph-node tissue[22].Furthermore, multinucleated cellshave been observed after hydroxyapatite implantation inrat periodontal tissues [39]. Bone marrow fibrosis might beinduced by marrow injury and inflammation[40]. Theseeffects are reported to be associated with surgical traumaduring insertion of implants [41]. Multinucleated giant cellscan be elicited by wear particles in periprosthetic tissuesin human specimens and these cells might contain phago-cytized wear particles [42]. These findings cited above arein accordance with the histological observations made inundecalcified, resin embedded human bone and implantsections in the present study. 5. Conclusion Ti can be released into human jawbone and jawbone marrowtissues from dental implants. Compared to all other investi-gated isotopes only 47Ti was observed in both the implants andhuman bone/bone marrow tissues near the implant but not inthe control samples. The intensity of 4 Ti in human jawboneincreased with decreasing distance to implant. Ti particles insize between 0.5 pm and 40 pm were observed in the jawbonemarrow tissues near the implant, which might induce fibro-sis and multinucleated cells. Based on our study with a smallsample size we suggest that Ti dental implants might have noadverse clinical effects. Acknowledgements This study was financially supported by the China ScholarshipCouncil (CSC, 201206220099). We would like to thank Mr. PeterGrill and Mr. Stefan Schulz for the technical support. ( REFERENCES ) ( [1] Lautenschlager EP, Monaghan P. Tit a nium a n d titaniumalloys as dental materials. I n t Dent J 1 9 93;43:245-53 . ) ( [2] Elias C, Lima J , Valiev R , Meyers M. Bio me dical applications of titanium and its alloys. JOM 2 008;60:46-9. ) ( [3] McCracken M . Dental implant m a terials: comm e rcially puretitanium and titanium alloys.J Prosthodont 1 9 99;8:40-3. ) ( [4] K asemo B. Biocompatibility o f titanium implants: surface science a spect s . J P r osthet Dent 1983;49:832-7 . ) ( [5] Schl i ephak e H, R eiss G, Urban R, Neukam F W , G u ckel S . Metal r el e ase f rom titaniu m f i xtures du ri n g placemen t i n the mandible: an e xper im ental stud y . Int JOral Maxillofac I mplants 1993;8:502-11. ) ( [6] Okabe T, Hero H. The use of ti t anium in dentistry. Cells Mater (USA) 1995;5:211-30. ) ( [7] FranchiM, Bacchelli B , Martini D, Pasquale VD, Orsini E, Ottani V , et al . Early detachment of titanium p a rtic l es fromvarious different surfaces o f endosseous dental implants. Biomaterials 2004;25:2239-46. ) ( [8] Adya N, Alam M , Ravindranath T, Mubee n A, Saluja B. Corrosion i n t itanium dental implants: l iterature review.J I ndian Prosthodont Soc 2005;5:127. ) ( [9] Vel a sco-Ortega E , Jos A, Camean AM, Pa to-Mour e lo J, Segura-Eg e a J J . In uitro evaluation of cytotoxicity and genotoxicity of a commercial titanium alloy f or dentalimplantology. Mutat Res/Genet Toxico l Environ Mutagen 2010;702:17-23. ) ( [10] Meachim G, Williams D. Changes in nonosseous tissue a d jacent to titanium implants.J Biomed M ater Res 1973;7:555 - 72. ) ( [11] Galante J O , L e m ons J , Spector M, Wilson J r P D, W right T M . The biologic e ffects of im p lant m aterials. J Orth o p Res 1991;9:760-75. ) ( [12] H a llab NJ, Mi k ecz K , Verm e s C , Skipor A, J acobs JJ.Orthop a edic implant related metal toxicity in terms o f human lymphocyte reactivity t o metal-protein complexes produced from