Many marine organisms have complex life histories, having sessile
adults and relying on the planktonic larvae for dispersal. Larvae swim
and disperse in a complex fluid environment and the effect of ambient
flow on larval behavior could in turn impact their survival and transport.
However, to date, most studies on larvae–flow interactions have
focused on competent larvae near settlement. We examined the
importance of flow on early larval stages by studying how local flowand
ontogeny influence swimming behavior in pre-competent larval sea
urchins, Arbacia punctulata. We exposed larval urchins to grid-stirred
turbulence and recorded their behavior at two stages (4- and 6-armed
plutei) in three turbulence regimes. Using particle image velocimetry to
quantify and subtract local flow, we tested the hypothesis that larvae
respond to turbulence by increasing swimming speed, and that the
increase varies with ontogeny. Swimming speed increased with
turbulence for both 4- and 6-armed larvae, but their responses
differed in terms of vertical swimming velocity.
方案详情

The CompanyofBiologistsC 2016. Published by The Company of Biologists LtdJournal of Experimental Biology (2016) 219, 1303-1310 doi:10.1242/jeb.129502RESEARCH ARTICLE RESEARCH ARTICLEJournal of Experimental Biology (2016) 219,1303-1310 doi:10.1242/jeb.129502 Ontogenetic changes in larval swimming and orientation ofpre-competent sea urchin Arbacia punctulata in turbulence Jeanette D. Wheeler1,*, Kit Yu Karen Chan1,2,3,*,, Erik J. Anderson3,4 and Lauren S. Mullineaux1 Many marine organisms have complex life histories, having sessileadults and relying on the planktonic larvae for dispersal. Larvae swimand disperse in a complex fluid environment and the effect of ambientflow on larval behavior could in turn impact their survival and transport.However, to date, most studies on larvae-flow interactions havefocused on competent larvae near settlement. We examined theimportance of flow on early larval stages by studying how local flow andontogeny influence swimming behavior in pre-competent larval seaurchins, Arbacia punctulata. We exposed larval urchins to grid-stirredturbulence and recorded their behavior at two stages (4- and 6-armedplutei) in three turbulence regimes. Using particle image velocimetry toquantify and subtract local flow, we tested the hypothesis that larvaerespond to turbulence by increasing swimming speed, and that theincrease varies with ontogeny. Swimming speed increased withturbulence for both 4- and 6-armed larvae, but their responsesdiffered in terms of vertical swimming velocity. 4-Armed larvae swammost strongly upward in the unforced flow regime, while 6-armed larvaeswam most strongly upward in weakly forced flow. Increasedturbulence intensity also decreased the relative time that larvae spentin their typical upright orientation. 6-Armed larvae were tilted morefrequently in turbulence compared with 4-armed larvae. Thisobservation suggests that as larvae increase in size and add pairs ofarms, they are more likely to be passively re-oriented by moving water,rather than being stabilized (by mechanisms associated with increasedmass), potentially leading to differential transport. The positiverelationship between swimming speed and larval orientation anglesuggests that there was also an active response to tilting in turbulence.Our results highlight the importance of turbulence to planktonic larvae,not just during settlement but also in earlier stages throughmorphology-flow interactions. KEY WORDS: Pluteus, Behavior, Hydrodynamics, Particle imagevelocimetry INTRODUCTION Many marine invertebrates have complex life histories in which theplanktonic larval phase acts as the vehicle to connect otherwise ( ' Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA02543, USA. D i vision of L i fe Science, Hong Kong University of Science andTechnology, Clear Water Bay, Kowloon, Hong Kong. A pplied Ocean Physics andEngineering Department , Woods H o le Oceanographic Institution, Woods Hole,MA 02543, USA. “Department of Mechani c al Engineering, Grov e City Coll e ge,Grove City, PA 16127, USA. ) ( *These authors contributed equally to this work ) ( +Author for correspondence (karenchan@ust.hk) ) ( T hi s i s an Open A ccess a r ticl e dist r ibuted under th e t er ms o f t h e Creativ e Commons Attribut i onLicense (http: / /crea t ivecommons.org/li c enses/by / 3.0), w h ic h permits unr e stricted us e , d istribution and reproduc t io n in any medium provided that the original w ork is p r operly at t ributed. ) disjointed benthic adult populations which are mostly non-mobile(Cowen and Sponaugle, 2009; Levin, 2006). Larval supply, interms of both the number of settlers and their condition, plays animportant role in determining population dynamics and communityinteractions (Pechenik, 2006; Pineda et al., 2007). Larval swimmingbehaviors in response to various chemical, biological and physicalcues have important implications for the adult populations (Metaxasand Saunders, 2009). For planktonic, pre-competent larvae,swimming behaviors significantly impact vertical distributionpatterns, which in turn shape dispersal (Clay and Griinbaum,2010; Sameoto et al.,2010). For older, competent larvae, behaviorsaround settlement sites could significantly affect recruitmentpatterns (Hadfield and Koehl, 2004; Wethey, 1986; Zimmer andButman, 2000). One such set of behavior-triggering cues are the hydromechanicalsignals associated with turbulent flow that larvae experience innature (Eckman, 1996; Roy et al., 2012). These hydromechanicalsignals can be interpreted as three interacting components, namelyacceleration, rotation and deformation (Vogel, 1994). Larvae mayrespond to combinations of these signals in an active and/or passivemanner. In the water column, strong swimming larvae such as crabzoea actively change their swimming speeds in response toturbulence intensity (Welch and Forward, 2001) and barnaclecypridsswimupwardstocounteract downwellingcurrents(DiBacco et al.,2011). For moderate swimming larvae such asoyster larvae, a plasticity in response to turbulence has beenobserved, where competent-to-settle larvae have been observed toboth sink (Fuchs et al.,2013) and swim upward (Fuchs et al., 2015;Wheeler et al.,2013) in high turbulence; these active behavioralresponses may be regulated by body size. For weaker swimmingplankton, such as larval sand dollars, it has been observed that theirmorphologies primarily interact passively with ambient flow (Chan,2012; Clay and Grinbaum, 2010). Plutei of larval sand dollarsrepresent an ‘armed morphology', for which organisms use ciliatedextensions (such as arms or lobes) for feeding and swimming. Thistype of dual-use structure is shared between many weaklyswimming