
方案详情
文
DSC is the most direct and sensitive approach for characterizing the thermodynamic parameters controlling noncovalent bond formation (and therefore stability) in proteins and other macromolecules. In an experiment requiring only a few micrograms of material, the protein is thermally unfolded, allowing the relationship between enthalpy and entropy of the denaturation process to be established in about one hour. Correlating thermodynamic properties to stability is necessary for the rational design of engineered proteins and protein therapeutics.
方案详情

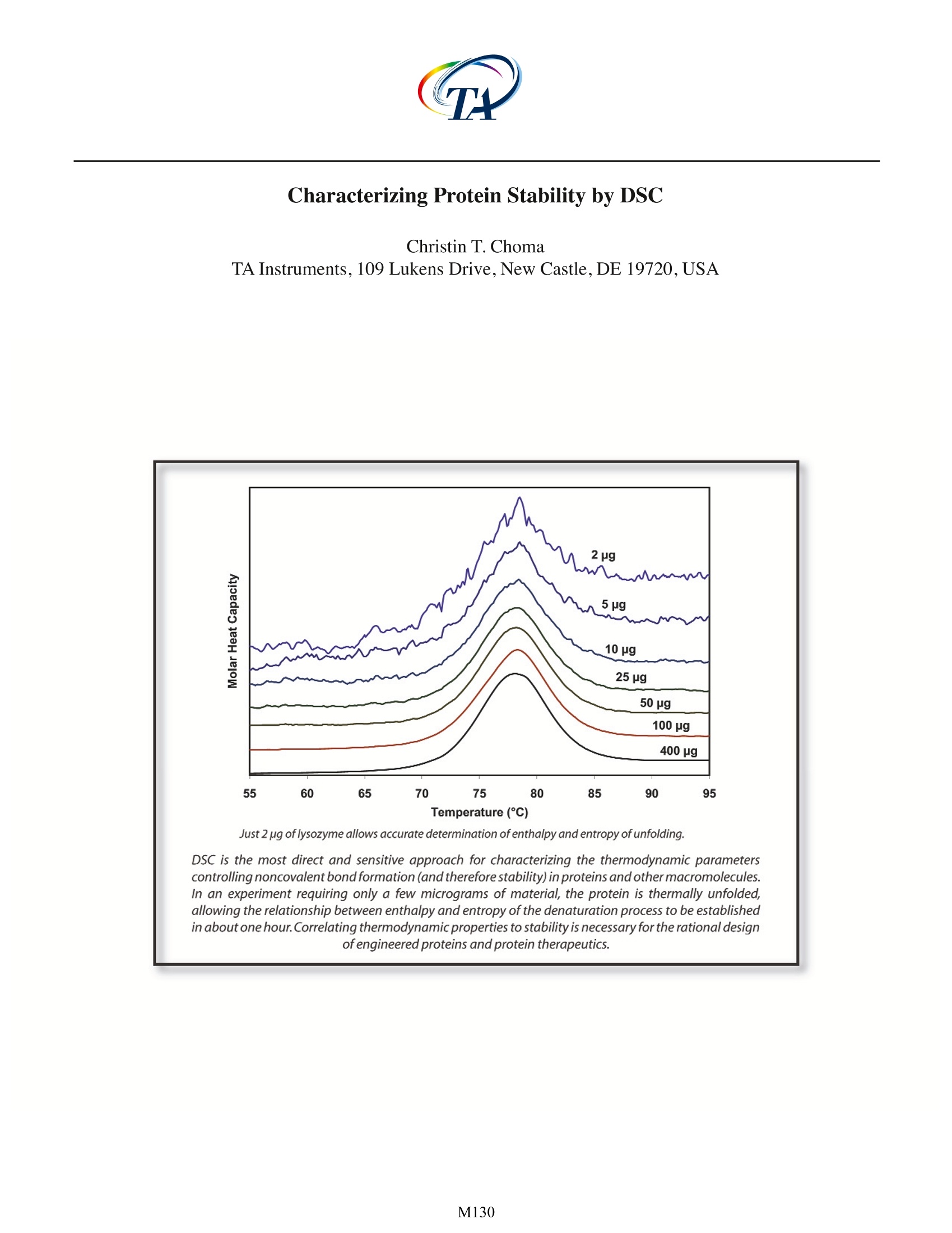
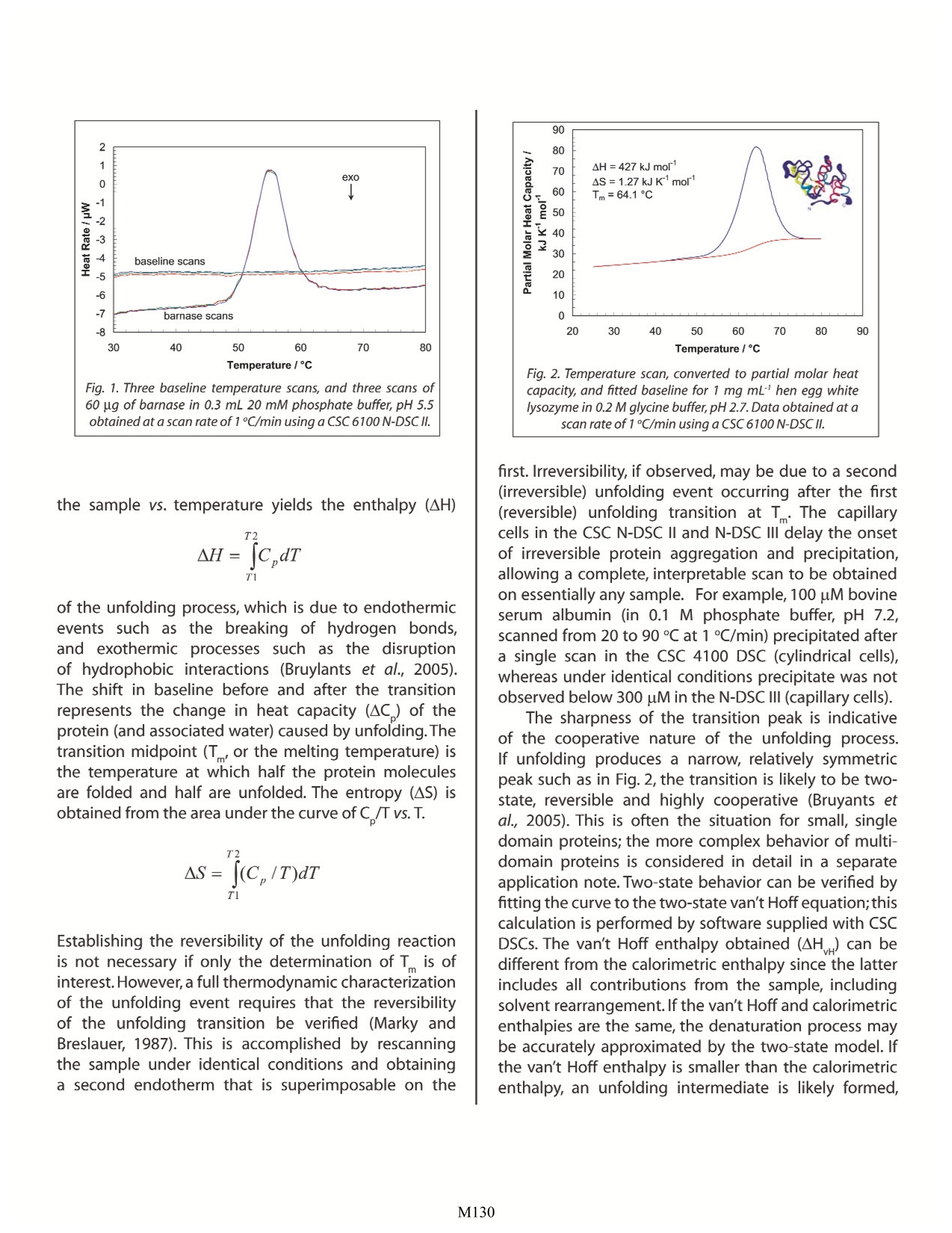
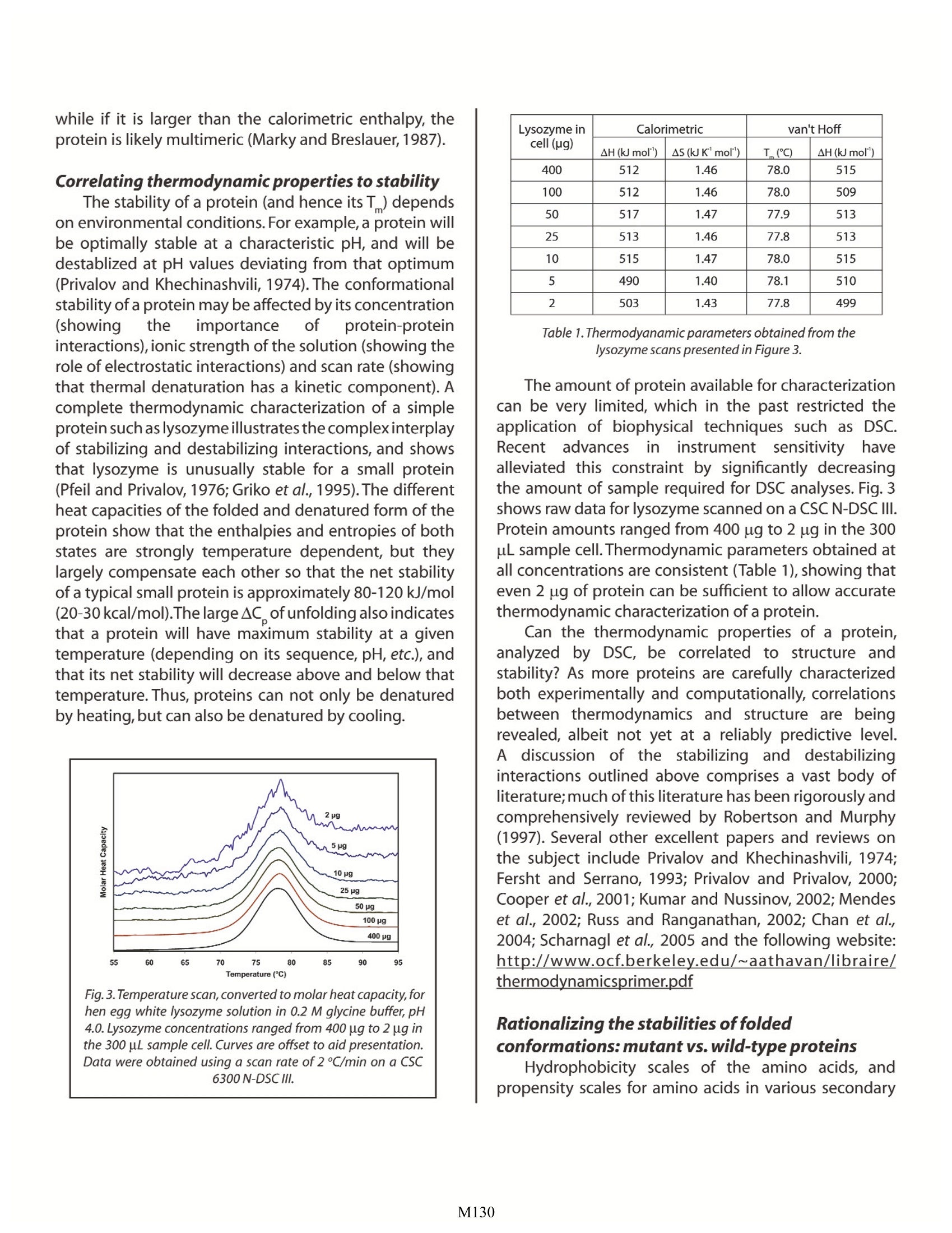
Characterizing Protein Stability by DSC Christin T. Choma TA Instruments, 109 Lukens Drive,New Castle, DE 19720, USA in about one hour.Correlating thermodynamic properties to stability is necessary for the rational design ofengineered proteins and protein therapeutics. ' ifty years ago,the elegant experiments of ChristianAnfinsen showed unequivocally that the linearsequence of amino acids of a protein (its primarystructure) encodes all the information for its threedimensional structure (Sela et al., 1957;Anfinsen, 1973).It is now clear that the sequence directs the interactionsboth between component amino acids, and betweenthe protein and the solvent, and that these interactionsare governed by thermodynamics. What is not clearis how the sequence encodes the complex structureof a protein. The thermodynamic balance in proteinsis delicate: the folded structure of a small protein isstabilized by approximately 80-120 kJ/mol (20-30 kcal/mol) compared to its unfolded structure, and manyproteins thermally unfold by 70C. If most proteins canbe denatured by relatively small increases in temperature,and if the balance between folded and unfolded states isheld by the equivalent of 5 -8 hydrogen bonds,how arethermophilic proteins stabilized, allowing organisms tothrive at elevated pressures and at temperatures above100℃(Perl and Schmid,2002)? In the absence of thermalchanges, what thermodynamic events trigger proteinsthat cause amyloid and prion diseases to switch from onestable conformation to another, with fatal consequences(Mastrianni, 2004)? Would a thorough comprehensionof the interactions controlling conformation permitthe full complement of three dimensional proteinstructures to be accurately predicted from an organism'sgenome (Webster 2000)? Achieving a fundamentalunderstanding of the thermodynamics directing theformation