
方案详情
文
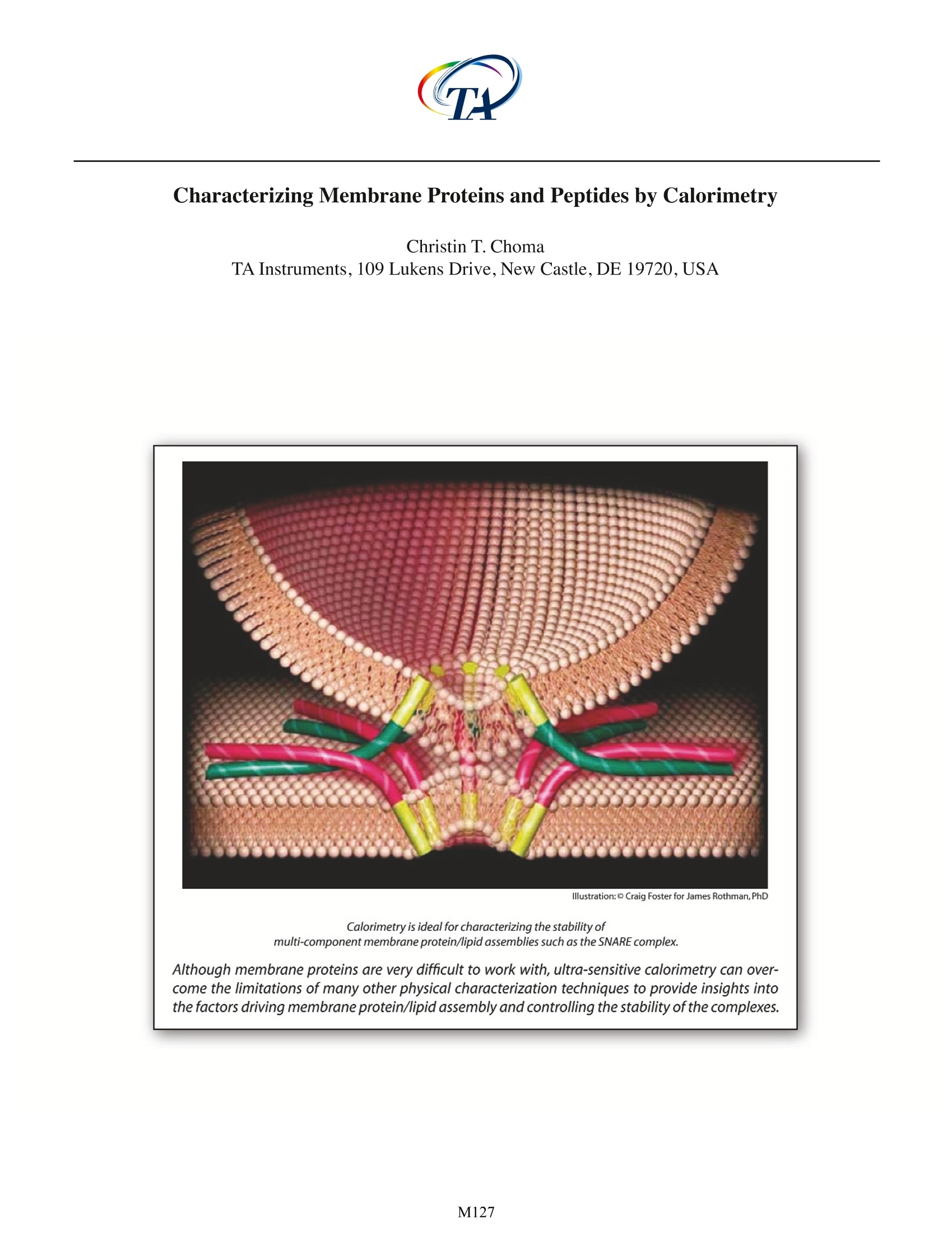
Although membrane proteins are very difficult to work with, ultra-sensitive calorimetry can overcome the limitations of many other physical characterization techniques to provide insights into the factors driving membrane protein/lipid assembly and controlling the stability of the complexes.
