方案详情
文
he nature of water's hydrogen-bonding network is a vital in
fluence on the chemistry that occurs at interfaces, but a
complete understanding of interfacial water has proven elusive.
Even-order nonlinear optical spectroscopies, such as vibrational sum
frequency generation (VSFG) spectroscopy and heterodyne detected
phase-sensitive sum frequency generation (PS-SFG) spectroscopy,
are inherently surface specific. With the advent of advances in these
spectroscopic techniques, researchers can now explore many longstanding
questions about the dynamics and structures present at the
vaporwater and watersolid interfaces. Of special interest to the
atmospheric chemistry community is the accommodation of ions and
solutes by water's hydrogen-bonding network. A better understanding
of how ions and solutes behave in hydrogen-bonded water has afforded
a fresh perspective of aqueous aerosols, because the interactions
involved therein drive phenomena such as the hydrolysis of atmospheric
chemical species. In this Account, we present work from our
laboratory focusing on applying VSFG and the recently developed PSSFG
techniques to probe the perturbation ofwater's hydrogen-bonding
network at the vaporwater interface by a variety of ions and solutes.
We also present very recent results from our laboratory on the direct
observation of the adsorption of ions at the waterCaF2 interface.
方案详情

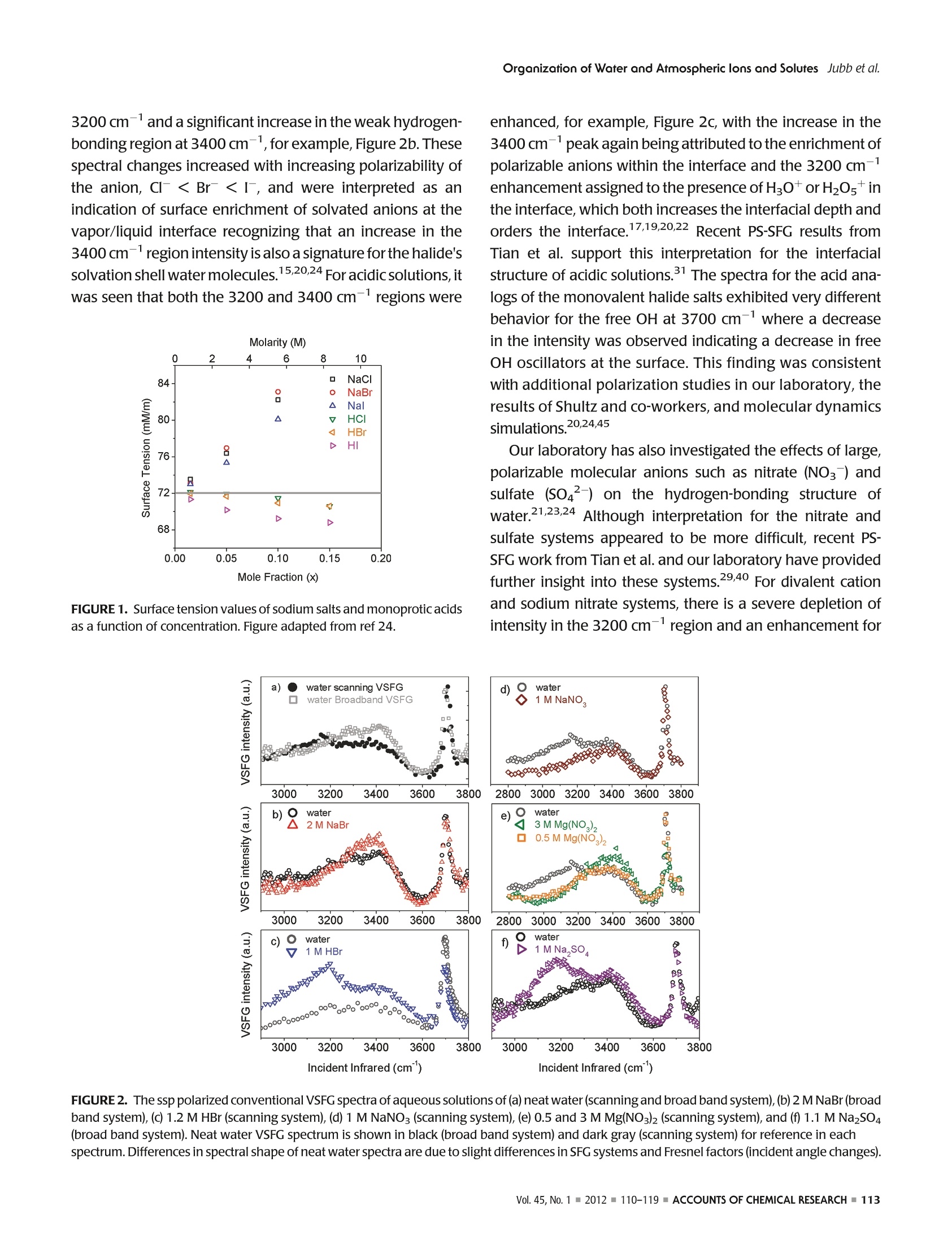
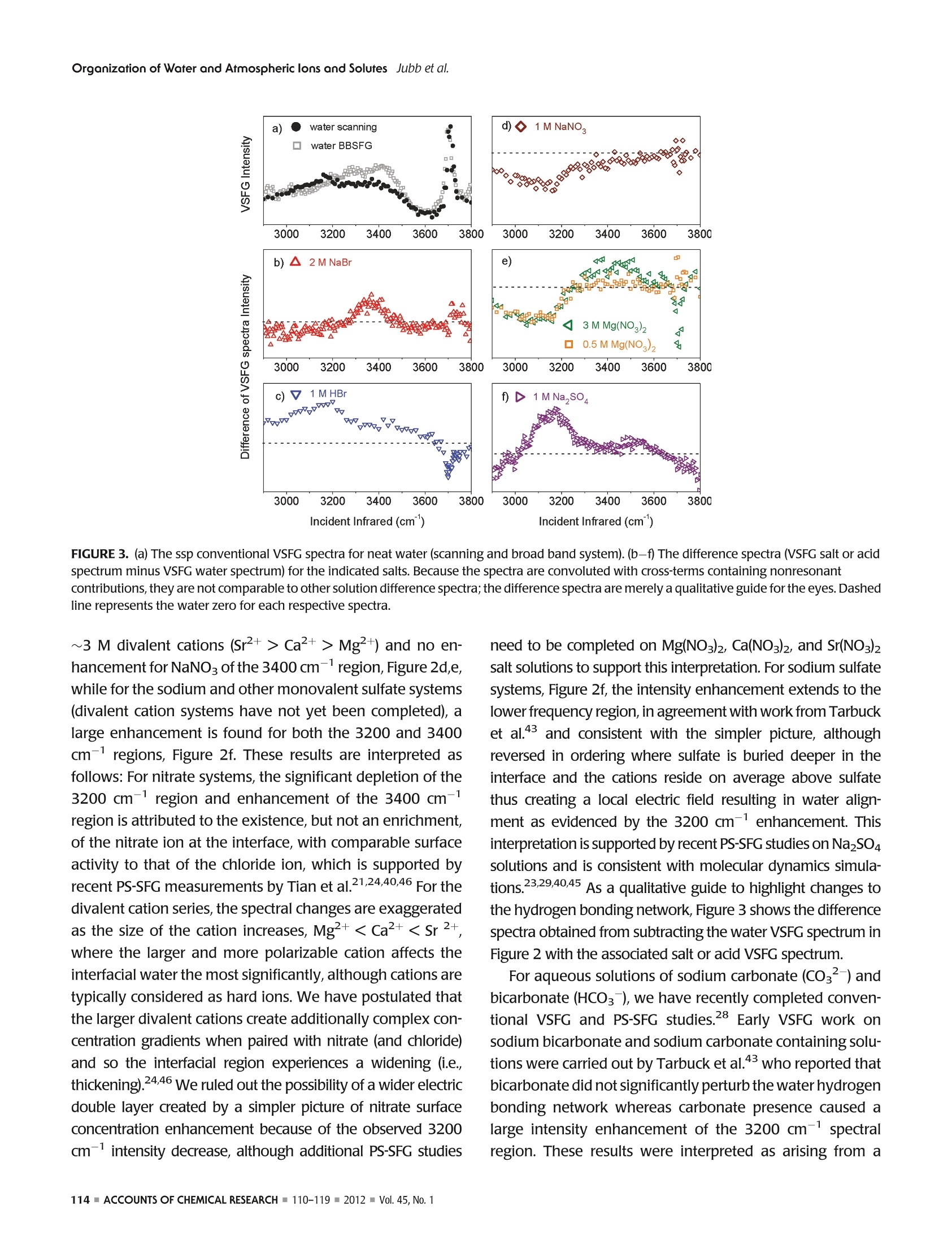
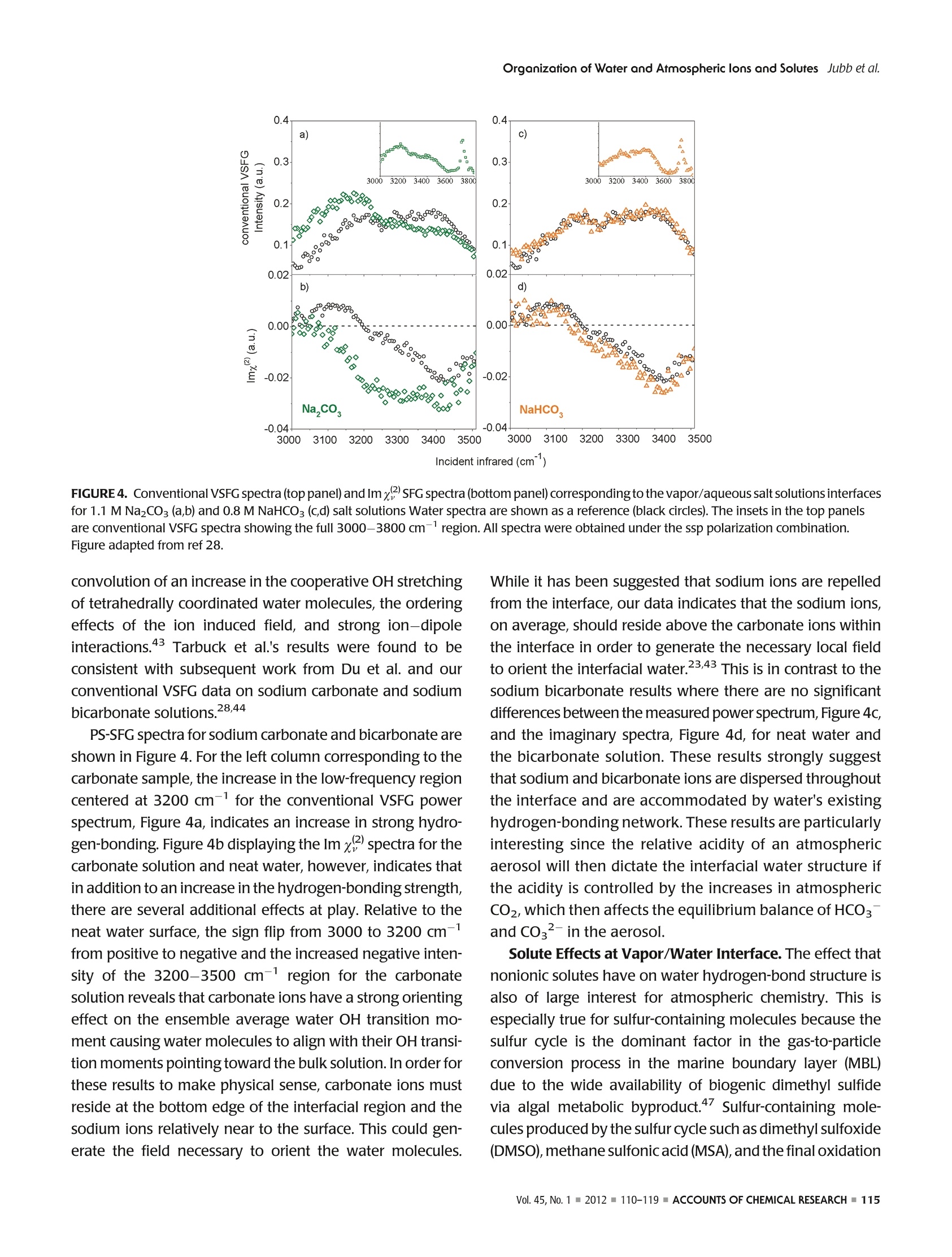
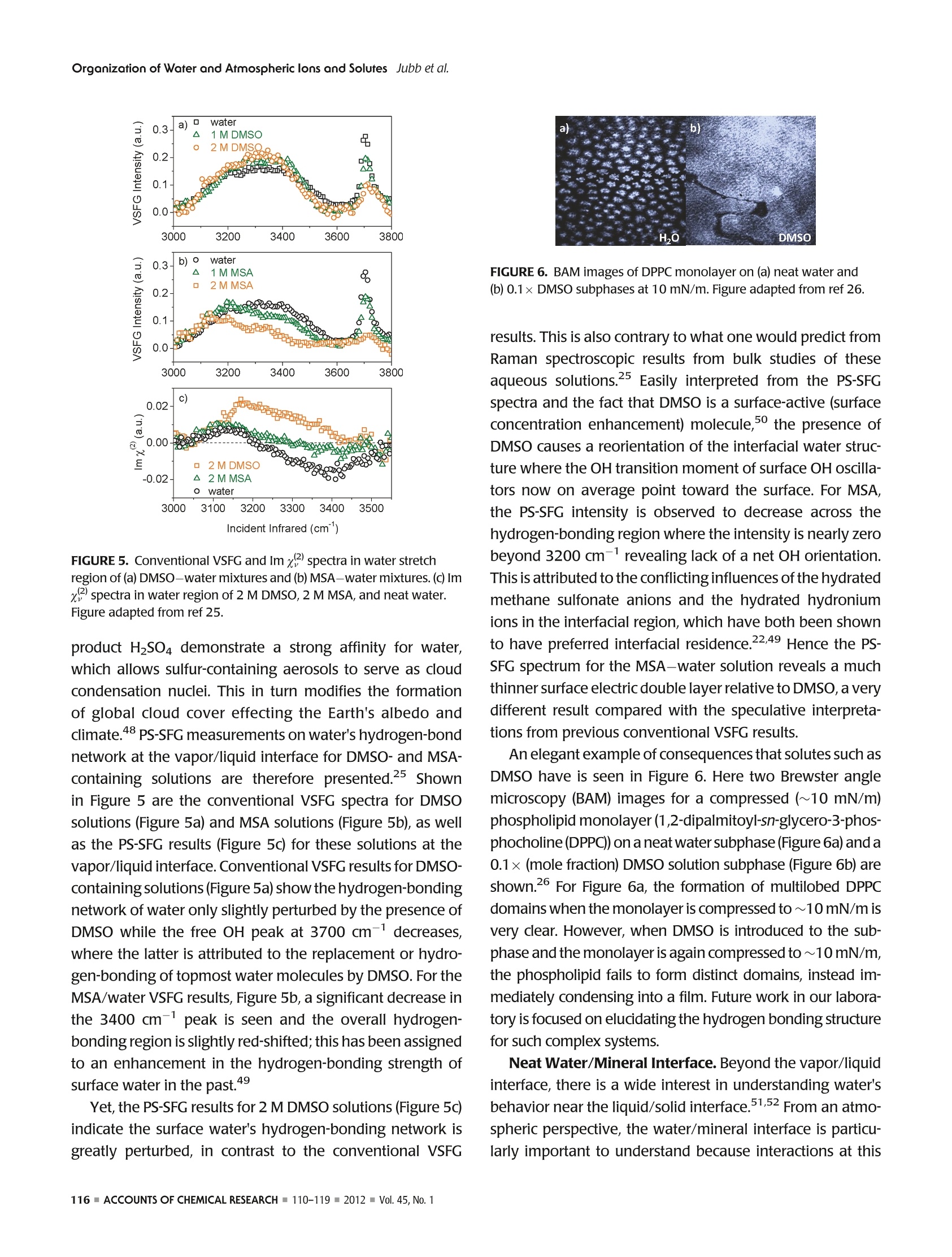
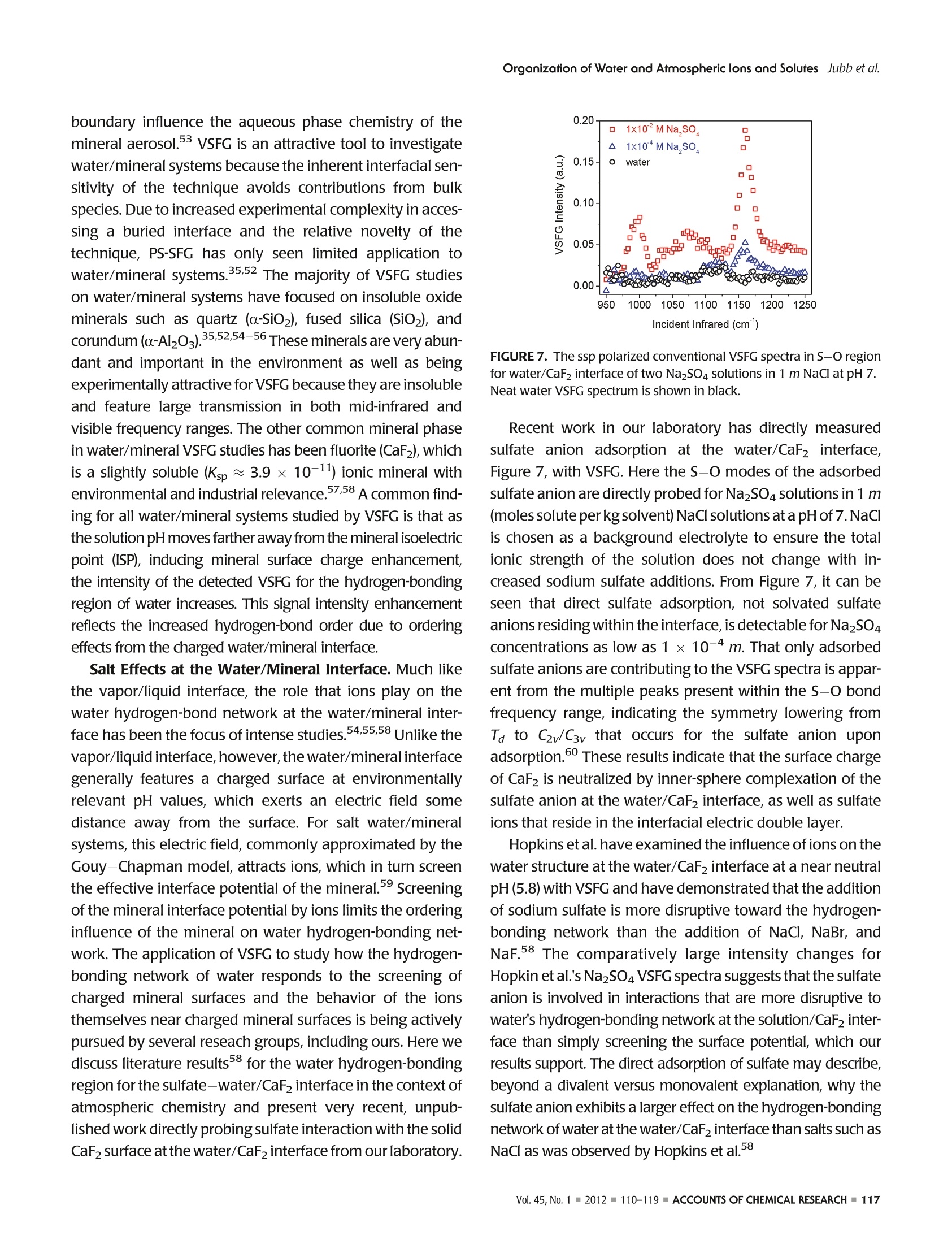
ResearchGateSee discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/51782891 ACCOUNTS-of chemical research Organization of Water and AtmosphericallyRelevant Ions and Solutes: Vibrational SumFrequency Spectroscopy at the Vapor/Liquid andLiquid/Solid Interfaces Article in Accounts of Chemical Research·November2011 DOI: 