
方案详情
文
通过活体组织提供一个直接的的定量检测同时测量热率产生和氧气利用率,意味着在代谢状态下面对改变生理和环境条件检测细微变化。The ratio of calorimetrically measured heat flux to the respirometrically measured oxygen flux is often called the “calorimetric-respirometric ratio” or CR ratio.
方案详情

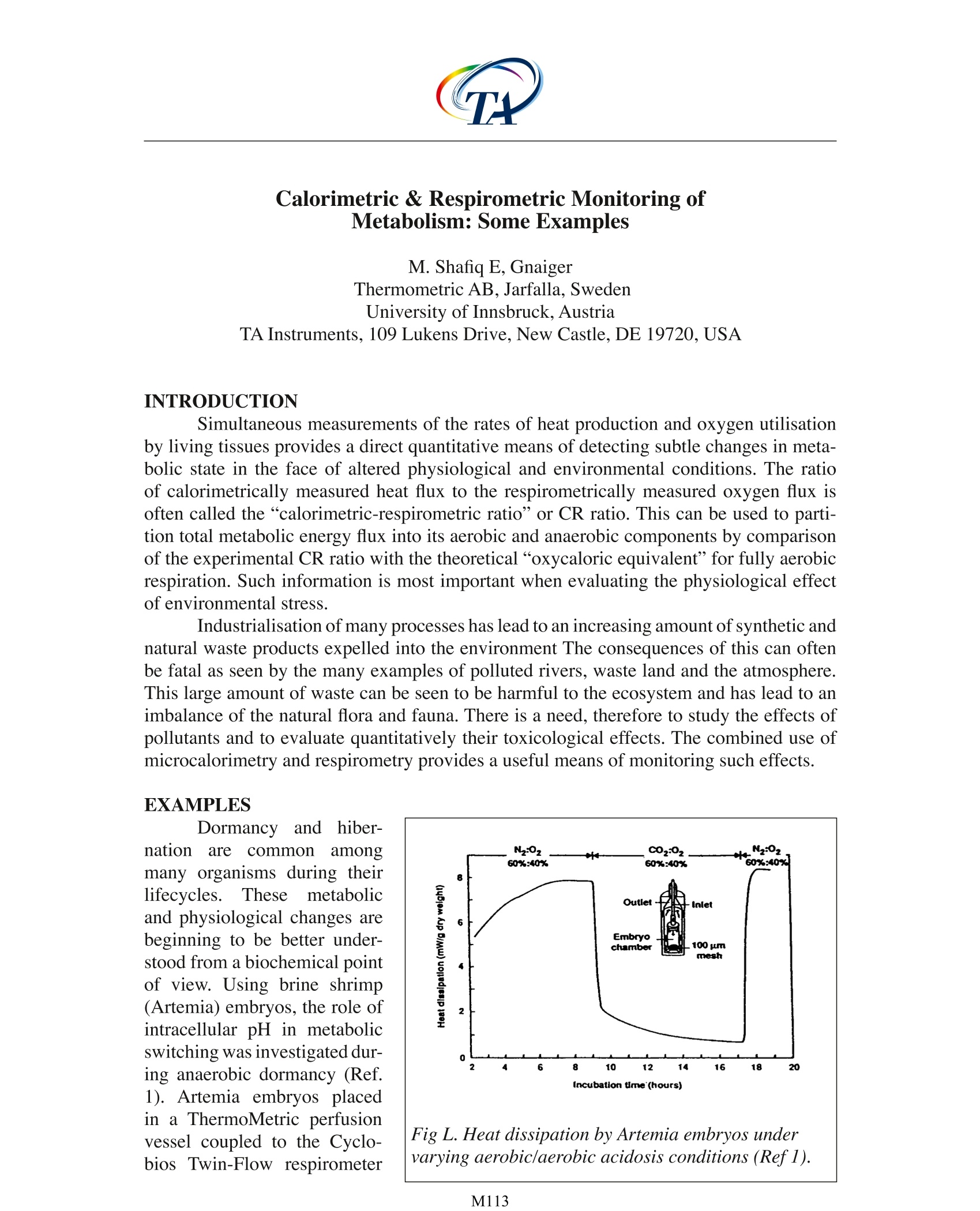
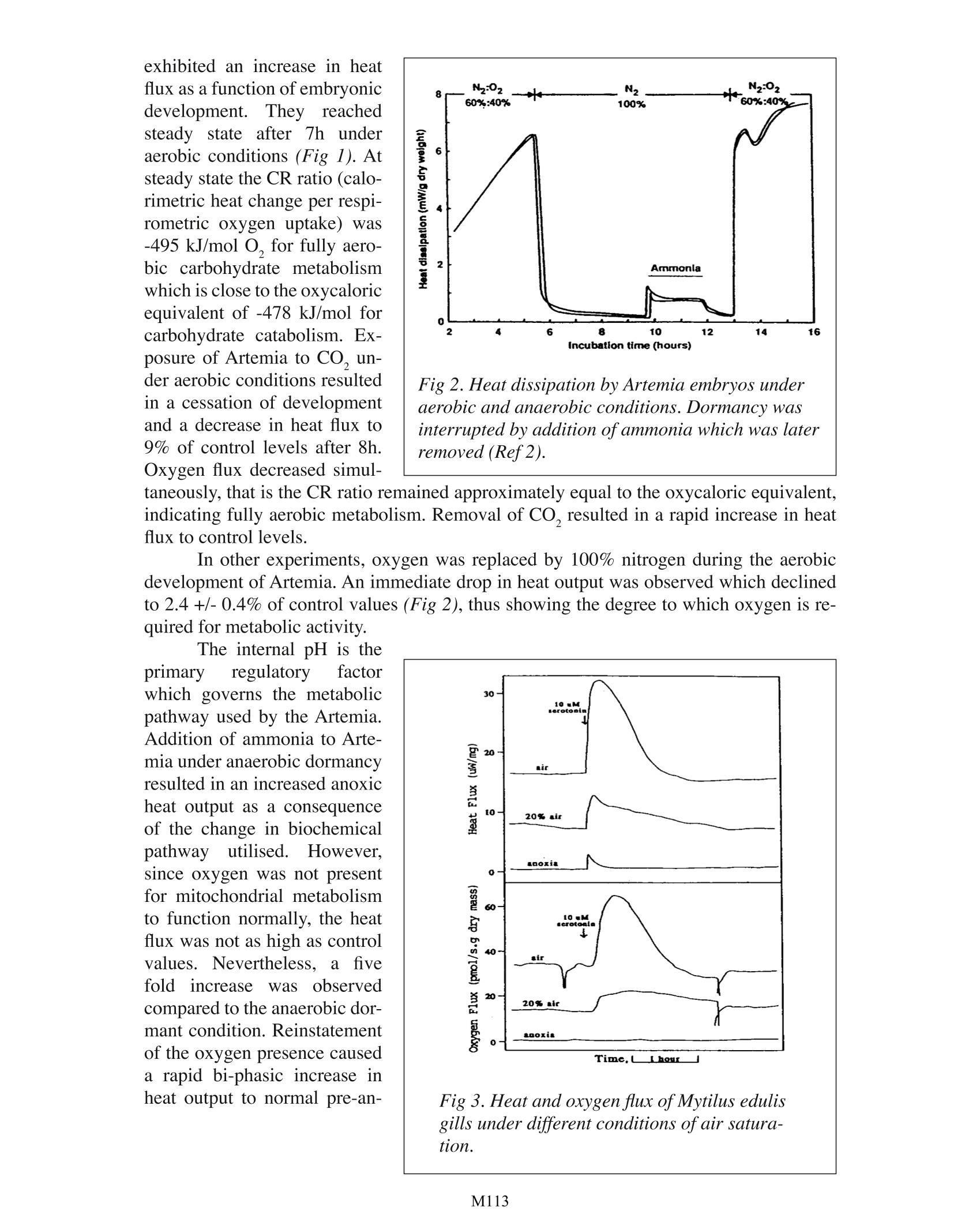
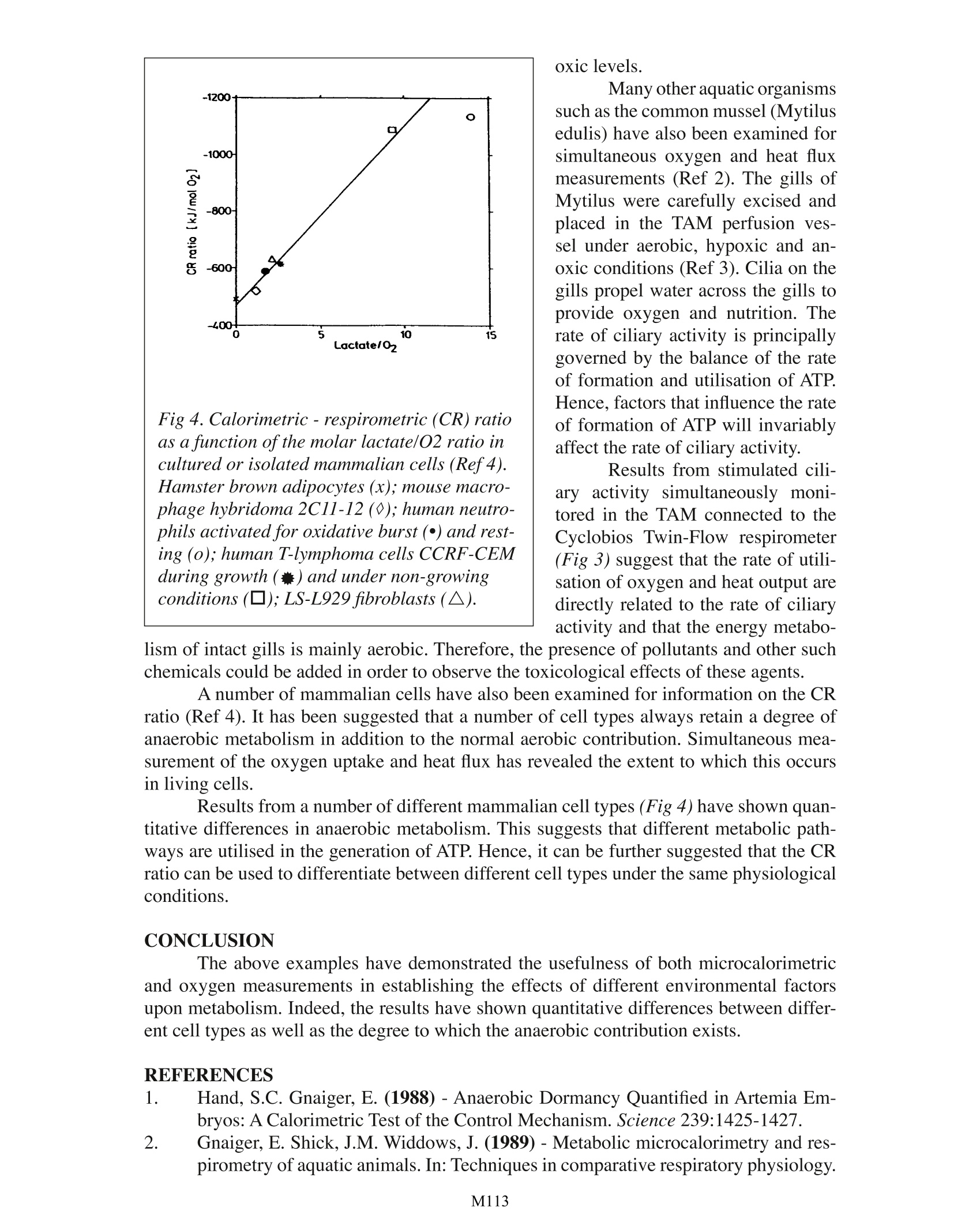
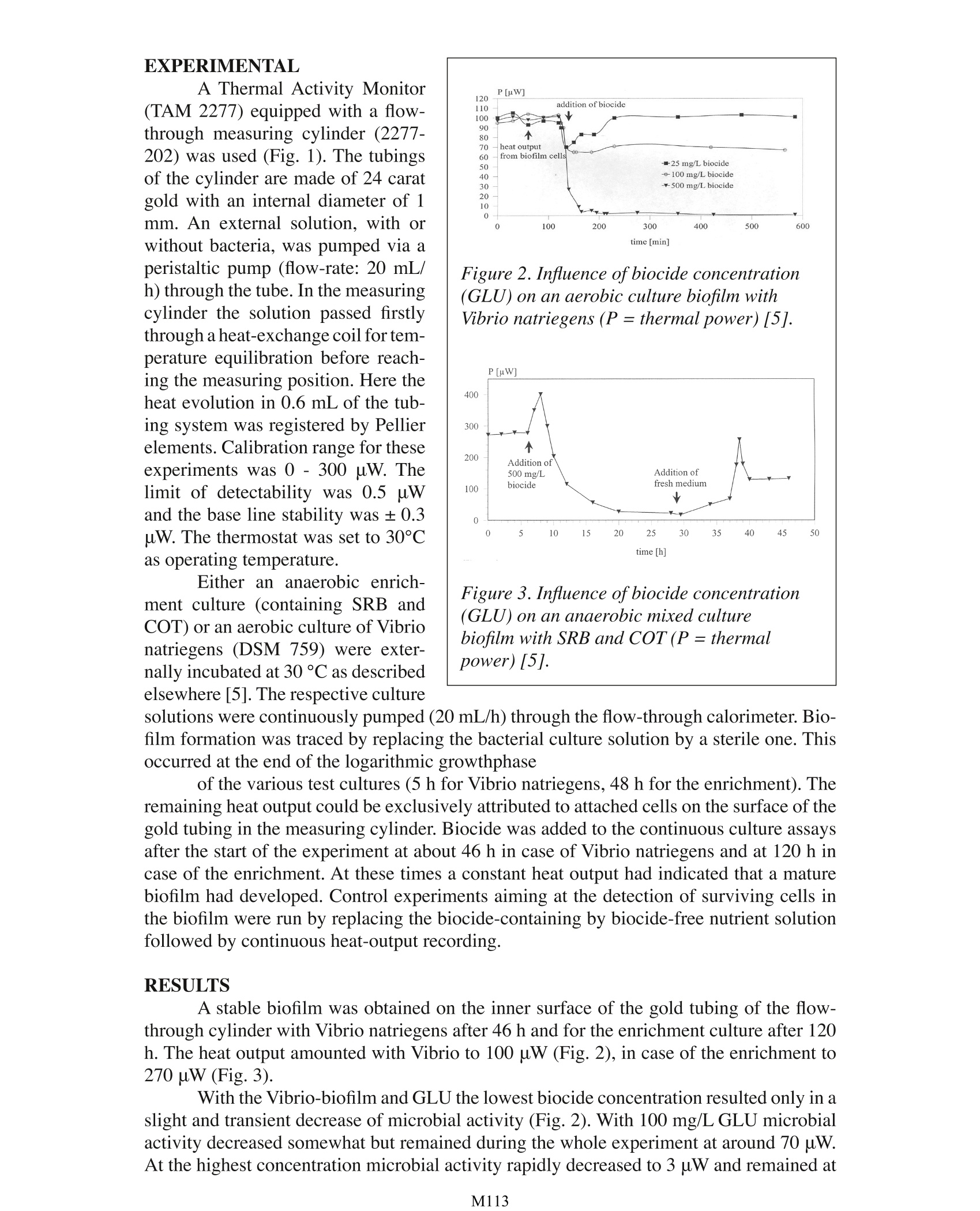
Calorimetric & Respirometric Monitoring ofMetabolism: Some Examples M. Shafiq E, Gnaiger Thermometric AB, Jarfalla,Sweden University of Innsbruck, Austria TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA INTRODUCTION Simultaneous measurements of the rates of heat production and oxygen utilisationby living tissues provides a direct quantitative means of detecting subtle changes in meta-bolic state in the face of altered physiological and environmental conditions. The ratioof calorimetrically measured heat flux to the respirometrically measured oxygen flux isoften called the “calorimetric-respirometric ratio”or CR ratio. This can be used to parti-tion total metabolic energy flux into its aerobic and anaerobic components by comparisonof the experimental CR ratio with the theoretical“oxycaloric equivalent”for fully aerobicrespiration. Such information is most important when evaluating the physiological effectof environmental stress. Industrialisation of many processes has lead to an increasing amount of synthetic andnatural waste products expelled into the environment The consequences of this can oftenbe fatal as seen by the many examples of polluted rivers, waste land and the atmosphere.This large amount of waste can be seen to be harmful to the ecosystem and has lead to animbalance of the natural flora and fauna. There is a need, therefore to study the effects ofpollutants and to evaluate quantitatively their toxicological effects. The combined use ofmicrocalorimetry and respirometry provides a useful means of monitoring such effects. EXAMPLES Dormancyand hiber-nation are common2amongmany organisms during theirlifecycles. These