cobalt-base and t itanium-base i mplant a lloyd e gradation. Mol Cell Biochem 2001;222:127-36. ) ( [13] Hallab N J, Jacobs JJ , Skipor A, Black J, Mikecz K, Galante JO. Systemic metal-protein binding associated wit h tot a l joint replacement arthroplasty. J Biomed Mater Res 2000;49:353-61. ) ( [14] J acobs JJ, Gilber t JL, Urban RM. Current concepts review -corrosion of metal orthopaedic i mplants. J Bone Jt Surg1998;80:268-82. ) ( [15] M a r t i ni D , Fini M, Franc h i M, P asquale V D, B acchell i B , GamberiniM , et al. D etachment of titanium and f luo r ohyd r oxyap a tite p art i cles in u nl oaded endosseous implants. Biomaterials 2003;24:1309-16. ) ( [16] Edwin SH. Characteri s tic s of t race ions released from embedded metal implants in the rabbit; 1962. ) ( [17] D u c heyne P,Willems G, Marten s M, He l sen J. I n vivo m etal-ion release from porous titanium-fiber m a terial. J Biom e d Mater Res 1984;18:293-308. ) ( [18] B rien WW, Salvati E A , B e tts F , B ullough P, W right T , RimnacC, et al. Metal level s in cemented t otal hip arthroplasty. A comparison of well - fixed a nd l o ose implants. C l in Orth o p Relat Res 1992:66-74. ) ( [19] U rb a n R M , Jacobs J, Tomlinson MJ, Gavrilovic J, Black J,Peoc'h M . D issemination of wea r particles t o the liver, spleen, and abdominal lymph nodes of patients with hip orknee r e pl a cement. J B one Jt Su r g Am 2000;82:457-76. ) ( [20] Sicilia A, Cuesta S, Coma G, Arregui I, Gu i sasola C , R uiz E,et al. Titanium al l ergy in d ental im pl ant pa t i ents: a c l i nical study o n 1500 consecutiv e p a tients. Clin Oral I mplants R e s2008;19:823-35. ) ( [21] Lalor PA, Revell PA, Gray AB, Wright S, Railto n G T , FreemanMA. S ensitivity t o t itanium. A caus e o f im p lant failure?J Bone Jt Surg Br 1991 ; 73:25-8. ) ( [22] Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, et a l . Widesprea d dissemi n ation of meta l debr i s fromimplants.J Bone Jt S urg Br 1 994;76:701-1 2. ) ( [23] A mstutz HC, Campbell P, Kossovsky N, Clarke IC.Mechanism and clinical significance of wear debris-inducedosteolysis. Clin Orthop R el a t Res 1 992:7-18. ) ( [24] Dannenmaier W C, Haynes DW,Nelson CL. Granulomatous reaction and cystic bony destruction a ssociated w ith high wear rate in a tota l knee prosthesis.Clin Orthop Relat Res1985;22:4 - 30 . ) ( [25] Kim JH, Ju n g Y, Kim B S , K im SH. S tem cell recru i tme n t and angiogenesis of neuropep t ide substance P coupled with self-assembling pe p tide nanofiber i n a mouse hind limb ischemia m odel. B iomateri a ls 2013;34:1657-68. ) ( [26] Milz S, P u tz R . Quantitative morp h ology o f the subchondralplate of the tibia l plateau. J Ana t 1994;185:103 - 10. ) ( [27] Gr u hl S, W itte F, Vogt J , Vogt C. Determin a tion o fconcentration grad i ents in b one tissue generated by a b i ologically degradable magnesium implant. J Anal Atomic S p ectrom 2009;24:181-8. ) ( [28] Niinomi M. M etals f o r biomedical devices. Elsevier; 2010. ) ( [29] Rae T . T he t o xicity of metals used in o r thopaedic pr o stheses. An e xper i mental study using cult u red human sy n ov i alfibroblasts. J Bone Jt Surg Br Vol 1981;63:435-40. ) ( [30] Saquib Q, A l -Khedhairy AA, Siddiqui MA, Abou - Tarboush FM, Azam A, Musarrat J. Titanium dioxide nanoparticles induced c ytotoxicity, oxidative stress and D N A d amage i n human amnion e pithelial (WISH) cells . T o xicol In