
方案详情
文
In the continuing search for novel compounds targeting serotonin 5-HT2A, 5-HT2C, and serotonin transporter, new arylpiperazine-containing pyrrole 3 carboxamide derivatives were synthesized and evaluated. Based on the lead reported previously, structural modifications regarding N-(3-(4-(2,3-dichlorophenyl) piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3 carboxamide 5, were accomplished for improvements in not only binding affinity against serotonin receptors and transporter, but also in hERG channel inhibition. Along the line, both the forced swimming tests and spontaneous locomotor activity tests were performed to distinguish between antidepressant activity and false positive results. As potential antidepressant agents, both 2,4-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide and 5-tert-butyl-2-methyl-1H-pyrrole-3-carboxamide derivatives exhibited favorable in vitro and in vivo activities, warranting further investigation around these scaffolds.
方案详情

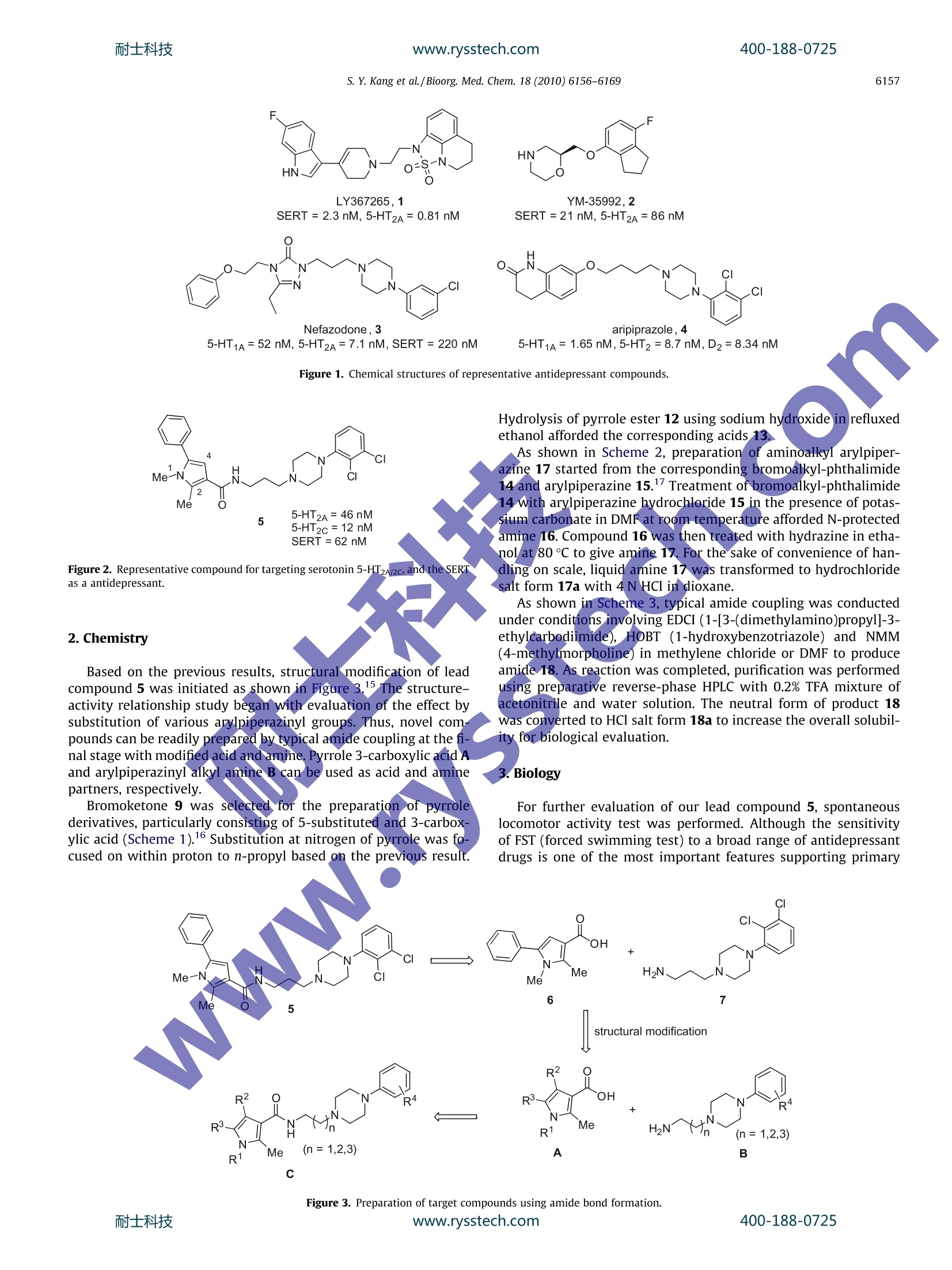
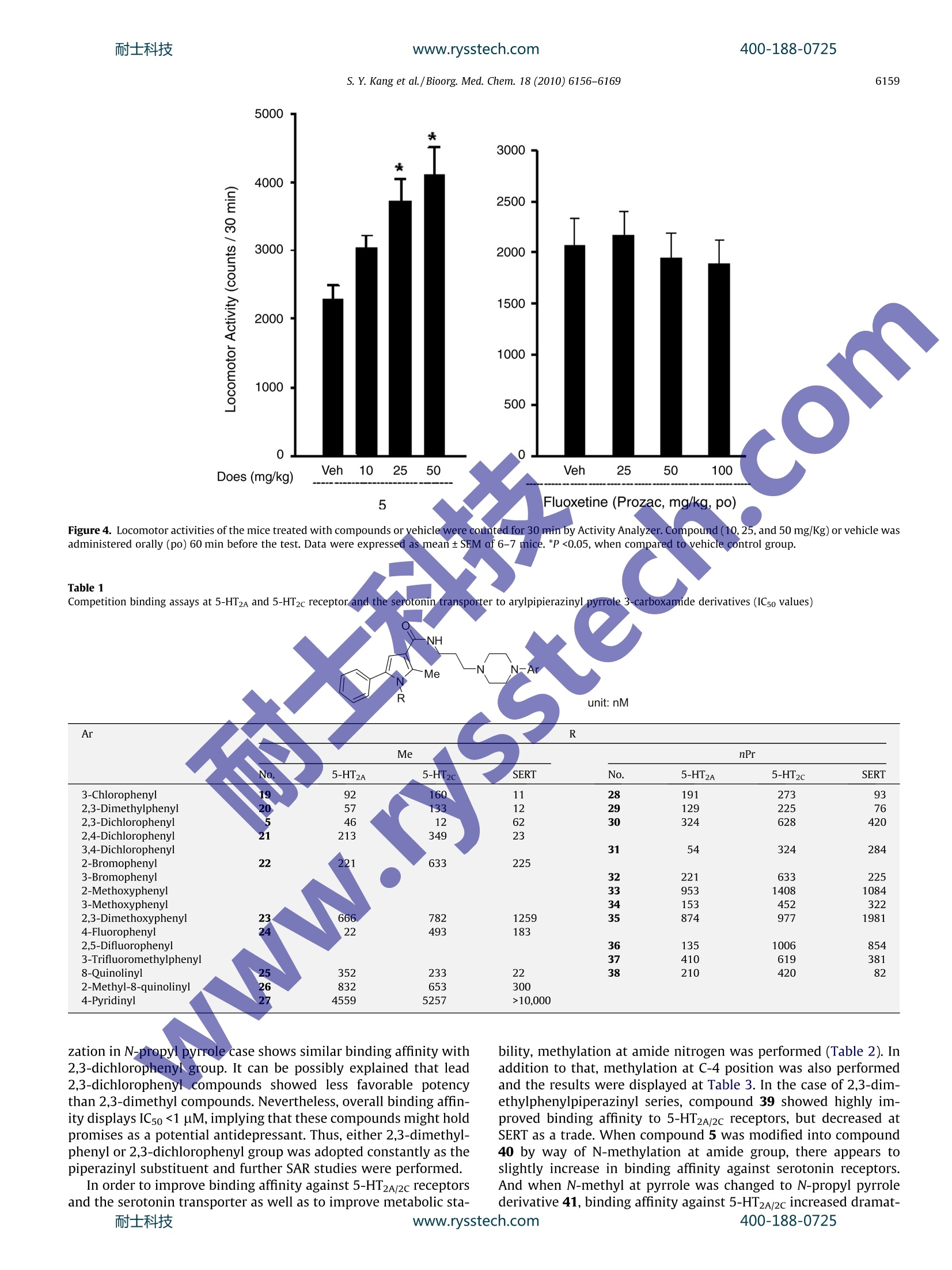
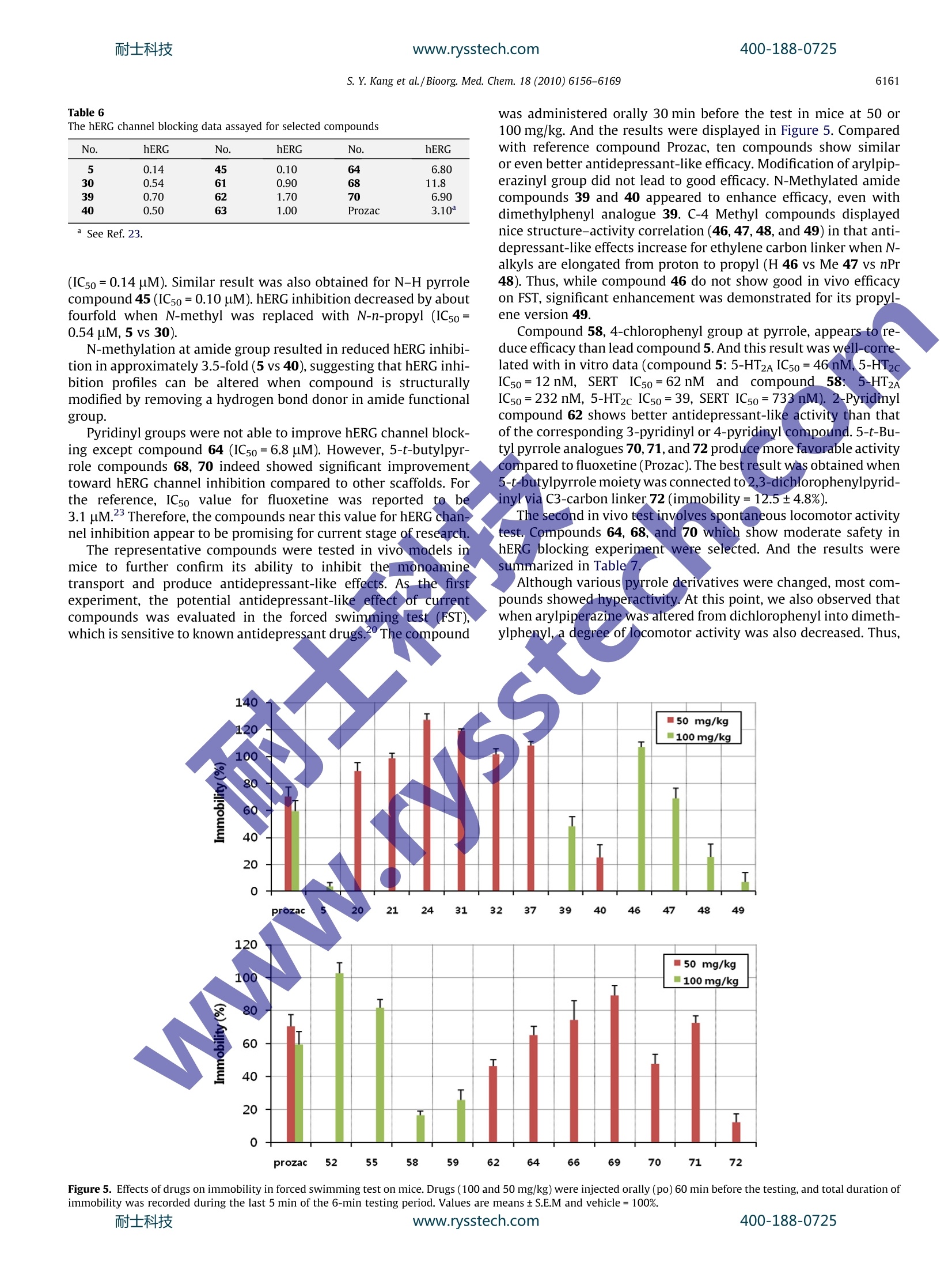
www.rysstech.comBioorganic & Medicinal Chemistry 18 (2010) 6156-6169耐士科技400-188-0725 耐士科技400-188-0725www.rysstech.comS. Y. Kang et al./ Bioorg. Med. Chem. 18 (2010) 6156-61696157 Contents lists available at ScienceDirect Bioorganic & Medicinal Chemistry journal homepage: www.elsevier.com/locate/bmc Further optimization of novel pyrrole 3-carboxamides for targeting serotonin5-HT2A, 5-HT2c,and the serotonin transporter as a potential antidepressant Suk Youn Kang , Eun-Jung Park, Woo-Kyu Park, Hyun Jung Kim, Gildon Choi, Myung Eun Jung, Hee Jeong Seo, Min Ju Kim, Ae Nim Pae, Jeongmin Kim, Jinhwa Lee a* Research Center, Green Cross Corporation, 303 Bojeong-Dong, Giheung-Gu, Yongin 446-770,Republic of Korea DPharmacology Research Center, Korea Research Institute of Chemical Technology (KRICT), 100 Jang-Dong, Yuseong-Gu, Daejeon 305-343, Republic of Korea ‘Biochemical Research Center, Korea Institute of Science and Technology, 39-1Hawolgok-Dong, Seongbuk-Gu, Seoul 136-791, Republic of Korea ABSTRACT ARTICLE INFO Pyrrole 1.Introduction Depression, especially major depression, is an extremely seriousdisease affecting about 121 million people and is one of the leadingcauses of disability worldwide. And unipolar depression is predicted to be the second main cause of disability in 2020 by theWorld Health Organization.2Over the past decades,the synapticactions of monoamine neurotransmitters such as norepinephrine(NE) and serotonin (SER, 5-HT) were considered as important indi-cations to psychiatric disease,34including anxiety and depression.And individually, various psychotropic drugs were developed asso-ciated with these neurotransmitters. After great success of a seriesof selective serotonin reuptake inhibitors (SSRI), it was rationalizedthe relationship between extracellular levels of serotonin and anumber of psychiatric indications, especially depressive disorder,by inhibiting serotonin transporters and consequently elevatingsynaptic serotonin levels. SSRIs such as fluoxetine, sertraline, par-oxetine, and citalopram have been the most widely prescribedantidepressants since 1980s.5 Despite numerous usage of SSRIs, they also have some sideeffects including anxiety, sedation, headache, tremor, and sexual ( * C orresponding a u thor. Tel.: +82 31 260 9892; fax: +82 3 1 2 609020. E -mail a ddresses: j i n hw a lee@g r ee nc r o ss.com, j i nhwa _ 2 _ l ee@ ho tmai l. com (J. Lee). ) dysfunction. In addition,SSRIs are troublesome in that it is gener-ally effective only less than two-third patients. Recently, there hasbeen achievement of important development for antidepressant,which interacts with dual or multiple targets. Because numerousside effects are associated with non-selective binding at post-syn-aptic 5-HT receptors, addition of 5-HT receptor antagonist compo-nent to 5-HT reuptake inhibitor has been proposed.8-10 With thisapproach, it was expected to increase the level of synaptic 5-HTand eventually achieve rapid onset time. Along the line, variouscompounds have been proposed and developed as potential anti-depressant with dual activity (Fig. 1).11-14 From the literature, common structure of antidepressantsknown to have dual activities appeared to exhibit combinationof two distinct structural motifs. Thus, aryl piperazine elementsand heterocyclic motifs were connected to each other using a lin-ker frequently to serve as SERT and 5-HT receptor inhibitors.As asimilar strategy, we recently disclosed a new class of antidepres-sant compounds involving pyrrole 3-carboxamide compounds.Particularly, compound 5 showed good in vitro activity against5-HT2A/2c receptors and serotonin reuptake inhibition, and alsodemonstrated excellent oral bioavailability and brain penetrativeproperties (Fig. 2). As a continuation of our investigation, we wishto describe further exploration of the structure-activity relation-ships (SAR) in this novel series. Evaluation of their in vitro andin vivo profiles was conducted in terms of efficacy versus sideeffects. Figure 2. Representative compound for targeting serotonin 5-HT2A/2c,and the SERTas a antidepressant. 2. Chemistry Based on the previous results, structural modification of leadcompound 5 was initiated as shown in Figure 3.15 The structure-activity relationship study began with evaluation of the effect bysubstitution of various arylpiperazinyl groups. Thus, novel com-pounds can be readily prepared by typical amide coupling at the fi-nal stage with modified acid and amine.Pyrrole 3-carboxylic acid Aand arylpiperazinyl alkyl amine B can be used as acid and aminepartners, respectively. Bromoketone 9 was selected for the preparation of pyrrolederivatives, particularly consisting of 5-substituted and 3-carbox-ylic acid (Scheme 1).16 Substitution at nitrogen of pyrrole was fo-cused on within proton to n-propyl based on the previous result. Figure 1. Chemical structures of representative antidepressant compounds. Hydrolysis of pyrrole ester 12 using sodium hydroxide in refluxedethanol afforded the corresponding acids 13. As shown in Scheme 2, preparation of aminoalkyI arylpiper-azine 17 started from the corresponding bromoalkyl-phthalimide14 and arylpiperazine 15. Treatment of bromoalkyl-phthalimide14 with arylpiperazine hydrochloride 15 in the presence of potas-sium carbonate in DMF at room temperature afforded N-protectedamine 16. Compound 16 was then treated with hydrazine in etha-nol at 80°C to give amine 17. For the sake of convenience of han-dling on scale, liquid amine 17 was transformed to hydrochloridesalt form 17a with 4 N HCl in dioxane. As shown in Scheme 3, typical amide coupling was conductedunder conditions involving EDCI (1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide), HOBT (1-hydroxybenzotriazole) and NMM(4-methylmorpholine) in methylene chloride or DMF to produceamide 18. As reaction was completed, purification was performedusing preparative reverse-phase HPLC with 0.2% TFA mixture ofacetonitrile and water solution. The neutral form of product 18was converted to HCl salt form 18a to increase the overall solubil-ity for biological evaluation. 