方案详情
文
固体在液体里的分散可以分为润湿,解聚,防止聚合三个阶段。其中润湿阶段是关键,我们把关注点放在润湿热力学,通过四个列子进行分析。
方案详情

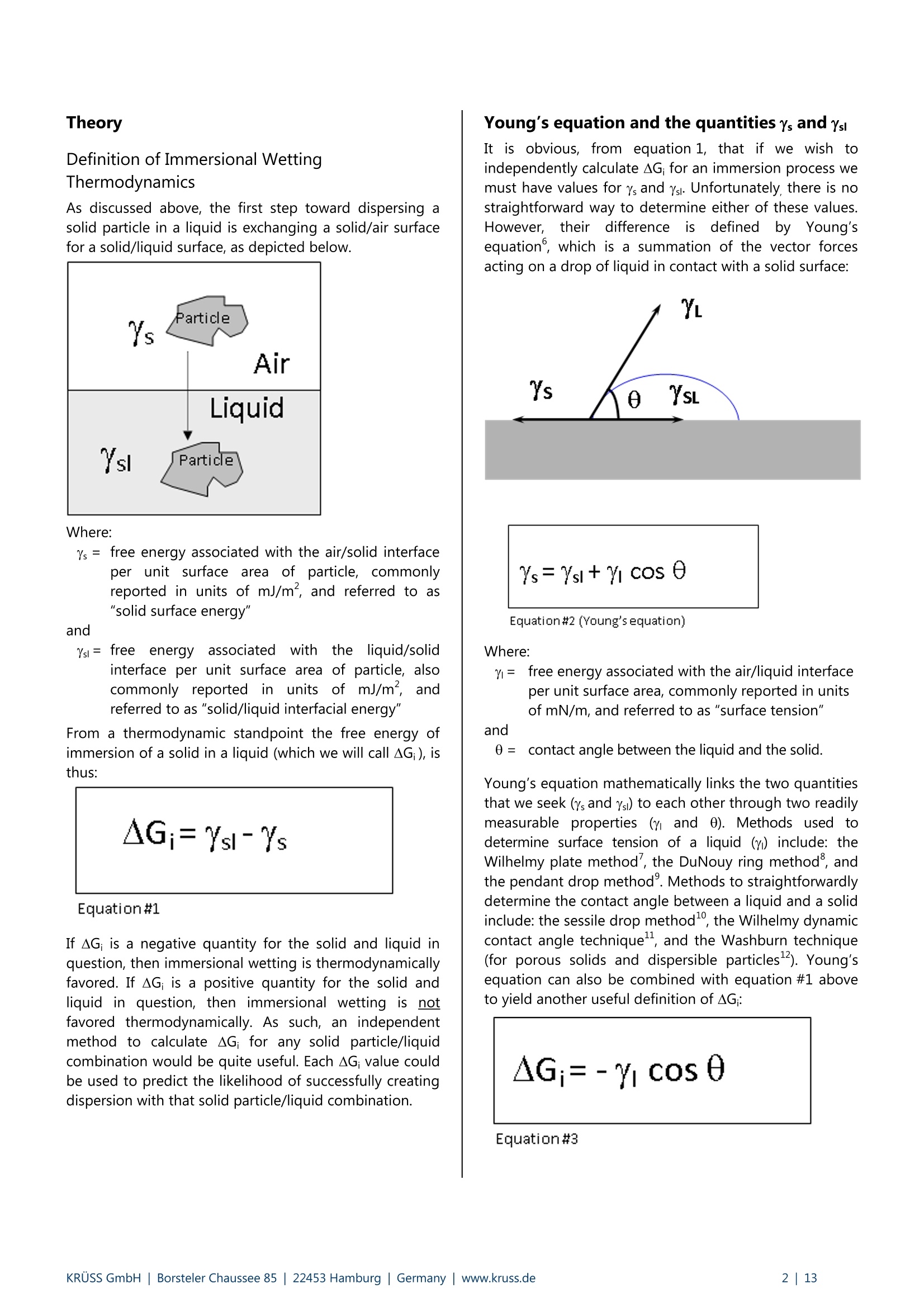
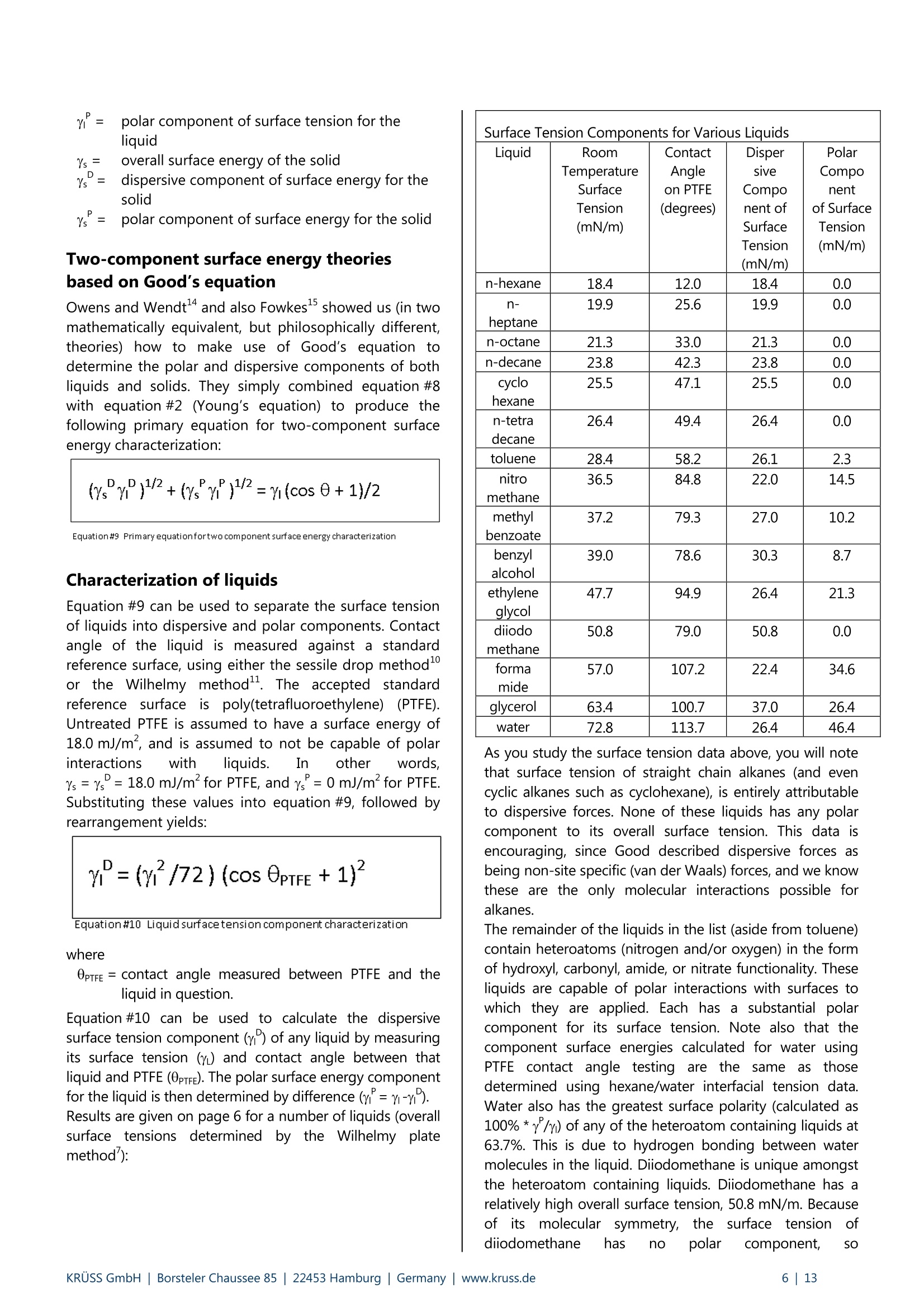
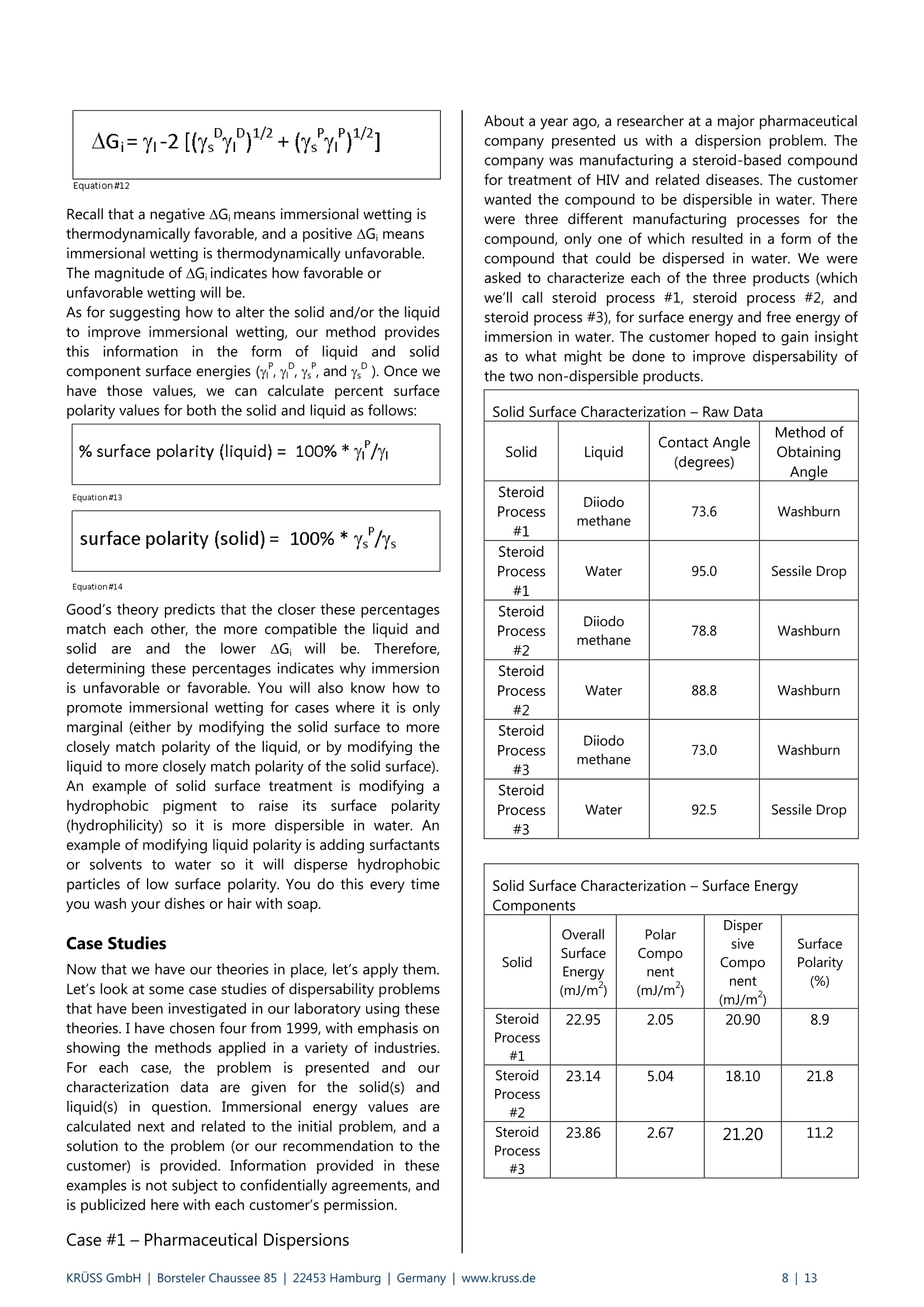
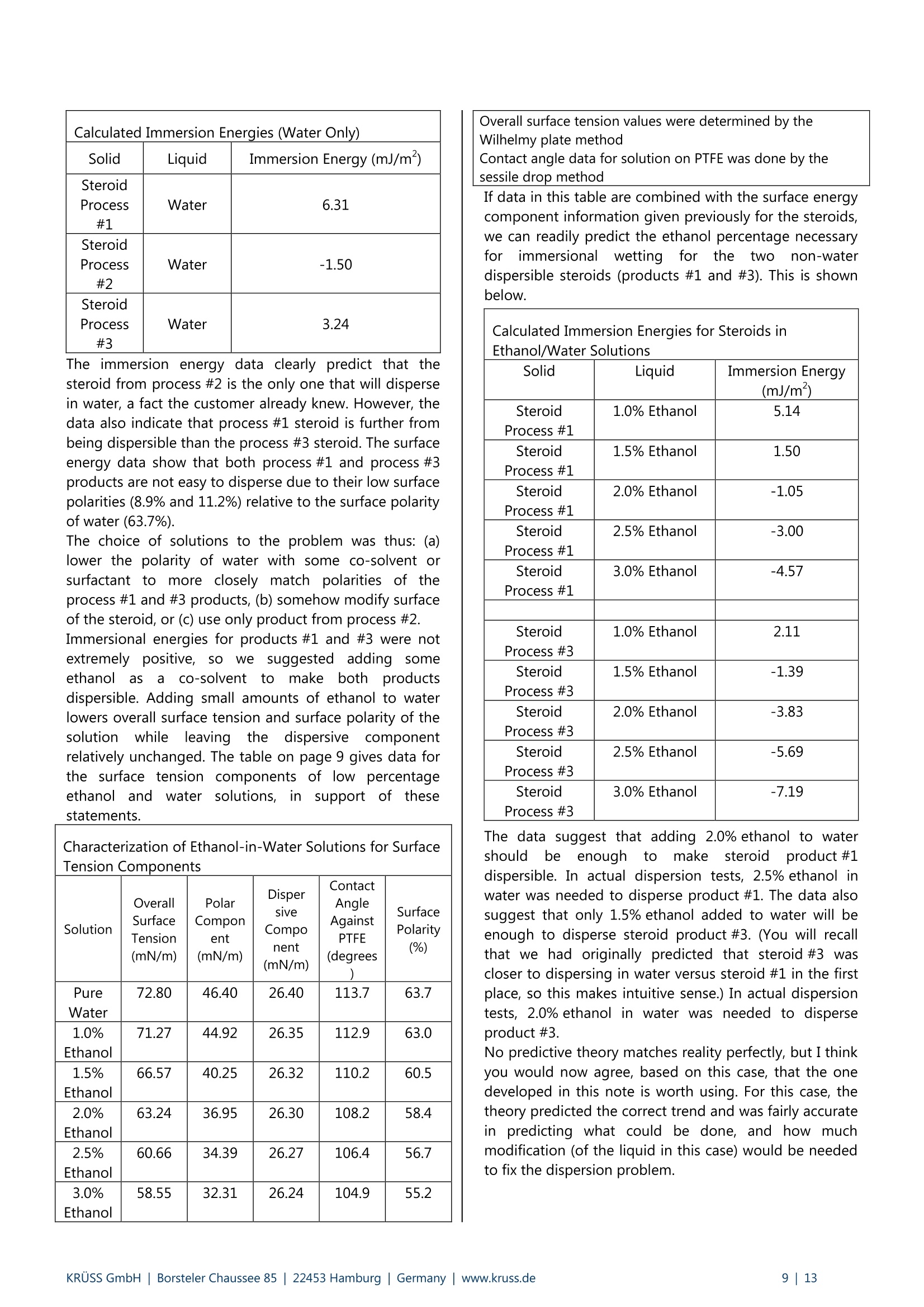
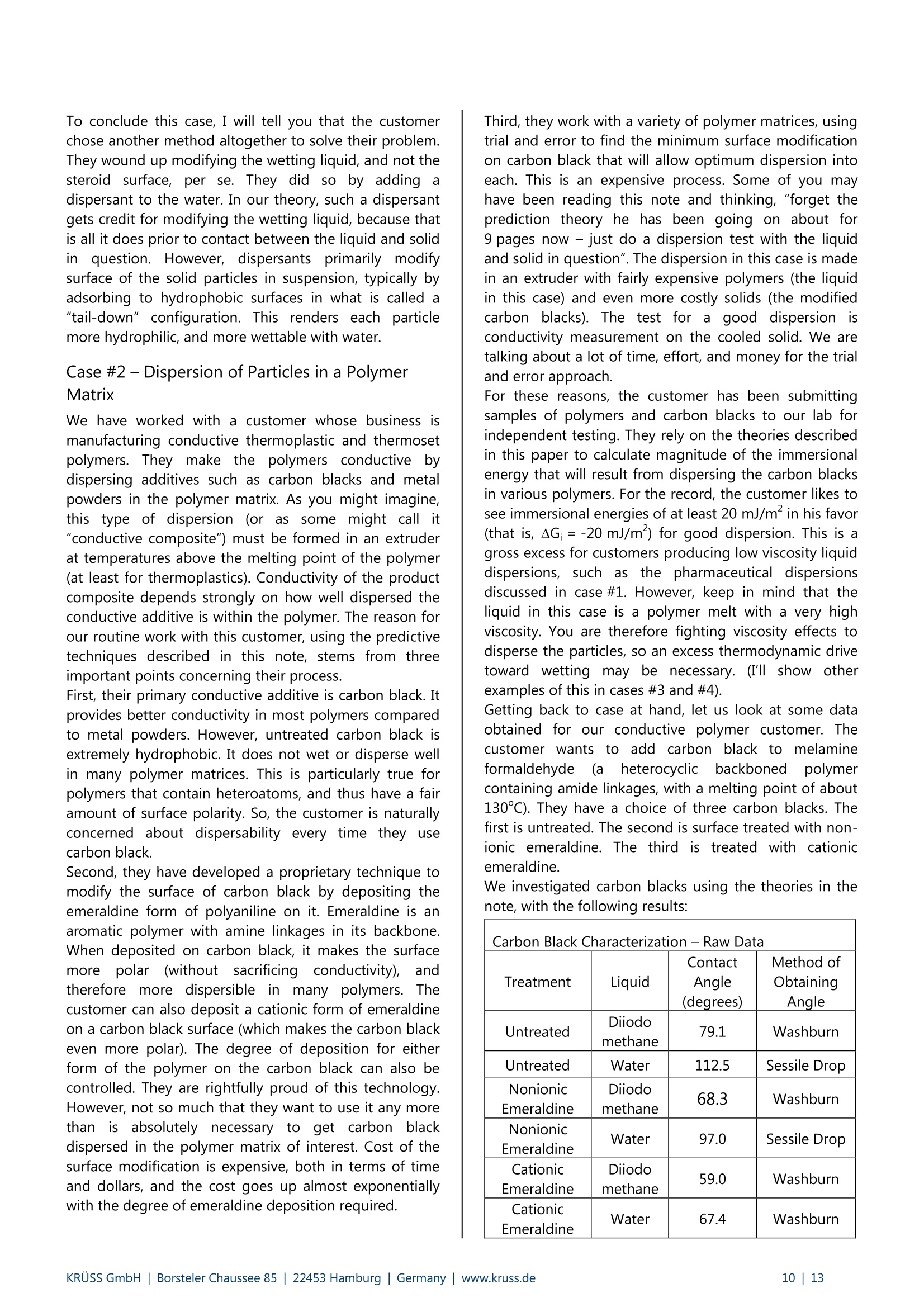
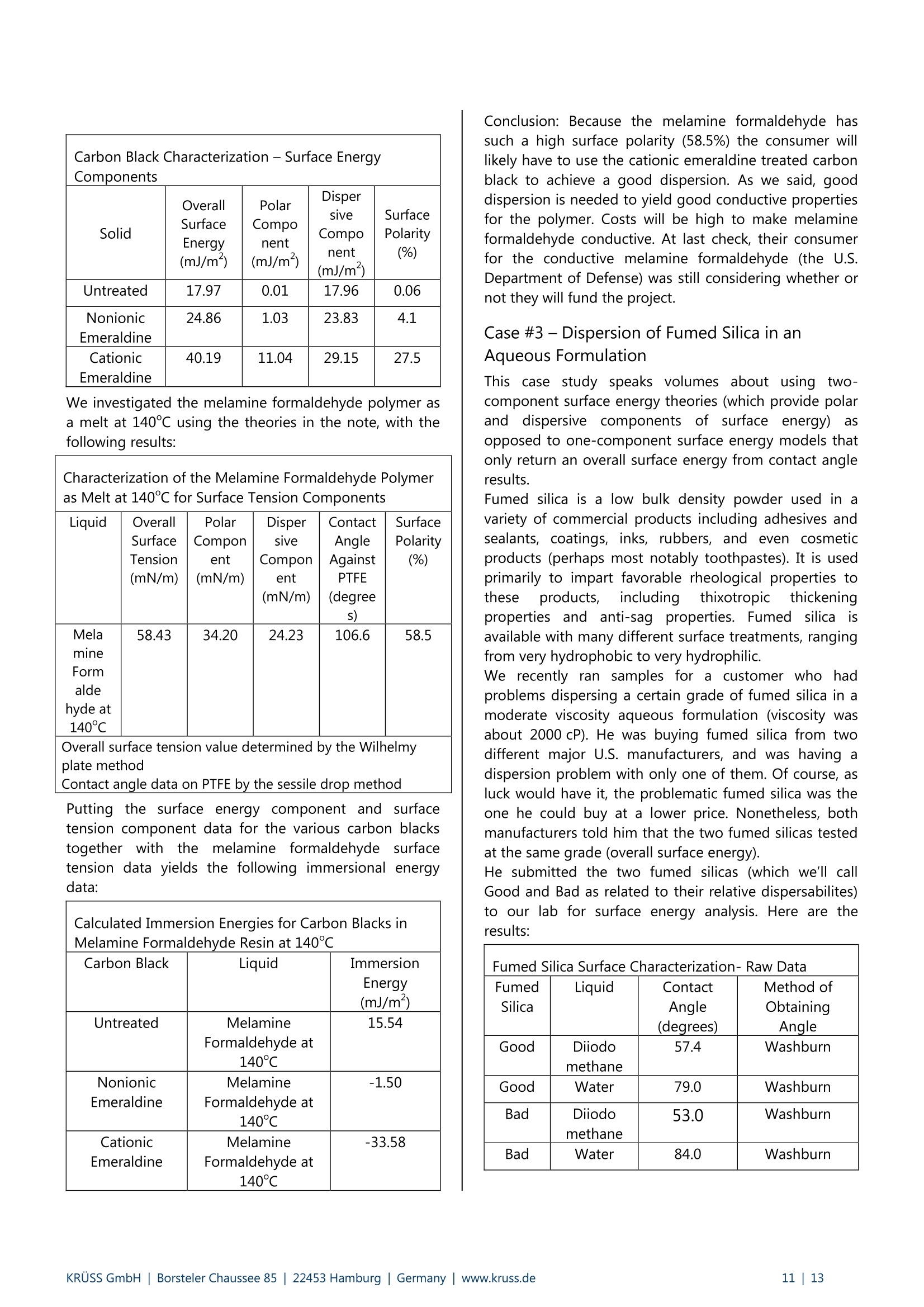
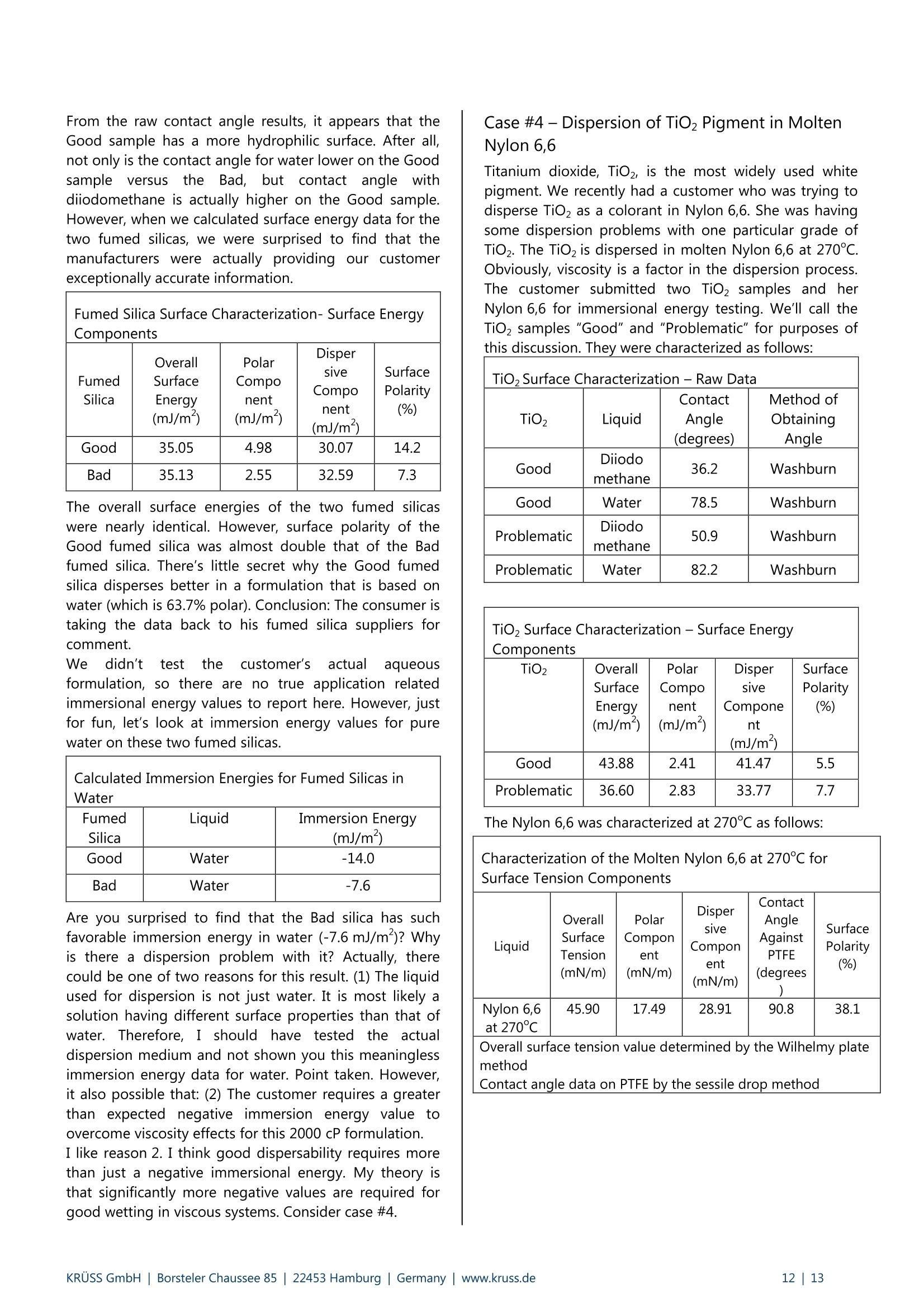
KRUSS Application Report Two-Component Surface Energy Characterization as a Predictor ofWettability and Dispersability Abstract The dispersal of a solid in a liquid has been described as a three-fold process: (1) wetting the particles and displacingentrapped air, (2) deaggregation and/or fragmentation of the particle clusters, and (3) prevention of reaggregation of thedispersed particles.23 Of these, the first is most critical. Wetting must take place in order for the solid to become evenpartially dispersed in the liquid. Deaggregation and fragmentation of the particle clusters (step 2) may be encouraged bymechanical means and the prevention of reaggregation (step 3), by steric stabilization, columbic’,and other effects. Thisis the subject of a vast amount of research in colloid and surface science. However, if wetting of the solid by the liquid isthermodynamically unfavorable (or only marginally favorable), then discussion of these latter steps in the dispersionprocess becomes irrelevant. The focus of this note is the thermodynamics of wetting as related to dispersability of particles in liquids. Four casestudies on dispersability problems solved in our laboratory will be discussed. In each case, we combined simple wettingthermodynamics with independent characterizations of the wetting liquid and the prospective dispersed solid to quantifythe thermodynamic drive toward immersional wetting. As you will see, the value of such work goes beyond predictingwhether or not a solid will wet. The two-component surface energy approach that is described also provides a "road map"for modification of either the solid or the liquid to enhance wetting in cases where it is unfavorable or only marginallyfavorable. Theory Definition of Immersional Wetting Thermodynamics As discussed above, the first step toward dispersing asolid particle in a liquid is exchanging a solid/air surfacefor a solid/liquid surface, as depicted below. Where: ys=free energy associated with the air/solid interfaceper unit surface area of particle, commonlyreported in units of mJ/m, and referred to as"solid surface energy" and Ysi= free energyassociatedwith the liquid/solidinterface per unit surface area of particle, alsocommonly reported in units of mJ/m, andreferred to as "solid/liquid interfacial energy" From a thermodynamic standpoint the free energy ofimmersion of a solid in a liquid (which we will call ^G;), isthus: Equation#1 If AG; is a negative quantity for the solid and liquid inquestion, then immersional wetting is thermodynamicallyfavored. If AG; is a positive quantity for the solid andliquid in question, then immersional wetting is notfavored thermodynamically. As such, an independentmethod to calculate AG; for any solid particle/liquidcombination would be quite useful. Each AG; value couldbe used to predict the likelihood of successfully creatingdispersion with that solid particle/liquid combination. Young's equation and the quantities Ys and Ysi It is obvious, from equation 1, that if we wishtiindependently calculate AG; for an immersion process wemust have values for ys and Ysi. Unfortunately there is nostraightforward way to determine either of these values.However,ttheirdifferenceiss defineddby Young'sequation, which is a summation of the vector forcesacting on a drop of liquid in contact with a solid surface: Equation#2 (Young'sequation) Where: y= free energy associated with the air/liquid interfaceper unit surface area, commonly reported in unitsof mN/m, and referred to as "surface tension" and 0=contact angle between the liquid and the solid. Young’s equation mathematically links the two quantitiesthat we seek (ys and Ysi) to each other through two readilymeasurable properties (yand 0). Methods used todetermine surface tension of a liquid (y) include: theWilhelmy plate method', the DuNouy ring method , andthe pendant drop method. Methods to straightforwardlydetermine the contact angle between a liquid and a solidinclude: the sessile drop method, the Wilhelmy dynamiccontact angle technique-, and the Washburn technique(for porous solids and dispersible particles). Young’sequation can also be combined with equation #1 aboveto yield another useful definition of AG: Equation#3 A quick inspection of equation #3 leads to the conclusionthat AG; will be negative (immersional wetting will befavored) if the contact angle (0) between the wettingliquid and the solid in question is less than 90°. Further,immersional wetting will be more strongly favored (AG;will be a larger negative value) the lower the contactangle (0) is, and the highery is. Equation #3 is therefore the fundamentalequationrelating particle dispersability to contact angle. Ourlaboratory conducts many experiments to determinecontact angle of a prospective dispersant liquid against asolid in order to predict its dispersability. The lower thecontact angle, the more favorable dispersion is likely tobe. Therefore,I do not want to make light of equation #3.However, our goal for this application note is bit more farreaching. We seek an independent method to calculateAG; for the particles we wish to disperse, without need ofan actual experiment that brings the liquid and the solidtogether. The calculation would be based solely onknown (or easily measurable) properties of the particlesthemselves and the perspective dispersant liquid. Why do we seek this? How will an independent methodbenefit us any more than a contact angle experimentdone between the solid we need to disperse and theprospective dispersant liquid? The answer to thisquestion will become clear as we go on. You will find thatthe independent method we develop provides guidance(ideas and options) for modification of either the solid orthe liquid to enhance wetting in cases were wetting isunfavorable, or not favorable enough. By contrast,straightforward contact angle experiments done onlybetween the liquid and the solid will only tell you how faryou have come toward achieving your goal of goodwetting. They do not