方案详情
文
识别和保护鲨鱼育苗区是重建过度捕捞种群常规策略,然而,我们对幼年和成年种群之间的联系知之甚少。通过分析鲨鱼锥软骨微量元素的组成,根据育苗区特有的化学特征可以推断它们的出生地。为了评估该方法的有效性,我们在2012年,2013年从墨西哥湾的四个区域采集了幼年黑尖鲨脊椎骨,并利用LA-ICP-MS进行分析。我们发现六种元素存在显著的区域差异,Ca元素2012年和2013年的比例。多元素化学特征在地区间和年龄段有显着性差异。尽管2012年没有从所有四个区域获得样本,通过年龄特异性对区域分类的准确性在2012为81%,在2013年为85%。从而证明了这种方法的有效性。这些结果是鼓舞人心的,但是也突出了需要更多的研究来更好地评估脊椎化学在研究elasmobranch种群的相关性。
方案详情

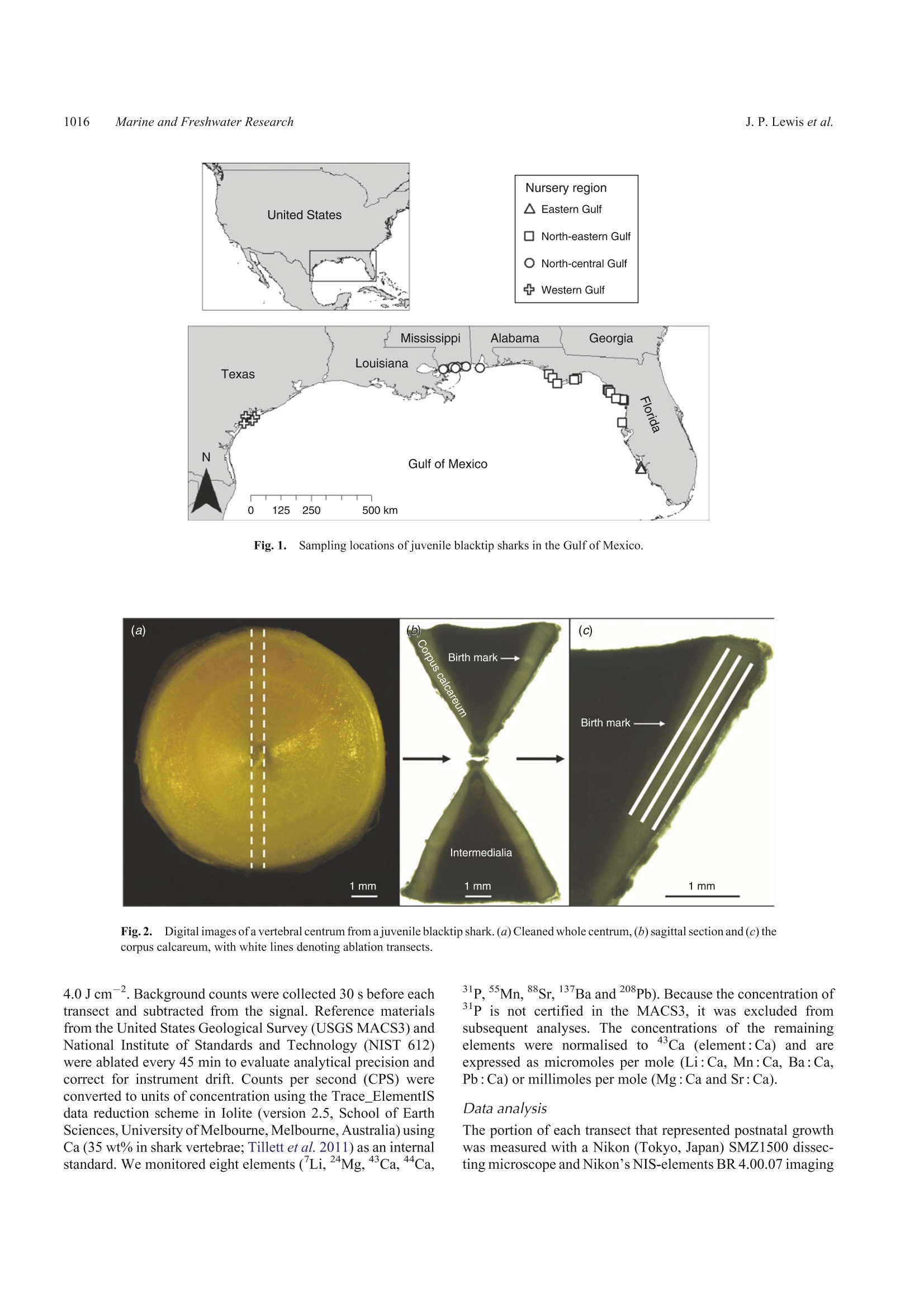
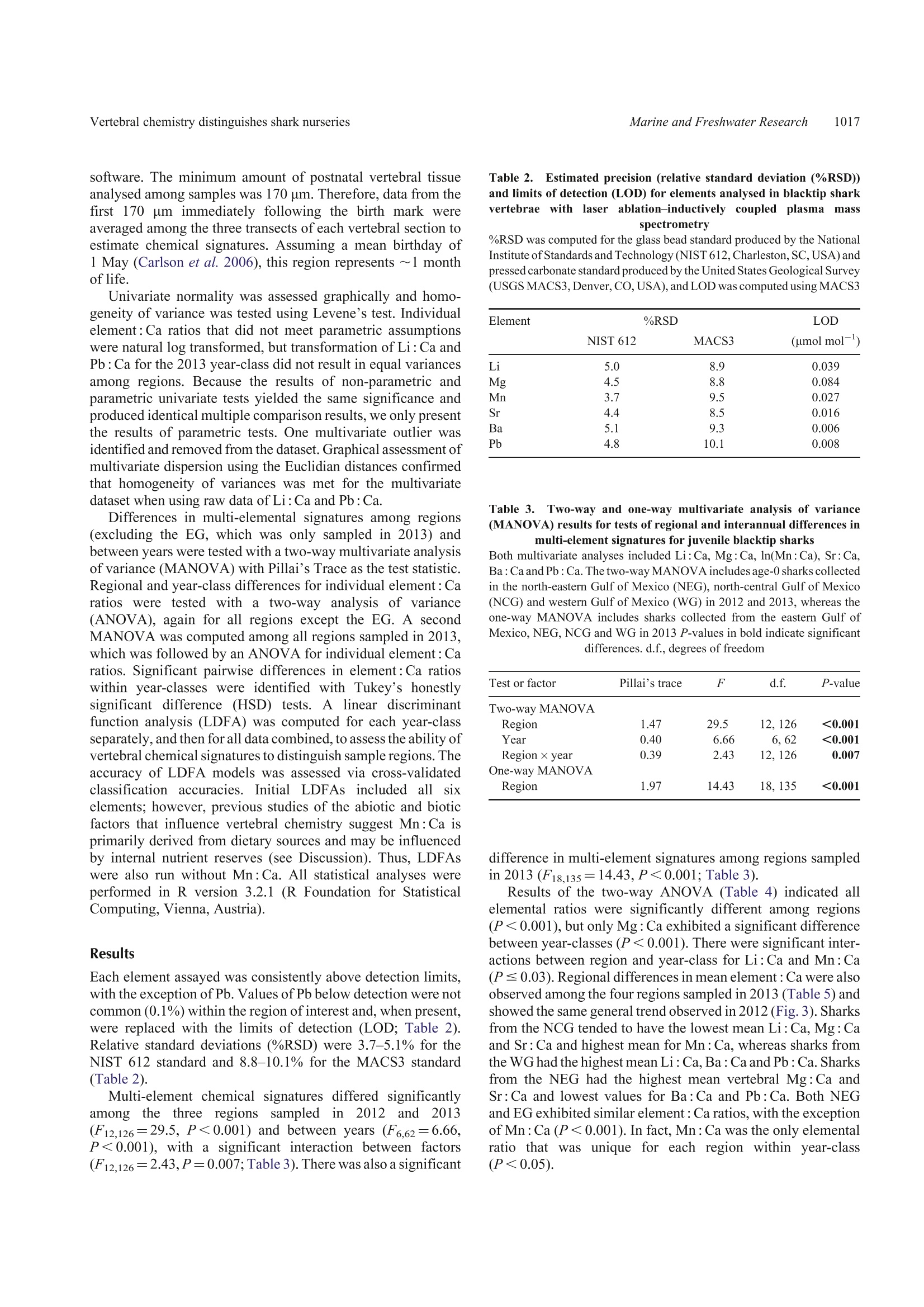
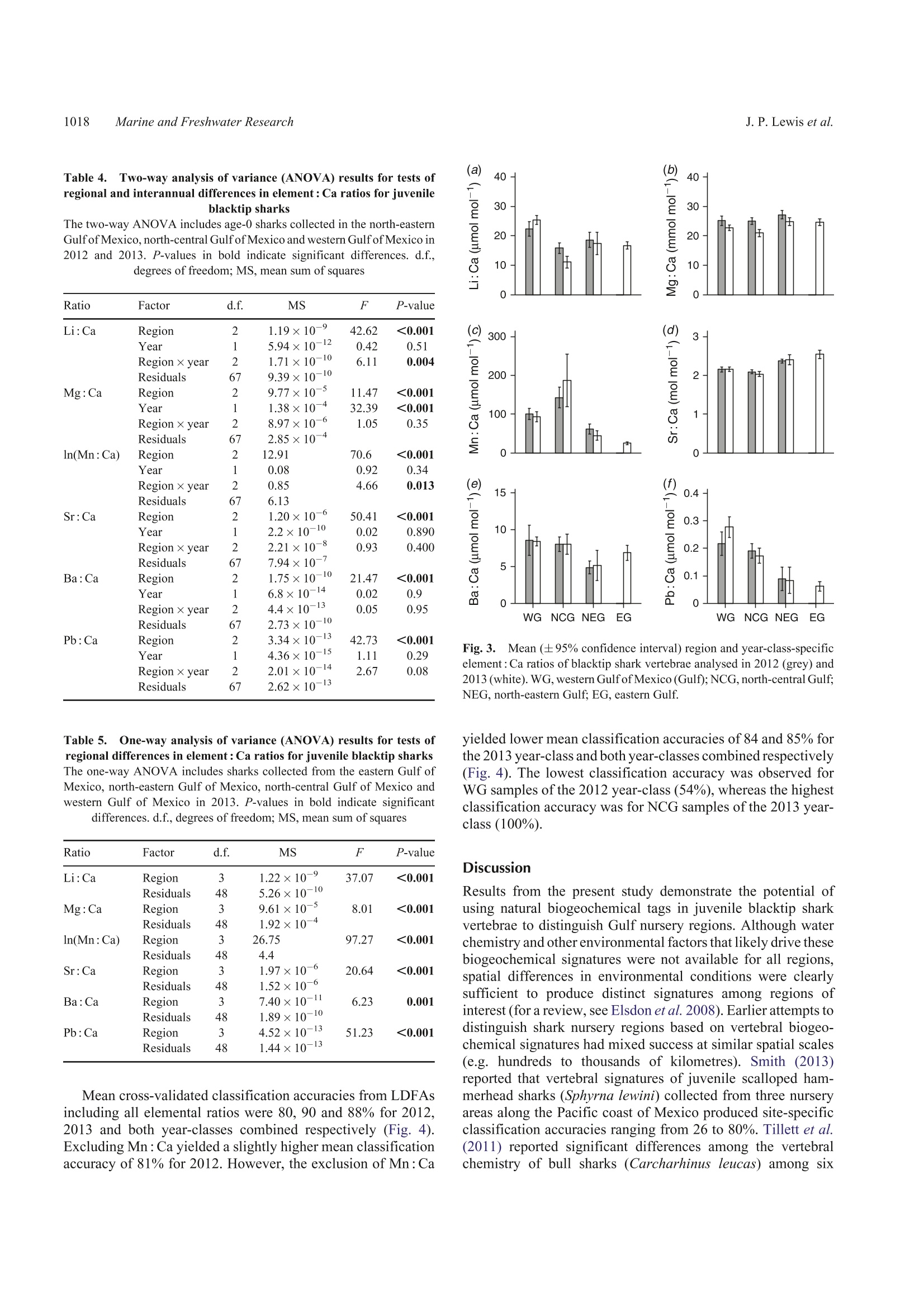
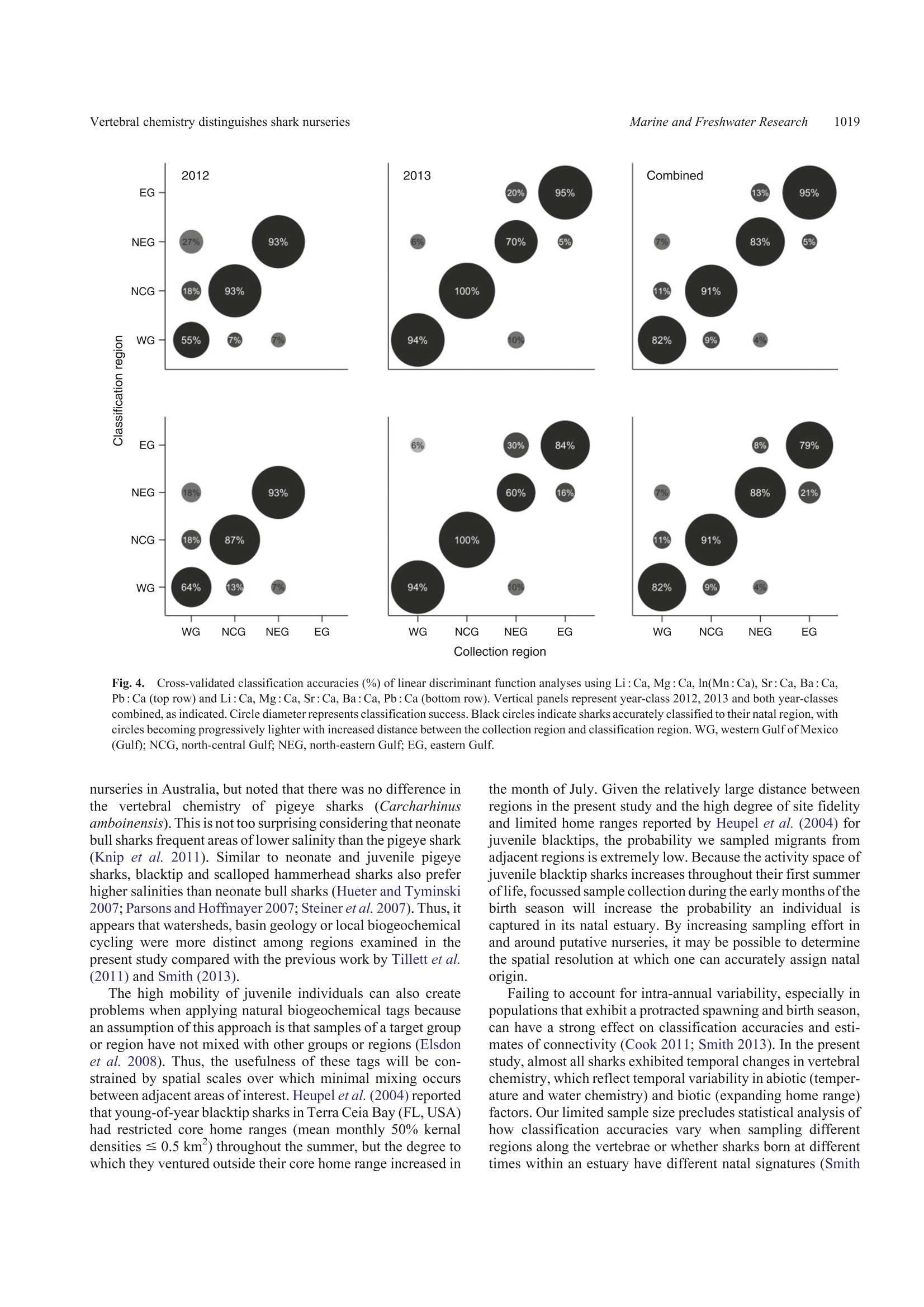
SPECIAL ISSUECSIRO PUBLISHINGMarine and Freshwater Research,2016,67,1014-1022 Vertebral chemistry distinguishes shark nurseries1015Marine and Freshwater Research http://dx.doi.org/10.1071/MF15088 Do vertebral chemical signatures distinguish juvenileblacktip shark (Carcharhinus limbatus) nursery regionsin the northern Gulf of Mexico? Justin P. LewisA.B.E William F. Patterson,IA,B. John K. CarlsonCand Katherine McLachlinD ^University of South Alabama, Department of Marine Sciences, 307 North University Boulevard,Mobile, AL 366888,USA. Dauphin Island Sea Lab, 101 Bienville Boulevard, Dauphin Island, AL 36528, USA National Marine Fisheries Service,Southeast Fisheries Science Center, Panama City Laboratory,3500 Delwood Beach Road, Panama City, FL 32408, USA.. Electro Scientific Industries, 685 Old Buffalo Trail, Bozeman, MT 59715, USA.Corresponding author. Present address: Center for Fisheries Research and Development,Gulf Coast Research Laboratory, The University of Southern Mississippi, 703 East Beach Drive,Ocean Springs, MS 39564, USA. Email: jplewis86@gmail.com Abstract.Identifying and protecting shark nurseries is a common management strategy used to help rebuild overfishedstocks, yet we know little about connectivity between juvenile and adult populations. By analysing trace metalsincorporated into vertebral cartilage, it may be possible to infer natal origin based on nursery-specific chemical signatures.To assess the efficacy of this approach, we collected juvenile blacktip sharks (Carcharhinus limbatus; n=93) from fourregions in the Gulf of Mexico in 2012 and 2013 and analysed their vertebral centra with laser ablation-inductively coupledplasma-mass spectrometry. We observed significant regional differences in six element: Ca ratios in both 2012 and 2013.Multi-element chemical signatures were significantly different among regions and between year-classes. Year-class-specificlinear discriminant function analysis yielded regional classification accuracies of 81% for 2012 and 85% for 2013, althoughsamples were not obtained from all four regions in 2012. Combining year-classes resulted in an overall classificationaccuracy of 84%, thus demonstrating the usefulness of this approach. These results are encouraging yet highlight a need formore research to better evaluate the efficacy of vertebral chemistry to study elasmobranch population connectivity. Additional keywords: laser ablation, natal origin, shark nurseries, vertebral chemistry. Received 1 March 2015, accepted 1 December 2015, published online 11 February 2016 Introduction Nurseries are areas that contribute a disproportionate number ofjuvenile recruits to adult populations, thus the conservationbenefit of protecting nurseries is evident. However, inconsistentapplication ofthe term‘nursery'prompted the development ofguidelines to empirically compare the value of different habitatsor systems in the context of population maintenance and growth(Beck et al. 2001; Heupel et al. 2007). Proxies that indicate anarea provides favourable conditions for subsequent recruitmentto the adult population (e.g. high juvenile density, growth rateand survival) are important for understanding the ecologicalprocesses of source versus sink regions (for a review, see Becketal.2001). However, the ultimate character that distinguishes afunctional nursery is a disproportionately high contribution ofrecruits to the adult population, thus determining where adultsspent their early life is the ultimate test of whether a givenhabitat or system is, in fact, a nursery. Identifying nursery areas based on estimates of connectivityto adult populations is particularly challenging for coastal sharkspecies. Conventional mark-recapture studies are limitedbecause recapture rates are typically low (e.g.=10%; Kohlerand Turner 2001) and home ranges in the order of tens of squarekilometres (e.g. Yeiser et al. 2008) make it difficult to mark.enough individuals to compensate for high mortality during thefirst year of life. In the case of bony fishes, natural biogeochemicaltags based on chemical constituents of calcified structures (e.gotoliths, scales, fin rays and bone) provide an alternative approachto study movement patterns and population connectivity (for areview, see Elsdon et al. 2008). During the biomineralisation2+.2+process, divalent cations, such as Sr+and Ba2+, substitute forCa relative to their environmental availability (Wells et al.2000), whereas other elements may become incorporated intersti-tially or associated with the protein matrix (Campana 1999). Thus,the chemical constituents of calcified structures are representative of environmental conditions at the time of deposition. In bonyfishes, otoliths are the preferred structure for chemical analysis ofnatural biogeochemical tags because they are inert once formedand their biomineralisation pattern produces alternating trans-lucent and opaque zones that provide a chronometer that accom-panies the recorded chemical histories (Campana 1999).Materialat the core of the otolith represents deposition during the first yearof life; thus, chemical signatures found in the core can act as anatural tag to be used to estimate the proportion of adults derivedfrom different nurseries (e.g. Vasconcelos et al. 2011). The incorporation of trace metal impurities into calcifiedstructures of other marine organisms has also been shown toreflect the environmental conditions at the time of mineralisa-tion (e.g. Pitts and Wallace 1994) and may be used to address avariety of ecological questions. Results from recent field(Scharer et al. 2012) and experimental (Smith et al. 2013)studies show some