marine invertebrate larvae, such as other echinoderms,mollusks and some polychaete larvae (Strathmann and Griinbaum,2006). Shuttlecock-like pluteus larvae have long ciliated armssupported by a calcite skeleton (Pennington and Strathmann, 1990),and add pairs of arms (from 4-armed, to 6-armed, and to 8-armed)through their development. Plutei are characterized by fore-aftasymmetry (Chan, 2012) and, in some species, a distinct densitydifferential in which mass is concentrated in the posterior (Mogamiet al., 2001). Both these morphological characteristics of pluteuslarvae induce passive reorientation in low Reynolds numberconditions, by two different mechanisms. Fore-aft asymmetrycoupled with negative buoyancy produces a gravity-inducedhydrodynamic torque (Roberts, 1970), while a differential densityproduces a torque proportional to the distance between the larva’scenter of gravity and center of buoyancy (Mogami et al., 2001; Roberts, 1970). Both mechanisms likely play a role in the observedreorientation of larvae in flow, but previous experimental evidencesuggests that urchin larvae primarily rely on non-homogeneousdensity, or ‘bottom heaviness’to reorient (Mogami et al., 2001).The larvae-turbulence interaction is therefore a combination of bothactive behavioral choices such as those of barnacle cyprids andlarval oysters and passive biomechanical limitations imposed by thepluteus morphologies of larval sand dollars and sea urchins. The three-way interactions between larval behavior, larvalmorphologyy aandttlhesurroundingflowhave ssignificantimplications for population distribution. Clay and Griinbaum(2010) reported that 4- and 8-armed larval sand dollars are morelikely to be passively reoriented into downwelling regions by tiltingin shear flow. This tilting in shear flow suggests an inability tomaintain a stable orientation (hereafter stability)-the larva’s usualvertically directed swimming with arms in an upright position. Suchontogenetic differences in stability could imply distinct passivemechanisms to mediate transport and vertical position throughontogeny, and, indeed, field observations of larval sand dollarssuggest that older larvae are more likely to be found in deeper waters(Pennington and Emlet, 1986). Swimming-flow interactions couldalso affect distribution patterns through aggregation. Some weaklyswimming plankton, e.g. Heterosigma akashiwo, exhibit strongspatial and temporal patchiness driven by gyrotactic motility inshear: directed motion resulting from the orientation of cell axesthrough the balance of viscous and gravitational torque (De Lilloet al., 2014; Durham et al., 2013; Durham and Stocker, 2012).Therefore, quantifying swimming behaviors of planktonic larvae inenvironmentally relevant flow fields is essential for understandingtransport and dispersal. To date, however, most studies have focused on larvae-turbulence interactions of competent larvae preparing to settle(but see Chan et al., 2015; McDonald, 2012; Roy et al., 2012).Various studies highlight that larvae actively respond to turbulenceor components of turbulence, e.g. larval boat snails Crepidulafornicata increase upward swimming with increasing turbulencelevel (Fuchs et al., 2010), larval sea slugs Phestilla sibogae retracttheir vela when encountering turbulent filaments containingchemical cues (Hadfield and Koehl, 2004), and larval easternoysters Crassostrea virginica dive when experiencing high fluidacceleration over short time intervals (Wheeler et al., 2015).Together with other modeling studies, these earlier works suggestturbulence enhances larval settlement (Eckman, 1990; Fuchs et al.,2015; but see Pearce et al., 1998, who suggested that an increasein turbulence reduces settlement in scallop larvae). Recently,deformation associated with horizontal shear has been suggested toinduce competency in larval urchins (Gaylord et al., 2013) and toinduce cloning in coral larvae (Heyward and Negri, 2012). Whilethere is increasing information about how late-stage larvae respondto realistic flow fields near settlement, there is still relatively littleunderstanding of how weakly swimming ciliated planktonic larvaerespond to turbulence in earlier life stages and how that responsemay change through ontogeny. In this study, we exposed the weakly swimming planktonic larvaeof the sea urchin Arbacia punctulata to three environmentallyrelevant turbulent flow regimes at two different developmentalstages (4- and 6-armed plutei). We hypothesized that (1) larvaeactively modify their swimming speeds in response to ambient flowconditions such that older, larger larvae swim faster and (2) larvaeare passively reoriented in flow such that older, larger larvae aremore stable as a result of bottom heaviness. Using non-invasivevideo tracking and flow subtraction techniques, we investigated the effect of turbulence on swimming speed and stability of larval4. punctulata through ontogeny. MATERIALS AND METHODS Study organism and larval culturing The study organism is the purple urchin A. punctulata (Lamarck1816), which has long been a focus species ofembryonic and larvaldevelopment studies (Harvey, 1932; Hinegardner, 1969) with well-established eco-toxicological responses (Nacci et al., 2000). Twomale and two female A. punctulata adults were procured from theMarine Biological Laboratory Animal Supply (Woods Hole, MA,USA) and were injected with 0.55 mol1-KCl to induce spawning(Strathmann, 1987). Oocytes collected were washed througha63 um mesh to remove debris and sperm were collected dry. Eggs ofeach female were fertilized in 0.22 um-filtered seawater (~18℃,32 psu) with sperm solutions from both males at concentrations ofapproximately 1000 sperm ml-. Fertilization success was visuallyconfirmed by the presence of a fertilization envelope in>95% of theeggs at 20 min post-fertilization. Maternal cultures were rearedseparately and later combined in equal proportions for theexperimental observations. At 24 h post-fertilization, hatched embryos from each femalewere transferred into six 16 1 plastic containers (12 containers intotal) holding filtered seawater (0.22 um filtered, 32 psu) at adensity of ~6 individuals ml-. This rearing density was chosen toprovide a sufficient number of larvae for subsequent videoobservations. These containers were aerated and kept in anenvironmental chamber maintained at 18±1°C. Larvae were fedad libitum with a combination of Isochrysis galbana and Dunaliellatertiolecta at 15,000 and 2500 cells ml, respectively, daily.Complete water changes were performed every 3 days. Daily 20 mlsubsamples were taken from each container and examined under amicroscope to confirm normal larval development. Video observations of larval swimming in turbulence Swimming behaviors of larvae