方案详情
文
采用立陶宛Ekspla公司的NL240型半导体泵浦的纳秒激光器泵浦染料激光器研究虾青素自由基清除和细胞抗氧化剂特性。
方案详情

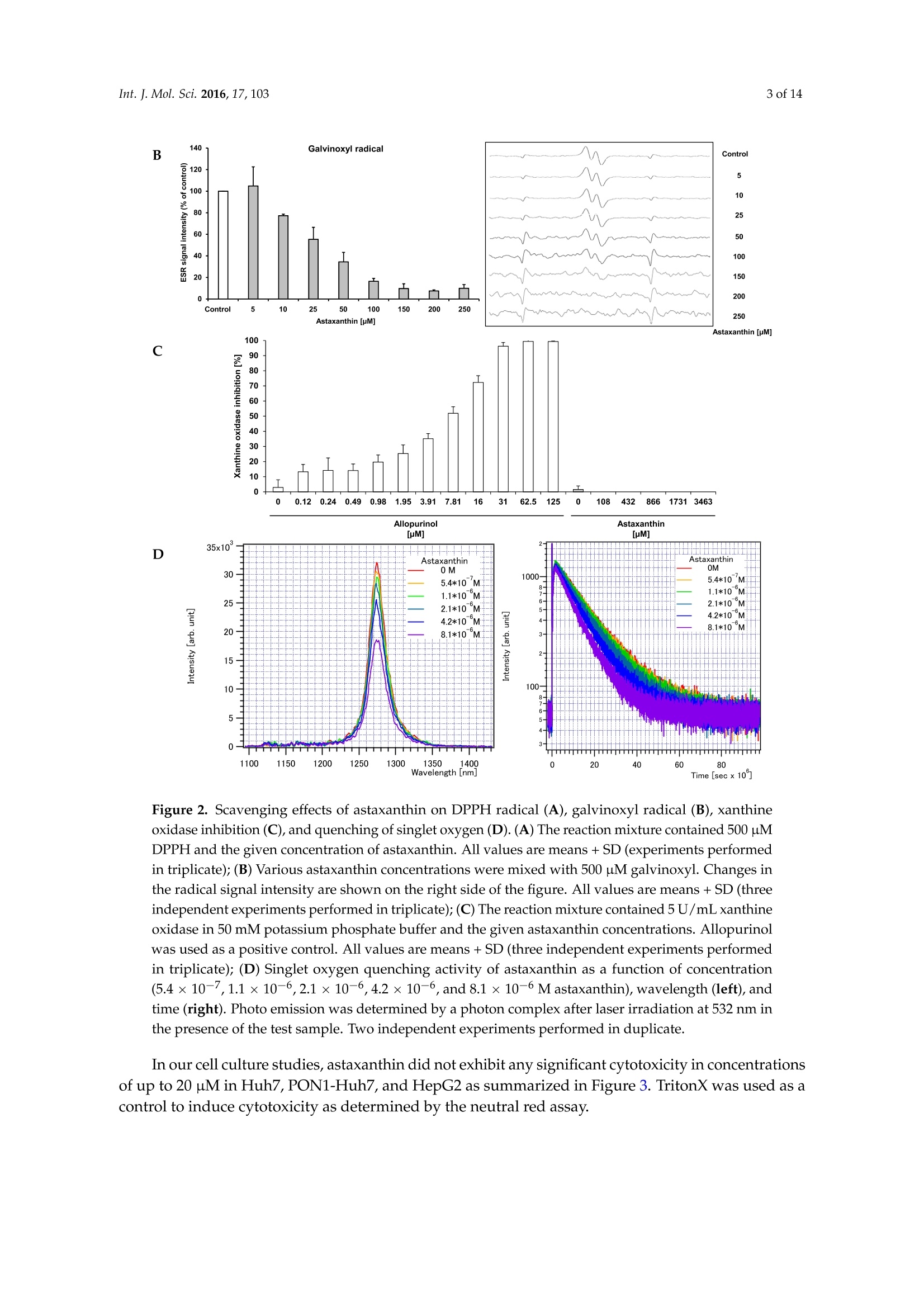
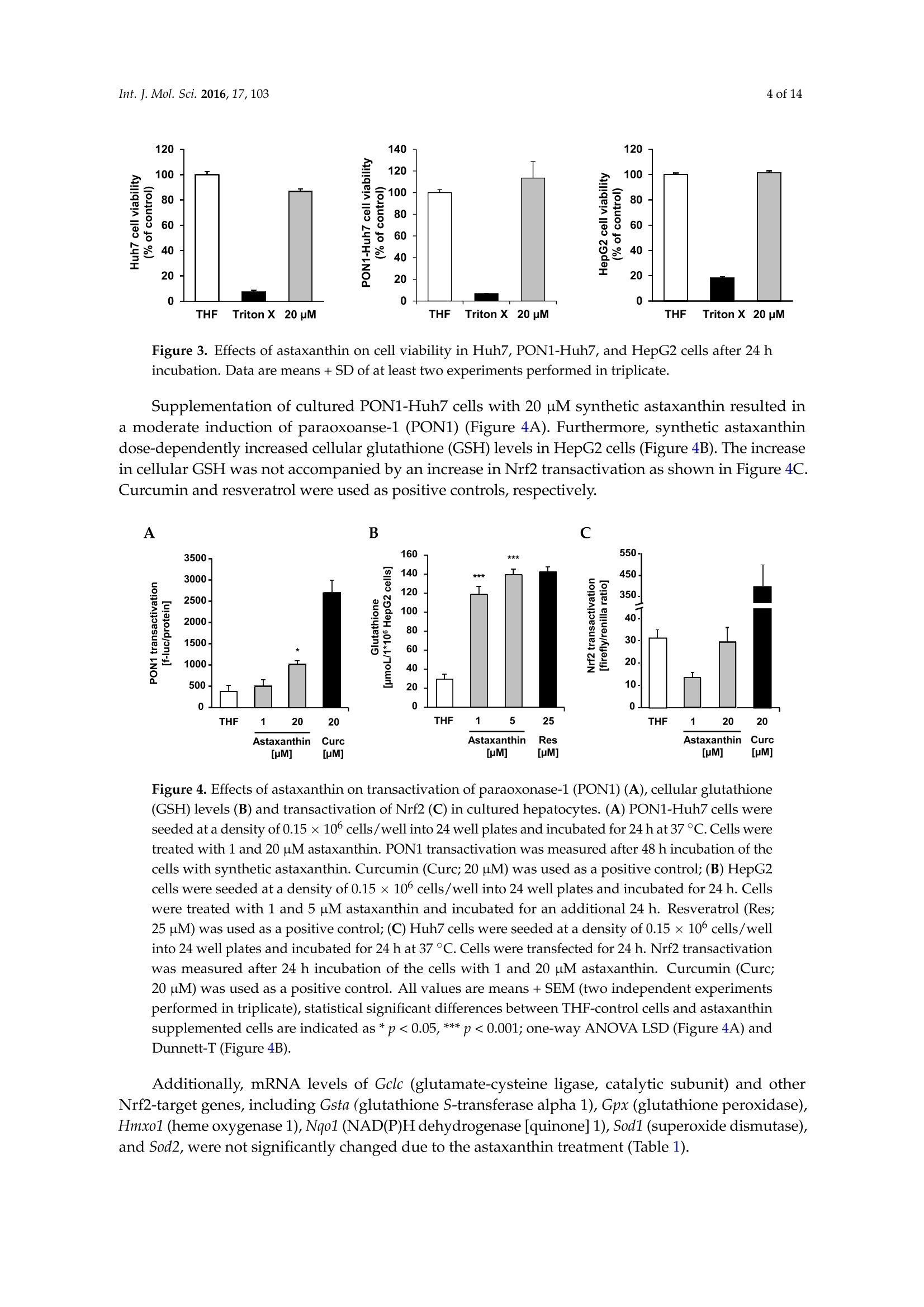
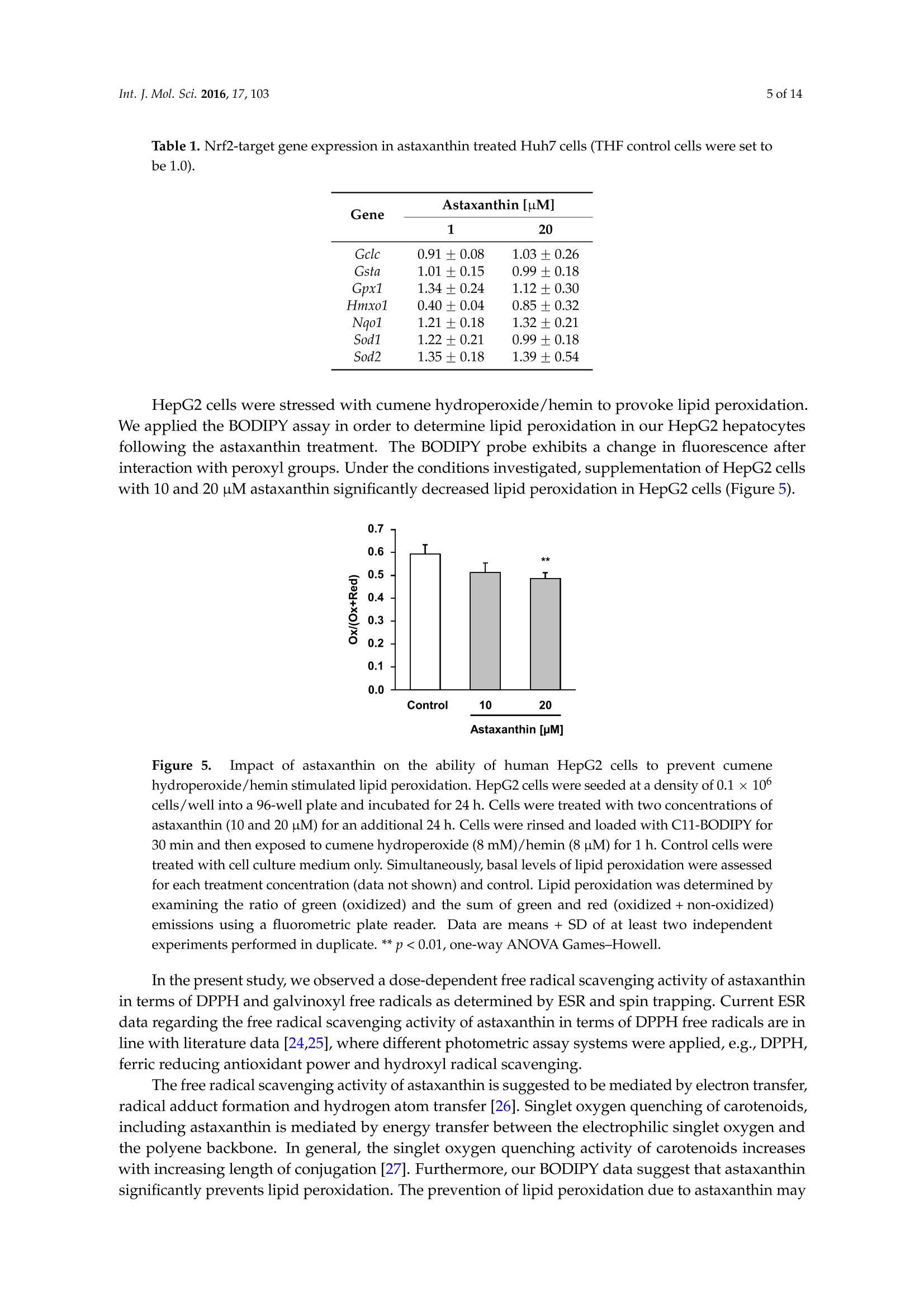
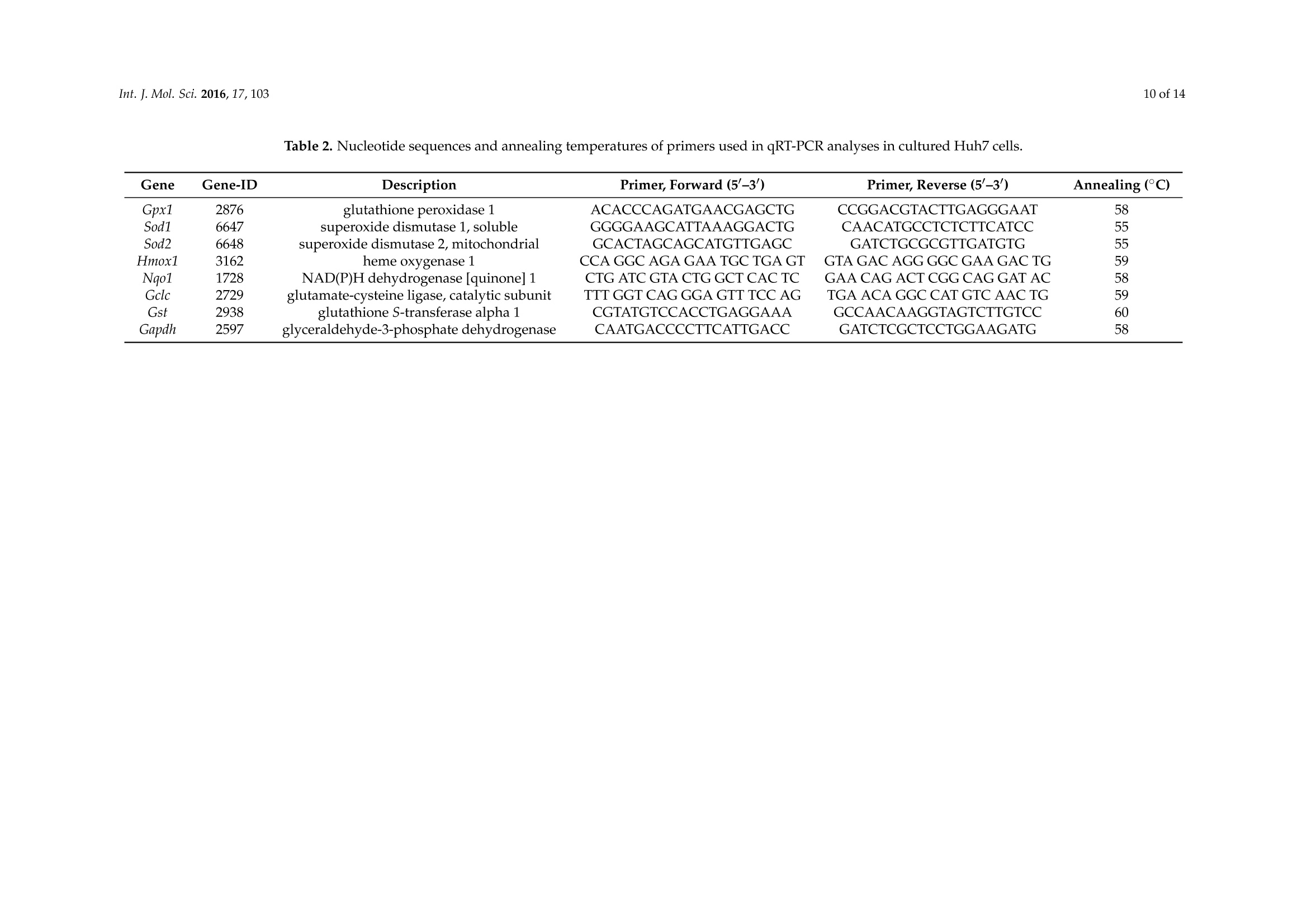
International Journal ofMolecular Sciences Int. J. Mol.Sci. 2016,17,1032 of 14 Int.J. Mol. Sci. 2016, 17,103; doi:10.3390/ijms17010103www.mdpi.com/journal/ijms Article Free Radical Scavenging and Cellular AntioxidantProperties of Astaxanthin Janina Dose 1, Seiichi Matsugo2, Haruka Yokokawa 2, Yutaro Koshida2, Shigetoshi Okazaki 3,Ulrike Seidel 1, Manfred Eggersdorfer 4, Gerald Rimbach 1 and Tuba Esatbeyoglu 1* Received: 12 October 2015; Accepted: 8 January 2016; Published: 14 January 2016 Academic Editor: Esra Capanoglu Institute of Human Nutrition and Food Science, University of Kiel, Hermann-Rodewald-Strafe 6,D-24118 Kiel,Germany; dose@foodsci.uni-kiel.de (J.D.); seidel@foodsci.uni-kiel.de (U.S.);rimbach@foodsci.uni-kiel.de (G.R.) 2 School of Natural System, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan; s-matsugoh@se.kanazawa-u.ac.jp (S.M.); haruside8133@gmail.com(H.Y.); koshi04015087@gmail.com (Y.K.) 3 Medical Photonics Research Center, Hamamatsu University School of Medicine, Handamachi 1-20-1,Higashi-ku, Hamamatsu, Shizuoka 431-3192,Japan; okazaki@hama-med.ac.jp 4 DSM Nutritional Products, P.O. Box 2676, 4002 Basel, Switzerland; manfred.eggersdorfer@dsm.com Correspondence: esatbeyoglu@foodsci.uni-kiel.de; Tel.: +49-431-880-5333 Abstract: Astaxanthin is a coloring agent which is used as a feed additive in aquaculture nutrition.Recently, potential health benefits of astaxanthin have been discussed which may be partly relatedto its free radical scavenging and antioxidant properties. Our electron spin resonance (ESR)and spin trapping data suggest that synthetic astaxanthin is a potent free radical scavenger interms of diphenylpicryl-hydrazyl (DPPH) and galvinoxyl free radicals. Furthermore, astaxanthindose-dependently quenched singlet oxygen as determined by photon counting. In addition to freeradical scavenging and singlet oxygen quenching properties, astaxanthin induced the antioxidantenzyme paroxoanase-1, enhanced glutathione concentrations and prevented lipid peroxidationin cultured hepatocytes. Present results suggest that, beyond its coloring properties, syntheticastaxanthin exhibits free radical scavenging, singlet oxygen quenching,and antioxidant activitieswhich could probably positively affect animal and human health. Keywords: astaxanthin;free radical scavenging; antioxidant; electron spin resonance spectroscopy 1. Introduction Astaxanthin (3,3'-dihydroxy-B,B'-carotene-4,4'-dione, for chemical structure see Figure 1) isa xanthophyll carotenoid [1] which naturally occurs in algae, krill, trout, crayfish, and salmon.Astaxanthin is widely used in aquaculture nutrition as a coloring agent [2]. In addition to its coloringproperties, astaxanthin may also affect immune status and reproduction [3,4]. Figure 1. Chemical structure of astaxanthin. Astaxanthin has two chiral centers at positions 3 and 3. The astaxanthin stereoisomer -3S,3S'-is the mainform found in wild salmon [5]. Most astaxanthin used in aquaculture nutrition is produced syntheticallywhich yields three different stereoisomers, including 3S,3'S;3R, 3'S; and3R, 3'R [1]. In 1981, Widmer et al. [6] synthesized astaxanthin from the educt 6-oxo-isophorone (3,5,5-trimethyl-2-cyclohexene-1,4-dione) via aseven-step synthesis. Over a Wittig reaction of two equivalents C15-phosphonium salt with C10-dialdehyde,astaxanthin was obtained with yields up to 50% yields [6]. Astaxanthin has also significant applications in the nutraceutical industry [7]. Various healthbenefits, including cardiovascular disease and cataract prevention, have been associated withastaxanthin consumption [3,8-10]. Although it has been suggested that potential health benefits of astaxanthin are, at least partly,mediated by its free radical scavenging [11], antioxidant [12,13], and gene-regulatory properties [14,15],systematic studies are scarce. In previous studies, free radical scavenging activity of astaxanthin has been determined by FRAP(ferric reducing antioxidant power) [16], TEAC (trolox equivalent antioxidant capacity) [17], andORAC (oxygen radical absorbance capacity) [18] assays, respectively. Furthermore, the decolorizationof the 2,2-diphenyl-1-picrylhydrazyl(DPPH) radical may be used to assess the free radical scavengingactivity of antioxidants, taking into account that alcohols as solvents may lead to an overestimation ofits free radical scavenging activity [19-21]. The different antioxidant test systems, as described in theliterature, differ in terms of underlying methodology, reagents, wavelength, pH, etc. It is suggestedthat a combination of various assays should be used in assessing antioxidant activities in vitro [22]. In addition to these rather indirect methods, electron spin resonance spectroscopy (ESR) combinedwith spin trapping has been established as a robust method for the direct assessment of free radicalreactions [23]. Therefore, in this study, we applied ESR, as well as photon counting methods, todetermine the free radical scavenging and singlet oxygen quenching activity of astaxanthin. Furthermore, we studied the cellular antioxidant activity of astaxanthin in cultured hepatocytes.We addressed the question, if and to what extend astaxanthin may induce enzymatic antioxidantdefense mechanisms, including paraoxoanase-1. Furthermore, we determined cellular glutathionelevels in response to an astaxanthin treatment, since glutathione is the most important endogenouscytosolic antioxidant centrally involved in redox signaling and stress response. Overall, this manuscript aims at providing comprehensive information in terms of the free radicalscavenging, antioxidant, and cell signaling modifying properties of astaxanthin. 