Vitro 2012;26:351-61. ) ( 31] H e X, Hartlie b E, Rothmund L, Waschke J, Wu X, Van Landuyt KL, et al . Intracellular u p t ake a n d toxicity of three di f ferent titanium part i cles. Dent M ater 2015;31:734-44. ) ( [32] Himml o va L, D o stalova T , Kacovsky A, Konvickova S. Influence o f implan t length and d i ame t er on st r ess ) ( distribution: a finite element analysis. J P rosthet Dent 2004;91:20-5. ) ( [33] Baggi L, Cappelloni I, Di Girolamo M, Maceri F , Vairo G. T heinf l uence of i m plant diameter and len g th on stressdistribution of osseointegrated impla n ts related t o c r estal bone geometry: a three-d i mensional fini te element analysis.J Prosthet Dent 2008;100:422 - 31 . ) ( [34] Prohask a T, Lat ko czy C, S ch u ltheis G, T eschler-Nicola M, Stingeder G. I nvestigation of Sr i sotope ratios in prehistorichuman bones and teeth using laser abl a tion ICP-MS a n d ICP-MS aft e r R b/Sr s epar a ti o n. J Anal Atomic Spectro m 2002;17:887-91. ) [35]Kang D, Amarasiriwardena D, Goodman AH. Application oflaser ablation-inductively coupled plasma-massspectrometry (LA-ICP-MS) to investigate trace metal spatialdistributions in human tooth enamel and dentine growthlayers and pulp. Anal Bioanal Chem 2004;378:1608-15. [36]Flatebo RS, Hol PJ, Leknes KN, Kosler J, Lie SA, Gjerdet NR.Mapping of titanium particles in peri-implant oral mucosaby laser ablation inductively coupled plasma massspectrometry and high-resolution optical darkfieldmicroscopy.JOral Pathol Med 2011;40:412-20. [37]Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependentinternalization of particles via the pathways of clathrin-andcaveolae-mediated endocytosis. Biochem J 2004;377:159-69. [38]Conner SD, Schmid SL. Regulated portals of entry into thecell. Nature 2003;422:37-44. ( [39] Kawaguchi H , Ogawa T, Shirakawa M, Okamoto H, Akisaka T. Ultrastru c tural and u ltracytochemical characteristics of multi n ucleated cells after hydroxyapatite i mplantati o n into rat periodontal t issue. J Periodontal Res 1992;27:48-54. ) [40]Travlos GS. Histopathology of bone marrow. Toxicol Pathol2006;34:566-98. 41]Piattelli A, Piattelli M, Mangano C, Scarano A. A histologicevaluation of eight cases of failed dental implants: is boneoverheating the most probable cause. Biomaterials1998;19:683-90. [42]Anazawa U, Hanaoka H, Morioka H, Morii T, Toyama Y.Ultrastructural cytochemical and ultrastructuralmorphological differences between human multinucleatedgiant cells elicited by wear particles from hip prostheses andartificial ligaments at the knee. Ultrastruct Pathol2004;28:353-9. Please cite this article in press as: He X, et al. Analysis of titanium and other metals in human jawbones with dental implants -A case seriesstudy. Dent Mater (,http://dx.doi.org/j.dental. 目标:本研究的目的是测量人类颌骨中钛的含量,并证明钛是从植入颌骨的牙齿中释放出来的。方法:以4例种植体患者7例为试验组,6例未种植体患者6例相似地形区骨标本为对照。采用电感耦合等离子体发射光谱法测定了人颌骨中各种元素的含量。用激光烧蚀-电感耦合等离子体质谱法(LA-ICP-MS)测定了人下颌骨不同同位素的分布。应用光镜对未脱钙、Giemsa-Eosin染色的下颌骨切片进行组织学分析,扫描电镜-能量色散x线对人骨髓颗粒进行鉴别。结果:在测试组相比,钛含量明显高于对照组。试验组Ti平均含量为400μg/kg,对照组为634μg/kg。人体下颌骨Ti含量最高,为37,700μg/kg。在样本4-7(人体受试者II-IV)中,在距种植体556-1587μm的下颌组织中,LA-ICP-MS检测到Ti强度增加,且强度随着距种植体距离的减小而增大。在4-7个样本中,距种植体60-700μm距离的人类颌骨骨髓组织中发现了0.5-40μm大小的颗粒。
确定






还剩8页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《人类颌骨与牙齿种植中钛和其他金属检测方案(激光剥蚀进样)》,该方案主要用于骨骼中钛和其他金属检测,参考标准--,《人类颌骨与牙齿种植中钛和其他金属检测方案(激光剥蚀进样)》用到的仪器有ESL213 灵活的激光剥蚀系统
推荐专场
相关方案
更多
该厂商其他方案
更多