方案详情

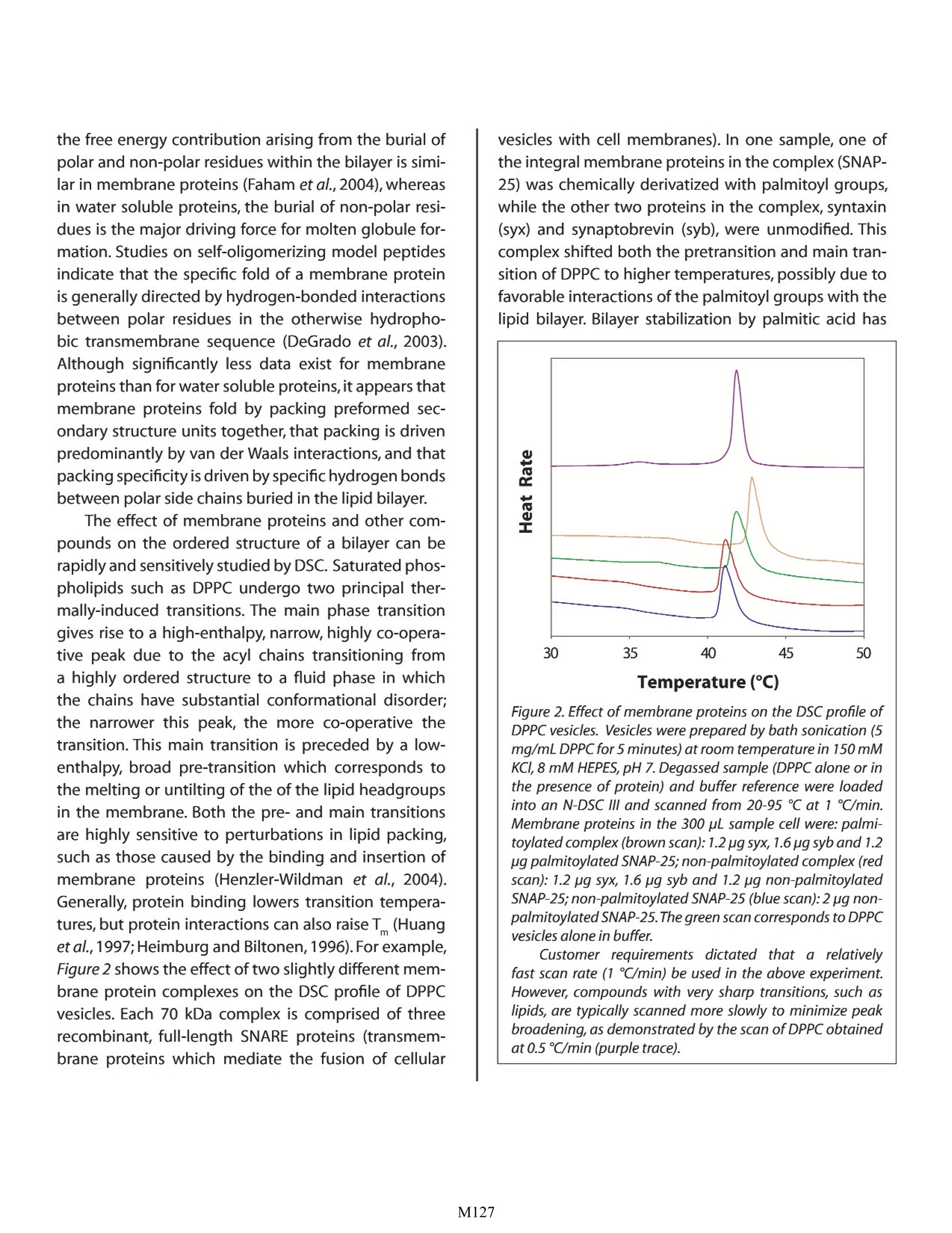
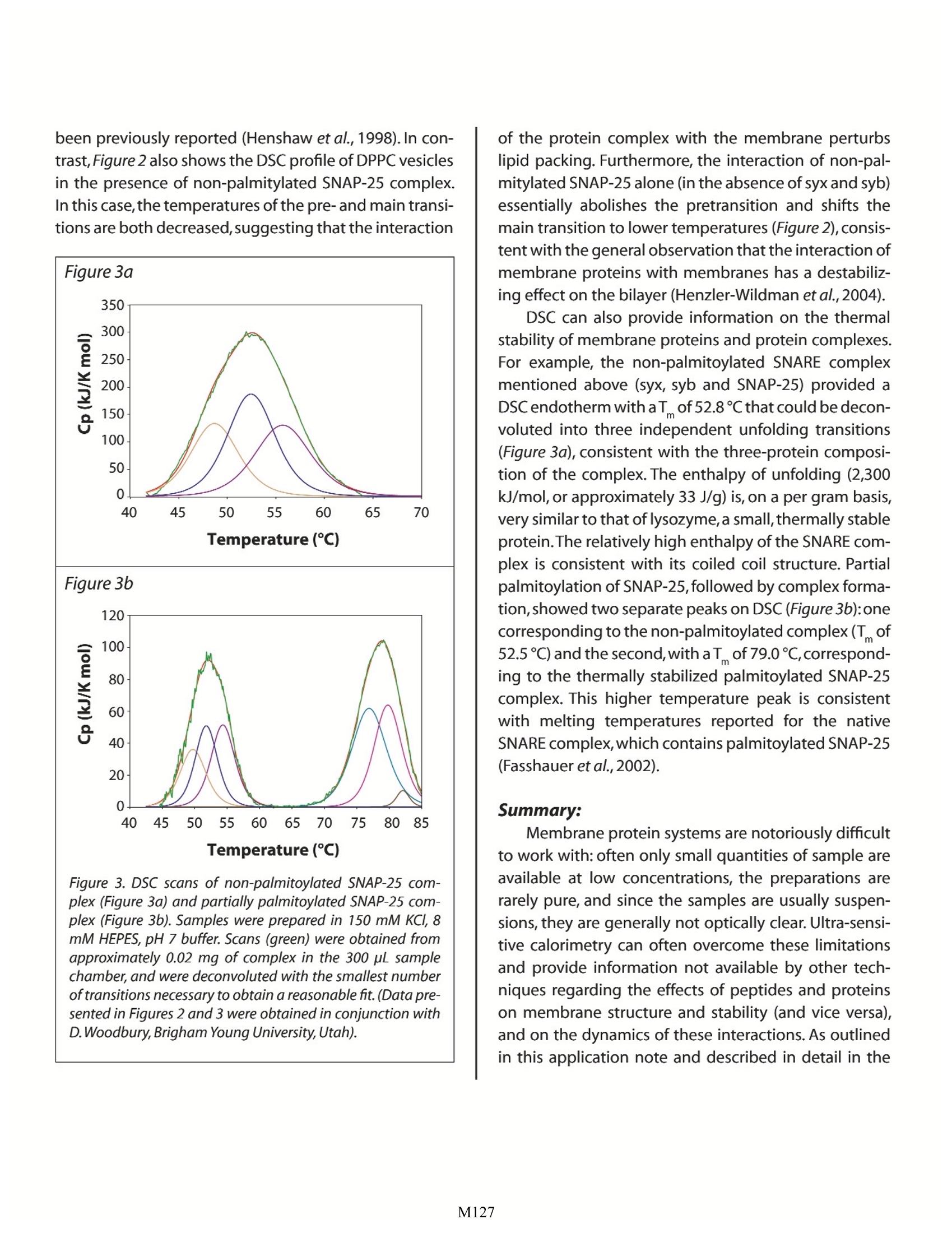
Characterizing Membrane Proteins and Peptides by Calorimetry Christin T. Choma TA Instruments, 109 Lukens Drive,New Castle, DE 19720, USA Calorimetry is ideal for characterizing the stability of multi-component membrane protein/lipid assemblies such as the SNARE complex. Although membrane proteins are very difficult to work with, ultra-sensitive calorimetry can over-come the limitations of many other physical characterization techniques to provide insights intothe factors driving membrane protein/lipid assembly and controlling the stability of the complexes. roteins and peptides are integral componentsDof all biological membranes. Embedded in themembrane or peripherally associated with thebilayer, membrane proteins and peptides have diversebiological functions ranging from receptors and ionchannels to antibiotics and fungicides. Insights intothe dynamics and interactions governing membraneprotein and peptide functionality require an under-standing of the molecular interactions between thelipid and polypeptide molecules (hydrophobic effects,hydrogen bonding and electrostatics), and the physi-cal properties of the system, such as membrane fluid-ity and protein structure and stability. Since thermody-namics fundamentally govern these interactions andphysical properties (Heerklotz,2004),techniques whichdirectly characterize the thermodynamic properties ofmembrane complexes provide a powerful approach forunderstanding the functionality of these systems. Thisapplication note explores the utility of differential scan-ning calorimetry (DSC) and isothermal titration calo-rimetry (ITC) for characterizing the energetics govern-ing membrane systems. For general descriptions of theprinciples behind DSC and ITC, please see CalorimetryScience Corporation's overview notes entitled LifeScience Applications of DSC and Life Science ApplicationsofITC. The Properties of Lipid Membranes Lipids can form a variety of phase structuresdepending on their chemical structures, hydrationstate and temperature. When lipid/membrane proteinsystems are studied, lipids are generally fully hydratedand assembled in either unilamellar or multi-lamellarvesicles.DSC can directly monitor the effect of proteinsand peptides on lipid phase structures by revealingboth changes in the apparent molar heat capacity ofthe lipid, and changes in the temperature and sharp-ness of the melting transition. Pure lipids usually havevery sharp, large enthalpy melting transitions whichare broadened by association with a protein or peptide.The melting transition takes the membrane from a low-temperature gel phase,characterized by predominantly ordered acyl chains,to a high-temperature fluid phasein which the acyl chains are conformationally disor-dered. During this transition, the surface area of themembraneincreases by about 25%and the membranebecomes thinner (Heimburg, 2000). In addition, satu-rated phosphatidylcholine membranes also exhibit alower temperature, broad, low enthalpy pretransitionin which the acyl chains go from fully ordered to pre-dominantly ordered (with local point defects charac-terized by disordered acyl chains; Heimburg, 2000).The pretransition can be shifted 10℃ or more, or becompletely abolished, by the addition of membraneproteins, peptides, or membrane-active drugs such asanesthetics (Fa et al.,2006). Model