2. Results and Discussion We applied ESR and spin trapping in order to directly quantify the free radical scavengingactivity of astaxanthin. Astaxanthin dose-dependently scavenged DPPH (Figure 2A) and galvinoxyl(Figure 2B) free radicals. However, astaxanthin did not scavenge superoxide anion free radicals (datanot shown). Accordingly, astaxanthin did not inhibit xanthine oxidase, which generates superoxideanion free radicals (Figure 2C). When singlet oxygen is relaxed to the ground state, oxygen photonemission is observed. Singlet oxygen quenching activity of astaxanthin as a function of emissionspectrum, concentration, and decay curve is given in Figure 2D. As shown in Figure 2D, astaxanthindose-dependently quenched singlet oxygen as determined by photon counting. A Figure 2. Cont B C D Wavelength [nm] Time [sec x 10] Figure 2. Scavenging effects of astaxanthin on DPPH radical (A), galvinoxyl radical (B), xanthineoxidase inhibition (C), and quenching of singlet oxygen (D). (A) The reaction mixture contained 500 pMDPPH and the given concentration of astaxanthin. All values are means + SD (experiments performedin triplicate); (B) Various astaxanthin concentrations were mixed with 500 uM galvinoxyl. Changes inthe radical signal intensity are shown on the right side of the figure. All values are means + SD (threeindependent experiments performed in triplicate); (C) The reaction mixture contained 5U/mL xanthineoxidase in 50 mM potassium phosphate buffer and the given astaxanthin concentrations. Allopurinolwas used as a positive control. All values are means + SD (three independent experiments performedin triplicate); (D) Singlet oxygen quenching activity of astaxanthin as a function of concentration(5.4×10-7,1.1×10-6,2.1×10-6,4.2×10-6, and 8.1×10-6M astaxanthin), wavelength (left),andtime (right). Photo emission was determined by a photon complex after laser irradiation at 532 nm inthe presence of the test sample. Two independent experiments performed in duplicate. In our cell culture studies, astaxanthin did not exhibit any significant cytotoxicity in concentrationsof up to 20 uM in Huh7, PON1-Huh7, and HepG2 as summarized in Figure 3. TritonX was used as acontrol to induce cytotoxicity as determined by the neutral red assay. Figure 3. Effects of astaxanthin on cell viability in Huh7,PON1-Huh7, and HepG2 cells after 24 hincubation. Data are means + SD of at least two experiments performed in triplicate. Supplementation of cultured PON1-Huh7 cells with 20 uM synthetic astaxanthin resulted ina moderate induction of paraoxoanse-1 (PON1) (Figure 4A). Furthermore, synthetic astaxanthindose-dependently increased cellular glutathione (GSH) levels in HepG2 cells (Figure 4B). The increasein cellular GSH was not accompanied by an increase in Nrf2 transactivation as shown in Figure 4C.Curcumin and resveratrol were used as positive controls, respectively. Figure 4. Effects of astaxanthin on transactivation of paraoxonase-1 (PON1) (A), cellular glutathione(GSH) levels (B) and transactivation of Nrf2 (C) in cultured hepatocytes.(A)PON1-Huh7 cells wereseeded at a density of 0.15×10cells/well into 24 well plates and incubated for 24 h at 37°C. Cells weretreated with 1 and 20 uM astaxanthin. PON1 transactivation was measured after 48 h incubation of thecells with synthetic astaxanthin. Curcumin (Curc; 20 pM) was used as a positive control;(B) HepG2cells were seeded at a density of 0.15 ×10cells/well into 24 well plates and incubated for 24 h. Cellswere treated with 1 and 5 uM astaxanthin and incubated for an additional 24 h. Resveratrol (Res;25 uM) was used as a positive control; (C) Huh7 cells were seeded at a density of 0.15 ×10 cells/wellinto 24 well plates and incubated for 24 h at 37 °C. Cells were transfected for 24 h. Nrf2 transactivationwas measured after 24 h incubation of the cells with 1 and 20 uM astaxanthin. Curcumin (Curc;20 uM) was used as a positive control. All values are means + SEM (two independent experimentsperformed in triplicate), statistical significant differences between THF-control cells and astaxanthinsupplemented cells are indicated as * p<0.05, *** p<0.001; one-way ANOVA LSD (Figure 4A) andDunnett-T (Figure4B). Additionally, mRNA levels of Gclc (glutamate-cysteine ligase, catalytic subunit) and otherNrf2-target genes, including Gsta (glutathione S-transferase alpha 1), Gpx (glutathione peroxidase),Hmxo1 (heme oxygenase 1), Nqo1 (NAD(P)H dehydrogenase [quinone] 1), Sod1 (superoxide dismutase),and Sod2, were not significantly changed due to the astaxanthin treatment (Table 1). Table 1.Nrf2-target gene expression in astaxanthin treated Huh7 cells (THF control cells were set tobe 1.0). Gene Astaxanthin [uM] 20 Gclc 0.91 ±0.08 1.03±0.26 Gsta 1.01±0.15 0.99±0.18 Gpx1 1.34±0.24 1.12±0.30 Hmxo1 0.40±0.04 0.85±0.32 Nqo1 1.21±0.18 1.32±0.21 Sod1 1.22±0.21 0.99±0.18 Sod2 1.35±0.18 1.39±0.54 HepG2 cells were stressed with cumene hydroperoxide/hemin to provoke lipid peroxidation.We applied the BODIPY assay in order to determine lipid peroxidation in our HepG2 hepatocytesfollowing the astaxanthin treatment. The BODIPY probe exhibits a change in fluorescence afterinteraction with peroxyl groups. Under the conditions investigated, supplementation of HepG2 cellswith 10 and 20 uM astaxanthin significantly decreased lipid peroxidation in HepG2 cells (Figure 