
方案详情
文
表面活性剂使用广泛但一般不单独使用,表面活性剂之间存在协同作用。本文以SDS和DTAB为例,利用CMC变化研究其热力学行为。
方案详情

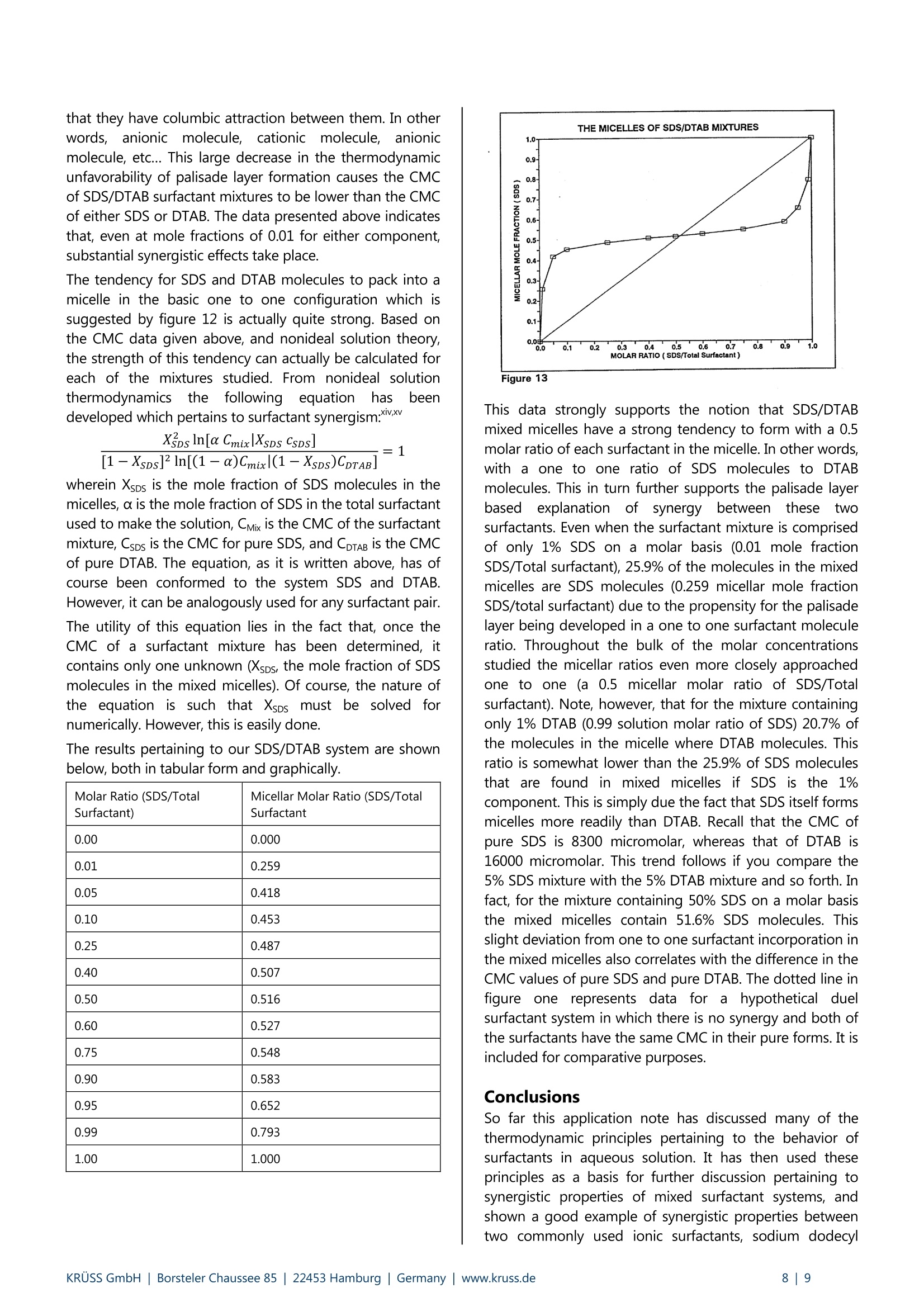
KRUSS Application Report Synergistic aspects of surfactant mixtures The anionic surfactant sodium dodecyl sulfate and the cationic surfactant dodecyltrimethylammonium bromide Background Surfactants are compounds which are structurally heterogeneous. Each surfactant molecule consists of a hydrophobic end(commonly referred to as the "tail") and a hydrophilic end (commonly referred to as the "head"). This heterogeneous nature iswhat makes surfactants so useful in industrial and commercial formulations. Surfactants are primary components of productssuch as cleansers, degreasers, emulsifiers, dispersants, and foamers and defoamers. In each of these products, surfactantsperform a specific function, and that function is only possible due the heterogeneous nature of surfactant molecules. Forcleansers and degreasers the function is to remove hydrophobic material from a solid substrate. For emulsifiers anddispersants the function is to stabilize either a hydrophobic or a hydrophilic material in a liquid medium which is of theopposite nature. For foamers and defoamers the function is either stabilization or destabilization of gas bubbles in a liquidmedium. However, heterogeneity is only the beginning of the surfactant story. Aggregation is the remainder. In many surfactant basedproducts, including most of those described above, surfactant molecules do not act individually to perform their functions.Rather, they act as aggregates. Aggregation of surfactant molecules in solution occurs because either their head group ortheir tail group is not soluble in the bulk solvent. This application note focuses largely on surfactants in aqueous solution. In aqueous solution, it is a surfactant's hydrophobictail group that is insoluble. The tail group is insoluble not because it dislikes water (as the misnomer "hydrophobic" implies),but because it is thermodynamically unfavorable for water molecules to associate with it. It is, however, favorable for watermolecules to associate with a surfactant's head group. Therefore, a certain thermodynamic conflict of interest is established onthe part of water with regard to surfactants. The most favorable solutions to this conflict are ones which allow water moleculesto interact with head groups of surfactant molecules and not interact with tail groups. One thermodynamically favorable solution is for thesolvent to drive a fraction of the surfactant molecules tothe solution's boundaries. The size of this fraction isdictated by considerations of chemical potential, which are governed by the structural makeup of the surfactant andthe solvent in question, as well as the concentration ofsurfactant in solution. Aggregation of surfactant moleculesat a solution's boundaries can be loosely viewed as form of phase separation (surface phase separation if you will). Thetwo dimensional surface phase of a surfactant solutionconsists of a disproportionate concentration of surfactantmolecules relative to the bulk solution phase. Aggregationof surfactant molecules at a solution's surface is alsoreferred to as monolayer formation. Monolayer formationis the first type of surfactant aggregation considered in thisnote because, when one starts with pure solvent andaugments surfactant concentration, it is generally the firstto occur. For aqueous surfactant systems, as a surfactant monolayeris established, surface tension of the solution decreases.This decrease in surface tension can be monitored as afunction of bulk solution surfactant concentration by anumber of methods. One typical method is the Wilhelmyplate method. Figure 1 shows data pertaining to decreasesin surface tension due to increases in bulk surfactantconcentration for a simple system containing only waterand a nonionic alcohol ethoxylate. The Wilhelmy platemethod with a platinum plate, and a Kruss model K12Tensiometerr equippedwith an automatedddosingaccessory, was used to obtain this data. (A completedescription of Wilhelmy plate techniques is beyond thescope of this text. Those interested in such a description arereferred to the Kruss K12 brochure.) Region 1 of figure 1corresponds to the region of surfactant concentrationsover which surface tension is logarithmically dependent onbulk surfactantconcentration. Suchaallogarithmicdependence is a common result of the chemical potentialwhichcausesadsorption ofsurfactant atsolutionboundaries. Figure 1 Throughout the range of concentrations