metabolicand physiological changes arebeginning to be better under-stood from a biochemical pointof view. Using brine shrimp(Artemia) embryos, the role ofintracellular pH in metabolicswitching was investigated dur-ing anaerobic dormancy (Ref.1). Artemia embryos placedin a ThermoMetric perfusionvessel coupled to the Cyclo-biosTwin-Flow respirometer Fig L. Heat dissipation by Artemia embryos under varying aerobic/aerobic acidosis conditions (Refl). exhibited an increase in heatflux as a function of embryonicdevelopment. They reachedsteady state eafter 7hh uunderaerobic conditions (Fig 1). Atsteady state the CR ratio (calo-rimetric heat change per respi-rometric oxygen uptake) was-495 kJ/mol O, for fully aero-bic carbohydrate metabolismwhich is close to the oxycaloricequivalent of -478 kJ/mol forcarbohydrate catabolism. Ex-posure of Artemia to CO, un-der aerobic conditions resultedin a cessation of developmentand a decrease in heat flux to9% of control levels after 8h.Oxygen flux decreased simul- Fig 2. Heat dissipation by Artemia embryos underaerobic and anaerobic conditions. Dormancy wasinterrupted by addition ofammonia which was laterremoved (Ref2). taneously, that is the CR ratio remained approximately equal to the oxycaloric equivalent,indicating fully aerobic metabolism. Removal of CO, resulted in a rapid increase in heatflux to control levels. In other experiments, oxygen was replaced by 100% nitrogen during the aerobicdevelopment of Artemia. An immediate drop in heat output was observed which declinedto 2.4 +/-0.4% of control values (Fig 2), thus showing the degree to which oxygen is re-quired for metabolic activity. The internal pH is theprimary regulatory factorwhich governs the metabolicpathway used by the Artemia.Addition of ammonia to Arte-mia under anaerobic dormancyresulted in an increased anoxicheat output as a consequenceof the change in biochemicalpathway/ lutilised. However,since oxygen was not presentfor mitochondrial metabolismto function normally, the heatflux was not as high as controlvalues. Nevertheless,a fivefold