5). Figure 5.Impact of astaxanthin on the ability of human HepG2 cells to prevent cumenehydroperoxide/hemin stimulated lipid peroxidation. HepG2 cells were seeded at a density of 0.1 ×10cells/well into a 96-well plate and incubated for 24 h. Cells were treated with two concentrations ofastaxanthin (10 and 20 uM) for an additional 24 h. Cells were rinsed and loaded with C11-BODIPY for30 min and then exposed to cumene hydroperoxide (8 mM)/hemin (8uM) for1 h. Control cells weretreated with cell culture medium only. Simultaneously, basal levels of lipid peroxidation were assessedfor each treatment concentration (data not shown) and control. Lipid peroxidation was determined byexamining the ratio of green (oxidized) and the sum of green and red (oxidized+non-oxidized)emissions using a fluorometric plate reader. Data are means + SD of at least two independentexperiments performed in duplicate. ** p<0.01, one-way ANOVA Games-Howell. In the present study, we observed a dose-dependent free radical scavenging activity of astaxanthinin terms of DPPH and galvinoxyl free radicals as determined by ESR and spin trapping. Current ESRdata regarding the free radical scavenging activity of astaxanthin in terms of DPPH free radicals are inline with literature data [24,25], where different photometric assay systems were applied, e.g.,DPPH,ferric reducing antioxidant power and hydroxyl radical scavenging. The free radical scavenging activity of astaxanthin is suggested to be mediated by electron transfer,radical adduct formation and hydrogen atom transfer [26]. Singlet oxygen quenching of carotenoids,including astaxanthin is mediated by energy transfer between the electrophilic singlet oxygen andthe polyene backbone. In general, the singlet oxygen quenching activity of carotenoids increaseswith increasing length of conjugation [27]. Furthermore, our BODIPY data suggest that astaxanthinsignificantly prevents lipid peroxidation. The prevention of lipid peroxidation due to astaxanthin may improve the sensory quality and shelve life of fish which warrants further investigation. However,it needs to be taken into account that the astaxanthin concentrations, as administered in our cell cultureassays, may be higher than astaxanthin concentrations in fish plasma [28]. We did not observe any inhibition of xanthine oxidase in response to the astaxanthin treatment.Thus, unlike other antioxidants including flavonoids [29,30], astaxanthin does not seem to mediateantioxidant activity via xanthine oxidase inhibition. For our cell studies, astaxanthin was dissolved in tetrahydrofuran (THF). We used THF sinceit has been previously shown by our group to be non-cytotoxic to HepG2 cells [31].Furthermore,astaxanthin dissolved in THF exhibits an appropriate stability in the cell culture medium [31] and iseffectively taken up by HepG2 and HT29 cultured cells [31,32]. Current cell culture data clearly suggest that synthetic astaxanthin does not only work as a freeradical scavenger, but also as an inductor of cellular antioxidant defense mechanisms, including PON1and reduced glutathione. PON1 is a hepatic enzyme which prevents and/or delays the oxidationof LDL [33]. Thus, the prevention of plasma lipid peroxidation in humans due to astaxanthin, asreported by Barlic and co-workers [34], may be partly mediated by an induction of paraoxonase-1.Recently, it has been shown that PON1 also counteracts oxidative stress induced by mercury chloride,thereby offering a beneficial strategy against HgCl2 toxicity [35] which may be of particular interestfor fish nutrition. We also determined the cellular activity of natural astaxanthin which was isolatedfrom Haematococcus pluvialis (data not shown). Importantly, natural source astaxanthin did not exhibita superior bioactivity in any of our test systems as also stated by others [36]. In terms of PON1, atransactivation occurred only in response to synthetic astaxanthin. Synthetic astaxanthin dose-dependently increased cellular GSH levels in HepG2 cells which isin line with findings by Saw and co-workers who found an induction of cellular GSH due to bothastaxanthin and omega-3 fatty acids [13]. Interestingly, in our study the increase in cellular GSH inHepG2 cells was, however, not accompanied by an increase in Nrf2 transactivation. Furthermore, Gclc,the rate limiting enzyme of GSH synthesis, was not induced by astaxanthin. Additionally, steady statelevels of other Nrf2 target genes, including Gsta, Gpx, Hmxo, Nqo1, Sod1, and Sod2 were not inducedin response to the astaxanthin treatment. Thus, it is suggested that the increase in cellular GSH dueto astaxanthin was not mediated by an Nrf2 dependent signal transduction pathway. It is worthmentioning that GSH synthesis is not only regulated by Nrf2, but also by other transcription factors,including activator protein 1 (AP-1) [37,38], specificity protein 1 (Sp1) [39,40], nuclear respiratory factor1 (Nrf1) [41,42], and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) [42,43].Based on the present data, it is suggested that elevated GSH concentration may be a sign of loweroxidative stress levels as a result of the astaxanthin treatment. 