in different levels of turbulence wereobserved at 8 and 23 days post-fertilization (4- and 6-armed larvalstages, respectively). These observations were made in a Plexiglastank (44.5x44.5x90 cm) equipped with two vertically oscillatinggrids identical to that detailed in Wheeler et al. (2013). Larvalswimming and fluid motion were observed in a vertical cross-section field of view (FOV) of 3.5×3.5 cm in the center of the tankusing a monochrome high-speed video camera (Photron FastcamSA3) at 60 frames s-l. The FOV was illuminated with a sheet oflight from a near-infrared laser (Firefly 300 W, 1000 Hz,808 nm;Oxford Lasers) set perpendicular to the optical axis of the camera. Four replicate trials were conducted for each stage using differentbatches of larvae, with the tank drained and rinsed between trials.Larvae were subsampled from all culturing vessels, i.e. larvae fromboth mothers were simultaneously used for these experimentaltrials. Larval urchins were gently poured into the tank from a beaker,with approximately 60,000 and 28,000 larvae introduced per trialat day 8and day 23, respectively, for larval densities of<0.5 individuals ml-. Neutrally buoyant polystyrene particles of3-3.4 um diameter (Spherotech, 2.5 cm’of a 10% w/v suspension)were injected into the tank as passive tracer particles for particleimage velocimetry (PIV). During each trial, larval urchins were exposed to three differenttreatment levels by oscillating the grids at 0, 0.25 and 0.5 Hz at afixed amplitude of5 cm. The energy dissipation rates of these threeregimes were previously computed using a separate set of PIVexperiments and the resultant 2D flow vectors as per Doron et al. (2001), and were estimated to be ~0, 0.002 and 0.017 cm²s-3(Wheeler et al.,2013).Kolmogorov length scales for the two higherflow regimes were 0.147 and 0.088 cm, respectively, and theintegral length scales were 3.023 and 3.649 cm, respectively,corresponding to the ranges of the smallest to dominant energy-containing eddies in the tank (Wheeler et al., 2015). The mean4- and 6-armed larval midline lengths, for comparison, wereapproximately 0.013 and 0.018 cm, respectively. Hereafter, thesethree turbulence treatments are referred to as unforced, low forcingand moderate forcing. The qualitative terms are intended to describethe relative intensity of the turbulence in the range of what larvaemight experience in field conditions. While surf zone conditions canreach energy dissipation rates of 10° to 104 cm²s-3(Gaylord et al.,2013), tidal and estuarine flows are calmer (10-2 to 10° cm²s-3),with energy dissipation rates decreasing further away from the coast(Gross and Nowell, 1985). Our turbulence forcing regimes reflectenergy dissipation rates of the open ocean and of calmer nearshorewaters. Larvae were exposed to increasing turbulence from unforcedflow to moderate forcing in a sequential manner. Five minutes ofspin up time at the beginning of each turbulence level allowed theflow to equilibrate before filming began, and multiple 45 s videoclips (5-11 clips) were collected at each turbulence level. Videoclips were collected until an adequate total number of larvae (>100)had been observed in the FOV. In between treatments of low andmoderate forcing, the grids were stopped to allow flows to dissipate.Additional unforced flow video clips were collected during thisinterval after transient net downward flow was no longer visible inthe FOV. All video clips were exported and saved as high-resolution(1024×1024 pixels) TIFF images and used for larval tracking andflow visualization. At the end ofeach trial, larval urchins were collected on a 100 ummesh and fixed in buffered 2% paraformaldehyde for latermicroscopy and image analysis. Body lengths, lengths of skeletalarm rods and stomach were measured using the software Fiji(Schindelin et al., 2012; Fig. 1). Larval tracking Observed larval movement is a combination of both individualswimming motion and advection by surrounding fluid, so we Fig. 1. A comparison of the relative size and morphology of the 4- and 6-armed larva. Scale bars (lower right), 100 um. PO, postoral arm; AL,anterolateral arm. Reported length scales are stomach length, mid-line bodylength and total length (where total length is defined as the distance from thebase of the body to the oral hood, neglecting arm length). adapted the flow subtraction method from Wheeler et al. (2013,2015) to test whether swimming behaviors alone vary with changesin flow conditions. To compute larval velocities in such a relativeframework (isolated from advection in flow), we first identifiedlarval positions in each frame and calculated absolute velocity ofindividuals in the flow field. We subsequently estimated local flowvelocities using PIV and subtracted these to isolate larval swimmingvelocities from advection. The flow velocities were calculated using LaVision DaVisimaging software (v7.9). Each high-resolution TIFF image (forexample, Fig. 2A) was subdivided into 16×16 pixel interrogationwindows with no overlap and the average displacement of particlesin each window between images was determined by a multi-pass 2Dfast Fourier transform (FFT) analysis scheme. Velocity fields wereconverted into MATLAB data files for use in the subsequent flowsubtraction, and smoothed with a 10 time step interval boxcar filterto eradicate high-frequency noise. Full details ofthe PIV procedureare presented in Wheeler et al. (2013). To track individual larvae, the TIFF images were filtered toremove background noise and thresholded for brightness, leavinglarvae to appear as white silhouettes on a black background. Thecentroid position of individual larvae was recorded frame by frameusing customized LabView 2010 (National Instruments) software.Larval tracks were then compiled with in-house MATLAB (v7.12.0R2011a) software by connecting centroids frame-by-frame within auser-defined threshold distance, and used to calculate larval velocityin the FOV. Flow subtraction and larval velocity computation Larval positional data were integrated with PIV flow field data inMATLAB, where an annulus of flow velocity vectors around eachlarva was identified in each frame (Fig. 2B). The annulus had aninner radius equal to the sum of the larval radius and the PIV spatialgrid interval, to mask poor PIV velocity estimates near the larvalposition. The outer radius was4 times the inner radius, toincorporate ~4-6 velocity vectors away from the larva in alldirections. The flow velocity at the larval position was thenestimated: a second-order two-dimensional Taylor expansion wasfitted to the annulus velocity vectors and interpolated to the larvalposition. Larval swimming velocities (independent of advection inthe flow) were calculated at each time step (Fig. 2C) by