increasewas observedcompared to the anaerobic dor-mant condition. Reinstatementof the oxygen presence causeda rapid bi-phasic increase inheat output to normal pre-an- Fig 3. Heat and oxygen flux of Mytilus edulisgills under different conditions ofair satura-tion. Many other aquatic organismssuch as the common mussel (Mytilusedulis) have also been examined forsimultaneous oxygen and heat fluxmeasurements (Ref2). The gills ofMytilus were carefully excised andplaced in the TAM perfusion ves-sel under aerobic, hypoxic and an-oxic conditions (Ref 3). Cilia on thegills propel water across the gills toprovide oxygen and nutrition. Therate of ciliary activity is principallygoverned by the balance of the rateof formation and utilisation of ATP.Hence, factors that influence the rateof formation of ATP will invariablyaffect the rate of ciliary activity.Results from stimulated cili-ary activity simultaneously moni-tored in the TAM connected to theCyclobios Twin-Flow respirometer(Fig 3) suggest that the rate of utili-sation of oxygen and heat output aredirectly related to the rate of ciliaryactivity and that the energy metabo-lism of intact gills is mainly aerobic. Therefore, the presence of pollutants and other suchchemicals could be added in order to observe the toxicological effects of these agents. Fig 4. Calorimetric-respirometric (CR) ratioas a function of the molar lactate/O2 ratio incultured or isolated mammalian cells (Ref4).Hamster brown adipocytes (x); mouse macro-phage hybridoma 2C11-12(0); human neutro-phils activated for oxidative burst (·) and rest-ing (o); human T-lymphoma cells CCRF-CEMduring growth (*) and under non-growingconditions (口); LS-L929 fibroblasts (A). oxic levels. A number of mammalian cells have also been examined for information on the CRratio (Ref 4). It has been suggested that a number ofcell types always retain a degree ofanaerobic metabolism in addition to the normal aerobic contribution. Simultaneous mea-surement of the oxygen uptake and heat flux has revealed the extent to which this occursin living