elements deposited in the hydroxyapatitematrix (Ca10(PO4)6(OH)2) of elasmobranch vertebrae are alsoinfluenced by abiotic factors (e.g. salinity, water chemistry andtemperature). These results indicate that chemical signatures invertebrae may serve as natural biogeochemical tags of elasmo-branch juvenile habitat that could be used to examine sources ofrecruits to adult populations. However, their usefulness forstudying connectivity between juvenile and adult populationsis contingent on our ability to accurately classify individuals totheir natal nurseries based on vertebral chemical signatures. In the present study, blacktip sharks (Carcharhinus limbatus)were examined as a model coastal shark species to test whethervertebral chemical signatures distinguish nursery regions in thenorthern Gulf of Mexico (Gulf) and whether differences can beused to infer nursery origin. The blacktip shark is a cosmopolitanspecies that is found throughout coastal waters ofthe Gulf and isone of the more economically important shark species in theregion (SoutheastData, Assessment, and Review 2012).Females reproduce biennially following a gestation period of~12 months, with parturition peaking from March to May incoastal nurseries throughout the Gulf (Baremore and Passerotti2013). Juvenile blacktips remain in natal coastal embayments upto the first 6 months of life before migrating to wintering grounds(Heupel et al. 2004; Hueter and Tyminski 2007). Tagging dataindicate individuals up to the age of 3 years return to theirnurseries during summer months (Heupel and Simpfendorfer2002; Hueter et al. 2005), whereas analysis of mitochondrial(mt)DNA and nuclear DNA microsatellites indicates restrictedgene flow that is consistent with female philopatry to natal regions(Keeney et al. 2005). Development of region-specific naturalbiogeochemical tags based on vertebral chemistry may permitfurther testing of site fidelity and philopatry for this species, aswell as facilitate examination of connectivity between juvenileand adult populations. Materials and methods Sample collection and preparation Juvenile blacktip sharks were collected opportunistically in 2012and 2013 (Table 1) during the Gulf of Mexico States SharkPupping and Nursery Survey (GULFSPAN). The GULFSPANsurvey is a fisheries-independent gill net survey that was initiatedby the National Marine Fisheries Service (NMFS) Laboratory in Table 1. Sample size and size ranges of juvenile blacktip sharks (2012and 2013 year-classes) from four nursery regions in the northern Gulf ofMexico (Gulf) Average size at birth for blacktip sharks in the Gulf is 380-mm fork length(FL) (Baremore and Passerotti 2013). EG, eastern Gulf; NEG, north-easternGulf; NCG, north-central Gulf; WG, western Gulf Year-class Region Sample size Size range (mm FL) 2012 NEG 14 465-610 NCG 15 530-660 WG 11 604-693 2013 EG 19 420-580 NEG 10 430-570 NCG 7 499-589 WG 16 503-666 Panama City (FL,USA) in 2003 and occurs annually withinseveral coastal areas ofthe Gulf. Samples were also obtained fromfisheries-independent surveys by the Mote Marine Laboratory(Sarasota, FL, USA), Florida State University Coastal and MarineLaboratory (St Teresa,FL,USA), Dauphin Island Sea Laboratory(Dauphin Island, AL, USA), University of Southern MississippiGulfCoast Research Laboratory (Ocean Springs, MS, USA) andTexas Parks and Wildlife Department (Port O'Connor, TX,USA)using gill nets or longlines. Samples of the 2012 and 2013 year-classes were collected from four regions in the Gulf (Fig. 1):(1) the eastern Gulf (EG) off the south-west coast of Florida;(2) the north-eastern Gulf (NEG) from the Big Bend west throughthe Florida panhandle; (3) the north-central Gulf (NCG) withinthe state boundaries of Alabama and Mississippi; and (4) thewestern Gulf (WG) in San Antonio Bay, Texas. Thoracic vertebrae were removed in the field or in thelaboratory and stored frozen until processed. Excess tissue andhaemal and neural arches were excised with a scalpel (Fig. 2a).Individual vertebrae were sectioned along the sagittal plane with aBuehler (Lake Bluff, IL, USA) Isomet low-speed saw, resulting in~0.5-mm sections (Fig. 2b). Vertebral sections were placed inacid-leached polystyrene cells filled with ultrapure water (18 MQcm) and sonicated for 1 h. Following sonication, sections wererinsed with ultrapure water, transferred to acid-leached cell wellsand dried under a Class 10 laminar flow clean hood. Sample analysis Vertebral chemistry wasanalysed1 withll;aser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS).The LA-ICP-MS system consisted of a Nd:YAG NWR213(Electro Scientific Industries, Portland,OR, USA)laser ablationsystem coupled with an Agilent Technologies (Santa Clara, CA,USA) 7700x ICP-MS. Helium (flow rate 0.7 L min-) was usedto sweep ablated material from the ablation cell then mixed withargon (flow rate 0.79 L min) and transported the ICP-MS.Sample order was randomised and LA-ICP-MS analysisconsisted of three parallel laser ablation transects through thecorpus calcareum (Fig. 2c). Transects were spaced 120 um apartand each transect was preablated (laser spot size 100 um, speed110 ums,repetition rate 5 Hz, and fluence 0.41 Jcm-2) beforeanalysis. Ablation transects consisted of a spot size of 80 um, ascan speed of 11 ums, arepetition rate of 10 Hz and fluence of Fig. 1..Sampling locations of juvenile blacktip sharks in the Gulf of Mexico. Fig.2.Digital images of a vertebral centrum from a juvenile blacktip shark. (a) Cleaned whole centrum, (b) sagittal section and (c) thecorpus calcareum, with white lines denoting ablation transects. 