
方案详情
文
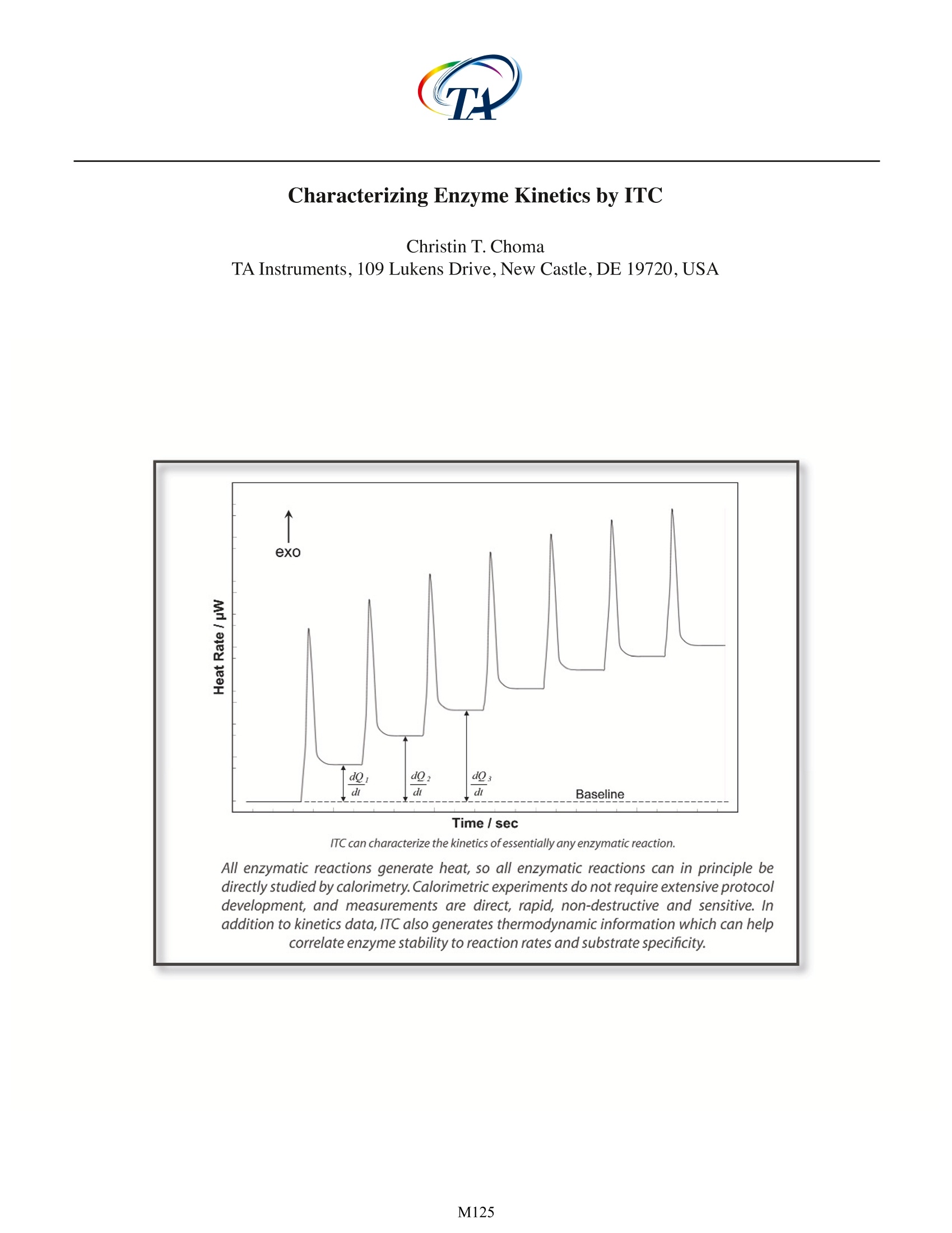
All enzymatic reactions generate heat, so all enzymatic reactions can in principle be directly studied by calorimetry. Calorimetric experiments do not require extensive protocol development, and measurements are direct, rapid, non-destructive and sensitive. In addition to kinetics data, ITC also generates thermodynamic information which can help correlate enzyme stability to reaction rates and substrate specificity.