cells. Results from a number of different mammalian cell types (Fig 4) have shown quan-titative differences in anaerobic metabolism. This suggests that different metabolic path-ways are utilised in the generation of ATP. Hence, it can be further suggested that the CRratio can be used to differentiate between different cell types under the same physiologicalconditions. CONCLUSION The above examples have demonstrated the usefulness of both microcalorimetricand oxygen measurements in establishing the effects of different environmental factorsupon metabolism. Indeed, the results have shown quantitative differences between differ-ent cell types as well as the degree to which the anaerobic contribution exists. REFERENCES 1. Hand, S.C. Gnaiger, E. (1988)- Anaerobic Dormancy Quantified in Artemia Em-bryos: A Calorimetric Test of the Control Mechanism. Science 239:1425-1427. 2 Gnaiger, E. Shick, J.M.Widdows,J. (1989)-Metabolic microcalorimetry and res-pirometry of aquatic animals. In: Techniques in comparative respiratory physiology. An experimental approach. (Bridges, CR. Butler, P.J. eds), Soc. Exp. Biol. SeminarSeries, Cambridge Univ. Press, London:113-135. 3. Doeller, J.E. Kraus,D. W.Gnaiger, E. Shick, J.M. (1990)-Calorespirometry andspectrophotometry of the ciliated gill of the marine mussel Mytilus edulis. Thermo-chim. Acta 172:171-178. 4. Gnaiger, E. Kemp,R.B. (1990)-Anaerobic metabolism in aerobic mammalian cells:information from the ratio of calorimetric heat flux and respirometric oxygen flux.Biochimica et Biophysica Acta 1016:328-332. NOTE Thermometric AB collaborates with Cyclobios (Austria) in the improvement ofCalor-respirometric systems. EXPERIMENTAL A Thermal Activity Monitor(TAM 2277) equipped with a flow-through measuring cylinder (2277-202) was used (Fig. 1). The tubingsof the cylinder are made of 24 caratgold with an internal diameter of 1mm. An external solution, with orwithout bacteria, was pumped via aperistaltic pump (flow-rate: 20 mL/h) through the tube. In the measuringcylinder the solution passed firstlythrough a