suggest means for improvement. Good's equation and the quantities Ys and Ysi Having Young's equation, which links the two quantitiesys and Ysl yet still having no experimental means toseparate the two quantities, Good- decided that perhapsthe interfacial free energy between a solid and a liquid(ysi) need not be such an elusive quantity. Formation of asolid/liquid interface can be considered analogous to theformation of a liquid/liquid interface. The difference isthat in formation of a liquid/liquid interface, all therelevant interfacial energies can be directly measured bystandard techniques. Methods, which will straightforwardlyand independently determine the surface tension of eachliquid (Yi and Y2) have been discussed earlier. Methods formeasurement of interfacial tension between two liquids(Y1,2) include: the DuNouy ring method, the pendantLOdrop method and the drop volume method. It is clear from the picture shown below that the freeenergy for formation of a liquid/liquid interface can beexpressed as: Liquid #1 Liquid #2 Where Y1= surface tension of liquid#1 Y2= surface tension of liquid #2 and Y1,2= interfacial tension between the two liquids Since all the above quantities are directly measurable,Good was quickly able to determine that free energy offormation for all liquid/liquid interfaces is negative. Examples: Hexane has a surface tension of 18.4mN/m at roomtemperature.Water has a surface tension of 72.8 mN/m at roomtemperature. The interfacial tension between hexane and water atroom temperature is 47.1 mN/m (when each phase issaturated with the other, that is, at equilibrium). Therefore,AGhex/wat=-44.1 mN/m (mJ/m) at roomtemperature. Benzene has a surface tension of 28.9 mN/m at roomtemperature. Water has a surface tension of 72.8 mN/m at roomtemperature. The interfacial tension between benzene and water atroom temperature is 34.1 mN/m (when each phase issaturated with the other, that is, at equilibrium). Therefore,AGben/wat=-67.6 mN/m(mJ/m) at roomtemperature. Benzyl alcohol has a surface tension of 39.0 mN/m atroom temperature. Water has a surface tension of 72.8 mN/m at roomtemperature. The interfacial tension between benzyl alcohol and waterat room temperature is 15.0 mN/m (when each phase issaturated with the other, that is, at equilibrium).Therefore, AGbza/wat=-96.8 mN/m (mJ/m) at roomtemperature. The formation of any liquid/liquid interface by elimi-nation of two gas/liquid interfaces is thermodynamicallyfavorable. This is because liquids exhibit cohesive inter-actions between their molecules while gases do not. Themolecules that make up the surface of a liquid aretherefore sacrificing cohesive interactions since they arein contact with a gaseous phase. This is what gives rise toliquid surface tension. Surface tension is the amount ofwork necessary to overcome liquid cohesive forces andform a unit area of liquid/gas interface. Molecules at thesurface of a liquid favor any interaction with anotherliquid over having to interact with a gas. This is true evenif the liquids are very incompatible. Consider hexanee andl w\ater for example..Hexanemolecules are only capable of relatively weak van derWaals type interaction with other molecules. Watermolecules are capable of van der Waals interactions aswell. In addition, they are capable of much strongerhydrogenbondinginteractions. Therefore,watermolecules would far prefer to interact with each otherthan to interact with hexane molecules. However, watermolecules at the surface of water are forced to interactwith a gas. They would happily trade their interactionwith a gas for interaction with any liquid offering at leastsome cohesive interactions. This is true even when theinteraction offers only van der Waals forces, as is the casefor hexane. For their part, hexane molecules at thehexane surface are less unhappy than water molecules atthe surface of water. They are only sacrificing weak vander Waals interaction to be at the surface. However, theytoo would be happier to interact with water, since wateroffers cohesive interactions. Therefore, the free energy offormation of ahexane/water interface isisnegative(favorable). The hexane/water interface (AGhex/wat = -44.1 mJ/m²) is anextreme example of two incompatible liquids in contact.When the liquids are more compatible, the free energy ofinterface formation is even more negative. Benzyl alcoholis capable of hydrogen bonding and -cloud relateddipole/dipole interactions. The free energy change whenbenzyl alcohol and water are put in contact (relative toeach having a surface with a gas) is much more negative(AGbza/wat=-96.8 mJ/m²). Even theie in-cloudrelateddipole/dipole interactions offered by benzene make thefree energy of forming a benzene/water interface(AGben/wat=-67.6 mJ/m²)even more favorable thanforming a hexane/water interface. A brief note would be prudent at this point beforeproceeding to discuss Good’s theory. Stating that thefree energy of formation for all liquid/liquid interfaces isnegative or favorable, as I have done above may causesome confusion. One may mistakenly get the impression that liquid/liquid interfaces form spontaneously in allsituations (that, for example, emulsification of one liquidin another is thermodynamically favored). This is untrue,and not attall whatislsbeing statedhere. Thethermodynamics of dispersing one liquid in another aredefined by an entirely different set of mathematics thanthose described in this note. The free energy offormation for a liquid/liquid interface is defined hererelative to the situation of initially having two gas/liquidsurfaces that are eliminated to form one liquid/liquidinterface. This interaction is always thermodynamicallyfavorable, for reasons discussed above. After noting that free energy of formation for aliquid/liquid interface is more negative (favorable) if thetwo liquids are compatible (capable of similar cohesiveinteractions), Good had the idea that perhaps this freeenergy could be predicted. If one had information aboutwhether or not strong cohesive interactions existedbetween molecules of each of the two liquids, thencompatibility of the liquids, and thus free energy due toformation of an interface between them, might bepredicted. This is the basis of what we call two-component surfaceenergy theory. Good noticed that every liquid with anoverall surface tension of 30 mN/m, for