方案详情
文
采用立陶宛Ekspla公司NT342B型纳秒(4ns)脉冲Nd:YAG可调谐激光器 输出的460nm激光对多种卤素吡啶样品进行了三阶非线性光学特性测量。研究了四种含芘查尔酮衍生物的合成。
方案详情

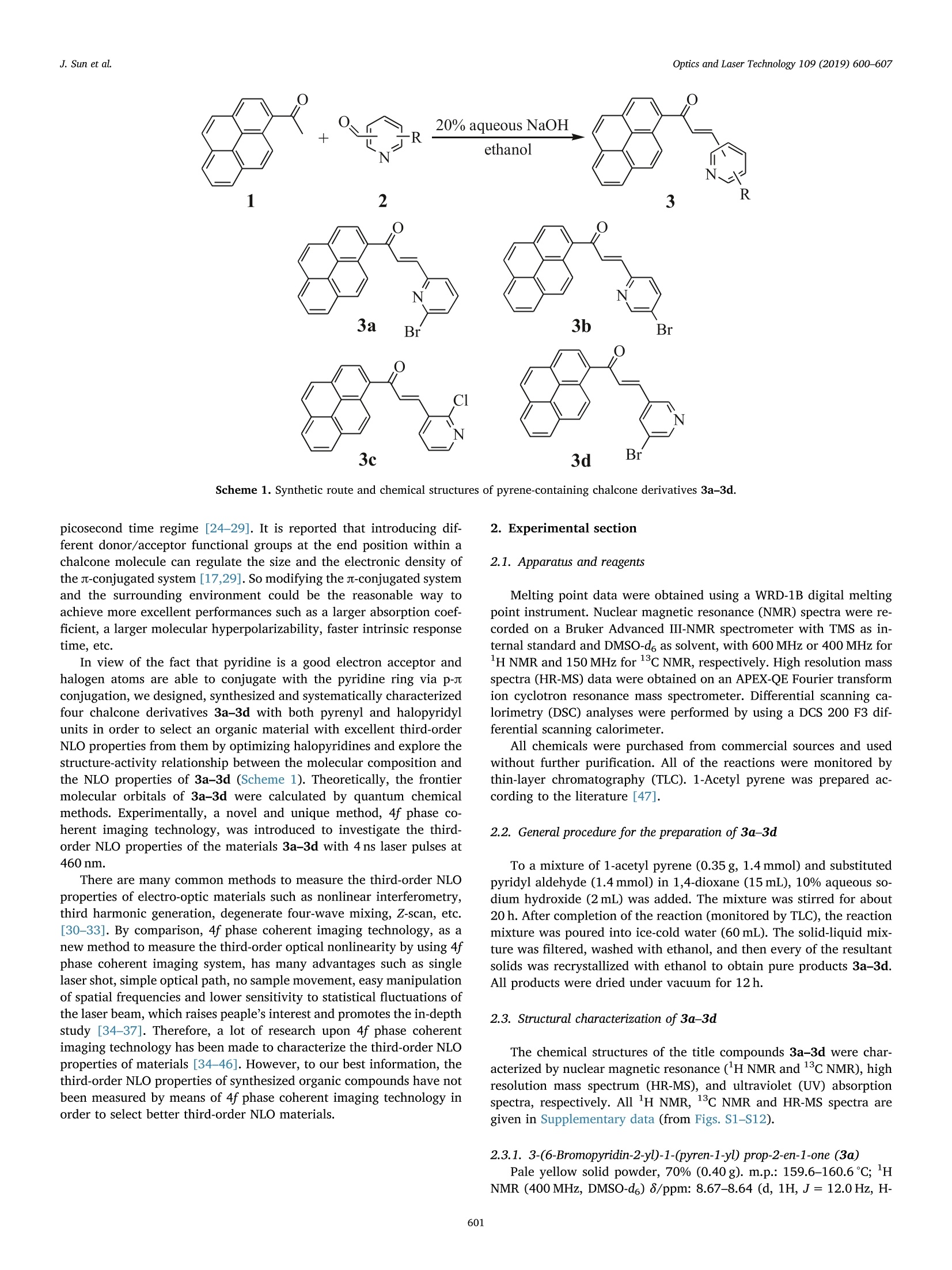
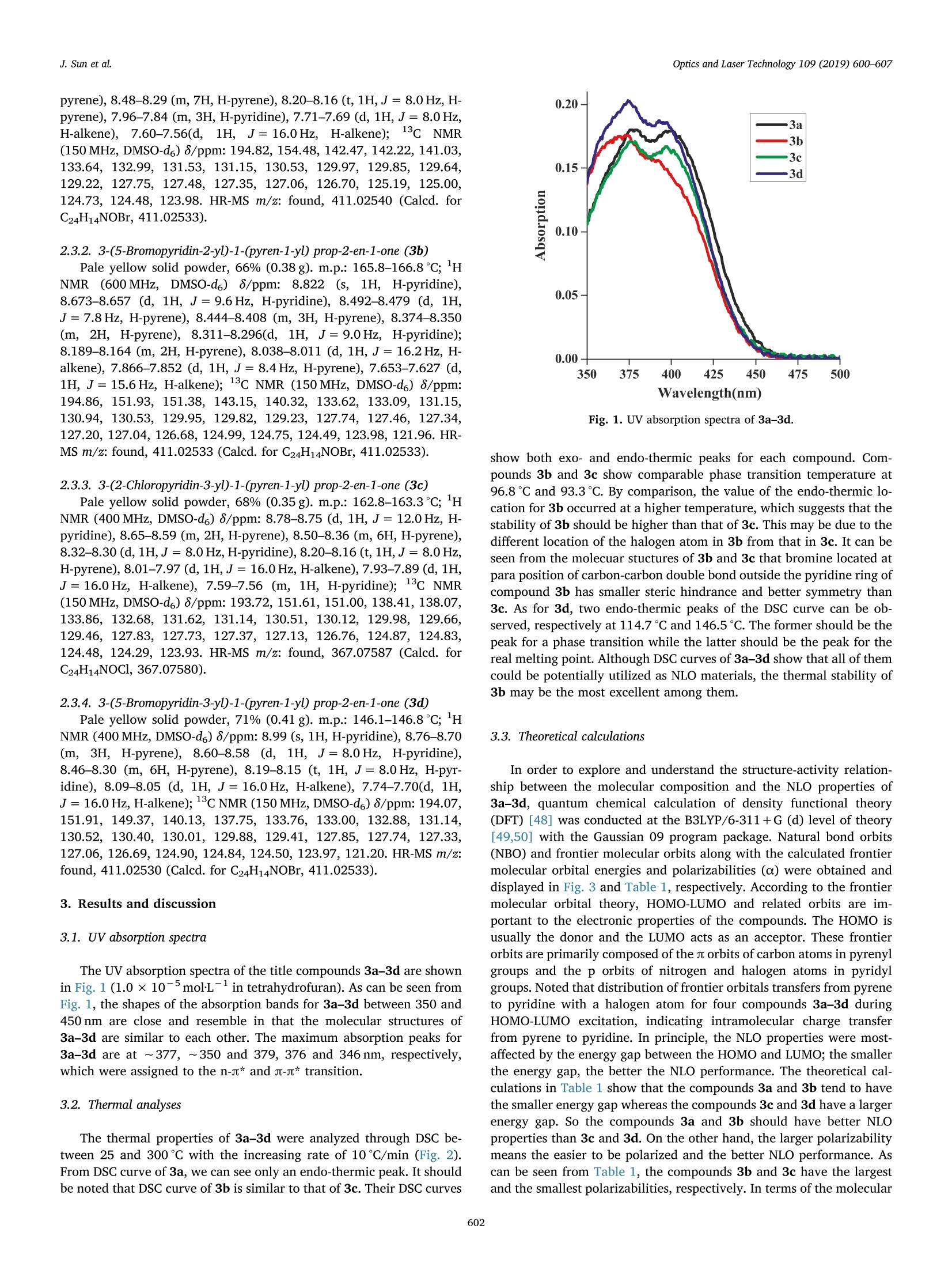
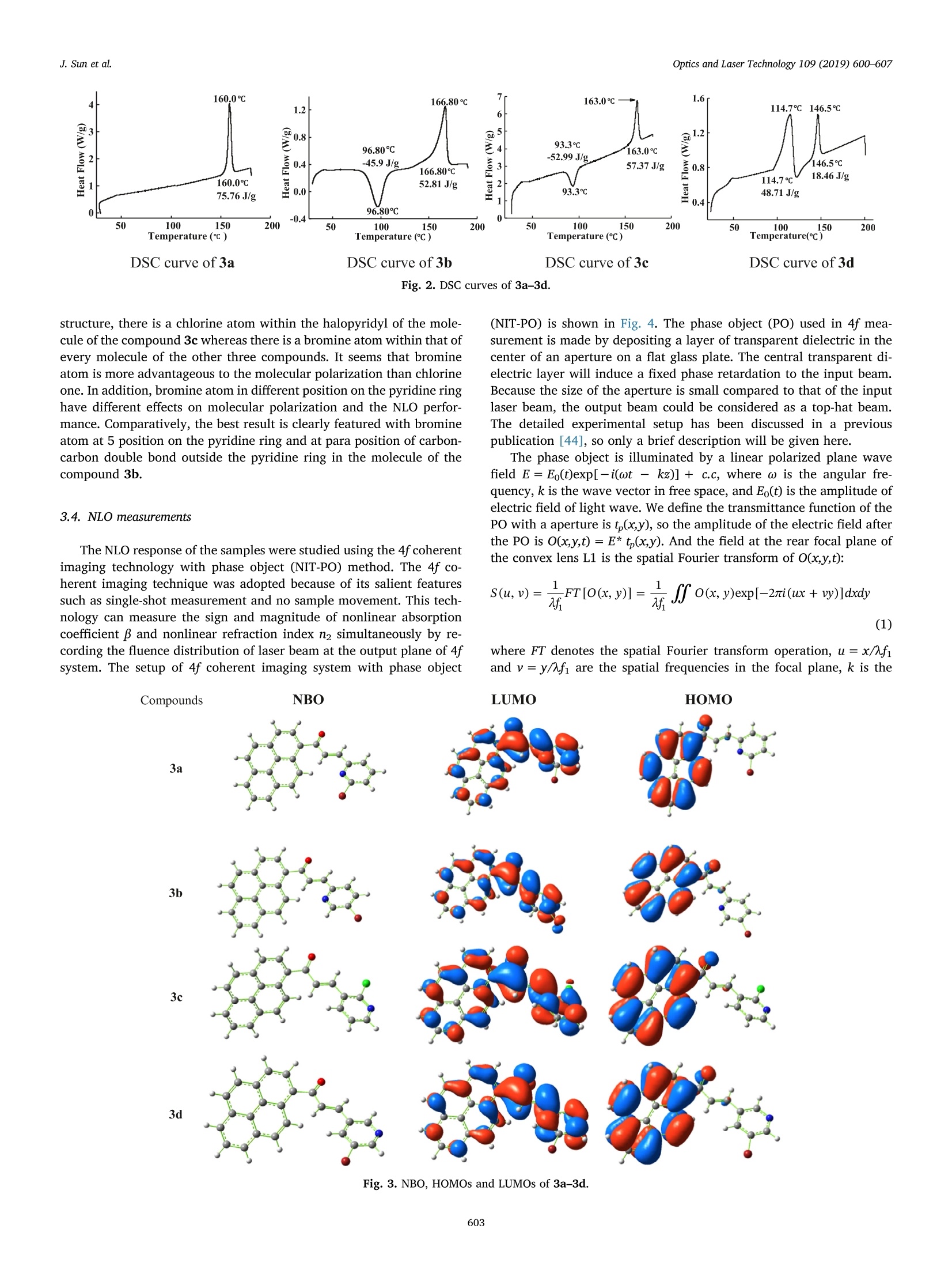
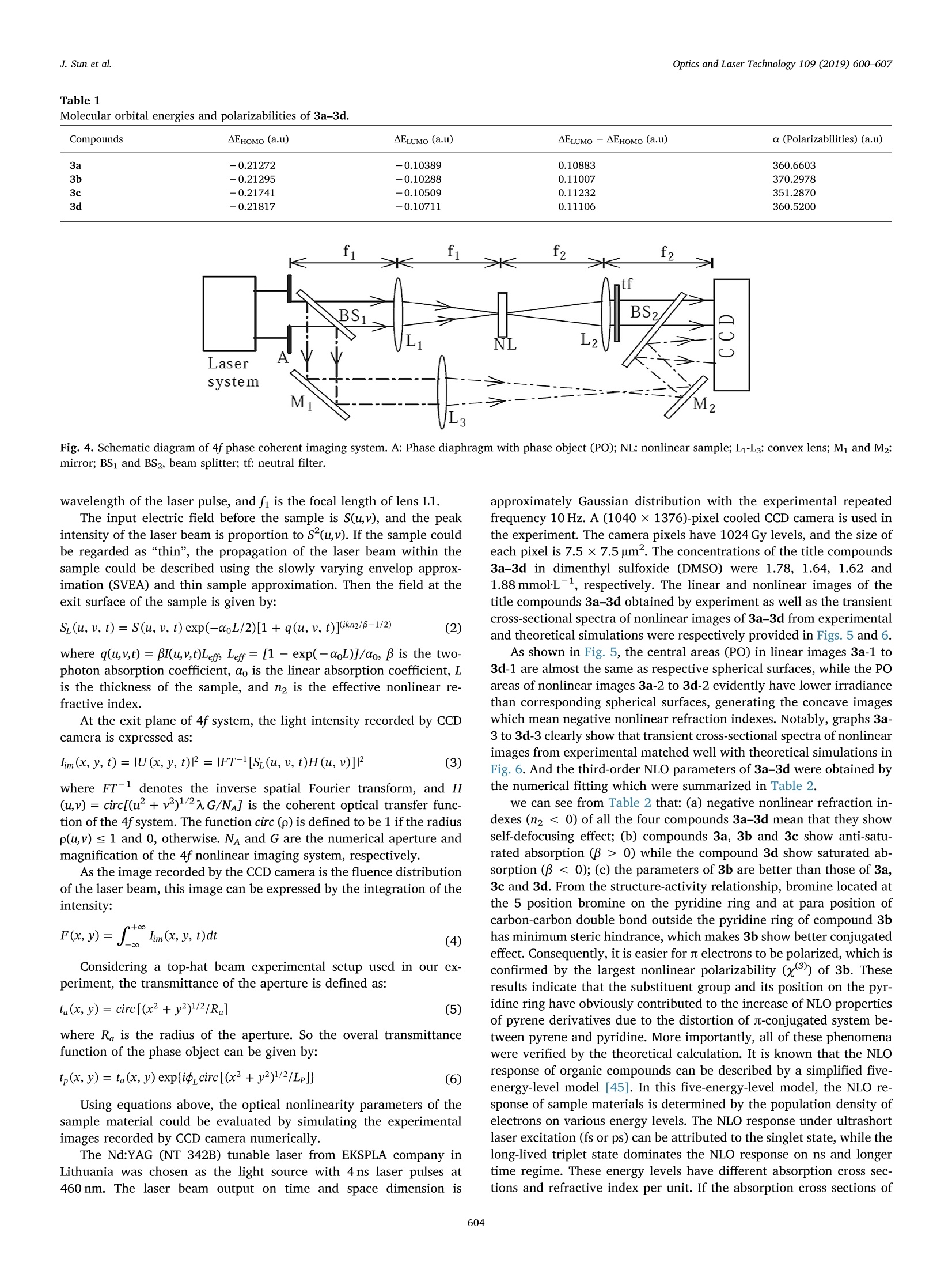
Optics and Laser Technology 109 (2019) 600-607 J. Sun et al.Optics and Laser Technology 109 (2019) 600-607 Contents lists available at ScienceDirect Optics and Laser Technology journal homepage: www.elsevier.com/locate/optlastec Full length article Synthesis of four pyrene-containing chalcone derivatives: Achievingexcellent third-order nonlinear optical properties by optimizinghalopyridines Jinyu Sun, Guilin Wang, Chengqi Liu, Yufang Shi*, Minggen Zhao Department of Chemistry, Xinzhou Teachers University, Xinzhou 034000, China HIGHLIGHTS · Four chalcone derivatives based on pyrenyl and halopyridyl were synthesized. · A novel and unique method, 4fphase coherent imaging technology, was used to test the optical nonlinearity. ·Four compounds can respond quickly to 4 ns laser pulses. The structure-activity relationship was discussed in detail. A RTICLEINFO ABSTRACT 1. Introduction Nonlinear optical (NLO) materials are of high scientific significancedue to their potential applications in optical switching, optical com-munications, optical limiting, data storage, dynamic holography, har-monic generator, etc. [1-8]. NLO properties of organic compounds, as awide category of materials, have been studied for a few decades [9-11].Compared with inorganic materials, organic NLO materials have manyadvantages such as large NLO absorption coefficients, large molecularhyperpolarizabilities, fast intrinsic response time, ease of processing,lower cost and so on [12-14]. On the other hand, organic compoundsused as NLO materials can more easily be modified by tuning the in-trinsic donors and/or acceptors in their parent molecules to obtaindesired NLO properties [15-17]. Because it may not be possible in most of the inorganic compounds for donor and acceptor functional groups tocombinate within the same molecules. Since the early 1990s, a largenumber of studies on the NLO properties of such organic NLO materialsas chalcone derivatives have been carried out [18-22], but there is onlyfew work upon pyrene-containing chalcone derivatives reported[23-29]. Pyrene is considered as one of the most promising nonlinear func-tional units with both a larger u-conjugated system and a larger elec-tron density, which makes pyrene-containing chalcone derivatives beable to exhibit excellent NLO properties [23]. It is known that the NLOresponse of materials depends on various parameters, such as pulseduration, input wavelength, light polarization, etc. Hence, a consider-able research efforts have been devoted to investigating the NLO re-sponse of pyrene and its derivatives, especially on femtosecond or ( h t t ps :// doi . org / 1 0.1016/ j .opt l a stec.20 1 8 . 08 . 0 3 8 ) ( Received 19 October 2 0 17; Received in revised f o rm 24 June 2018; Accepted 14 August 2018Available online 03 September 2018 ) ( 0030-3992/ C 2 018 Elsevier Ltd. All rights re s erved. ) R picosecond time regime [24-29]. It is reported that introducing dif-ferent donor/acceptor functional groups at the end position within achalcone molecule can regulate the size and the electronic density ofthe jt-conjugated system [17,29]. So modifying the u-conjugated systemand the surrounding environment could be the reasonable way toachieve more excellent performances such as a larger absorption coef-ficient, a larger molecular hyperpolarizability, faster intrinsic responsetime, etc. In view of the fact that pyridine is a good electron acceptor andhalogen atoms are able to conjugate with the pyridine ring via p-stconjugation, we designed, synthesized and systematically characterizedfour chalcone derivatives 3a-3d with both pyrenyl and halopyridylunits in order to select an organic material with excellent third-orderNLO properties from them by optimizing halopyridines and explore thestructure-activity relationship between the molecular composition andthe NLO properties of 3a-3d (Scheme 1). Theoretically, the frontiermolecular orbitals of 3a-3d were calculated by quantum chemicalmethods. Experimentally, a novel and unique method,4f phase co-herent imaging technology, was introduced to investigate the third-order NLO properties of the materials 3a-3d with 4 ns laser pulses at460nm. There are many common methods to measure the third-order NLOproperties of electro-optic materials such as nonlinear interferometry,third harmonic generation, degenerate four-wave mixing, Z-scan, etc.