of region 1,monolayer formation at the solution’s boundariess isincomplete. Monolayer formation becomes complete at acritical point in surfactant concentration. This concentrationcorresponds to the boundary between region 1 and region2 in figure 1. It is commonly termed the critical micelleconcentration or "CMC" of the surfactant. Please note thatthe words "complete" and "incomplete" is the last fewsentences are not to be taken as absolute terms. To yourauthor, "complete" gives the conceptual idea that everymolecule at the boundary of a solution is a surfactantmolecule. This iss most often not the case. Due to considerations of solvation of the head groups of thesurfactant, some solvent is typically interdispersed withsurfactant molecules in the monolayer, even at what hasbeen termeddheree"complete" monolayer formation.Therefore, by "complete monolayer" I mean only to expressthat a solution's boundaries are saturated with surfactant tothe point that it becomes more thermodynamicallyfavorable for interactions between the tail groups of asurfactant and the solvent to be diminished by othermeans. For most aqueous surfactant systems, this "othermeans" takes the form of aggregation of surfactantmolecules in the bulk solution to form what are termed"association colloids"." Once the necessary surfactantconcentration is reached such that formation of associationcolloids begins, further increases in the solution’s bulksurfactant concentration cause very minor further increasesin the concentration of surfactant in the solution's surfacephase. This corresponds to very little further decrease insurface tension at the solution's boundaries, as is indicatedin region 2 of figure 1. Prior to discussing surfactant association colloids however,it may be instructive to briefly discuss at least one industrialapplication in which monolayer formation is a fundamentalreason for the addition of surfactants to a formulation. Agood example of this is the addition of surfactants to sprayformulations. One of the large considerations in developinga product which is applied as a spray is "atomization". Theterm atomization relates to how well a liquid spray productdisperses into droplets as it leaves a sprayer and travelsthrough the atmosphere to a substrate at which it meant tobe applied. In most cases, it is desirable for spray dropletsto be as small as possible prior to contacting a substrate. Atspray manufacturing companies, many engineers workdiligentlyatestablishinggspraynozzleedesigns andoptimizingg sspprraayyer backpressuretto achieve betteratomization of their sprays. Better atomization meanssmaller, more uniform sized, droplets. The dispersion of asolution into droplets in a spray application is governed bymany effects which are difficult to model mathematically.These include Bernoulli effects, Rayleigh-Taylor instabilities,and Kelvin-Helmholtz instabilities. However, one of thefundamental factors governing atomization is surfacetension of the solution being sprayed. Surface tension isthe force necessary to create a new atmosphere/solutionsurface per unit area of surface created. Surfactantmonolayer formation lowers a solution's surface tension. Asa result, surfactants are often added to spray formulationsfor the purpose of forming complete (or even partial)monolayers on the surfaces of spray droplets, therebyallowing them to disperse into small droplets more easily.This concept is shown schematically in figure 2. Figure 2 Be aware that in some ways figure 2 presents anoversimplified explanation of the use of surfactants in sprayformulations, because spraying is an application which isdynamic in nature. Therefore, in deciding the propersurfactant type and concentration for a spray formulation, itis often necessary to also study the dynamics of surfactantmonolayer formation. A discussion of such a study isbeyond the scope of this text. However, for thoseinterested, Kruss does offer a dynamic surface tensioninstrument (the Bubble Pressure BP2) for these studies, andwe do have some expertise in this area. Sprays are only one industrial aapplication in whichsurfactants are added to a formulation because they tendto aggregate at solution boundaries. Many others exist.However, the purpose of this background section is not tofocus on any one set of applications for surfactants.Rather,it is to lay the ground work for a discussion of theidiosyncracies of multiple surfactant systems, by providingthe reader with a working level of understanding of the useof surfactants in general. Therefore, it