4.0 J cm. Background counts were collected 30 s before eachtransect and subtracted from the signal. Reference materialsfrom the United States Geological Survey (USGS MACS3) andNational Institute of Standards and Technology (NIST 612)were ablated every 45 min to evaluate analytical precision andcorrect for instrument drift. Counts per second (CPS) wereconverted to units of concentration using the Trace_ElementISdata reduction scheme in Iolite (version 2.5, School of EarthSciences, University of Melbourne, Melbourne,Australia) usingCa (35 wt% in shark vertebrae; Tillett et al. 2011) as an internalstandard. We monitored eight elements (Li,24Mg, 43Ca,44Ca, 31p,5Mn, 88Sr, 137Ba and 208Pb). Because the concentration ofP is not certified in the MACS3, it was excluded fromsubsequent analyses. The concentrations of the remainingelements were normalised to43Ca (element: Ca) and areexpressed as micromoles per mole (Li: Ca, Mn: Ca, Ba: Ca,Pb :Ca) or millimoles per mole (Mg :Ca and Sr : Ca). Data analysis The portion of each transect that represented postnatal growthwas measured with a Nikon (Tokyo, Japan) SMZ1500 dissec-ting microscope and Nikon’s NIS-elements BR 4.00.07 imaging software. The minimum amount of postnatal vertebral tissueanalysed among samples was 170 um. Therefore, data from thefirst 170 um immediately following the birth mark wereaveraged among the three transects of each vertebral section toestimate chemical signatures. Assuming a mean birthday of1 May (Carlson et al. 2006), this region represents ~1 monthof life. Univariate normality was assessed graphically and homo-geneity of variance was tested using Levene’s test. Individualelement: Ca ratios that did not meet parametric assumptionswere natural log transformed, but transformation of Li: Ca andPb : Ca for the 2013 year-class did not result in equal variancesamong regions. Because the results of non-parametric andparametric univariate tests yielded the same significance andproduced identical multiple comparison results, we only presentthe results of parametric tests. One multivariate outlier wasidentified and removed from the dataset. Graphical assessment ofmultivariate dispersion using the Euclidian distances confirmedthat homogeneity of variances was met for the multivariatedataset when using raw data of Li:Ca and Pb: Ca. Differences in multi-elemental signatures among regions(excluding the EG, which was only sampled in 2013) andbetween years were tested with a two-way multivariate analysisof variance (MANOVA)with Pillai’s Trace as the test statistic.Regional and year-class differences for individual element:Caratios were tested with a two-way analysis of variance(ANOVA), again for all regions except the EG. A secondMANOVA was computed among all regions sampled in 2013,which was followed by an ANOVA for individual element : Caratios. Significant pairwise differences in element: Ca ratioswithin year-classes were identified with Tukey’s honestlysignificant difference (HSD) tests. A linear discriminantfunction analysis (LDFA) was computed for each year-classseparately, and then for all data combined, to assess the ability ofvertebral chemical signatures to distinguish sample regions. Theaccuracy of LDFA models was assessed via cross-validatedclassification accuracies. InitialLDFAs included all sixelements; however, previous studies of the abiotic and bioticfactors that influence vertebral chemistry suggest Mn: Ca isprimarily derived from dietary sources and may be influencedby internal nutrient reserves (see Discussion). Thus, LDFAswere also run without Mn:Ca. All statistical analyses wereperformed in R version 3.2.1 (R Foundation for StatisticalComputing, Vienna, Austria). Results Each element assayed was consistently above detection limits,with the exception of Pb. Values of Pb below detection were notcommon (0.1%) within the region of interest and, when present,were replaced with the limits of detection (LOD; Table 2).Relative standard deviations (%RSD) were 3.7-5.1% for theNIST 612 standard and 8.8-10.1% for the MACS3 standard(Table 2). Multi-element chemical signatures differed significantlyamong the three regions sampled in 22012 and 2013(F12,126=29.5, P<0.001) and between years (F6,62=6.66,P<0.001), with a significant interaction between factors(F12,126=2.43,P=0.007;Table 3). There was also a significant Table 2.Estimated precision (relative standard deviation (%RSD))and limits of detection (LOD) for elements analysed in blacktip sharkvertebrae withlaser ablation-inductively coupled plasmai masS spectrometry %RSD was computed for the glass bead standard produced by the NationalInstitute of Standards and Technology (NIST612, Charleston, SC,USA) andpressed carbonate standard produced by the United States Geological Survey(USGS MACS3, Denver, CO,USA), and LOD was computed using MACS3 NIST 612 MACS3 (umol mol) Li 5.0 8.9 0.039 Mg 4.5 8.8 0.084 Mn 3.7 9.5 0.027 Sr 4.4 8.5 0.016 Ba 5.1 9.3 0.006 Pb 4.8 10.1 0.008 Table 3. Two-way and one-way multivariate analysis of variance(MANOVA) results for tests of regional and interannual differences inmulti-element signatures for juvenile blacktip sharks Both multivariate analyses included Li:Ca, Mg :Ca, ln(Mn: Ca), Sr:Ca,Ba:Ca and Pb : Ca. The two-way MANOVA includes age-0 sharks collectedin the north-eastern Gulf of Mexico (NEG), north-central Gulf of Mexico(NCG) and western Gulf of Mexico (WG) in 2012 and 2013, whereas theone-way MANOVA includes sharks collected from the eastern Gulf ofMexico, NEG, NCG and WG in 2013 P-values in bold indicate significant differences. d.f., degrees of freedom Test or factor Pillai's trace F d.f. P-value Two-way MANOVA Region 1.47 29.5 12,126 <0.001 Year 0.40 6.66 6,62 <0.001 Region ×year 0.39 2.43 12, 126 0.007 One-way MANOVA Region 1.97 14.43 18,135 <0.001 difference in multi-element signatures among regions sampledin 2013 (F18.135=14.43, P<0.001; Table 3). Table 4.Two-way analysis of variance (ANOVA) results for tests ofregional and interannual differences in element : Ca ratios for juvenileblacktip sharks The two-way ANOVA includes age-0 sharks collected in the north-easternGulfofMexico, north-central Gulfof Mexico and western Gulf of Mexico in2012 and 2013. P-values in bold indicate significant differences. d.f., degrees of freedom; MS, mean sum of squares Ratio Factor d.f. MS F P-value Li : Ca Region 2 1.19×10-9 42.62 <0.001 Year 1 5.94×10-12 0.42 0.51 Region × year 1.71×10-10 6.11 0.004 Residuals 9.39×10-10 Mg : Ca Region 9.77×10- 11.47 <0.001 Year 1.38×10-4 32.39 <0.001 Region × year 8.97×10° 1.05 0.35 Residuals 67 2.85×10 ln(Mn:Ca) Region 12.91 70.6 <0.001 Year 0.08 0.92 0.34 Region x year 2 0.85 4.66 0.013 Residuals 6.13 Sr: Ca Region 1.20×10-6 50.41 <0.001 Year 2.2×10-10 0.02 0.890 