3.Biology For further evaluation of our lead compound 5, spontaneouslocomotor activity test was performed. Although the sensitivityof FST (forced swimming test) to a broad range of antidepressantdrugs is one of the most important features supporting primary Figure 3. Preparation of target compounds using amide bond formation.www.rysstech.com 13 (R"=H, Me, Et, nPr) Scheme 1. Reagents and conditions: (a) NaH, THF; (b) NH40Ac, AcOH, 80℃; (c)NaH, Alkyl iodide, DMF; (d) NaOH, EtOH, reflux. / -N a Br N N R HN HCI 14 moc15 .i HN+ N 17 Scheme 2. Reagents and conditions: (a) K2CO3, DMF,rt; (b)NH2NHH20, EtOH,rt; (c) 4N HCl in dioxane. Scheme 3. Reagents and conditions: (a) EDCI, HOBT, NMM, CH2Cl2 or DMF; (b) HCl, MeOH,0 C. usage as a viable animal model, the activity in the FST is alsoknown to be detected by either anticholinergic or antihistaminedrugs depending on test conditions. The major false positives inthe FST are psychomotor stimulants, which affect immobility inthe FST. They are not anticipated to have clinical efficacy. Fromthese reasons, our lead compound 5 was subjected to screeningagainst spontaneous locomotor activity along with the FST. The re-sults were summarized in Figure 4. Fluoxetine, a clinically estab-lished reference, shows lower spontaneous locomotor activityregardless of dosing amounts. And compound 5 tends to displayincreasing locomotor activities with dose dependency. It meansthat our compound shows antidepressant-like activity by reducingimmobility in the FST, but its efficacy was falsely produced fromhyperactivity of the compounds. That is the reason why we focusedour efforts to remove spontaneous locomotor activity, while main-taining immobility in FST experiments. Biological evaluations were performed upon compounds pre-pared by the above synthetic pathway.The binding affinity of cur-renttcompoundsagainstit5-HT2A/2C receptor andserotonintransporter, stably expressed in CHO-K1 cells, were evaluated bydisplacement binding using[H]Ketanserin,[H]Mesulergine and,[H]Imipramine, respectively, as radioligands. Based on our previous report,derivatization of arylpiperazinewas expanded to various pyrroles 13 including 1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxylic acid and 2-methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxylic acid. The results are summarizedin Table 1. In the case of N-methyl pyrrole, overall derivatizationshowed less favorable binding affinity than that of 2,3-dichloro-phenyl group. Particularly,4-fluorophenyl and 8-quinolinyl groupshowed more potent binding affinity at 5-HT2A and SERT, respec-tively (24, 25, and 38). But it turned out to result in inferior activitywith consideration of other receptors. On the other hand, derivati- S. Y.Kang et al./Bioorg. Med. Chem. 18 (2010)6156-6169 Figure 4. Locomotor activities of the mice treated with compounds or vehicle were counted for 30 min by Activity Analyzer. Compound (10,25, and 50 mg/Kg) or vehicle wasadministered orally (po)60 min before the test. Data were expressed as mean+SEM of 6-7 mice. *P<0.05, when compared to vehicle control group. Table 1 ( Competition b i nding assays a t 5-HT2A and 5- H T2c receptor an d the serotonin transporter to arylpipierazinyl py r role 3-c a rboxamide derivatives (ICso va l ues) ) NH Me R unit: nM R Me nPr No. 5-HT2A 5-HT2C SERT No. 5-HT2A 5-HT2c SERT 3-Chlorophenyl 19 92 160 11 28 191 273 93 2,3-Dimethylphenyl 20 57 133 12 29 129 225 76 2,3-Dichlorophenyl 5 46 12 62 30 324 628 420 2,4-Dichlorophenyl 3,4-Dichlorophenyl 21 213 349 23 31 54 324 284 2-Bromophenyl 22 221 633 225 3-Bromophenyl 32 221 633 225 2-Methoxyphenyl 33 953 1408 1084 3-Methoxyphenyl 34 153 452 322 2,3-Dimethoxyphenyl 23 666 782 1259 35 874 977 1981 4-Fluorophenyl 24 22 493 183 2,5-Difluorophenyl 36 135 1006 854 3-Trifluoromethylphenyl 37 410 619 381 8-Quinolinyl 25 352 233 22 38 210 420 82 2-Methyl-8-quinolinyl 26 832 653 300 4-Pyridinyl 27 4559 5257 >10,000 zation in N-propyl pyrrole case shows similar binding affinity with2,3-dichlorophenyl group.It can be possibly explained that lead2,3-dichlorophenyl compounds showed less favorable potencythan 2,3-dimethyl compounds. Nevertheless, overall binding affin-ity displays IC50 <1 uM, implying that these compounds might holdpromises as a potential antidepressant. Thus, either 2,3-dimethyl-phenyl or 2,3-dichlorophenyl group was adopted constantly as thepiperazinyl substituent and further SAR studies were performed. In order to improve binding affinity against 5-HT2A/2c receptorsand the serotonin transporter as well as to improve metabolic sta- bility, methylation at amide nitrogen was performed (Table 2). Inaddition to that, methylation at C-4 position was also performedand the results were displayed at Table 3. In the case of 2,3-dim-ethylphenylpiperazinyl series, compound39 showed highly im-proved binding affinity to 5-HT2A/2C receptors, but decreased atSERT as a trade. When compound 5 was modified into compound40 by way of N-methylation at amide group, there appears toslightly increase in binding affinity against serotonin receptors.And when N-methyl at pyrrole was changed to N-propyl pyrrolederivative 41, binding affinity against 5-HT2A/2c increased dramat- 400-188-0725 Table 2 Binding affinities for 5-HT2A and 5-HT2c receptor and serotonin transporter ofarylpipierazinyl pyrrole 3-carboxamide derivatives No. R R² R3 5-HT2A 5-HT2c SERT 5 Me H Cl, Cl 46 12 62 20 Me H Me, Me 57 13 12 29 nPr H Me, Me 129 225 76 30 nPr H Cl, Cl 324 628 420 39 nPr Me Me, Me 17 12 163 40 Me Me Cl, Cl 39 35 98 41 nPr Me Cl, Cl 33 21 102 Table 3 Binding affinities against 5-HT2A and 5-HT2c receptors and inhibition againstserotonin transporter of arylpipierazinyl pyrrole 3-carboxamide derivatives R2 CI R1 HN+ Me unit: nM No. R1 R2 n 5-HT2A 5-HT2c SERT 42 H H 1 40 37. 87 43 Me H 1 175 15666 57 44 nPr H 1 408 310 45 H H 2 300 599 137 46 H Me 1 37 13 162 47 Me Me 1 33 16 78 48 nPr Me 1 25 75 78 49 H Me 2 39 16 36 ically, thereby displaying similar level of affinity as against seroto-nin transporter. As another practice, instead of N-methylation at amide group,methylation at C-4 position of pyrrole displayed somewhat differ-ent results (Table 3). Thus, it was observed that when 2,3-dichlor-ophenylpiperazinyl part and pyrrole 3-carboxilic acid part wasconnected with ethylene linker, and binding affinities were at levelof IC50 <80 nM for C-4 methylated analogues, except compound 46(IC5o=162 nM) at SERT. Favorable binding affinities against 5-HT,5-HT, and SERT were also observed for compound 49 as shownin Table 3. Limited exploration of the effects of substitution on the phenylof pyrrole C-5 position was also undertaken (Table 4). At N-alkyl-ated pyrrole series, 4-chlorophenyl modification tends to reducebinding affinity against 5-HT2A and inhibition against SERT but in-crease binding affinity against 5-HT2c receptor (51 vs 54 and 56 vs59). On the other hand, in the case of N-H pyrrole compounds, theexactly opposite trends are displayed (50 vs 52). With this in mind,replacement of phenyl group with more hydrophilic pyridinylgroup was also conducted. 2-Pyridinyl substituted compound 61is more potent than phenyl compound 50, especially against5-HT2A and SERT. However, when N-alkylation at pyrrole N-1 posi-tion was changed to methyl or propyl, binding affinity about5-HT2c and SERT decreased while maintaining 5-HT2A activity(61 vs 62 vs 63). 3-Pyridinyl and 4-pyridinyl compounds displayedsimilar level of activities. Most of pyridinyl compounds exhibited Table 4 Binding affinities against 5-HT2A and 5-HT2c receptors and inhibition againstserotonin transporter of arylpipierazinyl pyrrole 3-carboxamide derivatives unit: nM No. R1 R2 R 5-HT2A 5-HT2c SERT 50 H Phenyl Me, Me 300 615 77 51 Et Phenyl Me, Me 75 139 36 56 Et Phenyl Cl, Cl 376 66 182 52 H 4-Chlorophenyl Me, Me 92 189 19 53 Me 4-Chlorophenyl Me, Me 213 129 129 54 Et 4-Chlorophenyl Me, Me 151 39 93 55 nPr 4-Chlorophenyl Me,Me 192 26 131 57 H 4-Chlorophenyl Cl, Cl 105 106 56 58 Me 4-Chlorophenyl Cl, Cl 232 39 733 59 Et 4-Chlorophenyl Cl, Cl 153 22 629 60 nPr 4-Chlorophenyl Cl, Cl 333 41 1159 61 H 2-Pyridinyl Cl, Cl 21 221 28 62 Me 2-Pyridinyl Cl, cl 43 267 110 63 nPr 2-Pyridinyl Cl, Cl 58 639 397 64 Me 3-Pyridinyl Cl, Cl 209 92 7 65 nPr 3-Pyridinyl Cl, CI 46 344 133 66 Me 4-Pyridinyl Cl, 175 85 67 Cl, cl 34 420 78 4-Pyridinyl Table 5 Binding affinities for 5-HT2A and 5-HT2c receptor and serotonin transporter ofarylpipierazinyl pyrrole 3-carboxamide derivatives No. R R2 5-HT2A 5-HT2c SERT 68 Me Me, Me 1 36 2227 908 69 Me Me, Me 2 386 1145 252 70 Me Cl, Cl 1 50 109 45 71 H Cl, Cl 2 107 1392 860 72 Me Cl, Cl 2 163 1021 375 more favorable activity against 5-HT2A than against 5-HT2c andSERT. Introduction of t-butyl group at phenyl position of pyrrole wasalso studied, and the results were summarized in Table 6. Althoughits Clog P value is similar to that of phenyl compound (Clog P=6.60at 5) and t-butyl compound (Clog P=6.33 at 72), Compound 72which is t-butyl version of lead compound 5, showed poor bindingaffinity against 5-HT2c (IC50=1021 nM) and moderate activityagainst 5-HT2A (IC50=163nM) and SERT (IC50=375nM). 