subtractingthe flow velocity (interpolated to the larval position) from theabsolute velocity. For each larva, we denote swimming speed ateach time step as V,-[us, Ws], where V, is overall swimming velocityvector, V, is the vector magnitude, or speed, and us, Ws are horizontaland vertical velocities, respectively. Larval orientation To identify larval orientation, TIFF images were imported intoMATLAB and subsampled in time to 10 frames s-. A subset oflarvae was randomly selected (per video clip), and from larvalposition data these larvae were magnified individually to facilitatethe identification of the larval orientation axis. The larval orientationaxis was determined by extending the body midline to a pointequidistant between the visible pair of arms (anterolateral orposterodorsal), and this process was repeated frame by frame toconstruct a time series for the orientation angle of each larva. A totalof 16 larval trajectories (sampled at10 frames s-) in eachturbulence regime (3 levels) for each of the 4 trials were analyzedin this way, for a total of 192 larval trajectories in each of the 4- and6-armed groups. We computed the angular deviation betweenorientation axis and the true Cartesian vertical (defined here as .B 18 20 22 D 18 20 22 x (mm) x(mm) Fig. 2. Larval movement and orientation tracking. (A) Sample experimental image, where an in-focus larva (with arms clearly visible) is highlighted by the whitedashed box. Smaller white specks are passive particles and larger diffuse spots are out of focus larvae.(B)Close-up of the highlighted larva, with overlaid particleimage velocimetry (PIV) velocity field (white arrows) surrounding it. Passive particle intensity is dimmed for clarity. (C) Sample time series of an individual larva'svertical swimming velocity as it was tracked in the field of view. (D) Close-up of the highlighted larva, with overlaid Cartesian coordinate system and shaded rangeof angles(-25 to 25 deg from vertical) at which the larva was considered upright. (E) Sample time series of an individual larva's orientation angle from vertical as itwas tracked in the field of view. The shaded region denotes the range of orientation angles at which the larva was considered upright. 0 deg, using the FOV as the reference),hereafter referred to as larvalorientation angle (Fig. 2D). Using the orientation time series ofeachlarva (Fig. 2E), we computed the proportion of time spent upright bynormalizing the duration in which the orientation angle lies between±25 deg from the vertical with the total time the larva was tracked.The identification of orientation axes was not automated because ofthe highly variable appearance of the multi-armed larvae in twodimensions. Attempts to automate orientation computations led toheavy biases toward the angles of the arms that were closest to thefocal plane, which were much brighter than the more distant arms.While the range of angles from vertical defining an uprightorientation (±25 deg) was chosen somewhat arbitrarily, analysiswith various magnitudes of deviation showed that our subsequentstatistical conclusion is not overly sensitive to this parameter. Statistical analysis We compared changes in larval size between 4- and 6-armed stagesusing a t-test. We tested for the effect of turbulence level on verticaland horizontal swimming velocities, swimming speed and stabilityusing non-parametric statistical methods because the dataset did notmeet the assumption of equal variance. We compared the swimmingspeeds (V ) and velocities (us, Ws) across all forcing regimes betweenthe two stages with a Mann-Whitney test. We also compared the effectof the turbulence regime on the distributions of horizontal velocity(us), vertical velocity (ws) and speed (V ) within the 4- and 6-armedstages separately with Kruskal-Wallis tests. We compared the effect ofturbulence regime on stability at each of the two developmental stagesusing the proportion of time spent upright as a metric with Kruskal-Wallis tests, and identified possible directional biases in orientationangle with a Wilcoxon signed-rank test. We further explored therelationship between orientation angle and horizontal velocity (us),vertical velocity (ws) and swimming speed (V ) with a Spearman rankcorrelation for each of the developmental stages. RESULTS Larval size and swimming speed increased with ontogeny As larval urchins developed, their overall size significantly increased,with an average mid-line body length of 131.0± 10.1 um at the 4-armed stage and 175.8±15.5 um at the 6-armed stage (t-test,F=135.1, P<0.001; Table S1). Considering all turbulence regimes combined,swimming speed (V) increased through ontogeny, with an averageflow-subtracted swimming speed of 748.6±14.6 um s-at the 4-armed stage and 953.5± 18.9 um s12at the 6-armed stage(U=1,921,191, P<0.0001).).. HEowever, vertical andhorizontalvelocities varied with stage in a different pattern, such that therewas no significant effect of stage on horizontal velocity but asignificant effect on vertical velocity (U=1,212,659, P<0.0001). Swimming speeds changed with turbulence level butdiffered between stages Generally, larval urchins swam in relatively straight, upwardlydirected paths. Their swimming speeds varied significantly betweenturbulence regimes at both 4-armed (K=438.8, P<0.001; Fig. 3A)and 6-armed stages (K=542.1, P<0.001; Fig. 3B), where overallspeed (V ) increased monotonically with increasing turbulenceintensity for both stages. The turbulence regime had a significanteffect on vertical velocity (ws) for both 4-armed (K=6.446,P=0.040; Fig. 3C) and 6-armed stages (K=21.763, P<0.001;Fig. 3D). However, the two developmental stages demonstrateddistinct relationships between turbulence and vertical swimmingvelocity: at the 4-armed stage, vertical velocity decreased slightlywith increasing turbulence (Fig. 3C), such that vertical velocity wasfastest in the unforced regime with a median vertical velocity of445 um s-;at the 6-armed stage, vertical velocity increased andthen decreased with increasing turbulence (Fig. 3D), such thatvertical velocity was highest in the low forcing regime with amedian vertical velocity of 337 um s-, compared with 244 um s-in the unforced regime and 122 um s-at moderate forcing. Larvalhorizontal velocities (us), however, did not significantly changewith turbulence at either the 4-armed (K=4.896, P=0.086) or6-armed stage (K=0.784, P=0.676). Stability decreased with turbulence and age Larval orientation angle in all observed cases averaged close tovertical (0.9±16.5 deg), with >73% of all observations within±10 deg. The median angle did not significantly differ from zero(W=-472, P=0.914), demonstrating that the larvae as a populationhad no preferred direction (positive or