10.1021/ar200152v·Source:PubMed CITATIONS34 READS 68 3 authors: Aaron Jubb Wei Hua Oak Ridge National Laboratory The Ohio State University 20 PUBLICATIONS 333 CITATIONS 25 PUBLICATIONS 570 CITATIONS SEE PROFILE SEE PROFILE HeatherC AllenThe Ohio State University 153 PUBLICATIONS3,654 CITATIONS SEE PROFILE Organization of Water and AtmosphericallyPPHIECayRelevant Ions and Solutes: Vibrational SumFrequency Spectroscopy at the Vapor/Liquid andLiquid/Solid Interfaces AARON M. JUBB, WEI HUA, AND HEATHER C.ALLEN* Department of Chemistry, The Ohio State University, 100 West 18th Avenue, Columbus, Ohio 43210, United States RECEIVED ON JUNE 7, 2011 air/NaHCO;solution interface We begin by discussing the influence of ions and solutes on interfacial water structure. Results for halide salts and the acid analogs on interfacial water structure are shown to be quite different, aswould be expected from differences in surface tension measurements that have been known for a long time. Also examined are systemswith the largely polarizable molecular anions nitrate (NOg~), sulfate (SO4), carbonate (CO3), and bicarbonate (HCO).These systemsfeature more complicated influences on interfacial water structure than halide-containing solutions; however, our conventional VSFGresults for both nitrate and sulfate solutions are in agreement with recent PS-SFG results and molecular dynamics simulations. We also discuss recent PS-SFG work on carbonate and bicarbonate systems in which the accommodation of the bicarbonate ion at thevapor-water interface is in stark contrast to the carbonate results. Perturbation of interfacial water by solutes is examined for solutionsof dimethyl sulfoxide and methylsulfonic acid. PS-SFG results for these systems are striking: they illustrate the dramatic changes thatinterfacial water molecules undergo in the presence of solutes that are not observed with conventional VSFG. Finally, we discuss