3. Experimental Section 3.1. Radical Scavenging Measured by Electron Spin Resonance Spectroscopy (ESR) ESR measurements were performed using a JEOL JES-FR30EX free radical monitor (JEOL Ltd.,Akishima, Japan). The measurement conditions were as follows: magnetic field,337.394 ±7.5 mT;power, 4 mW; sweep time, 1 min; sweep width, 7.5; modulation, 100 kHz, 0.32 mT; amplitude, 400;and time constant, 0.3 s. Signal intensity was compared based on the ratio against the magnetic Mn+marker and was represented by relative height ratio. The ESR spectra were measured three times. Preparation of the astaxanthin stock solution. Astaxanthin (DSM, Kaiseraugst, Switzerland) wasdissolved in 21.8% tetrahydrofuran (THF) (Wako Chemicals, Osaka, Japan) and 78.2% ethanol (NacalaiTesque, Kyoto, Japan). The actual concentration of astaxanthin was determined by diluting the stocksolution with THF using the molar coefficient of astaxanthin (69,600 M-1. cm-1at 477 nm) [44]. Basedon this value, the stock solution of astaxanthin was determined as 30.56 mM. The astaxanthin stocksolution was further diluted with tert-butyl alcohol/THF (89.3:10.7,v/v). DPPH radical scavenging experiments. To a reaction mixture containing 80 uL distilled water, 20 uL500 uM DPPH (in methanol; Nacalai Tesque, Kyoto, Japan) and 100 uL astaxanthin (0, 50, 100, 200, 300,400,500,600,800,1000,1200, and1500 uM) were added and stirred for a few seconds. tert-Butyl alcohol(containing 10.7% THF) was used as a control. After1 min incubation the ESR spectra were measured. Galvinoxyl radical scavenging experiments. To a reaction mixture containing 80 uL distilled water,20 uL 500 uM galvinoxyl (in methanol; Nacalai Tesque, Kyoto, Japan) and 100 uL astaxanthin (0, 10, 20,50,100,200,300,400,500uM) were added and stirred for a few seconds. tert-Butyl alcohol (containing10.7% THF) was used as a control. After 1 min incubation the ESR spectra were measured. Superoxide radical scavenging experiments. To the reaction mixture of 30 uL of 5 mM hypoxanthine(dissolved in NaOH and then diluted with PBS (pH 7.4)), 40 pL 4 M5,5-dimethyl-1-pyrroline N-oxide(DMPO), 25 uL ultrapure water, and 10 uL of astaxanthin (0, 1, 7.5, 15 mM) were added to 5 uL of1 U/mL xanthine oxidase in 200 mM PBS buffer solution (pH 7.4). After 1 min incubation at 25 °C theESR spectra were measured. Three independent experiments were performed in triplicate. 3.2. Inhibition of Xanthine Oxidase The inhibition of xanthine oxidase was measured according to Braunlich et al. 2013 [45] with somemodifications. An enzyme solution consisting of 400 uL xanthine oxidase (5U/mL) in 7.6 mL 50 mMpotassium phosphate buffer (pH= 7.5) was prepared immediately before use. One hundred andfifty microliters of the prepared enzyme solution was added to each well of a 96 well microtiterplate.Astaxanthin (4 g/L in THF) was serially diluted in the wells (dilution factor 2). THF was used as blanksample. Allopurinol (500 uM; Sigma, Darmstadt, Germany) served as positive control and was seriallydiluted in the wells (dilution factor 2). The substrate hypoxanthine (40 ug/mL; dissolved in NaOH andthen in distilled water) was prepared daily and 80 pL were injected at cycle 10. The absorption wasmeasured at 290 nm (microplate reader Tecan infinite 200Pro, Crailsheim, Germany) over 110 cycles(~150 s) at 28°C. At least three independent experiments were performed in triplicate. 3.3. Quenching Experiments of Near-Infrared Emission Spectra of 102 by Astaxanthin The singlet oxygen scavenging activity of astaxanthin was determined by photon countingaccording to Shimizu et al. (2010) [46] with some modifications. Ten millimolar astaxanthin was prepared in THF/ethanol (20/80;v/v). After preparing 10 mMastaxanthin, 1 mM astaxanthin was prepared in THF/ethanol (10.7/89.3;v/v). The final concentrationof astaxanthin was confirmed by measuring the solution of 2 mL ethanol and 80 uL astaxanthin solution(1 mM) by UV-Vis spectrometer (Hitachi, Tokyo, Japan) using the molar coefficient of astaxanthin(69,600M-1.cm-1at 入=477 nm). The 102 formation was directly measured by the near-infrared luminescence at around 1270 nmfrom deactivated 1O2 which corresponds to the 102(A)-302() transition. The emission fromO2 was measured using an apparatus based on a commercially available apparatus and improvedfor high-sensitivity detection (NIR-PII System, Hamamatsu Photonics K.K., Hamamatsu, Japan).The excitation pulse was obtained using a dye laser (CL-EGC USHO Optical Systems Co., Ltd., Osaka,Japan) excited by an Nd-YAG Laser (NL 240/TH EKSPLA Lithuania). The excitation wavelength was530 nm, pulse width and intensity were approximately 7 ns and 17 mW, respectively, and the repetitionrate was 500 Hz. Emission of 102 was monitored using an infrared-gated image intensifier (NIR-PII,Hamamatsu Photonics) after passage through a polychromator (250 is, Chromex Inc., Albuquerque,NM, USA). Measurements started at 1 us after application of the excitation pulse and the exposuretime was 50 us. Signals were accumulated by repeated detection (500 Hz, 10s). The emission decaywas detected by the NIR-PII system (Hamamatsu Photonics) after passing through the bandpassfilter (1270 nm) and recorded by the multichannel scalar (NanoHarp 250, PicoQuant GmbH, Berlin,Germany). The measuring time was set at 16 ns/bin for ca. 260 us and accumulated for 2 min at500 Hz. The solution studies consist of 2 mL of 5 uM Rose Bengal (in ethanol) as a photosensitizer.The quenching of the singlet oxygen decay was examined by adding 109 uM astaxanthin as follows: addition of 10 uL, 10 uL (final 20 uL), 20 uL (final 40 uL), 40 uL (final 80 uL),and 80 uL (final 160 uL).The decay curve and reaction rate of astaxanthin were calculated using the decay curve and quenchingratio of the duplicate experiments. 