方案详情
文
二次和三次采油导致油包水乳液的形成。破乳剂可以分离乳液使原油脱水脱盐。使用震荡滴法可以测量界面流变为实际过程选择合适的破乳剂。
方案详情

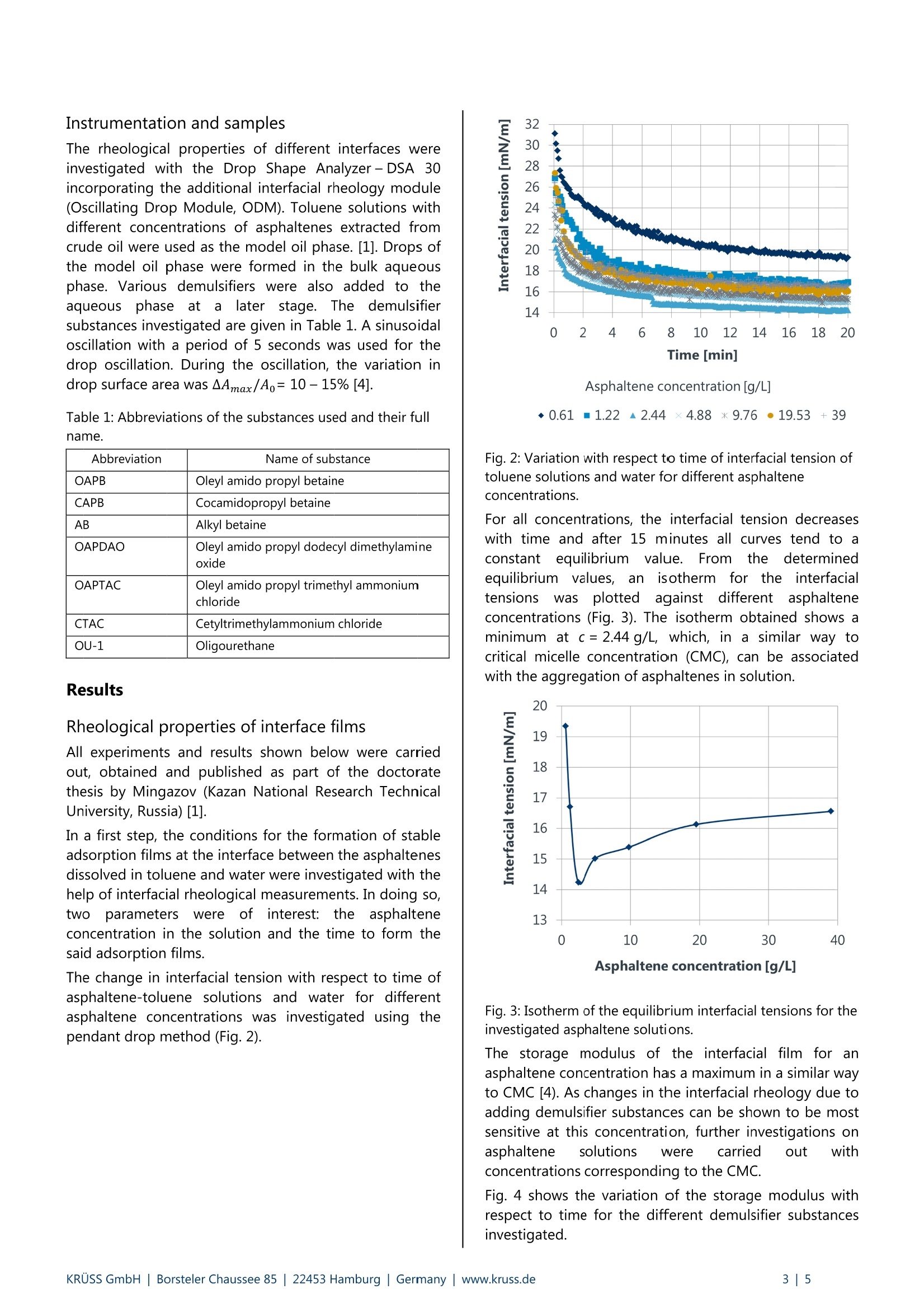
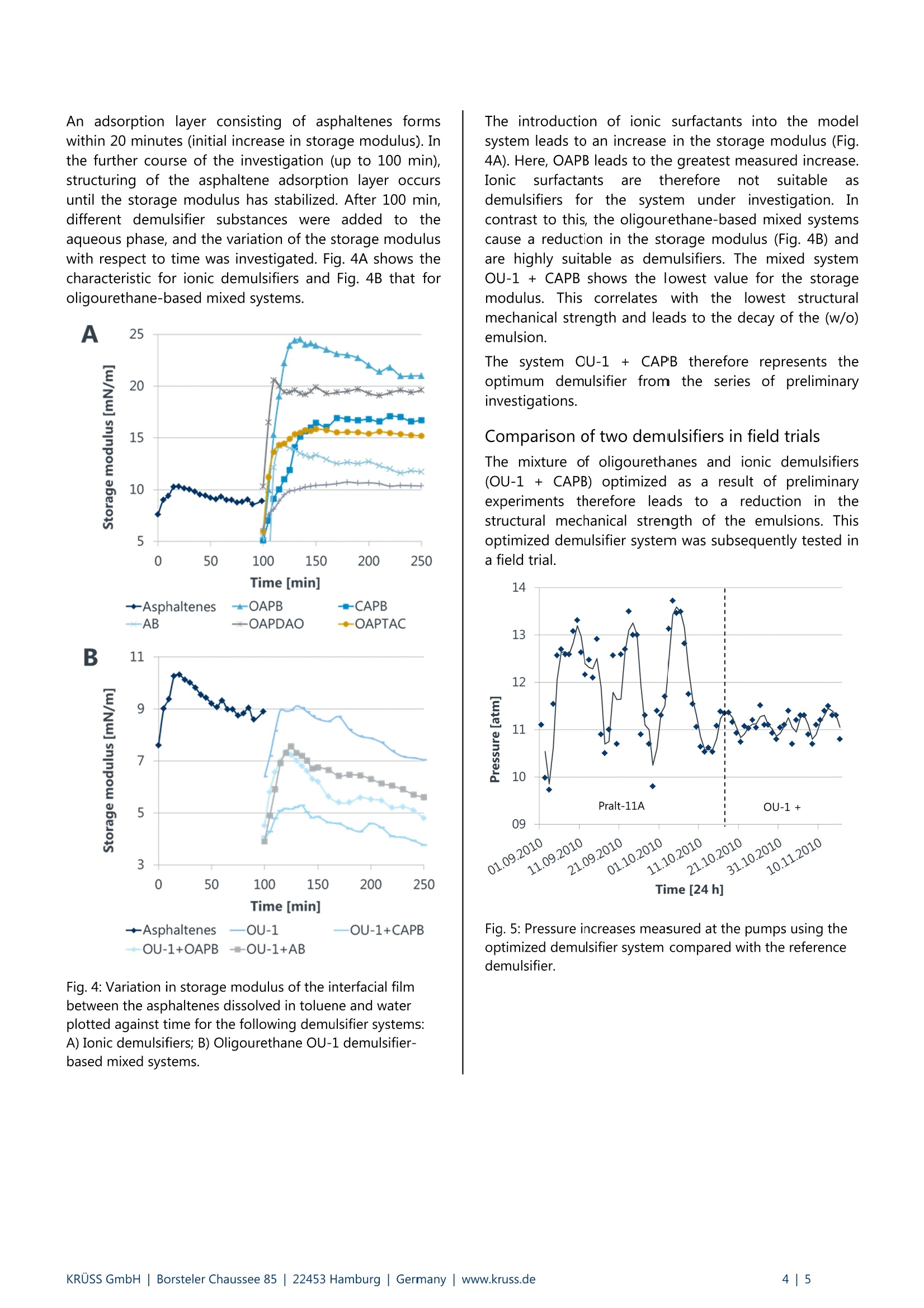
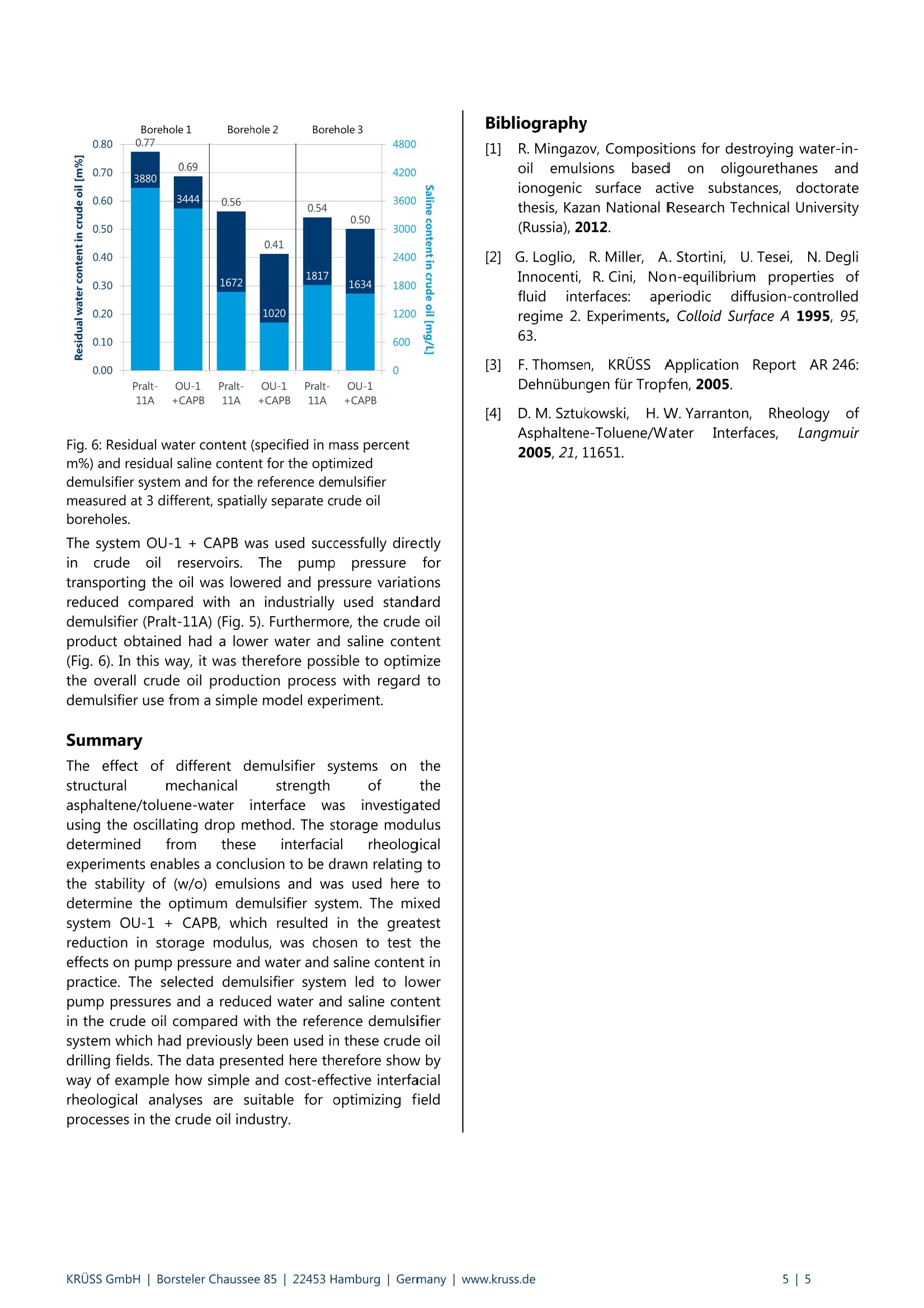
KRUSS Application Report Application report: AR276 Industry section: Oil recovery Author: IK, DF, RM,TW, MK Date: 07/2015 Method: Drop Shape Analyzer- DSA30R Keywords: Interfacial rheology,water-in-oil emulsions, demulsifier, crude oil processing, corrosion Development of customized demulsifiers Interfacial rheological measurements on crude oil emulsions The process of secondary and tertiary oil recovery leads to the formation of water-in-oil (w/o) emulsions. Demulsifierssplit these emulsions, thus enabling dewatering and desalination of the crude oil. Dewatering is necessary in order to saveenergy required for processing crude oil, to avoid corrosion problems during preparation for the refinery, and to reducepressure variations when transporting oil through pipelines Interfacial rheological measurements using the oscillating drop method were carried out in order to select suitabledemulsifiers for use in practice. This method predicts the structural mechanical stability and therefore the decay of thew/o emulsion when using demulsifiers. The optimized demulsifier was tested in practice in crude oil drilling fields and ledto a lower salinity of the oil and a reduction in pump pressure. Background The oil production process leads to the formation ofwater-in-oil (w/o) emulsions. These have essentially twomajor disadvantages in the subsequent oil recoveryprocess. 1) (w/o) emulsions are more viscous than the oil itselfand their transportation (e.g. from the production site tothe refinery) demands high pump powers which increasethe energy requirement and adversely affect pump life. 2) The dispersed phase of the(w/o) emulsions ischloride-containingi brinee whichhcauses corrosionproblems during preparation for the refinery. Asphaltenes present in crude oil make a significantcontribution to stabilizing such emulsions. Asphaltenesstabilize w/o emulsions as they form a "hard" molecularmesh at the oil-water phase boundary. Such