[30-33]. By comparison, 4f phase coherent imaging technology, as anew method to measure the third-order optical nonlinearity by using 4fphase coherent imaging system, has many advantages such as singlelaser shot, simple optical path, no sample movement, easy manipulationof spatial frequencies and lower sensitivity to statistical fluctuations ofthe laser beam, which raises peaple’s interest and promotes the in-depthstudy [34-37]. Therefore, a lot of research upon 4f phase coherentimaging technology has been made to characterize the third-order NLOproperties of materials [34-46]. However, to our best information, thethird-order NLO properties of synthesized organic compounds have notbeen measured by means of 4f phase coherent imaging technology inorder to select better third-order NLO materials. 2. Experimental section 2.1. Apparatus and reagents Melting point data were obtained using a WRD-1B digital meltingpoint instrument. Nuclear magnetic resonance (NMR) spectra were re-corded on a Bruker Advanced III-NMR spectrometer with TMS as in-ternal standard and DMSO-d6 as solvent, with 600 MHz or 400 MHz for1H NMR and 150 MHz for 13c NMR, respectively. High resolution massspectra (HR-MS) data were obtained on an APEX-QE Fourier transformion cyclotron resonance mass spectrometer. Differential scanning ca-lorimetry (DSC) analyses were performed by using a DCS 200 F3 dif-ferential scanning calorimeter. All chemicals were purchased from commercial sources and usedwithout further purification. All of the reactions were monitored bythin-layer chromatography (TLC). 1-Acetyl pyrene was prepared ac-cording to the literature [47]. 2.2. General procedure for the preparation of 3a-3d To a mixture of 1-acetyl pyrene (0.35 g, 1.4 mmol) and substitutedpyridyl aldehyde (1.4 mmol) in 1,4-dioxane (15 mL), 10% aqueous so-dium hydroxide (2mL) was added. The mixture was stirred for about20 h. After completion of the reaction (monitored by TLC), the reactionmixture was poured into ice-cold water (60mL). The solid-liquid mix-ture was filtered, washed with ethanol, and then every of the resultantsolids was recrystallized with ethanol to obtain pure products 3a-3d.All products were dried under vacuum for 12 h. 2.3. Structural characterization of 3a-3d The chemical structures of the title compounds 3a-3d were char-acterized by nuclear magnetic resonance (H NMR and 13cNMR), highresolution mass spectrum (HR-MS), and ultraviolet (UV) absorptionspectra, respectively. All lH NMR, 13c NMR and HR-MS spectra aregiven in Supplementary data (from Figs. S1-S12). 2.3.1. 3-(6-Bromopyridin-2-yD-1-(pyren-1-yl prop-2-en-1-one (3a)Pale yellow solid powder, 70% (0.40g). m.p.: 159.6-160.6℃; H NMR (400 MHz, DMSO-d6) 8/ppm: 8.67-8.64 (d, 1H, J= 12.0 Hz, H- pyrene), 8.48-8.29 (m,7H,H-pyrene), 8.20-8.16 (t, 1H,J= 8.0 Hz, H-pyrene), 7.96-7.84 (m, 3H, H-pyridine), 7.71-7.69 (d, 1H,J=8.0Hz,H-alkene),7.60-7.56(d, 1H, J=16.0Hz,H-alkene););13CNMR(150MHz, DMSO-d6) 8/ppm: 194.82, 154.48, 142.47,142.22,141.03,133.64, 132.99,131.53, 131.15,130.53,129.97, 129.85,129.64,129.22,127.75,127.48,127.35,127.06,126.70,125.19,125.00,124.73,124.48,123.98. HR-MS m/z: found, 411.02540 (Calcd. forC24H14NOBr,411.02533). 2.3.2. 3-(5-Bromopyridin-2-yD-1-(pyren-1-yl prop-2-en-1-one (3b) Pale yellow solid powder, 66% (0.38g). m.p.: 165.8-166.8℃; HNMR((600MHz, DMSO-d6)8/ppm: 8.822 (S, 1H, H-pyridine),8.673-8.657 (d, 1H, J=9.6 Hz, H-pyridine), 8.492-8.479 (d, 1H,J= 7.8 Hz, H-pyrene), 8.444-8.408 (m, 3H, H-pyrene), 8.374-8.350(m, 2H, H-pyrene), 8.311-8.296(d, 1H, J= 9.0 Hz, H-pyridine);8.189-8.164 (m, 2H, H-pyrene), 8.038-8.011 (d, 1H, J= 16.2Hz, H-alkene), 7.866-7.852 (d, 1H, J=8.4 Hz, H-pyrene), 7.653-7.627 (d,1H, J=15.6 Hz, H-alkene); 13C NMR (150MHz, DMSO-d6) 8/ppm:194.86,151.93,151.38,143.15, 140.32,133.62,133.09, 131.15,130.94,130.53,129.95,129.82,129.23,127.74,127.46,127.34,127.20,127.04,126.68,124.99,124.75,124.49,123.98,121.96. HR-MS m/z: found, 411.02533 (Calcd. for C24H14NOBr, 411.02533). 2.3.3. 3-(2-Chloropyridin-3-yD-1-(pyren-1-yD prop-2-en-1-one (3c) Pale yellow solid powder, 68% (0.35g). m.p.: 162.8-163.3℃; HNMR (400 MHz, DMSO-d6) 8/ppm: 8.78-8.75 (d, 1H,J=12.0 Hz, H-pyridine), 8.65-8.59 (m, 2H, H-pyrene), 8.50-8.36 (m, 6H, H-pyrene),8.32-8.30(d, 1H,J= 8.0 Hz,H-pyridine), 8.20-8.16 (t, 1H,J=8.0 Hz,H-pyrene), 8.01-7.97(d, 1H, J= 16.0 Hz, H-alkene), 7.93-7.89 (d,1H,J=16.0Hz, H-alkene), 7.59-7.56 (m, 1H, H-pyridine); 13c NMR(150 MHz, DMSO-d6) 8/ppm: 193.72,151.61,151.00,138.41,138.07,133.86,132.68,131.62,131.14,130.51,130.12,129.98,129.66,129.46,127.83,127.73,127.37,127.13,126.76,124.87,124.83,124.48,124.29, 123.93. HR-MS m/z: found,367.07587 (Calcd. forC24H14NOCl, 367.07580). 2.3.4. 