3.4. Cell Culture Human Huh7 hepatic cellular carcinoma cells (Institute of Applied Cell Culture, Munich,Germany) were cultivated in high glucose(4.5g/L) Dulbecco's modified Eagle’s medium (DMEM, withsodium pyruvate, L-glutamine and 3.7 g/L NaHCO3 supplemented with 10% (v/v) fetal bovine serum(FBS), 100 U/mL penicillin and 100 ug/mL streptomycin (all PAN-Biotech, Aidenbach, Germany)at 37°℃ in a 5% CO2 setting. For the preparation of PON1-Huh7 cells, Huh7 cells were stablytransfected with a 1009bp[-1013, -4] fragment of the human PON1 promoter (kindly provided byX. Coumoul and R. Barouki,INSERM UMR-S, Paris, France[47]).PON1-Huh7 cells were cultured inhigh glucose (4.5 g/L)DMEM (without L-glutamine) supplemented with 10% heat-inactivated FBS,2 mmol/L glutamine, 100 U/mL penicillin, 100 ug/mL streptomycin, and 100 ug/mL G418 sulfate(PAN-Biotech, Aidenbach, Germany) in a humidified atmosphere of 5% CO2 at 37 C. Human HepG2hepatocellular carcinoma cells (Institute of Applied Cell Culture, Munich, Germany) were cultured inRPMI 1640 (with L-glutamine and 2.0 g/L NaHCO3;PAN-Biotech, Aidenbach, Germany) containing10%FBS,100 U/mL penicillin and 100 ug/mL streptomycin in an incubator (95% air, 5% CO2) at 37°C.At least two independent experiments were performed in triplicate. All cell culture experiments wereconducted at non-cytotoxic concentrations of astaxanthin. 3.5. Neutral Red Cell Viability Assay PON1-Huh7,Huh7, and HepG2 cells were seeded at a density of 1.5 ×105 cells per well in 24-wellplates. Cells were treated with 1-20 pM astaxanthin for 48 (PON1-Huh7) or 24 h (Huh7,HepG2).Afterwards, the culture medium was replaced with neutral red solution (50 ug/mL, prepared in cellculture medium) and incubated for 2 h at 37°C. Cells were extracted with ethanol:water:glacial aceticacid (50:49:1, v/v/v; glacial acetic acid purchased from Carl Roth, Karlsruhe, Germany) and incubatedat room temperature for 15 min under continuous shaking. The absorbance was measured at 540 nmin a plate reader (Labsystems iEMS Reader, Helsinki, Finland). At least two independent experimentswere performed in triplicate. 3.6. Paraoxonase Activity PON1-Huh7 cells were seeded at a density of0.15 ×10° cells per well in 24-well plates andincubated for 24 h. Subsequently, cells were treated with 1 and 20 uM synthetic (DSM, Kaiseraugst,Switzerland) versus algae-based (BioAstin, Cyanotech Corporation, Kailua-Kona,HI, USA) astaxanthin.Curcumin (20 uM) served as positive control. After 48 h incubation, cells were washed with PBS andlysed with cell culture lysis reagent (1:5, v/v; Promega, Mannheim, Germany). Luciferase activity(Luciferase Assay System; Promega, Mannheim, Germany) was measured by luminescence reading(Infinite 200 microplate reader, Tecan, Crailsheim, Germany). Results were normalized to total proteincontent, determined by the BCA assay (Pierce, Bonn, Germany) according to the manufacturer’sinstructions. At least two independent experiments were performed in triplicate. 3.7. Glutathione Assav HepG2 cells (0.15 ×10°cells/well) were incubated for 24 h at37°C. Cells were treated with 1and 5 uM astaxanthin, respectively and incubated for an additional 24 h. Glutathione measurementswere performed according to Esatbeyoglu et al. (2014)[48] and Vandeputte et al. (1994) [49]. 3.8. Nrf2 Transactivation Huh7 cells (0.15 ×106cells per well) were incubated in 24-well plates for 24 h. Cellswere transiently transfected with pARE_GIGPx_Luc according to Wagner et al. (2010) [50]. After24 h-transfection, cells were incubated with 1 and 20 uM astaxanthin for further 24 h. Curcumin(20 uM) served as positive control. Cell lysis and measurement of Nrf2 transactivation were conductedas reported previously [50]. At least two independent experiments were performed in triplicate. 3.9. RNA Isolation and qRT-PCR Cells were harvested with TriFAST (VWR International GmbH, Erlangen, Germany) andRNA was isolated according to the manufacturer’s protocol. RNA concentration and qualitywere determined using the NanoDrop ND-2000 spectrophotometer (VWR International GmbH,Erlangen, Germany). Aliquots of RNA samples were stored at -80 ℃ until PCR analyses.mRNA expression levels of glutathione peroxidase 1 (Gpx1), superoxide dismutase 1, soluble(Sod1), superoxide dismutase 2, mitochondrial (Sod2), heme oxygenase 1 (Ho-1), NAD(P)Hdehydrogenase [quinone] 1, (Nqo1), glutamate-cysteine ligase, catalytic subunit (Gclc), glutathioneS-transferase alpha 1 (Gsta), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were determinedusing target-specific primers. Primer3 Input software version 0.4.0 was used for primer design(http://bioinfo.ut.ee/primer3-0.4.0/primer3/). Primer sequences are depicted in Table 2. Primerswere purchased from Eurofins MWG (Ebersberg, Germany). qRT-PCR was carried out as a one-stepprocedure using the SensiFASTTM SYBR No-ROX One-Step Kit (Bioline, Luckenwalde, Germany) withSYBR Green detection on a Rotorgene 6000 cycler (Corbett Life Science, Sydney, Australia). Calculationof results was done by an external standard curve. Transcription levels of target genes were normalizedby the transcription of Gapdh which served as housekeeping gene in qRT-PCR analyses. 3.10. Lipid Peroxidation Determined by BODIPY Assay C11-BODIPY (581/591) (Life technologies, Darmstadt, Germany) fluorescent dye served as alipid peroxidation sensor. C11-BODIPY is a lipophilic fatty acid analog which incorporates intobiomembranes and shifts its fluorescent properties from reduced (Xex=540 nm/入em=595 nm7ac) tooxidized (Xex=480 nm/入em =520 nm) status. Adjusted index of BODIPY oxidation was calculatedas follows: HepG2 cells (density of 0.15 ×10 cells per well in 24-well plates and incubated for 24 h) wereincubated with 10 and 20 pM astaxanthin for 24 h. Cells were treated with 10 pM C11-BODIPY(581/591) in medium without FBS for 30 min. BODIPY was removed and cells were stressed with80 uM cumene hydroperoxide/80 nM hemin in PBS for 1 h. Afterwards, PBS was refreshed andfluorescence was measured in adherent cells. 