2,3-Dimethylphenyl compound 69 appeared to show similar level ofbinding affinity. To date the best result of t-butyl scaffold was ob-tained when 2,3-dichlorophenylpiperazinyl group was connectedwith C2 linker (compound 70) (see Table 5). For further evaluation of this series of compounds, severalselected compounds were subjected to hERG channel inhibition as-say.1 Patch-clamp technique method was used for in vitro assayfor hERG blocking. And the results are summarized in Table 6. Leadcompound 5 turned out be a significant inhibitor of hERG channel Table 6The hERG channel blocking data assayed for selected compounds No. hERG No. hERG No. hERG 5 0.14 45 0.10 64 6.80 30 0.54 61 0.90 68 11.8 39 0.70 62 1.70 70 6.90 40 0.50 63 1.00 Prozac 3.10 See Ref. 23. (IC50=0.14uM). Similar result was also obtained for N-H pyrrolecompound 45 (IC50=0.10 uM). hERG inhibition decreased by aboutfourfold when N-methyl was replaced with N-n-propyl (IC50=0.54 uM, 5 vs 30). was administered orally 30 min before the test in mice at 50 or100 mg/kg. And the results were displayed in Figure 5. Comparedwith reference compound Prozac, ten compounds show similaror even better antidepressant-like efficacy. Modification of arylpip-erazinyl group did not lead to good efficacy.N-Methylated amidecompounds 39 and 40 appeared to enhance efficacy, even withdimethylphenyl analogue 39. C-4 Methyl compounds displayednice structure-activity correlation (46, 47,48, and 49) in that anti-depressant-like effects increase for ethylene carbon linker when N-alkyls are elongated from proton to propyl (H 46 vs Me 47 vs nPr48).Thus, while compound 46 do not show good in vivo efficacyon FST, significant enhancement was demonstrated for its propyl-ene version 49. N-methylation at amide group resulted in reduced hERG inhibi-tion in approximately 3.5-fold (5 vs 40), suggesting that hERG inhi-bition profiles can be altered when compound is structurallymodified by removing a hydrogen bond donor in amide functionalgroup. Pyridinyl groups were not able to improve hERG channel block-ing except compound 64 (IC50=6.8 uM). However, 5-t-butylpyr-role compounds 68, 70 indeed showed significant improvementtoward hERG channel inhibition compared to other scaffolds. Forthe reference, ICso value for fluoxetine was reported tobe3.1 uM.3 Therefore, the compounds near this value for hERG chan-nel inhibition appear to be promising for current stage of research. Compound 58, 4-chlorophenyl group at pyrrole, appears to re-duce efficacy than lead compound 5. And this result was well-ll-Corree-lated with in vitro data (compound 5:5-HT2A IC50=46 nM,,5-HT2cIC50=12nM, SERT IC50=62nM and compound58:5-HT2AIC50=232 nM, 5-HT2c IC50=39, SERT IC50=733 nM). 2-Pyridinylcompound 62 shows better antidepressant-like activity than thatof the corresponding 3-pyridinyl or 4-pyridinyl compound.5-t-Bu-tyl pyrrole analogues 70,71, and 72 produce more favorable activitycompared to fluoxetine (Prozac). The best result was obtained when5-t-butylpyrrole moiety was connected to 2,3-dichlorophenylpyrid-inylvia C3-carbon linker 72 (immobility=12.5±4.8%). The second in vivo test involves spontaneous locomotor activitytest. Compounds 64, 68, and70 which show moderate safety inhERG blocking experiment were selected. And the results weresummarized in Table 7. The representative compounds were tested in vivo models inmice to further confirm its ability to inhibit themonoaminetransport and produce antidepressant-like effects. As the firstexperiment, the potential antidepressant-like effectof currentcompounds was evaluated in the forced swimming testt (FST),which is sensitive to known antidepressant drugs.The compound Although various pyrrole derivatives were changed, most com-pounds showed hyperactivity. At this point, we also observed thatwhen arylpiperazine was altered from dichlorophenyl into dimeth-ylphenyl, a degree of locomotor activity was also decreased. Thus, Figure 5. Effects of drugs on immobility in forced swimming test on mice. Drugs (100and 50 mg/kg) were injected orally (po)60 min before the testing, and total duration ofimmobility was recorded during the last 5 min of the 6-min testing period. Values are means±S.E.M and vehicle=100%.www.rysstech.com Locomotor activities of the mice treated with compounds (25 mg/kg) or vehicle werecounted for 30 min by Activity Analyzer No. Locomotor activity Hyperactivity sign 64 Hyperactive 68 7302±2414 Mid-hyperactive 70 14860±1531 Hyperactive 72 15804±5162 Hyperactive Effexor 7158±484 Mid-hyperactive Prozac” 5931±1979 Normal Vehicle -5000 Normal Data were expressed as mean ±S.E.M of 6-7 mice. See Ref. 22. In-house data. in order to reduce hyperactivity with maintaining antidepressantactivity, 3-chloro-2-methylphenyl piperazine was introduced as anovel arylpepierazine moiety, and with consideration of hERGblocking issue,t-butylpyrrole analogues were selected. Derivativesof compound 68 or 70 were evaluated and the in vitro and in vivoresults are shown in Table 8. In summary, we investigated new arylpiperazine-containingpyrrole 3-carboxamide series of compounds as our continuing ef-fort to identify novel compounds targeting 5-HT2A/2C and SERTfor the treatment of depressive disorders. Based on the lead com-pound 5, a number of structural modifications are presentedincluding modification of arylpiperazinyl group, N-alkylation ofamide group,C-4 methylation at pyrrole, and replacement of phe-nyl group of pyrrole with p-chlorophenyl, pyridinyl, or t-butyl. Allthe prepared compounds were evaluated by competition bindingassays against 5-HT2A/2c and SERT. Structure-activity relationships(SARs) were established. hERG blocking assay was conducted forfurther evaluation of this series. A few of selected compounds weretested in vivo FST animal models. More than ten compounds in thisseries produced similar or better antidepressant-like activity com-pared with fluoxetine, well-established reference antidepressant.Spontaneous locomotor activity test was conducted to distinguishthe false positive effects in the FST. In order to overcome hyperac-tivity issue, 3-chloro-2-methylphenyl group was introduced intothe series. Through a series of lead optimization efforts, compound74I was identified to possess good bindingaffinity (5-HT2AIC50=54nM, 5-HT2c IC50=210 nM, SERT IC50=315nM) in addi-tion to relatively safe hERG inhibition (IC50=3.6 uM). By in vivoefficacy study,,ccompound 74 also showed 1reasonable antidepres-sant activity without exhibiting hyperactivity. Thus, novel pyrrole3-carboxamides targeting serotonin 5-HT2A, 5-HT2c,and the sero-tonin transporter may have potential as a pharmacotherapy forthe treatment of depressive disorders. Although compound 5 in our prior workdemonstrated goodin vitro and in vivo antidepressant-like activity, 5 also sufferedfrom negative aspects including strong hERG inhibition and falsepositive effects. Current research successfully identified compound74 bearing t-butyl group at pyrrole C-5, overcoming the inherentproblems of compound 5. 5. Experimental 5.1. General methods All reactions are conducted under an inert atmosphere at roomtemperature,unless otherwise noted. All reagents were purchasedat the highest commercial quality and used without further purifi-cation,unless otherwise indicated. Microwave reaction was con-ducted with a Biotage Initiator microwave reactor. NMR spectrawere obtained on a Varian 400-MR (400 MHz H, 100 MHz 13c)spectrometer. NMR spectra were recorded in ppm (8) relative totetramethylsilane (8=0.00) as an internal standard unless statedotherwise and are reported as follows: chemical shift, multiplicity(s=singlet, d=doublet, t=triplet, q=quartet, quint=quintet,sext= sextet, m=multiplet, and br=broad), coupling constant,and integration. 13c NMR spectra were referenced to the residualCDCl3(8=77.0) or DMSO-d6(8=39.7).Mass spectra were obtainedwith an Agilent 6110 quadruple LC-MSD (ESI+). Preparative HPLCpurifications were performed on a Gilson purification system. Forpreparative HPLC, ca. 100 mg of a product was injected in 1 ml ofmethanol onto a SunFire Prep C18 OBD 5 um 30×100 mm Column Table 8Binding affinities against 5-HT2A and 5-HT2c receptors and inhibition against serotonin transporter of compound 73 and 74 and effects on immobility in forced swimming test onmice and locomotor activities R No. 5-HT2A(nM) 5-HT2c(nM) SERT (nM) FST (%) SLA(cm) Phenyl 73 152 233 64 86.15±10.89 7096±1926 t-Butyl 74 54 210 315 49.12±14.50 6217±2903 For FST, drugs (25 mg/kg) were injected orally (po) 60 min before the testing, and total duration of immobility was recorded during the last 5-min of the 6-min testing period.For locomotor activities, locomotion of mice treated with compounds or vehicle were counted for 30 min by Activity Analyzer. Data were expressed as mean ±S.E.M. with a 30 min gradient from 5% to 90% acetonitrile in water and a45 ml/min flow rate. Biotage SP1 and Isolera purification systemswere used for normal phase column chromatography with ethylacetate and hexane. Flash chromatography was performed usingE. Merck 230-400 mesh silica gel according to the procedure ofStill et al. Reactions were monitored by either thin-layer chroma-tography (TLC) on 0.25 mm E. Merck silica gel plates (60F-254)using UV light and p-anisaldehyde solution as visualizing agentsor HPLC analysis on an Agilent 1200 series system. 