3-(5-Bromopyridin-3-yD-1-(pyren-1-yl prop-2-en-1-one (3d) Pale yellow solid powder, 71% (0.41 g). m.p.: 146.1-146.8℃; HNMR (400 MHz, DMSO-d6)8/ppm: 8.99 (s, 1H, H-pyridine), 8.76-8.70(m, 3H, H-pyrene), 8.60-8.58 (d, 1H, J=8.0 Hz, H-pyridine),8.46-8.30 (m, 6H, H-pyrene), 8.19-8.15 (t, 1H, J=8.0Hz, H-pyr-idine), 8.09-8.05 (d, 1H, J=16.0Hz, H-alkene), 7.74-7.70(d, 1H,J=16.0Hz, H-alkene); 13C NMR (150MHz, DMSO-d6) 8/ppm: 194.07,151.91,149.37,140.13, 137.75,133.76,133.00,132.88,131.14,130.52,130.40,130.01,129.88,129.41,127.85,127.74, 127.33,127.06,126.69,124.90,124.84,124.50,123.97,121.20.HR-MS m/z:found, 411.02530 (Calcd. for C24H14NOBr,411.02533). 3. Results and discussion 3.1. UV absorption spectra The UV absorption spectra of the title compounds 3a-3d are shownin Fig. 1 (1.0×10-5molL-1 in tetrahydrofuran). As can be seen fromFig. 1, the shapes of the absorption bands for 3a-3d between 350 and450nm are close and resemble in that the molecular structures of3a-3d are similar to each other. The maximum absorption peaks for3a-3d are at ~377, ~350 and 379, 376 and 346 nm, respectively,which were assigned to the n-n* and J-* transition. 3.2. Thermal analyses The thermal properties of 3a-3d were analyzed through DSC be-tween 25 and 300℃ with the increasing rate of 10℃/min (Fig. 2).From DSC curve of 3a, we can see only an endo-thermic peak. It shouldbe noted that DSC curve of 3b is similar to that of 3c. Their DSC curves Fig. 1. UV absorption spectra of 3a-3d. show both exo- and endo-thermic peaks for each compound. Com-pounds 3b and 3c show comparable phase transition temperature at96.8℃ and 93.3℃. By comparison, the value of the endo-thermic lo-cation for 3b occurred at a higher temperature, which suggests that thestability of 3b should be higher than that of 3c. This may be due to thedifferent location of the halogen atom in 3b from that in 3c. It can beseen from the molecuar stuctures of 3b and 3c that bromine located atpara position of carbon-carbon double bond outside the pyridine ring ofcompound 3b has smaller steric hindrance and better symmetry than3c. As for 3d, two endo-thermic peaks of the DSC curve can be ob-served, respectively at 114.7℃ and 146.5℃. The former should be thepeak for a phase transition while the latter should be the peak for thereal melting point. Although DSC curves of 3a-3d show that all of themcould be potentially utilized as NLO materials, the thermal stability of3b may be the most excellent among them. 3.3. Theoretical calculations In order to explore and understand the structure-activity relation-ship between the molecular composition and the NLO properties of3a-3d, quantum chemical calculation of density functional theory(DFT) [48] was conducted at the B3LYP/6-311+G (d) level of theory[49,50] with the Gaussian 09 program package. Natural bond orbits(NBO) and frontier molecular orbits along with the calculated frontiermolecular orbital energies and polarizabilities (a) were obtained anddisplayed in Fig.3 and Table 1, respectively. According to the frontiermolecular orbital theory, HOMO-LUMO and related orbits are im-portant to the electronic properties of the compounds. The HOMO isusually the donor and the LUMO acts as an acceptor. These frontierorbits are primarily composed of the s orbits of carbon atoms in pyrenylgroups and the p orbits of nitrogen and halogen atoms in pyridylgroups. Noted that distribution of frontier orbitals transfers from pyreneto pyridine with a halogen atom for four compounds 3a-3d duringHOMO-LUMO excitation, indicating intramolecular charge transferfrom pyrene to pyridine. In principle, the NLO properties were most-affected by the energy gap between the HOMO and LUMO; the smallerthe energy gap, the better the NLO performance. The theoretical cal-culations in Table 1 show that the compounds 3a and 3b tend to havethe smaller energy gap whereas the compounds 3c and 3d have a largerenergy gap. So the compounds 3a and 3b should have better NLOproperties than 3c and 3d. On the other hand, the larger polarizabilitymeans the easier to be polarized and the better NLO performance. Ascan be seen from Table 1, the compounds 3b and 3c have the largestand the smallest polarizabilities, respectively. In terms of the molecular Fig. 2. DSC curves of 3a-3d. structure, there is a chlorine atom within the halopyridyl of the mole-cule of the compound 3c whereas there is a bromine atom within that ofevery molecule of the other three compounds. It seems that bromineatom is more advantageous to the molecular polarization than chlorineone. In addition, bromine atom in different position on the pyridine ringhave different effects on molecular polarization and the NLO perfor-mance. Comparatively, the best result is clearly featured with bromineatom at 5 position