membranes more closely approximatingthe properties of biological membranes are generallycomposed of several types of lipids, including cho-lesterol and sphingomyelin. In these more complexsystems, groups of specific lipids may aggregate intopatches or domains which have physical propertiesdistinctly different from those of other lipid domainspresent elsewhere in the membrane.In addition,somelipids can exist either in a fluid or a gel phase at physi-ological temperature, resulting in distinct domains of asingle lipid type that coexist in a membrane (Almeidaet al., 2005). Most proteins and peptides interact withspecific membrane domains through electrostaticattraction with the lipid headgroups, followed by par-titioning into the bilayer,although if a peptide containsno charged groups, interaction is strictly by adsorp-tion and partitioning. In either case, interaction withthe bilayer often results in substantial conformationalchanges in peptides and, to a lesser extent, proteins(Seelig, 2004). The effect of bilayer structure on thestructure and activity of membrane proteins (and theeffect of integral membrane proteins on membranestructure) has been extensively reviewed (Lee,2004). Characterizing Vesicle Binding Using IsothermalTitration Calorimetry Unlike site-specific binding (such as between amacromolecule and a ligand) the partitioning of a peptide or protein into a membrane does not neces-sarily result in the formation of a specific complex witha defined stoichiometry. The thermodynamics andstoichiometry of membrane protein/lipid interactionsare more akin to sorption isotherms than to site-spe-cific binding. Nonetheless, the binding of peptidesand proteins to membranes can be quickly and easilycharacterized by ITC, allowing determination of thebinding constant (K ) and reaction enthalpy. Figure 1illustrates a simple example, the binding of cyclosporinA to dipalmitoyl phosphatidylcholine (DPPC) vesicles.Cyclosporin A is a hydrophobic 11 residue cyclic pep-tide used clinically as an immunosuppressive agent.Since vesicles hold promise as potential drug carriers,the binding of hydrophobic drugs such as cyclosporinA to vesicles has relevance for clinical applications.The Figure 1. Cyclosporin A titrated with DPPC vesicles in a CSCmodel 5300 ITC-/II.DPPC was sonicated in 1 mL water for 2 hrat 60℃ to provide a 70 mM suspension of small unilamellarvesicles. Cyclosporin A (1.8 mg), dissolved in 100 pL ethanol,was slowly added to 100 mL rapidly-stirred water (final con-centration: 15x10M cyclosporin A). The cyclosporin A solu-tion was loaded into the 1.0 mL sample cell,and the DPPCsuspension was loaded into a 250 pL syringe. Twenty-five, 10pL aliquots of DPPC were titrated into the cyclosporin A solu-tion while stirring at 200 rpm and maintaining the tempera-ture of the system at 28℃. Data were fit to an independentbinding model; the graph shows the pJ from each injectionplotted against the mole fraction of DPPC to cyclosporin A. ITC data show that, on average, six DPPC moleculesinteract with each bound cyclosporin A, that theK of binding is approximately 390 M, and that theenthalpy of binding is -61 kJ/mol DPPC. In contrast to completely hydrophobic peptideslike cyclosporin, peptides and proteins containingboth charged and hydrophobic residues initiallyinteract with vesicles via electrostatic interactionsbetween their charged residues and the charged lipidheadgroups, followed