方案详情
文
将氧化铁纳米粒子与银纳米粒子、金纳米粒子、银磁铁矿和金磁铁矿分别通过内吞作用引入到成纤维细胞中,形成多功能复合纳米粒子。含有无毒纳米粒子的细胞在外部磁场中是可置换的,可以在微流控通道中进行操作。在表面增强拉曼散射(SERS)映射、激光消融电感耦合等离子体质谱(LA-ICP-MS)微研磨和低温软x射线断层扫描(cryo soft- xrt)的基础上,得到了内体系统中复合纳米结构的分布。完整的玻璃化细胞冷冻软x光检查显示复合纳米粒子包裹体形成内聚体。纳米粒子提供了内质网环境中表面生物分子组成的SERS信号。SERS数据表明,纳米粒子在内质粒和溶酶体的恶劣环境中具有较高的稳定性和等离子体性质。该光谱指向银磁铁矿和金磁铁矿纳米结构表面的分子组成,与其他复合结构非常相似,但不同于研究细胞内纯银和金SERS纳米组成所得到的结构。由LA-ICP-MS数据可知,磁铁矿复合材料的吸附效率大约是纯金、银纳米颗粒的2 - 3倍。
方案详情

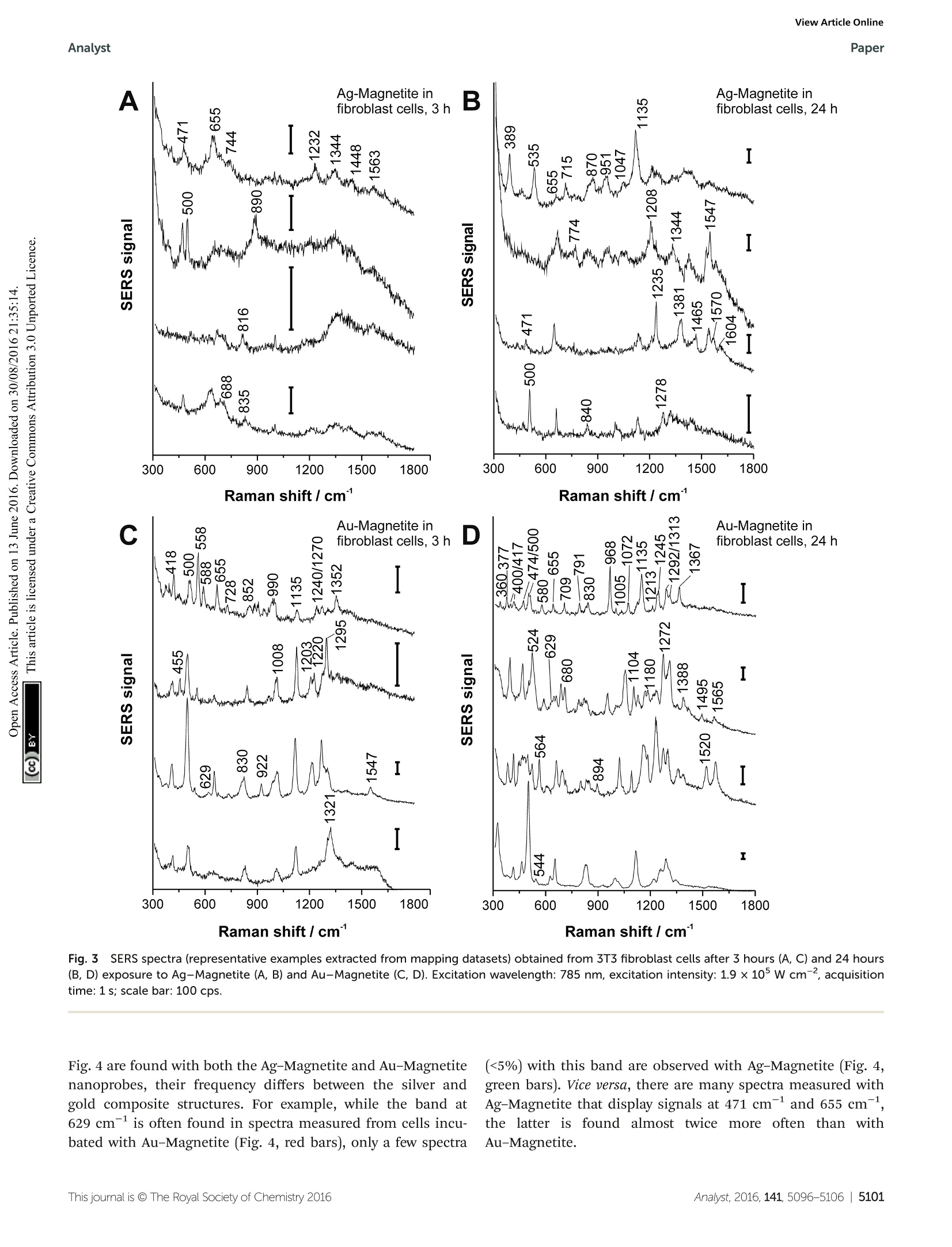
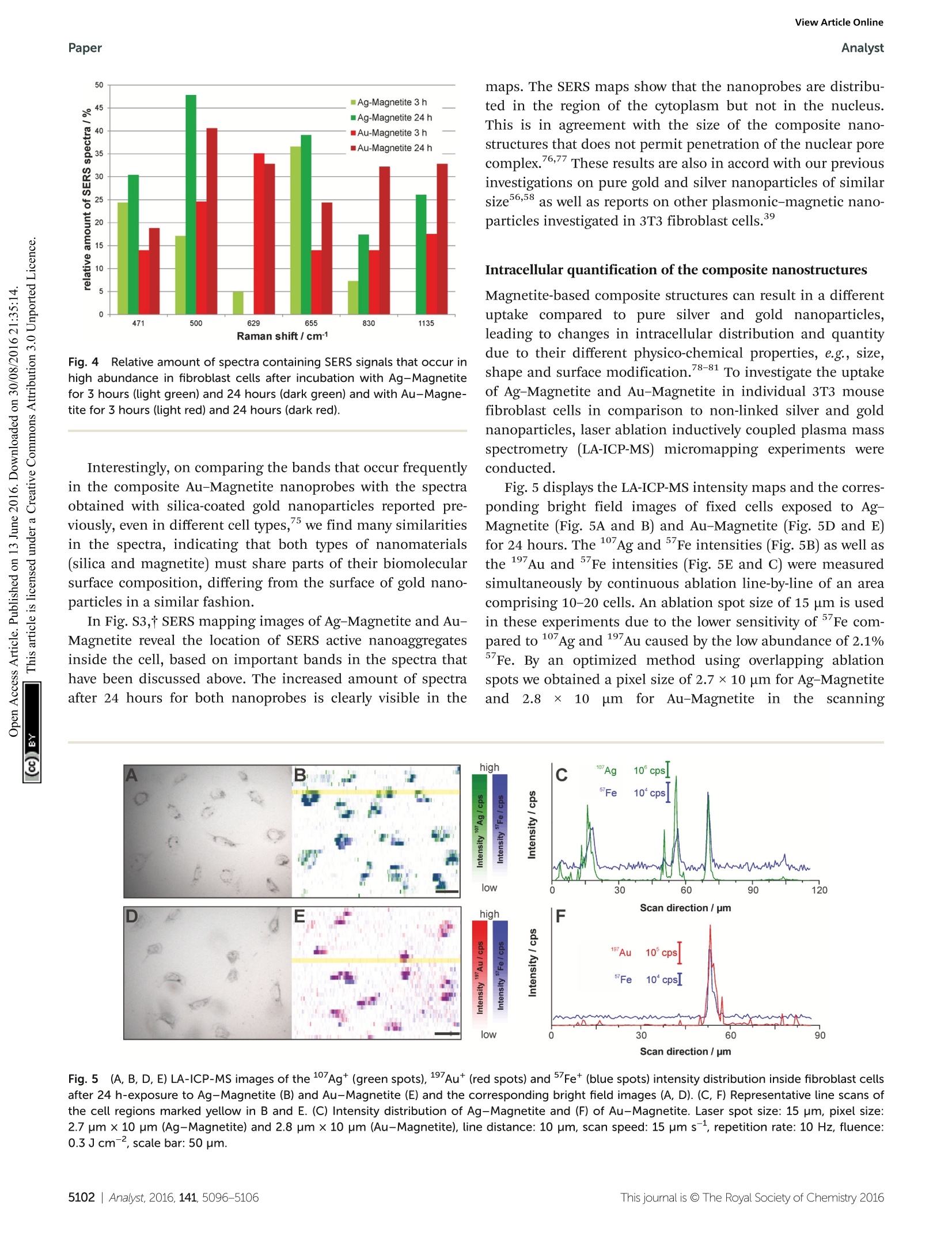
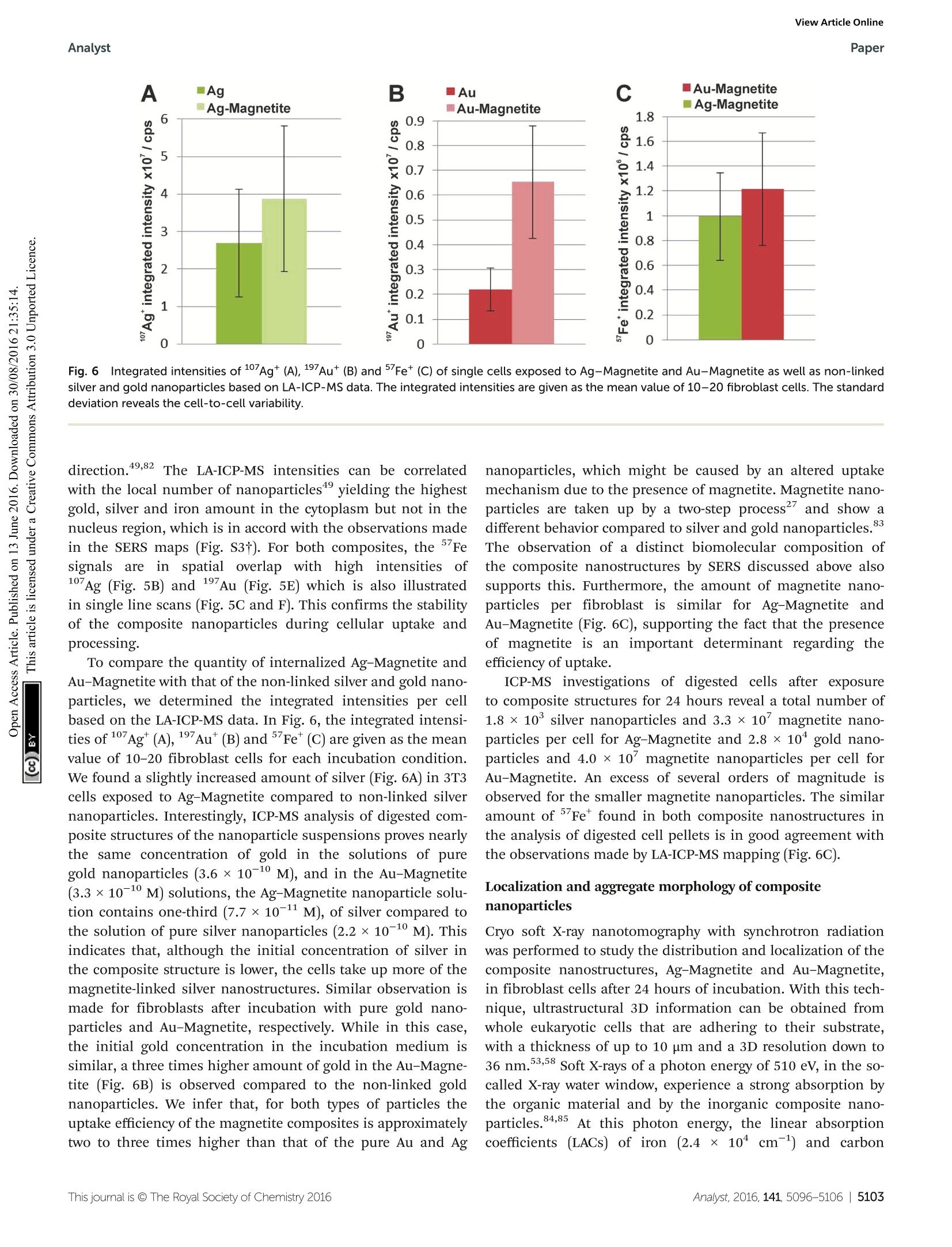
View Article OnlineView Journal|View Issue View Article OnlineAnalystPaper Analyst Themed issue: Surface-enhanced Raman scattering ISSN 0003-2654 PAPER Janina Kneipp et al. Biomolecular environment, quantification, and intracellularinteraction of multifunctional magnetic SERS nanoprobes CrossMark 10 umfor Au-Magnetitee iin theescanning Fig.5(A, B, D, E) LA-ICP-MS images of the 107Ag* (green spots),197Au*(red spots) and 5Fet (blue spots) intensity distribution inside fibroblast cellsafter 24 h-exposure to Ag-Magnetite (B) and Au-Magnetite (E) and the corresponding bright field images (A, D). (C, F) Representative line scans ofthe cell regions marked yellow in B and E. (C) Intensity distribution of Ag-Magnetite and (F) of Au-Magnetite. Laser spot size: 15 pm, pixel size:2.7 pm x 10 pm (Ag-Magnetite) and 2.8 pm x10 pm (Au-Magnetite), line distance: 10 um, scan speed: 15 um s-1, repetition rate: 10 Hz, fluence:0.3Jcm-, scale bar: 50 pm. Fig.6Integrated intensities of 107Ag* (A),197Au*(B) and 5Fe*(C) of single cells exposed to Ag-Magnetite and Au-Magnetite as well as non-linkedsilver and gold nanoparticles based on LA-ICP-MS data. The integrated intensities are given as the mean value of 10-20 fibroblast cells. The standarddeviation reveals the cell-to-cell variability. direction.49,82 The LA-ICP-MS intensities can be correlatedwith the local number of nanoparticles4 yielding the highestgold, silver and iron amount in the cytoplasm but not in thenucleus region, which is in accord with the observations madein the SERS maps (Fig. S3t). For both composites, the 5Fesignals are in spatial overlap withhigh intensities of107Ag (Fig. 5B) and 197Au (Fig. 5E) which is also illustratedin single line scans (Fig. 5C and F). This confirms the stabilityof the composite nanoparticles during cellular uptake andprocessing. To compare the quantity of internalized Ag-Magnetite andAu-Magnetite with that of the non-linked silver and gold nano-particles, we determined the integrated intensities per cellbased on the LA-ICP-MS data. In Fig. 6, the integrated intensi-ties of 17Ag(A),197Au (B) andFe*(C) are given as the meanvalue of 10-20 fibroblast cells for each incubation condition.We found a slightly