on the pyridine ring and at para position of carbon-carbon double bond outside the pyridine ring in the molecule of thecompound 3b. 3.4. NLO measurements The NLO response of the samples were studied using the 4f coherentimaging technology with phase object (NIT-PO) method. The 4f co-herent imaging technique was adopted because of its salient featuressuch as single-shot measurement and no sample movement. This tech-nology can measure the sign and magnitude of nonlinear absorptioncoefficient B and nonlinear refraction index n2 simultaneously by re-cording the fluence distribution of laser beam at the output plane of 4fsystem. The setup of 4f coherent imaging system with phase object (NIT-PO) is shown in Fig. 4. The phase object (PO) used in 4f mea-surement is made by depositing a layer of transparent dielectric in thecenter of an aperture on a flat glass plate. The central transparent di-electric layer will induce a fixed phase retardation to the input beam.Because the size of the aperture is small compared to that of the inputlaser beam, the output beam could be considered as a top-hat beam.The detailed experimental setup has been discussed in a previouspublication [44], so only a brief description will be given here. The phase object is illuminated by a linear polarized plane wavefield E=Eo(t)exp[-i(ωt - kz)]+c.c, where ω is the angular fre-quency, k is the wave vector in free space, and Eo(t) is the amplitude ofelectric field of light wave. We define the transmittance function of thePO with a aperture is t,(x,y), so the amplitude of the electric field afterthe PO is O(x,y,t)= E*t,(x,y). And the field at the rear focal plane ofthe convex lens L1 is the spatial Fourier transform of O(x,y,t): where FT denotes the spatial Fourier transform operation, u = x/Afiand v=y/Afi are the spatial frequencies in the focal plane, k is the Fig. 3. NBO, HOMOs and LUMOs of 3a-3d. Compounds AEHoMo (a.u) AELuMo (a.u) AELUMO -AEHOMO(a.u) a (Polarizabilities) (a.u) 3a -0.21272 -0.10389 0.10883 360.6603 3b -0.21295 -0.10288 0.11007 370.2978 3c -0.21741 -0.10509 0.11232 351.2870 3d -0.21817 -0.10711 0.11106 360.5200 Fig. 4. Schematic diagram of 4f phase coherent imaging system. A: Phase diaphragm with phase object (PO); NL: nonlinear sample; Li-L: convex lens; Mi and M2:mirror; BSi and BS2, beam splitter; tf: neutral filter. wavelength of the laser pulse, and fi is the focal length of lens L1. The input electric field before the sample is S(uv), and the peakintensity of the laser beam is proportion to S(uv). If the sample couldbe regarded as “thin”,the propagation of the laser beam within thesample could be described using the slowly varying envelop approx-imation (SVEA) and thin sample approximation. Then the field at theexit surface of the sample is given by: where q(uv,t)=BI(u,v,t)Leff, Leff=[1 - exp(-aoL)]/ao,B is the two-photon absorption coefficient, ao is the linear absorption coefficient, Lis the thickness of the sample, and n2 is the effective nonlinear re-fractive index. where FT-1 denotes the inverse spatial Fourier transform, and H(u,v)= circ[(u?+v2)1/2a. G/N] is the coherent optical transfer func-tion of the 4f system. The function circ (p) is defined to be 1 if the radiusp(u,v) ≤ 1 and 0, otherwise. NA and G are the numerical aperture andmagnification of the 4f nonlinear imaging system,respectively. As the image recorded by the CCD camera is the fluence distributionof the laser beam, this image can be expressed by the integration of theintensity: Considering a top-hat beam experimental setup used in our ex-periment, the transmittance of the aperture is defined as: where Ra is the radius of the aperture. So the overal transmittancefunction of the phase object can be given by: Using equations above, the optical nonlinearity parameters of thesample material could be evaluated by simulating the experimentalimages recorded by CCD camera numerically. The Nd:YAG (NT 342B) tunable laser from EKSPLA company inLithuania was chosen as the light source with 4 ns laser pulses at460 nm. The laser beam output on time and space dimension is approximately Gaussian distribution with the experimental repeatedfrequency 10 Hz. A (1040×1376)-pixel cooled CCD camera is used inthe experiment. The camera pixels have 1024 Gy levels, and the size ofeach pixel is 7.5 ×7.5 um2. The concentrations of the title compounds3a-3d in dimenthyl sulfoxide (DMSO) were 1.78, 1.64,1.62 and1.88 mmolL-, respectively. The linear and nonlinear images of thetitle compounds 3a-3d obtained by experiment as well as the transientcross-sectional spectra of nonlinear images of 3a-3d from experimentaland theoretical simulations were respectively provided in Figs. 5 and 6. As shown in Fig. 5, the central areas (PO) in linear images 3a-1 to3d-1 are almost the same as respective spherical surfaces, while the POareas of nonlinear images 3a-2 to 3d-2 evidently have lower irradiancethan corresponding spherical surfaces, generating the concave imageswhich mean negative nonlinear refraction indexes. Notably, graphs 3a-3 to 3d-3 clearly show that transient cross-sectional spectra of nonlinearimages from experimental matched well with theoretical simulations inFig. 