by at least partial penetrationof their hydrophobic residues into the hydropho-bic bilayer. ITC provides a straightforward approachfor quantitatively characterizing this partitioning, asillustrated by the interaction of a cationic peptide,nisin Z, with membranes composed of different molarratios of POPC and POPG (Breukink et al., 2000). NisinZ binding to neutral POPC vesicles yielded weak, butquantifiable,binding heats and a partition constant ofapproximately 540 M1. Although the peptide carriesa +3.8 charge, the ITC binding isotherm could be bestfit with a model assuming a charge of +1, suggestingthat hydrophobic interactions dominate during bind-ing and that the charged residues remain somewhatdistant from the membrane.However,the addition ofvarious amounts of negatively charged POPG resultedin substantially tighter binding and correspondingdecreases in the intrinsic partition coefficient,indicat-ing that the hydrophobic contribution to the bindingprocess was much smaller compared to the case withneutral vesicles. By comparing the binding character-istics of several nisin Z mutants differing in their over-all charge,the authors were able to separate electro-static and hydrophobic contributions to the bindingprocess, the partition constants, and the free energyand entropy of binding. Many peptides undergo extensive conformationalchanges upon binding to a membrane, going from arandom coil in solution to folded when bound.As withother physical techniques used to study conforma-tional changes and binding of β-structure peptides tovesicles, the interpretation of ITC data of, for example,B-amyloid peptide binding to POPC/POPG vesicles, is not straightforward.The analysis is complicated by thefact that the heat measured by the titration isotherm iscomprised of conformational changes, a binding reac-tion, and an aggregation/dissociation reaction (Terziet al., 1995).In contrast, the investigation of a-heli-cal peptide binding is relatively facile. For example, tostudy membrane-induced a-helix formation, the heli-cal propensity of magainin (Weiprecht et al.,1999) anda mitochondrial leader sequence peptide (Wieprechtet al., 2000) were studied in POPC/POPG membranes.The helical propensity of magainin was systematicallyaltered by substituting two adjacent amino acids withtheir D-enantiomers at various locations in the pep-tide.The substitution of residues near the center of thehelix reduced helical content substantially more thansubstitutions near the termini. Using ITC, the bindingenthalpies of the various magainin peptides were mea-sured; when plotted as a function of helical content, anapproximate straight line was obtained, allowing thebinding enthalpy of an entirely random coil magaininpeptide to be estimated from the y-axis intercept. Theaverage contribution of a residue in the magaininsequence to the binding enthalpy (AHe) could thenbe calculated (-3.0 kJ/mol residue).Comparable experi-ments with a leader sequence peptide (Wieprecht etal.,2000) provided a very similar value for AH. ITC binding studies with a given peptide or pro-tein using either sonicated small unilamellar vesicles orextruded large unilamellar vesicles provide very similarbinding constants and free energies, but significantlyhigher enthalpies of binding are observed with smallvesicles,perhaps due to the different packing densitiesof small versus large vesicles (Wieprecht et al., 2002;Seelig, 