
方案详情
文
The expression or replication of genes is affected by the binding of small molecule ligands and proteins to nucleic acid sequences. Such binding events are critical for the physiological integrity of organisms and therefore are of fundamental interest to life scientists. Recently, the thermodynamics driving these interactions have also become important to pharmaceutical scientists investigating the anticancer, antibacterial and antiviral potential of nucleic acid/ligand interactions. In addition, as the number of diseases identified as being due to a malfunction of cellular control processes increases, the possibility of treating disorders by manipulating gene expression is further focusing attention on the thermodynamics underlying nucleic acid binding affinity and specificity.
方案详情

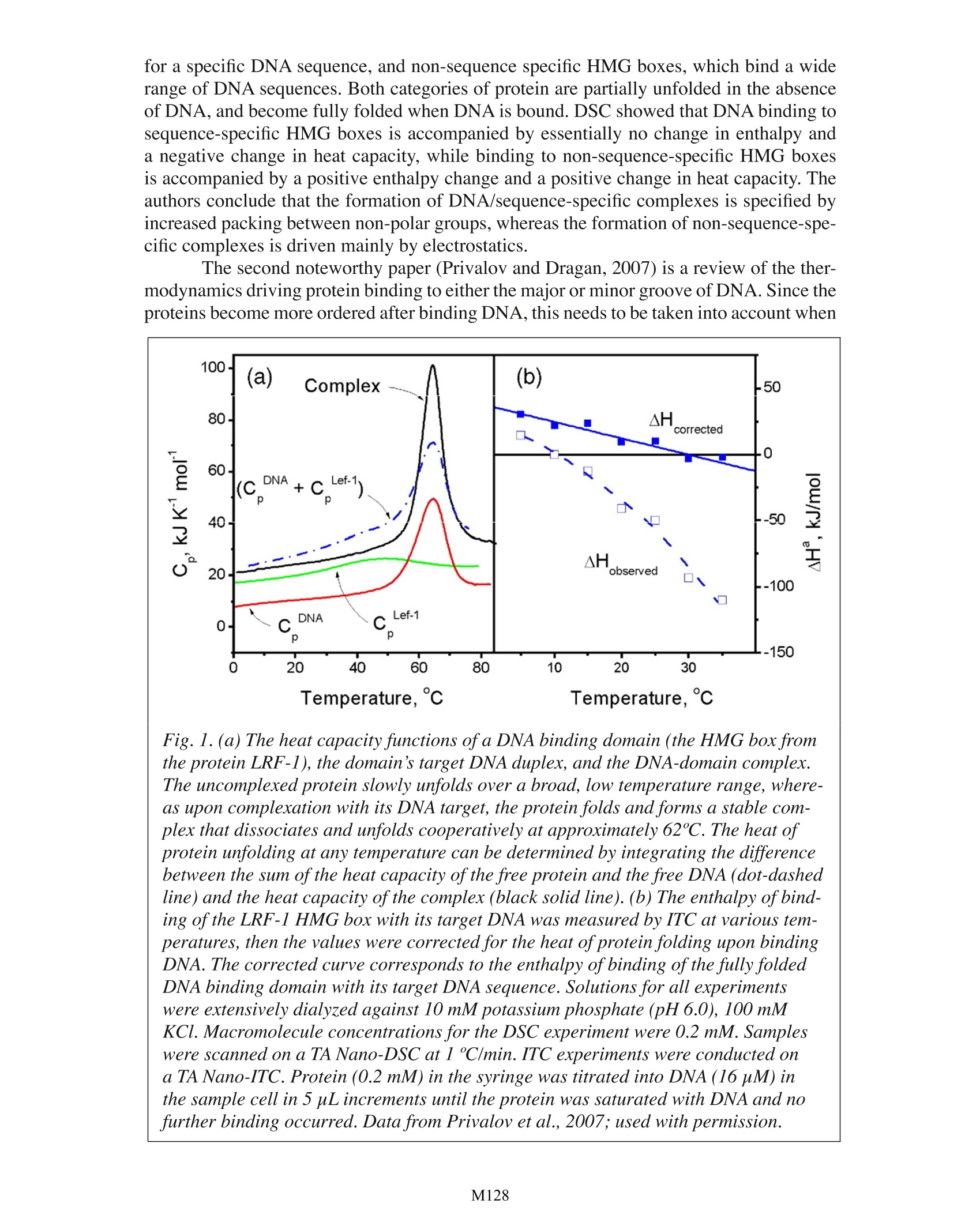
Characterizing non-covalent nucleic acid interactionswith small molecules and proteins by calorimetry Christin T. Choma TA Instruments, 109 Lukens Drive, New Castle, DE 19720,USA The expression or replication of genes is affected by the binding of small mol-ecule ligands and proteins to nucleic acid sequences. Such binding events are critical forthe physiological integrity of organisms and therefore are of fundamental interest to lifescientists.Recently, the thermodynamics driving these interactions have also become im-portant to pharmaceutical scientists investigating the anticancer, antibacterial and antiviralpotential of nucleic acid/ligand interactions. In addition, as the number of diseases identi-fied as being due to a malfunction of cellular control processes increases, the possibilityof treating disorders by manipulating gene expression is further focusing attention on thethermodynamics underlying nucleic acid binding affinity and specificity. Calorimetry is the most accurate and rapid approach for obtaining direct ther-modynamic information which, in combination with high resolution structural data andmechanistic studies, provides the most complete picture possible of the factors involvedin the recognition and binding of nucleic acids to ligands. This application note examinesthe utility of differential scanning calorimetry (DSC) and isothermal titration calorimetry(ITC) for characterizing the energetics governing nucleic acid/ligand complexes. For gen-eral descriptions of the principles behind DSC and ITC, please see TA’s overview notesentitled Life Science Applications ofDSC and Life Science Applications of ITC. Non-covalent nucleic acid/small molecule binding interactions Since the impetus for understanding the rules governing recognition and bindingto nucleic acids largely arises from interest in designing drugs capable of sequence- andstructure-specific nucleic acid recognition, most thermodynamic binding studies on nucleicacids have focused on small molecule, drug-like ligands binding to DNA. The enthalpicand entropic contributions to nucleic acid binding are the same as those driving protein/ligand interactions: increased number of hydrogen bonds, more favorable van der Waalsinteractions, and electrostatic and polar interactions (all enthalpic factors), and confor-mational changes and the release of bound solvent (entropic factors). The physical basisand relative importance of these contributions are discussed in Gohlke and Klebe (2002).However, although these interactions are the same as those driving protein/ligand binding,the models used for ligand binding to proteins cannot be used for ligand binding to nucleicacids. This is primarily because the three dimensional structures of nucleic acids generallylack the intricacy of protein structures, requiring that nucleic acids be modeled as a lattice.Thus, the statistics governing ligand binding to proteins and nucleic acids are different, adistinction that is not always appreciated in the published literature. Small molecules usually bind non-covalently to double-stranded DNA either byintercalation or minor groove binding (Haq, 2002). Intercalation generally occurs when theligand contains flat heteroaromatic rings that can insert between two adjacent base pairs and be stabilized by J-t