example, doesnot have the same free energy of interface formationwith water. He decided to assign component surfacetensions to every liquid. He defined liquids that have onlythecapability ofvanCderrWaalsilrinteractions as"dispersive"because their entire surface tension is due tothe loss of van der Waals interactions from the moleculeson their surfaces. All other interactions were described as"polar", to include dipole/dipole interactions, hydrogenbonding, n-cloud n-cloud interactions, and so on. Liquidscapable of such interactions are said to be both polarand dispersive, since all liquids exhibit van der Waalsinteractions between their molecules. Good reasonedthat surface tensions of such liquids could be separatedinto two components, since molecules on their surfacesshow a loss of both polar and dispersive interactions,relative to molecules in the bulk. Liquids such as water,benzyl alcohol, and even benzene are therefore said tohave two components to their overall surface tensionwhich, when added together, total their overall surfacetension. Free energy of formation for a liquid/liquid interface islikely to be most negative in cases where the two liquidsare most compatible. Based on this assumption, Goodproposed that surface tension components of variousliquids be defined such that free energy of formation foran interface between two liquids could be predicted bythe following equation: Equation#5 Where Y1"=dispersive component for the surface tension ofliquid#1 Y2 = ddispersive component for the surface tension ofliquid #2 Y1=polar component for the surface tension ofliquid #1 Y2=ppolar component for the surface tension ofliquid #2 Good chose the mathematical form given above on anempirical basis (based on fitting a variety of data), butalso because it represents what is known in statistics as a'geometric mean" approach. This approach was naturalfor Good's theories and observations that the free energyis lowest when the liquids are most compatible. If theliquids are completelyyincompatible (liquid one iscompletely dispersive Yi=Y1(Yi=0) and the other iscompletely polar y2 =Y2(Y2=0)), then the free energyof interface formation (AG12) is zero. This is never thecase for real liquids however, since every liquid has adispersive component to its surface tension. Therefore,this theory agrees with the observation that formation ofall liquid/liquid interfaces is favorable (AG1,2 is alwaysnegative). It can also be shown (albeit with more effort) that, for anypair of liquids having overall surface tensions of Yi and Y2,equation #5 predicts a minimum free energy for the casewhere Yi/Y1=Y2 /Y2. In other words, free energy isminimized when the two liquids have the same fractionalsurface polarity. Fractional surface polarity is defined asYi/y!(and oftenreported as percent surfacepolarity=100%*Y1/Yi). This is also in keeping withobservations thatfreeenergy of 1formation forliquid/liquid interfaces is most favorable (most negative)where the liquids arceapable of similar cohesiveinteractions. Good combined equation #5 with equation #4 to furthershow that interfacial tension between two liquids can bepredicted if one has prior knowledge of the polar anddispersive components of each liquid's surface tension.This takes the form of equation #6. Equation#6 Equation #6 can also be used in reverse to calculate thecomponent surface tensions of liquids. All that need beknown is surface tensions of the liquids (Yi and y2) andinterfacial tension between the two liquids (Y12). Animportant example would be determining componentsfor the surface tension of water (liquid 1) based onhaving measured interfacial tension between water andhexane (liquid 2). this case: y1=72.8 mN/m,Yi=unknown, Yi'= unknown, Y2=Y2=18.4mN/m, andY12=47.1 mN/m as reported above. Since hexane is onlycapable of vanP der Waals interactions, Y2 =0. Therefore,the polar component term drops out of equation #6, andY1'1 can be calculated as 26.4 mN/m (equation #7). Equation#7-Calculationof the dispersive component of the surfacetension of water The polar component for water is thus: Y1 =(Yi-yi)=(72.8-26.4)=46.4 mN/m. In other words,46.4/72.8=63.7% of the surface tension of water is dueto its polar interaction capability. The characterization of water given above will be usedfor further calculations shortly. However, we first need toreiterate the primary objective of this note. Ourobjectivewas to find a means of determining values for interfacialenergy between a liquid and a solid (Ysi) and values forsolid surface energy (ys). We could then use equation #1to predict solid particle wettability and dispersability.During our discussion, we found that values for Ys are notdirectly measurable. So, we considered that liquid/liquidinterfaces might be analogous to liquid/solid interfaces,and followed Good's observations and theories. This ledus to equation #6 for interfacial tension between twoliquids. Now we turn the analogy around (as Good did). If equation #6 applies to liquid/liquid interfacial tensions,it should also apply to liquid/solid interfacial energies.Therefore, Good has given us a definition of liquid/solidinterfacial energy (Ysl) which depends only on individualproperties of the liquid and solid of interest (and not onproperties which can only be determined by actuallybringing the liquid and solid into contact). Good’sequation for solid/liquid interfaces is formally written asfollows: Equation#8-Good's Equation Where Yi= overall surface tension of the liquid D=ddispersive component of surface tension for theliquid y= ppolar component of surface tension for theliquid Ys= overall surface energy of the solid = ddispersive component of surface energy for thesolid y=polar component of surface energy for the solid Two-component surface energy theoriesbased on Good's equation Owens and Wendt and also Fowkesshowed us (in twomathematically equivalent, but philosophically different,theories) how to make use of Good’s equation todetermine the polar and dispersive components of bothliquids and solids. They simply combined equation #8with equation #2 (Young's equation) to produce thefollowing primary equation for two-component surfaceenergy characterization: Equation#9 Primaryequationfortwo component surface energy characterization Characterization of liquids Equation #9 can be used to separate the surface tensionof liquids into