
方案详情
文
能源消耗增加需要提高原油回收率。由于化学试剂和环境问题费用高昂,优化采油过程和减少化学物质使用将有效节省成本。由于原油间的界面张力极低,乳液之间的界面张力测量需要用到特殊仪器。
方案详情

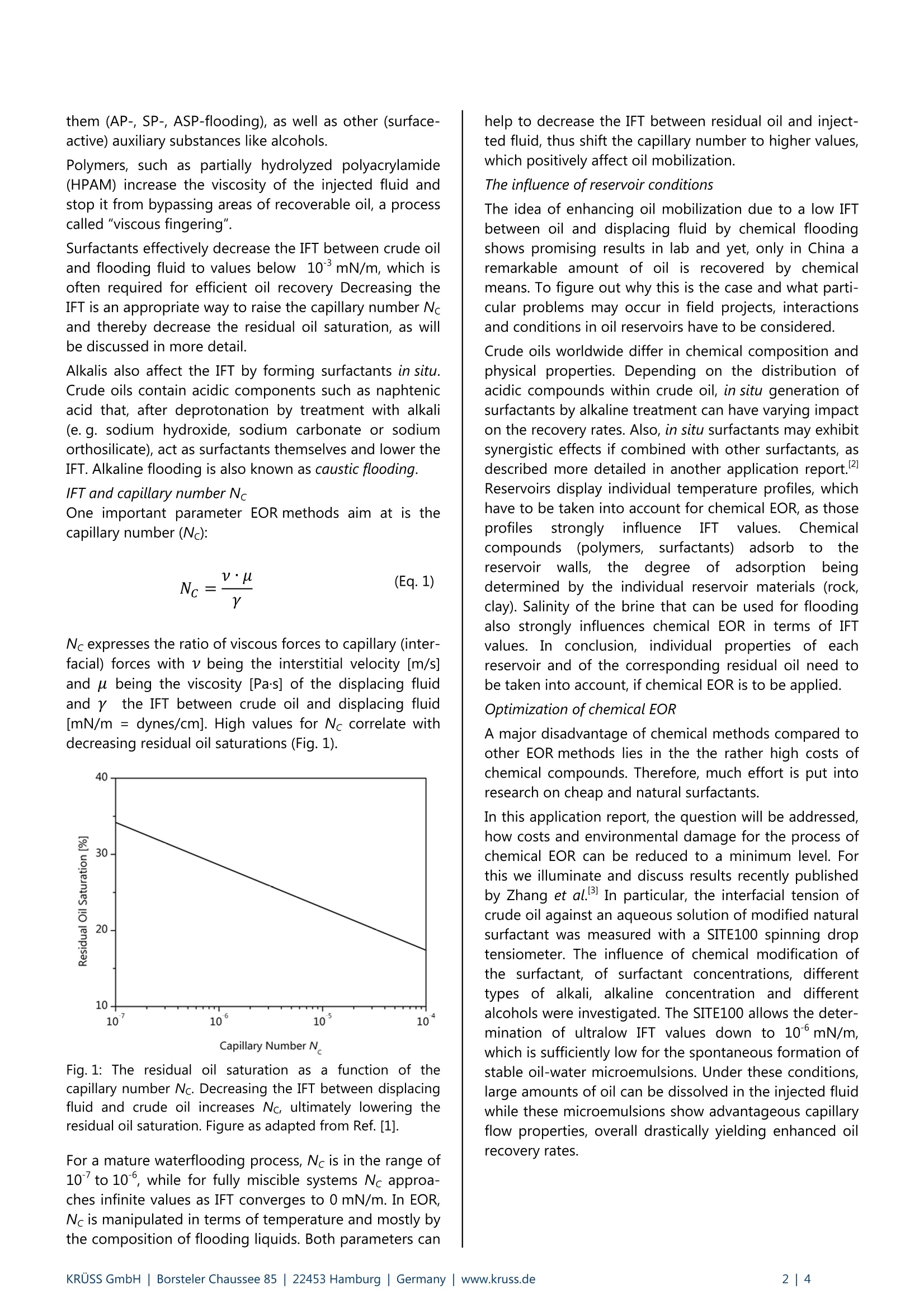
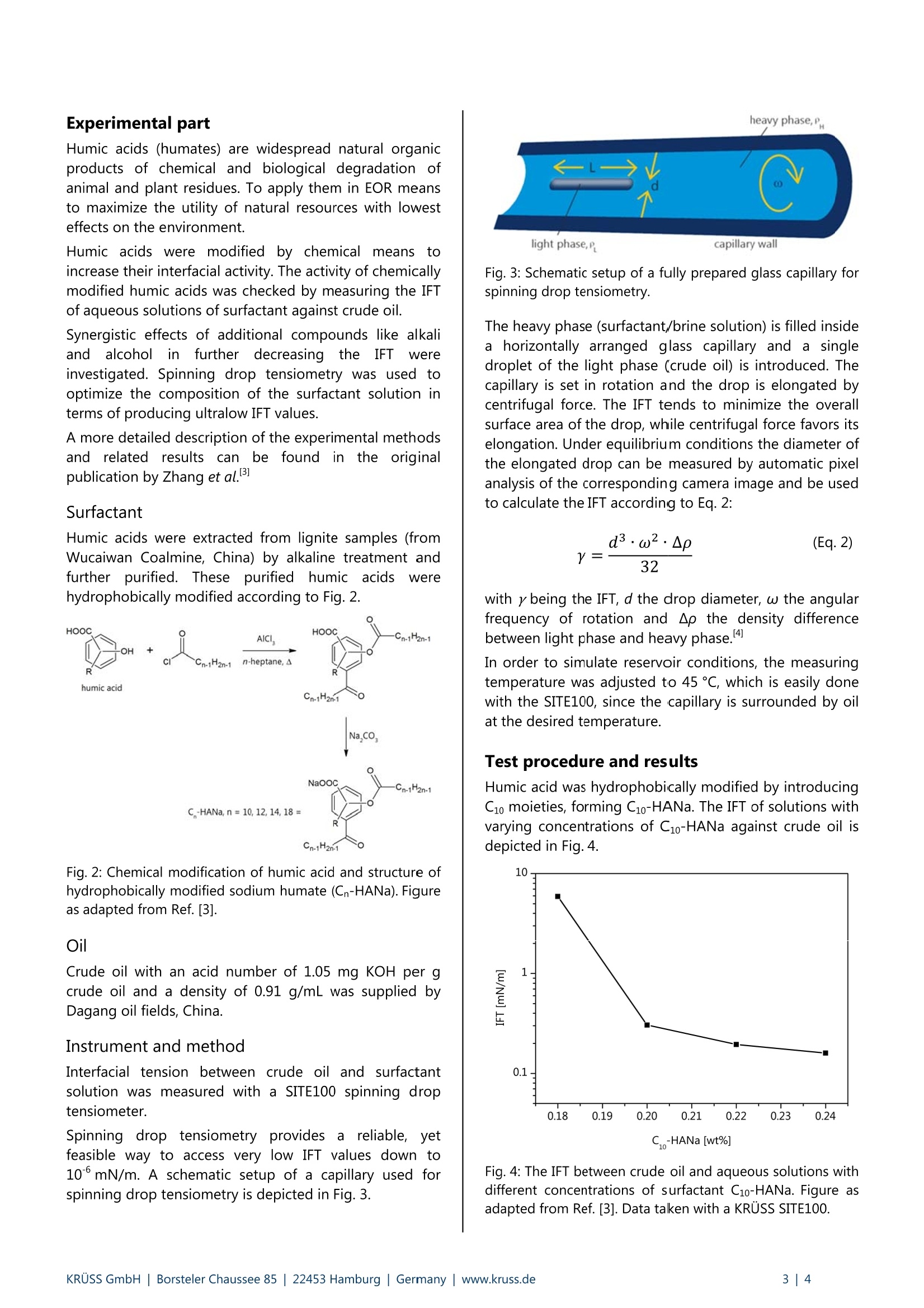
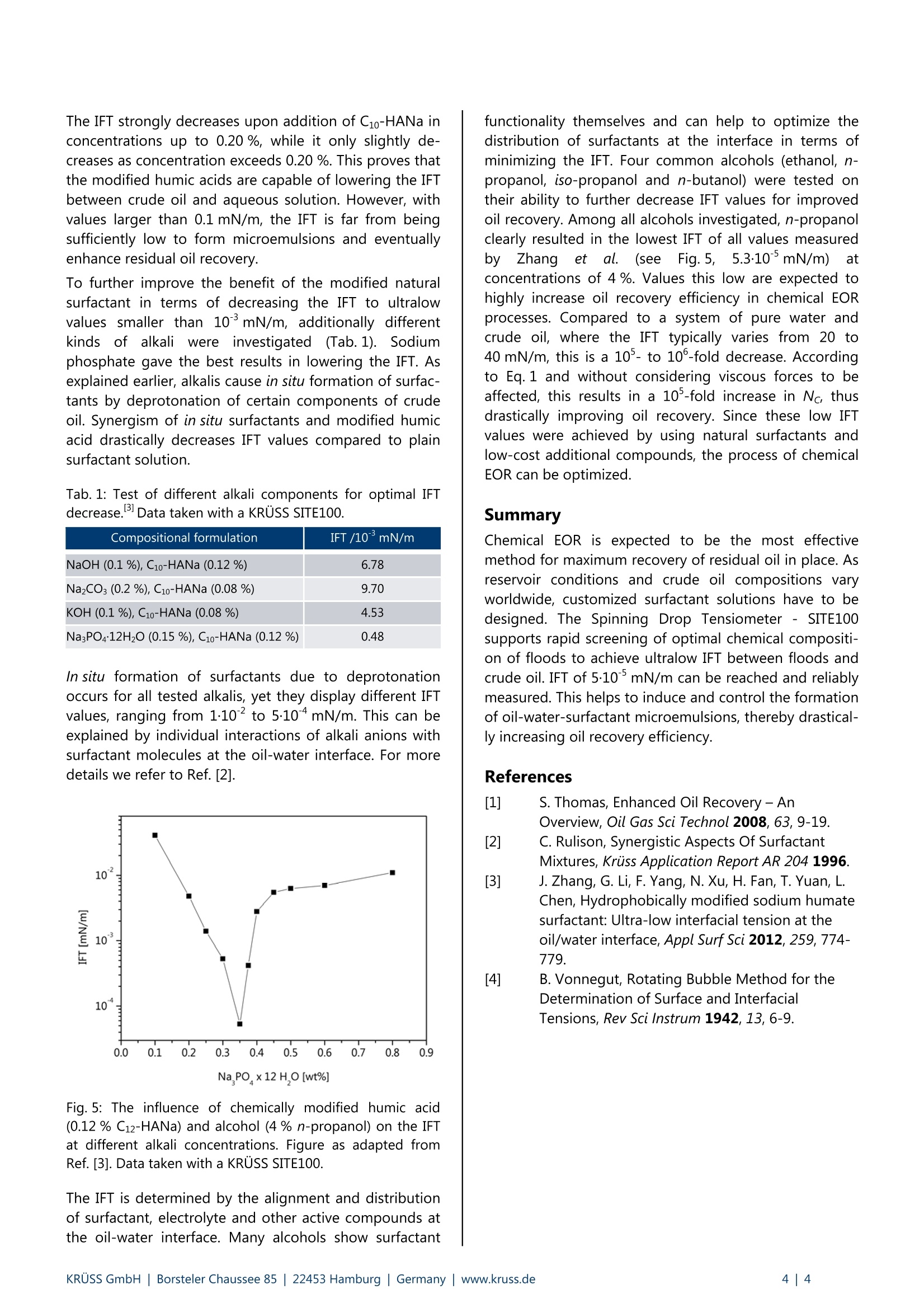
Application Report Ultralow Interfacial Tension in EOR UItralow interfacial tension in enhanced oil recovery (EOR) Keywords: enhanced oil recovery (EOR), ultralow interfacial tension, surfactant, chemical flooding (S, SP, ASP, micellar),crude oil, microemulsion, emulsion, brine Abstract Increasing energy demands require improved methods for enhanced oil recovery (EOR). Chemical EOR methods are mostpromising for maximum oil recovery, but, so far, limited due to high costs of chemicals and environmental concerns.Therefore, there is a huge interest in optimizing procedures for chemical EOR that minimize the amount of chemicalsneeded and rely on natural compounds, thus decreasing costs and environmental stress. In this application report we review how spinning drop tensiometry can be used to optimize liquid compositions forimproved oil recovery yields. Interfacial tension (IFT) between crude oil and aqueous solution of varying compositiondown to 5-10mN/m were reliably measured with a Spinning Drop Tensiometer- SITE100. At such low IFT values,microemulsions are formed that dramatically increase residual oil recovery. Background In oil recovery, conventional methods include the use ofthe reservoir's natural pressure (primary phase) and theinjection of water or gas (secondary phase) to push outthe crude oil to the surface. However, it is well knownthat reservoirs on average still contain about two-thirdsof the original oil-in-place (OOIP) after conventionalmethods have been applied and thus approximately2·102 barrels of oil remain unrecovered."To gain accessto this residual oil-in-place, much effort has been spentondeveloping tertiary phaseiettechniques,,:so-calledenhanced oil recovery (EOR) methods. These methodsmake use of heat or floods of well-defined compositionto mobilize residual oil. EOR methods A broad range of EOR methods have been introduced,several of which being tested in field projects. An over- view of EOR methods can be found in a review byS. Thomas. So far, due to high costs and environmentalconcerns, EOR only constitutes a small percentage oftotal oil production. However, improved technology andrising oil prices driven by tight supply and high energydemand are expected to increase tertiary oil