interactions. Intercalation structurally perturbs the DNA, caus-ing it to unwind and lengthen slightly. In contrast, ligands which bind in the minor grooveoften contain several aromatic groups connected by rotationally-flexible linkers, allowingthe ligand to insert and follow the curve of the groove without disturbing packing. Thebinding of these ligands is stabilized by van der Waals interactions, hydrogen bonds andhydrophobic interactions, in particular between the ligand and A-T base pairs (Haq et al.,2000). General thermodynamic considerations: The energetics involved in reversible reactions can be most directly determinedby calorimetry because calorimetry alone directly measures the enthalpy of a reaction; allother techniques require that enthalpy be calculated from measurements, which introducesa degree of inaccuracy. The enthalpy, entropy, free energy and binding constant of thereaction are all related and defined at equilibrium by the standard thermodynamic relation-ship: where AG°, AH° and AS° are the changes in the equilibrium free energy, standard enthalpyand standard entropy,respectively, R is the gas constant, T is the absolute temperature andK is the equilibrium concentration of the complex divided by the concentrations of the freereactants. The effect of temperature on the free energy is described by: where AC is the change in heat capacity and T is the reference temperature. At constantpressure, the heat capacity can be defined in terms of the change in enthalpy or entropywith temperature: The binding constant and the stoichiometry of a reaction can be determined from a singleITC experiment, but probing the temperature dependence of the equilibrium, which is re-flected in the enthalpy change for the binding, requires titrations at at least two (and prefer-ably several) temperatures. Measuring AH as a function of temperature by ITC allows, inprinciple, the change in the heat capacity for the binding reaction to be determined, as dis-cussed below. Guidelines for designing DNA-drug ITC binding experiments are outlinedin detail in the review by Haq et al. (2001), while more general considerations for experi-ment design are presented in the TA application note Characterizing binding interactionsby ITC, and in O’Brien et al. (2001). A review of current approaches for analyzing complexDNA/ligand ITC binding data is provided by Buurma and Haq, 2007. Heat capacity The heat capacity of a compound or complex arises from vibrations and rotations ofall the molecules in the reaction, including the solvent.The heat capacity of a biopolymerclearly will change as it changes conformation upon ligand binding, and this change can bemeasured as the temperature dependence of the enthalpy or entropy. As equation 3 implies,AH and AS for a given transition change together, so transitions with large changes in heatcapacity could exhibit a degree of enthalpy/entropy compensation. However, care must betaken not to over-interpret the data: since the enthalpy and entropy values are derived fromthe same data, apparent compensation could merely be an artifact of statistical coupling between the calculated values. The relative contributions of different physical processes to heat capacity changesin proteins have been studied extensively (Cooper, 2005), but there have been fewer com-parable studies on nucleic acids. Given the highly ordered localized, repetitive structureof nucleic acids, and their very ionic nature (resulting in a large number of associatedcounterions), the relative importance of each factor to the heat capacity of nucleic acids islikely different from what it is for proteins (Mikulecky and Feig,2006). However, whetherheat capacity effects in nucleic acids are due primarily to differential solvation of polar andnonpolar surfaces, or are the general result of order-disorder transitions in a system stabi-lized by many weak interactions (Cooper, 1999), the heat capacity of nucleic acids is ob-served to change non-linearly with temperature, and increases during the binding reaction.Therefore, binding studies conducted over even a relatively narrow temperature range needto take into account the