3.11. Statistical Analysis Statistical analysis was conducted using SPSS software version 23.0 (SPSS Inc., Munich, Germany).All cell culture data were tested for normal distribution (Kolmogorow-Smirnow). One-way ANOVAwas applied. In case of homogeneous variances, LSD and Dunnett-T post hoc test and, in case ofheterogeneous variances, Games-Howell post hoc test was conducted to test significant differencesbetween the control group and the test groups. All data are expressed as the mean +SD or SEM.Significance was accepted at p<0.05. Gene Gene-ID Description Primer, Forward (5'-3') Primer, Reverse (5'-3') Annealing (C) Gpx1 2876 glutathione peroxidase 1 ACACCCAGATGAACGAGCTG CCGGACGTACTTGAGGGAAT 58 Sod1 6647 superoxide dismutase 1, soluble GGGGAAGCATTAAAGGACTG CAACATGCCTCTCTTCATCC 55 Sod2 6648 superoxide dismutase 2, mitochondrial GCACTAGCAGCATGTTGAGC GATCTGCGCGTTGATGTG 55 Hmox1 3162 heme oxygenase 1 CCA GGC AGA GAA TGC TGA GT GTA GAC AGG GGC GAA GAC TG 59 Nqo1 1728 NAD(P)H dehydrogenase [quinone]1 CTG ATC GTA CTG GCT CAC TC GAA CAG ACT CGGCAG GAT AC 58 Gclc 2729 glutamate-cysteine ligase, catalytic subunit TTT GGT CAG GGA GTT TCC AG TGA ACA GGC CAT GTC AAC TG 59 Gst 2938 glutathione S-transferase alpha 1 CGTATGTCCACCTGAGGAAA GCCAACAAGGTAGTCTTGTCC 60 Gapdh 2597 glyceraldehyde-3-phosphate dehydrogenase CAATGACCCCTTCATTGACC GATCTCGCTCCTGGAAGATG 58 4. Conclusions Collectively, our data suggest that, beyond its coloring properties, synthetic astaxanthin exhibitsimportant free radical scavenging, oxygen quenching, and antioxidant activities. The direct free radicalscavenging activity of astaxanthin was determined by ESR and spin trapping and its oxygen quenchingactivity by photon counting. The cellular antioxidant activity of astaxanthin was examined in variousbioassays (e.g.,transactivation of PON1, cellular GSH levels, transactivation of Nrf2 and expressionof its target genes, lipid peroxidation) in cultured cell lines. Since we worked with cell lines, ourfindings can not be generalized to primary cells and animal models, respectively, and should thereforebe verified in appropriate in vivo models in the future. Additionally, it would be interesting to testwhether there are synergistic interactions between astaxanthin and other lipid (e.g., vitamin E) andwater soluble antioxidants (e.g., ascorbic acid, flavonoids) in terms of their free radical scavenging,antioxidant and gene-regulatory activity. Acknowledgments: We thank Christoph Plieth (Center of Biochemistry and Molecular Biology, University of Kiel)for providing the microplate reader (Tecan infinite 200Pro, Crailsheim, Germany) and for the fruitful discussions.We also thank Vivien Schmuck for excellent technical assistance. Author Contributions: Janina Dose, Gerald Rimbach, and Tuba Esatbeyoglu designed the study and wrote themanuscript. Seiichi Matsugo, Haruka Yokokawa, and Yutaro Koshida conducted the electron spin resonancespectroscopy experiments. Shigetoshi Okazaki conducted the singlet oxygen quenching activity experiments.Ulrike Seidel performed the BODIPY assay. All other experiments were conducted by Janina Dose andTuba Esatbeyoglu. All authors read, edited and gave feedback for the final manuscript. Conflicts of Interest: Manfred Eggersdorfer is an employee of DSM. References ( 1. Ambati, R . R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sourc e s, extraction, stability,biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128-152. [ C rossRef ] [ PubMed ] ) ( 2. J iao, G.; H ui, J.P.M.; Burton, I . W.; Thibault, M . -H.; Pelletier, C.; B o udreau, J.; Tch o ukanova, N.;Subramanian, B.; Djaoued, Y.; E w art, S.; et al. Characterization of shrimp oil from Pandalus b o realis byhigh performance liquid chromatography and h i gh r e solution m ass spectrometry. Mar. D rugs 2015, 13,3849-3876. [ CrossRef ] [PubMed] ) ( 3. Higuera-Ciapara, I.; F elix-Valenzuela, L .; Goycoolea, F.M. Astaxanthin: A re v iew of its chemistry andapplications. C rit. Rev. Food Sci. Nutr. 2006, 46, 1 85-196. [C r o ssRef] [ Pu bMed] ) ( 4. Otton, R.;Marin, D.P.; Bolin, A.P.; de Cassia Santos Macedo,R.; Campoio, T. R .; Fineto, C.; Guerra,B.A.; Leite, J.R. ; Barros, M.P. ; Mattei, R . Combined f i sh oil a nd a s taxanthin supplementation modulates ratlymphocyte function. Eur. J. Nut r . 2012,51,707-718. [ Cross R e f ] [PubMed] ) ( 5. Megdal, P . A.; Craft, N.A.;Handelman, G.J. A simplified method to di s tinguish farmed (Salmo salar) fromwild s almon: Fatty acid ratios versus astaxanthin chiral isomers. Lipids 2009,44,569-576. [Cr o s s R ef] P ubMe d ) ( 6. Widmer, E. ; Zell, R. ; Broger, E.A.; Crameri, Y.; Wagner, H.P.; Din k el,J.; Schlageter, M.; Luka e , T. TechnischeVerfahren zur Synthese von Carotinoiden und verwandten Verbindungen aus 6-Oxo-isophoron. II. Ein neuesKonzept fiir die Synthese von (3RS,3RS)-Astaxanthin. Helv. Chi m . Acta 1981, 64, 2436-2446. (In German). [ CrossRef l ) ( 7. Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: A p plications for human health andnutrition. Trends Biotechnol. 2003,21,210-216.