ofmacromolecular noncovalent:bondswould answer these questions, and critically impactsuch diverse endeavors as the self-assembly of protein-inspired supramolecular structures (Yeates and Padilla,2002),the composition of shape-memory biocompatiblepolymers (Langer and Tirrell, 2004), the rational designof protein-specific small molecule drugs (Waldron andMurphy, 2003) and the formulation of stable proteintherapeutics (Remmele and Bombotz,2000). The thermodynamic paran rstthatt controlnoncovalentabbondfformation in proteins andother macromolecules can be directly studied andquantified by differential scanning calorimetry (DSC).This Application Note examines the utility of DSC forcharacterizing protein stability. For a general descriptionof the principles behind DSC, how a DSC experimentis conducted and interpreted, and the technique's versatility for studying biological problems, please seeCSC's Overview Note entitled 'Life science applicationsof DSC. A second calorimetric approach,isothermal titrationcalorimetry (ITC), is particularly suited to measuringdynamic events such as binding and kinetics. The ITCtechnique and its utility in biophysical research aredescribed in a separate set of CSC application notes. Analyzing protein thermalstability As described in the DSC Overview Note, analyzingthe stability of a protein in dilute solution involvesdetermining changes in the partial molar heat capacityof the protein at constant pressure (AC). The changein heat capacity of a compound reflects its ability toabsorb heat and experience a defined increase intemperature. Because of its extensive hydrogen bondnetwork, water has a substantially higher heat capacitythan organic compounds such as proteins. In addition,water is more ordered and tightly packed adjacent tohydrophobic patches on the protein's surface: sincewater cannot make hydrogen bonds; to nonpolarmoieties, hydrogen bonding between water moleculesis maximized (clathrate formation) at the protein-solventinterface (Shinoda, 1977). As the temperature is raised,the ordered water shell around the protein starts todisorder and become more like bulk water.This 'melting'produces the anomalously large heat capacity of proteinaqueous solutions.The contribution of the protein to thecalorimetrically measured heat capacity (its partial C) isdetermined by subtracting a scan of a buffer blank fromthe sample data prior to analysis. The partial C is notjust the heat capacity of the protein, but also includesany effects the protein had on the solvent, such aspromoting