non-linear change in heat capacity of the nucleic acid as well aspotential temperature-dependant changes in structure (Mikulecky and Feig, 2006). Inher-ent difficulties and necessary precautions when performing calorimetric studies on varioustypes of nucleic acids are comprehensively summarized by Mikulecky and Feig (2006). Traditionally,AC is determined by measuring AH as a function of temperature andtaking the slope of AHvs. T. In practice, this provides only an estimate ofAC due to themagnification of any errors in AH on taking the derivative. In addition, the heat of as-sociation measured by ITC includes heats associated with conformational rearrangementof the reactants, which should be subtracted from the heat of binding. The change in heatcapacity is most accurately determined from DSC scans conducted on an instrument withan ultra-stable and reproducible baseline, such as the TA Nano-DSC (Privalov and Dragan,2007). The change in molar heat capacity of each reactant due to conformational changesand hydration effects is measured by DSC, then the individual molar heat capacities of thereactants are subtracted from the molar heat capacity of the complex to provide the changein heat capacity for the binding reaction. These measurements are direct, do not involvetaking derivatives, and allow the heat of solute and solvent rearrangement to be separatedfrom the heat of binding. An excellent review of the importance of heat capacity in proteinthermodynamic studies is provided by Privalov (2007). Estimating the enthalpic and entropic contributions of DNA-ligand binding Two ligands can bind to a nucleic acid target with similar affinity and yet, due todifferent enthalpic and entropic contributions, the energetics driving the reaction can bequite different. In general, an interaction dominated by enthalpic contributions (arisingfrom an increased number of hydrogen bonds, favorable van der Waals interactions andpolar effects) would give rise to more specific binding than an equally tight associationdominated by entropic contributions (conformational changes and release of bound wateras hydrophobic groups interact). Complete dissection of all the enthalpic and entropic con-tributions to a binding reaction is not presently possible, but the problem can be partiallydeconvoluted using calorimetry together with computational approaches. Calculations of binding parameters from an ITC experiment generally assume thatbinding only involves the target molecules in the syringe and calorimeter cell, whereasin fact there are also interactions between the reactants and ions, solvent and protons.The binding constant is therefore dependant on salt concentration and pH, as well as ontemperature and pressure. Since nucleic acids are polyanions, the binding of a positivelycharged ligand to its target sequence will displace cations clustered around the binding sitephosphate groups. Nucleic acid binding reactions are therefore generally highly sensitiveto salt concentration. The free energy of binding is comprised of two terms: an electrostaticcomponent (generally favorable and largely due to the ionic nature of the nucleic acid, which can be quantified from the dependence of the equilibrium constant on salt concen-tration; Chaires,1996), and a non-electrolytic component. The non-electrolytic componentis comprised of free energy contributions from conformational changes in the nucleic acidand ligand upon binding, losses in rotational and translational freedom upon complex for-mation, hydrophobic transfer of the ligand from solution to the binding site, and from non-covalent ligand-nucleic acid interactions (Chaires, 1998). Conformational changes in DNA upon ligand binding, and the loss of rotationaland conformational degrees of freedom, present free energy barriers to binding that mustbe overcome by favorable contributions from hydrophobic, non-covalent and electrostaticcomponents. The change in free energy due to groove binding has been reported to beclose to zero (Haq, 2002), which could suggest (but does not prove) that groove bindingdemands little conformational change in either the nucleic acid or the ligand. In