6. And the third-order NLO parameters of 3a-3d were obtained bythe numerical fitting which were summarized in Table 2. we can see from Table 2 that: (a) negative nonlinear refraction in-dexes (n2 < 0) of all the four compounds 3a-3d mean that they showself-defocusing effect; (b) compounds 3a, 3b and 3c show anti-satu-rated absorption (B> 0) while the compound 3d show saturated ab-sorption (β<0); (c) the parameters of 3b are better than those of 3a,3c and 3d. From the structure-activity relationship, bromine located atthe 5 position bromine on the pyridine ring and at para position ofcarbon-carbon double bond outside the pyridine ring of compound 3bhas minimum steric hindrance, which makes 3b show better conjugatedeffect. Consequently, it is easier for s electrons to be polarized, which isconfirmed by the largest nonlinear polarizability (x) of 3b. Theseresults indicate that the substituent group and its position on the pyr-idine ring have obviously contributed to the increase of NLO propertiesof pyrene derivatives due to the distortion of ut-conjugated system be-tween pyrene and pyridine. More importantly, all of these phenomenawere verified by the theoretical calculation. It is known that the NLOresponse of organic compounds can be described by a simplified five-energy-level model [45]. In this five-energy-level model, the NLO re-sponse of sample materials is determined by the population density ofelectrons on various energy levels. The NLO response under ultrashortlaser excitation (fs or ps) can be attributed to the singlet state, while thelong-lived triplet state dominates the NLO response on ns and longertime regime. These energy levels have different absorption cross sec-tions and refractive index per unit. If the absorption cross sections of Fig.5. Experimental images of 3a-3d. (1): The linear images 3a-1 to 3d-1 obtained by experiment. (2): The nonlinear images 3a-2 to 3d-2 obtained by experiment,central area means PO area. Fig. 6. The profile 3a-3 to 3d-3 of transient cross-sectional spectra of nonlinear images of (2) in Fig. 5 and the simulated results. Solid lines represent the profiles ofimages, while dots stand for the theoretical fitting results. Summary of the nonlinear parameters of 3b compared to some literature data. Compounds Absorption coefficient β (10-1m/W) Refraction index n2 (10-17m²/W) Polarizability x3)(10-11 esu) Experimental condition References 3b 20.5 -15.7 9.61 4 ns, 460 nm This work 1 7.3 -3.6 2.56 4 ns, 500 nm 23 PMAK 7.0 -3.5 2.54 4 ns, 532 nm 51 1 5.5 -2.1 1.58 4 ns, 440 nm 52 ( Supplementary data associated with thi s article can be found, in th e online version, a t h ttps: //d oi.org /1 0 .1016/j. o pt l astec.2018.0 8 .038. ) ( References ) ( [ 1 ] N . V enka t ra m , M . A . A ku ndi, R . D . N a r a y a na, No n lin e a r abs o rp ti on , sc a t tering an d o ptical l i m iting studi es o f CdS nanoparti c les , O p t . Expres s 1 3 ( 2 005) 867 - 8 7 2 . ) ( [2] J . Su n , H . F a n, X . W an g, Q . R en , J. Che n, Q . S un, G. Zh a n g , D. Xu , S t udy o n opt ic al l i m iting p r op e rt y of o rg a no m e t a l lic c ompo un d, C h i n. J . L a se rs 3 6 ( 2009) 2417 -2 421. ) ( [3] A . N. P ra bhu, A . J a y a ra m a , B.K . Su b ra h m anya , V . U p a dh ya ya, G ro w t h , c har a c t er - i za t i on a nd s t ructur a l i n ve sti g a t ion of a nov e l n o nlinear o p ti c a l c r y s ta l , J . M ol . S truc t. 10 3 1 ( 2013 ) 7 9- 8 4. ) ( [ 4] S .K. P a r k, J. Y. D o , J. J . J u, S . P a rk , M .S. K i m , M . H. L e e , No n l i n e ar op t i c al po lyme r a p plic ab l e f o r a ll- o p t i c a l w a ve l e ng t h co nv e rt e r s i n co mmunica tio ns b a nd s n e a r 1.5 u m, M ater. L e t t . 59 ( 20 05 ) 2 872- 2 87 5. ) ( [5] H . Liu , A . R u a, O . V as q u e z , V. S. V i khn i n, F . E. Fe rn a n d e z , L. F. F o n s e ca , O . Re s to, S .Z. W ei s z , O p ti c a l a nd nonli ne ar o p t i c a l r esp o nse of light senso r th i n f i l m s , S en s o r s 5 (2005)185- 1 98. ) ( [ 6] S . Bug a y c huk i, G. K limus h eva, Y . G ar b o vsk i y, T . M irnay a, A . I s c h en k o , No nlinea r o p tica l pr o per t i es o f c om po s i te s b as e d o n c on d uc t i v e m et a l- al k an o a t e liq u i d cr y s - t als, Opto - Elec t ro n . R e v . 