clathrate formation (Freire,1995).Heating theprotein sample initially produces a slightlyincreasingbaseline but as heating progresses, heat is absorbedby the protein and causes it to thermally unfold over atemperature range characteristic for that protein, givingrise to an endothermic peak (Fig. 1).During the unfoldingprocess, water molecules around the protein reorganizeand restructure asiSmore non-polar sidechains areexposed. Once unfolding is complete, heat absorptiondecreases and a new baseline is established. Afterblank subtraction, the data can be analyzed to providea complete thermodynamic characterization of theunfolding process. Integration of the heat capacity of Fig. 1. Three baseline temperature scans, and three scans of60 pg of barnase in 0.3 mL 20 mM phosphate buffer, pH 5.5obtained at a scan rate of 1C/min using a CSC 6100 N-DSCⅡ. the sample vs. temperature yields the enthalpy (AH) of the unfolding process, which is due to endothermicevents such as the breaking of hydrogen bonds,and exothermic processes such as the disruptionof hydrophobic interactions (Bruylants et al., 2005).The shift in baseline before and after the transitionrepresents the change in heat capacity (AC ) of theprotein (and associated water) caused by unfolding. Thetransition midpoint (T, or the melting temperature) isthe temperature at which half the protein moleculesare folded and half are unfolded. The entropy (AS) isobtained from the area under the curve of C /T vs. T. Establishing the reversibility of the unfolding reactionis not necessary if only the determination ofT is ofinterest. However, a full thermodynamic characterizationof the unfolding event requires that the reversibilityof the unfolding transition be verified (Marky andBreslauer, 1987). This is accomplished by rescanningthe sample under identical conditions and obtaininga second endotherm that is superimposable on the Fig. 2. Temperature scan, converted to partial molar heatcapacity, and fitted baseline for 1 mg mL’hen egg whitelysozyme in 0.2 M glycine buffer, pH 2.7. Data obtained at ascan rate of 1C/min using a CSC 6100 N-DSC/. first.Irreversibility, if observed, may be due to a second(irreversible) unfolding event occurring after the first(reversible) unfolding transition at T . The capillarycells in the CSC N-DSC II and N-DSC III delay the onsetof irreversible protein aggregation and precipitation,allowing a complete, interpretable scan to be obtainedon essentially any sample. For example, 100 uM bovineserum albumin (in 0.1 M phosphate