
方案详情
文
Continuous isothermal titration calorimetry (cITC) is ideal for studying rapid binding events typical of many biological recognition and binding reactions. The binding constant, stoichiometry and enthalpy of the reaction are accurately determined in significantly less time than that required using incremental isothermal titration calorimetry.
方案详情

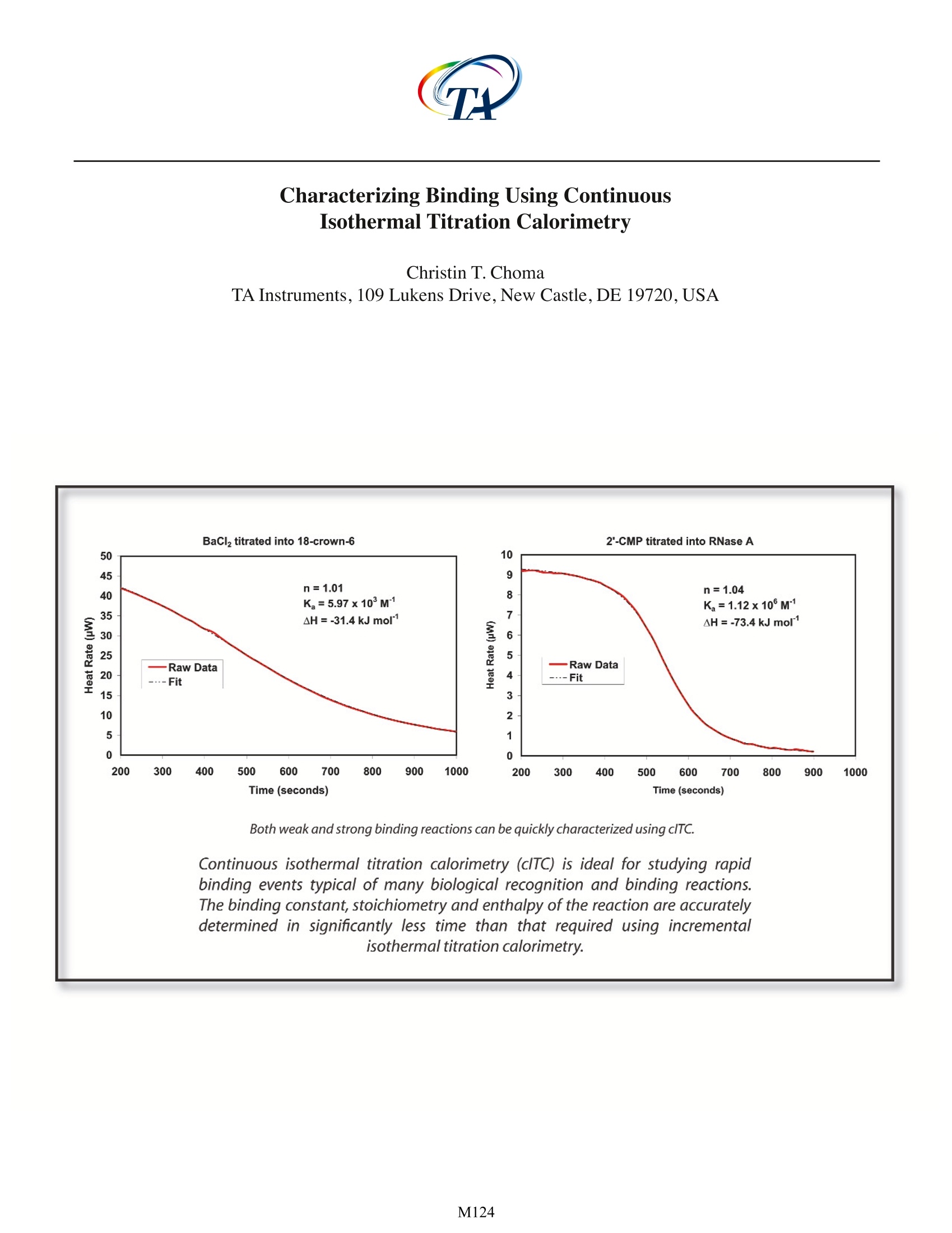
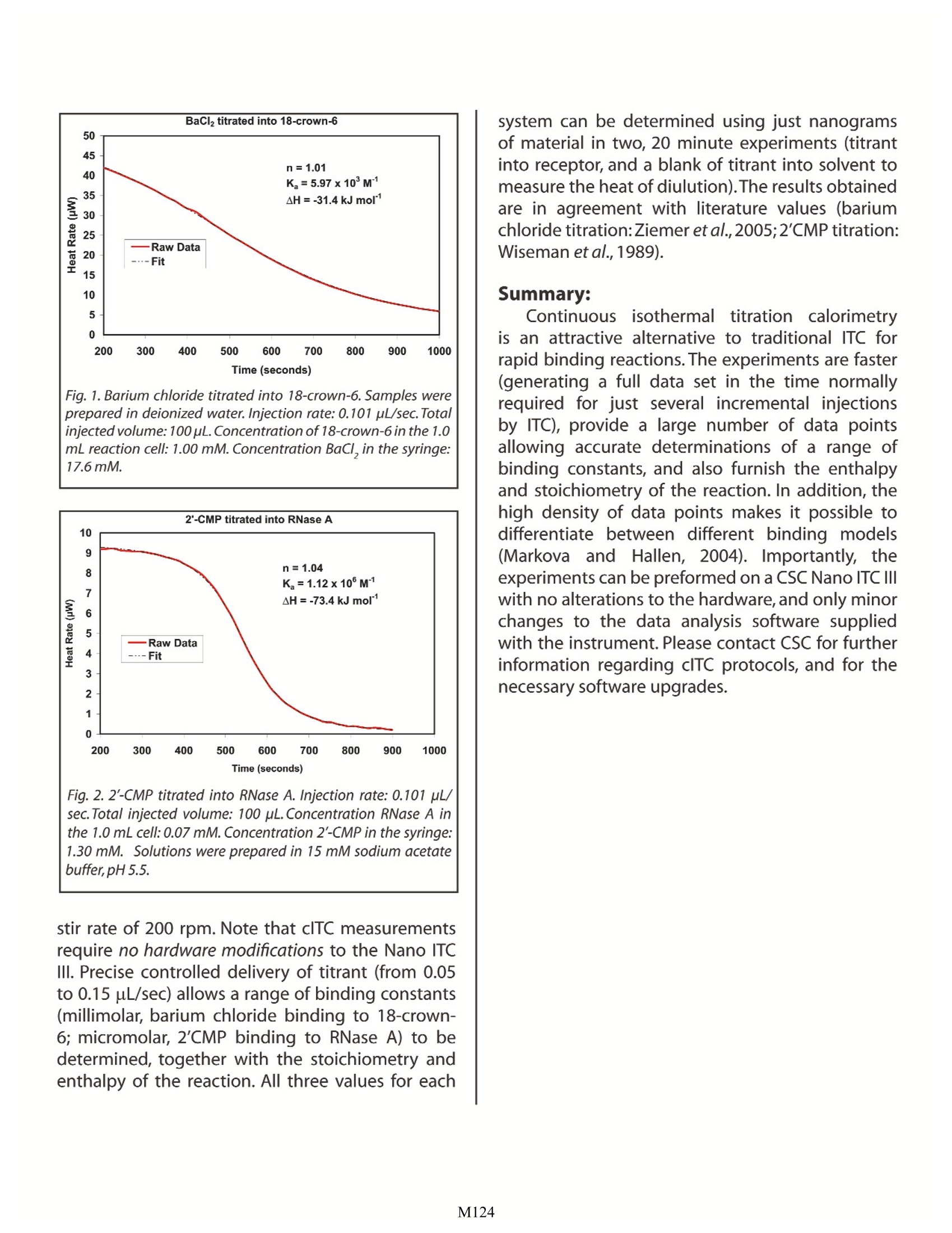
Characterizing Binding Using ContinuousIsothermal Titration Calorimetry Christin T. Choma TA Instruments, 109 Lukens Drive,New Castle, DE 19720, USA tructuralbiologyanddpproteomicshaveprovided an architecturalframeworkfforunderstanding how the three dimensionalstructure of a protein, controlled by an intricatenetworkofrnumerous weak non-covalentinteractions, directstherecognition, binding,catalysis and transport reactions characteristicof living systems. As the number and diversityof high resolutionn sStructuress increasesitisbecomingl apparent thatunderstanding themolecular mechanisms regulating these dynamicintermolecular events requires not only structuralknowledge of the participating species, but also anunderstanding of the underlying thermodynamicsand kinetics controlling each process. Isothermal titration calorimetry (ITC) is a rapid,sensitive and universally-applicable technique forstudying the thermodynamics controlling bindingequilibria. By investigating the thermodynamicproperties of a system as a function of temperature,pH, ionic strength or ligand concentration in aseries of ITC experiments, the contributions ofspecific intermolecular interactions to the overallfree energy of a process can be revealed. Specificrecognition and binding reactions between a ligand(e.g., a drug) and receptor (target macromolecule)are fundamental to the development of efficaciouspharmaceuticals, so methodological developmentswhich enhance the sensitivity or versatility ofITC measurements are of considerable practicalinterest. This Application Note describes the utility of onesuch development, continuous isothermal