directsulfate ion adsorption for the aqueous sodium sulfate-CaFz interface, with the goal of elucidating water behavior at this surface. Introduction Water's ubiquity on Earth and the vital role it plays in allaspects of life make the necessity of scientific inquiry into its properties both obvious and practical. Beyond practicalconcerns, however, water is a fascinating subject that dis-plays many unique and subtle properties, which are still not completely understood and are the subject of much diverseand intense experimental and theoretical work. -10 Manyof water's important properties stem from its large hydro-gen-bonding nature such as its uncommonly high boilingpoint to the ability to solvate ions and solutes. The hydrogen-bond network at the asymmetric two-dimensional vapor/water interface is particularly interesting because muchchemistry is controlled by interactions at this interface.12The advancement of surface-specific even-order nonlinearoptical spectroscopic techniques, such as VSFG and surfacesecond harmonic generation (SHG), in the past two decadesalong with theoretical work has enabled elucidation ofmany questions on interfacial water.10,13-20 Of particularimportance to fields such as atmospheric chemistry is theaccommodation of ions and solutes by water's hydrogen-bond network. Work in the Allen laboratory has focused onelucidating the behavior of water's hydrogen-bonding net-work in the bulk and at the vapor/liquid and liquid/solidinterfaces for a variety of systems relevant to atmosphericaerosol chemistry,geochemistry, and biology through the useof vibrational linear (Raman and infrared) and nonlinear(VSFG) optical spectroscopies.17,21-29 Here we present primarily our most recent work,publishedand unpublished, on the roles that ions and solutes play inperturbing the hydrogen-bond network at the vapor/liquidand solid/liquid interfaces,as well as comment on contribu-tions from other