production. Chemical EOR Of all reported methods, chemical EOR (flooding withwater containing chemical additives) is often declared tobe the most promising one for maximum recovery of the2.102 barrels of oil left underground.Chemical floodscontaining agents that increase oil mobilization areinjected into reservoirs and push out residual oil whichotherwise could not be recovered using primary orsecondary phase techniques. These floods may containsurfactant (S), alkali (A), polymer (P) or combinations of them (AP-, SP-, ASP-flooding), as well as other (surface-active) auxiliary substances like alcohols. Polymers, such as partially hydrolyzed polyacrylamide(HPAM) increase the viscosity of the injected fluid andstop it from bypassing areas of recoverable oil, a processcalled "viscous fingering". Surfactants effectively decrease the IFT between crude oiland flooding fluid to values below 10mN/m, which isoften required for efficient oil recovery Decreasing theIFT is an appropriate way to raise the capillary number Ncand thereby decrease the residual oil saturation, as willbe discussed in more detail. Alkalis also affect the IFT by forming surfactants in situ.Crude oils contain acidic components such as naphtenicacid that, after deprotonation by treatment with alkali(e. g. sodium hydroxide, sodium carbonate or sodiumorthosilicate), act as surfactants themselves and lower theIFT. Alkaline flooding is also known as caustic flooding. IFT and capillary number Nc One important parameter EOR methods aim at is thecapillary number (Nc): Nc expresses the ratio of viscous forces to capillary (inter-facial) forces with v being the interstitial velocity [m/s]and u being the viscosity [Pa·s] of the displacing fluidand ythe IFT between crude oil and displacing fluid[mN/m= dynes/cm]. High values for Nc correlate withdecreasing residual oil saturations (Fig. 1). Fig. 1: The residual oil saturation as a function of thecapillary number Nc.Decreasing the IFT between displacingfluid and crude oil increases Nc, ultimately lowering theresidual oil saturation. Figure as adapted from Ref.[1]. For a mature waterflooding process, Nc is in the range of10"’to 10, while for fully miscible systems Nc approa-ches infinite values as IFT converges to 0 mN/m. In EOR,Nc is manipulated in terms of temperature and mostly bythe composition of flooding liquids. Both parameters can help to decrease the IFT between residual oil and inject-ted fluid, thus shift the capillary number to