contrast,DNA melting studies clearly show that intercalation carries a penalty of between 5 to 10kcal/mol for formation of the intercalation cavity (Chairs, 1998). For both intercalators andgroove binders, the rotational and translational cost of ligand binding to DNA is approxi-mately 15 kcal/mol (Spolar and Record,1994). The remaining non-electrolytic components, together with the (generally favor-able) electrolytic contribution, must overcome unfavorable entropy due to losses in con-formational, rotational and translational freedom. For both intercalating and groove bind-ing ligands, the major favorable contribution is due to hydrophobic effects arising fromthe transfer of the ligand from aqueous solution to the interior of the DNA molecule or toits minor groove. Changes in the solvent accessible surface area have been correlated withheat capacity changes, allowing the free energy contribution from the hydrophobic interac-tions to be estimated using heat capacity measurements from ITC or DSC (Chaires, 1998).Evaluation of the last contribution to the free energy of binding, non-covalent interactionssuch as hydrogen bonds and van der Waals interactions, is best addressed by systematicallyaltering specific ligand functional groups followed by ITC binding studies. Although thisprocess is laborious, the hope is that, once a database of binding free energies correlatedwith specific interactions is established, this data base will provide a training set againstwhich computational approaches can be tested, eventually eliminating the need to experi-mentally determine this complex component of the free energy of binding (Chaires, 1998;Haq and Ladbury, 2000). Practical considerations regarding heat capacity and other calorimetric measure-ments of DNA/ligand interactions are presented in Haq etal. (2000). Detailed calorimetric,structural and theoretical studies on a number of ligands have focused on identifying spe-cific differences in the binding modes of intercalators and groove binders, and the targetingof ligands to duplex, triplex and tetraplex nucleic acid structures. Thermodynamics driving minor groove binding and intercalation, studied by ITC The best studied minor groove binder is the dye Hoechst 33258, which binds to AT-rich domains of double-stranded DNA. ITC studies in conjunction with other techniques(Haqet al., 1997; Han et al.,2005) have shown that at physiologically-relevant tempera-tures, the binding of Hoechst 33258 is entropically driven, with a free energy change of-11.7 kcal mol at 25 oC, and a large negative change in heat capacity (-330 cal molK-1).Hydration provides a large contribution to the binding entropy, about 71 kcal mol-1 K-1,consistent with each bound Hoechst 33258 displacing about 55 water molecules from theminor groove of the DNA (Han et al., 2005). Configurational, rotational and translationalcontributions to entropy from both the dye and the DNA were determined to be insignifi-cant, and the binding of the ligand is accompanied by the displacement of only a singlecation (typically Nat) from the minor groove (Han et al., 2005). These analyses show that the binding of Hoechst 33258 to DNA is overwhelmingly driven by hydrophobic effects,with new hydrogen bonds between the ligand and the DNA replacing hydrogen bonds fromthe displaced water molecules. This conclusion was somewhat unexpected, as NMR andcrystallographic structures indicated a fairly optimized network of hydrogen bonds and vander Waals interactions between the ligand and DNA. In the event, these interactions wereactually shown to be slightly unfavorable. Much of the impetus behind studies such as those on Hoechst 33258 is the desire tounderstand binding thermodynamics sufficiently so that therapeutic agents can be designedto bind tightly to specific stretches of DNA. The results from the Hoechst 33258 studiesserve to highlight the difficulty of designing sequence-specific nucleic acid ligands, sinceaffinity may often be driven primarily by (relatively non-specific) hydrophobic interac-tions. In addition, small molecules can only interact with a few base pairs, which is insuf-ficient to direct them