2004). It is also clear that amphipathic peptidesbind exothermically to small unilamellar vesicles, andthat enthalpy, rather than entropy, is the major driv-ing force (Seelig and Ganz, 1991;Wieprecht et al., 2000;Abraham et al.,2005). Characterizing Membrane Polypeptide Stabilityand Structure by Differential Scanning Calorimetry DSC provides information on the thermal stabil- ity of membrane peptides and proteins, the domainand subunit structure of membrane proteins, and theeffect that the interaction of a membrane protein orpeptide has on the physical properties of the bilayer.The DSC analysis of membrane protein thermal stabil-ity and tertiary/quaternary structure is conducted in amanner analogous to that for water soluble proteins,and is described in detail in the CSC application notesentitled Characterizing Protein Stability by DSC andCharacterizing Protein Structure by DSC. Specific exam-ples of stability and structure studies on membranepeptides and proteins are reviewed in Shnyrov et al.(1997),Lee (2004) and Minetti and Remeta (2006). DSC allows evaluation of the magnitude of intra-molecular interactions (the unfolding enthalpy, AH)and the temperature-dependent exposure of polar andnonpolar residues to the solvent during unfolding (thechange in heat capacity,AC).The principle differencesobserved between water soluble and membrane solu-ble proteins arise from the membrane-spanning por-tions of membrane proteins. Transmembrane a-heli-ces and p-barrels tend to be very thermally stable andretain their secondary structure even after the extra-membranous portions of the protein have unfolded.Consequently,AC is considerably lower for membraneproteins than for water soluble proteins because mini-mal hydrophobic surface area is exposed to the solventupon unfolding. The unfolding enthalpy also tends tobe lower than for water soluble proteins, indicatingthat the transmembrane segments of unfolded mem-brane proteins retain significant structure (Minetti andRemeta, 2006). Although solvent-exposed loops ofmembrane proteins unfold with enthalpies and heatcapacities comparable to that observed for water sol-uble proteins, they nonetheless apparently contributesignificantly to the stabilization of transmembrane a-helices and B-barrels (Minetti and Remeta,2006). Perhaps surprisingly, the overall stability of mem-brane proteins is comparable to that of water solubleproteins: approximately 4 KJ/mol per 10 A of buriedprotein surface area (Faham et al., 2004). Extensivemutational studies on bacteriorhodopsin indicate that the free energy contribution arising from the burial ofpolar and non-polar residues within the bilayer is simi-lar in membrane proteins (Faham et al., 2004), whereasin water soluble proteins, the burial of non-polar resi-dues is the major driving force for molten globule for-mation.Studies on self-oligomerizing model peptidesindicate that the specific fold of a membrane proteinis generally directed by hydrogen-bonded interactionsbetween polar residues in the otherwise hydropho-bic transmembrane sequence (DeGrado et al., 2003).Although significantly less data exist for membraneproteins than for water soluble proteins, it appears thatmembrane proteins fold by packing preformed sec-ondary structure units together, that packing is drivenpredominantly by van der Waals interactions, and thatpacking specificity is driven by specific hydrogen bondsbetween polar side