is important toproceed to a discussion of surfactant "associative colloids"and their utility in industrial and commercial productformulations. Recall that surfactant associative colloidsbegin to form in aqueous surfactant solutions as asecondary means of diminishing interactions betweenwater and surfactant tail groups. The most widely studied surfactant associative colloids arespherical micelles. This is because, in many surfactantsystems, they are the first type of associative colloid toform bulk solution surfactant concentrationisaugmented beyond what is necessary to "complete" amonolayer at the solution's boundaries. A schematic of asurfactant micelle in aqueous solution is shown in figure 3.Be aware that this schematic is meant to represent a crosssection of a spherical entity. Figure 3 Micelles are generally spherical due to two opposingforces. The first force, which causes surfactant tail groups toassociate, has to do with the entropic and enthalpicunfavorability of the association of water molecules withhydrophobic moieties. This principle was discussedpreviously. The second force, the one which gives a micelleits spherical nature, has to do with interactions between thehead groups of surfactant molecules in a micelle, and withsolvation effects. In general, the spherical shape of micellesis due to the thermodynamic favorability of keeping thehead groups of surfactants solvated, even when surfactantmolecules are associated due to their tail groups. This is thesame effect that causes surfactant "monolayers" at solutionboundaries to not be completely devoid of solventmolecules. The spherical conformation allows space forwater molecules to solvate the surfactant head groups of amicelle. The region of surfactant head groups in a micelle(or other surfactant associative colloid) is termed the'palisade layer". The nature of developed palisade layers isperhaps the most significant factor in determining howassociative colloids are developed in aqueous surfactantsolutions. Ironically, it is, in many cases, much moreimportant than the nature of a particular surfactant'shydrophobic tail, which is, after all, the portion of thesurfactant that causes colloidalaassociations to bedeveloped. We shall consider this concept in some detailshortly, since the main focus of this application note issurfactant synergy. The synergistic effects observed inmany multi-surfactant systems are due to advantageousinteractions between the head groups of surfactantmolecules in palisade layers. However, before we discusssynergistic effects, it is instructive to briefly expand ourdiscussion of surfactant association colloids to includeimportant aggregations which are of a higher order thanspherical micelles. An abbreviated list" of relevant surfactant associationcolloids that may exist in aqueous surfactant systems isprovided in figure 4. For each type of association aschematic of the structure of the colloid is provided. Figure 4 Please make particular attention to the descriptions at thebottom of figure 4, which pertain to variables that cancause transitions between phases. These descriptions onlyrepresent general trends and are not necessarily to beregarded as absolute. As we will see in a moment, even"simple" single surfactant aqueous solutions can havecomplex phase diagrams which are dependent on thesevariables. Nonetheless, our discussion to this point hasfollowed the trend of increasing surfactant concentration.With that in mind, note that, according to figure 4.increasing surfactant concentration will generally promotespherical micelles to be reformed into hexagonal phaseassociation colloids, and then into llamellar phaseassociations. In a hexagonal phase, the head groups ofsurfactant molecules are closer packed (in the palisadelayer) than they are in spherical micelles. In the lamellarphase, head groups are even closer packed in the palisadelayer. Why do these association colloids becomethermodynamically favorable in surfactant solutions, evenin light of the fact that micelles have a spherical shape toprovide for solvation of surfactant head groups? Theanswer is that, once the concentration of surfactant in asolution becomes high enough, the formation of sphericalmicelles is no longer an efficient way to eliminate contactsbetween surfactant tails and the water structure. There arejust so many tails that need to be associated that moreefficient packing becomes favorable. This means makingthe thermodynamic sacrifice of forcing surfactant headgroups into close proximity (often diminishing theirsolvation sheafs),), for thethermodynamicgainassociating more tailgroups. Atwhatpointconcentration does this spherical micelle to hexagonal orlamellar phase transition occur? This is largely dependenton how compliant or resistant the head groups of thesurfactant are to being close packed. For some surfactantsystems these transitions never occur. In others they occurat low concentrations just above (or even at) the CMC ofthe surfactant. It depends largely on the nature of thepalisade layer that would be developed if the colloid wasformed. Shown below is a rough phase diagram for the nonionicsurfactant polyoxyethylene(8)cetylether(chemicalstructure:C16H33(OCH2CH2):OH) in water. Figure 5 This phase diagram shows the general trends discussedabove, although the transitions between regions based onchanges in surfactant concentration and temperature arecomplex as promised. The region labelled "isotropic" in thisdiagram is most likely a reverse micellar region. (It may alsobe a cubic phase, the description of which is beyond thescope of this text.) Reverse micelles occur at extremesurfactant concentrations in aqueous solutions. In fact,when they occur the solution is phase inverted and oilcontinuous. The region labelled "monomer and crystals"may also contain some reverse hexagonal phase. A reversehexagonal phase is also an oil continuous phase. The otherlabelled regions have been discussed previously. Industrial and commercial products are formulated in eachof these associative colloidal phases,and one basic reasonfor using mixtures of surfactants in a formulation is topromote the development of one or more of these phases.Micelles act as stabilizers for disperse oils and colloids. Thehydrophobic oils and/or particles simply reside in theinterior of micelles with the surfactant tail groups. This isuseful for products such as detergents, emulsifiers, anddispersants.The hexagonal phase is also capable ofdispersephaseSstabilization,bybasically theessamemechanism. However, in addition, the hexagonal phasetends to close pack as shown in figure 4. This providesrheological properties whichare often desirableincosmetics and other products. Lamellar phases are similarlyimportant as cleaners and stabilizers, but have muchdifferent rheological properties than the hexagonal phase.Lamellar sheets tend to slip pass one another if thestructure is sheared. Lamellar phases are also, in general,the best foam stabilizers. The reverse hexagonal phase isused for degreasers, and also in cosmetics. It typically hasrheological properties analogous to those of the hexagonalphase. Reverse micelles are used to stabilize hydrophilicmaterials in a hydrophobic medium. Figure 6 illustratessome of these applications. Figure 6 As was stated previously, the nature of palisade layers isoften key to determining the type of colloidal associationsthat will occur in a solution containing surfactants. In orderto consider placide layers further, let's consider figure 7 inlight of some literature data pertaining to micelles ofvarious surfactants. Figure 7 The schematics in figure 7 represent two very differentmicelles. Micelle #1 has a typical spherical shape, with wellsolvated surfactantthheadgroups.For purposes otillustration we will say that it is composed of nonionicsurfactant molecules. Micelle#2 iss c(omprised ofahypothetical surfactant with the exact same tail group asthe surfactant used to make micelle #1. However, thesurfactant in micelle #2 is anionic. Its head group containsan ionic bond which dissociates in aqueous solution torender the surfactant head group negatively charged. Thecation to the surfactant diffuses some distance from themicelle due to Donnan equilibrium effects." Ionic surfactanthead groups not only need to be solvated by water (as dononionic head groups), but they also repel each othercoulombically. If you think of each surfactant head groupoccupying a certain surface areraed oon1 the spherical micelle,ionics take up more surface area per head group versusnonionics as result of columbic repulsion.This is, of course,assuming a single surfactant system in which all of thehead groups havelike charges. FFurther, for ionicsurfactants, columbic repulsion ttends to resist theformation of micelles. Remember that the hydrophobiceffect and the association of surfactant tails is what drives micelle formation. The palisade (head group) layer is forcedto form as a consequence. Shown below is data pertaining to formation of micelles forsome select ionic and nonionic