to a unique binding site on the human genome (which would requireon the order of 15 base pairs). Studies comparable to those conducted on Hoechst 33258have been reported for four other minor groove binding ligands (propamidine, netropsin,distamycin and berenil; Haq, 2002) and show that in all cases, binding is accompanied bya large negative change in heat capacity, consistent with changes in solvent accessible sur-face. However, whereas the binding of Hoechst 33258 is entopically driven, of these fourligands all but berenil are enthalpically driven, suggesting that it may not yet be possible togeneralize about the binding mechanism of small minor groove ligands. Although the binding of intercalators is also accompanied by a negative change inheat capacity, ITC experiments with five intercalating ligands indicated that this change isin general smaller than that for minor groove binding ligands (Ren et al., 2000). The changein free energy due to hydration effects was large, showing that the transfer of the ligandfrom solvent to the intercalation site was a significant factor driving the binding process.However, in contrast to minor groove binding, which is largely driven by hydrophobicinteractions, analysis of all the components contributing to the overall free energy changeaccompanying intercalator binding showed that non-covalent interactions, and in particularhydrogen bonding, contribute as much as hydrophobic interactions to binding (Haq, 2002).There is indeed some evidence that intercalators can be tailored to target DNA sequence-specifically more readily than minor groove binding ligands (Ren et al., 2000). However,caution is required when designing and interpreting ITC experiments with intercalators, asat high concentrations, intercalators can stack and form self complexes. DNA sequences designed to fold into triplexes which recognize gene-specificstretches of DNA, or which interact with transcription factors that bind DNA, have beencharacterized thermodynamically (Jenkins, 2000), as have G-rich single-stranded DNAmolecules that fold into tetraplex conformations and target telomerase, an enzyme over-expressed in tumor cells (Olsen et al., 2006). To date, however, too few thermodynamicstudies have been completed to allow general conclusions regarding the energetics drivingthese higher-order structure interactions to be drawn. DSC studies of DNA-ligand binding. Although ITC is generally the most direct approach for measuring binding con-stants, there are situations when estimating binding constants by DSC is preferable: name-ly, when the binding constant is very high, or when the ligand is too poorly soluble toprepare a solution to titrate into a DNA sample. The principle and experimental approachbehind estimating binding constants from DSC scans of ligand/receptor complexes is de-scribed in detail in the TA application note entitled Characterizing protein/ligand bindingby DSC. Revisiting these principles in the context of nucleic acids, if a small molecule suchas a groove binder or intercalator binds preferentially to duplex DNA, it will stabilize the duplex structure and elevate its melting temperature (alternatively, if the ligand binds pref-erentially to single-stranded DNA, the melting temperature of the sample will decrease asthe equilibrium shifts towards the single-stranded structure). If the double-stranded DNAis saturated with ligand, if the ligand does not bind to single-stranded DNA, and if the en-thalpy of the melting of the DNA is known, the shift in the midpoint of the thermal unfold-ing can be used to estimate the binding constant of the ligand: where T and Tm are the melting temperature in Kelvin of the DNA and the DNA-ligandcomplex, respectively, R is the gas constant, AHis the enthalpy of melting a DNA basepair, K is the binding constant for the ligand at T, L is the ligand concentration and n isthe size of the binding site expressed in base pairs (Crothers, 1971; McGee, 1976; Spinkand Wellman, 2001). AHis determined from the number of base pairs and the enthalpyof denaturation of the DNA alone, while n is determined either by ITC or from fitting melt-ing curves at