negative) in orientation angle Fig. 3. Larval swimming speed and time spent in vertical orientation atdifferent turbulence intensities. (A,B) Median larval swimming speed (V)with 95% confidence intervals, with respect to the turbulence regime, for4-armed larvae (A) and 6-armed larvae (B). (C,D) Median larval verticalswimming velocity (Ws) with 95% confidence intervals, with respect to theturbulence regime, for 4-armed larvae (C) and 6-armed larvae(D). (E,F) Meanproportion of time spent by larvae within ±25 deg of the vertical orientation with95% confidence intervals, with respect to the turbulence regime for 4-armedlarvae (E) and 6-armed larvae (F). from vertical. As expected, turbulence did not bias larval orientationangle, as the flow was near isotropic and homogeneous. Thedistribution of the median angle was unimodal, demonstrating thatthere were not multiple groups of larvae with preferred directionalangles that averaged zero. However, transient tilting occurredfrequently in turbulence. Larvae at both developmental stages spentover 95% of the time in the upright position in the unforced regime(Fig. 3E,F), but time spent upright decreased significantly withturbulence for both 4-armed (K=17.33, P<0.001; Fig. 3E) and6-armed larvae (K=27.62,P<0.001; Fig. 3F). Older larvae appearedmore susceptible to tilting than younger larvae: in the moderateforcing regime, the younger 4-armed larvae were upright on average87.6±2.9% (±s.e.) of the observed time compared to 69.7±4.1%(ts.e.) at the 6-armed stage. Swimming speed correlated with orientation angle We explored the relationship between larval orientation (measuredfrom the vertical, 0 deg) and swimming speed across all flow regimesand found a considerable difference between the 4- and 6-armedstages. At the 4-armed stage, there was no significant relationshipbetween the orientation angle and overall swimming speed (V),horizontal velocity (us) or vertical velocity (ws)(P>0.126). In contrast, at the 6-armed stage, the slight deviations from vertical orientation(Fig. 4A, data points at lower left) were significantly correlated withincreased swimming speed (Spearman rank p=0.163, P=0.002;Fig. 4A). There was no significant correlation between horizontalvelocity and orientation angle (Spearman rank p=0.0716, P=0.325).Vertical velocity, however, was negatively correlated with orientationangle (p=-0.221,P-0.002; Fig. 4B), i.e. tilted larvae were more likelyto sink than upright larvae. The sinking of larvae as a result ofnegativebuoyancy explains how it is possible to have negative verticalvelocities even though orientation angles are less than 90 deg (Fig. 4B).Observed variance in swimming speed is due in part to the range inbody size of observed larvae (in addition to behavioral components)and the variance may partially obscure the changes in larval behaviorwhen tilted. Probability distributions of swimming speed (Fig. 4C,D)and vertical velocity (Fig. 4E,F) for upright versus tilted larvaehighlight the changes in behavior: median swimming speed in uprightlarvae was 624.1±34.8 pum s- (Fig. 4C) and increased to 817.3+116.5 ums-in tilted larvae (Fig. 4D). Upright larvae had a medianvertical velocity of 346.5±31.3 um s(Fig. 4E), while tilted larvaehad a median vertical velocity of 91.2±87.6 um s-(Fig.4F). DISCUSSION Marine invertebrate larvae swim in a complex fluid environment,and their responses to hydromechanical signals during theirplanktonicaand near-settlementitstages have significantimplications for transport, survival and recruitment. By exposingtwo different stages of larval urchin A. punctulata to differentturbulence regimes in the laboratory, we found that larval swimmingspeed increased and vertical velocity changed with increasingturbulence. Vertical velocity of the older 6-armed larvae increasedat low forcing but decreased when they experienced moderateforcing, suggesting more downward movement. This is consistentwith ourobservations that 6-armed larvae were more unstable whenexposed to turbulence and spent less time in the upright positioncompared with 4-armed larvae. Such changes in stability throughontogeny can potentially lead to changes in depth distributions aslarvae age: larvae may occupy wider or narrower bands of depth, orshift from surface to deeper water. Such changes to verticaldistributions may in turn impact larval survival and transport. The swimming speed and vertical velocity of larval urchinschanged significantly with age and turbulence regime (Fig. 3). Theolder, larger, 6-armed larval urchins had higher swimming speedsthan the 4-armed larvae, and this increase is consistent with earlierhydromechanical model predictions that weight-carrying capacity isproportional to total arm length (Griinbaum and Strathmann, 2003).Here, weight-carrying capacity is defined as the maximum netdownward force, i.e. the difference between gravitational pull andbuoyancy sustained by a larva at zero velocity. However, this increasein swimming speed with turbulence did not translate to an increase invertical velocity. For 4-armed larvae, the median vertical velocity ofthe population was smaller in both the forced regimes compared withthe unforced regime. For 6-armed larvae, median vertical velocitypresented a dome-shaped response with increasing turbulence, suchthat vertical velocity was largest at low forcing and smallest atmoderate forcing. Contrary to findings in other marine invertebratelarvae, e.g. mollusk veligers (Fuchs and DiBacco, 2011; Wheeleret al., 2013), the vertical velocity of larval urchins did not increasewith increased turbulence. However, these results are consistent withprevious observations of other echinoderms through ontogeny: olderlarval sand dollars and larval green urchins have a lower verticalvelocity under no flow or shear conditions compared with youngerlarvae (Chan et al., 2015,2011). 