Region × year 2.21×10-8 0.93 0.400 Residuals 67 7.94×10-' Ba: Ca Region 2 1.75×10-10 21.47 <0.001 Year 1 6.8×10-14 0.02 0.9 Region × year 2 4.4×10-13 0.05 0.95 Residuals 67 2.73×10-10 Pb : Ca Region 2 3.34×10-13 42.73 <0.001 Year 4.36×10-1 1.11 0.29 Region × year 2 2.01×10-4 2.67 0.08 Residuals 67 2.62×10-13 Table 5. One-way analysis of variance (ANOVA) results for tests ofregional differences in element : Ca ratios for juvenile blacktip sharksThe one-way ANOVA includes sharks collected from the eastern Gulf ofMexico, north-eastern Gulf of Mexico, north-central Gulf of Mexico andwestern Gulf of Mexico in 2013. P-values in bold indicate significant differences. d.f., degrees of freedom; MS, mean sum of squares Ratio Factor d.f. MS F P-value Li: Ca Region 3 1.22×10- 37.07 <0.001 Residuals 5.26×10-10 Mg :Ca Region 9.61×10- 8.01 <0.001 Residuals 1.92×10-4 ln(Mn: Ca) Region 26.75 97.27 <0.001 Residuals 4.4 Sr: Ca Region 1.97×10-6 20.64 <0.001 Residuals 1.52×10- Ba : Ca Region 3 7.40×10-11 6.23 0.001 Residuals 1.89×10-10 Pb: Ca Region 4.52×10-13 51.23 <0.001 Residuals 1.44×10-13 Mean cross-validated classification accuracies from LDFAsincluding all elemental ratios were 80, 90 and 88% for 2012,2013 and both year-classes combined respectively (Fig. 4).Excluding Mn: Ca yielded a slightly higher mean classificationaccuracy of 81% for 2012. However, the exclusion of Mn : Ca 40- WG NCG NEG EG WG NCG NEG EG Fig. 3.Mean (±95% confidence interval) region and year-class-specificelement: Ca ratios of blacktip shark vertebrae analysed in 2012 (grey) and2013 (white). WG, western GulfofMexico (Gulf); NCG,north-central Gulf;NEG, north-eastern Gulf; EG, eastern Gulf. yielded lower mean classification accuracies of 84 and 85% forthe 2013 year-class and both year-classes combined respectively(Fig. 4). The lowest classification accuracy was observed forWG samples of the 2012 year-class (54%), whereas the highest.classification accuracy was for NCG samples of the 2013 year-class (100%). Discussion Results from the present study demonstrate the potential ofusing natural biogeochemical tags in juvenile blacktip sharkvertebrae to distinguish Gulf nursery regions. Although waterchemistry and other environmental factors that likely drive thesebiogeochemical signatures were not available for all regions,spatial differences in environmental conditions were clearlysufficient to produce distinct signatures among regions ofinterest (for a review, see Elsdon et al. 2008). Earlier attempts todistinguish shark nursery regions based on vertebral biogeo-chemical signatures had mixed success at similar spatial scales(e.g. hundreds to thousands of kilometres). Smith (2013)reported that vertebral signatures of juvenile scalloped ham-merhead sharks (Sphyrna lewini) collected from three nurseryareas along the Pacific coast of Mexico produced site-specificclassification accuracies ranging from 26 to 80%. Tillett et al(2011) reported significant differences among the vertebralchemistry of bull sharks (Carcharhinus leucas) among six Fig.4.+.Cross-validated classification accuracies (%) of linear discriminant function analyses using Li : Ca, Mg :Ca, In(Mn:Ca), Sr :Ca, Ba :Ca,Pb : Ca (top row) and Li: Ca, Mg: Ca, Sr: Ca, Ba : Ca, Pb : Ca (bottom row). Vertical panels represent year-class 2012, 2013 and both year-classescombined, as indicated. Circle diameter represents classification success. Black circles indicate sharks accurately classified to their natal region, withcircles becoming progressively lighter with increased distance between the collection region and classification region. WG, western Gulf of Mexico(Gulf);NCG, north-central Gulf; NEG, north-eastern Gulf; EG, eastern Gulf. nurseries in Australia, but noted that there was no difference inthevertebral chemistry of pigeye sharks (Carcharhinusamboinensis). This is not too surprising considering that neonatebull sharks frequent areas of lower salinity than the pigeye shark(Knip et al. 2011). Similar to neonate and juvenile pigeyesharks, blacktip and scalloped hammerhead sharks also preferhigher salinities than neonate bull sharks (Hueter and Tyminski2007; Parsons and Hoffmayer 2007; Steiner et al.2007). Thus, itappears that watersheds, basin geology or local biogeochemical)cal b1(cycling were more distinct among regions examined in thepresent study compared with the previous work by Tillett et al.(2011) and Smith (2013). The high mobility of juvenile individuals can also createproblems when applying natural biogeochemical tags becausean assumption of this approach is that samples of a target groupor region have not mixed with other groups or regions (Elsdonet al. 2008). Thus, the usefulness of these tags will be con-strained by spatial scales over which minimal mixing occursbetween adjacent areas of interest. Heupel et al. (2004) reportedthat young-of-year blacktip sharks in Terra Ceia Bay (FL, USA)had restricted core home ranges (mean monthly 50% kernaldensities ≤0.5 km) throughout the summer, but the degree towhich they ventured outside their core home range increased in the month of July. Given the relatively large distance betweenregions in the present study and the high degree of site fidelityand limited home ranges reported by Heupel et al. (2004) forjuvenile blacktips, the probability we sampled migrants fromadjacent regions is extremely low. Because the activity space ofjuvenile blacktip sharks increases throughout their first summerof life, focussed sample collection during the early months ofthebirth season will increase the probability an individual iscaptured in its natal estuary. By increasing sampling effort inand around putative nurseries, it may be possible to determinethe spatial resolution at which one can accurately assign natalorigin. Failing to account for intra-annual variability, especially inpopulations that exhibit a protracted spawning and birth season,can have a strong effect on classification accuracies and esti-mates of connectivity (Cook 2011; Smith 2013). In the presentstudy, almost all sharks exhibited temporal changes in vertebralchemistry, which reflect temporal variability in abiotic (temper-ature and water