[C rossRef] ) ( 8. Fassett, R.G.; Coombes, J.S.Astaxanthin: A potential therapeutic a g ent in c a rdiovascular disease. Mar. Drugs2011, 9,447-465. [ CrossRef ] [PubMed] ) ( 9. Hashimoto,H.;Arai, K.; Hayashi, S.;Okamoto,H.; Takahashi,J.;Chikuda,M.;Obara, Y. Eff e cts of astaxanthinon antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013,53, 1-7. [CrossRef ][ PubMed] ) ( 10. Fassett, R.G.; Healy, H.; Driver, R. ; Robertson, I.K.; Geraghty,D.P.; Sharman,J.E.; Coombes,J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008,9,17. [C r ossRef] [P ub M e d] ) ( 1 1 . L . Rodrigues,E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactiveoxygen and nitrogen species i n a membrane-mimicking system. Mar. Drugs 2012, 10,1784-1798. [CrossR e f ] [ PubMed l ) ( 12. Rao, A.R.; Sarada, R.; S hylaja, M.D.; Ravishankar, G.A. Evaluation of h epatoprotective and antioxidantactivity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Technol.2015,52,6703-6710. [ CrossRef ][ P ubMed] ) ( 13. Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.N.T. Astaxanthin and omega-3 fatty a c ids individually and incombination protect against oxidative stress via th e Nrf2-ARE pathway. F ood Chem. Toxicol. 2013, 62,869-875.[ CrossRe f ] [PubMed ] ) ( 14. Kavitha, K.; Kowshik, J; Kishore, T.K.K.; Baba, A .B.; Nagini, S . Astaxanthin i nhibits NF-kB and Wnt/B-catenin signaling pathways via i n activation of E rk/MAPK and PI3K/Akt to in d uce intri n sicapoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta 2013 , 1830, 4433 - 4444. [Cros s R ef] [ PubMed l ) ( 15. Lee, S.-J. ; Bai, S.-K . ; Lee, K.- S .; Namkoong, S. ; Na, H.J; Ha, K.-S.; Han, J.-A.; Yim, S.-V.; Chang, K.; Kwon, Y.-G.; et al . Astaxanthin inhibits nitr i c oxide production an d inflammatory gene expression by suppressing IkB kinase-dependent NF-kB activation. Mol. Cells 2003,16,97-105. [PubMed] ) ( 16. Polotow, T.G.;Vardaris,C.V.;Mihaliuc, A.R.;Goncalves, M.S.; Pereira, B.; Ganini,D.; Barros,M.P. Astaxanthinsupplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus musclesof Wistar rats. Nutrients 2014, 6,5819-5838. [CrossRe f] [PubMed ] ) ( 17. Regnier, P.; Bastias,J.;Rodriguez-Ruiz, V.; Ca b allero-Casero,N.; Caballo, C.; Sicilia, D.; Fuentes, A.;Maire, M.;Crepin, M .; L etourneur, D.; et al. Astaxanthin from H aematococcus pluvialis prevents oxidative stress onhuman endothelial cells without toxicity. Mar. Drugs 2015,13,2857-2874.[C r o ssRef] [P ubMed] ) ( 18. Sueishi, Y.; Ishikawa,M.;Yoshioka, D.;En d oh, N.; Oowada,S.; Shimmei, M.; Fujii, H.; Kota k e, Y. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flav o noids, resveratrol and astaxanthin as measured w i th t h e ORAC-EP R method. J. Clin. Biochem. Nu t r. 2012,50, 127-132.[Cro ssRef][Pu bM ed] ) ( 19. Litwinienko, G.; Ingold, K.U. Abnormal s o lvent effects on h ydrogen atom abstractions. 1 . The reactionsof phenols with 2,2-diphenyl-1-picrylhydrazyl (dpph*) in alcohols. J. Org. Chem. 20 0 3, 68 , 3433-3438.[ CrossRef ] [PubMed ] ) ( 20. Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen at o m abstraction. 2. Resolution of the curcumin antioxidant controversy. The r ole of sequential proton loss electron transfer. J. Org. Chem. 2004, 69,5888-5896. [ C rossRef] [ PubMed] ) ( 21. Litwinienko, G.; Ingold, K.U. Solvent effects on the rates and mechanisms of reaction of phenols with freeradicals. Acc. Chem. Res. 2007,40,222-230. [C rossR e f] [P ubMed] ) ( 22. Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of b ioactivecomponents in plant foods. M utat. R es.2003,523-524,9-20. [Cr ossRef] ) ( 23. Rimbach, G.;Ho h ler, D.; Fi s cher, A.;Roy, S.; Virgili,F;Pa l lauf, J.; Packer,L. M e thods to assess free radicalsand oxidative stress in biological systems. Arch . Tierernah r . 1999,52, 203-222. [CrossRef ] [PubMed] ) ( 24. Dong, S.; Huang, Y.; Z hang, R . ; Wang, S.; L i u, Y. F our different m ethods c o mparison for extraction of astaxanthin f rom green alga Haematococcus pluvialis. Sci. World J.2014,2014,694305.[Cr ossRef] [ Pu bMed] ) ( 25. Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability a nd antioxidant activity of complex of astaxanthin withhydroxypropyl-B-cyclodextrin. Carbohydr. Polym. 2013,91,385-389.[Cro ssRef] [P ub Med] ) ( 26. Zhang,J;Sun, Z.; Sun,P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in humanhealth. Food Funct. 2014,5,413-425. [CrossRe f] [PubMed] ) ( 27. Papa, T.B.R.;Pinho, V.D.; do Nascimento,E.S.P.; Santos, W.G.;Burtoloso, A.C.B.;Skibsted, L.H.; Cardoso, D.R. Astaxanthin diferulate as a bifunctional antioxidant. Fr e e Radic. Res. 2015, 49, 102-111.[Cr o s sRef] [Pu bM ed] ) ( 28. Ytrestoyl, T.; Bjerkeng, B . I ntraperitoneal a nd d i etary administration of astaxanthin in rainbow trout(Oncorhynchus m y kiss)—Plasma uptake and tissue distribution of geo m etrical E/Z isomers. Comp. Biochem.Physiol. B 2007,147,250-259. [ C rossRe f ] [PubMed ] ) ( 29. Mladenka, P.; Zatloukalova, L . ; F ilipsky, T.;Hrdina, R. Cardiovascular effects of flavonoids are not causedonly by direct antioxidant activity . Free Radi c . Bi o l. Med. 2010, 49,963-975 . [CrossRe f ][Pu b Med] ) ( 30. Moini, H .; G uo, Q.; Packer, L. X a nthine ox i dase an d xanthine deh y drogenase inhibition by t h eprocyanidin-rich French m a ritime pine bark extract, py c nogenol: A p r ot e in bind i ng effect. Adv. Exp. Med. Biol. 2002,505,141-149. [PubMed] ) ( 31. Boesch-Saadatmandi, C.; Rimbach, G.; Jungblut, A.; Frank, J. Comparison of tetrahydrofuran, fetal ca l fserum, and Tween 40 fo r the delivery of astaxanthin and canthaxanthin to HepG2 cells. Cytotechnology 2011,63,89-97. [CrossRe f] [PubMed ] ) ( 32. Briviba, K.; Bornemann, R.; Lemmer, U . Visualization of astaxanthin l ocalization in HT29 human colon adenocarcinoma cells by combined confocal resonance Raman and fluorescence microspectroscopy. Mol. Nutr.Food Res. 2006,50, 991-995. [CrossR e f] [PubMed] ) ( 33. Schrader, C.; Rimbach, G . D e terminants of paraoxonase 1 s t atus: Ge n es, dr u gs and nutrition.Curr. Med. Chem. 2011, 18,5624-5643. [ C rossRef] [ PubMed] ) ( 34. Baralic, I;Djordjevic, B.; D i kic, N.; Kotur-Stevuljevic, J.; Sp a sic, S.; Je l ic-Ivanovic, Z.; Ra d ivojevic, N.; Andjelkovic, M.; Pejic,S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother . Res.2013,27,1536-1542.