dispersive and polar components. Contactangle of the liquid is measured against a standardreference surface, using either the sessile drop methodor the Wilhelmy method-. The accepted standardreference surface is poly(tetrafluoroethylene) (PTFE).Untreated PTFE is assumed to have a surface energy of18.0 mJ/m, and is assumed to not be capable of polarinteractions with liquids. In other words,ys=Ys=18.0 mJ/mfor PTFE, and ys=0 mJ/mfor PTFE.Substituting these values into equation #9, followed byrearrangement yields: where 0pe =contact angle measured between PTFE and theliquid in question. Equation #10 can be used to calculate the dispersivesurface tension component (y) of any liquid by measuringits surface tension (y) and contact angle between thatliquid and PTFE (0pTFe). The polar surface energy componentfor the liquid is then determined by difference (y"=Y-Y).Results are given on page 6 for a number of liquids (overallsurface tensions determined by the Wilhelmy platemethod ): Surface Tension Components for Various Liquids Liquid Room TemperatureSurfaceTension (mN/m) ContactAngleon PTFE (degrees) DispersiveComponent ofSurfaceTension (mN/m) Polar Componentof SurfaceTension(mN/m) n-hexane 18.4 12.0 18.4 0.0 n-heptane 19.9 25.6 19.9 0.0 n-octane 21.3 33.0 21.3 0.0 n-decane 23.8 42.3 23.8 0.0 cyclohexane 25.5 47.1 25.5 0.0 n-tetradecane 26.4 49.4 26.4 0.0 toluene 28.4 58.2 26.1 2.3 nitromethane 36.5 84.8 22.0 14.5 methylbenzoate 37.2 79.3 27.0 10.2 benzyalcohol 39.0 78.6 30.3 8.7 ethyleneglycol 47.7 94.9 26.4 21.3 diiodo methane 50.8 79.0 50.8 0.0 Tormamide 57.0 107.2 22.4 34.6 glycerol 63.4 100.7 37.0 26.4 water 72.8 113.7 26.4 46.4 As you study the surface tension data above, you will notethat surface tension of straight chain alkanes (and evencyclic alkanes such as cyclohexane), is entirely attributableto dispersive forces. None of these liquids has any polarcomponent to its overall surface tension. This data isencouraging, since Good described dispersive forces asbeing non-site specific (van der Waals) forces, and we knowthese are the only molecular interactions possible foralkanes. The remainder of the liquids in the list (aside from toluene)contain heteroatoms (nitrogen and/or oxygen) in the formof hydroxyl, carbonyl, amide, or nitrate functionality. Theseliquids are capable of polar interactions with surfaces towhich they are applied. Each has a substantial polarcomponent for its surface tension. Note also that thecomponent surface energies calculated for water usingPTFE contact angle testingare the sameasis thosedetermined using hexane/water interfacial tension data.Water also has the greatest surface polarity (calculated as100%*y/y) of any of the heteroatom containing liquids at63.7%. This is due to hydrogen bonding between watermolecules in the liquid. Diiodomethane is unique amongstthe heteroatom containing liquids. Diiodomethane has arelatively high overall surface tension, 50.8 mN/m. Becauseof its molecularr symmetry, the: surface tension oofdiiodomethane has no polar component, SO y=YP=50.8 mN/m. This makes diiodomethane very usefulas a probe liquid to characterize solid surfaces forcomponent surface energies, as we will see shortly. It is also interesting that toluene (a benzene ring with onemethyl substituent) has a surface polarity of about 8%,although it has no heteroatom. The polarity can beattributed to polarizability of the n-cloud electrons on thebenzene ring. This n-cloud effect also contributes to the22% surface polarity obtained for benzyl alcohol. The purpose of this section was to show that surfacetension of and contact angle data for a dispersant liquidagainst PTFE is all the data needed to characterize theliquid part of a prospective solid/liquid wetting problem.Discussion of tthe various liquids given aaboveisbackgroundiinformation to (give you a betterunderstandingiof two-component surface tensioncharacterization. Characterization of solids Equation #9 can be used to characterize solids for overall,polar, and dispersive components of surface energy. TheOwens and Wendt theory and the Fowkes theory differon how to apply equation #9 to determine the values ysand Ys. We will stick with the Fowkes theory in this text,for two reasons. First, we have had good success usingthe Fowkes theory for a number of dispersion problems,as the case studies will demonstrate. Second, the Fowkestheory is simpler to apply. If you are interested inlearning more about the Owens and Wendt theory, anoverview has been written. The first step in determining a solid surface energy usingthe Fowkes theory is to test the solid for contact angleusing a liquid which has only a dispersive component to itssurface tension (that is, a liquid for which y=0, so thaty=y). In this case, equation #9 reduces to: and yscan be calculated directly from contact angle datafor the probe liquid on the solid. The second step is to test the solid for contact angle usinganother liquid which has both a dispersive component anda non-dispersive (polar) component to its surface tension.With data on surface tension components of the liquid,contact angle between the liquid and solid, and ys(ascalculated in step 1), one can calculate Ys as the onlyunknown in equation #9. Overall surface energy of thesolid,Ys, is then calculated as Ys =Ys+Ys . Typically, Fowkes theory is applied using contact angle datafrom only two liquids. The recommended liquids arediiodomethane and water. As stated above, diiodomethanehas a relatively high surface tension of 50.8 mN/m, all ofwhich arises from dispersiveinteractions. Sincey=YP=50.8 mN/m for diiodomethane, it is used as probeliquidfor thefirst stepdescribedaabove. Water (y"=46.4mN/m, and y=26.4 mN/m) is then used as testliquid for the second step. Descriptions of methods by which contact angles aretypically obtained for diiodomethane and water on solidparticles are beyond the scope of this text. As notedpreviously,such methodsinclude: the sessile dropmethod, the Wilhelmy plate method, and, mostnotably for powder characterization, the Washburnmethod. All these methods have been described inother notes that are referenced in the footnotes. In thecase studies that will be presented, the method used isnoted for each diiodomethane and water contact angleobtained. The Goal Realized -A Useful Guide toImmersional Wetting Some pages ago we set the goal for this application