researchers working in this field. Motivationfor this work stems from a knowledge gap with regard toatmospheric particulate matter (aerosols), both liquid, inclu-sive of water, and solid phase matter, primarily mineral, andhow the surfaces of these aerosols influence chemistry thatoccurs in the atmosphere. Surface effects of atmosphericaerosols have been the focus of scientific inquiry since the1990s, with emphasis on tropospheric aerosols. Because probing the asymmetrictwo-dimensional hydro-gen bond network of water found at interfaces necessitatesa surface-specific technique, VSFG is widely utilized withinour laboratory because it is an even-order nonlinear opticalspectroscopy that provides information on the vibrationalcharacter of molecules at surfaces. Conventional VSFG in-tensity is proportional to the square modulus of the second-order nonlinear susceptibility,lx2)1, multiplied by the inten-sities of the input visible and infrared beams, eq 1. Here IsFG lvis, and li are the intensities of the output sumfrequency beam, the visible excitation beam, and theinfrared excitation beam, respectively, and x andxrefer to the nonresonant and resonant components ofthe second-order nonlinear susceptibility. Equations 2aand 2b reveal the Lorentzian line-shape character of thesecond-order nonlinear susceptibility when the VSFGresonances are discrete where A, is the SFG transitionmoment strength,w, is the frequency of the SFG activevibration,wir is the frequency of the incident infraredlaser beam,andT,, is the line-width of the VSFG transition.Equation 2b is the imaginary term from the expansion ofeq 2a. For continuum modes, such as the collective OHvibrations of interfacial water, eq3 more accuratelyrepresents xwhere p(ω,) is the density of VSFG modesatω.31 While eqs 2a and 2b inherently contain information onthe sign of the VSFG transition moment and thus theorientation of the induced dipolemoment, we