3000 2000 A o B 2500弓>o 1500 2000 1000 00 88 1500 500 88 . c9 8 8.88 o 1000 0 8 0 do c0 0 500 -500 0 0 10 20 30 40 50 60 70 -1000L0 10 20 30 40 50 60 70 Track-averaged angle from vertical (deg) F Probability Fig. 4. Correlation between swimming speed and orientation angle. (A,B) Track-averaged orientation angle (0 deg is vertical) versus track-averagedswimming speed (V;A) and track-averaged vertical swimming velocity (w ;B). Each point represents an individual 6-armed larva, using larvae from all turbulenceregimes. (C-F) Probability distribution of track-averaged swimming speed in upright larvae (within ±25 deg of vertical; C) and tilted larvae (outside ±25 deg ofvertical; D), and probability distribution of track-averaged vertical swimming velocity in upright larvae (E) and tilted larvae (F). Asterisks denote median speed orvelocity for each distribution. Note that the x-axes for the tilted larvae (D and F) are reversed,so that they are mirror images of C and E, respectively. The observed differences in vertical velocity between 4- and 6-armedlarvae may stem from a changing morphology as they age. While theincreased number and length of arms could provide more weight-carrying capacity (Grinbaum and Strathmann, 2003), the associatedincrease in the calcite skeleton structure could potentially counteractthis increase in weight-carrying capacity and incur metabolic costs forupward swimming while carrying additional weight (Pennington andStrathmann, 1990). The importance of this second factor is supportedby our observations of lower vertical swimming velocities in 6-armedlarvae when compared with 4-armed larvae, across all turbulenceregimes. However, as the functional response of vertical swimmingvelocity to turbulence regime also differed between thetwodevelopmental stages, we conclude that the ambient flow must alsoplay a role, and that observed changes in swimming velocity are notsolely driven by the balance between weight-carrying capacity and size.Some potential explanations for the between-stage differences arestructural: the additional pair of posterodorsal arms in the 6-armedlarvae have fenestrated skeletal rods, providing drag to reduce passivesinking speeds (Emlet, 1983), and these arms are connected to thelarval body by muscles that allow larvae to orient these arms to redirect local flow fields or permit reverse swimming (Lacalli and Gilmour,1990). The ability to increase drag and redirect flow might help 6-armedlarval urchins to maintain to a certain degree their vertical velocities atlow forcing, in comparison to unforced or moderate forcing regimes,where they may not engage these strategies. Like swimming velocity, larval orientation also changedsignificantly with age and turbulence regime. Larvae of both agegroups spent significantly less time upright as turbulence intensityincreased, and 4-armed larvae were significantly more stable than6-armed larvae in all turbulence regimes. Ontogenetic changes inlarval shape may play a role in the larval ability to maintain anupright orientation and, hence, stability in flow. Older, heavierlarval urchins have increased skeleton weight as ballast, whichcould enhance stability (Gruinbaum and Strathmann, 2003; Mogamiet al.,2001). However, the addition of arms and increased size couldalso compromise stability by affecting the separation distancebetween the center of gravity and center of buoyancy (Grunbaumand Strathmann, 2003). Individuals with a longer or wider spread ofarms might also more readily cross stream lines, thus experiencing alarger fluid torque (Chan, 2012; Clay and Gruinbaum,2010). These biomechanical constraints may help account for the observation that6-armed larvae were more prone to tilting in higher turbulencecompared with smaller 4-armed larvae. Our results highlight the importance of morphology in creatingbiomechanical constraints for movement. The pluteus morphology ofechinoids varies significantly between families, but differentmorphological structures could serve ia similar purpose: forexample, the arbaciids studied here have posterolateral arms;spatangoids have both posterolateral arms and an apical process (DeAmaralet al.,2007). In both cases, these extensions could potentiallyact to counterbalance larvae in moving water. Computational fluiddynamics modeling work on functional morphology and associatedtradeoffs of ‘armed’larvae would significantly contribute to ourunderstanding of the ecology and evolution of larval forms (Chan,2012; Kiorboe et al., 2014; Miller et al.,2012). Ourobservations suggest that pre-competent larval urchins respondto turbulence with both active and passive mechanisms: they activelyincrease their swimming speed in increased turbulence and arepassively reoriented through morphology-flow interactions, whichcompromise their ability to maintain directed swimming. Whenexposed to increased turbulence, 6-armed larvae had higherswimming speeds but lower vertical swimming velocities than 4-armed larvae. It is important to note that a decrease in such time-averaged vertical velocity in the 6-armed larvae does not necessarilyrepresent a reduction in movement with turbulence. Instead, thischange may reflect an increase in the proportion of time spentswimming in directions other than the preferred vertical orientation,suggesting a passive response to turbulence. This is supported by thesignificant negative correlation between orientation angle and verticalswimming velocity. However, the observed increase in swimmingspeed in higher turbulence regimes demonstrates that larvae are notmerely being reoriented and persisting in a default swimming mode:the positive correlation between orientation angle and swimmingspeed demonstrates that larvae swim faster when they are tilted, orpotentially that stronger swimmers are more prone to tilting. The first interpretation, that tilted larvae swim faster, suggests anintriguing possibility that an active behavior is triggered by a passivelarval response to local flow conditions.While tilting andreorientation are likely passive responses to increased shear inturbulence, an increased swimming speed subsequent to tilting is anactive response. Larval responses to ambient flow may involve moresuch interactions between active and passive responses than havepreviously been investigated. Despite the absence of a statocyst-likestructure in larval urchins, our results support early observations thatthey have mechanoreception ability and are capable of adjusting theirpropulsive behaviors accordingly (Mogami et al., 2001,1988; Wadaet al.,1997). Further work on ciliary motion control (Gustafson et al.,1972; Shiba et al., 2002) and gene expression (e.g. orthologs ofvertebrate mechanosensory genes, such as Sp-Usherin and TRPA1;Sodergren et al., 2006;M. Volnoukhin, Mechanosensory cells andswimming behaviour ofembryos ofthe sea urchin Strongylocentrotuspurpuratus, MSc thesis, Simon Fraser University, 2010) could helpus to understand the biophysical mechanisms of behavioral controlunder natural flow conditions. The second interpretation, that faster swimming larvae are moreprone to tilting, also has a sound biomechanical basis. Gallager(1993) demonstrated using tethered swimming shipworm larvae thatthe flows generated by swimming larvae decay more rapidly awayfrom the larval body as swimming speed increases. Self-inducedflows may shield larvae from background turbulence, and so slowerswimming may confer greater stability. Future investigations of larvalswimming in moderate and high turbulence regimes where tilting is most prevalent will help to further elucidate the relationship betweenorientation angle and swimming speed, and to