heat-exchange coil for tem-perature equilibration before reach-ing the measuring position. Here theheat evolution in 0.6 mL of the tub-ing system was registered by Pellierelements. Calibration range for theseexperiments was 0 - 300 uW. Thelimit of detectability was 0.5 pWand the base line stability was ±0.3uW. The thermostat was set to 30°Cas operating temperature. Either an anaerobic enrich-ment culture (containing SRB andCOT) or an aerobic culture of Vibrionatriegens (DSM 759) were exter-nally incubated at 30 °C as describedelsewhere [5]. The respective culture Figure 2. Influence of biocide concentration(GLU) on an aerobic culture biofilm withVibrio natriegens (P=thermal power) [5]. Figure 3. Influence of biocide concentration(GLU) on an anaerobic mixed culturebiofilm with SRB and COT (P= thermalpower)[5]. solutions were continuously pumped (20 mL/h) through the flow-through calorimeter. Bio-film formation was traced by replacing the bacterial culture solution by a sterile one. Thisoccurred at the end of the logarithmic growthphase of the various test cultures (5 h for Vibrio natriegens, 48 h for the enrichment). Theremaining heat output could be exclusively attributed to attached cells on the surface of thegold tubing in the measuring cylinder. Biocide was added to the continuous culture assaysafter the start of the experiment at about 46 h in case of Vibrio natriegens and at 120 h incase of the enrichment. At these times a constant heat output had indicated that a maturebiofilm had developed. Control experiments aiming at the detection of surviving cells inthe biofilm were run by replacing the biocide-containing by biocide-free nutrient solutionfollowed by continuous heat-output recording. RESULTS A stable biofilm was obtained on the inner surface of the gold tubing of the flow-through cylinder with Vibrio natriegens after 46 h and for the enrichment culture after 120h. The heat output amounted with Vibrio to 100 pW (Fig. 2), in case of the enrichment to270 pW (Fig.3). With the Vibrio-biofilm and GLU the lowest biocide concentration