can see fromeq 1 that this information is lost when collecting the con-ventional VSFG intensity spectrum. It becomes impossiblethen to directly measure the orientation (phase) of the VSFGtransition moment utilizing conventional VSFG techniques.This issue has been recognized for many years as a shortcoming of the conventional VSFG technique and, previous todirect measurement of SFG phase, researchers have at-tempted to address this both experimentally and throughtheoretical simulations.32,33 The first direct measurement ofthe phase of x) was completed using SHG in 1986 byKemnitz et al. 8 It was not until 1990 that the phase of x(2)was measured with VSFG by Superfine et al. for a pentade-canoic acid monolayer on water;4 however, this methodsaw limited adoption due to the added experimental com-plexity. In the following years, several groups reported theImx(eq2b) spectra for water at a variety of interfaces;32,35however, a widely applicable experimental approach todirectly measure Im x was lacking until recently. Thistechnique, pioneered by Ji et al. and referred to from hereas heterodyne detected phase-sensitive sum frequency gen-eration (PS-SFG), allows the measurement of the Imx spectra for molecules at the interface of two centrosym-metric media.36 This marks a huge step forward for VSFGinstrumentation, and the ideas of Ji et al. are being quicklyadopted by both experimentalists and theoreticians withinthe surf e spectroscopy community.9,10,25,27-29,37-40pc PS-SFG is an excellent tool to investigate the effect thations and solutes exert upon the hydrogen-bond network ofwater because PS-SFG can show whether the transitiondipole moment of water reorients due to the presence ofan ion or solute, even if the VSFG power spectrum remainsunchanged. As such, the majority of the work presented inthis Account utilizes the PS-SFG technique where an existingconventional femtosecond broad-band VSFG