buffer, pH 7.2,scanned from 20 to 90 C at 1C/min) precipitated aftera single scan in the CSC 4100 DSC (cylindrical cells),whereas under identical conditions precipitate was notobserved below 300 uM in the N-DSC III (capillary cells). The sharpness of the transition peak is indicativeof the cooperative nature of the unfolding process.If unfolding produces a narrow, relatively symmetricpeak such as in Fig. 2, the transition is likely to be two-state, reversible and highly cooperative (Bruyants etal., 2005). This is often the situation for small, singledomain proteins; the more complex behavior of multi-domain proteins is considered in detail in a separateapplication note. Two-state behavior can be verified byfitting the curve to the two-state van't Hoff equation;thiscalculation is performed by software supplied with CSCDSCs. The van’t Hoff enthalpy obtained (AH ) can bedifferent from the calorimetric enthalpy since the latterincludes all contributions from the sample, includingsolvent rearrangement. If the van't Hoff and calorimetricenthalpies are the same, the denaturation process maybe accurately approximated by the two-state model. Ifthe van't Hoff enthalpy is smaller than the calorimetricenthalpy, an unfolding intermediate is likely formed, while if it is larger than the calorimetric enthalpy, theprotein is likely multimeric (Marky and Breslauer,1987). Correlating thermodynamic properties to stability The stability of a protein (and hence its T) dependson environmental conditions. For example,a protein willbe optimally stable at a characteristic pH, and will bedestablized at pH values deviating from that optimum(Privalov and Khechinashvili, 1974). The conformationalstability of a protein may be affected by its concentration(showing the: iimportanceoff protein-proteininteractions), ionic strength of the solution (showing therole of electrostatic interactions) and scan rate (showingthat thermal denaturation has a kinetic component). Acomplete thermodynamic characterization of a simpleprotein such as lysozyme illustrates the complexinterplayof stabilizing and destabilizing interactions, and showsthat lysozyme is unusually stable for a small protein(Pfeil and Privalov, 1976; Griko et al.,1995). The differentheat capacities of the folded and denatured form of theprotein show that the enthalpies and entropies of bothstates are strongly temperature dependent, but theylargely compensate each other so that the net stabilityof a typical small protein is approximately 80-120 kJ/mol(20-30 kcal/mol).The