resulted only in aslight and transient decrease of microbial activity (Fig.2). With 100 mg/L GLU microbialactivity decreased somewhat but remained during the whole experiment at around 70 uW.At the highest concentration microbial activity rapidly decreased to 3 uW and remained at that level. If in the latter case the biocide-containing medium was replaced by a biocide-free one, metabolic activity became restored to 85 pW after 10 h (not shown). Obviously,several bacteria survive the biocidal action (probably those which are deeply embedded inthe biofilm and thus are protected) and start to regrow. The biofilm of the enrichment culture was tested in the same way. In these experi-ments the biocide concentrations 25 and 100 mg/L remained without any influence on mi-crobial activity (not shown). Only at a concentration of 500 mg/L GLU microbial activity(after a short transient increase) was strongly reduced to 3 uW after 23 h (Fig. 3). Afternutrient solution exchange, biofilm regrowth also occurred. A heat output of 110 pW wasnoted after 18 h of consecutively culturing. DISCUSSION For testing biocide efficacy many techniques are in use. However, plate counts andMPN-techniques remain to be the most widely used ones because of their low cost and theunequivocal results concerning the killing effect of the biocidal agents. The results present-ed here indicate that microcalorimetry has considerable advantages over those techniques.Although the latter is primarily more expensive than the classical methods, it may replacethe former, because it allows to measure rapidly, even online, an inhibition of microbialactivity by biocidal action. Furthermore, in combination with the classical techniques itbecomes possible to differentiate between killing or inhibition as a result of a biocidalaction. Even a remaining effect, due to adsorbed biocide to the biofilm matrix, becomesdetectable. Thus, the time span until a further biocide application becomes necessary, canbe determined. This may be of considerable importance for industries such as