14 ( 2006) 2 75- 2 7 9. ) ( [7]] B . C ham p ag n e , A . P l a q u et, J.L . P o z zo, V . R odrigue z , F. C a s tet, No nli ne a r o p t ic a l m olec u l a r s w it c h e s a s s e l e c t i ve cat i o n s e n s o rs, J . A m . C h e m . S o c . 13 4 ( 2 0 12 ) 8101-810 3 . ) ( [ 8] Z . Q in, Y . Wen, Y . S ha n g, Y . S ong, Y . Wa n , S tudy o f a n organic n o nl i near op tical m at e r i a l f o r na n o s ca l e d at a s to ra g e b y s ca n n ing tunneling micro s c o pe , A p p l . P h ys. A 8 7 ( 2 0 0 7) 27 7 - 2 80. ) ( [ 9 ] M . M a t s u ok a , S p ecial i s su e on n o n l i n ea r - o pt ic w a v e g u i de m a t er i als an d d evi ce s. O r gani c ch ro m ophor es f or n onlin e ar o p t i ca l m ater i a l s, Re v. L a ser E ng . 2 1 ( 19 9 3 ) 1 106 - 1115. ) ( [10] R . R a j endr a n, T. H. F r e e d a, U . L . K ala s ek a r, R . N . P er u m a , S y nt h e sis, c r ystal growth a nd ch a r a cter i za t io n o f o r ga nic NL O m a t e rial: M -N i tro a ce t ani l i d e , A dv. M ate r. P hy s . C h em. 1 ( 2 011) 3 9-43. ) ( [11] W . H e n i , C . Ha f f n er, D.L . E l d e r, A .F . T i ll a ck , Y . F e d orys hyn , R . C o ttier, Y . S a l a m in, C. Hoess bach er, U . Koch, B . C heng, B . R obinso n , L . R. D alton, J . L e ut ho l d, N o n l in e ar i ti e s of o r ga n ic e le c tro - o p t i c m ate r ia l s i n n a n osc ale s l o t s a nd implic a ti o n s f o r the o pt im um m odulato r de sig n , O pt . E xp r e ss 25 ( 201 7 ) 2 627- 2 6 53. ) ( [12 ] C . H u, Z . C hen, H . X i a o , Z . Z h en, X . L i u, S . B o, S ynt h esis a nd c h ar a cte r i z a t i o n o f a n ovel i ndo l ine b as ed n o n line ar o p t i ca l c hromopho r e w i t h e x c ellen t e lec t ro - o p t ic a c ti v i t y a nd hig h therma l sta b ili t y b y m o d i f yi n g t h e J -co n juga te d bridges, J . Ma t e r . C hem. C 5 ( 2 01 7 ) 5 111 - 5 1 1 8. ) ( [1 3 ] S .R . M ar d er , Org a nic n on l i ne a r op t i c a l m a t e r i a l s : wh er e w e ha ve b e en a nd wh e r e we a re g o i ng , C hem. Commun. 3 7 (200 6 ) 1 3 1-1 3 4 . ) ( [14 ] R . K a lai v a n an, K . Sr inivas a n , Sy nth e sis, g r owth a nd c h ar a cterizat i on o f o r ga n ic n o n linea r op tic a l mat eri a l : N-b e n z y l- 2 - met h yl - 4 -nit roani line ( BN A), Opt . L a se r T e c h nol. 90 (2017) 2 7- 3 2. ) ( [15]] L . Xu, D. Z ha n g, Y . Z h o u , Y . Z h eng , L . C a o , X. J i a ng, F . L u , 4- N , N - b is ( 4 -m eth ox - y lphen y l ) a n i li n e s u b st i t u t e d an th raqu i no n e: X-r ay c ryst a l s t r u c t u r e s , t heo r e t ic a l c a l c u la t io n s a nd t h ird- o r d er n o n l i ne a r op t i ca l p r o pe r ti e s, Op t . M a t e r . 70 (2 0 1 7) 1 3 1 - 1 3 7. ) ( [16] S . M u h am m a d, A . G. Al s eh e m i , Z . Su , H . X u , A . I rf a n , A . R . Cha u dhr y , Fi r st p r i n c i p l e s s tudy f o r t h e k ey e lec t ron i c, o p t i c a l and no n linear o ptica l pro p erti e s o f no vel do n o r- a ccepto r c hal c ones, J . M ol. G raph . Model l . 7 2 ( 20 17 ) 58- 69 . ) ( [17] M . Torres, S . S e m i n, I. R azd o l s k i , J . L . X u, J . A . A . W . E l e m a n s , T . R a s i n g, A . E. Ro w a n, R . J . M . No l t e , Exten d ed s - co njug ated ruthe n i u m z i nc-p o r p hy r i n co mpl e xe s wi t h e nhance d nonli ne a r-op t ical p roperti es , Ch em. C o mmun. 1 4 ( 2015) 2 855-2 8 58. ) ( [18] Nal Y . K i t a o ka, T. S a sak i, S . N ak a i , A . Yo k o t a n i, Y . Go t o, M . N a k ay a m a , L as e r p ro perti es o f n ew o r g a n i c n o n linear opt i c al cr y s ta l s ch a l c o n e d er i v at iv e s , Ap pl . Ph y s . L ett. 5 6 (1990) 2 07 4 - 2 076. ) ( [19] B . G u , W . J i , X . Q . H uan g , P . S . P a t i l , S. M . Dha r m aprak ash, N o n linear o p tical p ro p er t i e s o f 2 , 4 , 5-Trime t h oxy-4-nit ro c h a lc one: o b s er v at io n o f t w o - p h o t on-i n d uce d e xcit ed - s ta t e n on linea r i t ie s , Op t . E x press 1 7 ( 2 009) 1 1 2 6-1 13 5. ) ( [20] E . D . D ’si lva, G . K. Podag a tl a pa l l i, S . V. Rao , S . M . Dharm a pr a k a sh , St u dy on t hir d - o r de r n o n linear o pti c a l p roperties o f 4-me t hylsul f a n yl c h alc one de r i v a t i v e s u s i ng p ic os ec o n d pu l ses , M at e r. Res. Bull . 47 (2012) 3 552- 3 5 5 7. ) ( [21] . A . N. P r a bhu, V . U p adhya y a , A. J a y a r a m a, K.S. Bh a t , S y n th es is , gr o w th an d c har- a c te ri z a tion o f n c onj ug ate d o rg a n i c n onli n e ar o p ti cal c h a l c o ne de r ivat ive, Ma te r Chem. P h ys . 