various non-saturating ligand concentrations (Chaires 1998). At non-saturat-ing concentrations, complex DSC thermograms are obtained arising from the dissociationof ligand from a thermally-unfolded portion of the DNA molecule, followed by transientbinding to an intact portion of the sequence (Crothers, 1971; McGee, 1976; Spink andChaires, 1997; Leng et al., 1998). Obtaining thermograms over a range of DNA-ligandconcentrations provides detailed thermodynamic information on the binding process, asdescribed in Spink and Wellman (2001). Although the DSC approach is less direct than ITC, DSC can measure extremelyhigh binding constants. This is because DSC does not depend on a signal from the ligand,and so the ligand can be present at an extremely low concentration. In addition, since sam-ples can be prepared by equilibrating DNA solutions with solid ligand, the binding of verysparingly soluble compounds can be studied. However, a drawback of the DSC approachis that the binding constant can only be estimated at the temperature of unfolding of thecomplex, which is unlikely to be the temperature of interest (often 37 oC). Extrapolationfrom the unfolding temperature to the temperature of interest requires knowledge of thechange in heat capacity for binding over that temperature range, or making the assumption(usually incorrect) that the heat capacity does not change over that temperature range. Nucleic acid/protein binding interactions In 1994, Spolar and Record published a study of the forces driving DNA site-spe-cific recognition and binding by proteins. Prior to this it was known that structural changesoccurred to double-stranded DNA when it bound to a protein, ranging from relatively mi-nor deformations to sharp bending of the helical axis and disruption of base-pair stacking.Spolar and Record showed that site-specific protein-DNA recognition is accompanied by alarge negative change in heat capacity, resulting from the removal of a large amount of wa-ter from non-polar surfaces during the binding event and a significant increase in the orderof the protein at the binding site. Numerous studies since have demonstrated the general-ity of these conclusions, as reviewed in Jayaram and Jain (2004) and von Hippel (2007),amongst others. Arguably the most detailed analyses of the thermodynamics driving DNA/proteinbinding have been by Privalov and coworkers. From a large body of publications, two areparticularly noteworthy. In the first paper (Dragan et al., 2004), DSC was used to study thethermodynamics driving DNA binding to two versions of a high-affinity DNA binding do-main called the HMG box: sequence-specific HMG boxes, which show a strong preference for a specific DNA sequence, and non-sequence specific HMG boxes,which bind a widerange of DNA sequences. Both categories of protein are partially unfolded in the absenceof DNA, and become fully folded when DNA is bound. DSC showed that DNA binding tosequence-specific HMG boxes is accompanied by essentially no change in enthalpy anda negative change in heat capacity, while binding to non-sequence-specific HMG boxesis accompanied by a positive enthalpy change and a positive change in heat capacity. Theauthors conclude that the formation of DNA/sequence-specific complexes is specified byincreased packing between non-polar groups, whereas the formation of non-sequence-spe-cific complexes is driven mainly by electrostatics. The second noteworthy paper (Privalov and Dragan, 2007) is a review of the ther-modynamics driving protein binding to either the major or minor groove of DNA. Since theproteins become more ordered after binding DNA, this needs to be taken into account when Fig. 1. (a) The heat capacity functions ofa DNA binding domain (the HMG box fromthe protein LRF-1), the domain's target DNA duplex, and the DNA-domain complex.The uncomplexed protein slowly unfolds over a broad, low temperature range, where-as upon complexation with its DNA target, the protein folds and forms a stable com-plex that dissociates and unfolds cooperatively at approximately 62C. The heat ofprotein unfolding at any temperature can be determined by integrating the differencebetween the sum ofthe heat capacity of the free protein and the free DNA (dot-dashedline) and the heat capacity ofthe complex (black solid line).