interfacefilms have a visco-elastic character and have a highstorage modulus (= modulus of elasticity). This studytherefore focuses on the effect of asphaltenes on filmstability. Demulsifiers are used in order to avoid problems whichoccur due to the formation of (w/o) emulsions in crudeoil production. These split the emulsions and makedewatering and desalination of the crude oil possible [1]. The choice of a suitable demulsifier system must beadapted to suit the globally varying composition of crudeoil and to whether onshore or offshore drilling isinvolved..AAs aconsequence offtthis. extensiveoptimization of the demulsifier system isi nnecessarywhich, depending on the price of oil, often makes the useof demulsifiers uneconomic. To achieve individual matching, demulsifiers are initiallyanalyzed in the laboratory using small sample quantities.If this is satisfactory, it is sufficient to check the result justonce based on more extensive investigations in a realenvironment, i.e. at the oilfield. Exclusive optimization ofdemulsifiers in the reservoir would be considerably moretime-consumingand cost-intensive.Ininthis case,interfacial rheology is a suitable method for investigatingand optimizingddemulsifier systems in modelexperiments. The following explains the methodology of interfacialrheology and shows how to arrive at the optimal resultsin real crude oilfields from the model experiment. Thedemulsifier (OU-1 + CAPB) optimized in the modelexperiment is compared with an established industrialdemulsifier (Pralt-11A).. Here, it is sshown that theoptimized demulsifier leads to improved dewatering anddesalination and to a reduction in pump pressurescompared with Pralt-11A. Experimental part Interfacial rheological measurements using theoscillating drop method Measurement of the dynamic interfacial tension while thedrop is simultaneously oscillated forms the basis fordetermining dilativee rheologicalproperties of theinterface [2, 3]. Here, the volume of a pendant drop isvaried periodically and the resulting interfacial tensioncalculated as a function of time based on the opticallydetected drop profile. The characteristic