higher values,which positively affect oil mobilization. The influence of reservoir conditions The idea of enhancing oil mobilization due to a low IFTbetween oil and displacing fluid by chemical floodingshows promising results in lab and yet, only in China aremarkable amount of oil is recovered by chemicalmeans. To figure out why this is the case and what parti-cular problems may occur in field projects, interactionsand conditions in oil reservoirs have to be considered. Crude oils worldwide differ in chemical composition andphysical properties. Depending on the distribution ofacidic compounds within crude oil, in situ generation ofsurfactants by alkaline treatment can have varying impacton the recovery rates. Also, in situ surfactants may exhibitsynergistic effects if combined with other surfactants, asdescribed more detailed in another application report.Reservoirs display individual temperature profiles, whichhave to be taken into account for chemical EOR, as thoseprofiles stronglyiiinfluenceIFT values. Chemicalcompounds(polymers,surfactants)) iadsorbi to thereservoirrwalls,theedegree:eoff aadsorption1beingdetermined by the individual reservoir materials (rock,clay). Salinity of the brine that can be used for floodingalso strongly influences chemical EOR in terms of IFTvalues. In conclusion, individualproperties of eachreservoir and of the corresponding residual oil need tobe taken into account, if chemical EOR is to be applied. Optimization of chemical EOR A major disadvantage of chemical methods compared toother EOR methods lies in the the rather high costs ofchemical compounds. Therefore, much effort is put intoresearch on cheap and natural surfactants. In this application report, the question will be addressed,how costs and environmental damage for the process ofchemical EOR can be reduced to a minimum level. Forthis we illuminate and discuss results recently publishedby Zhang et al.3 In particular, the interfacial tension ofcrude oil against an aqueous solution of modified naturalsurfactant was measured with a SITE100 spinning droptensiometer. The influence of chemical modification ofthe surfactant, of surfactant concentrations, differenttypes of alkali, alkaline concentration and differentalcohols were investigated. The SITE100 allows the deter-mination of ultralow IFT values down to 10mN/m,which is sufficiently low for the spontaneous formation ofstable oil-water microemulsions. Under these conditions,large amounts of oil