(b) The enthalpy of bind-ing of the LRF-1 HMG box with its target DNA was measured by ITC at various tem-peratures, then the values were corrected for the heat of protein folding upon bindingDNA. The corrected curve corresponds to the enthalpy of binding of the fully foldedDNA binding domain with its target DNA sequence. Solutions for all experimentswere extensively dialyzed against 10 mM potassium phosphate (pH6.0), 100 mMKCl. Macromolecule concentrations for the DSC experiment were 0.2 mM. Sampleswere scanned on a TA Nano-DSC at 1 ℃/min. ITC experiments were conducted ona TA Nano-ITC. Protein (0.2 mM) in the syringe was titrated into DNA (16 uM) inthe sample cell in 5 uL increments until the protein was saturated with DNA and nofurther binding occurred. Data from Privalov et al., 2007; used with permission. analyzing temperature-dependant ITC data, as the observed titration heats are comprisedof both the heat of binding and the heat of refolding. Following this correction (essentiallyaccounting for the change in heat capacity of the protein), the enthalpies measured fromITC data at different temperatures followed a straight line when plotted vs. temperature,rather than a curved function (Fig.1). The results from over 20 DNA binding proteinsshow, without exception, that the enthalpy change for protein binding to the minor grooveis always positive, while the enthalpy change for binding to the major groove is alwaysnegative. However, the free energy of binding is similar for both categories of protein, in-dicating that the enthalpic differences are balanced by entropic factors. In fact, the entropiccontribution of minor groove binding is larger than that of major groove binding. The dif-ferent energy profiles for these two classes of protein are apparently due to differences inhydration of the major and minor grooves, with water being highly ordered in the minorgroove, and substantially less so in the major groove. Thus, the removal of water fromthe minor groove upon protein binding is a major driving force for minor groove binding,while major groove binding appears to be driven by increased hydrogen bonding and vander Waals interactions. CONCLUSIONS The non-covalent interactions between nucleic acids and their small molecule andmacromolecule ligands are governed by the same physical-chemical factors as those gov-erning protein-small molecule and protein-protein interactions. However, due to the highlyionic nature of nucleic acids, and the partially unfolded structures of nucleic acid bindingproteins in the absence of their ligand,studying these systems can be challenging. Accord-ingly, the models used for more ‘typical' proteins cannot be applied indiscriminately tonucleic acid systems. Used correctly, however, calorimetry provides the most sensitive andreliable approach available for understanding the thermodynamics controlling the expres-sion and regulation of genes. REFERENCES: 1. Buurma, N. J. and I. Haq(2007). Advances in the analysis of isothermal titrationcalorimetry data for ligand-DNA interactions. Methods 42,162-172. 2. Chaires,J. B. (1996).Dissecting the free energy of drug binding to DNA. Anti Can-cer Drug Des. 11,569-580. 3. Chaires,J. B. (1998). Energetics of drug-DNA interactions. Biopolymers 44, 201-215. 4. Cooper, A. (1999).Thermodynamic analysis of biomolecular interactions. Curr.Opin. Chem. Biol. 3,557-563. 5. Cooper, A. (2005). Heat capacity effects in protein folding and ligand binding: are-evaluation of the role of water in biomolecular thermodynamics. Biophys. Chem.115,89-97. 6. Crothers, D. M. (1971). Statistical thermodynamics of nucleic acid melting transi-tions with coupled binding equilibria. Biopolymers 10, 2147-2160. .7 Dragan, A. I. et al. (2004). DNA binding and bending by HMG boxes: energeticdeterminants of specificity. J. Mol. Biol. 343, 371-393. 8. Gohlke, H. and G. Klebe (2002). Approaches to the description and prediction ofthe binding affinity of small-molecule ligands to macromolecular receptors. Angew.Chem.Int. Ed. 41,2644-2676. 