increased amount of silver (Fig. 6A) in 3T3cells exposed to Ag-Magnetite compared to non-linked silvernanoparticles. Interestingly, ICP-MS analysis of digested com-posite structures of the nanoparticle suspensions proves nearlythe same concentration of gold in the solutions of puregold nanoparticles (3.6 × 10-10 M), and in the Au-Magnetite(3.3×10-10 M) solutions, the Ag-Magnetite nanoparticle solu-tion contains one-third (7.7×10-11 M), of silver compared tothe solution of pure silver nanoparticles (2.2×10-10 M). Thisindicates that, although the initial concentration of silver inthe composite structure is lower, the cells take up more of themagnetite-linked silver nanostructures. Similar observation ismade for fibroblasts after incubation with pure gold nano-particles and Au-Magnetite, respectively. While in this case,the initial gold concentration in the incubation medium issimilar, a three times higher amount of gold in the Au-Magne-tite (Fig. 6B) is observed compared to the non-linked goldnanoparticles. We infer that, for both types of particles theuptake efficiency of the magnetite composites is approximatelytwo to three times higher than that of the pure Au and Ag nanoparticles, which might be caused by an altered uptakemechanism due to the presence of magnetite. Magnetite nano-particles are taken up by a two-step process and show adifferent behavior compared to silver and gold nanoparticles.The observation of a distinct biomolecular composition ofthe composite nanostructures by SERS discussed above alsosupports this. Furthermore, the amount of magnetite nano-particles per fibroblast is similar for Ag-Magnetite andAu-Magnetite (Fig. 6C), supporting the fact that the presenceof magnetite is an important determinant regarding theefficiency of uptake. ICP-MS investigations of digested cells after exposureto composite structures for 24 hours reveal a total number of1.8 × 10’silver nanoparticles and 3.3 × 10’ magnetite nano-particles per cell for Ag-Magnetite and 2.8 x 10 gold nano-particles and 4.0 ×10 magnetite nanoparticles per cell forAu-Magnetite. An excess of several orders of magnitude isobserved for the smaller magnetite nanoparticles. The similaramount ofFe found in both composite nanostructures inthe analysis of digested cell pellets is in good agreement withthe observations made by LA-ICP-MS mapping (Fig. 6C). Localization and aggregate morphology of compositenanoparticles Cryo soft X-ray nanotomography with synchrotron radiationwas performed to study the distribution and localization of thecomposite nanostructures, Ag-Magnetite and Au-Magnetite,in fibroblast cells after 24 hours of incubation. With this tech-nique, ultrastructural 3D information can be obtained fromwhole eukaryotic cells that