chains buried in the lipid bilayer. The effect of membrane proteins and other com-pounds on the ordered structure of a bilayer can berapidly and sensitively studied by DSC. Saturated phos-pholipids such as DPPC undergo two principal ther-mally-induced transitions. The main phase transitiongives rise to a high-enthalpy, narrow, highly co-opera-tive peak due to the acyl chains transitioning froma highly ordered structure to a fluid phase in whichthe chains have substantial conformational disorder;the narrower this peak, the more co-operative thetransition. This main transition is preceded by a low-enthalpy, broad pre-transition which corresponds tothe melting or untilting of the of the lipid headgroupsin the membrane. Both the pre- and main transitionsare highly sensitive to perturbations in lipid packing,such as those caused by the binding and insertion ofmembrane proteins (Henzler-Wildman et al., 2004).Generally, protein binding lowers transition tempera-tures, but protein interactions can also raiseT (Huanget al., 1997;Heimburg and Biltonen,1996).For example,Figure 2 shows the effect of two slightly different mem-brane protein complexes on the DSC profile of DPPCvesicles. Each 70 kDa complex is comprised of threerecombinant, full-length SNARE proteins (transmem-brane proteins which mediate the fusion of cellular vesicles with cell membranes). In one sample, one ofthe integral membrane proteins in the complex (SNAP-25) was chemically derivatized with palmitoyl groups,while the other two proteins in the complex, syntaxin(syx) and synaptobrevin (syb), were unmodified. Thiscomplex shifted both the pretransition and main tran-sition of DPPC to higher temperatures, possibly due tofavorable interactions of the palmitoyl groups with thelipid bilayer. Bilayer stabilization by palmitic acid has Figure 2. Effect of membrane proteins on the DSC profile ofDPPCvesicles. Vesicles were prepared by bath sonication (5mg/mL DPPCfor 5 minutes) at room temperature in 150 mMKCI, 8 mM HEPES, pH7. Degassed sample (DPPC alone or inthe presence of protein) and buffer reference were loadedinto an N-DSC III and scanned from 20-95 ℃ at 1 ℃/min.Membrane proteins in the 300 pL sample cell were: palmi-toylated complex (brown scan): 1.2 pg syx, 1.6 pg syb and 1.2ug palmitoylated SNAP-25;non-palmitoylated complex (redscan): 1.2 pg syx, 1.6 pg syb and 1.2 pg non-palmitoylatedSNAP-25;non-palmitoylated SNAP-25 (blue scan):2 pg non-palmitoylated SNAP-25.The green scan corresponds to DPPCvesicles alone in buffer. Customer requirements dictated that a relativelyfast scan rate (1℃/min) be used in the above experiment.However, compounds with very sharp transitions, such aslipids, are typically scanned more slowly to minimize peakbroadening, as demonstrated by the scan of DPPC obtainedat 0.5 ℃/min (purple trace). been previously reported (Henshaw et al., 1998). In con-trast,Figure 2 also shows the DSC profile of DPPC vesiclesin the presence of non-palmitylated SNAP-25 complex.In this case,the temperatures of the pre- and main transi-tions are both decreased,suggesting that the interaction Fiqure 3. DSC scans of non-palmitoylated SNAP-25 com-plex (Figure 3a) and partially palmitoylated SNAP-25 com-plex (Figure 3b). Samples were prepared in 150 mM KCl, 8mM HEPES, pH 7buffer. Scans (green) were obtained fromapproximately 0.02 mg of complex in the 300 pL samplechamber,and were deconvoluted with the smallest