paper indus-try, where biocide actions can only reduce but not totally remove microbial biofilm-boundactivity. CONCLUSION Microcalorimetry allows by rapid and online tests to screen biocides for their effi-cacy under a manifold of conditions. However, until now, it is not possible to test biofilmson other surfaces than gold with the flow-through system. Because of the direct response ofthe instrument to any heat evolution, the experimental time becomes considerably reduced.In times, where economical considerations together with environmental responsibility areof utmost importance, the novel technique will surely f nd its place in the biocide busi-ness. REFERENCES 1 Flemming,H.-C. (1995). Chemie Ingenieur Technik 67: 1425-1430. 2Donlan, R.M., Pipes, T.I. and Yohe, T.L. (1994). Wat. Res 28: 1497-1503. 3 Ridgway, H.F., Kellyr A.r Justice, C. and Olson. B.H. (1983). Appl. Environ. Mi-crobiol. 45: 1066-1084 4Foley, I. and Gilbert, P. (1996). Biofouling 10:331-346 5 von Rege, H. and Sand, W. (1998). Evaluation of biocide efficacy by microcalo-rimetric determination of microbial activity in biofilms; J. Microbiol. Methr 33:227-235 M Simultaneous measurements of the rates of heat production and oxygen utilization by living tissues provides a direct quantitative means of detecting subtle changes in metabolic state in the face of altered physiological and environmental conditions. The ratio of calorimetrically measured heat flux to the respirometrically measured oxygen flux is often called the “calorimetric-respirometric ratio” or CR ratio. This can be used to partition total metabolic energy flux into its aerobic and anaerobic components by comparison of the experimental CR ratio with the theoretical “oxycaloric equivalent” for fully aerobic respiration. Such information is most important when evaluating the physiological effect of environmental stress.
确定


还剩4页未读,是否继续阅读?
TA仪器为您提供《蛋白质中热量检测方案(差示扫描量热)》,该方案主要用于其他中热量检测,参考标准--,《蛋白质中热量检测方案(差示扫描量热)》用到的仪器有TA仪器+Affinity ITC+等温滴定微量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多