chemistry) and biotic (expanding home range)factors. Our limited sample size precludes statistical analysis ofhow classification accuracies vary when sampling differentregions along the vertebrae or whether sharks born at differenttimes within an estuary have different natal signatures (Smith 2013). The duration of the birth season for blacktip sharks in theGulf is ~2 months (Baremore and Passerotti 2013), which ismuch shorter than that of the scalloped hammerhead populationalong the Pacific coast ofMexico, which occurs between May andOctober(Smith 2013). Hameret al. (2003) reported that separatingjuveniles into two intra-annual cohorts had a negligible effect onclassification accuracies for age-0+ individuals of the snapper(Pagrus auratus) that settle in coastal nurseries of Victoria,Australia, between mid-December and March. Therefore, intra-annual variability may have little effect on classification accu-racies of blacktip sharks in the Gulf given the short birth season. The usefulness of natural biogeochemical tags derived fromcalcified structures stems from the relationship between waterchemistry, other environmental factors and element: Ca ratiosin the structures themselves. Under estuarine conditions, bonyfish otolith Sr : Ca generally shows a positive correlation withsalinity, whereas Ba :Ca typically shows a negative relationshipwith salinity (Limburg 1995; Elsdon and Gillanders 2005;Macdonald and Crook 2010). Because a large portion of otolithSr and Ba is derived from the surrounding water (Walther andThorrold 2006; Webb et al. 2012; Izzo et al. 2015), fluctuationsin Sr: Ca and Ba:Ca can be used to infer the relative salinity ofenvironments experienced by a fish during its lifetime (e.g.Limburg 1995). Similarly, Scharer et al. (2012)reported that theSr: Ca in vertebral sections of the smalltooth sawfish (Pristispectinata) had a positive correlation with salinity, and Smithet al.(2013) reported that the Ba: Ca in the vertebrae of roundstingray (Urobatis halleri) was correlated with Ba in seawater.For juvenile blacktip sharks examined here, there were regionaldifferences in Sr:Ca and Ba : Ca that were inversely related.However, it is difficult to attribute this pattern to regionaldifferences in salinity without water chemistry data becausethe amount of Sr and Ba in freshwater end members will affectthe amount of Sr and Ba available at a particular salinity (Wellset al. 2003; Kraus and Secor 2004). Moreover, several factors,including growth rate, ontogeny, temperature and diet, canaffect the relationship between water chemistry and element:Ca deposition in calcified structures (for a review, see Sturrocket al. 2012). Growth rate does not appear to affect Li: Ca,Mg :Ca, Mn: Ca, Sr: Ca and Ba: Ca deposition in the roundstingray; however, Mg:Ca, Mn: Ca and Ba: Ca were sig-nificantly affected by water temperature (Smith et al. 2013).The work of Smith et al. (2013) represents the only study tofocus on the relationship between water chemistry and vertebralelement: Ca in elasmobranchs; thus, we know little about theinfluences mentioned above and the extent of interspeciesvariability. It is not necessary to fully elucidate the underlying abioticand biotic factors driving regional variability in element: Caratios of calcified structures to use them as natural biogeo-chemical tags to examine population connectivity. However,other biologically relevant questionsn l can be addressed if theprimary factors influencing vertebral chemistry can be deter-mined. Previously, regional differences in otolith Mn: Ca in gaggrouper (Mycteroperca microlepis) and red snapper (Lutjanuscampechanus) across the Gulf were attributed to latitudinaldifferences in soil chemistry (Hanson et al. 2004; Sluis et al.2012). However, the majority of Mn in elasmobranch soft tissueappears to be derived from dietary sources (Mathews and Fisher 2009), which may explain observed enrichment in vertebralMn: Ca relative to water Mn: Ca (Smith et al. 2013). Anincrease in Mn: Ca immediately following birth was present inthe vertebrae of sharks from all regions in the present study,thussuggesting a common process influencing vertebral Mn: Ca. It. is not clear to what degree the distribution of Mn is driven by theprotein content of specific portions of vertebrae (Sturrock et al.2012). Such transition metals do show an affinity for proteinbinding sites (Miller etal. 2006) and~28%ofotolith Mn can bebound to water soluble proteins (Izzo et al. 2016). However,there may also be a physiological process at play. The neonatallife stage is particularly stressful for elasmobranchs and can beassociated with a decline in body mass (Duncan and Holland2006) and high mortality (Heupel and Simpfendorfer 2002;Duncan and Holland 2006). Neonates rely on maternal nutrientreserves in the form of an enlarged liver to compensate for thelack of foraging experience (Hussey et al. 2010;Olin et al.2011). Because the liver plays an important role in Mn homeo-stasis (Aschner and Aschner 2005; Madejczyk et al. 2009),metabolising liver tissue may result in excess Mn entering thebloodstream that becomes deposited in the vertebrae during thefirst weeks or months of life. Although Mn: Ca was the onlyelemental ratio that was significantly different among regions inboth years in the present study, the possible link to foodavailability and maternal investment may result in intra-annualvariability in vertebral Mn: Ca in a given area as less fitindividuals perish. Thus, it is unknown whether vertebralMn:Ca ratios of sharks collected early in the year wouldaccurately reflect that of the surviving members of a cohortExcluding Mn:Ca demonstrated that, although useful fordiscriminating groups, one can still achieve moderate to highclassification accuracies using an array of other minor and traceelements. Results from the present study suggest that examiningconnectivity between blacktip shark nurseries and offshore adultpopulations based on vertebral biogeochemical signatures holdssome promise,but one factor that may compromise the effec-tiveness of such an approach is the unknown metabolic stabilityof vertebral tissue. Bone in higher vertebrates