surfactants. All data wasobtained from literature and pertains to the surfactants inaqueous solution near room temperature. Surfactant Structure CMC(Micromolar) AggregationNumber Sodium DodecylSulfate C12H25SO4 Na 8200° 64 Dodecyl Trimethyl ammoniumBromide C12H25N*(CH3)3Br 16000 55 Tetradecyl Trimethylammonium Bromide C14H25N*(CH3)3Br 3600i 70 HexadecylTrimethylammonium Bromide C16H25N*(CH3)3 Br 920* 89 Polyoxyethylene(6) Dodecyl Ether C12H25(OC2H4)6OH 87* 400* Polyoxyethylene(8) Dodecyl Ether C12H25(OC2H4):OH 100* 123* Based on the explanations put forth above, pertaining tofigure 7, it might be expected that an ionic surfactantwould have a higher CMC and a lower number ofmolecules per micelle (aggregation number) than anonionic surfactant with the same size hydrophobic tailgroup. The data above supports this. The CMC's of bothsodium dodecyl sulfate and dodecyl trimethylammoniumbromide are approximately two orders of magnitudehigher those of the nonionic polyoxyethylene dodecylethers listed. Comparing the same surfactants, aggregationnumbers at the CMC are at least twice as large for thenonionics as they are for the ionics. Data pertaining to tetradecyl trimethylammonium bromideand hexadecyl trimethylammonium bromidee are alsoincluded for comparison with the dodecyltrimethylammonium bromide data. This series shows theeffect of increasing the size of a surfactant's tail groupwhile holding the head group constant. As expected, theeffect is that CMC decreases and aggregation numberincreases. More hydrophobe equals more thermodynamicdrive toward association. However, this data also suggeststhat the effects of increasing a surfactant's tail length byfour alkyl units are only to decrease CMC by a little morethan an order of magnitude and increase aggregationnumber by less than a factor of two. This justifies previousstatements that the head group of a surfactant, and thenature of the palisade layer, can have even more influenceon aggregation properties of the surfactant than does thenature of the surfactant's tail group. Thisbackground sectionhas focused largely onaggregation properties of single surfactants in aqueoussolution. It has been mentioned that many industrial andcommercial systems contain multiple surfactants for thepurpose of tailoring their properties to the system'sapplication. As a result, effective formulators of multi-surfactant systems understand, not only the informationpresented thus far, but also that if more than onesurfactant is present in a solution, then the surfactantassociation colloids formed can be quite different fromthose formed with any of the surfactants individually.Mixing justtwo surfactants cannproduce surfactantassociations in concentration and temperature regimeswherein such associations are not possible for either of thesurfactants individually. This concept is generally known as"surfactant synergism". Asan example of surfactantsynergism, we have recently studied micelle formation inaqueous solutions containing the anionic surfactantsodium dodecyl sulfate and the cationic surfactant dodecyltrimethylammonium bromide. Experimental Both sodium (dodecyl sulfate (SDS) ddodecyltrimethylammonium bromide(DTAB)were obtainedcommercially in powdered form. Each surfactant had areported purity of 99%, and each was used without furtherpurification. Thirteen aqueous solutions wereprepared from drysurfactant powders. Each was prepared in distilled water.The total concentration of surfactant in each of thesesolutions was varied as necessary by dilution with puredistilled water. However, each solution contained a specificmolar ratio of SDS relative to the total amount of surfactantin the system (SDS + DTAB). The following SDS/surfactantmolar ratios were studied. Solution # Molar Ratio (SDS/TotalSurfactant) 1 0.00 2 0.01 3 0.05 4 0.10 5 0.25 6 0.40 7 0.50 8 0.60 9 0.75 10 0.90 11 0.95 12 0.99 13 1.00 Solution #1 was of course an aqueous solution containingonly DTAB, while solution #13 was an aqueous solutioncontaining only SDS. Solutions #1 and #13 were used, individually, as dosingsolutions to determine CMC values for SDS and DTAB, bythevWilhelmy plateenmethod.The KrussProcessorTensiometer modelK12! with an automated dosingaccessory and a roughened platinum Wilhelmy plate wasused for this and all of the remaining CMC work reportedin this text. The initial solution in both cases was purewater. (Those who are unfamiliar with the operation of aK12 in the automated CMC mode are referred to the K12brochure.) The results of these initial