5.2. Chemistry 5.2.1. 1,2-Dimethyl-5-phenyl-1H-pyrrole-3-carboxylic acid (6) 5.2.1.1. Step 1: Ethyl 2-acetyl-4-oxo-4-phenylbutanoate. To asolution of ethyl acetoacetate (10g,76.8 mmol) in tetrahydrofuran(50 ml) at 0℃ was added sodium hydride (4 g, 60% in mineral oil,100 mmol) portionwise. The reaction mixture was stirred for30 min, and 2-bromoacetophenone (16.8 g, 84.5 mmol) in tetrahy-drofuran (20 ml) was added dropwisely. After warming the mix-ture to room temperature, it was stirred for 4h. The resultingsolution was quenched with water and normal work-up was con-ducted with diethyl ether. The organic layer was dried with MgSO4,and purified by silica gel column chromatography (EtOAc/Hex=1:5) to produce the desired compound (19g, 98%) as lightvellow color oil.MH+ 249. 5.2.1.2. Step2: Ethyl 2-methyl-5-phenyl-1H-pyrrole-3-carboxyl-ate. Ethyl 2-acetyl-4-oxo-4-phenylbutanoate (19g,76.8mmol)was treated with NH40Ac (29.4g, 384mmol) in acetic acid(100 ml) at room temperature. After stirring 10 min, the reactionmixture was heated to 80℃ for overnight. After cooling to roomtemperature, acetic acid was evaporated under reduced pressureand subsequently water (50 ml) was added. The organic layerwas separated and the aqueous layer was extracted with diethylether. Purification by normal phase preparative LC provided thedesired compound (13.3g,76%)asyyellow solid.1H NMR(400 MHz, CDCl3) 8 8.45 (br s, 1H), 7.45 (dd,l,J=8.4,1.6 Hz, 2H),7.36 (t, J=7.6Hz, 2H),7.20 (dd,J=7.2,, 1.1 Hz, 1H), 6.84 (d,J=2.8Hz, 1H), 4.29 q, 2 Hz,2H), 2.59 (s, 3H),1.36(tJ=6.8 Hz, 3H). MH+230. 5.2.1.3. Step 3: Ethyl 1,2-dimethyl-5-phenyl-1H-pyrrole-3-car-boxylate. To ethyl 2-methyl-5-phenyl-1H-pyrrole-3-carboxylate(3 g, 13mmol) in THF (50 ml) was added iodomethane (4ml,65 mmol) and sodium hydride (630 mg,60% in mineral oil,15.6 mmol) at 0°C. After warming the reaction mixture to roomtemperature, it was stirred for 1 day. Water (20ml) was addedand extracted the resulting mixture with diethyl ether. With nor-mal phase preparative LC, purification was conducted to gave thetitle compound (3 g, 94%) as yellow solid. H NMR (400 MHz,CDCl3) 8 7.42-7.31(m, 5H), 6.58 (s, 1H), 4.28(q,J=7.2 Hz, 2H),3.50 (s, 3H), 2.60 (s,3H), 1.34 (t,J=7.2Hz, 3H). MH+ 244. 5.2.1.4. Step 4: 1,2-Dimethyl-5-phenyl-1H-pyrrole-3-carboxylicacid (6). To the solution of ethyl 1,2-dimethyl-5-phenyl-1H-pyr-role-3-carboxylic acid (3g, 13 mmol) in EtOH (50 ml) was addedNaOH (1.6 g,39 mmol) at room temperature. The reaction mixturewas refluxed for 1 day with LC-MS monitoring. After the reactionwas completed, EtOH was evaporated under reduced pressure.Water (50 ml) was added and extracted the aqueous layer withdiethyl ether twice. 1 N HCl solution was added to the aqueouslayer until the solution became acidic (pH <3). And extractionwas accomplished with EtOAc. The organic layer was dried withMgSO4 and the filtered organic volatiles were evaporated in vacuoto provide the title compound (2.6 g,95%) as yellow solid. Withoutfurther purification, the acid was used for the next amide coupling. 5.2.2. 2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethanaminedihydrochloride (7) 5.2.2.1. Step 1: 2-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)pro-pyl)isoindoline-1,3-dione. 1-(2,3-Dichlorophenyl)piperazinehydrochloride (3.16 g,g,111.8 mmol) and potassium carbonate(4.08 g, 29.5 mmol) were added to the solution of 2-(3-bromopro-pyl)isoindoline-1,3-dione (3.16 g, 11.8 mmol) in DMF (20 ml). Thereaction mixture was stirred for overnight at room temperature.After reaction was completed, water (40 ml) was added and thennormal work-up was conducted. The residue was purified withnormal phase preparative column chromatography to afford the ti-tle compound (4g, 81%yield) as white solid. MH+ 418. 5.2.2.2. Step 2: 3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propan-1-amine dihydrochloride. To a stirred solution of 2-(3-(4-(2,3dichlorophenyl)piperazin-1-yl)propyl)isoindoline-1,3-dione(4g,9.57 mmol) in ethanol (50 ml) was added hydrazine (3 ml) at roomtemperature. The reaction mixture was heated to 80℃ and stirredfor 1 day at that temperature. The resulting solution was cooleddown to room temperature, and the volatiles were evaporated un-der reduced pressure. The residue was work-up with EtOAc andsaturated sodium bicarbonate solution. After evaporation of organ-ic layer under reduced pressure, it was poured into 1 N HCl solu-tion. The aqueous solution was washed with ethyl ether, andthen basified with aqueous ammonia. CH2Cl2 was used for work-up organic layer, dried with MgSO4. After solvent was removed un-der reduced pressure, 4 N HCl in dioxane (5ml) was added at 0 ℃and stirred 10 min to produce HCl salt form. Light yellow solid titlecompound(3.1 g, 89%) was obtained by evaporation and drying invacuo volatile compounds. MH+274(-2HCl). 5.2.3.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide (5) To the mixture of 1,2-dimethyl-5-phenyl-1H-pyrrole-3-carbox-ylicacid (100 mg, 0.46 mmol) and 3-(4-(2,3-dichlorophenyl)pipera-zin-1-yl)propan-1-amine dihydrochloride (166 mg, 0.46 mmol) inCH2Cl2(5ml) were added EDCI (178 mg, 0.92 mmol), HOBT(130 mg, 0.9 mmol) and NMM (0.3 ml, 1.8 mmol). After stirring for1! day at room temperature, MeOH was added to the resulting solu-tion, and filter off. After evaporation under reduced pressure, theresidue was purified by reverse-phase preparative HPLC to providethe title compound (149 mg, 71%) as white solid. 1H NMR(400 MHz, CDCl;) 8 7.46 (br s, 1H), 7.24-7.13 (m, 6H), 6.87-6.73(m, 4H), 6.27 (s, 1H), 3.54 (dd,J=11.2, 5.2 Hz, 2H), 3.48 (s, 3H),3.25-3.19 (m, 5H), 2.64 (s, 3H), 2.60-2.44 (m, 6H), 1.80 (m, 2H).MH+451. 5.2.4.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide hydrochloride(5a) HCl solution (4 N in dioxane, 0.5 ml) was added to the solutionof N-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimeth-yl-5-phenyl-1H-pyrrole-3-carboxamide (100mg)in methanol(5 ml). After stirring for 10 min, the volatiles were evaporated un-der reduced pressure and dried in vacuo to produce HCl salt formas light yellow solid. 5.2.5.N-(3-(4-(3-Chlorophenyl)piperazin-1-yl)propyl)-1,2-dimeth-yl-5-phenyl-1H-pyrrole-3-carboxamide (19) Compound 19 was obtained by repeating the procedure ofcompound 5. 1H NMR (400MHz, CDCl3) 8 7.46 (br s, 1H), 7.24-7.13 (m, 6H), 6.87-6.73 (m, 4H), 6.27 (s, 1H), 3.54 (dd,J=11.2,5.2 Hz, 2H), 3.48 (s, 3H), 3.25-3.19 (m, 5H), 2.64 (s, 3H), 2.60-2.44 (m, 6H), 1.80(m,2H). MH+ 451. 5.2.6. N-(3-(4-(2,3-Dimethylphenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide (20) Compound 20 was obtained by repeating the procedure of com-pound5. HNMR(400 MHz, CDCl3) 8 7.43 (br s, 1H), 7.39-7.31(m,5H), 6.94 (t,J=7.6 Hz,1H), 6.88(d,J=7.2 Hz, 1H), 6.40(s,1H),3.54(dd,J=11.2,5.6 Hz,2H), 3.49 (s,3H), 2.92 (t,J=4.0Hz,4H), 2.65(s,3H), 2.60 (t,J=5.2 Hz, 2H), 2.26 (s, 3H), 2.19 (s, 3H),1.83-1.79 (m,2H). MH+ 445. 5.2.7. N-(3-(4-(2,4-Dichlorophenyl)piperazin-1-yl)propyl)-1,2- dimethyl-5-phenyl-1H-pyrrole-3-carboxamide hydrochloride(21)Compound 21 was obtained by repeating the procedure of com-pound 5a. H NMR (400 MHz, DMSO-d6)8 10.95(br s, 1H), 7.91 (brs, 1H), 7.57 (d,J=2.0 Hz, 1H), 7.43-7.28(m,6H), 7.19 (d,J=8.4 Hz,1H), 6.58 (s, 1H), 4.38-4.28 (m,4H), 3.54-3.52(m,2H), 3.44(s,3H),3.38-3.35 (m,2H), 3.25-3.13 (m,4H), 2.51 (S, 3H), 1.94-1.91 (m,2H). MH+ 485 (-HCl). 5.2.8. N-(3-(4-(2-Bromophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide (22) Compound 22 was obtained by repeating the procedure of com-pound 5. 