numberoftransitions necessary to obtain a reasonable fit. (Data pre-sented in Figures 2 and 3 were obtained in conjunction withD. Woodbury,Brigham Young University, Utah). of the protein complex with the membrane perturbslipid packing. Furthermore, the interaction of non-pal-mitylated SNAP-25 alone (in the absence of syx and syb)essentially abolishes the pretransition and shifts themain transition to lower temperatures (Figure 2), consis-tent with the general observation that the interaction ofmembrane proteins with membranes has a destabiliz-ing effect on the bilayer (Henzler-Wildman et al.,2004). DSC can also provide information on the thermalstability of membrane proteins and protein complexes.For example, the non-palmitoylated SNARE complexmentioned above (syx, syb and SNAP-25) provided aDSCendotherm with aT of52.8℃that could be decon-voluted into three independent unfolding transitions(Figure 3a), consistent with the three-protein composi-tion of the complex. The enthalpy of unfolding (2,300kJ/mol, or approximately 33 J/g) is, on a per gram basis,very similar to that of lysozyme, a small,thermally stableprotein.The relatively high enthalpy of the SNARE com-plex is consistent with its coiled coil structure. Partialpalmitoylation of SNAP-25, followed by complex forma-tion,showed two separate peaks on DSC (Figure 3b):onecorresponding to the non-palmitoylated complex(T of52.5℃) and the second,with aT of 79.0℃,correspond-ing to the thermally stabilized palmitoylated SNAP-25complex. This higher temperature peak is consistentwith melting temperatures reported for the nativeSNARE complex,which contains palmitoylated SNAP-25(Fasshauer et al.,2002). Summary: Membrane protein systems are notoriously difficultto work with: often only small quantities of sample areavailable at low concentrations, the preparations arerarely pure, and since the samples are usually suspen-sions, they are generally not optically clear.Ultra-sensi-tive calorimetry can often overcome these limitationsand provide information not available by other tech-niques regarding the effects of peptides and proteinson membrane structure and stability (and vice versa),and on the dynamics of these interactions. As outlinedin this application note and described in detail in the references cited below, calorimetry provides the mostdirect approach for understanding the thermodynam-ics governing the stability of structures and the mecha-nisms by which membrane protein systems function. References: (Preference has been given to current references. Citationdoes not imply that a paper is necessarily the original refer-ence to a study.) Abraham, T., R. Lewis, R. S. Hodges and R. McElhaney(2005). Isothermal titration calorimetry studies of thebinding of the antimicrobial peptide Gramicidin S tophospholipid bilayer membranes. Biochemistry 44,11279-11285. ( Almeida,P.F.F.,A. Pikorny and A. Hinderliter (2005).Ther- modynamics of membrane domains.Biochim.Biophys. Acta 1720,1-13. ) ( Breukink, E ., P. Ganz, B. de Kruijff and J. Seelig (2000). Binding o f n isin Z to bilayer vesicles as determined with isothermal titration calorimetry.Biochemistry 39, 10247-10254. ) ( DeGrado,W. F., H. Gratkowski and J.D. Lea r (2003).How do h elix-helix interactions help determine the folds of membrane proteins? P e rspectives from t h e s t udy of homo-oligomeric helical b u ndles. Protein Science 