is constantlyremodelled owing to its high healing potential (Kalfas 2001)The limited physiological response of elasmobranchs followingnatural (Officer et al. 1995) and experimentally induced(Ashhurst2004) trauma suggests elasmobranchs may not be capable ofextensive skeletal remodelling. However, unsuccessful attemptsto age sharks based on radionuclide decay (226Ra:210Pb) mayindicate metabolic instability of the tissue, but another explanationwould be exogenous uptake of 210Pb during growth bandformation (Welden et al. 1987; Fenton 2001). Furthermore,the success of bomb radiocarbon age validation (e.g. Campanaet al. 2002; Passerotti etal. 2010, 2014), use of stable isotopes todetect ontogenetic shifts in diet (Estrada et al. 2006) andverification of annual banr· (ds using Sr:Ca patterns that corres-pond to known life history characteristics (Scharer et al. 2012)indicates the corpus calcareum likely remains relatively inertonce formed. Important questions remain surrounding the mechanismsgoverning mineral dynamics and sites of inclusion (e.g. substi-tution in calcium phosphate hydroxyapatite matrix, bound toproteins or incorporated interstitially), but the results of the present study demonstrate that vertebral biogeochemical tagscan be used to accurately distinguish nursery regions of blacktipsharks and may provide a means to estimate the relativecontribution of different areas to the adult stock. By buildingan atlas of cohort-specific vertebral chemical signatures fromdifferent areas, it may be possible to infer the natal origin ofadult sharks. This type of information would be particularlyuseful because some coastal sharks possess life history traits thatmake them vulnerable to overharvest. Whether protectingnursery areas should be the most important aspect of a manage-ment plan will depend on the biology ofthe species and life stageremoved by the fishery (Kinney and Simpfendorfer 2009).However, estimates of connectivity between juvenile and adultpopulations can provide the insight necessary to evaluate theeffectiveness of nursery conservation as a management tool. Acknowledgements Funding support for this project was provided by National Oceanic andAtmospheric Administration (NOAA) Fisheries, Panama City Laboratory,the Highly Migratory Species Division of NOAA Fisheries and the NationalScience Foundation (grant number DBI-1319188 to W.F. Patterson III). Theauthors thank D. M. Bethea and K. Smith (NOAA Fisheries), W. J. Bubley(Texas Parks and Wildlife Department), J. M. Drymon, M. N. Schrandt,A. M. Koretz and K. C. Gregalis (Dauphin Island Sea Lab) R. D. Grubbs,C. T. Peterson and J. L. Imhoff (Florida State University Coastal and MarineLaboratory), J. M. Hendon, J. M. Higgs and S. Ashworth (Gulf CoastResearch Laboratory),R. J. D. Wells (Texas A&M University Galveston),N. M. Whitney and J. Morris (Mote Marine Laboratory) and the many staffmembers, students and volunteers who participated in field collection. Theauthors are also grateful for the guidance and advice provided by N. R. Miller(University of Texas Austin) during LA-ICP-MS analyses, and instrumenttime and technical support provide by C. J. P. O’Connor, J. Wilkins and thestaff of the Scientific Division of Electro Scientific Industries (Bozeman,MT,USA). References ( Aschner, J . L., and Aschner, M. ( 2005). N utritional aspects of manganesehomeostasis. Molecular Aspects of Medicine 26, 353-3 6 2. doi:10 .1 01 6/ J . MAM .20 05 .0 7 . 0 03 ) ( Ashhurst, D . E . (2004). The cartilaginous skeleton of a n elasmobranch fi s h does not heal. Matrix Biolog y 23, 1 5 -22. do i:10.1 0 1 6 / J .M A TBIO .20 04 .0 2.0 01 ) ( Baremore, I. E. , and Passerotti, M. S. (2013).Reproduction of the blacktip shark i n the Gulf of Mexico. M arine and Coastal Fisheries 5, 1 2 7-138 . doi:10. 1 08 0/ 1 94 2 5120.201 2.7 58204 ) ( Beck,M. W., Hec k , K. L., Jr, Able, K. W., Childers,D. L.,Eggleston, D. B. , Gillanders, B. M.,Halpern, B., Hays, C. G., Hoshino, K.,Minello,T.J . ,Orth, R. J., Sheridan, P. F .,and Weinstein, M. P. (2001). The identifica- tion, conservation, and management o f estuarine and m a rine nurseries for fish and invertebrates . Bioscience 51, 633 - 641. doi:1 0 .16 41/0006 - 3568(2 0 01)051[0633 : T I C A MO ] 2 . 0.C O;2 ) ( C a mpana, S. E. (1999). Chemistry and composition of fish otol i ths: path-ways , mechanisms and applications. Marine E cology P rogress S eries 188, 263-297. doi :10 . 33 5 4 /M E P S 188263 ) ( Campana,S. E. , Natanson, L. J ., and Myklevoll, S. (2002). Bomb dating and age determination of large pelagic sharks. Canadian Journal of Fisheries and Aquatic Sciences 59, 450-455. doi: 10 .1 139/ F 0 2-0 2 7 ) ( Carlson, J. K., Sulikowski, J. R., and Baremore,I. E. ( 2 006).Do differences in l ife history trait s exis t fo r blacktip sharks, Carcharhinus limbatus, f rom the United States South Atlantic Bight an d Eastern GulfofMexico? Environmental B i ology of F i shes 7 7 , 2 7 9-292. do i :10 .10 0 7/S10641- 0 06- 91 29-X ) ( Cook, G. S. (2011). Changes in otolith m i crochemistry ov e r a protractedspawning s e ason i n fluence assignment of natal origin. Marine EcologyProgress Series 423, 197 - 209. doi:1 0.3 354 / M EPS08 9 42 ) ( Duncan, K . M . , a n d Holland, K. N. (2 0 06). Ha b itat us e , growth rates and dispersal patterns of juvenile scalloped hammerhead sh a rks Sp h yrnalewini i n a n ursery habitat. Marine Ecology P rogress S eries 3 12,211-221. doi:10 .3354 /M EPS31 2 211 ) ( Elsdon,T. S . , and Gillanders,B. M. (2005). Alternative life-history patterns of e stuarine f ish: b arium i n o t oliths e l ucidates freshwater residency. Canadian Journal of Fisheries a nd Aquatic Sciences 62, 1143-115 2 . doi: 1 0 . 113 9 /F05 - 0 2 9 ) ( Elsdon, T . S., W ells, B. K., Campana, S. E., Gillanders, B. M., Jon e s, C. M., L imburg,K. E. , Secor,D. H.,Th o rrold, S.R., and Walther, B. D. (20 0 8). Otolith chemistry to describe movements and life- h istory parameters of fishes: hypotheses, assumptions, limitations and inferences.Oceanographyand M arine B iology a n Annual R e view 4 6 , 2 9 7-330. d o i:10 . 