1 3 8 (2 013 ) 1 7 9 - 1 85 . ) ( [22] S . R. M aidur,P. S . P a t il, S . V . Rao, M. Sh k ir, S.M . Dha r m a pr a ka s h, E xperimen t al a n d ) ( c o m p ut at i on a l s t u d ies on s e co n d-a n d t h ird - o r d e r n on l inear o pt i c al p r o p e rtie s of a n ovel D - 一 -A ty p e c h alc on e d er i v a tiv e: 3 - (4-met h oxy ph enyl) -1 - ( 4 -nitrop he n y l ) prop - ) 2-en-1-one, Opt. Laser Technol. 97 (2017) 219-228. ( [ 23] J . S un, Y . S hi , G . Wa n g, M . Z h ao, S y n t h esis an d three - ord e r no n linear o p t ica l p roper t y o f p y re n yl c ha lcon e , C h i n . J . App l . Chem . 3 2 ( 201 5 ) 1 1 3 4 -1 1 3 8 (I n Chine s e ) . ) ( [24 ] Z . X ia o , Y . S hi , R . S un, J . Ge, Z . L i , Y . F a ng, X . W u , J . Y ang, M . Z h a o , Y . S o n g , U l t rafas t broadband o p tical l i miting i n si m p l e p y rene-b a sed m ol e c u le s wit h hig h t r a nsmittance f r om visi b le to in f r ared r e gi ons , J . M at er . C h e m . C 4 ( 20 1 6 ) 46 4 7-4653 . ) ( [ 25] Y. S h i, Z. L i , Y. F a ng, J . Sun, M . Zh a o , Y . S o ng, U l t r af a st t h ir d - ord e r no n l inear o ptical response of pyrene d e r iv a tives , Opt . Lase r T e c hnol . 90 ( 2017) 1 8- 21 . ) ( [26] Z . J in,D. W a n g , X . W ang , P . L i a n g, Y.M i , H . Y a n g , E f f ici en t m od i f ic atio n o f py r e n e - deriv ati ve f e a t u ri n g t h ir d -o rd er no n li n e a r o p t ic s v i a t he c li c k p os t -fun c t ional i za- t ion , T e tra h e d ron L e tt . 54 (2013) 4859- 48 64. ) ( [27] C .L. D e v i, K . Ye s ud a s, N. S . Ma k a r ov, V . J. Ra o, K . Bh an upra ka sh , J. W. P e rr y , F l u ore n yl et hyn y l p y re n e d e riv a ti v e s w i th st r o n g t w o - p ho t on a bs o r pti o n: i n f lu e nce o f s ubstituent s on opt i ca l p r opertie s , J . Mate r . C h em . C 3 ( 20 1 5 ) 3730 -37 44 . ) ( [28]X 1 . W u, J. X i ao , R . Sun, J. JJ 1 i 8 a , J. Y an g , G . Sh i , Y . W a n g , X . Zh a ng , Y. So n g , P y rene deriv at i ve s a s b ro a db a n d nonlinear op tic a l m ater i a l : M ag ni t ud e a nd mechani s m of tran si e n t r e fracti o n, Dye s Pi g . 1 4 3 (20 1 7 ) 1 6 5 -1 7 2 . ) [29] IP.J. Tejkiran, M.S.B. Teja, P.S.S. Kumar, P. Sankar, R. Philip, S. Naveen,N.K. Lokanath, G.N. Rao, D-A-n-D Synthetic approach for thienyl chalcones-NLO0-a structure activity study, J. Photochem. Photobiol. A 324 (2016) 33-39. ( [ 30] J . W . H ah n , E.S . L e e, M ea s ure m ent o f nonreso n ant t h i rd - or d er sus cep t ib i litie s o f v a rio u s g ases b y t he n o n line ar i nt e rf ero me t r i c t ec h nique, J . O pt . S o c . A m. B 1 2 1 8 ( 1 9 95) 1 021 - 1027. ) ( [31] U . G u b l er, C . B osshar d , Optical t hird- h ar m onic g e n e r ation of f u sed s i lic a i n gas a tmos p h e r e : A b s ol ut e v alu e o f th e t h ird - o r d e r n o nl i near o pt i c a l su scept ib i l i ty x , P hy s. Re v. B 61 (2000)10702- 1 0710. ) [32] H. Zhao, Z. Cheng,D. Shi, Influence of third-order nonlinear and phase variationson third harmonic generation, Optik 119 (2008) 161-166. ( [33 S ] . V . R ao, N . K . M . N . S ri niva s , D. N . R a o , L. G i r i b abu, B .G . Ma i y a, R . P h i l i p, G . R . K umar , St udies o f t h ird-o r de r op t ic al n o nline a r i ty a n d n o n li n ear a bsor p t io n in t e t r a tol y l p o r ph yr i n s us i n g d e g e ner at e fo ur w a v e mi x i ng an d Z - s ca n, O p t . C o mm u n. 1 82 (20 0 0 ) 2 55 -2 6 4 . ) ( [ 3 4] S . C he r ukulap p urat h , G . B o udebs , A . M o n teil, 4 f coher e n t i m age r sy stem and it sa p p li c atio n t o n on l i n ea r opt i c a l meas u r e m en t s , J . Opt . S o c . A m. B 2 1 ( 20 04) 273 - 2 79. ) ( [ 3 5] Y . L i , X. Zhang , Y. Wang, K . Yang , Y . So n g, O p t imization o f p has e o bjects in 4 f c o h e r en t i m a g i ng sy stem f or no n li ne a r re fr acti o n mea s u rements , Op t . C o m m u n . 2 6 6 ( 2 006 ) 686-690. ) ( [ 36] K . F edus , G . B oud e b s , Se n s i t ivity of th e 4f coh e r e nt i mag i n g syst e m u s ed i n de - g ene rat e mul t i w a ve m i xing e x per i me nt s , J. O p t. S o c . A m . B 26 (20 09) 2 4 4 -2 48. ) ( [37] K . F edus , G . B oud e bs , H . L e bl o n d, D e g en er a te mu l ti-wav e m i xi ng i n s i d e a 4f ima -g ing s y s t em i n p r e s ence o f n on l ine a r a bsor p t ion , Ap p l. Ph ys . B 10 0 ( 2 01 0 ) 82 7 - 8 3 1 . ) ( [38]Z . Ni e, G . S hi , Z . L i , G. A o, Y . W an g, X . Z h a ng , M. S hu i , J. Y a n g , Y . S o ng , Y. F a ng , I n ve st ig atio n o f t h e t h ird-ord e r n online ar re fr actio n us i ng 4f c oh erent i m a g i ng s y st e m w i th p o s i tiv e-neg a t iv e b ar pha s e o bje c ts , Op t . Lase r E ng . 