noteas finding an independent method to calculate the freeenergy of immersional wetting (AG;) for solid particles wehoped to disperse in some liquid. We hoped todetermine AG; without actually doing an experiment thatbrought the liquid and the solid together. Instead, wewanted our value of AG; to predict the likelihood ofobtaining a good dispersion for the solid in the liquid.And, we wanted AG to be obtained from known (oreasily measurable) properties of the particles themselvesand the prospective dispersant liquid. We thought such a method might benefit us more thanresults of a contact angle measurement between thesoliddweiehhopedto disperseeandtheperspectivedispersant liquid. It should also provide direction formodifying either the solid or the liquid to enhancewetting in cases where it is not favorable. By contrast,contact angle data for the liquid and solid in questiononly tell us how far we have progressed toward achievingfavorable immersional wetting. They provide no guidanceas to how improvement might be achieved. This goal has been achieved! In the previous two sectionswe have described methods which can be used todetermine surface tension of any liquid, separated intopolar and dispersive components. Using the sametheories, surface energy of any solid, with polar anddispersive components can also be determined. Once wehave this information about a liquid and a solid (whichwere tested independently on each other), we can useGood's equation (equation #8) to predict the interfacialfree energy (Ysi) between them when they are broughtinto contact. The fundamental thermodynamic definitionof immersional wetting (equation #1) can then be usedto predict free energy of immersional wetting (AG;) forthe solid being dispersed in the liquid. We can alsocombine equations #8 and #1 to produce a single masterequation for immersional wetting (equation #12): Equation#12 Recall that a negative AG;means immersional wetting isthermodynamically favorable, and a positive AG; meansimmersional wetting is thermodynamically unfavorable.The magnitude of AG indicates how favorable orunfavorablewetting will be. As for suggesting how to alter the solid and/or the liquidto improve immersional wetting, our method providesthis information in the form of liquid and solidcomponent surface energies (YY, Ys, andYs). Once wehave those values, we can calculate percent surface polarity values for both the solid and liquid as follows: % surface polarity (liquid)=100%*y/y Equation#13 surface polarity (solid)=100%* Ys lYs Equation#14 Good’s theory predicts that the closer these percentagesmatch each other, the more compatible the liquid andsolidareand the lower AG willbe. Therefore,determining these percentages indicates why immersionis unfavorable or favorable. You will also know how topromote immersional wetting for cases where it is onlymarginal (either by modifying the solid surface to moreclosely match polarity of the liquid, or by modifying theliquid to more closely match polarity of the solid surface).An example of solid surface treatment is modifying ahydrophobic pigment to raise its surface polarity(hydrophilicity) so it is more dispersible in water. Anexample of modifying liquid polarity is adding surfactantsor solvents to water so it will disperse hydrophobicparticles of low surface polarity. You do this every timeyou wash your dishes or hair with soap. Case Studies Now that we have our theories in place, let’s apply them.Let’s look at some case studies of dispersability problemsthat have been investigated in our laboratory using thesetheories. I have chosen four from 1999, with emphasis onshowing the methods applied in a variety of industries.For each case, the problem is presented and ourcharacterization data are given for the solid(s)andliquid(s) in question. Immersional energy values arecalculated next and related to the initial problem, and asolution to the problem (or our recommendation to thecustomer) is provided. Information provided in theseexamples is not subject to confidentially agreements, andis publicized here with each customer's permission. Case #1 -Pharmaceutical Dispersions About a year ago, a researcher at a major pharmaceuticalcompany presented us with a dispersion problem. Thecompany was manufacturing a steroid-based compoundfor treatment of HIV and related diseases. The customerwanted the compound to be dispersible in water. Therewere three different manufacturing processes for thecompound, only one of which resulted in a form of thecompound that could be dispersed in water. We wereasked to characterize each of the three products (whichwe'll call steroid process #1, steroid process #2, andsteroid process #3), for surface energy and free energy ofimmersion in water. The customer hoped to gain insightas to what might be done to improve dispersability ofthe two non-dispersible products. Solid Liquid Contact Angle(degrees) Method ofObtainingAngle SteroidProcess#1 Diiodomethane 73.6 Washburn SteroidProcess#1 Water 95.0 Sessile Drop SteroidProcess#2 Diiodomethane 78.8 Washburn SteroidProcess#2 Water 88.8 Washburn SteroidProcess #3 Diiodomethane 73.0 Washburn SteroidProcess#3 Water 92.5 Sessile Drop Solid Surface Characterization-Surface Energy Components Solid OverallSurfaceEnergy(mJ/m) PolarComponent (mJ/m) Disper siveComponent(mJ/m) SurfacePolarity(%) SteroidProcess#1 22.95 2.05 20.90 8.9 SteroidProcess#2 23.14 5.04 18.10 21.8 SteroidProcess#3 23.86 2.67 21.20 11.2 Calculated Immersion Energies (Water Only) Solid Liquid Immersion Energy (mJ/m) SteroidProcess#1 Water 6.31 SteroidProcess#2 Water -1.50 SteroidProcess #3 Water 3.24 The immersion energy data clearly predict that thesteroid from process #2 is the only one that will dispersein water, a fact the customer already knew. However, thedata also indicate that process #1 steroid is further frombeing dispersible than the process #3 steroid. The surfaceenergy data show that both process #1 and process #3products are not easy to disperse due to their low surfacepolarities (8.9% and 11.2%) relative to the surface polarityof water (63.7%). The choice of solutions to the problem was thus: (a)lower the polarity of water with some co-solvent orsurfactant to more closely match polarities of theprocess #1 and #3 products, (b) somehow modify surfaceof the steroid, or (c) use only product from process #2.Immersional energies