of the interfacialtension in response to the drop oscillation is to a greatextent sensitive to demulsifiers at the liquid boundary.For this reason, investigation of the drop oscillationenables a judgment to be made regarding the thicknessand strength of an interfacial layer. The measuringprinciple is shown in Fig. 1. Time Fig. 1: Schematic diagram of the measurement of visco-elastic properties of interfaces using the oscillating dropmethod. The profile of a pendant drop is shown on the left.The volume is varied periodically at constant amplitude(shown by the red lines). The sinusoidal characteristic curvesfor the interfacial tension (Ao) and the surface area (AA) areshown on the right along with the phase shift (p) and theoscillation period (2Tt/w). The complex visco-elastic modulus E* can be calculatedfrom the change in drop surface area (^A), the meandrop surface area (Ao) and the change in interfacialtension (Ao): The visco-elastic modulus can be divided into the storagemodulus E’ and loss modulus E" by means of thedetermined phase shift (: Instrumentation and samples The rheological properties of different interfaces wereinvestigated with the Drop Shape Analyzer-DSA 30incorporating the additional interfacial rheology module(Oscillating Drop Module, ODM). Toluene solutions withdifferent concentrations of asphaltenes extracted fromcrude oil were used as the model oil phase. [1]. Drops ofthe model oil phase were formed in the bulk aqueousphase. Various demulsifiers were also added to theaqueous phase at a later stage. The demulsifiersubstances investigated are given in Table 1. A sinusoidaloscillation with a period of 5 seconds was used for thedrop oscillation. During the oscillation, the variation indrop surface area was AAmax/Ao=10-15%[4]. Table 1: Abbreviations of the substances used and their fullname. Abbreviation Name of substance OAPB Oleyl amido propyl betaine CAPB Cocamidopropyl betaine AB Alkyl betaine OAPDAO Oleyl amido propyl dodecyl dimethylamineoxide OAPTAC Oleyl amido propyl trimethyl ammoniumchloride CTAC Cetyltrimethylammonium chloride OU-1 Oligourethane Results Rheological properties