experiments are shown in figures8 and 9. Figure 8 Figure 9 From these CMC curves, it is evident that both the SDS andthe DTAB used contained some impurities. Impurities cancause pre-CMC dips in a surfactant's CMC curve, if theimpurities are more surface active than the main surfactant.For example, the impurity in sodium dodecyl sulfate is mostoften the lauryl alcohol from which it is synthesized. It isoften difficult to obtain a CMC value for a surfactant that ishighly contaminated, but that was not the case with thesesurfactants. In order to avoid the impurity dips in these setsof data, the CMC for each surfactant was simply taken asthe point in concentration at which each curve reached aplateau after the impurity dip. See figures 8 and 9. Thismethod seems to work quite well. The CMC valuesobtained from figures 8 and 9 were 8300 and 16000 micromolar respectively. The literature values, as reportedpreviously, are 8200 micromolar for SDS and 16000micromolar for DTAB. Based on this data we were, I believe,justified in using the same CMC evaluation technique fordata pertaining to solutions containing both SDS andDTAB. Therefore, by these techniques, CMC data was obtainedfrom solutions #2 through #12. Figure 10 is one of theeleven resultant CMC curves, which is shownas anexample. It shows that the CMC of an aqueous solutioncontaining a 0.5 molar fraction of SDS to total surfactant is120 micromolar. Figure 10 Results and discussion Shown below, in both tabular and graphical form, is all ofthe CMC data obtained from the study. Molar Ratio (SDS/TotalSurfactant) Critical Micelle Concentration(Micromoles of Total Surfactant) 0.00 16000 0.01 8000 0.05 1200 0.10 490 0.25 270 0.40 180 0.50 120 0.60 150 0.75 220 0.90 390 0.95 700 0.99 5000 1.00 8300 Figure 11 The dotted line in figure 11 represents CMC values whichmight be expected for SDS/DTAB mixtures if synergism didnot occur. In other words, in the absence of synergism, theCMC of a surfactant mixture might be expected to simplybe the average of the CMC values of the componentsurfactants weighted by their respective molar ratios withinthe mixture. It is evident that this is by no means the casefor SDS/DTAB mixtures. The data shows a synergisticpattern, with all CMC data for the mixtures falling belowthe dotted line. The synergistic effects reach a maximum ata 0.5 molar ratio of SDS (and correspondingly a 0.5 molarratio of DTAB) in the surfactant mixture. At this point theCMC is 120 micromolar of total surfactant, which is almosta full two orders of magnitude lower the CMC of eithersurfactant in pure form. What causes this level of synergism? Quite simply the factthat mixed surfactant systems have the thermodynamicalternative to form micelles in which the head groups ofthe palisade layer are not repulsive. (In fact, for SDS/DTABsystems, they are attractive.) Recall our backgrounddiscussion of how and why CMC values varied in nonionicversus ionic surfactants, and consider figure 12. Figure 12 The micelle depicted in figure 12 is formed from a mixtureof cationic and anionic surfactant molecules, like theSDS/DTAB systems we are discussing here. It is stillfavorable for head groups of such a micelle to be solvated,but the thermodynamic drive to keep them apart is greatlydiminished by the fact that they can pack into an array such that they have columbic attraction between them. In othermolecule, etc... This large decrease in the thermodynamicunfavorability of palisade layer formation causes the CMCof SDS/DTAB surfactant mixtures to be lower than the CMCof either SDS or DTAB. The data presented above indicatesthat, even at mole fractions of 0.01 for either component,substantial synergistic effects take place. The tendency for SDS and DTAB molecules to pack into amicelle in the basic one to one configuration which issuggested by figure 12 is actually quite strong. Based onthe CMC data given above, and nonideal solution theory,the strength of this tendency can actually be calculated foreach of the mixtures studied. From nonideal solutionthermodynamics the followingequation Ihas beendeveloped which pertains to surfactant synergism:x = 1 wherein Xsps is the mole fraction of SDS molecules in themicelles, a is the mole fraction of SDS in the total surfactantused to make the solution, CMix is the CMC of the surfactantmixture, Csps is the CMC for pure SDS, and CDTAB is the CMCof pure DTAB. The equation, as it is written above, has ofcourse been conformed to the system SDS and DTAB.However, it can be analogously used for any surfactant