for products #1 and #3 were notextremely positive, so we suggested addinggsomeethanol as a co-solvent to make bothproductsdispersible. Adding small amounts of ethanol to waterlowers overall surface tension and surface polarity of thesolution whileleaving the dispersive componentrelatively unchanged. The table on page 9 gives data forthe surface tension components of low percentageethanoland water solutions, in support of thesestatements. Characterization of Ethanol-in-Water Solutions for SurfaceTension Components Solution OverallSurfaceTension(mN/m) PolarComponent(mN/m) DispersiveComponent(mN/m) ContactAngleAgainstPTFE(degrees) SurfacePolarity(%) PureWater 72.80 46.40 26.40 113.7 63.7 1.0%Ethanol 71.27 44.92 26.35 112.9 63.0 1.5%Ethanol 66.57 40.25 26.32 110.2 60.5 2.0%Ethanol 63.24 36.95 26.30 108.2 58.4 2.5%Ethanol 60.66 34.39 26.27 106.4 56.7 3.0% Ethanol 58.55 32.31 26.24 104.9 55.2 Overall surface tension values were determined by theWilhelmy plate methodContact angle data for solution on PTFE was done by thesessile drop method If data in this table are combined with the surface energycomponent information given previously for the steroids,we can readily predict the ethanol percentage necessaryfor immersionalwetting1for the twonon-waterdispersible steroids (products #1 and #3). This is shownbelow. Calculated Immersion Energies for Steroids inEthanol/Water Solutions Solid Liquid Immersion Energy(mJ/m‘) SteroidProcess#1 1.0% Ethanol 5.14 SteroidProcess #1 1.5% Ethanol 1.50 Steroid Process #1 2.0% Ethanol -1.05 SteroidProcess #1 2.5% Ethanol -3.00 SteroidProcess #1 3.0% Ethanol -4.57 SteroidProcess #3 1.0% Ethanol 2.11 SteroidProcess #3 1.5% Ethanol -1.39 Steroid Process #3 2.0% Ethanol -3.83 Steroid Process #3 2.5% Ethanol -5.69 Steroid Process #3 3.0% Ethanol -7.19 The data suggest that adding 2.0% ethanol to watershould be enoughhto makee steroid product #1dispersible. In actual dispersion tests, 2.5% ethanol inwater was needed to disperse product #1. The data alsosuggest that only 1.5% ethanol added to water will beenough to disperse steroid product #3. (You will recallthat we had originally predicted that steroid #3 wascloser to dispersing in water versus steroid #1 in the firstplace, so this makes intuitive sense.) In actual dispersiontests, 2.0% ethanol in water was needed to disperseproduct #3. No predictive theory matches reality perfectly, but I thinkyou would now agree, based on this case, that the onedeveloped in this note is worth using. For this case, thetheory predicted the correct trend and was fairly accuratein predicting what could be done, and how muchmodification (of the liquid in this case) would be neededto fix the dispersion problem. To conclude this case, I will tell you that the customerchose another method altogether to solve their problem.They wound up modifying the wetting liquid, and not thesteroid surface, per se. They did so by adding adispersant to the water. In our theory, such a dispersantgets credit for modifying the wetting liquid, because thatis all it does prior to contact between the liquid and solidinquestion. However, dispersants primarily modifysurface of the solid particles in suspension, typically byadsorbing to hydrophobic surfaces in what is called a"tail-down" configuration. This renders each particlemore hydrophilic, and more wettable with water. Case #2- Dispersion of Particles in a PolymerMatrix We have worked with a customer whose business ismanufacturing conductive thermoplastic and thermosetpolymers. They make thepolymers conductive bydispersing additives such as carbon blacks and metalpowders in the polymer matrix. As you might imagine,thisittype of dispersion (or as some might call it"conductive composite") must be formed in an extruderat temperatures above the melting point of the polymer(at least for thermoplastics). Conductivity of the productcomposite depends strongly on how well dispersed theconductive additive is within the polymer. The reason forour routine work with this customer, using the predictivetechniques described in this note, stems from threeimportant points concerning their process. First, their primary conductive additive is carbon black. Itprovides better conductivity in most polymers comparedto metal powders. However, untreated carbon black isextremely hydrophobic. It does not wet or disperse wellin many polymer matrices. This is particularly true forpolymers that contain heteroatoms, and thus have a fairamount of surface polarity. So, the customer is naturallyconcerned about dispersability every time they usecarbon black. Second, they have developed a proprietary technique tomodify the surface of carbon black by depositing theemeraldine form of polyaniline on it. Emeraldine is anaromatic polymer with amine linkages in its backbone.When deposited on carbon black, it makes the surfacemorepolar (without sacrificing conductivity),), andtherefore more dispersiblein many polymers. TThecustomer can also deposit a cationic form of emeraldineon a carbon black surface (which makes the carbon blackeven more polar). The degree of deposition for eitherform of the polymer on the carbon black can also becontrolled. They are rightfully proud of this technology.However, not so much that they want to use it any morethan isisaabsolutely necessary toget carbon blackdispersed in the polymer matrix of interest. Cost of thesurface modification is expensive, both in terms of timeand dollars, and the cost goes up almost exponentiallywith the degree of emeraldine deposition required. Third, they work with a variety of polymer matrices, usingtrial and error to find the minimum surface modificationon carbon black that will allow optimum dispersion intoeach. This is an expensive process. Some of you mayhave been reading this note and thinking, "forget theprediction theory he has been going on about for9 pages now - just do a dispersion test with the liquidand solid in question". The dispersion in this case is madein an extruder with fairly expensive polymers (the liquidin this case) and even more costly solids (the modifiedcarbon blacks). Thetest for a good dispersion isconductivity measurement on the cooled solid. We aretalking about a lot of time, effort, and money for the trialand error approach. For these reasons, the customer has been submittingsamples of polymers and carbon blacks to our lab forindependent testing. They rely on the theories describedin this paper to calculate magnitude of the immersionalenergy that will result from dispersing the carbon blacksin various polymers. For the record, the customer likes tosee immersional energies of at least 20 mJ/m² in his favor(that is, AG;=-20 mj/m’) for good dispersion. This is agross excess for customers producing low viscosity liquiddispersions, suchas the pharmaceutical dispersionsdiscussed in case #1. However, keep in mind that theliquid in this case is a polymer melt with a very highviscosity. You are therefore fighting viscosity effects todisperse the particles, so an excess thermodynamic drivetoward wetting may be necessary..