方案详情

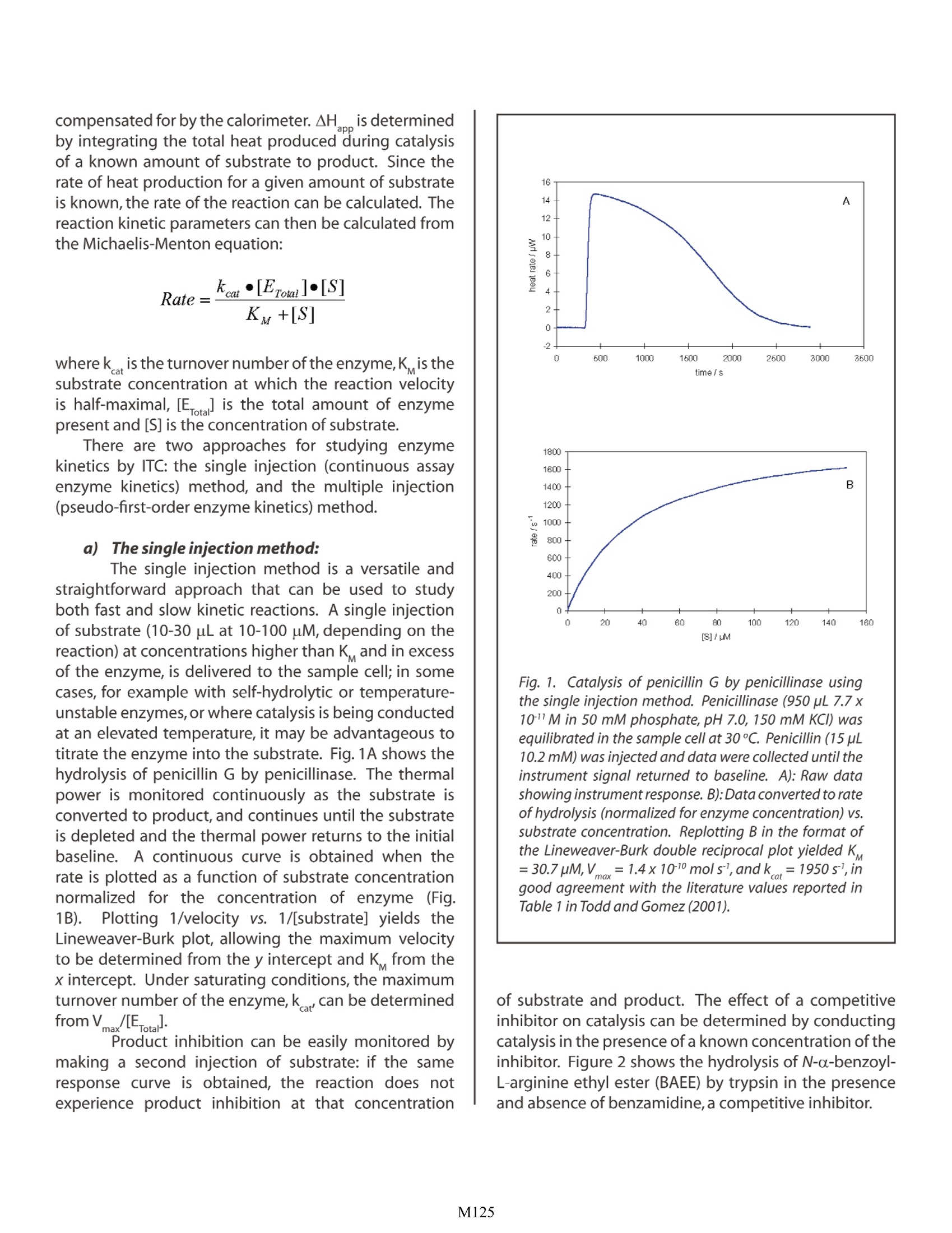
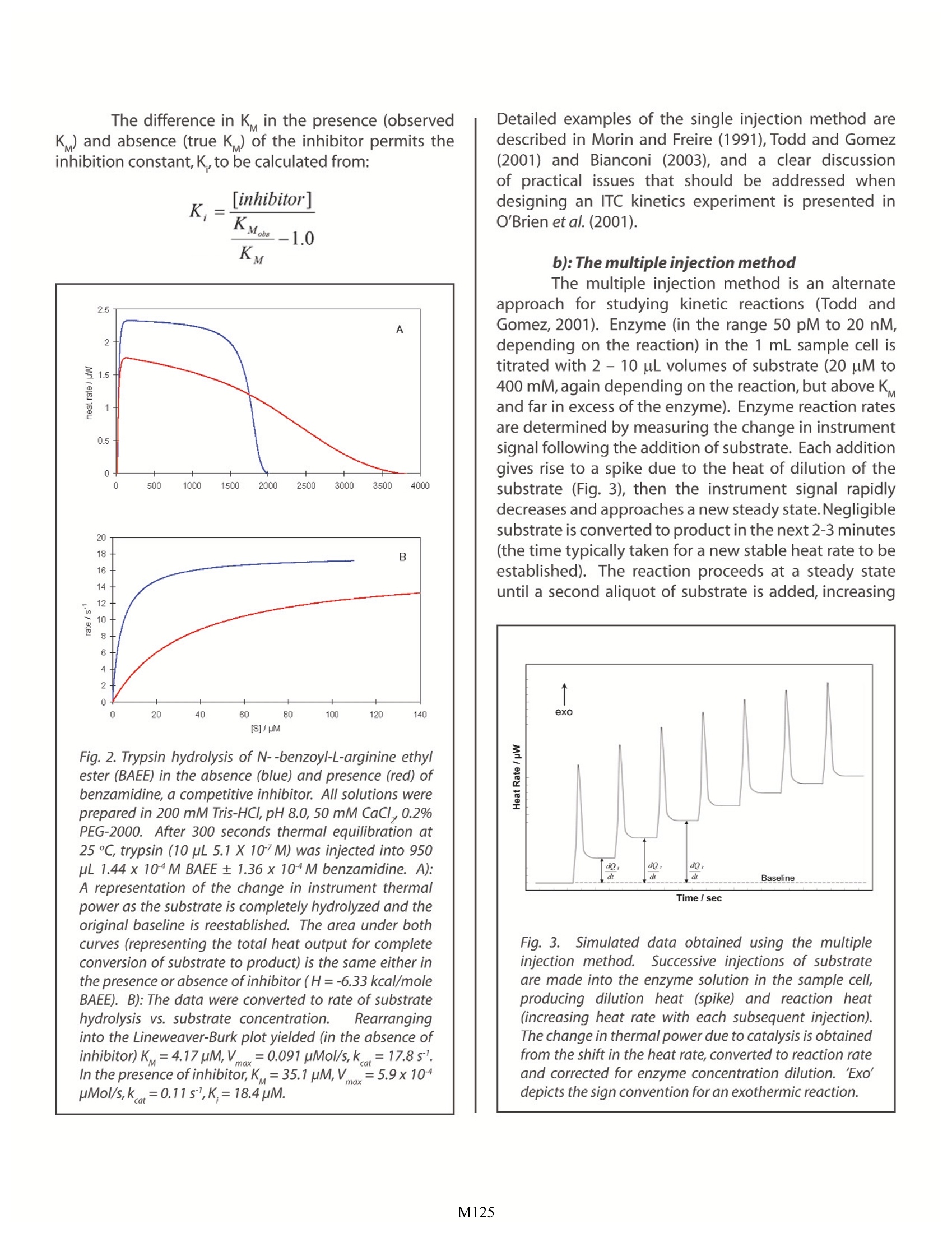
Characterizing Enzyme Kinetics by ITC Christin T. Choma TA Instruments, 109 Lukens Drive,New Castle, DE 19720, USA t has long been believed that enzymes catalyticallycontrol all biochemical processes, and that eachenzyme is specific for a particular substrate andcatalyzes only one reaction.While the first belief remainstrue, recent proteomics and functional genomics studieshave shown that many enzymes have capabilitiesseemingly unrelated to their primary catalytic function,and that some of these secondary capabilities maybe connected to various diseases. For example, someenzymes can accept a range of substrates and directseemingly unrelated catalytic chemistries (‘promiscuity';Bornscheuer and Kazlauskas,2004).In addition,enzymescan exhibit structural or regulatory functions that areunrelated to their primary catalytic role ('moonlighting';Copley, 2003). These findings, coupled with an evolvingunderstanding of the chemical tbasisfor enzymecatalysis (Benkovic andHammes-Schiffer,2003)highlight the necessity of understanding not only howenzymes catalyze reactions, but also why. How doesthermodynamics drive binding and specificity? Whatthermodynamic processes control the conformationalrearrangements necessary to produce the transitionstate? What factors control an enzyme'spromiscuity,anddetermine whether or not it engages in ‘moonlighting'?lf the thermodynamic basis for these various functionscould be established, it might be possible to controlfunctionality (particularly of enzymes involvedindiseases)using, for examplee,, riationally-designedfunction-specific inhibitors. The internal motions of an enzyme are reducedupon substrate binding and transition state formation:hydrogen bonds form and are optimized between thebinding site and the substrate,thus reducing the internalmotions of the enzyme-substrate complex. Importantly,transition state formation improves non-covalent bondsthroughout the entire enzyme,stabilizing the structure.Itappears that this positive change in enthalpy is sufficientto offset the entropic penalty of reducing the dynamicbehavior of the enzyme in the transition state.Enzymesfor which data areavailable show that enzyme-catalyzedreactions are greatly favored in enthalpy, and thatenthalpic factors are largely responsible for the observedcatalytic rate enhancement (Williams et al.,2004). Calorimetry is a direct approach for studying thethermodynamics of enzyme function. There are twogeneral itypes of calorimetry: differential scanning calorimetry (DSC) and isothermal titration calorimetry(ITC). DSC, describedin an accompanying set ofapplication notes,is a powerful approach for determiningthe stability of macromolecules such as enzymesand enzyme-inhibitor complexes. In contrast, ITC isparticularly suited to measuring dynamic events