of interface films All experiments and results shown below were carriedout, obtained and published as part of the doctoratethesis by Mingazov (Kazan National Research TechnicalUniversity, Russia) [1]. In a first step, the conditions for the formation of stableadsorption films at the interface between the asphaltenesdissolved in toluene and water were investigated with thehelp of interfacial rheological measurements. In doing so,tWoparameters were ofinterest: the asphalteneconcentration in the solution and the time to form the. said adsorption films. The change in interfacial tension with respect to time ofasphaltene-toluene solutions and water for differentasphaltene concentrations was investigated using thependant drop method (Fig.2) Asphaltene concentration [g/L] ◆0.61m1.22▲ 2.44×4.88*9.76●19.53 +39 Fig. 2: Variation with respect to time of interfacial tension oftoluene solutions and water for different asphalteneconcentrations. For all concentrations, the interfacial tension decreaseswith time and after 15 minutes all curves tend to aconstant equilibrium value. From the determinedequilibrium values, anisotherm for the interfacialtensionswasplottedagainst different asphalteneconcentrations (Fig. 3). The isotherm obtained shows aminimum at c= 2.44 g/L, which, in a similar way tocritical micelle concentration (CMC), can be associatedwith the aggregation of asphaltenes in solution. Fig. 3: Isotherm of the equilibrium interfacial tensions for theinvestigated asphaltene solutions. The storage modulus of the interfacial film for anasphaltene concentration has a maximum in a similar wayto CMC [4). As changes in the interfacial rheology due toadding demulsifier substances can be shown to be mostsensitive at this concentration, further investigations onasphaltene solutions were carried out withconcentrations corresponding to the CMC. Fig. 4 shows the variation of the storage modulus withrespect to time for the different demulsifier substancesinvestigated. An adsorption layer consisting of asphaltenes formswithin 20 minutes (initial increase in storage modulus). Inthe further course of the investigation (up to 100 