spectrometerin our laboratory was adapted for phase detection using thedesign demonstrated first by Nihonyanagi et al.In thisdesign,collecting both conventional VSFG (power spectrum)and PS-SFG is possible. The broad-bandwidth VSFG spectro-meter used for this work has been described in greater detailelsewhere along with a more recent description of the PS-SFG setup utilized for this work.25,27 Briefly, a titanium:sapphire oscillator (792 nm)/double regenerative amplifier(1 kHz) (Spectra-Physics) generates both the picosecondvisible and femtosecond infrared light. The full spectralbandwidth for the infrared beam was 800 and 500 cm-in the water hydrogen-bonding region for the conventionalVSFG and PS-SFG setups, respectively. Average energies forthe visible and infrared beams were ~300 and ~10 uJ,respectively. For the liquid/solid work, a scanning VSFGspectrometer (20 Hz, Ekspla) was used. Details of the 20 Hzscanning VSFG system can be found elsewhere.21 Briefly, aNd:YAG laser is used to produce the visible beam (532.1 nm,~500 J) and to pump an OPA/OPG/DFG system, which. produces the tunable infrared beam (2.5-12 um,>50 uJ). Results and Discussion Vapor/Neat Water Interface. In order to understand therole that solvated ions and solutes play in perturbing waterstructure at the vapor/liquid interface, it is vital to have anunderstanding of neat water structure both in the bulk and atthe vapor/water surface.Bulk vibrational studies usingRaman and infrared spectroscopies are useful in helping toassign the complicated hydrogen-bonding continuum regionlocated from 3000 to 3650 cm-. This region is generallyattributed to the collective OH stretching modes of hydrogen-bonded water molecules, from a more strongly to a moreweakly hydrogen-bonding network. The most apparent dif-ference between VSFG spectra for the vapor/liquid interfaceand Raman and infrared spectra for bulk water occurs at 3700 cm, where a sharp, narrow