are adhering to their substrate,with a thickness of up to 10 um and a 3D resolution down to36 nm.53,58 Soft X-rays of a photon energy of 510 eV, in the so-called X-ray water window, experience a strong absorption bythe organic material and by the inorganic composite nano-particles.84,85At this photon energy, the linear absorptioncoefficients (LACs) of iron (2.4 x 1104cm-1) and carbon (4.4×10 cm-l) are similar. In contrast, the LACs of the goldor silver particles are ten times higher (1.8 ×10 cm- forsilver and 2.2×10cm-for gold). Fig. 7 displays the X-ray microscopy images from tilt seriesof cells incubated with Ag-Magnetite (Fig. 7A) and Au-Magne-tite (Fig. 7C), respectively, and a slice of the respectivetomographic reconstructions (Fig. 7B and D). The -ray micro-graphs, as well as Movie S4 and S5t of microscopic tilt seriesin the supporting material show the presence of nanoaggre-gates in the cytoplasm but not in the nucleus for both types ofnanocomposite materials, in agreement with the results of theSERS and LA-ICP-MS mapping experiments described above.Also TEM micrographs of magnetite nanostructures reportedpreviously86 show that magnetite can easily enter the cell inde-pendent of the particle size and for a large range of particlesizes of 70-500 nm. The analysis of the projection images/tomographic slices based on the contrast of the plasmonicparts of the aggregates showed that the sizes of the Ag-Magne-tite and Au-Magnetite nanostructures formed inside the endo-somal structures of the cells are similar (264± 148 nm forAg-Magnetite and 193 ± 133 nm for Au-Magnetite), with sizescovering a wide range between 50 nm and submicron size(Fig. S4t). This resembles the sizes of aggregates of unlinkedsilver and gold nanoparticles (253 ±109 nm for silver nano-particles and 223±135 nm for gold nanoparticles). This is incontrast to other, pure magnetite nanoparticles, which formagglomerates of >600 nm in human breast cells. The tomographic slices (Fig. 7B and D) also display small,nm-sized gaps between the silver and gold nanoparticlesfor both composite substrates. Thus, based on our knowledgefrom electron microscopy of the nanostructures (Fig. 1Aand B), we assume that these gaps correspond to the linkedmagnetite nanoparticles around the small silver and gold Fig. 7 X-ray microscopy images (A, C) and tomographic slices (B, D) ofvitrified 3T3 fibroblast cells after incubation with Ag-Magnetite (A, B)and Au-Magnetite (C, D) for 24 hours. The images were acquired with a25 nm zone plate (9.8 nm pixel size). Arrows indicate the presence ofcomposite nanoprobes. Scale bar: 1 um. Abbreviations: Nu, nucleus; Nc,Nucleolus; NM, nuclear membrane; V, vesicle; PM, plasma membrane. nanoparticles and nanoaggregates, and that the