1H NMR (400 MHz, CD30D) 8 7.61-7.59 (m, 1H), 7.43-7.32 (m, 6H), 7.23-7.21(m, 1H), 7.05-7.02 (m, 1H), 3.63 (s, 3H),3.54-3.45 (m, 4H), 3.33-3.15(m, 8H), 2.59 (s, 3H), 2.07 (m, 2H).MH+495. 5.2.9.N-(3-(4-(2,3-Dimethoxyphenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide (23) Compound25 was obtained by repeating the procedure of com-pound 5. HNMR (400 MHz, CDCl3)8 8.88 (dd,J=1.6, 4.0 Hz, 1H),8.10 (dd,J=1.6,8.0Hz, 1H), 7.45-7.31 (m, 8H), 7.13 (dd,J=3.6,5.2 Hz, 1H), 6.46 (br s, 1H), 6.30(s,1H), 3.58 (q,J=6.0Hz, 2H),3.50 (s, 3H), 3.46 (br s, 3H), 2.87(br s, 3H), 2.72 (t, J=6.0 Hz,2H), 2.64 (s, 3H), 1.58-1.52(m,2H).MH+ 467. ( 5.2.12. 1 ,2-Dimethyl-N-(3-(4-(2-methylquinolin-8-yl)piperazin- 1-yl)propyl)-5-phenyl-1H- p yrrole - 3-carboxamide (26) ) Compound 26 was obtained by repeating the procedure of com-pound 5.H NMR (400 MHz, CDCl3) 8 8.12 (dd,J=1.6, 8.0 Hz, 1H),7.44-7.32(m,8H), 7.15(dd,J=3.6,5.2 Hz,1H), 6.46 (br s, 1H), 6.31(s, 1H), 3.57(q,J=6.0 Hz,2H), 3.52 (s, 3H), 3.46 (br s, 3H), 2.87 (brs, 3H), 2.72 (t,J=6.0Hz, 2H), 2.64 (s, 3H), 2.53 (s, 3H), 1.58-1.52(m, 2H). MH+ 481. ( 5.2.13. 1,2-Dimethyl-5-phenyl-N-(3-(4-(pyridin-4-yl)piperazin-1-yl)propyl)-1H-pyrrole-3-carboxamide di h ydrochloride (27) ) ( Compound 27 was obtained by repeating the procedure of com-pound 5a. H NMR (400 MHz, DMSO-d6) 8 8. 3 2 (d, J=7.6 Hz, 2H), ) ( 7.92-7.87 (m, 1H), 7.43-7.35(m, 4H), 7.32-7.28(m, 1H), 7.25 (d, J =7.6Hz,2H), 6.58 (s, 1H), 3 . 44(s , 3 H ), 4.21-3.88 (m, 4H), 3.34- 3.22 (m, 6 H), 3.08-3.04(m, 2H), 2.50 (s, 3 H ), 1.94-1.92 (m,2H). MH+ 418 (-2HCl). ) ( 5.2.14.N-(3-(4-(3-Chlorophenyl)piperazin-1-yl)propyl)-2- methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (28) ) ( Compound 28 was obtained by repeating the procedure of com- pound 5. HNMR(400 MHz, CDCl3)8 7.40-7.28 (m,2H), 7.24-7.20 (m , 1H), 7.18-7.13 (m,5H), 6.87-6.71(m,4H), 6.23 (s, 1H), 3.80(t, J =7.6 Hz, 2 H), 3 .53 ( dd,J= 1 1.6, 5 . 6 Hz, 2H), 3.22-3.18 (m, 4H), 2.64 ( s, 3H), 2.64-2.56 (m, 5H), 1.80 (t, J=4.0Hz, 2H), 1.61-1.51 (m, 2H), 0.73 (t,J=7.2Hz, 3H). MH+479. ) ( 5.2.15.N-(3-(4-(2,3-Dimethylphenyl)piperazin-1-yl)propyl)-2- methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (29) ) Compound 29 was obtained by repeating the procedure of com-pound 5.1H NMR(400 MHz, CDCl3) 87.38-7.26(m,6H),6.96-6.87(m, 3H), 6.63 (d,J=7.6Hz, 1H), 6.36 (s, 1H), 3.84-3.80 (m, 3H),3.53 (t,J=6.0 Hz, 2H), 2.91-2.88(m, 6H), 2.65 (s, 3H), 2.61-2.57(m, 5H), 2.25 (s,3H), 2.19 (s, 3H), 1.79-1.77 (m, 2H), 1.55-1.54(m, 2H), 0.74 (t,J=7.6Hz, 3H). MH+ 473. ( 5 .2.16 . N- ( 3-(4-(2,3-Dichlorophenyl)piperazin- 1 -yl)propyl)-2- methyl-5- p henyl-1-propyl-1H-pyrrole-3-carboxamide (30) ) ( C o mp ound 30 was obtained by repeating the procedure of com- pound 5. 1 H NMR (400 MHz, CDCl) 87 .41 - 7.27 (m, 6H ) , 7.14 (dd, J = 8. 4 , 1 . 6H z ,1H ) , 7.00(t , J=8.0Hz, 1H) , 6.65 (dd,J=8.4, 1.6 Hz, 1H), 6.34 (s, 1H), 3.8 2 (t , J=8.0Hz, 2H), 3.56-4.51( m , 2H), 3.20 (m, 4H), 2.66 (s, 3H), 2 .59 ( t , J=6. 4 Hz, 3H), 1.83-1 . 74(m,3H), 1.60-1.51(m,5H),0. 8 1- 0 .73 ( m, 4H). MH+513. ) ( 5.2.17. N -(3-( 4 -(3,4-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl- 5 - p henyl-1-propyl-1H-pyrrole-3-carboxamide h y dro- chloride (31 ) ) ( C om p ound 31 was obtained by repeating the procedure of com- pound 5 a. HNMR(400 MHz, DMSO-d6)8 10.98 (br s, 1H), 7.90 (br, 1H ), 7 . 43- 7 .30(m, 6H), 7 .21 (d,J=2.8Hz, 1H), 6 . 97 (dd,J=8.8, 2.8Hz, 1H), 6 .53 (s, 1H), 3.87-3.79 ( m, 4 H), 3.53-3.47(m, 2H), 3. 2 2 - 3 .13(m,4H), 3.10-3.02 (m, 4H), 2.52 (s, 3H), 1.93-1.90(m,2H ) , 1.46-1.37 (m,2H), 0.63 (t,J = 7.2 Hz,3H). MH+ 513 (-HCl). ) ( 5.2.18. N-(3-(4-(2-Bromophenyl)piperazin-1-yl)propyl)-2- ) methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (32) ( Compound 32 was obtained by repeating the procedure of com- pound 5.HNMR (400 MHz, CDCl3) 8 7.53 (dd,J=8.0, 1.2 Hz, 1 H), 7.34 (m, 5 H), 7.12 (td,J=8.0, 1.2 Hz, 1H), 6.89 (td,J= 7 .6, 1.2 Hz,1H), 6.74 (dd, J=8.0, 1.6Hz, 1H), 3.82 (t, J =7.6 H z , 2 H ), 3.55-3.51 (m, 2 H), 3.05 ( m, 4H), 2 .65 ( s, 3H), 2.60 (t,J=6.0Hz, 3H), 1.82-1.76(m,2H), 1.63-1.50(m, 5H), 0.74 ( t,J=7.6Hz, 3H). M H+ 523. ) ( 5.2.19.N-(3-(4-(2-Methoxyphenyl)piperazin-1-yl)propyl)-2- methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (33) ) Compound 33 was obtained by repeating the procedure of com-pound 5. 1H NMR(400 MHz, DMSO-d6)8 7.72 (br s, 1H), 7.39-7.28(m,5H), 6.92-6.86 (m,2H), 6.83-6.78 (m,2H), 6.46 (s, 1H), 3.80 (d,J=8.0 Hz, 2H), 3.72 (s,3H), 3.18(q,J=6.8 Hz, 2H), 2.95-2.87 (m,4H), 2.50 (s, 3H), 2.52-2.42 (m,4H), 2.36-2.32 (m, 2H), 1.64-1.60(m, 2H), 1.43-1.37(m,2H), 0.62 (t,J=7.2Hz, 3H). MH+ 475. ( 5.2.20.N-(3-(4-(3-Methoxyphenyl)piperazin-1-yl)propyl)-2-methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (34) ) Compound 34 was obtained by repeating the procedure of com-pound 5. HNMR(400 MHz, DMSO-d6) 8 7.73 (br s, 1H), 7.36-7.29(m,5H),7.08 (t,J=8.0Hz, 1H), 6.50(d,J=7.6 Hz,1H), 6.46 (s,1H),400-188-0725 6.42(s, 1H), 6.35 (d,J=6.0Hz, 1H), 3.80(d,J=7.6 Hz, 2H), 3.68 (s,3H), 3.21-3.19 (m, 2H), 3.14-3.02 (m, 4H), 2.51 (s, 3H), 2.52-2.35(m,6H), 1.72-1.65 (m, 2H), 1.46-1.36 (m, 2H), 0.63 (t,J=7.2 Hz,3H).MH+ 475. 5.2.21. N-(3-(4-(2,3-Dimethoxyphenyl)piperazin-1-yl)propyl)-2-methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (35) Compound 35 was obtained by repeating the procedure of com-pound 5. 1H NMR (400MHz, CDCl) 87.35-7.25 (m, 6H), 6.90(t,J=8.0Hz, 1H), 6.61 (dd,J=8.4, 1.2 Hz, 1H),6.38 (dd,J=8.4,1.2 Hz, 1H), 6.31 (s, 1H), 3.87-3.78(m, 8H), 3.53 (quartet,J=5.6Hz, 2H), 3.17 (br s, 4H), 2.69-2.55(m, 9H), 1.78 (quartet,J=6.0Hz, 2H), 1.62-1.49(m,2H), 0.74 (t, J=7.2 Hz, 3H). MH+505. 5.2.22.N-(3-(4-(2,4-Difluorophenyl)piperazin-1-yl)propyl)-2-methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide hydro-chloride (36) Compound 36 was obtained by repeating the procedure of com-pound 5a.HNMR (400 MHz,DMSO-d6)8 11.02(br s, 1H), 7.91 (br,1H), 7.43-7.40 (m, 2H), 7.34-7.30 (m, 3H), 7.25-7.19 (m, 1H),7.14-7.09 (m, 1H), 6.53 (s, 1H), 3.81(t,J=7.6 Hz, 2H), 3.53-3.46(m, 2H), 3.37-3.32(m, 2H), 3.23-3.10(m,8H), 2.52 (s, 3H), 1.96-1.90 (m, 2H), 1.46-1.37 (m,2H), 0.63 (t,J=7.6 Hz, 3H). MH+ 481(-HCl). 5.2.23.2-Methyl-5-phenyl-1-propyl-N-(3-(4-(3-(trifluoromethyl)-phenyl)piperazin-1-yl)propyl)-1H-pyrrole-3-carboxamide hydro-chloride (37) Compound 37 was obtained by repeating the procedure of com-pound 5a. H NMR (400 MHz, CD30D) 8 7.48-7.39 (m,3H),7.35-7.33 (m, 3H), 7.28-7.27 (m, 2H), 7.19-7.18 (m, 1H), 3.96-3.88(m, 4H), 3.73-3.64(m, 3H), 3.50-3.47 (m, 2H), 3.26-3.17 (m,4H), 2.57 (s, 3H), 2.11-2.08 (m, 2H), 1.53-1.44 (m, 2H),0.69 (t,J=7.6Hz, 3H). MH+513(-HCl). 5.2.24.2-Methyl-5-phenyl-1-propyl-N-(3-(4-(quinolin-8-yl)pipe-razin-1-yl)propyl)-1H-pyrrole-3-carboxamide (38) Compound 38 was obtained by repeating the procedure of com-pound 5. 1H NMR (400 MHz, CDCl) 8 8.90 (dd,J=1.6, 4.0 Hz, 1H),8.12(dd,J=1.6, 8.0 Hz, 1H)1), 7.46-7.30 (m, 8H), 7.14 (dd,j=4.0,5.2 Hz, 1H), 6.50 (br s, 1H), 6.31 (s, 1H), 3.86 (t,J=7.2 Hz, 2H),3.55 (q,J=5.6Hz, 2H), 3.08(br s, 4H), 2.69 (br s, 4H), 2.61 (t,J=6.0Hz, 2H), 1.83-1.77 (m, 2H), 1.71-1.61 (m, 3H), 0.79 (t,J=7.6Hz, 3H).MH+467. 5.2.25.N-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)-2-methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (39) Compound 39 was obtained by repeating the procedure of com-pound 5.'H NMR(400 MHz, CDCls)87.43-7.40(m,2H), 7.35-7.31(m,3H),7.28-7.26(m,2H), 7.19-7.16(m,1H), 6.45 (br s,1H), 3.90(t, J=7.2 Hz, 2H), 3.82 (d,J=11.6Hz, 2H), 3.72 (t, J=5.6 Hz, 2H),3.55 (d,J=13.2Hz, 2H), 3.50-3.11(m, 8H), 1.53-1.44 (m, 2H),0.70 (t, J=7.6Hz,3H). MH+499. ( 5.2.26.N-(3-(4-(2, 3 -Dichlorophenyl)piperazin-1-yl)propyl)- N,1,2-trimethyl-5-phenyl-1H-pyrrole-3-carboxamide (40) ) ( Compound 4 0 wa s obtained by repeating the procedure of com- pound 5.H NM R (40 0 MHz, CDCl;) 87.43-7.26 (m, 6H), 7.19-7.04 (m,2H), 3.68-3.64(m, 2 H), 3.52 (s, 3H), 3.33 (s,3H), 3.30-3.22(m, 8H),2.38 (s,3H). MH+499. ) ( 5.2.27. N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-N,2- dimethyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide hydro- chloride (41) ) ( Compound 41 was obtained by repeating the procedure of com-pound 5a. H NMR (400 MHz, MeOH-d4) 8 7.46-7.37 (m, 5H ) , 7.06 ) ( (t,J=7.5 Hz, 1H), 7.01(m,2H),4.26(t,J=6.9 Hz, 2H), 4.13 (s, 3H), 3.70-3.64 (m, 2 H ), 3 . 