peak is observed for thevapor/water interface VSFG spectrum. This peak is assigned tothe free OH stretch of water molecules that straddle the vapor/liquid interface. These water molecules have one OH bondpointing toward the vapor phase and the other OH bondpointing toward the liquid where it is free to interact with otherwater molecules via hydrogen-bonding. “ A precise assignment for the origin of the hydrogen-bonding continuum from 3000 to 3650 cm- has provento be elusive and as such is a large source of controversywithin the community.9,10,14,17,32,39,41 Explanations for thisregion range from the presence of several hydrogen-boundwater species, to the prevailing effects of large dynamicfluctuations in water, to intramolecular coupling betweenthe overtone of the OH bending mode and the OH stretchfundamental.7,39,41 The recent development of PS-SFG, aswell as thorough theoretical studies, has provided muchadditional information toward resolving this issue;9,10,36however the controversy persists. Ion Effects at Vapor/Water Interface. The relationshipand effect that solvated ions have upon the structure of water'shydrogen-bonding network is of special interest to the atmo-spheric chemistry community because aqueous aerosols areinvolved in a wide range of atmospherically important pro-cesses such as the heterogeneous hydrolysis of atmosphericchemical species.11,12 The use of surface specific vibrationalspectroscopic techniques such as VSFG has been activelypursued by a number of groups to help elucidate long-stand-ing questions on the surface structure of water containing aiWviIde variety of ions.615-17,19,20,24,29,31,40,42-44Provided hereis a brief synopsis of the major findings from work on vapor/ion-water interfaces using VSFG completed by our laboratoryin the past as well as the major unresolved questions from thiswork that we seek to address using the PS-SFGtechnique. Veryrecent results