large ACofunfolding also indicatesthat a protein will have maximum stability at a giventemperature (depending on its sequence,pH, etc.), andthat its net stability will decrease above and below thattemperature. Thus, proteins can not only be denaturedby heating, but can also be denatured by cooling. Fig. 3. Temperature scan, converted to molar heat capacity, forhen egg white lysozyme solution in 0.2 M glycine buffer, pH4.0. Lysozyme concentrations ranged from 400 pg to 2 pg inthe 300 uL sample cell. Curves are offset to aid presentation.Data were obtained using a scan rate of2 C/min on a CSC6300 N-DSCIII. Lysozyme incell (pg) Calorimetric van’t Hoff AH (kJ mol') AS (kJ K'mol) T(℃) AH (kJ mol') 400 512 1.46 78.0 515 100 512 1.46 78.0 509 50 517 1.47 77.9 513 25 513 1.46 77.8 513 10 515 1.47 78.0 515 5 490 1.40 78.1 510 2 503 1.43 77.8 499 Table 1.Thermodyanamic parameters obtained from thelysozyme scans presented in Figure 3. The amount of protein available for characterizationcan be very limited, which in the past restricted theapplication of biophysical techniques such as DSC.RecentadvancesSinninstrumentsensitivityhavealleviated this constraint by significantly decreasingthe amount of sample required for DSC analyses. Fig. 3shows raw data for lysozyme scanned on a CSC N-DSCIII.Protein amounts ranged from 400 pg to 2 pg in the 300uL sample cell. Thermodynamic parameters obtained atall concentrations are consistent (Table 1), showing thateven 2 pg of protein can be sufficient to allow accuratethermodynamic characterization of a protein. Can the thermodynamic properties of a protein,analyzed by DSC, be correlated to structure andstability? As more proteins are carefully characterizedboth experimentally and computationally, correlationsbetween thermodynamics and structure are beingrevealed, albeit not yet at a reliably predictive level.A(discussion of the stabilizing and destabilizinginteractions outlined above comprises a vast body ofliterature;much of this literature has been rigorously andcomprehensively reviewed by Robertson and Murphy(1997). Several other excellent papers and reviews onthe subject include Privalov and Khechinashvili, 1974;Fersht and Serrano, 1993; Privalov and Privalov, 2000;Cooper et al.,2001; Kumar and Nussinov, 2002; Mendeset al., 2002; Russ and Ranganathan, 2002; Chan et al.,2004; Scharnagl et al., 2005 and the following website:http://www.ocf.berkeley.edu/~aathavan/libraire/thermodynamicsprimer.pdf Rationalizing the stabilities of foldedconformations: mutant vs. wild-type