suchas binding and kinetics.This Application Note examinesthe utility of ITC for the analysis of enzymatic reactions.For a general description of the principles behind ITC,its versatility and the types of biological problems thatcan be addressed by this technique, please see CSC'sOverview Note entitled Life science applications of ITC. ITC is widely applicable to the study of enzymekinetics: The power of ITC derives from the universality of thetechnique: every reaction generates or absorbs heat, soevery reaction can in principle be studied by calorimetry.In practiceithasbeen shown that representative enzymesfrom every EC classification can be analyzed kineticallyusing ITC (Todd and Gomez, 2001). In addition, ITCanalyses are rapid, precise,nondestructive, compatiblewith both physiological and synthetic substrates, andare as sensitive as spectroscopic techniques but do notrequire a spectroscopic label or chemical tag. Importantly, ITC analyses of enzyme kinetics arealso straightforward. The sum of the enthalpy (AH) andentropy (AS) of a spontaneous reaction such as enzymecatalysis must result in a decrease in the free energy (AG)of the system: where the enthalpic component is observed as heat.ITC experiments are designed to measure the rate ofheat generation (i.e., dQ/dt, or the thermal power) thusallowing the rate of the reaction to be calculated: whereV is the volume of the sample in the reaction celland AHis the experimentally-determined change inenthalpy. In a typical ITC experiment, buffered substrateis injected into enzyme (dissolved in the same buffer)in the sample cell. Catalytic conversion of the substrateinto product generates heat, which is detected and compensated for by the calorimeter. AH is determinedby integrating the total heat produced during catalysisof a known amount of substrate to product. Since therate of heat production for a given amount of substrateis known,the rate of the reaction can be calculated. Thereaction kinetic parameters can then be calculated fromthe Michaelis-Menton equation: where k is the turnover number of the enzyme,K is thesubstrate concentration at which the reaction velocityis half-maximal, [Ea] is the total amount of enzymepresent and [S] is the concentration of substrate. There are two approaches for studying enzymekinetics by ITC: the single injection (continuous assayenzyme kinetics) method, and the multiple injection(pseudo-first-order enzyme kinetics) method. a))The single injection method: The single injection method is a versatile andstraightforward approach that can be used to studyboth fast and slow kinetic reactions. A single injectionof substrate (10-30 uL at 10-100 uM, depending on thereaction) at concentrations higher than Kand in excessof the enzyme, is delivered to the sample cell; in somecases, for example with self-hydrolytic or temperature-unstable enzymes, or where