pair. The utility of this equation lies in the fact that, once theCMC of a surfactant mixture has been determined, itcontains only one unknown (Xsps, the mole fraction of SDSmolecules in the mixed micelles). Of course, the nature ofthe equation is such that Xsps must be solved fornumerically. However, this is easily done. The results pertaining to our SDS/DTAB system are shownbelow, both in tabular form and graphically. Molar Ratio (SDS/TotalSurfactant) Micellar Molar Ratio (SDS/TotalSurfactant 0.00 0.000 0.01 0.259 0.05 0.418 0.10 0.453 0.25 0.487 0.40 0.507 0.50 0.516 0.60 0.527 0.75 0.548 0.90 0.583 0.95 0.652 0.99 0.793 1.00 1.000 Figure 13 This data strongly supports the notion that SDS/DTABmixed micelles have a strong tendency to form with a 0.5molar ratio of each surfactant in the micelle. In other words,with a one to one ratio of SDS molecules to DTABmolecules. This in turn further supports the palisade layerbasedexplanation of synergybetween thesetwosurfactants. Even when the surfactant mixture is comprisedof only 1% SDS on a molar basis (0.01 mole fractionSDS/Total surfactant), 25.9% of the molecules in the mixedmicelles are SDS molecules (0.259 micellar mole fractionSDS/total surfactant) due to the propensity for the palisadelayer being developed in a one to one surfactant moleculeratio. Throughout the bulk of the molar concentrationsstudied the micellar ratios even more closely approachedone to one (a 0.5 micellar molar ratio of SDS/Totalsurfactant). Note, however, that for the mixture containingonly 1% DTAB (0.99 solution molar ratio of SDS) 20.7% ofthe molecules in the micelle where DTAB molecules. Thisratio is somewhat lower than the 25.9% of SDS moleculesthat are found in mixed micelles if SDS is the 1%component. This is simply due the fact that SDS itself formsmicelles more readily than DTAB. Recall that the CMC ofpure SDS is 8300 micromolar, whereas that of DTAB is16000 micromolar. This trend follows if you compare the5% SDS mixture with the 5% DTAB mixture and so forth. Infact, for the mixture containing 50% SDS on a molar basisthe mixed micelles contain 51.6% SDS molecules. Thisslight deviation from one to one surfactant incorporation inthe mixed micelles also correlates with the difference in theCMC values of pure SDS and pure DTAB. The dotted line infigure one represents data for a hypothetical duelsurfactant system in which there is no synergy and both ofthe surfactants have the same CMC in their pure forms. It isincluded for comparative purposes. Conclusions So far this application note has discussed many of thethermodynamic principles pertaining to the behavior ofsurfactants in aqueous solution. It has then used theseprinciples as a basis for further discussion pertaining tosynergistic properties of mixed surfactant systems, andshown a good example of synergistic properties betweentwo commonly used ionic surfactants, sodium dodecyl sulfate and dodecyl trimethylammonium bromide. Thenote itself is particularly designed for individuals who workwith products containing multiple surfactants. It is meant toteach, and also to inform such people that Kruss offers aninstrument (the K12 Processor Tensiometer) which canassist them in their study of surfactant interactions in multi-surfactant systems. This brings up a couple of issues whichrequire a bit of closure. First, having read this application note, you may bethinking that we chose a relatively easy pair of surfactantswith which to work. Once you understand the importanceof the palisade layer in determining how surfactantsaggregate in solution, you will realize that a cationic and ananionic surfactant are almost bound to have synergisticrelations. This is, of course, completely true. In industry it isperhaps more common to deal with ionic/nonionicmixtures or evennonionic/nonionic mixtures. However, youmight be surprised to find that these mixtures often havesynergistic properties as well. For example, nonionics oftenform mixed aggregates with ionics because the presence ofnonionic head groups in the palisade layers of aggregatesbuffers columbic repulsions between the ionic headgroups. Rosen discusses the synergistic properties of awide variety of surfactants in chapter 11 of his bookentitled Surfactants and Interfacial Phenomena, which wehighly recommend to those who have had their curiosityinspired by this application note. There are also othermeans of causing surfactant systems to alter theiraggregation properties aside from adding a cosurfactant.One of the most common is the