58 (s, 3H), 3.48-3.23 (m, 8H), 2.09-2.06 (m, 4H), 1.10 (t,J=6.8 Hz,3H). MH+5 2 7 (-HCl). ) ( 5.2.28.N-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)-2- methyl-5-phenyl-1H-pyrrole-3-carboxamide (42) ) ( Compound 42 was obtained by repeating the procedure of com- pound 5. 1HNMR(400 MHz, CDCl3)87.57-7.55 (m,2H), 7.35-7.27 (m, 4 H), 7.20-7.16 (m, 2H), 3 . 83 (d,J=12.0Hz, 2H) , 3.7 6 (t, J =5 . 2 Hz, 2 H), 3.56 (t, J=13.6Hz, 2H), 3. 4 5 (t , J= 5 .6 Hz, 2H),3.36(t,J=9.6 Hz, 2H), 3.20 (t,J=9.6Hz,2H), 2.56(S,3H).MH+ 457. ) ( 5.2.29. N -(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)-1- ethyl-2-methyl-5-phenyl-1H-pyrrole-3-carboxamide hydro chloride (43) ) Compound 43 was obtained by repeating the procedure of com-pound 5a. H NMR (400MHz, MeOH-d4)8 7.43-7.26 (m, 7H), 7.17(dd,J=6.4,2.8 Hz, 1H), 3.96(q,J=6.8 Hz, 2H), 3.82 (d,J=12.4Hz,2H), 3.73 (t,J=5.2Hz, 2H), 3.55 (d,J=13.2Hz, 2H), 3.43 (t,J=5.6 Hz, 3.38-3.15 (m, 4H), 2.61(s, 3H),1.12 (t, J=7.2Hz, 3H).MH+ 485 (-HCl). ( 5.2. 3 0. N -(2-(4-(2,3-Dichlorophenyl)piperazin-1- y l)ethyl)-2- ) methyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide (44)Compound 44 was obtained by repeating the procedure of com-pound 5. HNMR (400MHz, CDCl3)87.43-7.40(m,2H), 7.35-7.31(m,3H), 7.28-7.26(m,2H),7.19-7.16 (m, 1H), 6.45 (br s, 1H), 3.90(t,J=7.2Hz, 2H), 3.82 (d,J=11.6Hz, 2H), 3.72 (t,J=5.6 Hz, 2H),3.55 (d,J=13.2 Hz, 2H), 3.50-3.11 (m, 8H), 1.53-1.44 (m, 2H),0.70 (t,J=7.6 Hz, 3H). MH+ 499. ( 5.2.31. N-(3 - (4 - (2 , 3-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-5-phenyl-1H-pyrrole-3-carboxamide (45) ) ( Com po und 45 was obtained by repeating the p rocedure of com- pound 5.H N MR(400 MHz, CDCl3)8 8.46 (br s, 1H), 7.53(m, 1H), 7 .3 9( d ,J = 7.6 Hz, 2H), 7 . 21-7.13(m, 2H), 6.98 (t , J=8.0 Hz, 1H), 6.81 (d,J=8.0Hz, 1 H ), 6 . 62 (d,J=2.4 Hz, 1 H ), 3.57-3.53(m, 2H), 3 . 1 3 ( br s , 4H), 2 .71 (br s, 5 H),2 . 63 (s, 3 H ), 1. 8 4-1.79 (m, 2H) . M H+ 471. ) ( 5 .2.32. N-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)-2,4- dimethyl-5-phenyl-1H-pyrrole-3-carboxamide hydrochloride(46) ) Compound 46 was obtained by repeating the procedure of com-pound5a. H NMR (400 MHz, MeOH-d4)87.40-7.31 (m, 4H), 7.30-7.28(m, 2H), 7.26-7.18(m,1H), 7.16-7.15(m, 1H), 3.84-3.80(m,4H),3.56(d,J=12.8Hz,2H), 3.49-3.43(m,2H), 3.38(d,J=11.2 Hz,2H), 3.21 (t, J=11.2 Hz, 2H), 2.45 (s, 3H), 2.30 (s, 3H). MH+ 471(-HCl). ( 5.2.33.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2,4- trimethyl-5-phenyl-1H-pyrrole-3-carboxamide hydrochloride (47) ) Compound 47 was obtained by repeating the procedure of com-pound 5a.HNMR(400 MHz, MeOH-d4)87.46-7.42(m,2H),7.38-7.31 (m, 1H), 7.30-7.23 (m, 4H), 7.20-7.15 (m, 1H), 3.70 (d,J=11.6 Hz, 2H), 3.58-3.53 (m, 4H), 3.37-3.10 (m, 6H), 3.35 (s,3H), 2.42 (s, 3H), 2.19-2.12 (m, 2H),2.06 (s,3H). MH+ 499 (-HCl). ( 5.2.34. N -(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2,4- dimethyl-5-phenyl-1-propyl-1H-pyrrole-3-carboxamide h y dro- chloride (48) ) Compound 48 was obtained by repeating the procedure of com-pound 5a. H NMR (400MHz, MeOH-d4)87.45-7.42 (m,2H), 7.39-7.37 (m, 1H), 7.35-7.18 (m,4H), 7.17-7.18 (m, 1H), 3.76-3.68 (m,4H), 3.57-3.53(m,4H), 3.36-3.18 (m, 4H), 2.44 (s,3H), 2.18-2.11(m,2H), 2.02 (s, 3H), 1.47-1.40 (m, 2H), 0.66 (t,J=7.2Hz,3H). 13c400-188-0725 5.2.35. N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2,4-dimethyl-5-phenyl-1H-pyrrole-3-carboxamide hydrochloride (49) Compound 49 was obtained by repeating the procedure ofcompound 5a. HNMR (400 MHz, MeOH-da) 8 7.41-7.35 (m, 4H),7.31-7.28(m,2H), 7.26-7.20(m, 1H), 7.18-7.16 (m, 1H), 3.70 (d,J=11.6Hz, 2H), 3.58-3.53 (m, 4H), 3.36-3.19 (m, 6H), 2.43 (s,3H), 2.28 (s, 3H), 2.20-2.12 (m, 2H). MH+ 485 (-HCl). 5.2.36.N-(3-(4-(2,3-Dimethylphenyl)piperazin-1-yl)propyl)-2-methyl-5-phenyl-1H-pyrrole-3-carboxamide (50) Compound 50 was obtained by repeating the procedure of com-pound 5. H NMR (400 MHz, CDCl3)8 8.41 (br s, 1H), 7.58(m,1H),7.44-7.42(m,2H),7.32-7.26(m,2H), 7.23-7.19(m,1H), 6.96-6.81(m, 3H), 6.67 (d,J=2.4Hz, 1H), 3.55 (dd,J=11.6, 5.6 Hz, 2H), 2.98(t,J=4.8 Hz, 4H), 2.64 (s,3H), 2.62-2.57 (m, 2H), 2.26 (s, 3H), 2.21(S, 3H), 1.84-1.78 (m, 2H), 1.63-1.60 (m, 2H). MH+ 431. 5.2.37.N-(3-(4-(2,3-Dimethylphenyl)piperazin-1-yl)propyl)-1-ethyl-2-methyl-5-phenyl-1H-pyrrole-3-carboxamide (51) Compound 51 was obtained by repeating the procedure of com-pound 5. 1H NMR (400 MHz, CDCl3) 8 7.03-7.35 (m, 7H), 6.96-6.87(m, 2H), 6.64 (d,J=7.6 Hz, 1H), 6.37 (s,1H), 3.91(q,J=7.2 Hz,2H)3.54 (dd,J=11.6,5.6Hz,2H), 2.92-2.89 (m, 3H), 2.67 (s,3H), 2.60(t,J=6.0Hz, 3H), 2.56 (s, 3H), 2.19(s,3H), 1.81-1.77(m,2H), 1.19(t,J=7.2 Hz,3H). MH+459. Compound 53 was obtained by repeating the procedure of com-pound 5a.H NMR(400 MHz,MeOH-d4)8 7.38 (dd,J=8.0,20.8 Hz,4H), 7.07 (d,J=7.6Hz, 1H), 7.03-6.97 (m, 2H), 3.66 (br s, 1H),3.51-3.48 (m,5H), 3.38-3.37 (br s, 1H), 3.33-3.32 (m, 1H), 2.26(d, J=1.6 Hz, 3H), 2.26 (d, 2.4Hz, 6H), 2.13-2.10 ((m, 2H). MH+479 (-HCl). 5.2.40.5-(4-Chlorophenyl)-N-(3-(4-(2,3-dimethylphenyl)pipera-zin-1-yl)propyl)-1-ethyl-2-methyl-1H-pyrrole-3-carboxamidehydrochloride (54) Compound 54 was obtained by repeating the procedure ofcompound 5a. H NMR(400 MHz, MeOH-d4)8 7.43-7.41(m,2H),7.36-7.34(m,2H), 7.07 (t,J=7.6Hz, 1H), 6.99-6.95 (m, 2H), 3.95(q,J=6.8 Hz, 2H), 3.64(d,J=10.0Hz, 2H), 3.48 (t, J=6.0Hz, 2H),3.33-3.28(m,2H), 3.26-3.16(m,6H), 2.59(d,J=2.0 Hz, 3H), 2.25(s, 6H), 2.10-2.07 (m,2H),1.13(t,J=7.2Hz,3H). MH+493 (-HCl). ( 5.2.41.5-(4-Chlorophenyl)-N-(3-(4-(2,3-dimethylphenyl)pipera- zin-1-yl)propyl)-2-methyl-1-propyl-1H-pyrrole-3-carboxamidehydrochloride(55) ) Compound 55 was obtained by repeating the procedure of com-pound 5a.HNMR(400 MHz,MeOH-d4)87.43-7.41 (m,2H), 7.35-7.32 (m, 2H), 7.10-7.05 (m, 1H), 6.99-6.95(m, 2H), 3.89 (t, ( J =7.6 Hz, 2 H), 3 .64 ( d, J = 10.8 Hz, 2H), 3.48 ( t, J=6.4Hz, 2H), 3.36-3.17 (m, 6H), 2.59 ( s , 3 H), 2.25 (s, 6H), 2.12-2.05 (m, 2H), 1.52-1.46(m,2H), 0.71 (t,J=7.2 Hz,3H). MH+ 5 0 7(-HCl). ) ( 5.2.42.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1-ethyl-2-methyl-5-phenyl-1H-pyrrole-3-carboxamide (56) ) ( Compound 56 was obtained by repeating the procedure of com- pound 5. HNMR (400 MHz, CDCl3)8 7.38-7.32 (m, 5H), 7.14 (dd, J =8.4, 1 .6 Hz, 1H), 7.01 (t,J=7.6 Hz, 1H), 6.66 (dd,J=8.0,1.6 H z, 1H), 6.34 (s, 1H), 3.91 (q,J=7.2 Hz, 2H), 3.53 (dd,J=11.2,5 . 6 Hz,2H), 3.06 (m, 4 H), 2 .67 (s, 3H), 2.61 (t,J=6.0Hz, 2 H ), 1.83-1.73 (m, 3H), 1.37-1.25(m,2H), 1 .19 (t,J= 7 .6 Hz, 3H). MH+ 499. ) ( 5.2.43. 5 -(4-Chlorophenyl)-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-1H-pyrrole-3-carboxamide hydroch-loride (57) ) ( Compound 57 was obtained by repeating the procedure o f com-pound 5 a. HN M R (400MHz, MeOH-d4) 8 7.53 (d,J = 8 . 4 Hz, 2H ) , 7.35-7.28 ((m, 3H), 7.18 8 ( dd, J=6.4, 3.2 Hz, : . 2 2H), 3 . 68 ( d, J=12.0 Hz, 2H), 3.37 (d,J=13.2Hz, 2 H), 3, 5 0 ( t ,J = 6 .0 H z,2H), 3.36-3.18(m,5H), 2.26(s,3H), 2.13-2.07( m ,2H).MH+505 ( -HCl). ) 5.2.44.5-(4-Chlorophenyl)-N-(3-(4-(2,3-dichlorophenyl)pipera- zin-1-yl)propyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide (58)Compound 58 was obtained by repeating the procedure of com-pound 5. 1H NMR(400MHz, CDCl3)8 11.46 (br s, 1H), 7.32-7.21(m, 5H), 7.13 (t,J=8.0Hz, 1H), 6.93 (d,J=7.6 Hz, 1H), 6.82 (s,1H), 6.55 (br s, 1H), 3.67-6.64 (m, 3H), 3.50-3.43(m, 1H), 3.44(s, 3H), 3.33-3.31(m,4H), 3.14 (br s, 2H), 2.56 (s, 3H), 2.31 (br s,2H). 1'C NMR (100 MHz,, CDCl:)8 167.3,148.8,137.2,134.1,133.4,133.2,130.7,130.2,128.7,127.8,127.5,126.0,119.2,108.1,54.6,52.5,48.0,37.5,32.0,23.9, 12.2. MH+ 519. 5.2.45.5-(4-Chlorophenyl)-N-(3-(4-(2,3-dichlorophenyl)pipera-zin-1-yl)propyl)-1-ethyl-2-methyl-1H-pyrrole-3-carboxamidehydrochloride (59) Compound 59 was obtained by repeating the procedure of com-pound5a.HNMR(400 MHz,MeOH-d4)87.43-7.41(m,2H),7.36-7.34(m, 2H), 7.30-7.29 (m, 2H), 7.18-7.16 (m, 2H), 3.95 (q,J=6.8Hz, 2H), 3.67 (d, J=12.0 Hz, 2H), 3.54 (d,J=12.4Hz, 2H),3.47 (t,J=6.4 Hz,2H), 3.34-3.17(m, 6H), 2.60 (s, 3H), 2.10-2.06(m, 2H), 1.13 (t, J=7.2 Hz, 3H). 1c NMR (100 MHz, MeOH-d4)8170.0,150.8,136.6,135.2,134.8,133.6,133.0,131.9,129.9,129.5,128.7,127.2,120.7,114.4,55.5,53.5,40.0,37.4,25.7,16.4,11.9.MH+534 (-HCl). ( 5.2.46.5-(4-Chlorophenyl)-N-(3-(4-(2,3-dichlorophenyl)pipera- zin-1-yl)propyl)-2-methyl-1-propyl-1H-pyrrole-3-carboxamidehydrochloride (60) ) Compound 60 was obtained by repeating the procedure of com-pound 5a.HNMR(400 MHz, MeOH-d4)87.43-7.41 (m,2H), 7.34-7.32 (m, 2H), 7.30-7.26(m, 2H), 7.20-7.16 (m, 1H), 3.89 (t,J=7.6Hz, 2H), 3.67 (d,J=11.6Hz, 2H), 3.54 (d,J=12.4Hz, 2H),3.47 (t, J=6.0 Hz, 2H), 3.34-3.17(m, 4H), 2.59 (s, 3H), 2.11-2.07(m,2H), 1.54-1.44(m,2H), 0.71 (t,J=7.2 Hz,3H).MH+ 547 (-HCl). 