12,647-665. ) ( Fa, N.,A. Schanck, M. Deleu, A. Gaigneaux,E.Goormagh- tigh and M . P . M ingeot-Leclercq ( 2 006). Effect of the antibiotic azithromycin on the thermotropic behav- ior of DOPC or DPPC bilayers. Chem. Phys. Lipids 1 4 4, 108-116. ) ( Faham, S. , D. Yang, E. Bare, S. Yohannan,J. P. Whitelegge and J . U. Bowie (2004). Side chain contributions to membrane protein structure and stability.J. Mol.Biol. 335,297-305. ) ( Fasshauer, D., W. Antonin,V. Subramaniam and R. Jahn (2002). SNARE a ssembly a nd disassembly e xhibit a pronounced hysteresis. Nature Structural Biology 9 , 144-151. ) ( Heerklotz, H. (2004).The microcalorimetry of lipid mem-branes.J.Phys.:Condens. Matter 16,R441-R467. ) Heimburg, T. and R. L. Biltonen (1996). A Monte Carlosimulation study of protein-induced heat capacitychanges and lipid-induced protein clustering. Bio-phys J.70,84-96. Heimburg, T. (2000). A model for the lipid pretransition:coupling of ripple formation with the chain-meltingtransition.Biophys.J.78,1154-1165. ( Henzler-Wildman,K.A.,G.V.Martinez,M. F.Brown and A. Ramamoorthy (2004). Perturbation o f the hydropho- bic core of lipid bilayers by th e humam antimicrobial peptide LL-37.Biochemistry 43,8459-8469. ) ( Huang,W., L. P.Vernon,L. D. H ansen,and J. D. Bell ( 1997). Interactions of thionin from Pyrularia pubera with di-palmitoylphosphatidylglycerol large unilamellar vesi- cles. Biochemistry 36,2860-2866. ) ( Jolene B., J. B. Henshaw, C. A. Olsen, A. R . Farnbach, K. H. Nielson and J. D. B e ll.( 1 998). D e finition of th e spe-cific roles of lysolecithin and palmitic a c id in altering the s usceptibility of dipalmitoylphosphatidylcho- line bilayers to phospholipase A 2. B iochemistry 3 7 , 10709-10721. ) Lee, A. G. (2004). How lipids affect the activities of inte-gral membrane proteins.Biochim. Biophys.Acta 1666,62-87. ( Minetti, C. and D. P. Remeta (2006). Energetic s o f mem- brane protein folding and stability. Archives Biochem. Biophys. 453,32-53. ) Seelig,J.and P.Ganz(1991).Nonclassical hydrophobic ef-fect in membrane binding equilibria.Biochemistry 30,9354-9359. Seelig,J.(2004).Thermodynamics of lipid-peptide inter-actions.Biochim.Biophys.Acta 1666, 40-50. Shnyrov,V.L.,J.M. Sanchez-Ruiz,B. N. Boiko,G.G.Zhadanand E. A. Permyakov (1997). Applications of scanningmicrocalorimetry in biophysics and biochemistry.Thermochem.Acta 302,165-180. Terzi, E., G. Holzemann and J. Seelig (1995). Self-associa-tion of beta-amyloid peptide (1-40) in solution andbinding to lipid membranes.J.Mol.Biol. 252,633-642. ( Wieprecht, T ., O.Apostolov, M.Beyermann a n d J. Seelig (1999). Thermodynamics of the co il -alpha-helix transition o f amphipathic peptides in a membrane ) environment:implications for the peptide-membranebinding equilibrium.J.Mol.Biol. 294,785-794. ( Wieprecht, T., O . Apostolov, M. Beyermann and J . Seelig (2000). Interaction of a mitochondrial p resequence with lipid membranes: role of helix f o rmation formembrane bi n ding an d per t urbation. Biochemistry 39,15297-15305. ) Wieprecht, T., M. Beyermann and J. Seelig (2002). Ther-modynamics of the coil-alpha-helix transition of am-phipathic peptides in a membrane environment: therole of vesicle curvature.Biophys.Chem. 96,191-201. M
确定





还剩6页未读,是否继续阅读?
TA仪器为您提供《通过量热法表征膜蛋白质和多肽》,该方案主要用于其他中检测,参考标准--,《通过量热法表征膜蛋白质和多肽》用到的仪器有NANO等温差示扫描量热仪、TA仪器+Affinity ITC+等温滴定微量热仪、TA仪器+等温滴定微量热仪+ NANO ITC、TA仪器+差示扫描量热仪+NANO DSC
推荐专场
相关方案
更多
该厂商其他方案
更多


