12 01/ 9 7814 200 65756.CH7 ) ( Estrada, J . A . ,Rice, A. N., Natanson, L. J., and Skomal, G.B. ( 2 006). Use ofisotopic analysis ofv e rtebrae i n r e constructing ontogenetic fe e ding ecology in w hite sharks. Ecology 87, 829-834. doi:10 . 18 90/0 0 12 - 9 6 5 8 ( 2 006)87 [ 8 2 9 : UO I A OV]2 .0. CO; 2 ) ( Fenton, G. E. (2001). Radiometric Ageing of Sharks. FRDC Final R e port1994/021 . Fisheries Research a nd D evelopment Corporation, Canberra,Australia. ) ( Hamer,P. A., Jenkins,G. P., a n d G i llanders, B . M . ( 2 003). Otolith chemistryof juvenile snapper Pagrus auratus in Victorian waters: natural chemicaltags and their temporal variation. Marine Ecology Progress Series 263,261-273. doi:1 0.3354 / MEPS2 632 61 ) ( Hanson, P . J ., Koenig, C. C., and Zdanowicz, V . S . (2004). E l emental composition of otoliths u s ed to trace estuarine habitats of juvenile ga g Mycteroperca m i crolepis a l ong t h e west c o ast o f Fl o rida. M a rine Ecology Progress Series 267, 253 - 265. doi: 1 0.3 354 /ME PS2 6 7 253 ) ( Heupel, M. R., and Simpfendorfer, C. A. (2002). Estimation of mortality of j uvenile blacktip sharks, Carcharhinus limbatus, within a nursery area using telemetry data. Canadian Journal of F i sheries a nd A q uaticSciences 59, 624-632. doi:1 0. 113 9/F02-036 ) ( H e upel,M. R . , Simpfendorfer, C. A . , a n d Hueter,R. E. ( 2 004). E s timation ofshark home ranges using passive m onitoring techniques. Environmental Biology of Fishes 71, 1 35-142. doi:10 .10 2 3/B : EBFI.00000 4 5710 . 18 9 9 7 .F 7 ) ( Heupel, M. R., Carlson, J. K. , and Simpfendorfer, C. A. (2007). Shark n u rseryareas: c oncepts, d efinition, characterization and assumptions. M a rine Ecology Progress Series 337, 28 7 -297. do i : 1 0 .3354/ME P S 33 7 2 8 7 ) ( Hueter,R . E . , and Tyminski, J. P . (2007). Species-specific distribution and habitat characteristics of shark nurseries in Gulf of Mexico waters off peninsular Florida and Texas. American F isheries Society Symposium 50, 193-223. ) ( Hueter, R. E ., Heupel, M . R . , H eist, E . J ., and K eeney, D . B . ( 2 005). E vidence of philopatry in sharks and implications for the management of shark f isheries. Journal of Northwest A tlantic Fishery Science 35, 239- 2 47. doi: 10.2960/ J .V 3 5 .M 4 93 ) ( Hussey, N . E., W intner, S. P ., Dudley, S. F ., Cliff, G., Cocks, D. T . , and A aron M a cNeil, M . (2 0 10). Ma t ernal inv e stment and size-specific reproductive output in carcharhinid sharks. Journal of Animal Ecology 79, 184-193. doi: 10. 111 1 / J . 1 365-2656. 2 0 09.016 2 3 .X ) ( Izzo, C . , Doubleday,Z.A., S chultz, A . G . , Woodcock,S. H., and Gillanders, B . M . (2015). C o ntribution o f water chemistry and fish condition to otolith chemistry: c omparisons across salinity environments. Journal ofFish Biology 86, 1680-1698. doi : 1 0.1 1 1 1/ JFB . 1 26 72 ) ( Izzo, C.,Doubleday,Z. A. , and Gillanders,B.M. (2016). Where do elements b ind w ithin the otoliths of fish? Marine and Freshwater Research 67. 1072- 1 076. doi: 10 . 107 1 /MF15064 ) ( Kalfas, I. H. (2001). P rinciples of bone healing. Neurosurgical Focus 10,1 - 4. doi:10 . 3 171/FOC. 20 0 1. 10.4.2 ) ( Keeney, D. B . , Heupel, M . R. , Hueter, R. E. , and He i st, E. J . (2005).Microsatellite and m itochondrial DNA analyses of the genetic structureof blacktip s hark (Carcharhinus limbatus) nurseries in the northwestern Atlantic, Gulf of Mexico, and Caribbean Sea. Molecula r Ecology 14, 1911-1923. d oi:1 0. 1 1 1 1/J . 1 365 - 2 9 4 X .2005 . 025 49 .X ) ( Kinney, M. J . , and Simpfendorfer, C. A. (2009). Re a ssessing the value of nursery areas to shark conservation and m anagement. ConservationLetters 2, 53-60 . do i :1 0 .1 1 1 1 / J . 1 75 5- 2 63 X .2008.00046 .X ) ( Knip, D. M ., H e upel, M . R. , S i mpfendorfer, C . A., T ob in, A. J. , a n dMoloney, J. (2011) . W e t-season effects on the distribution of juvenilepigeye sharks, Carcharhinus amboinensis, in tropical nearshore wa t ers. Marine and Freshwater Research 62,658-667. doi: 1 0 . 1 0 7 1 / M F10 1 36 ) ( Kohler, N . E., a nd T u rner, P . A . (2 0 01). Sh a rk ta g ging: a rev i ew ofconventional methods and studies. Environmental B iology o f Fishes 60, 191 - 224. doi: 1 0.102 3/ A: 10 07 67 9 303 0 8 2 ) ( Kraus, R . T. , a nd S e cor, D . H. ( 2 0 04). Incorporation of s tr o ntium into otoliths of an estuarine fish . Journal of Experimental Marine Bi o logy and Ecology302, 85-106. doi:10 . 10 1 6/ J .JEMBE . 2003 . 1 0. 0 0 4 ) ( Limburg, K. E . ( 1 995). O t olith s t rontium t r aces e n vironmental historyof s ubyearling American s h ad A l osa s a pidissima. M a rine Ec o logy Progress S eries 119, 25-35. doi: 10. 3 3 5 4 / MEP S1 1902 5 ) ( Macdonald, J. I., and Crook,D. A. ( 2 010). Variability in Sr: Ca and Ba : Ca ratios in water and f ish o toliths across a n e stuarine salinity gradient. Marine Ecology Progress Series 413, 147-161 . doi: 10.3 35 4 /ME PS 08703 ) ( Madejczyk, M . S., B o yer, J. L . , and B a llatori, N . ( 2009). H epatic uptake and b iliary excretion of manganese i n t h e l i ttle skate, Leucorajaerinacea. Comparative Biochemistry and Physiology Part C Toxicology & Pharmacology 149, 5 66-571. d oi: 1 0 . 1 01 6 / J . CBP C .2008. 1 2. 0 09 ) ( Mathews, T., and Fisher, N. S . (2009). Dominance of dietary int a ke of me t alsin marine e lasmobranch a n d teleost f ish. T he S cience o f the T otalEnvironment 407,5156- 5 161.doi:10 .1 01 6 / J.S C IT O TE N V .2009 .06. 00 3 ) ( Miller, M. B . , C lough, A. M . , B a tson, J. N . , and Vachet, R. W. (20 0 6). T ransition metal binding to cod otolith proteins. Journal ofExperimental Marine Biology a nd E c ology 32 9 , 13 5 -14 3. doi:10. 1 0 1 6 /J . J EM B E. 2 005.08.01 6 ) ( Officer, R. A., Clement, J . G ., a nd R owler, D . K . (1 9 95). Ve r tebral deformities in a s chool s hark, G aleorhinus galeus: circumstantialevidence f or e ndoskeletal r esorption? J ournal of Fish B i ology 46,85-98.doi:1 0. 1 111 / J. 109 5 -86 4 9 . 199 5 .TB 05 948 . X ) ( Olin, J. A . , Hussey, N. E., Fritts,M., Heupel, M. R., Simpfendorfer, C. A.,Poulakis, G. R ., and Frisk, A. T. (2011). Maternal meddling in neonatalsharks: i mplications f o r i n terpreting stable isotopes in young animals.Rapid C ommunications i n i M ass S pectrometry 25, 1008- 1 016.doi: 10 . 