5.2.47.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-5-(pyridin-2-yl)-1H-pyrrole-3-carboxamide dihydrochlo-ride (61) Compound 61 was obtained by repeating the procedure of com-pound 5a. 1H NMR(400 MHz, MeOH-d4) 8 8.55 (dd,J=8.0, 6.0 Hz,1H), 8.45 (dt,J=1.6,8.8 Hz, 1H), 8.13 (d,J=8.4Hz, 1H), 7.68 (dt,J=1.2, 7.2Hz, 1H), 7.59-7.56(m, 1H),7.31-7.28(m,2H), 7.15-7.10 (m, 1H), 3.70 (d,J=12.0Hz, 2H), 3.54 (d,J=13.6 Hz, 2H),3.50 (t,J=6.8 Hz, 2H), 3.37-3.31(m, 4H), 3.21 (d,J=12.4Hz,2H), 2.46 (s, 3H), 2.14-2.11(m,2H).MH+ 472(-2HCl). 400-188-0725 5.2.48.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-(pyridin-2-yl)-1H-pyrrole-3-carboxamide dihydro-chloride (62) Compound 62 was obtained by repeating the procedure of com-pound 5a. H NMR (400 MHz,MeOH-da) 8 8.74 (d,J=6.0Hz, 1H),8.58 (dt, J=1.6, 8.0Hz, 1H), 8.13 (d, J=8.4Hz, 1H), 7.90 (t,J=6.4Hz, 1H), 7.31-7.28 (m, 2H), 7.26-7.25 (m,1H), 7.18-7.15(m, 1H), 3.73 (s, 3H), 3.68 (d,J=12.0 Hz,2H), 3.53 (d,J=12.4Hz,2H), 3.48 (t,J=6.4 Hz, 2H), 3.33-3.28(m,2H), 3.23-3.19 (m, 2H),2.65 (s, 3H),2.13-2.09 (m, 2H). 1c NMR (100 MHz, CDCl;) 8167.7,152.2,151.0,148.8,137.2,136.5,134.0,131.3,127.3,124.5,121.8,120.7,118.4,115.2,108.5,58.4,53.5, 51.3, 39.7,32.5,25.0, 11.3. MH+ 486 (-2HCl). 5.2.49.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-1-propyl-5-(pyridin-2-yl)-1H-pyrrole-3-carboxamidedihydrochloride (63) Compound 63 was obtained by repeating the procedure of com-pound 5a. H NMR(400 MHz, MeOH-d4) 8 8.77 (dd, J=1.2, 6.0 Hz,1H), 8.61 (dt,J=1.6, 8.4Hz, 1H), 8.12 (d,J=8.0 Hz, 1H), 7.94 (dt,J=1.2, 7.2 Hz, 1H), 7.31-7.28 (m, 2H), 7.26-7.25 (m,1H), 7.18-7.15 (m, 1H), 4.14 (t, J=7.2Hz, 2H), 3.79 (d,J=12.4Hz, 2H), 3.54(d,J=13.6 Hz, 2H), 3.48 (t, J=6.4Hz, 2H), 3.33-3.28(m, 2H),3.21 (d,J=12.0 Hz, 2H), 2.45 (s, 3H), 2.12-2.10 (m, 2H), 1.59-1.56 (m, 2H), 0.76 (t, J=7.2Hz, 3H). MH+514(-2HCl). 5.2.50.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-(pyridin-3-yl)-1H-pyrrole-3-carboxamide(64) Compound 64 was obtained by repeating the procedure of com-pound 5.H NMR (400 MHz, CDCl3)8 8.64 (d,J=1.6 Hz, 1H), 8.55(dd,J=1.2, 4.8 Hz, 1H), 7.62 (td,J=2.0, 8.0 Hz, 1H), 7.26-7.24(m, 1H), 7.16-7.13(m, 1H), 7.02 (t,J=8.0Hz, 1H), 6.65 (dd,J=1.2,8.0 Hz, 1H), 6.44 (s,1H), 3.55-3.52(m,3H), 3.51 (s,,3H),3.04 (br s,4H), 2.67 (br s, 2H), 2.65 (s, 3H), 2.61 (t,J=5.6 HZ,2H), 1.83-1.77 (m, 2H). 13C NMR (100MHz, CDCl3) 8 165.46150.9,149.5,148.2,135.9,135.8.,1133.9,129.5,1128.8,127.5,124.6,123.3,118.3,115.4,107.5,77.3,58.1,53.5,51.3,39.4,31.7,25.1, 11.4. MH+ 486. 5.2.51.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-1-propyl-5-(pyridin-3-yl)-1H-pyrrole-3-carboxamide (65) Compound 65 was obtained by repeating the procedure of com-pound 5. 1H NMR (400 MHz, CDCl3) 8 8.63 (d,J=2.0 Hz, 1H), 8.57(dd,J=1.6, 4.8 Hz, 1H), 7.60 (td,J=2.0, 8.0 Hz, 1H), 7.26-7.22(m,1H), 7.14 (dd,J=1.2, 8.0Hz, 1H), 7.03 (t, J=8.0 Hz, 1H), 6.64 (dd,J=1.2,8.0Hz, 1H), 6.40 (s, 1H), 3.81 (t,J=8.0Hz, 2H), 3.53 (t,J=6.0Hz, 2H), 3.03 (br s, 4H), 2.67 (br s, 3H), 2.66(s, 3H), 2.60(t, J=6.0 Hz, 2H), 1.83-1.77 (m, 2H), 1.60-1.50 (m, 2H), 0.76 (t,J=7.2 Hz, 3H).MH+514. 5.2.52.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-5-(pyridin-4-yl)-1H-pyrrole-3-carboxamide (66) Compound 66 was obtained by repeating the procedure of com-pound 5. 'H NMR (400 MHz, CDCl3) 8 8.64 (d, J=1.2Hz, 1H), 8.55(dd,J=1.6, 4.8Hz, 1H),7.61 (td,J=2.0, 8.0 Hz, 1H), 7.26-7.24(m, 1H), 7.14 (dd,J=2.8,6.8 Hz, 1H), 7.02 (t, J=8.4 Hz, 1H), 6.65(dd,J=1.2, 8.0Hz, 1H), 6.43 (s, 1H), 3.55-3.51 (m, 2H), 3.51 (s,3H), 3.04 (br s, 4H), 2.67 (br s, 2H), 2.65 (s, 3H), 2.611 (t,J=6.4Hz,2H), 1.83-1.77(m,2H). MH+486. ( 5.2.53.N-(3-(4-(2,3-Dichlorophenyl)piperazin-1-yl)propyl)-2-methyl-1-propyl-5-(pyridin-4-yl)-1H-pyrrole-3-carboxamide (67) ) Compound 67 was obtained by repeating the procedure of com-pound 5. H NMR (400 MHz, CDCl3) 8 8.62 (d, J=1.2Hz, 1H), 8.56(dd,J=1.6, 4.8 Hz, 1H), 7.60 (td,J=2.0, 7.6 Hz, 1H), 7.26-7.24(m, 1H), 7.14 (dd,J=1.2, 8.0 Hz, 1H), 7.03 (t, J=8.0 Hz, 1H), 6.64 ( (dd,J= 1 .2, 8 . 0 Hz, 1H), 6 . 40 ( s ,1 H ), 3 . 81 ( t ,J=8.0 Hz, 2H ) , 3. 5 3(t, J=6.0Hz, 2H), 3 . 03 ( b r s , 4H), 2 . 67 ( b r s, 4H), 2 . 66 ( s , 3H),2.60 (t,J=6.0 Hz, 2H), 1.81 - 1.78(m, 2 H), 1 . 58-1.52(m,2H), 0.76(t,J=7.2 Hz, 3H). MH+514. ) ( 5.2.54. 5-tert-Butyl-N-(2-(4-(2,3-dimethylphenyl)piperazin-1-yl)ethyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide ( 6 8) ) ( Compound 68 was obtained by repeating the procedure of com-pound 5.H NM R (400 MHz, CDCl3) 8 7.08 (t,J=7.6 Hz, 1H), 6.93- 6.89 ( m, 2H), 6.31 ( b r s , 1H), 6.01 (s , 1 H ), 3.59(s, 3 H ), 3 . 51 ( q , J =5.2 Hz, 2H), 2.92 (br s, 4 H), 2.65 ( br s , 4H), 2.63 (q,J=6.0 Hz,2H), 2 .54 (s, 3H), 2 .27 ( s,3H), 2.23 ( s , 3H), 1.37 (s, 9 H). MH+ 410. ) ( 5.2.55. 5-tert-Butyl-N-(3-(4-(2,3-dimethylphenyl)piperazin- 1 - ) yl)propyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide (69) ( Compound 69 was obtained by repeating the procedure of com- pound 5. 1 H NMR (400MHz, CDCl;) 8 7.05 (t, J=7.6 Hz, 1H ) , 6.93- 6.87 (m,2H),6.02 (s,1H), 3.58 (s, 3H), 3.50-3.42( m ,2 H ) , 2 . 94(br s,4H), 2.64(br s, 4H), 2.57 (t,J=6.0Hz,2H), 2.55 (s , 3H),2.26(s , 2H),2.21(s, 3H) 1.80-1.77(m, 2H), 1.35(s,9H ) .MH+ 4 2 4. ) ( 5 .2.56.5-tert-Butyl-N-(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide h y drochlo- r ide (70) ) Compound 70 was obtained by repeating the procedure of com-pound 5a. 'H NMR (400 MHz, MeOH-d4)87.28-7.26(m,2H), 7.17-7.14 (m, 1H), 3.80(d,J-12.4Hz, 2H), 3.72 (t,J=5.2Hz, 2H), 3.56(d,J-13.6Hz, 2H), 3.42 (t, J=5.6Hz, 2H), 3.39-3.18 (m, 3H),2.48 (s, 3H), 1.35 (s, 9H). 130 NMR (100MHz,MeOH-d4) 8 169.1,149.3,140.7,135.8,133.7,128.0,127.2,125.6,119.2,110.8,57.4,52.4,52.4,31.9,31.3,29.1,10.3. MH+ 450 (-HCl). ( 5.2.57.5-tert-Butyl-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)- propyl)-2-methyl-1H-pyrrole-3-carboxamide hydrochloride (71) C o mpound 71 was obtained by repeating the procedure of com- p oun d 5. 1 H N MR (400 MHz, CDCl3) 8 7.78 ( b r s,1H), 7.19-7.10 (m, 2 H ), 6 .99 (br s, 1H), 6.93 (dd,J=2.4, 2.0 Hz , 1H) , 5.98(d , J= 2 .8 Hz, 1 H) , 3 .50 (q,J = 6.0 Hz, 2H ) , 3. 1 3 (br s, 4H), 2. 6 9(br s, 4H), 2.59 (t , J =6.4Hz, 2H), 2. 5 4 (s , 3H), 1.82-1.76 (m, 2H) , 1.2 3 (s, 9H). M H + 451(-HCl). ) 5.2.58.5-tert-Butyl-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide (72) Compound 72 was obtained by repeating the procedure of com-pound 5. 1HNMR (400 MHz, CDCl3) 87.17-7.10 (m, 2H), 6.92 (dd,J=2.0, 2.4 Hz,1H), 6.87 (br s, 1H), 5.99 (s 1H), 3.58 (s, 3H), 3.49 (q,J=5.6 Hz, 2H), 3.11 (br s,4H), 2.68 (br s, 4H), 2.58 (t,J=6.8Hz,2H),2.55 (s, 3H), 1.80-1.77 (m, 2H), 1.33 (s, 9H). 13C NMR (100 MHz,CDCl3) 8 166.1 151.0,140.2 134.8,134.0,127.3,127.3,124.5,118.5,112.9,102.1,58.1,53.5,51.3,39.2,32.6,31.7,30.3,25.6,11.2.MH+ 465. 5.2.59.5-tert-Butyl-N-(2-(4-(3-chloro-2-methylphenyl)pipera-zin-1-yl)ethyl)-1,2-dimethyl-1H-pyrrole-3-carboxamide (74) Compound 74 was obtained by repeating the procedure of com-pound 5.1H NMR (400 MHz,CDCl3)8 7.10-7.06(m, 2H),6.95-6.92(m, 1H), 6.25 (br s, 1H), 6.00 (s, 1H), 3.59 (s, 3H), 3.51 (q,J=6.4 Hz,2H), 2.92 (t,J=4.4 Hz, 4H), 2.67-2.62(m, 6H), 2.54(s,3H), 2.34 (s,3H),1.36(s,9H). MH+ 431. 