proteins Hydrophobicity scales of the amino acids, andpropensity scales for amino acids in various secondary structures (Koehl and Levitt, 1999; Acevedo and Lareo,2005),are used in conjunction with molecular modelingto predict the effect of specific mutations. Systematicmutation of proteins with known three-dimensionalstructures often aims to quantify the resultant stabilitychanges in terms of the energetics of unfolding. Asingle site-specific mutation can give rise to a largeincrease or decrease in stability, but many mutationsproduce only small effects. The ability of proteins tomake subtle solvation and conformational changes inorder to compensate for the enthalpic and entropicdisturbances caused by a mutation can result in onlysmall free energy changes being observed upon thermaldenaturation of the protein. Nonetheless, the growingdatabase of proteins thoroughly characterized by DSCis aiding comprehension of mutational effects on thestability of proteins, although care must be exercised inthe analyses (Sturtevant, 1994;Plum and Breslauer, 1995;Robertson and Murphy, 1997; Brylants et al., 2005). Therecent redesign of proteins for increased stability (Fowleret al.,2005; Lee et al.,2005), and in particular the rationalredesign ofenzymes for both enhanced entropic stabilityand catalytic function (O'Fagain, 2003;Eijsinket al.,2004),illustrate that as long as the mechanisms controllingthe stability and thermal unfolding of a protein aresufficiently understood, it is possible to logically andpredictably alter the properties of proteins of knownstructure. Summary Understanding the thermodynamics controllingthe formation of macromolecular noncovalent bonds isnecessary if the promise of diverse endeavors such as thedesign of supramolecular assemblies and therapeuticproteins are to be realized. Many factors are responsiblefor the stability of a protein, including hydrogen bondsto the solvent and intramolecular interactions. Rigorouscharacterization of the thermodynamic basis of aprotein's stability is prerequisite for enhancing its thermaland functional characteristics by sequence alteration.DSC is the most direct approach for determining theintrinsic stability of a protein in dilute solution, allowingthe relationship between enthalpy and entropy of thedenaturation process to be quickly established. Thesuperior sensitivity of the CSC N-DSC II or N-DSC III allowsaccurate thermodynamic parameters to be obtainedfrom just a few micrograms of protein References (Preference has been given to current references. Citation doesnot imply that a paper is necessarily the original reference to astudy.) Acevedo,O. E. and L. R. Lareo.