determine larvalresponse times for an active swimming response to tilting. The observed changes in response to turbulence intensity throughlarval developmenthave significant implications ffo)rrlarvaldistribution. If our observations in the laboratory can translate to thewater column in the coastal ocean, older, 6-armed larvae would bemore likely to be tilted, have slower vertical velocity, and move intodownwelling regions to be downwardly transported in these energeticenvironments (Clay and Griinbaum, 2010). This differentialswimming could result in selective transport, leading to ontogeneticdifferences in depth distribution (Chan et al., 2011; Griinbaum andStrathmann, 2003). One possible scenario is that the differentialtransport helps younger individuals to maintain themselves in thesurface water where food abundance is higher and help olderindividualstoaapproach their settlement sites. Alternatively,differences in response and distribution could reflect differentstrategies to avoid predation. One possible hypothesis is that smaller,4-armed larvae are less vulnerable to predators relying on vision and,hence, can survive even in well-illuminated surface waters. Our resultssuggest that the observation of early stage pre-competent larvae inrealistic flow conditions, in addition to competent larvae seekingsettlement sites, is important for understanding the populationdynamics of marineinvertebrates.. cOur observationsalsodemonstrateddthat agyrotaxis-like reorientation responsettoturbulence present in multicellularswimminggplankton.Turbulence, therefore, not only impacts larval transport but alsocould potentially shape the aggregation and distribution of otherzooplankton and thereby impact ecological interactions through themodulation of patch dynamics. Acknowledgements We thank Susan Mills for larval culturing and experimental assistance, and KarlHelfrich for assistance with video set-up and for thoughtful manuscript comments. Weare grateful for the valuable discussion on data analysis and interpretation that VictoriaStarczak and Jesús Pineda kindly provided. We also thank the facilities engineers,especially Richard Galat, at WHOI for their assistance in experimental setup. Competing interests The authors declare no competing or financial interests. Author contributions J.D.W., K.Y.K.C., and L.S.M. conceived and designed the study. J.D.W. and E.J.A.developed larval tracking and fluid motion analysis approach. J.D.W. and K.Y.K.C.conducted the experiments, performed data analysis, and drafted the manuscript.J.D.W.,K.Y.K.C., E.J.A., and L.S.M. edited the manuscript. All authors gave finalapproval for publication. Funding This work was supported by the National Science Foundation [OCE-0850419] andthe National Oceanic and Atmospheric Administration Sea Grant [NA14OAR4170074]. K.Y.K.C. was supported by the Postdoctoral Scholar Programat the Woods Hole Oceanographic Institution (WHOI), with funding provided by theCoastal Ocean Institute, the Croucher Foundation and the Royal Swedish Academyof Sciences.K.Y.K.C. is currently funded by the Croucher Foundation. Additionalfunding was provided to L.S.M. through the WHOI Ocean Life Fellowship anddiscretionary WHOI funds, and to E.J.A. through the faculty sabbatical program atGrove City College. Deposited in PMC for immediate release. Supplementary information Supplementary information available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.129502/-/DC1 References Chan, K. Y.K. (2012). Biomechanics of larval morphology affect swimming: insightsfrom the sand dollars Dendraster excentricus. Integr. Comp. Biol. 52, 458-469. Chan, K. Y. K., Grunbaum, D. and O'Donnell, M. J. (2011). Effects of ocean-acidification-induced morphological changes on larval swimming and feeding.J. Exp. Biol. 214, 3857-3867. Chan, K. Y. K., Garcia,E. Dupont, S. (2015). Acidification reduced growth rate butnot swimming speed of larval sea urchins. Sci. Rep.5, 9764. Clay, T. W. and Grunbaum, D.(2010). Morphology-flow interactions lead to stage-selective vertical transport of larval sand dollars in shear flow. J. Exp. Biol. 213,1281-1292. Cowen, R. K. and Sponaugle, S. (2009). Larval dispersal and marine populationconnectivity. Annu. Rev. Mar. Sci.1, 443-466. De Amaral, P. Nunes, C. and Jangoux, M. (2007). Larval growth andperimetamorphosis in the echinoid Echinocardium cordatum (Echinodermata):the spatangoid way to become a sea urchin.Zoomorphology 126, 103-119. De Lillo,F., Cencini, M., Durham, W. M., Barry, M., Stocker, R., Climent, E. andBoffetta, G. (2014). Turbulent fluid acceleration generates clusters of gyrotacticmicroorganisms. Phys. Rev. Lett.112,044502. DiBacco,C., Fuchs, H. L., Pineda, J. and Helfrich, K. (2011). Swimming behaviorand velocities of barnacle cyprids in a downwelling flume. Mar. Ecol. Prog. Ser.433,131-148. Doron, P., Bertuccioli, L., Katz, J. and Osborn, T. R. (2001). Turbulencecharacteristics and dissipation estimates in the coastal ocean bottom boundarylayer from PIV data.J. Phys. Oceanogr.31, 2108-2134. Durham, W. M. and Stocker, R. (2012). Thin phytoplankton layers: characteristics,mechanisms, and consequences. Annu. Rev. Mar. Sci. 4, 177-207. Durham,W. M., Climent, E., Barry, M., De Lillo, F., Boffetta, G., Cencini, M. andStocker, R. (2013). Turbulence ddrives microscale patches of motilephytoplankton. Nat. Commun.4,2148. Eckman, J. E. (1990). A model of passive settlement by planktonic larvae ontobottoms of differing roughness. Limnol.Oceanogr. 35, 887-901. Eckman,J.E.(1996). Closing the larval loop: linking larval ecology to the population dynamics of marine benthic invertebrates. J.Exp. Mar. Biol. Ecol. 200, 207-237.Emlet,R. B. (1983). Locomotion, drag, and the rigid skeleton of larval echinoderms.Biol. Bull. 164,433-445. Fuchs, H. L. and DiBacco, C. (2011). Mussel larval responses to turbulence areunaltered by larval age or light conditions. Limnol. Oceanogr. Fluids Environ. 1,120-134. Fuchs, H. L., Solow, A. R. and Mullineaux, L. S. (2010). Larval responses toturbulence and temperature in a tidal inlet: habitat selection by dispersinggastropods? J. Mar. Res. 68,153-188. Fuchs, H. L., Hunter, E. J., Schmitt, E. L. and Guazzo, R. A. (2013). Activedownward propulsion byoyster larvae in turbulence. J.Exp. Biol. 216, 1458-1469. Fuchs, H. L., Gerbi, G. P., Hunter, E. J., Christman, A. J. and Diez, F. J. (2015).Hydrodynamic sensing and behavior by oyster larvae in turbulence and waves.J.Exp. Biol. 218, 1419-1432. Gallager, S. M. (1993). Hydrodynamic disturbances produced by smallzooplankton: case study for the veliger larva of a bivalve mollusc. J. PlanktonRes. 15, 1277-1296. Gaylord, B., Hodin, J. and Ferner, M. C. (2013). Turbulent shear spurs settlementin larval sea urchins. Proc. Natl. Acad. Sci. USA 110,6901-6906. Gross, T. F. and Nowell, A. R.M.