5.3. Biology 5.3.1. Measurement of binding affinity for serotonin 5-HT2A and5-HT2c receptors Receptor binding affinities of the compounds for serotoninreceptors were measured by the method described in the litera- 耐士科技 www.rysstech.com 4 00-188-0725 ture.18 For serotonin 5-HT2A binding, an aliquot of human recombi-nant serotonin 5-HT2A receptor (PerkinElmer Life and AnalyticalSciences, USA) expressed in CHO-K1 cells (5 ug/well) and 1 nM[H]Ketanserin (PerkinElmer) were used in the presence of mian-serin (20 uM) as nonspecific. The reaction mixture was incubatedfor 60 min at 27°C using 50 mM Tris-HCl (pH 7.4) buffer contain-ing 4 mM CaCl2 and 0.1% ascorbic acid, and harvested through fil-termate A glass fiber filter (Wallac, Finland) presoaked in 0.5%polyethyleneimine (PEI) by microbeta filtermate-96 harvester(PerkinElmer) to terminate the reaction, and then washed withi:c.e cold 50 mM Tris-HCl buffer solution (pH 7.4). The filter wasthen covered with MeltiLex, sealed in a sample bag, dried in anoven. The radioactivity retained in the filter was finally countedusing MicroBeta Plus(Wallac). The binding affinity (IC50) of a com-pound for the receptor was calculated by computerized nonlinearregression analysis (GraphPad Prism Program, San Diego, USA)using 7-8 varied concentrations of the compound run in duplicatetubes. For serotonin 5-HT2c binding, frozen membranes from stableCHO-K1 cell line expressing the human recombinant 5-HT2c recep-tor (PerkinElmer,4 pg/well),[H]Mesulergine (Amersham, 1.3nM)and test compounds were added into 50 mM Tris-HCl (pH 7.4) buf-fer containing 0.1% ascorbic acid and 4mM CaCl2. Nonspecificbinding was determined using 100 uM mianserin. The incubationswere performed for 60 min at 27C, and these were terminated byrapid filtration through filtermate.A glass fiber filter presoaked in0.5% PEI. 5.3.2. Measurement of binding affinity for serotonin transporter For serotonin transporter binding assays, a reaction mixturewith a final volume of 0.25 ml was prepared by mixing a test com-pound, human serotonin transporter membrane expressed in HEK-293 cells (PerkinElmer, 5 ug/well),[H]Imipramine (PerkinElmer,2 nM) and 50 mM Tris-HCl (pH 7.4) buffer containing 120 mMNaCl and 5mM KCl. The reaction mixture was incubated for30 min at 27°C, and harvested through filtermate.A glass fiber fil-ter presoaked in 0.5% PEI with ice cold 50 mM Tris-HCl buffer (pH7.4) containing 0.9% NaCl. 5.3.3.[’H] Astemizole binding to hERG potassiumion channelFor hERG K+ channel binding, an aliquot of frozen membranefrom HEK293 cell line expressing the recombinant human ether-a-go-go related gene (hERG) (Millipore Coorperation, MA, USA)and [H] Astemizole 3 nM (PerkinElmer Life and AnalyticalSciences, USA) were mixed in the presence of astemizole(10 uM)as nonspecific. The reaction mixture was incubated for 60 min at27 C using 10 mM Hepes (pH 7.4) buffer containing 130 mM NaCl,5 mM KCl, 0.8 mM MgCl2, 1 mM NaEGTA, 10 mM glucose, 0.1%BSA, and harvested through Filtermat A glass fiber filter presoakedin 0.3% PEI using 25 mM Tris (pH7.4) washing buffer containing130 mM NaCl, 5 mM KCl, 0.8 mM CaCl2 and 0.1% BSA. 5.3.4. Measurement of antidepressants activity in forcedswimming test To evaluate the antidepressants activity of the compounds, theinhibitory effects on immobility in forced swimming test in micewere measured according to the methods described by Porsoltet al.2 Each mouse was placed in a 25-cm glass cylinder (10 cmdiameter) containing 15 cm of water maintained at 22±1C, andwas forced to swim for 10 min. Twenty-four hours later, the mousewas replaced into the cylinder and the total duration of immobilitywas recorded during the last 5 min of the 6-min testing period.Mice are judged immobile when they float in an upright positionand make only small movements to keep their head above water. Test drugs were suspended in 3% Tween 80 solution, and adminis-tered orally (po) 60 min before the testing. 5.3.5. Measurement of locomotor activity (for Figure 4) One day before the test, mice were placed in transparent poly-carbonate cages located in activity chambers and were allowed tohabituate for 20 min. For locomotor activity test, compound wassuspended in 3% tween80 solution, and administered orally60 min before the test. Spontaneous locomotor activity was re-corded every 10 min for 30 min using Activity Analyzer (AM1052Activity Monitor, Benwick, UK). Unit is count/30 min. 5.3.6. Measurement of locomotor activity (for Tables 7 and 8) We measured using an automated video tracking system (Nol-dus Information Technology, Wagenin, the Netherlands) to exam-ine locomotor patterns. Mice was treated with test compounds(25mg/kg B.W., po) and acclimated to the test chamberfor10 min before starting the experiment. Locomotor activity wasthen measured for 30 min. The distance travelled in centimetersby the animals in horizontal locomotor activity was analyzed.Datawere collected and analyzed between 09:00 and17:00h. Acknowledgments We are grateful to Dr. Eun Chul Huh for his leadership at R&Doperation, Green Cross Corporation (GCC). This work is in part sup-ported by Korea Ministry of Knowledge Economy. References and notes 1. Murray, C. J.; Lopez, A. D. Lancet 1997,a;349,1436. 2.5-Hydroxyltryptamine Mechanisms in Primary Headaches; Olesen, J., Saxena, P.,Eds., 1st ed., Raven Press: New York, 1992. 3. The Global Burden of Disease: A Comprehensive Assessment of Mortality andDisability from Disease, Injuries and Risk Factors in 1990 and Projected to 2020;Murray, C. J.. L.,Lopez, A. D., Eds.; Harvard University Press: Cambridge, MA,1996; p38. 4 WHOThe World Health Report 2001, Mental Health: New Understanding, NewHope, Fact Sheet No. 265. 5Blier, P.; Bergeron, R. J. Clin. Psychiatr. 1998, 59, 16.6 Katz, M. M.; Tekell, J. L.; Bowden, C. L.; Brannan, S.; Houston, J. P.; Berman, N.;Frazer, A. Neuropsychopharmacology 2004,29,566. and references cited therein. 7.(a) Morphy, R.; Kay, C.; Rankovic, Z. Drug Discovery Today 2004, 9, 641. andreference cited therein; (b) Pacher, P.; Kecskemeti, V.Curr.Med. Chem. 2004, 11,925. 8.Ballesteros,J; Callado, L. F. J. Affect. Disord. 2004, 79,137. and reference citedtherein.9. Romeo, L.; Arigas, F. J. Neurochem. 1997, 68, 2593. 10.1Berman, R. M.; Anand, A.; Cappiello, A.; Miller, H. L; Xu, X. S.; Oren, D. A.;Charney, D. S. Biol. Psychiatry 1999, 45, 1170. 11. Pullar, I. A.; Carney, S. L.; Colvin, E. M.; Lucaites, V. L.; Nelson, D. L.; Wedley,S.Eur. J. Pharmacol. 2000,407, 39. 12.H1atanaka, K.; Nomura, T.; Hidaka, K.; Takeuchi, H.; Yatsugi, S.; Fujii, M.;Yamaguchi, T. Neuropharmacology 1997, 35, 1621. 13.((a) Rush, A. J.; Armitage, R.; Gillin, J. C.; Yonkers, K. A.; Winokur, A.; Moldofsky,H.; Vogel, G. W.; Kaplita,S. B.; Fleming, J. B.; Montplaisir, J.; Erman, M. K.;Albala, B. J; McQuade, R. D. Biol. Psychiatry 1998, 44,3; (b) Avila, A.; Vardona,X.; Martin-Baranera, M.; Maho, P.; Sastre, F.; Bello, J. J. Clin. Psychopharmacol.2003,23,509;(c) Greene, D.; Barbhaiya, R. H. Clin. Pharmacokinet. 1997, 33,260;(d) Kent, J. M. Lancet 2000, 355, 911. 14.(a) Lawler, C. P.; Prioleau, C.; Lewis, M. M.; Mak, C.; Jian, D.; Schetz, J. A.;Gonzalez, A. M.; Sibley, D. R.; Mailman, R. B. Neuropsychopharmacology 1999,20, 612; (b) Burstein, e. S.; Ma,J.; Wong, S.; Gao, Y.; Pahrm, E.; Knapp, A. E.;Nash, N. R.; Olsson, R.; Davis, R. E.;Hacksell, U.; Weiner, D. M.; Brann, M. R. J.Pharmacol. Exp. Ther. 2005,315,1278. 15. Kang,S. Y.; Park, E.-J.; Park, W.-K.; Kim, H. J;Jeong, D.; Jung,M. E.; Song, K.-S.;Lee, S. H.; Seo, H. J.; Kim, M. J.; Lee, M.; Han,H.-K.; Son, E.J.; Pae, A. N.; Kim,J.;Lee,J. Bioorg. Med. Chem. Lett. 2010,20, 1705. 16i.. VVanotti,E.; D'alessio,R.; Tibolla, M.; Varasi, M.; Montagnoli, A.; Santocanale, C.;Martina, K.; Menichincheri, M. WO2005/013986,2005. 17. Robarge, M. J; Husbans, S. M.; Kieltyka, A.; Brodbeck, R.; Thurkauf, An.;Newman, A. H. J. Med. Chem. 2001,44,3175. 18. PFark, W.-K.;Jeong, D.; Cho, H.; Lee, S. J.; Cha, M. Y.; Pae, A. N.; Choi, K. I.; Koh, H.Y.;Kong, J. Y. Pharmacol. Biochem. Behav. 2005,82, 361. 19. Kerns,E. H.; Di, D. Drug-like Properties: Concepts, Structure Design and Methods:From ADME to Toxicity Optimization; Academic Press: Burlington,MA, 2008. ( 20. Po rsolt, R . D . ; B e rtin, A.; Jalfre, M. Eur. J. Pharmacol. 1978, 51,291. ) ( 21. ( (a) Cryan, J . F.; Valentino, R . J.; L ucki, I. Neurosci. Biobehav. Rev. 2005, 29,547; ( b) K ulkarni, S. K .; D hir, A. Prog. Neuropsychopharmacol.Biol . Psy c hiatry 2007, 3 1, 1 248; (c) B y master, F. P.; L e e, T. C.; Knadler, M. P. ; Detke, M. J.; I tengar, S.Curr. Pharm. D es. 2005, 11, 1 475. ) ( 22. A lthough spontaneous locomotor activity of compound 64 was n o t measured by A ctivity Analyzer, hyperactivity was observed b y its b ehavior s uch as c limbing strongly, etc. during forced swimming t e st. ) 23. D1ierk,T.; Gut, B.; Wendt-Nordahl, G.; Kiehn,J.J. Pharmacol. Exp. Ther. 2002,300,543. S- see front matter @ Elsevier Ltd. All rights reserved.doi:j.bmc.ww.rysstech.com耐士科技 耐士科技
确定











还剩12页未读,是否继续阅读?
上海鑫欣生物科技有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药新药研发中其他检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有
相关方案
更多
该厂商其他方案
更多