50 ( 2012) 140 5- 14 09. ) [39] R. Sharma, J. Solomon Ivan, C.S. Narayanamurthy, Comparative statistical analysisof phase profiles of a pseudo random phase plate with Kolmogorov phase screens,Optik 126 (2015) 4195-4201. ( [40 ] . A . S agan , S . Now i ck i, R . Bu c z yn s ki , M. K o wa l c z y k , T . S z o p l i k , Imaging p h a se o b - S K1 j e cts with s q u a re-r oot , F o uca u lt, a nd H o f f m a n r e a l f il te rs: a co m p a r i s o n , A p pl . Op t . 42 ( 200 3)5 816- 5 824. ) [41] J.L. Godeta, H. Derbal, S. Cherukulappurath, G. Boudebs, Optimization and limits ofoptical nonlinear measurements using imaging technique, Eur. Phys. J. D39 (2006)307-312. ( [42] N 1 . L i u , G. S h i , J. Y a ng , Y. So ng, E x c i t e d s t a t e r e f r a c t i o n of C70 /t ol u e n e st ud i ed b y u s i ng 4 f c o he re n t i ma gi ng s y st e m with ph a s e o b je c t, C hin. P hys . B 2 0 ( 20 11) 0 1 31 0 3 . ) [43] Z. Nie, Z. Li, X. Zhang, G. Ao, G. Shi, Y. Wang, Y. Song, Symmetrical positive-ne-gative annular phase object for optical nonlinearity characterization, Phys. Lett. A378 (2014) 131-138. ( [ 44] C . H e, J. F a n, Z . L i , Y. G a o, Z . C hen, Y . Song, Y . W u, B. Wa n g , Se lf-as s embled m ul t i l a y e r fi l ms c o n t a i nin g 1 ,8,1 5,22 - tetra k i s ( 8 -qu i no l i n e ox y - 5 -su l fo ni c a c i d )- p ht h alocy an i n e c o p pe r : P r e p a r at i on, a n d thir d o r der n o n l i near op t i cal p r opert i e s , O pt . M ate r . 36 ( 2014 ) 74 6 - 7 52 . ) ( [45] 1 G . Shi , C . He, Y . Li , R. Z ou , X . Z ha ng , Y . W a ng , K . Yang, Y . S ong, C .H . W an g , E x c i te d - s t a t e nonline a ri ty m easurement s o f Z nPcBr 4 / D M SO, J. O p t . So c . A m. B 2 6 (20 0 9)7 54-761 . ) ( [46] Y . W ang, C. Q uan, C. J. T a y, Ne w m e tho d o f a t t a ck a n d s e cu r it y e n han c e men t o n an a s ym met r i c c ry p t os y ste m b a s ed on e q u a l m o d u lu s d ec o mp o sit i on, A pp l. O pt. 5 5 ) ( (2016) 6 7 9- 6 86. ) ( [47 ] J . W e ng , Q . M ei , Q . L i n g , Q . F a n , W . H uan g, A n e w co l o r imetri c a n d f l u or e s cen t r atio m etr i c s ens o r f or H g 2 + b a se d o n 4-pyr e n-1-y l - py rimi di ne, T et r ah e d r on 6 8 ( 2 012) 3129- 31 34. ) ( [48] M .J . F r i s ch , G .W. T r u c k s, H .B. Sch l eg e l , G . E. Sc useri a , M . A. Ro b b, J .R. C h e e s ema n, G . S calmani, V . B arone, B . M e n n u cci, G . A . P eters s on, H . N a k atsuji, M . C a r i c a to , X . L i, H. P . Hratchian , A.F . I z may l o v , J . B l o ino, G. Z h eng , J.L . So nnen b e r g, M. H ad a , M . E h a r a , K . T oy o t a , R. F u k ud a, J. H a segaw a , M . I s h ida, T. N aka j ima , Y . H on d a, O . K i t a o , H . N aka i , T . V r even , J .A . M o n tgome ry , J. E. P e r a l ta, F . O g l i aro , M . B ea r park, J. J. Hey d , E . Bro t h e rs , K . N . K u din, V.N . S ta r o ve r ov , R . Kobayashi , J . No rm an d , K . R a g h a va c h ar i, A . R e n d el l , J .C . B u ran t , S . S . I y enga r , J. Tomas i , M . C o s s i , N . R eg a, J . M . M i lla m , M . K l ene, J . E. K n o x , J. B . C ros s, V . Ba k k e n , C . A damo , J . Ja ramillo, R . G o mpert s , R .E. S t ra tmann, O. Yaz y ev , A. J . A ust in, R . C a mm i , C . P omelli, J .W. O chter s ki , R . L . M a r t i n, K . Moroku m a , V .G. Zakrze w ski, ) Four chalcone derivatives 3a–3d based on pyrenyl donor and different halopyridyl acceptors were synthesized successfully. The resultant chromophores 3a–3d were characterized by nuclear magnetic resonance (NMR), high resolution mass spectra (HR-MS) and ultraviolet (UV) spectra. The theoretical calculations and thermal properties of the materials 3a–3d were systematically studied to illustrate structure-performance relationship. Further, a novel and unique method, 4f phase coherent imaging technology, was introduced to investigate thethird-order nonlinear optical properties of title compounds 3a–3d with 4 ns laser pulses at 460 nm. The thermal properties, the theoretical calculations and the experimental results consistently indicate that the compound 3b, namely 3-(5-bromopyridin-2-yl)-1-(pyren-1-yl) prop-2-en-1-one, is a more promising candidate for optoelectronic and nonlinear optical devices. Its third-order nonlinear absorption coefficient (β), refraction index (n2)and polarization (χ(33)) are found to be up to such order of magnitude as 10−9 m/W, 10−16m2/W and 10−11 esu, respectively.
确定



还剩6页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《卤素吡啶中三阶非线性光学性能检测方案(激光产品)》,该方案主要用于其他中可靠性能检测,参考标准--,《卤素吡啶中三阶非线性光学性能检测方案(激光产品)》用到的仪器有Ekspla NT340 高能量可调谐激光器(OPO)
推荐专场
该厂商其他方案
更多
