[Cr ossRef] [ Pu bMed] ) ( 35. Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibitionof skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the greenalga Haematococcus pluvialis. J. Agric. Food Che m . 2013, 61,3842-3851. [CrossRe f] [PubMed] ) ( 36. Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthinas an antioxidant and may not be suitable as a human nutraceutical supplement. Nu t rafoods 2014, 12,145-152. 「 C r ossRef l ) ( 37. Morales, A.; Garcia-Ruiz, C.; Miranda, M.;Mari, M.; Colell, A. ; Ardite, E.; F e rnandez-Checa,J.C. Tumornecrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chainof y-glutamylcysteine synthetase. J . B iol. Chem. 1997,272,30371-303 7 9. [Cro ssRef] [P u b M ed] ) ( 38. Morales, A.;Miranda,M.; Sanchez-Reyes, A.; Colell, A.; Biete, A.; Fe r nandez-Checa,J.C. Transcriptionalregulation of the heavy subunit chain of y-glutamylcysteine synthetase by ionizing radiation. FEBS Lett. 1998,427,15-20. [ CrossRef ) ( 39. Moffat, G .J.; McLaren, A . W.; Wolf, C.R. Sp1-mediated transcriptional activation of the human Pi class glutathione S-transferase promoter. J . Biol. Che m .1996,271,1054-1060. [ CrossRef ] [ P ubMed] ) ( 40. Ye,Q.;Zhang,X.;Huang, B.; Zhu, Y.; Chen,X. Astaxanthin suppresses MPP+-induced oxidative damage inPC12 cells through a Sp1/NR1 signaling pathway. Mar. Dr u gs 2013, 11, 1019-1034.[Cro ssRef] [P ub Med] ) ( 41 L . . Myhrstad,M . C.; H u sberg, C.; Murphy, P. ; Nordstrom, O.; Bl o mhoff, R. ; M o skaug, J.O.; Kolsto, A . B. T CF11/Nrf1 o v erexpression increases t h e i n tracellular g lutathione level and c an t ransactivate the y-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim. Biophys. Acta 2001, 1517,212-219. [ CrossRef l ) ( 42. Yang, H.; M agilnick,N.; Le e , C. ; Kalmaz, D.; Ou, X.; Cha n , J.Y.; Lu, S.C. Nrfl and Nrf2 regu l ate ratglutamate-cysteine ligase catalytic subunit transcription indirectly via NF-KB and AP-1 . Mol. Cell. Biol . 2005,25,5933-5946. [ CrossRef ] [PubMed] ) ( 43. Kurozumi, R.; Kojima, S. Increase of intracellular glutathione by low-level NO mediated by transcription factor NF-kB in RAW 264.7 ce l ls. B i ochim. Biophys. Acta 2005, 1744,58-67.[Cro ssRef] [P u b M ed] ) ( 44. Lababpour, A.; Lee, C.-G. Simultaneous measurement of chlorophyll and astaxanthin in Haematococcus pluvialis cells by first-order derivative ultraviolet-visible spectrophotometry.J. B iosci. B i oeng. 20 0 6,101, 104-110.[ CrossRef ] [P u bMed ] ) ( 45. Braunlich,M.; Slimestad, R.; Wangensteen,H . ;Brede, C.;Malterud, K.E.; Barsett, H. Extracts, anthocyaninsand procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5,663-678. [ C rossRef ] [ PubMed] ) ( 46. Shimizu, T.;Nakanishi, Y .;Nakahara, M.; Wada, N.;Moro-oka, Y. Radical on structure effect antioxidant activity of catecholamines toward singlet oxygen and othe r reactive oxyg e n species in vit r o. J. C l in.Biochem. Nutr. 2010, 47, 181-190. [CrossRef ] [ P ubMed] ) ( 47. Boesch-Saadatmandi, C.; Egert, S.; Sc h rader, C.; Coumoul, X.; Barouki, R.; M u lle r , M.J.; Wolff r am, S.; Rimbach, G. Effect of quercetin on paraoxonase 1 activity-studies in cultured cells, mice a n d humans. J. Physiol. Pharmacol . 2010,61,99-105. [ PubMed] ) ( 48. Esatbeyoglu, T.; Wagner, A .E.; Motafakkerazad, R . ; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radicalscavenging and antioxidant activity of betanin: El e ctron spinresonance spectroscopy studies and studies incultured cells. Food Chem. Toxicol. 2014,73 , 119-126. [ C rossRef][ Pu bMed] ) 49. Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for totalglutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a newminiaturized protocol. Cell Biol. Toxicol. 1994,10,415-421.[CrossRef] [PubMed] 50. Wagner, A.E.; Ernst, I.; Iori, R.; Desel, C.; Rimbach,G. Sulforaphane but not ascorbigen, indole-3-carbinoleand ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes inhuman keratinocytes in culture. Exp. Dermatol.2010,19,137-144.[CrossRef] [PubMed] @ 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open accessarticle distributed under the terms and conditions of the Creative Commons by Attribution(CC-BY) license (http://creativecommons.org/licenses/by/4.0/). Astaxanthin is a coloring agent which is used as a feed additive in aquaculture nutrition.Recently, potential health benefits of astaxanthin have been discussed which may be partly relatedto its free radical scavenging and antioxidant properties. Our electron spin resonance (ESR)and spin trapping data suggest that synthetic astaxanthin is a potent free radical scavenger interms of diphenylpicryl-hydrazyl (DPPH) and galvinoxyl free radicals. Furthermore, astaxanthindose-dependently quenched singlet oxygen as determined by photon counting. In addition to freeradical scavenging and singlet oxygen quenching properties, astaxanthin induced the antioxidantenzyme paroxoanase-1, enhanced glutathione concentrations and prevented lipid peroxidationin cultured hepatocytes. Present results suggest that, beyond its coloring properties, syntheticastaxanthin exhibits free radical scavenging, singlet oxygen quenching, and antioxidant activitieswhich could probably positively affect animal and human health.
确定










还剩12页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《虾青素中自由基、细胞抗氧化剂特性检测方案(激光产品)》,该方案主要用于其他中自由基、细胞抗氧化剂特性检测,参考标准--,《虾青素中自由基、细胞抗氧化剂特性检测方案(激光产品)》用到的仪器有Ekspla NL200型 紧凑纳秒激光器
推荐专场
相关方案
更多
该厂商其他方案
更多
