can be dissolved in the injected fluidwhile these microemulsions show advantageous capillaryflow properties, overall drastically yielding enhanced oilrecovery rates. Experimental part Humic acids (humates) are widespread natural organicproducts of chemical and biological degradation ofanimal and plant residues. To apply them in EOR meansto maximize the utility of natural resources with lowesteffects on the environment. Humic acids were modified by chemical means toincrease their interfacial activity. The activity of chemicallymodified humic acids was checked by measuring the IFTof aqueous solutions of surfactant against crude oil. Synergistic effects of additional compounds like alkaliand alcohol in further decreasingthe IFTWereinvestigated. Spinning drop tensiometry was used tooptimize the composition of the surfactant solution interms of producing ultralow IFT values. A more detailed description of the experimental methodsand related results can be found in the originalpublication by Zhang et al. Surfactant Humic acids were extracted from lignite samples (fromWucaiwan Coalmine, China) by alkaline treatment andfurther purified. These purifiedhumic acids werehydrophobically modified according to Fig. 2. Fig. 2: Chemical modification of humic acid and structure ofhydrophobically modified sodium humate (Cn-HANa). Figureas adapted from Ref. [3]. Oil Crude oil with an acid number of 1.05 mg KOH per gcrude oil and a density of 0.91 g/mL was supplied byDagang oil fields, China. Instrument and method Interfacial tension between crude oil and surfactantsolution was measured with a SITE100 spinning droptensiometer. Spinning(drop tensiometry provides a reliable, yetfeasible way to access very low IFT values down to10°mN/m. A schematic setup of a capillary used forspinning drop tensiometry is depicted in Fig. 3. Fig.3: Schematic setup of a fully prepared glass capillary forspinning drop tensiometry. The heavy phase (surfactant/brine solution) is filled insidea horizontally arranged glass capillary and a singledroplet of the light phase (crude oil) is introduced. Thecapillary is set in rotation and the drop is elongated bycentrifugal force. The IFT tends to minimize the overallsurface area of the drop, while centrifugal force favors itselongation. Under equilibrium conditions the diameter ofthe elongated drop can be measured by automatic pixelanalysis of the corresponding camera image and be usedto calculate the IFT according to Eq. 2: with