titrationcalorimetry (clTC). For a general description of theprinciples behind isothermal titration calorimetryand the types of biological problems that can beaddressed, please see CSC's Overview Note entitledLife Science Applications of ITC. The principle behind clTC: As described in CSC’s Application Note entitled'Characterizing Binding Interactions by ITC, ITCallows the binding constant (K ),enthalpy (AH) andstoichiometry (n) of a reaction to be calculated froma single experiment provided the concentration of both the macromolecule and ligand are accuratelyknown and fall within the range: where [M.]isthe: totalconcentration (macromolecule in the sample cell titrated by ligand(Wiseman et al., 1989). In an ITC experiment, 20-30 small aliquots of titrant are added in discreteincremental steps over a period of 50-80 minutes.In contrast, in a clTC experiment, binding betweena ligand and receptor molecule is studied by slowlyandcontinuouslytitratingonereactantintotheotherover a period of 15-20 minutes.Although traditionalITCremains the method of choice for characterizingbinding events occurring over a period of seconds,clTC can be advantageous for studying very rapidbinding reactions typical of many biologicalinteractions. The primary advantage of clTC is thatapproximately a thousand data points are obtainedfrom a single titration experiment, compared to the20-30 data points obtained from an ITC experiment.Since the binding constant has a profound effecton the shape of the titration curve (see Fig.2 inthe CSC application note entitled 'CharacterizingBinding Interactions by ITC'), sufficient data pointsare necessary to allow precise description of thecurve and accurate determination of K and n.Using incremental titration, a single data point lyingabove or below the curve could be regarded as ananomaly and discarded, whereas the multiple datapoints obtained by clTC would clearly show anydeviation from the main trend of the curve.Thus,clTC can be invaluable for discriminating betweenvarious binding models, or identifying the correctmulti-component