min),structuring of the asphaltene adsorption layer occursuntil the storage modulus has stabilized. After 100 min,different demulsifier substances were added to theaqueous phase, and the variation of the storage moduluswith respect to time was investigated. Fig. 4A shows thecharacteristic for ionic demulsifiers and Fig. 4B that foroligourethane-based mixed systems. A B 11 Fig. 4: Variation in storage modulus of the interfacial filmbetween the asphaltenes dissolved in toluene and waterplotted against time for the following demulsifier systems:A) Ionic demulsifiers; B) Oligourethane OU-1 demulsifier-based mixed systems. The introduction of ionic surfactants into the modelsystem lead to an increase in the storage modulus (Fig.4A). Here, OAPB leads to the greatest measured increase.IonicICsurfactants are therefore not suitable asdemulsifiers for the system under investigation. Incontrast to this, the oligourethane-based mixed systemscause a reduction in the storage modulus (Fig. 4B) andare highly suitable as demulsifiers. The mixed systemOU-1 + CAPB shows the lowest value for the storagemodulus. This correlates with the lowest structuralmechanical strength and leads to the decay of the (w/o)emulsion. The system OU-1 + CAPB therefore represents theoptimum demulsifier from the series of preliminaryinvestigations. Comparison of two demulsifiers in field trials The mixture of oligourethanes and ionic demulsifiers(OU-1 + CAPB) optimized as a result of preliminaryexperiments therefore leads to0a reduction in thestructural mechanical strength of the emulsions. Thisoptimized demulsifier system was subsequently tested ina field trial. Fig. 5: Pressure increases measured at the pumps using theoptimized demulsifier system compared with the referencedemulsifier. Fig. 6: Residual water content (specified in mass percentm%) and residual saline content for the optimizeddemulsifier system and for the reference demulsifiermeasured at 3 different, spatially separate crude oilboreholes. The system OU-1+ CAPB was used successfully directlyincrude: oilreservoirs.Theiepump pressurefortransporting the oil was lowered and pressure variationsreduced