magnetitestructures are not visible due to the magnetite's LAC beingsimilar to that of the cellular ‘background'. Similar obser-vations concerning spaces between the silver and gold nano-particles:s were made for these pplasmonic nanoparticleswith silica shells that could be observed by cryo soft-XRT. Incontrast, in non-linked silver and gold nanoparticles, verycompact nanoaggregates without gaps are observed.58 Thedata show that the composite structures remain stable also inthe harsh environment of the late endosomes and lysosomes,with the separation of the silver and gold nanoparticles,respectively, from one another by the magnetite nanoparticlesbeing maintained. On the other hand, the results suggest thatinside the cells the individual composite structures are presentas aggregates and agglomerates, which would lead to moreplasmonic nanoparticles coming into close proximity, therebyincreasing the number of spots with high local fields. Thisis supported by the higher number of SERS spectra obtainedfor longer incubation, specifically after 24 hours (compare,e.g., Fig. 4, Fig. S3t). Conclusions In conclusion, the results presented here show that Ag-Magne-tite and Au-Magnetite composite nanoprobes that havemagnetic as well as plasmonic properties can be taken up byeukaryotic cells. The nanoprobes, both Ag-Magnetite and Au-Magnetite, are non-toxic and enable the mechanical manipu-lation of the cells, for example the transport in magnetic fieldsapplied to microfluidic channels. As was revealed by the cryosoft-XRT data, the composite nanoparticles are contained inendosomes inside the cells, indicating their endocytic uptake.The nanostructures are stable even in the harsh environmentof the late endos)ommeess anadn dlysosomes, suggesting theirapplication as versatile optical and magnetic probes in thecharacterization of live cells. The high stability is specificallysupported by the SERS data. The spectra provide informationabout the composition of the biomolecules at the nanoparticlesurface. From the SERS spectra we infer that the surface com-position of Ag-Magnetite and Au-Magnetite is different fromthat of pure gold or silver nanoparticles and influenced by theinteraction of cellular biomolecules with the magnetite partsof the nanoprobes. LA-ICP-MS micromapping of intact cellsand ICP-MS experiments on cellular extracts suggest that theendosomal uptake is determined by the magnetite componentof the composite nanoprobes. Acknowledgements ( 1 C. Fang and M. Zhang, J. Mater. Chem.,2009,19, 6258- 6266. ) ( .2 O. M . B . M cBride, C. Berry, P . Burns, R. T . A. Chalmers, B. I Doyle, R. F orsythe, O. J. Garden, K. G oodman,C. Graham, P . H o skins, R . H o ldsworth, T. J. M a cGillivray,G. McKillop, G. Murray, K. O a