addition of small moleculeelectrolytes (salts) to ionic surfactants solutions. This causescondensation of counterions onto the palisade layer ofionic surfactant aggregates, which in turn causes a phasetransition toward a phase wherein the palisade layer lesssolvated (for example, a micellar to lamellar transition.) Thiseffect is used by shampoo manufacturers as a thickeningmechanism. Second, you may be concerned by the fact that this notediscusses a wide variety of surfactant colloidal associationsand then promotes only the study of micelle formation as atechnique for understanding the synergistic aspects ofmultiple surfactant systems. CMC studies are obviouslydirectly helpful to formulators who wish to produceproducts in the micellar region.It is obvious that takingadvantage of synergy can save surfactant cost and increasea product's performance to cost ratio for micellar basedproducts. The data presented here shows that a smallamount of either DTAB or SDS can save a large amount ofthe opposite surfactant, if micelle formation is the goal.However, the utility of CMC based studies to people whoformulateepproducts inhexagonal, lamellar,, or otherassociative regions may not yet be completely clear. The identification of hexagonal, lamellar, and many otherassociative surfactant phasesisscommonly donebypolarized light microscopy. The phases are identified bycharacteristicdiffraction patterns. However. thecharacterization of head group interactions in the palisade layers of these phases is quite difficult. In fact, no scientifictechnique has been established to routinely do so. Theby the formation of phasesdiagrams, such as figure 5 shown above, in combinationwith polarized light microscopy identification. Prediction ofconcentration and temperature regimes in which certainphases will occur can be made based on nonideal solutionthermodynamics and a thorough understanding3ofinteractions between surfactant molecules. CMC measurements do not directly provide information onassociative colloids of higher order than micelles. However,they do a good job of characterizing interactions betweensurfactants in mixed surfactant solutions. This informationcan be used to predict how the phase diagram of asurfactant mixture will differ from phase diagrams of thecomponent surfactants. Synergistic effects tend to shift aphase diagram to lower overall surfactant concentrations.In other words, the same synergistic effects that causemixed micelles to form at lower surfactant concentrationsalso cause hexagonal and lamellar phases to form at lowerconcentrations. As a result, people who formulatesurfactant systems, even in higher order phases than themicellar, rely on CMC measurements as predictors ofsurfactant phase behavior. Sorry, phase diagrams still needto be constructed. However CMC measurements can serveas a guide to which phase diagrams should be constructedand which are more likely to not be worthwhile. This is theirutility. References i. Tanford, C.; The Hydrophobic Effect, Wiley, New York,(1980). ii. Myers, D.; Surfaces, Colloids and Interfaces, VCH Publishers,New York, (1991). iii. Mittal, K.L. and Lindman B., editors; Surfactants in Solution1, Plenum Press, New York, (1984). ( IV. Shaw, D .J.; Colloid and S urface C hemistry, Butterworth- Heinemann Ltd,Oxford, (1992). ) ( v. Elworthy, P . H.; Mysels, K.J.; J. C o lloid Sci., 21, 3 3 1, (1966). ) ( vi. Lianos, P .; Zana, R .;J.Colloid Interface Sci., 8 4, 1 00, ( 1981). ) ( vii. Klevens, H.B.;J. Phys . Colloid Chem., 52,130 , (1948). ) ( viii. Lianos, P.;Zana, R.;J. Colloid Interface Sci. , 88 , 594 , (1982). ) ( IX. Czerniawski, M., Roczn. C hem., 40, 1935,(1966). ) ( X. Corkill, J.M. ; Goodman, J . F.; Ottewill, R.H. ; Trans. F araday Soc., 57, 1627,(1961). ) ( xi . Balmbra, R.R.; Clunie, J.S.; Corkill, J.M . ; Goodman,J.F.; Trans.Faraday Soc., 58, 1661, (1962). ) ( xii. Rosen, M.J.;Cohen, A.W.; Dahanayake, M.; Hua, X.Y.;J. Phys. Chem., 86,541, (1982). ) xiii.Becher, P.;J. Colloid Sci., 16, 49, (1961). xiv.Rosen, M.J.; Hua, X.Y.; J. Colloid Interface Sci., 86, 164,(1982). ( XV. Rubingh, D . N. in Solution Chemistry of Surfactants, Mittal, K.L., ed., Vol. 1 , Plenum, New York, (1979). ) ( xvi . Rosen M . J.; S urfactants and I nterfacial Phenomena, 2ndedition, John Wiley & Sons, New Y ork, (1989). ) KRUSS GmbH|Borsteler Chaussee Hamburg|Germany|www.kruss.de|
确定




还剩7页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《表面活性剂中协同作用检测方案(表面张力仪)》,该方案主要用于其他中协同作用检测,参考标准--,《表面活性剂中协同作用检测方案(表面张力仪)》用到的仪器有
相关方案
更多
该厂商其他方案
更多