for the aqueous sodium carbonate and sodiumbicarbonate systems utilizing PS-SFG are also presented andsummarized.28 Previous work has primarily focused on the effect thathalide ions (CI, Br, and I) with mono- and divalentcountercations (Nat and Mg,Ca+, and Sr+) have uponthe water structure near the vapor/liquid interface and howthese results compare with results for acidic solutions (HCl,HBr, and HI).19,20,22,24 From surface tension measurements,Figure 1, it is known that acidic solutions exhibit verydifferent behavior than solutions of the salt analogue, andaddressing this behavior was a central goal in past workIntroduction of the halide salts was seen to manifest as aslight decrease in the strong hydrogen-bonding region at 3200 cm-anda significant increase in the weak hydrogen-bonding region at 3400 cm-, for example, Figure 2b. Thesespectral changes increased with increasing polarizability ofthe anion, Cl- Ca2+> Mg) and no en-hancement for NaNO3 of the 3400 cm-region, Figure 2d,e,while for the sodium and other monovalent sulfate systems(divalent cation systems have not yet been completed), alarge enhancement is found for both the 3200 and 3400cm- regions, Figure 2f. These results are interpreted asfollows: For nitrate systems, the significant depletion of the3200 cm-region and enhancement of the 3400 cm-region is attributed to the existence, but not an enrichment,of the nitrate ion at the interface, with comparable surfaceactivity to that of the chloride ion, which is supported byrecent PS-SFG measurements by Tian et al.21,24,40,46 For thedivalent cation series, the spectral changes are exaggeratedas the size of the cation increases, Mg²+< Ca²+
确定





还剩9页未读,是否继续阅读?
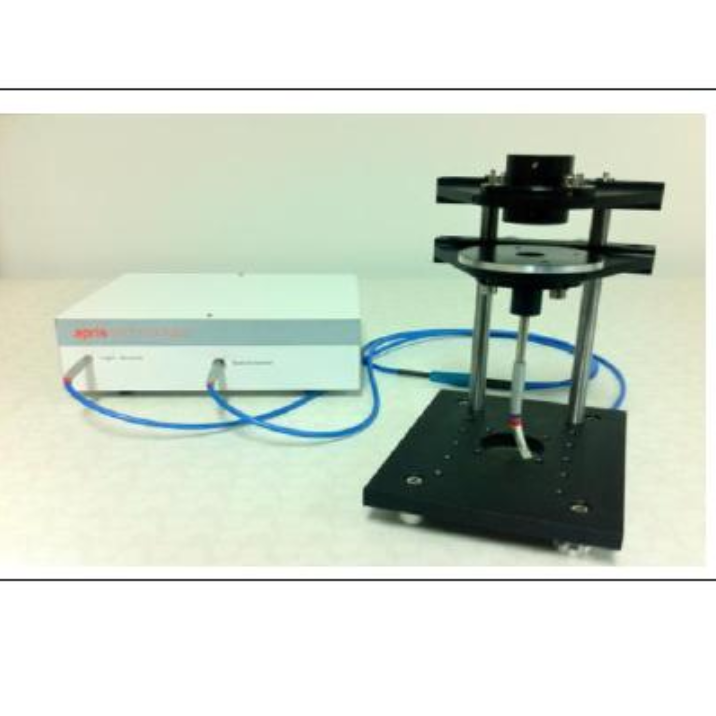
北京欧兰科技发展有限公司为您提供《空气/水界面中和频光谱检测方案(激光拉曼光谱)》,该方案主要用于其他中和频光谱检测,参考标准--,《空气/水界面中和频光谱检测方案(激光拉曼光谱)》用到的仪器有Ekspla CARS 相干反斯托克斯拉曼显微光谱仪、Ekspla SFG 表面和频光谱分析系统、Ekspla PL2230型高能量皮秒激光器
相关方案
更多
该厂商其他方案
更多