tey, J.M. J. Robson,G. Rod i ti,S. Semple, W. S tuart, E. J. R. van Beek, A. Vesey and D. E. Newby, O pen Heart, 2015,2. ) ( 3 Y. T. Lim, M. Y. Cho, J. K. Kim, S. Hwangbo and B. H. Chung, ChemBioChem, 2007,8,2204-2209. ) ( 4 A. L. Timothy, B. James, A. Jesse and S. Konstantin, Nano- technology,2007, 18,325101. ) ( 5 Z . Fan, M. S helton, A. K. Singh, D. Senapati, S. A . Khan andP. C. Ray, ACS Nano, 2012, 6,1065-1073. ) ( 6 C. Hoskins, S, Y. Min, M. G ueorguieva, C. McDougall, A. Volovick, P . Prentice, Z. Wang, A. M e lzer, A. Cuschieri and L . Wang,J. N anobiotechnol.,2 0 12,10,27 . ) ( 7 M. Abdulla-Al-Mamun, Y. Kusumoto, T. Z annat, Y. Horie and H. Manaka, RSC Adv.,2013,3,7816-7827. ) ( 8 X. Shi, H. Gong, Y. Li , C . Wang, L. Cheng and Z. Liu, Biomaterials, 2013,34,4786-4793. ) ( 9 Y . L iu, H. Y uan, A. M. Fales, J . K. Register and T . V o -Dinh, Front. Chem., 2015,3. ) ( 10 C . B lanco-Andujar, D. O r tega, P . S o uthern, S. A . N e sbitt,N. T. K. Thanh and Q . A. P a nkhurst, Nanomedicine, 20 1 5, 11,121-136. ) ( 1 1 T. Y. Ohulchanskyy, A . Kopwitthaya, M . J eon, M . Guo, W.-C. L aw, E . P . F urlani, C. Kim and P . N . P r asad, Nano- medicine,2013, 9, 1192-1202. ) ( 12 M. T akahashi, P . M ohan, A . N a kade, K. Higashimine,D. Mott, T. Hamada, K . Matsumura, T. T aguchi a ndS. Maenosono, Langmuir,2015,31,2228-2236. ) ( 13 I. Urries, C. Munoz, L. Gomez, C. Marquina, V. Sebastian,M. A rruebo and J . Santamaria, Nanoscale, 2014, 6, 9 230- 9240. ) ( 14 Q. Mu, F . M. Kievit, R. J. Kant, G. Lin, M. J eon andM. Zhang, Nanoscale, 2 015, 7,18010-18014. ) ( 15 K . E . M cCloskey, J. J . Chalmers a n d M. Zb o rowski, Anal.Chem.,2003,75,6868-6874. ) ( 16 B . P olyak, I. F ishbein, M. C h orny, I. Alferiev, D. Williams, B. Yellen, G. Friedman a nd R . J . Levy, Proc. N atl. Acad.Sci. U . S. A.,2008,105,698-703. ) ( 17 Y. Jing, N. Mal, P. S. Williams, M. M ayorga, M . S . P e nn, J . J . Chalmers and M. Z borowski, FASEB J . , 2008, 22, 4239- 4247. ) ( 18 Q . R amadan an d M. M . G ijs, Mic r ofluid. Na n ofluid., 2012, 13,529-542. ) ( 19 D. Zhang, 5 7 J. P P. Berry, D. Zhu, Y. W ang, Y Y. Chen, B. Jiang, S. Huang, H. Langford, G . Li, P . A. Davison,J. Xu, E. Aries and W. E . H uang, ISME J., 2015, 9, 6 03- 614. ) ( 20 N . X ia, T . H unt, B. M a yers, E . A l sberg, G. Wh i tesides, R. Westervelt and D. Ingber, Biomed.Mi c rodevices, 2006, 8,299-308. ) ( 21 K . K im, H. J. Jang a nd K. S. Shin, Analyst, 2009, 134,308- 313. ) ( 22 N. Pamme and C . W ilhelm, Lab Chip, 2006,6,974-980. ) ( 23 J. D. Adams, U. K im a n d H. T. Soh, Pr o c. Natl. Acad Sci. U. S. A., 2008,105,18165-18170. ) ( 24 H .-S. Kim, O.-T. Son, K.-H. Kim, S.-H. Kim, S. Maeng andH.