9. Han, F., N. Taulier and T. V. Chalikian (2005). Association of the minor groovebinding drug Hoechst 33258 with d(CGCGAATTCGCG)2: volumetric, calorimet-ric and spectroscopic characterizations. Biochemistry 44,9785-9794. 10. Haq, I., J. E. Ladbury, B. Z. Chowdhry, T. C. Jenkins and J. B. Chaires (1997). Spe-cific binding of Hoechst 33258 to the d(CGCAAATTGCG)2 duplex: calorimetricand spectroscopic studies. J. Mol. Biol. 271,244-257. 11. Haq, I. and J. Ladbury (2000). Drug-DNA recognition: energetics and implicationsfor drug design. J. Mol. Recognit. 13, 188-197. 12. Haq, I., T. C. Jenkins, B. Z. Chowdhry, J. Ren andJ. B. Chaires (2000). Parsing freeenergies of drug-DNA interactions. Meth. Enzymol.323, 373-405. 13. Haq, I., B. Z. Chowdhry and T. C. Jenkins (2001). Calorimetric techniques in thestudy of high-order DNA-drug interactions. Meth. Enzymol.340, 109-149. 14. Haq, I. (2002). Thermodynamics of drug-DNA interactions. Archives Biochem. Bio-phys. 403,1-15. 15. Jayaram, B. and T. Jain (2004). The role of water in protein-DNA recognition.Annu.Rev. Biophys. Biomol. Struct. 33, 343-361. ( 16 Jenkins, T. C. (2000). Targeting multi-stranded DNA structures. Curr. Med. Chem. 7,99-115. ) 17. Leng, F., W. Priebe and J. B. Chaires (1998). Ultratight DNA binding of a new bisin-tercalating anthracycline antibiotic. Biochemistry37,1743-1753. 18. McGee,J.D.(1976). Theoretical calculations of the helix-coil transistion of DNA inthe presence of large, cooperatively binding ligands. Biopolymers 15, 1345-1375. 19. Mikulecky, P.J. and A. L. Feig (2006). Heat capacity changes associated with nu-cleic acid folding. Biopolymers 82, 38-58. 20. O'Brien,R., J.E. Ladbury and B. Z. Chowdry. (2001) Isothermal titration calorim-etry of biomolecules. p. 263-286. In S. E. Harding and B. Z. Chowdry (Eds.) Pro-tein-Ligand Interactions: hydrodynamics and calorimetry. Oxford University Press,Oxford. .21. Olsen, C. M., W. H. Gmeiner and L. A. Marky (2006). Unfolding of G-quadru-plexes: Energetic, and ion and water contributions of G-quartet stacking. J. Phys.Chem.B, 110, 6962-6969. 22. Privalov, P. L. (2007). Thermodynamic problems in structural molecular biology.Pure Appl. Chem.79, 1445-1462. 23. Privalov, P. L. and A. I. Dragan (2007). Microcalorimetry of biological macromol-ecules. Biophys. Chem. 126,16-24. ( 24. Privalov, P. L. et al. (2007). What drives proteins into the major or minor grooves of DNA?J.Mol. Biol. 365.1-9. ) 25 Ren, J., T. C. Jenkins and J. B. Chaires (2000). Energetics of DNA intercalationreactions. Biochemistry 39, 8439-8447. 26. Spink, C. H. andJ. B. Chaires (1997). Thermodynamics of the binding of a cationiclipid to DNA. J. Am. Chem. Soc. 119, 10920-10928. .27. Spink, C. H. and S. E. Wellman (2001). Thermal denaturation as a tool to studyDNA-ligand interactions. Methods Enzymol. 340,193-211. 28. Spolar, R. S. and M. T. Record (1994). Coupling of local folding to site-specificbinding of proteins to DNA. Science 263, 777-784. 29. von Hippel, P. H. (2007). From ‘simple'DNA-protein interactions to the macro-molecular machines of gene expression. Annu. Rev. Biophys. Biomol. Struct. 36,79-105. M The expression or replication of genes is affected by the binding of small molecule ligands and proteins to nucleic acid sequences. Such binding events are critical for the physiological integrity of organisms and therefore are of fundamental interest to life scientists. Recently, the thermodynamics driving these interactions have also become important to pharmaceutical scientists investigating the anticancer, antibacterial and antiviral potential of nucleic acid/ligand interactions. In addition, as the number of diseases identified as being due to a malfunction of cellular control processes increases, the possibility of treating disorders by manipulating gene expression is further focusing attention on the thermodynamics underlying nucleic acid binding affinity and specificity.
确定








还剩7页未读,是否继续阅读?
TA仪器为您提供《蛋白质中结合反应检测方案(差示扫描量热)》,该方案主要用于其他中结合反应检测,参考标准--,《蛋白质中结合反应检测方案(差示扫描量热)》用到的仪器有TA仪器+Affinity ITC+等温滴定微量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多