(1985). Spectral scaling in a tidal boundary layer.J.Phys. Oceanogr. 15, 496-508. Grunbaum,D. and Strathmann, R. R.(2003). Form, performance and trade-offs inswimming and stability of armed larvae. J. Mar. Res. 61,659-691. Gustafson, T., Lundgren, B. and Treufeldt, R. (1972). Serotonin and contractileactivity in the echinopluteus: a study of the cellular basis of Larval behaviour. ExpCell Res.72, 115-139. Hadfield, M. G. and Koehl, M. A. R.(2004). Rapid behavioral responses of aninvertebrate larva to dissolved settlement cue. Biol. Bull.207,28-43. Harvey, E. B. (1932). The development of half and quarter eggs of Arbacia punctulata and of strongly centrifuged whole eggs. Biol. Bull.62, 155-167. Heyward, A. J. and Negri, A. P. (2012). Turbulence, cleavage, and the nakedembryo: a case for coral clones. Science 335, 1064-1064. Hinegardner, R.T.(1969). Growth and development of the laboratory cultured seaurchin. Biol. Bull. 137,465-475. Kiorboe, T., Jiang, H., Goncalves, R. J., Nielsen, L. T. and Wadhwa, N. (2014).Flow disturbances generated by feeding and swimming zooplankton. Proc. Natl.Acad. Sci. USA 111, 11738-11743. Lacalli, T.C. and Gilmour, T.H.J.(1990). Ciliary reversal and Locomotory control inthe Pluteus Larva of Lytechinus pictus. Philos. Trans. R Soc. B Biol. Sci. 330,391-396. Levin, L. A. (2006). Recent progress in understanding larval dispersal: newdirections and digressions. Integr. Comp. Biol. 46, 282-297. McDonald, K. A. (2012). Earliest ciliary swimming effects vertical transport ofplanktonic embryos in turbulence and shear flow. J. Exp. Biol.215, 141-151. Metaxas, A. and Saunders, M. (2009). Quantifying the “bio-"components inbiophysical models of larval transport in marine benthic invertebrates: advancesand pitfalls. Biol. Bull. 216, 257-272. Miller, L. A., Goldman, D. I., Hedrick, T. L., Tytell, E.D., Wang, Z. J., Yen, J. andAlben,S. (2012). Using computational and mechanical models to study animallocomotion. Integr.Comp. Biol.52,553-575. Mogami, Y., Oobayashi, C.,Yamaguchi, T., Ogiso, Y. and Baba, S. A. (1988).Negative Geotaxis in Sea Urchin Larvae: a possible role of mechanoreception inthe late stages of development. J. Exp. Biol. 137, 141-156. Mogami, Y., Ishii, J. and Baba, S. A. (2001). Theoretical and experimentaldissectionof gravity-dependent mechanicalorientation inGravitacticmicroorganisms. Biol. Bull. 201, 26-33. Nacci, D., Serbst, J., Gleason, T. R., Cayula, S., Thursby, G., Munns, W. R., Jr.and Johnston, R. K. (2000). Biological responses of the sea urchin, Arbaciapunctulata, to lead contamination for an estuarine ecological risk assessment.J. Aquat. Ecosyst. Stress Recovery 7,187-199. Pearce, C. M., Gallager, S. M., Manuel, J. L., Manning, D. A.,O'Dor, R. K. andBourget, E. (1998). Effect of thermoclines and turbulence on depth of larvalsettlement and spat recruitment of the giant scallop Placopecten magellanicus in9.5 m deep laboratory mesocosms. Mar. Ecol. Prog. Ser. 165, 195-215. Pechenik,J.A.(2006). Larval experience and latent effects—metamorphosis is nota new beginning. Integr. Comp. Biol. 46, 323-333. Pennington, J. T. and Emlet,R. B. (1986). Ontogenetic and diel vertical migrationof a planktonic echinoid larva, Dendraster excentricus (Eschscholtz): occurrence,causes, and probable consequences.J. Exp. Mar. Biol. Ecol.104, 69-95. Pennington, J. T. and Strathmann, R. R. (1990). Consequences of the calciteskeletons of planktonic echinoderm larvae for orientation, swimming, and shape.Biol. Bull. 179, 121-133. Pineda,J.,Hare,J.A. and Sponaugle, S.(2007). Larval transport and dispersal in thecoastal ocean and consequences for population connectivity. Oceanography 20,22-39. Roberts, A. (1970). Geotaxis in motile micro-organisms. J. Exp. Biol.53,687-699.Roy?,y ,A., Metaxas, A. and Ross, T. (2012). Swimming patterns of larvalStrongylocentrotus droebachiensis in turbulence in the laboratory. Mar. Ecol.Prog. Ser. 453, 117-127. Sameoto, J. A., Ross, T. and Metaxas, A. (2010). The effect of flow on larvalvertical distribution of the sea urchin, Strongylocentrotus droebachiensis. J. Exp.Mar. Biol. Ecol. 383, 156-163. Schindelin,J.,Arganda-Carreras,I.,Frise, E.,Kaynig, V.,Longair, M., Pietzsch,T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B. et al. (2012). Fiji: an . open-source platform for biological-image analysis. Nat. Methods 9, 676-682. Shiba, K., Mogami, Y. and Baba, S. A. (2002). Ciliary movement of sea-urchinembryos. Nat. Sci. Rep. Ochanomizu Univ. 53, 49-54. ( Sodergren, E. W einstock, G. M. Davidson, E. H. Cameron, R. A. Gibbs, R . A.Angerer, R . C. Angerer, L. M. Arnone, M. I. Burgess, D. R . Burke, R. D. e t al. (2006). The Genome of the Sea Urchin Strongylocentrotus purpuratus. Science 314,941-952. ) ( Strathmann, M.F.(1987). R eproduction and Development of Marine Invertebratesof the Northern Pacific Coast: Data and Methods for the Study of Eggs, Embryos, a nd Larvae. Seattle: U niversity of Washington Press. ) Strathmann, R. R. and Grunbaum, D. (2006). Good eaters, poor swimmers:compromises in larval form. Integr. Comp. Biol. 46, 312-322. Vogel, S. (1994). Life in Moving Fluids: The Physical Biology of Flow. Princeton: Princeton University Press. Wada, Y., Mogami, Y. and Baba, S. (1997). Modification of ciliary beating in sea urchin larvae induced by neurotransmitters: beat-plane rotation and control of frequency fluctuation. J. Exp. Biol.200,9-18. Welch, J. and Forward, R. (2001). Flood tide transport of blue crab, Callinectessapidus, postlarvae: behavioral responses to salinity and turbulence. Mar. Biol.139,911-918. Wethey, D. S. (1986). Ranking of settlement cues by barnacle larvae: influence ofsurface contour. Bull. Mar. Sci. 39, 393-400. Wheeler,J. D., Helfrich, K. R., Anderson,E.J., McGann, B., Staats, P., Wargula,A. E., Wilt, K. and Mullineaux, L. S. (2013). Upward swimming of competentoyster larvae Crassostrea virginica persists in highly turbulent flow as detected byPIV flow subtraction. Mar. Ecol. Prog. Ser. 488, 171-185. Wheeler, J. D., Helfrich, K. R., Anderson, E. J. and Mullineaux, L. S. (2015).Isolating the hydrodynamic triggers of the dive response in eastern oyster larvae.Limnol.Oceanogr. 60,1332-1343. Zimmer, R. K. and Butman, C. A. (2000). Chemical signaling processes in themarine environment. Biol.Bull. 198,168-187. Received August Accepted February
确定



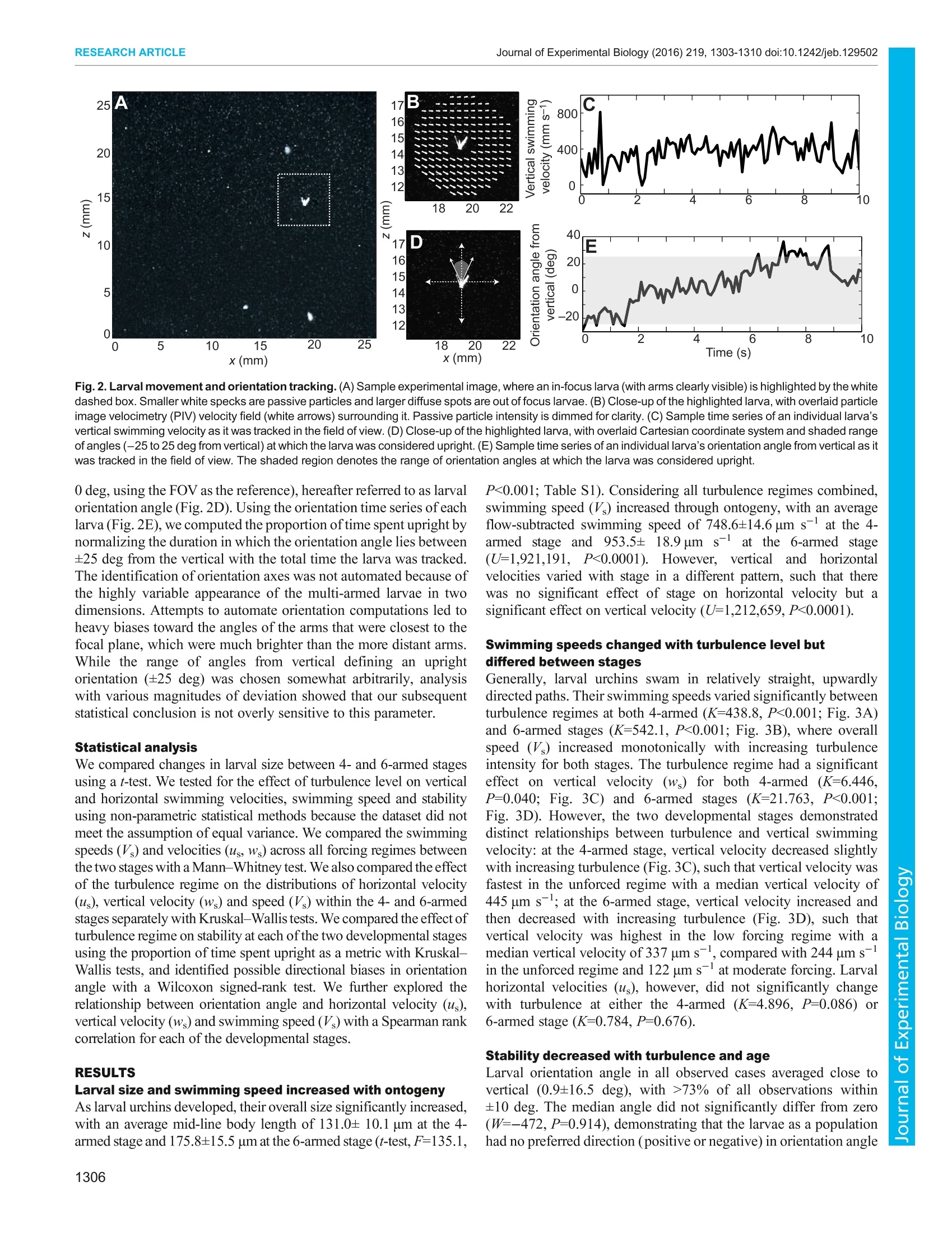
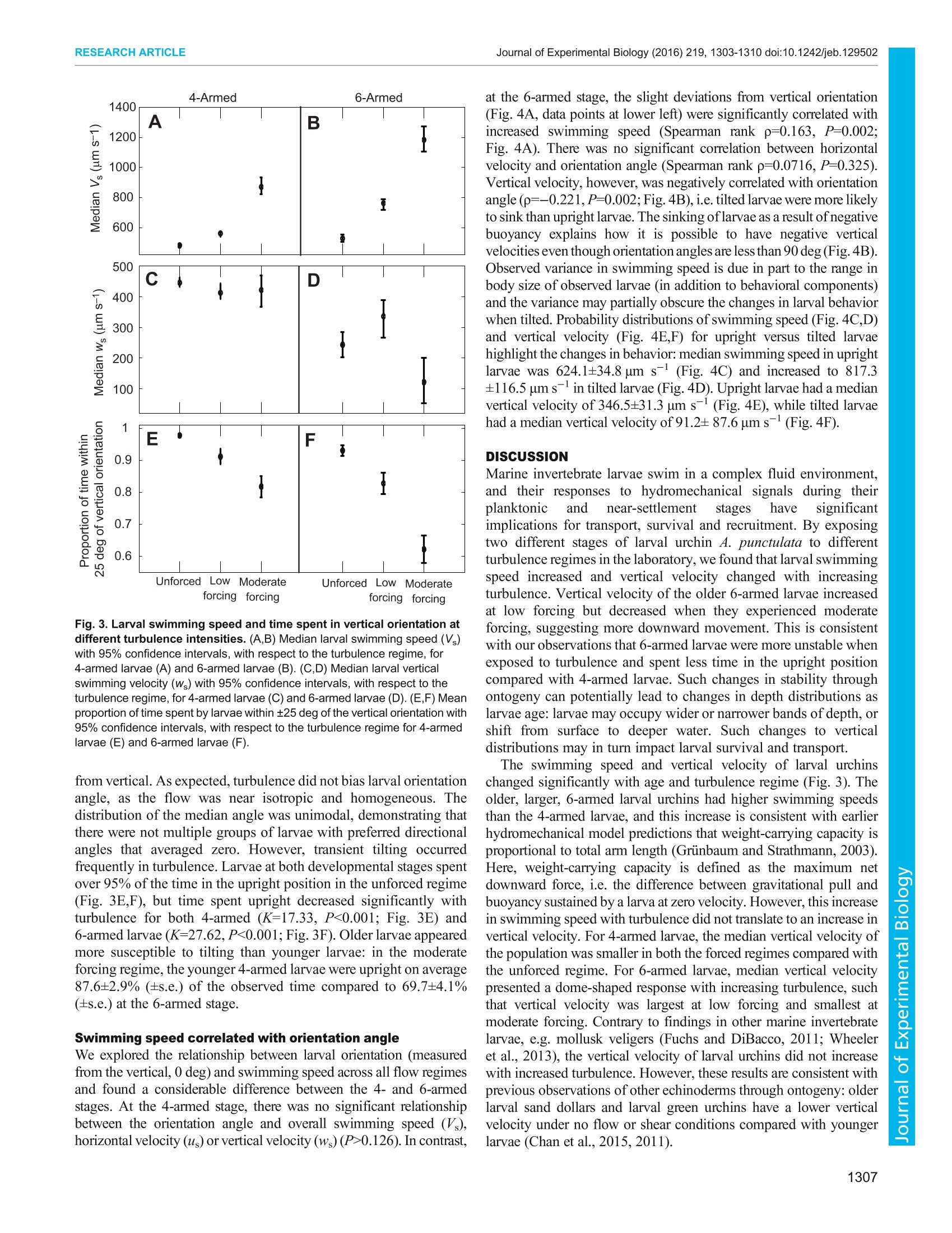
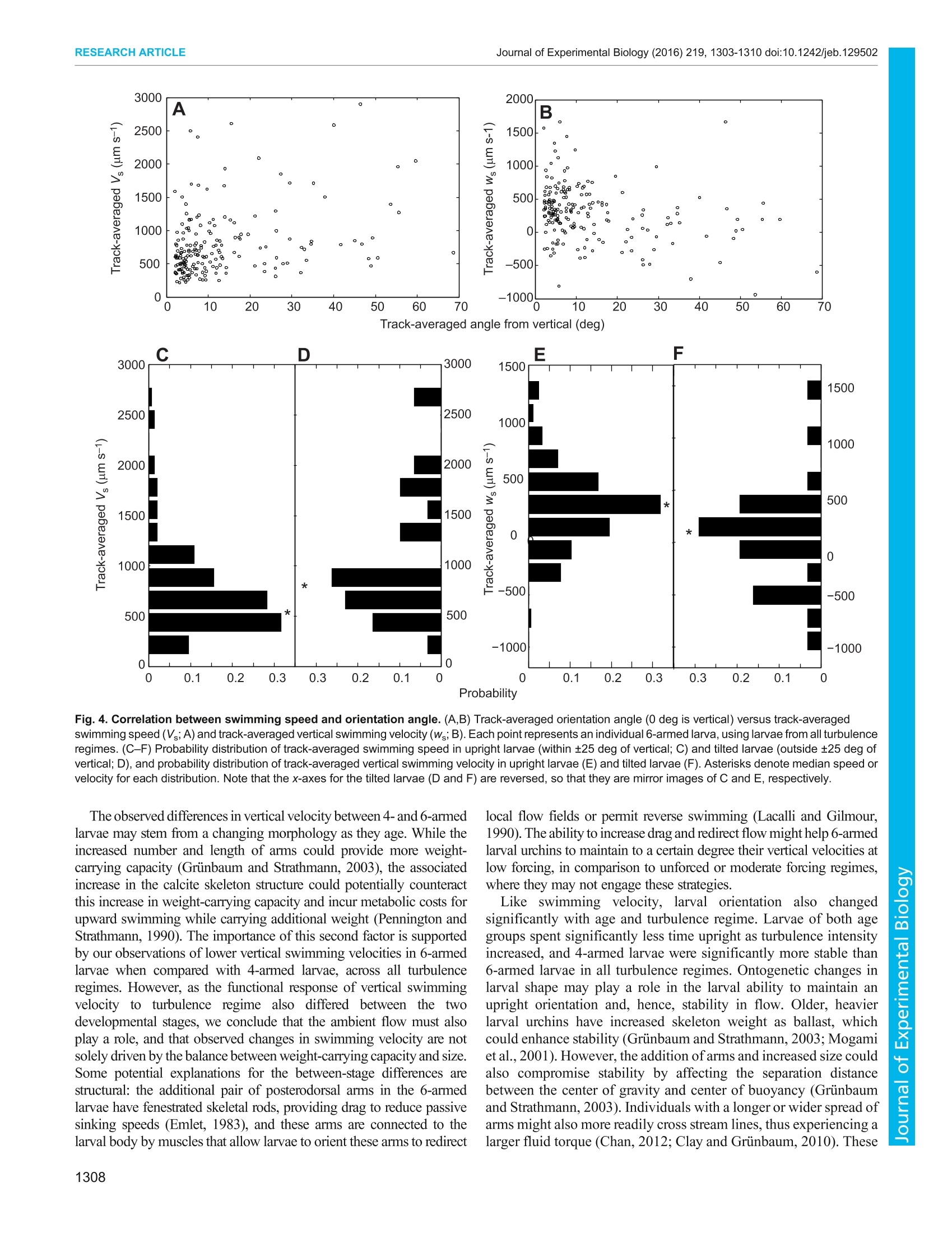


还剩6页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《游动的未成熟海胆幼虫中个体发育变化和湍流中的取向检测方案(粒子图像测速)》,该方案主要用于其他中个体发育变化和湍流中的取向检测,参考标准--,《游动的未成熟海胆幼虫中个体发育变化和湍流中的取向检测方案(粒子图像测速)》用到的仪器有德国LaVision PIV/PLIF粒子成像测速场仪、Imager sCMOS PIV相机
推荐专场
相关方案
更多
该厂商其他方案
更多
