y being the IFT, d the drop diameter, w the angularfrequency of rotation and Ap the density differencebetween light phase and heavy phase. In order to simulate reservoir conditions, the measuringtemperature was adjusted to 45℃, which is easily donewith the SITE100, since the capillary is surrounded by oilat the desired temperature. Test procedure and results Humic acid was hydrophobically modified by introducingC1o moieties, forming C10-HANa. The IFT of solutions withvarying concentrations of C10-HANa against crude oil isdepicted in Fig.4. Fig. 4: The IFT between crude oil and aqueous solutions withdifferent concentrations of surfactant C10-HANa. Figure asadapted from Ref. [3]. Data taken with a KRUSS SITE100. The IFT strongly decreases upon addition of C10-HANa inconcentrations up to 0.20 %, while it only slightly de-creases as concentration exceeds 0.20 %. This proves thatthe modified humic acids are capable of lowering the IFTbetween crude oil and aqueous solution. However, withvalues larger than 0.1 mN/m, the IFT is far from beingsufficiently low to form microemulsions and eventuallyenhance residual oil recovery. To further improve the benefit of the modified naturalsurfactant in terms of decreasing the IFT to ultralowvalues smaller than 10mN/m, additionally differentkinds;(ofalkaliwereirinvestigated(Tab.1). Sodiumphosphate gave the best results in lowering the IFT. Asexplained earlier, alkalis cause in situ formation of surfac-tants by deprotonation of certain components of crudeoil. Synergism of in situ surfactants and modified humicacid drastically decreases IFT values compared to plainsurfactant solution. Tab. 1: Test of different alkali components for optimal IFTdecrease.B Data taken with a KRUSS SITE100. Compositional formulation IFT /10mN/m NaOH (0.1%), C10-HANa (0.12 %) 6.78 NazCO3(0.2%), C10-HANa (0.08%) 9.70 KOH (0.1%), C10-HANa (0.08%) 4.53 Na3PO412H2O (0.15%), C10-HANa (0.12%) 0.48 In situ formation of surfactants due to deprotonationoccurs for all tested alkalis, yet they display different IFTvalues, ranging from 1.10to 5·10mN/m. This can beexplained by individual interactions of alkali anions withsurfactant molecules at the oil-water interface. For moredetails we refer to Ref. [2]. Fig. 5: The influence of chemically modified humic acid(0.12 % C12-HANa) and alcohol (4% n-propanol) on the IFTat different alkali concentrations. Figure as adapted fromRef. [3]. Data taken with a KRUSS SITE100. The IFT is determined by