compared with an industrially used standarddemulsifier (Pralt-11A) (Fig. 5). Furthermore, the crude oilproduct obtained had a lower water and saline content(Fig. 6). In this way, it was therefore possible to optimizethe overall crude oil production process with regard todemulsifier use from a simple model experiment. Summary The effect of different demulsifier systems5or thestructural mechanical strength of theasphaltene/toluene-waterrinterfacewas investigatedusing the oscillating drop method. The storage modulusdetermined from tlthese interfacial rheologicalexperiments enables a conclusion to be drawn relating tothe stability of (w/o) emulsions and was used here todetermine the optimum demulsifier system. The mixedsystem OU-1+ CAPB, which resulted in the greatestreduction in storage modulus, was chosen to test theeffects on pump pressure and water and saline content inpractice. The selected demulsifier system led to lowerpump pressures and a reduced water and saline contentin the crude oil compared with the reference demulsifiersystem which had previously been used in these crude oildrilling fields. The data presented here therefore show byway of example how simple and cost-effective interfacialrheological analyses are suitable for optimizing fieldprocesses in the crude oil industry. [1] R. Mingazov, Compositions for destroying water-in-oilemulsions basedon oligourethanesandionogenic surface active substances, doctoratethesis, Kazan National Research Technical University(Russia), 2012. [2]G. Loglio, R. Miller, A. Stortini, U. Tesei, N. DegliInnocenti, R. Cini, Non-equilibrium properties offluid interfaces: aperiodicdiffusion-controlledregime 2. Experiments, Colloid Surface A 1995, 95,63. [3] F. Thomsen, KRUSS Application Report AR 246:Dehnubungen fur Tropfen,2005. [4] D. M. Sztukowski,H. W. Yarranton,RheologyofAsphaltene-Toluene/WaterInterfaces, Langmuir2005,21, 11651. KRUSS GmbH|Borsteler Chaussee Hamburg| Germany |www.kruss.de|
确定


还剩3页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《乳液中破乳检测方案(接触角测量仪)》,该方案主要用于其他中破乳检测,参考标准--,《乳液中破乳检测方案(接触角测量仪)》用到的仪器有KRUSS DSA25标准型接触角测量仪
推荐专场
相关方案
更多
该厂商其他方案
更多