catalysis is being conductedat an elevated temperature, it may be advantageous totitrate the enzyme into the substrate. Fig. 1A shows thehydrolysis of penicillin G by penicillinase. The thermalpower is monitored continuously as the substrate isconverted to product, and continues until the substrateis depleted and the thermal power returns to the initialbaseline. A continuous curve is obtained when therate is plotted as a function of substrate concentrationnormalized for the concentration of enzyme (Fig.1B). Plotting 1/velocity vs. 1/[substrate] yields theLineweaver-Burk plot, allowing the maximum velocityto be determined from the y intercept and K from thex intercept. Under saturating conditions, the maximumturnover number of the enzyme,k, can be determinedfrom Vmax/[Erotal. Product inhibition can be easily monitored bymaking a second injection of substrate: if the sameresponse curve is obtained, the reaction does notexperience product inhibition at that concentration Fig. 1. Catalysis of penicillin G by penicillinase usingthe single injection method. Penicillinase (950 pL 7.7 x10M in 50 mM phosphate, pH 7.0, 150 mM KCI) wasequilibrated in the sample cell at 30℃. Penicillin (15 uL10.2 mM) was injected and data were collected until theinstrument signal returned to baseline. A): Raw datashowing instrument response. B):Data converted to rateof hydrolysis (normalized for enzyme concentration) vs.substrate concentration. Replotting B in the format of.the Lineweaver-Burk double reciprocal plot yielded K=30.7uM,V==1.4x1010 mols,andk =1950 sl, ingood agreement with the literature values reported inTable 1 in Todd and Gomez (2001). of substrate and product. The effect of a competitiveinhibitor on catalysis can be determined by conductingcatalysis in the presence of a known concentration of theinhibitor. Figure 2 shows the hydrolysis of N-a-benzoyl-L-arginine ethyl ester (BAEE) by trypsin in the presenceand absence of benzamidine, a competitive inhibitor. The difference in K in the presence (observedK ) and absence (true K) of the inhibitor permits theinhibition constant, K., to be calculated from: Fig. 2. Trypsin hydrolysis of N--benzoyl-L-arginine ethy/ester (BAEE) in the absence (blue) and presence (red) ofbenzamidine, a competitive inhibitor. All solutions wereprepared in 200 mM Tris-HCl, pH 8.0, 50 mM CaCl, 0.2%PEG-2000. After 300 seconds thermal equilibration at25 ℃, trypsin (10 uL 5.1 X 10M) was injected into 950uL 1.44 x 10M BAEE±1.36x 10^M benzamidine. A):A representation of the change in instrument thermalpower as the substrate is completely hydrolyzed and theoriginal baseline is reestablished. The area under bothcurves (representing the total heat output for completeconversion of substrate to product) is the same either inthe presence or absence of inhibitor (H=-6.33 kcal/moleBAEE). B): The data were converted to rate of