(I'l show otherexamples of this in cases #3 and #4). Getting back to case at hand, let us look at some dataobtained for our conductive polymer customer. Thecustomer wants to add carbon black to melamineformaldehyde (a heterocyclicic backboneddppolymercontaining amide linkages, with a melting point of about130℃). They have a choice of three carbon blacks. Thefirst is untreated. The second is surface treated with non-ionic emeraldine. The third is treated with cationicemeraldine. We investigated carbon blacks using the theories in thenote, with the following results: Carbon Black Characterization - Raw Data Treatment Liquid ContactAngle(degrees) Method ofObtainingAngle Untreated Diiodomethane 79.1 Washburn Untreated Water 112.5 Sessile Drop NonionicEmeraldine Diiodomethane 68.3 Washburn Nonionic Emeraldine Water 97.0 Sessile Drop CationicEmeraldine Diiodomethane 59.0 Washburn Cationic Emeraldine Water 67.4 Washburn Carbon Black Characterization - Surface EnergyComponents Solid OverallSurfaceEnergy(mJ/m) PolarComponent(mJ/m) DispersiveComponent(mJ/m) SurfacePolarity(%) Untreated 17.97 0.01 17.96 0.06 NonionicEmeraldine 24.86 1.03 23.83 4.1 CationicEmeraldine 40.19 11.04 29.15 27.5 We investigated the melamine formaldehyde polymer asa melt at 140℃ using the theories in the note, with thefollowing results: Characterization of the Melamine Formaldehyde Polymeras Melt at 140℃ for Surface Tension Components Liquid OverallSurfaceTension(mN/m) PolarComponent(mN/m) Disper siveComponent(mN/m) ContactAngleAgainstPTFE(degrees) SurfacePolarity(%) MelamineFormaldehyde at140℃ 58.43 34.20 24.23 106.6 58.5 Overall surface tension value determined by the Wilhelmyplate method Contact angle data on PTFE by the sessile drop method tension component data for the various carbon blackstogether with the melamine formaldehyde surfacetension data yields the following immersionalenergydata: Calculated Immersion Energies for Carbon Blacks inMelamine Formaldehyde Resin at 140℃ 1Carbon Black Liquid ImmersionEnergy (mJ/m) Untreated Melamine Formaldehyde at140℃ 15.54 Nonionic Emeraldine Melamine Formaldehyde at140°C -1.50 Cationic Emeraldine Melamine Formaldehyde at140℃ -33.58 Conclusion: Because the melamine formaldehyde hassuch a high surface polarity (58.5%) the consumer willlikely have to use the cationic emeraldine treated carbonblack to achieve a good dispersion. As we said, gooddispersion is needed to yield good conductive propertiesfor the polymer. Costs will be high to make melamineformaldehyde conductive. At last check, their consumerfor the conductive melamine formaldehyde (the U.S.Department of Defense) was still considering whether ornot they will fund the project. Case #3 -Dispersion of Fumed Silica in anAqueous Formulation This case study speaks volumes about usingtwO-component surface energy theories (which provide polarand (dispersive components of surface energy))asopposed to one-component surface energy models thatonly return an overall surface energy from contact angleresults. Fumed silica is a low bulk density powder used in avariety of commercial products including adhesives andsealants, coatings, inks, rubbers, and even cosmeticproducts (perhaps most notably toothpastes). It is usedprimarily to impart favorable rheological properties tothese products,including thixotropicthickeningproperties and anti-sag properties. Fumed silica isavailable with many different surface treatments, rangingfrom very hydrophobic to very hydrophilic. We recently ran samples for a customer who hadproblems dispersing a certain grade of fumed silica in amoderate viscosity aqueous formulation (viscosity wasabout 2000 cP). He was buying fumed silica from twodifferent major U.S. manufacturers, and was having adispersion problem with only one of them. Of course, asluck would have it, the problematic fumed silica was theone he could buy at a lower price. Nonetheless, bothmanufacturers told him that the two fumed silicas testedat the same grade (overall surface energy). He submitted the two fumed silicas (which we'll callGood and Bad as related to their relative dispersabilites)to our lab for surface energy analysis. Here are theresults: FumedSilica Liquid ContactAngle (degrees) Method ofObtaining Angle Good Diiodo methane 57.4 Washburn Good Water 79.0 Washburn Bad Diiodomethane 53.0 Washburn Bad Water 84.0 Washburn From the raw contact angle results, it appears that theGood sample has a more hydrophilic surface. After all,not only is the contact angle for water lower on the GoodsampleversusttheBad, but: contact angle withdiiodomethane is actually higher on the Good sample.However, when we calculated surface energy data for thetwo fumed silicas, we were surprised to find that themanufacturers were actually providing our customerexceptionally accurate information. Fumed Silica Surface Characterization- Surface EnergyComponents FumedSilica OverallSurfaceEnergy(mJ/m) PolarComponent(mJ/m) DispersiveComponentmJ/m) SurfacePolarity(%) Good 35.05 4.98 30.07 14.2 Bad 35.13 2.55 32.59 7.3 The overall surface energies of the two fumed silicaswere nearly identical. However, surface polarity of theGood fumed silica was almost double that of the Badfumed silica. There's little secret why the Good fumedsilica disperses better in a formulation that is based onwater (which is 63.7% polar). Conclusion: The consumer istaking the data back to his fumed silica suppliers forcomment. We(didn'ttest the customer's aactual aqueousformulation, so there are no true application relatedimmersional energy values to report here. However, justfor fun, let’s look at immersion energy values for purewater on these two fumed silicas. Calculated