(2005) Amino acid propensitiesrevisited.OMICS 9,391-399. Anfinsen, C. B. (1973) Principles that govern the folding ofprotein chains.Science 181,223-230. ( Bruylants, G., J. Wouters a nd C . M i chaux.(20 0 5) Differential scanning calorimetry i n l i fe s ciences: t hermodynamics, stability, molecular recognition and applications in drug design. Curr. Med.Chem.12,2011- 2 020. ) ( Chan, H . S ., S. Shimizu and H . K aya. ( 2004) Cooperativity principles in protein folding. Methods Enzymol. 380, 350- 379. ) ( Cooper, A ., M. A. Nutley a nd A . Wadood. (2001) Differentialscanning c alorimetry. p. 287-318. I n S. E. H arding and B . Z . C howdry (E d s.) P r otein-Ligand I n teractions:hydrodynamics and calorimetry.Oxford Un i versity Press, Oxford . ) Eijsink, V. G. H. et al. (2004) Rational engineering of enzymestability.J.Biotechnol.113,115-120.p. ( Fersht,A.R. and L. Serrano.(1 9 93) Principles of protein stability derived from protein engineering experiments.Curr.Opin. Struct.Biol . 3,75-83. ) ( Fowler,S.B.etal.(2005)Rational design of aggregation-res i stant bioactive peptides: reengineering human calcitonin.PNAS 102,10105-10110. ) ( Freire,E. ( 1995)Thermal denaturation methods in the study ofprotein folding. Methods Enzymol.259,144-168. ) ( Griko, Y.V., E. Freire, G . Privalov, H. Van Dael and P. L . Privalov. (1995)The unfolding gt thermodynamics s o f c-typelysozymes: a calorimetric study of the heat denaturation of equine lysozyme.J.Mol.Biol.252,447- 4 59. ) Koehl, P. and M. Levitt. (1999) Structure-based conformationalpreferences of amino acids.PNAS 96, 12524-12529. Langer, R. and D. A. Tirrell. (2004) Designing materials forbiology and medicine. Nature 428,487-492. ( Lee, C-F. , G . I. Makhatadze and K-B W ang. (2005) Effects of charge-to - alanine substitution s on the stability of ribosomal protein L 30e f r om T he rmococcus ce l er. Biochemistry 44,16817- 1 6825. ) ( Marky,L . A.andK.J. B reslauer.(1987)Calculatingthermodynamicdata f or transitions of any molecularity from e q uilibrium melting c u rves. Biopolymers 26,1601-1620. ) Mastrianni, J. A. (2004) Prion diseases. Clinical Neurosci.Res. 3,469-480. ( Mendes,J. , R. Guerois and L. Serrano. (2002) Energy estimationin protein d esign.Curr. Opin.Struct. Biol. 1 2,4 4 1- 4 46. ) ( O'Fagain,C. (2003) Enzyme stabilization: r e cent experimentalprogress.Enzyme Microb.Technol. 33, 1 37-149. ) ( Perl,D. and F. X . Schmid.