-I. Jung, Biotechnol. Lett., 2007, 29, 1659-1663. ) ( 25 M. G . M auk, B . L . Ziober, Z. Chen, J. A. Thompson and H. H . Bau, Ann. N . Y . Acad. S c i., 2007, 1098, 467-475. ) ( 26 J . . H. Kang, S. Krause, H. Tobin. , A. Mammoto. M. K anapathipillai and D . E . I ngber, L ab C hip, 2012, 12,2175-2181. ) ( 27 C . Wilhelm, F. Gazeau, J.Roger, J. N. Pons and J. C . Bacri,Langmuir, 2002, 18,8148-8155. ) ( 28 P. A. Valberg and H. A. Feldman, Biophys.J., 1987,52,551- 561. ) ( 29 D . A. Mbeh,T . Javanbakht, L. T abet, Y. Merhi, K. Maghni, E. Sacher and L . H . Yahia, J. B i omed. Na n otechnol., 20 1 5, 11,828-840. ) ( 30 I . I. S. Lim, N. N. Peter, P. Hye-Young, W. Xin, W. Lingyan, M. D errick and Z . Chuan-Jian, Nanotechnology, 2008, 19,305102. ) ( 31 M. S puch-Calvar, L. Rodriguez-Lorenzo, M. P. Morales,R. A. Alvarez-Puebla and L. M. Liz-Marzan, J. Phys. Chem. C, 2009,113,3373-3377. ) ( 32 M . G uhlke, S . S e lve and J . K n eipp, J. R a man Spectrosc., 2012,43,1204-1207. ) ( 33 X X. . X. ] Han, A.M. Schmidt, G. Marten, A. A. F Fischer, I. M. Weidinger a nd P. H ildebrandt, ACS Nano, 2013, 7,3212-3220. ) ( 34 T . Donnelly, W. E. Smith, K. Faulds and D. Graham, Chem. Commun.,2014,50,12907-12910. ) ( 35 A. L a P o rta, A. Sanchez-Iglesias, T. A l tantzis, S . B als,M. G rzelczak a nd L. M. L i z-Marzan, Nanoscale, 2015, 7, 10377-10381. ) ( 36 M . S. Noh, B .-H. Jun, S. Kim, H. Kang, M.-A. Woo, A. Minai- Tehrani, J.-E. Kim, J. Kim, J. Park a nd H.-T. L i m, Biomater- ials,2009,30,3915-3925. ) ( 37 B .-H. J un, M . S. Noh, J.Kim, G . Kim, H. Kang, M. - S. Kim,Y.-T. Seo, J. Baek, J.-H. Kim, J . P ark, S . K im, Y . -K. Kim,T. Hyeon, M.-H. C ho, D. H . Jeong and Y.-S. L ee, Small, 2010,6,119-125. ) ( 38 Y. Liu, Z. Chang, H. Yuan, A. M. F ales and T . Vo-Dinh, Nanoscale, 2013,5, 1 2126-12131. ) ( 39 S. Charan, C . W. K u o, Y.-W. K u o, N. Singh, P. Dr a ke,Y.-J. Lin, L. Tay and P . C h en, J. Appl. Phys., 20 0 9, 10 5 , 07B310. ) ( 40 F. Bertorelle, M. Ceccarello, M. Pinto, G. F FracassO, D. Badocco, V . Amendola, P . Pastore, M . Colombatti a n d M. Meneghetti, J. Phys. Chem . C, 2014,118,14534-14541. ) ( 41 L . Zhang,J. Xu, L. M i, H . Gong, S. Jiang and Q. Yu, Biosens.Bioelectron.,2012, 31,130-136. ) ( 42 Z. Fan, D. Senapati, S. A. Khan , A. K. Singh, A . Hamme, B. Yust, D. Sardar and P . C . Ray, Chem. - Eur.J., 2013, 19, 2839-2847. ) ( 43 U . Tamer, D. Cetin, Z. Suludere, I. H. Bo y aci, H. T. Te m iz,H. Y egenoglu, P. D aniel, I. Dincer and Y. Elerman, Int. J. Mol . Sci., 2013,14,6223-6240. ) ( 44 P . C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391-3395.45 N . Leopold and B . Lendl, J. Phys. Chem. B, 2003, 107,5723- 5727. ) ( 46 R .-P. Liang, G.-H. Yao, L .-X. Fan and J.