Immersion Energies for Fumed Silicas in Water FumedSilica Liquid Immersion Energy(mJ/m‘) Good Water -14.0 Bad Water -7.6 Are you surprised to find that the Bad silica has suchfavorable immersion energy in water (-7.6 mJ/m)? Whyis there a dispersion problem with it? Actually, therecould be one of two reasons for this result. (1) The liquidused for dispersion is not just water. It is most likely asolution having different surface properties than that ofwater. Therefore, I should have tested the actualdispersion medium and not shown you this meaninglessimmersion energy data for water. Point taken. However,it also possible that: (2) The customer requires a greaterthan expected negative immersion energy value toovercome viscosity effects for this 2000 cP formulation.I like reason 2. I think good dispersability requires morethan just a negative immersional energy. My theory isthat significantly more negative values are required for good wetting in viscous systems. Consider case #4. Case #4-Dispersion of TiO2 Pigment in MoltenNylon 6,6 Titanium dioxide, Tio, is the most widely used whitepigment. We recently had a customer who was trying todisperse TiO2 as a colorant in Nylon 6,6. She was havingsome dispersion problems with one particular grade ofTiO. The TiO2 is dispersed in molten Nylon 6,6 at 270℃.Obviously, viscosity is a factor in the dispersion process.The customer submitted two TiO samples andherNylon 6,6 for immersional energy testing. We'll call theTiO2 samples "Good" and "Problematic" for purposes ofthis discussion. They were characterized as follows: TiO, Surface Characterization- Raw Data TiO2 Liquid ContactAngle (degrees) Method ofObtainingAngle Good Diiodomethane 36.2 Washburn Good Water 78.5 Washburn Problematic Diiodomethane 50.9 Washburn Problematic Water 82.2 Washburn TiO2 Surface Characterization- Surface Energy Components TiO2 OverallSurfaceEnergy (mJ/m) Polar Component (mJ/m‘) Disper siveComponent (mJ/m‘) SurfacePolarity(%) Good 43.88 2.41 41.47 5.5 Problematic 36.60 2.83 33.77 7.7 The Nylon 6,6 was characterized at 270℃ as follows: Characterization of the Molten Nylon 6,6 at 270℃ forSurface Tension Components Liquid OverallSurfaceTension(mN/m) PolarComponent(mN/m) DispersiveComponent(mN/m) ContactAngleAgainstPTFE(degrees) SurfacePolarity(%) Nylon 6,6at 270°C 45.90 17.49 28.91 90.8 38.1 Overall surface tension value determined by the Wilhelmy platemethod Contact angle data on PTFE by the sessile drop method Contact angle data on PTFE by the sessile drop method Immersional energy values wereethen calculated asfollows: Calculated Immersion Energies for TiO2’s in Nylon 6,6 at 270℃ TiO2 Liquid Immersion Energy(mJ/m) Good Nylon 6,6 at270°℃ -35.73 Problematic Nylon 6,6 at270°℃ -30.12 Nylon 6,6 was found to have a surface polarity of 38.1%.Both the TiO2 samples must have been hydrophobicallymodified grades, because their surface polarities arequite low (5.5% and 7.7% respectively). High dispersivecomponent values for both TiO2 samples show that theimmersional energy values are favorable for dispersion ofboth. The dispersive component is particularly high forthe “Good" TiO2, which supports a more favorableimmersional energy and keeps the data trend correct. Based on this data, the customer has since switched toan even more hydrophilic (higher % surface polarity)grade of TiO2 than either of the ones tested here, and isnow having good success. I included this case because itis striking that she was having problems with a dispersionfor which the immersion energy was -30.12 mJ/m. AgainI attribute this to viscosity of the liquid, which is certainlyhigh for Nylon 6,6 at 270C. The customer needs a largenegative immersion energy (thermodynamic driving forcefor wetting) to overcome the viscous resistance towetting. Perhaps someday I will have enough viscosity versusimmersion energy data to write another note correlatingthe two in terms of what immersion energy is necessaryto overcome what magnitude of viscous effect. However,that day is not here yet. Summary My intent in writing the application note was to showthat a simple theory does exist to predict whether or nota solid particle can be dispersed in a liquid based only oneasily measured properties of the liquid and solid. Thetheory may be used to predict dispersability for anyliquid/solid pair. These predictions can prevent a greatdeal of expensive trial and error dispersion testing. In addition, the method also serves as a guide for caseswhere dispersability is poor or completely unfavorable. Ittells you, in the form of percent surface polarity valuesfor the liquid and the solid, why the liquid and the solidare incompatible. You can then make an informeddecision on which of the two phases you will alter toimprove dispersability. I hope this note is useful for those of you who formdispersions of solids in liquids. If you have questions orcomments concerning the note, or you wish to have ourlaboratory study your dispersion problem, please meknow. Literature .1. Parfitt, G.D.; Dispersion of Powders in Liquids, 2Edition, Wiley, New York, (1973). .2. Parfitt, G.D. and Picton, N.H.; Trans. Faraday Soc., 64,1955,(1968). 3 Parfitt,G.D. and Wharton, D.G.; Jour. Coll. InterfaceSci., 38,431, (1972). 4. Napper, D.H.; Polymeric Stabilization of ColloidalDispersions, Academic Press, (1983). 5. Overbeek, J. Th. G.; Kinetics of Flocculation, Chapter7, of Colloid Science, H.R. Kruyt, editor, 278-283,(1965). .6 Bartell, F.E. and Bartell L.S.; Jour. Amer. Chem. Soc.56,2205, (1934). 7. Kruss Product Literature - Brochure for DigitalTensiometer K10 8. Kruss Product Literature - Brochure for ProcessorTensiometer K12 9 Kruss Product Literature - Brochure for AutomatedGoniometer DSA10 Kruss Technical Note #303 L.Kruss Product Literature- Brochure for ProcessorTensiometer K12 ( 12. . Kruss Technical Note #302 ) 13. Good, R.J. and Girifalco, L.A.; Jour. Phys. Chem, 64,561, (1960). 14.+.Owens, D.K. and Wendt,R.C.; Jour. Applied PolymerSci., 13,1741, (1969). 155.. Fowkes, F.M.; Industrial and Engineering Chemistry,56, 12,40,(1964). 16.KKruss Technical Note #302 KRUSS GmbH| Borsteler Chaussee Hamburg| Germany| www.kruss.de|
确定





还剩11页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《混合材料,碳黑,氧化硅,二氧化钛,尼龙66中表面能检测方案(接触角测量仪)》,该方案主要用于其他中表面能检测,参考标准--,《混合材料,碳黑,氧化硅,二氧化钛,尼龙66中表面能检测方案(接触角测量仪)》用到的仪器有KRUSS DSA100接触角测量仪
推荐专场
相关方案
更多
该厂商其他方案
更多