the alignment and distributionof surfactant, electrolyte and other active compounds atthe oil-water interface. Many alcohols show surfactant functionality themselves and can help to optimize thedistribution of surfactants at the interface in terms ofminimizing the IFT. Four common alcohols (ethanol, n-propanol, iso-propanol and n-butanol) were tested ontheir ability to further decrease IFT values for improvedoil recovery. Among all alcohols investigated, n-propanolclearly resulted in the lowest IFT of all values measuredby/Zhangetetal. (seeFig.5,5.3.10*5mN/m) atconcentrations of 4 %. Values this low are expected tohighly increase oil recovery efficiency in chemical EORprocesses. Compared to a system of pure water andcrude oil, where the IFT typically varies from 20 to40 mN/m, this is a 10-to 10-fold decrease. Accordingto Eq.1 and without considering viscous forces to beaffected, this results in a 10-fold increase in Nc, thusdrastically improving oil recovery. Since these low IFTvalues were achieved by using natural surfactants andlow-cost additional compounds, the process of chemicalEOR can be optimized. Summary Chemical EOR is expected to be the most effectivemethod for maximum recovery of residual oil in place. Asreservoir conditions and crude oil compositions varyworldwide, customized surfactant solutions have to bedesigned. The Spinning Drop Tensiometer - SITE100supports rapid screening of optimal chemical compositi-on of floods to achieve ultralow IFT between floods andcrude oil. IFT of 510mN/m can be reached and reliablymeasured. This helps to induce and control the formationof oil-water-surfactant microemulsions, thereby drastical-ly increasing oil recovery efficiency. References [1] S. Thomas, Enhanced Oil Recovery - An Overview, Oil Gas Sci Technol 2008,63,9-19. [21 C.Rulison, Synergistic Aspects Of SurfactantMixtures, Kruss Application Report AR 2041996. [3] J. Zhang,G. Li, F. Yang, N. Xu, H. Fan, T. Yuan, L.Chen, Hydrophobically modified sodium humatesurfactant: Ultra-low interfacial tension at theoil/water interface, Appl SurfSci 2012, 259,774-779. [4] B. Vonnegut, Rotating Bubble Method for theDetermination of Surface and InterfacialTensions, Rev Sci Instrum 1942, 13, 6-9. RUSS GmbH|Borsteler Chaussee | Hamburg| Germany|www.kruss.de
确定
还剩2页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《原油中界面张力检测方案(表面张力仪)》,该方案主要用于原油中界面张力检测,参考标准--,《原油中界面张力检测方案(表面张力仪)》用到的仪器有KRUSS SDT旋转滴界面张力仪
推荐专场
相关方案
更多
该厂商其他方案
更多