model. Continuous titration calorimetry has been anestablished technique since the 1960's (lzatt et al.,1966) but until recently (Markova and Hallen, 2004)was rarely applied to biological samples despitethe physiologically-relevant range of equilibriumconstants accessible by the technique. Two datasets illustrating applications of the clTC techniqueare presented in Figs. 1 and 2. All experiments wereperformed on a CSC Nano ITC III at 25 ℃ and a Fig. 1. Barium chloridetitrated into 18-crown-6. Samples wereprepared in deionized water. Injection rate: 0.101 pL/sec.Totalinjected volume: 100 pL.Concentration of 18-crown-6 in the 1.0mL reaction cell: 1.00 mM. Concentration BaCl, in the syringe:17.6mM. Fig. 2. 2'-CMP titrated into RNase A. Injection rate: 0.101 pL/sec.Total injected volume: 100 pL.Concentration RNase A inthe 1.0 mL cell: 0.07 mM. Concentration 2'-CMP in the syringe:1.30 mM. Solutions were prepared in 15 mM sodium acetatebuffer,pH 5.5. stir rate of 200 rpm. Note that clTC measurementsrequire no hardware modifications to the Nano ITCⅢI. Precise controlled delivery of titrant (from 0.05to 0.15 uL/sec) allows a range of binding constants(millimolar, barium chloride binding to 18-crown-6; micromolar,2'CMP binding to RNase A) to bedetermined, together with the stoichiometry andenthalpy of the reaction. All three values for each system can be determined using just nanogramsof material in two, 20 minute experiments (titrantinto receptor, and a blank of titrant into solvent tomeasure the heat of diulution).The results obtainedare in agreement with literature values (bariumchloride titration:Ziemer et al.,2005;2'CMP titration:Wiseman et al.,1989). Summary: Continuousisothermal titration calorimetryis an attractive alternative to traditional ITC forrapid binding reactions. The experiments are faster(generating a full data set in the time normallyrequired for just several incremental injectionsby ITC), provide a large number of data pointsallowing accurate determinations of a range ofbinding constants, and also furnish the enthalpyand