substratehydrolysis vs. substrate concentration. Rearranginginto the Lineweaver-Burk plot yielded (in the absence ofinhibitor)K=4.17pM,V=0.091 pMol/s,k=17.85.In the presence of inhibitor, K =35.1pM,Vmox=5.9x10pMol/s, k=0.11s,K=18.4uM Detailed examples of the single injection method aredescribed in Morin and Freire (1991), Todd and Gomez(2001) and Bianconi (2003), and a clear diof practical issues that should be addressed whendesigning an ITC kinetics experiment is presented in.O'Brien et al. (2001). b): The multiple injection method The multiple injection method is an alternateapproach for studying kinetic reactions (Todd andGomez, 2001). Enzyme (in the range 50 pM to 20 nM,depending on the reaction) in the 1 mL sample cell istitrated with 2- 10 pL volumes of substrate (20 uM to400 mM, again depending on the reaction, but above Kand far in excess of the enzyme). Enzyme reaction ratesare determined by measuring the change in instrumentsignal following the addition of substrate. Each additiongives rise to a spike due to the heat of dilution of thesubstrate (Fig. 3), then the instrument signal rapidlydecreases and approaches a new steady state.Negligiblesubstrate is converted to product in the next 2-3 minutes(the time typically taken for a new stable heat rate to beestablished). The reaction proceeds at a steady stateuntil a second aliquot of substrate is added, increasing Fig. 3. Simulated data obtained using the multipleinjection method..Successive injections of substrateare made into the enzyme solution in the sample cell,producing dilution heat (spike) and reaction heat(increasing heat rate with each subsequent injection).Thechange in thermal power due to catalysis is obtainedfrom the shift in the heat rate,converted to reaction rateand corrected for enzyme concentration dilution. Exo'depicts the sign convention for an exothermic reaction. both the concentration of substrate and the thermalpower generated by the enzymatic reaction (dQ,/dt).Typically 10 - 30 injections are made at 2 - 5 minuteintervals, and the enzyme reaction rate is calculatedas above. The catalysis of penicillin G by penicillinase,followed using the multiple injection method (Fig. 4),provided a K value of 101uM. This is very similar to theliterature value (120 uM) obtained using this technique;see Todd and Gomez (2001) for a complete description ofexperiment design and data analysis using the multipleinjection method. Fig.4. Catalysis of penicillin G by penicillinase using themultiple injection method. Penicillinase (950 pL 7.7 x10"M in 50 mM phosphate, pH 7.0, 150 mM KCI) wasequilibrated in the sample cell at 30 ℃. Penicillin (30.6mM) was injected in 20, 5 pL aliquots at 5 min intervals.Making the conversions described in Fig. 3 transformedthe data into the format shown in Fig. 4,yielding a K of101 pM, similar to the value (120 pM) obtained by thesame technique by Todd