-D. Qiu, Anal. Chim. Acta,2012, 737, 2 2-28. ) ( 47 V . Joseph, A. Matschulat,J. P olte, S. Rolf , F. Emmerling and J. Kneipp,J. Raman Spectrosc., 2011, 42,1736-1742. ) ( 48 D . D rescher, G . O rts-Gil, G. Laube, K. Natte, R. W. Veh, W. O sterle and J. Kneipp, Anal. Bioanal . Chem., 2011,400, 1367-1373. ) ( 49 D . D rescher, C . G iesen, H . T raub, U. Panne, J. Kneipp andN. Jakubowski, Anal. Chem.,2012,84,9684-9688. ) ( 50 W . S. R a sband, Im a geJ, ht t p://imagej.nih.gov/ij/, accessed date 13.04.2016. ) ( 51 G. Schneider, P. Guttmann, S. R ehbein, S. Werner and R. Follath, J . Struc t . Biol., 2012,177,2 1 2-223. ) ( 52 W. G. M uller, J. Bernard H eymann, K. N a gashima, P. Guttmann, S. Werner, S . R ehbein, G. Schneider and J . G. McNally,J. Struct. B iol., 2012,1 7 7, 179-192. ) ( 53 G. S chneider, ,P. G uttmann, l, S. Heim, S.Rehbein,F. Mueller, K. Nagashima,J. B. Heymann, W. G. Muller and J.G. M cNally, Nat. M ethods, 2010, 7,985-U116. ) ( 54 J. R. Kremer, D. N. M astronarde a n d J. R. M c Intosh,J. Struct. Biol., 1996,116, 71-76. ) ( 55 D . S. Wang and M. K e rker, Phys. R ev. B, 1981,24,1777. ) ( 56 J. Kneipp , H. Kneipp, M. McLaughlin, D. Brown and K. Kneipp, Nano Lett., 2006,6,2225-2231. ) ( 57 A . M atschulat, D . D rescher and J. K neipp, ACS Nano, 2010, 4, 3259-3269. ) ( 58 D. D rescher, , P P . . Guttmann, T. Bichner, S.Werner. G. Laube, A. H ornemann, B. Tarek, G. Schneider a n d J. Kneipp, Nanoscale, 2013, 5, 9193-9198. ) ( 59 K. Niemirowicz, I. Swiecicka, A A. Z. Wilczewska,I. Misztalewska, B. Kalska-Szostko, K. Bienias, R. Bucki andH. Car, Int. J. Nanomed.,2014,9,2217-2224. ) ( 60 I . Mellman, R . Fuchs and A. H elenius, Annu. Rev. Biochem., 1986,55,663-700. ) ( 61 S . K ornfeld and I. Mellman, Annu. Rev. Cell Biol., 1989,5, 483-525. ) ( 62 D . D rescher, T. Buchner, D. McNaughton a n d J. K n eipp, Phys. Chem. Chem. Phys.,2013,15,5364-5373. ) ( 63 T . Bichner, , D D. D rescher. H. Traub. , P. Schrade.S. Bachmann, N. Jakubowski a n d J. Kneipp, Anal. B ioanal.Chem., 2014,406,7003-7014. ) ( 64 D . Drescher and J. Kneipp, Chem. So c . Rev., 2012, 4 1 ,5780-5799. ) ( 65 N. M. S. Sirimuthu, C. D. Syme and J. M. Cooper, Anal. Chem.,2010, 82,7369-7373. ) ( 66 H .-W. Tang, X. B. Yang, J. Kirkham a n d D. A. S m ith, Anal.Chem.,2007,79,3646-3653. ) ( 67 G 3 . . L Diaz Fleming, J. J. Finnerty, M. C ampos-Vallette,F. Célis, A . E . Aliaga, C. Fredes an d R . Koch, J. RamanSpectrosc., 2009,40, 632-638. ) ( 68 F . S . P arker, Applications ofInfrared, R aman, a n d Resonance Raman Spectroscopy in Biochemistry, Plenum Press, New York, 1983. ) ( 69 E. P odstawka, Y . Ozaki i and L L. M . P r oniewicz, Appl.Spectrosc.,2005,59,1516-1526. ) ( 70 E . C asals, T. P faller , A . Duschl , G. J . Oostingh andV. F. Puntes, Small, 2011, 7, 3479-3486. ) ( 71 A. Jedlovszky-Hajdú, F. B. Bombelli, M. P . Monopoli,E. Tombacz and K. A. D awson, Langmuir, 2012, 28, 14983- 14991. ) ( 72 M. S afi, J. Courtois , M. Seigneuret , H. Conjeaud and J. F. Berret, Biomaterials, 2011, 32,9353-9363. ) ( 73 J. Gruenberg a n d K. E. Howell, Annu. Re v . Ce l l Biol., 1989, 5, 453-481. ) ( 74 J. Huotar i and A. Helenius, EMBO J.,2011,30, 3 481- 3500. ) ( 75 D . D rescher, I. Z eise, H . T raub, P. Guttmann, S. Se i fert, T. Buchner, N. Jakubowski, G. Schneider and J . Kneipp, Adv. Funct. Mater., 2014,24,3765-3775. ) ( 76 S . I . Dworetzky, R . E. L anford a n d C. M. Fel d herr, J. C ellBiol.,1988,107,1279-1287. ) ( 77 N. Pante and M . K ann, Mol. B iol. Cell, 2002,13,425- 434. ) ( 78 B. D. Chithrani, A . A. Ghazani and W. C. W. Chan, Nano Lett. , 2006,6,662-668. ) ( 79 S . E . A. G ratton, P . A. Ropp, P . D . P o hlhaus, J. C. Luft,V. J . Madden, M. E . Napier and J . M. DeSimone, Proc. N atl.Acad. Sci. U. S. A., 2008 , 105,11613-11618. ) ( 80 A . E . N el, L. M adler, D . V e legol, T. X ia, E. M . V . H o ek, P. Somasundaran, ,F K laessig, V. Castranova and M. Thompson,Nat. Mater., 2009, 8, 543-557. ) ( 81 C. B randenberger, C. Muhlfeld, z. Ali, A. G . l Lenz, O. Schmid, W. J. Parak, P. Gehr and B. Rothen-Rutishauser,Small, 2010, 6,1669-1678. ) ( 82L.Miller, H. T raub, N. J akubowski, L D.. Drescher. V. I. Baranov and J. Kneipp, Anal. Bioanal. Chem., 2014, 406,6963-6977. ) ( 83 K. K. C omfort, E. I . M aurer, L. K . B raydich-Stolle and S. M. Hussain, ACS Nano, 2011,5,10000-10008. ) ( 84 R . F eder and D. Sayre, Ann. N.Y. Acad. Sci., 1980,342,213-229. ) ( 85 D. Weif, G. S chneider, B. Niemann, l P. ?.Guttmann, D. R udolph and G . Schmahl, Ultramicroscopy, 2000, 84, 185-197. ) ( 86 D. M. P . F rayssinet, Maryle Combacau and N . Rouquet, in Biomaterials Applications J for r N Nanomedicine, ed. R. Pignatello, 2011, ch. 12, DOI: 1 0.5772/23173. ) ( 87 M . Chiappi, J. J. Conesa, E . Pereiro, C. O. S. Sorzano, M. J . Rodriguez, K . Henzler, G. Schneider, F. J. Chichonand J. L. Carrascosa,J. Nanobiotechnol., 2016, 14,1-10. ) Analyst, his journal is @ The Royal Society of Chemistry 将氧化铁纳米粒子与银纳米粒子、金纳米粒子、银磁铁矿和金磁铁矿分别通过内吞作用引入到成纤维细胞中,形成多功能复合纳米粒子。含有无毒纳米粒子的细胞在外部磁场中是可置换的,可以在微流控通道中进行操作。在表面增强拉曼散射(SERS)映射、激光消融电感耦合等离子体质谱(LA-ICP-MS)微研磨和低温软x射线断层扫描(cryo soft- xrt)的基础上,得到了内体系统中复合纳米结构的分布。完整的玻璃化细胞冷冻软x光检查显示复合纳米粒子包裹体形成内聚体。纳米粒子提供了内质网环境中表面生物分子组成的SERS信号。SERS数据表明,纳米粒子在内质粒和溶酶体的恶劣环境中具有较高的稳定性和等离子体性质。该光谱指向银磁铁矿和金磁铁矿纳米结构表面的分子组成,与其他复合结构非常相似,但不同于研究细胞内纯银和金SERS纳米组成所得到的结构。由LA-ICP-MS数据可知,磁铁矿复合材料的吸附效率大约是纯金、银纳米颗粒的2 - 3倍。
确定








还剩10页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《细胞中SERS纳米粒子生物分子环境、细胞内相互作用检测方案(激光剥蚀进样)》,该方案主要用于其他中SERS纳米粒子生物分子环境、细胞内相互作用检测,参考标准--,《细胞中SERS纳米粒子生物分子环境、细胞内相互作用检测方案(激光剥蚀进样)》用到的仪器有ESL213 灵活的激光剥蚀系统
推荐专场
相关方案
更多
该厂商其他方案
更多