(2002) Some like it hot:the m olecular determinants o f protein stability. ChemBiochem. 3,39-44. ) Pfeil,W.and P.L.Privalov.(1976)Thermodynamic investigationsof proteins ⅢI. Thermodynamic description of lysozyme.Biophys.Chem.4,41-50. ( Plum, G. E. and K. J. Breslauer.(1995) C a lorimetry of proteinsand nucleic a c ids. Curr.O p in. Struct.Biol. 5,682-690. ) ( Privalov,P.L.and N.N.Khechinashvili.(1974) A thermodynamic approach t o t he p roblem o f s t abilization of globular protein structure: a calorimetric study.J.Mol.Biol.86,665- 684. ) Privalov,G.P.and P.L.Privalov.(2000) Problems and perspectivesinmicrocalorimetry of bbiologicalrmacromolecules.Methods Enzymol.323,31-62. Remmele,R.L.and W.R. Gombotz.(2000) Differential scanningcalorimetry: A practical tool for elucidating stability ofliquid pharmaceuticals. BioPharm. 13,36-46. Robertson, A. D.and K. P. Murphy.(1997) Protein structure andthe energetics of protein stability. Chem. Rev. 97, 1251-1267. Russ, W. P. and R. Ranganathan.(2002) Knowledge-basedpotential functions in protein design. Curr. Opin. Struct.Biol.12,447-452. ( Scharnagl,C.,M.Reif and J.Friedrich.(2005) Stability of proteins: temperature, p ressure a nd t h e r o le of s olvent. Biochim. Biophys.Acta.1749,187- 2 13. ) ( Sela , M., F. W . White and C. B. Anfinsen. (1957) Reductive cleavage of disulfide bridges i n ribonuclease. Science 125, 691-692. ) ( Shinoda, K. (1977) 'lceberg'formation and solubility. J. Phys. Chem.81,8069-8072. ) ( Sturtevant, J. M. (1994 ) The thermodynamic e f fects of protein mutations.Curr.Opin. Struct. Biol.4,69-78. ) Yeates,T.O. and J. E. Padilla.(2002) Designing supramolecularprotein assemblies.Curr. Opin. Struct. Biol.12,464-470. ( Webeter, D . M . ( E d.) (2000) P r o tein structure pr e diction.Humana Press. ) M DSC is the most direct and sensitive approach for characterizing the thermodynamic parameters controlling noncovalent bond formation (and therefore stability) in proteins and other macromolecules. In an experiment requiring only a few micrograms of material, the protein is thermally unfolded, allowing the relationship between enthalpy and entropy of the denaturation process to be established in about one hour. Correlating thermodynamic properties to stability is necessary for the rational design of engineered proteins and protein therapeutics.
确定



还剩4页未读,是否继续阅读?
TA仪器为您提供《蛋白质中稳定性检测方案(差示扫描量热)》,该方案主要用于其他中稳定性检测,参考标准--,《蛋白质中稳定性检测方案(差示扫描量热)》用到的仪器有NANO等温差示扫描量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多