and Gomez (2001). Essentially any catalytic system can be studiedby ITC. Measurements are direct, fast, reliable andstraightforward. The amount of biological materialrequired is similar to that for spectroscopic methods,but there is no need for optical transparency or samplehomogeneity, and impurities are tolerated. Importantly,thermodynamic information is generated in addition tokinetics data. As ITC becomes increasingly prominentin the study of enzyme kinetics, the correlation ofstability and reaction rates will help elucidate the subtlestructural changes that thermodynamically control the(often multiple) functionalities of an enzyme. References: (Preference has been given to current references. Citation doesnot imply that a paper is necessarily the original reference to astudy.) ( Benkovic , S. J. and S . H ammes-Schiffer. ( 2003) A perspective o n enzyme ca t alysis. S cience 301, 1 1 96-1202. ) ( Bianconi , M. L. (2003) Calorimetric determination of thermodynamic p arameters of reaction revealsdifferent enthalp i c compensations of the y east hexokinase i sozymes. J .Biol.Chem.278,18709-18713. ) Bornscheuer,U. T. and R. J. Kazlauskas. (2004) Catalyticpromiscuity in biocatalysis: using old enzymes to formnew bonds and follow new pathways. Angew. Chem.Int.Ed.43,6032-6040. ( Copley , S. D. (2003) Enzymes w ith e xtra t alents:moonlighting f u nctions a n d ca t alytic promiscuity. Curr.Opin.Chem.Biol.7,265-272. ) ( Morin,P.E.and E.F r eire.(1991) Direct calorimetric analysis of the enzymatic a ctivity of yeast cytochrome coxidase. Biochemistry 30,8494-8500. ) ( O'Brien, R., J . E . Ladbury and B . Z . Chowdry. ( 2 001) Isothermal t itration calorimetry of b iomolecules. p. 2 63-286. In S. E . Harding a nd B . Z . Chowdry ( E d s.) Protein-Ligan d Interaction s : hydrodynamic s andcalorimetry.Oxford University P ress,Oxford. ) ( Todd, M. J. and J. Gomez. ( 2001) E nzyme kinetics determined u s ing c a lorimetry: a general a s say for enzyme activity? Anal.Biochem. 296,1 7 9-187. ) ( Williams , D . H., E . Stephen s and M. Zhou. ( 2004)C o ntribution t o t he catalytic efficiency of enzymes,and t he b inding o f l igands t o r eceptors, from improvements in packin g withi n enzyme s and receptors. Methods Enzymol.380,3-19. ) M All enzymatic reactions generate heat, so all enzymatic reactions can in principle be directly studied by calorimetry. Calorimetric experiments do not require extensive protocol development, and measurements are direct, rapid, non-destructive and sensitive. In addition to kinetics data, ITC also generates thermodynamic information which can help correlate enzyme stability to reaction rates and substrate specificity.
确定



还剩4页未读,是否继续阅读?
TA仪器为您提供《酶中酶动力学检测方案(差示扫描量热)》,该方案主要用于其他中酶动力学检测,参考标准--,《酶中酶动力学检测方案(差示扫描量热)》用到的仪器有TA仪器+Affinity ITC+等温滴定微量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多