1002 /R C M.49 4 6 ) ( Parsons, G. R., and Hoffmayer, E. R. (2007).Identification a nd charac-terization of shark nursery grounds along the Mississippi and Alabamagulf coasts. American Fisheries Socie t y Symposium 5 0 , 301-316. ) ( Passerotti, M. S.,Carlson,J. K., Piercy, A. N., and Campana, S. E. (2010).Age validation o f great hammerhead shark (Sphyrna mokarran), deter- mined by bomb radiocarbon an a lysis. Fishery Bulletin 108, 346-351. ) ( Passerotti, M. S., Andrews,A. H., Carlson,J. K., W inter, S.P., G o ldman, K.J., and N atanson, L. J. ( 2014). M aximum age and missing time in thevertebrae of sand tiger shark (Carcharias taurus): validated lifespan frombomb radiocarbon dating in t he western North Atlantic and southwesternIndian Oceans . Marine and Freshwater Research 65,674- 6 87.doi: 10 . 1071/ M F1 321 4 ) ( P itts, L. C . , and Wallace, G.T . ( 1 994). L e ad deposition in the sh e ll of the bivalve, Mya a renaria: a n indicator of d issolved l ead i n seawater. Estuarine, Coastal and Shelf Science 39, 93-104. doi :10.1006 / ECSS1994 . 1 05 1 ) ( Scharer, R. M., P a tterson, W . F. , III, Carlson, J. K . , a n d Poulakis, G. R.(2012). Age a nd growth o f endangered sm a lltooth sawfish (Pristis ) pectinata) verified with LA-ICP-MS analysis of vertebrae. PLoS One7, e47850. doi:10.1371/JOURNAL.PONE.0047850 ( Sluis, M. Z., B arnett,B. K . , Pa tterson, W.F., I II, Cowan, J. H . ,Jr, and Shiller,A. M . (2012). D i scrimination of juvenile red snapper otolith chemical s ignatures from G ulf of M exico n u rsery r e gions. M a rine and Coastal F isheries 4, 587-598. doi : 10 . 108 0/ 1 9 42 5 1 2 0 . 2 0 12. 7 0 3 16 3 ) ( Smith, W. D. ( 2 013). Vertebral e lemental m arkers i n elasmobranchs:potential f or reconstructing environmental history and population structure. Ph.D. Thesis, Oregon State Un i versity, Corvallis. ) ( S mith, W.D. , Miller, J. A., and H eppell,S. S. (2013). Elemental markers in elasmobranchs: effects ofenvironmental history and g rowth on vertebralchemistry. PLoS One 8, e62423. do i: 10. 13 71 /JOURN AL .PONE.006 24 23 ) ( Southeast D ata, Assessment, and Review (2012). Stock Assessment Reportof SEDAR 2 9 , H M S Gulf of Mexico Bla c ktip Shark. (Southeast Fisheries Science C enter: C harleston, S C.) Available at http://sedarweb. org/docs/sar/S29 _ GOM%20blacktip%20report _ SAR _ fin al .pdf [Verified8 November 20151. ) ( Steiner, P. A., Michel, M., and O’Donnell, P. M . ( 2007). N o tes o n the occurrence and d istribution of elasmobranchs in the Ten T housand Islands Estuary, Florida. American Fisheries Society Symposium 5 0 ,237- 2 50. ) ( S turrock,A. M., Trueman, C. N., Darnaude, A . M . , a n d H u nter, E . (2012). Can otolith elemental chemistry retrospectively track migrations in fully marine f ishes? Journal o f Fish B iology 81, 7 66-7 9 5. d o i:10 .1 1 11 / J.1 095 - 8 64 9.20 1 2 .0 3 3 72.X ) ( Tillett , B . J., Meekan, M. G. , Parry, D., Munksgaard, N . , Fi e ld, I . C., Thorburn, D., and B r adshaw, C. J. ( 2 0 11). De c oding fingerprints:elemental c omposition of vertebrae co r relates to age-related ha b itatuse i n t wo morphologically similar sharks. Marine Ecology ProgressSeries 434, 133-142 . do i : 10.3 3 5 4/MEPS09 222 ) ( Vasconcelos, R . P . , R eis-Santos, P., Costa, M. J ., and Cabral, H. N. (2011). Connectivity b etween e s tuaries and m a rine environment: Integratingmetrics t o a ssess estuarine nursery function. E cological Indicators 1 1 ,1123- 1 133. doi:10.10 16/ J .ECO L IND .2 01 0.12. 0 12 ) ( W alther, B. D ., and T horrold,S.R . ( 2 006). Water, not food, c o ntributes the majority of s trontium and barium de p osited in the otoliths of a m a rinefish . Marine E cology P rogress S eries 3 11, 1 25-130. doi:10 .33 5 4 /M EP S 311 125 ) ( Webb, S. D . , Woodcock, S. H., an d Gill a nders, B. M. ( 2012). So u r ces of otolith barium a nd strontium i n e s tuarine f i sh and t h e i n fluence o f salinit y and temperature. Marine E cology P r ogress S e ries 4 5 3, 189- 1 99. doi : 10.33 5 4 /MEPS0965 3 ) ( Welden, B. A., Cailliet, G. M., and Flegal , A. R . (1987) . Comparison of r adiometric with vertebral band age estimates in fo u r California elasmo-branchs. In ‘ Age and Growth of fish’. (Eds R. C. S u mmerfelt and G. E. H all.) pp. 301-315. (Iowa State University P r ess: Ames, IA . ) ) ( Wells, B . K ., B ath, G . E ., T horrold, S . R., and J ones, C . M . ( 2 000). Incorporation of s trontium, cadmium, a n d ba r ium in j uv e nile spot(Leiostomus x anthurus) s cales r eflects water chemistry. Canadian Journal of Fisheries and Aquatic Sciences 57, 2122-21 2 9. doi:10. 1 1 39 / F00-1 7 8 ) ( Wells, B. K., Rieman, B. E. , Clayton, J. L . , H oran, D. L., an d Jones, C. M. (2003) . Relationships between water, o tolith, and scale chemistries of westslope c utthroat trout from t he C oeur d ' Alene Ri v er, Id a ho: th e potential application of hard-part chemistry to describe movements in f reshwater. Transaction s of the American Fisheries Society 132, 409-424. doi :10.15 7 7/15 4 8 -8 659( 20 0 3 )132< 0 409: R BWOAS>2. 0 . C O ; 2 ) ( Y eiser, B. G., Heupel, M. R., and Simpfendorfer, C. A. (2008). Occurrence,home range a n d m o vement patterns of juvenile b u ll ( C archarhinusleucas) and l emon ( N egaprion br e virostris) sharks w i thin a Florida estuary. Marine and Freshwater R esearch 59, 4 89- 50 1. d oi:1 0 .1 071 / MF 07181 ) Journal compilation @ CSIRO Open Accesswww.publish.csiro.au/journals/mfr www.publish.csiro.au/journals/mfr 识别和保护鲨鱼育苗区是重建过度捕捞种群常规策略,然而,我们对幼年和成年种群之间的联系知之甚少。通过分析鲨鱼锥软骨微量元素的组成,根据育苗区特有的化学特征可以推断它们的出生地。为了评估该方法的有效性,我们在2012年,2013年从墨西哥湾的四个区域采集了幼年黑尖鲨脊椎骨,并利用LA-ICP-MS进行分析。我们发现六种元素存在显著的区域差异,Ca元素2012年和2013年的比例。多元素化学特征在地区间和年龄段有显着性差异。尽管2012年没有从所有四个区域获得样本,通过年龄特异性对区域分类的准确性在2012为81%,在2013年为85%。从而证明了这种方法的有效性。这些结果是鼓舞人心的,但是也突出了需要更多的研究来更好地评估脊椎化学在研究elasmobranch种群的相关性。
确定





还剩7页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《墨西哥湾北部幼年黑尖鲨中脊椎化学特征检测方案(激光剥蚀进样)》,该方案主要用于其他中脊椎化学特征检测,参考标准--,《墨西哥湾北部幼年黑尖鲨中脊椎化学特征检测方案(激光剥蚀进样)》用到的仪器有ESL213 灵活的激光剥蚀系统
推荐专场
相关方案
更多
该厂商其他方案
更多