stoichiometry of the reaction. In addition, thehigh density of data points makes it possible todifferentiate between different binding models(Markova and Hallen, 2004). Importantly, theexperiments can be preformed on a CSC Nano ITC IIIwith no alterations to the hardware, and only minorchanges to the data analysis software suppliedwith the instrument. Please contact CSC for furtherinformation regarding clTC protocols, and for thenecessary software upgrades. References: (Preference has been given to current references. Citation doesnot imply that a paper is necessarily the original reference to astudy.) Izatt, R. M., J. H. Rytting,L. D. Hansen and J.J. Christensen.(1966) Thermodynamics of proton dissociationin dilute aqueous solution. V. An entropy studyof adenosine, pentoses,s, hexosesand relatedcompounds.J.Am.Chem.Soc.88,2641-2645. Markova, N. and D. Hallen.(2004) The development of acontinuous isothermal titration calorimetric methodfor equilibrium studies. Anal. Biochem. 331, 77-88. Wiseman, T., S. Williston, J. F. Brants and L. N. Lin. (1989)Rapid measurement of binding constants and heatsof binding using a new titration calorimeter. Anal.Biochem.179,131-137. Ziemer, S. P., T. L. Niederhauser, E. M. Woolley. (2005)Thermodynamics of complexation of aqueous 18-crown-6 with barium ion; apparent molar volumesand apparent molar heat capacities of aqueous (18-crown-6+ barium nitrate) at temperatures (298.15 to393.15) K, at molalities (0.02 to 0.33) mol· kg,and atthe pressure 0.35 MPa, J. Chem. Thermodynamics 37,984-995. M Continuous isothermal titration calorimetry (cITC) is ideal for studying rapid binding events typical of many biological recognition and binding reactions. The binding constant, stoichiometry and enthalpy of the reaction are accurately determined in significantly less time than that required using incremental isothermal titration calorimetry.
确定


还剩2页未读,是否继续阅读?
TA仪器为您提供《蛋白质中结合检测方案(差示扫描量热)》,该方案主要用于其他中结合检测,参考标准--,《蛋白质中结合检测方案(差示扫描量热)》用到的仪器有TA仪器+Affinity ITC+等温滴定微量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多















