
采用由立陶宛Ekspla公司的PL2231型高功率皮秒激光器和光学参量发生器等设备构成的振动和频光谱测量系统,对重负离子复合物在软电荷界面形成独特的水结构的现象进行了实验研究。
方案详情

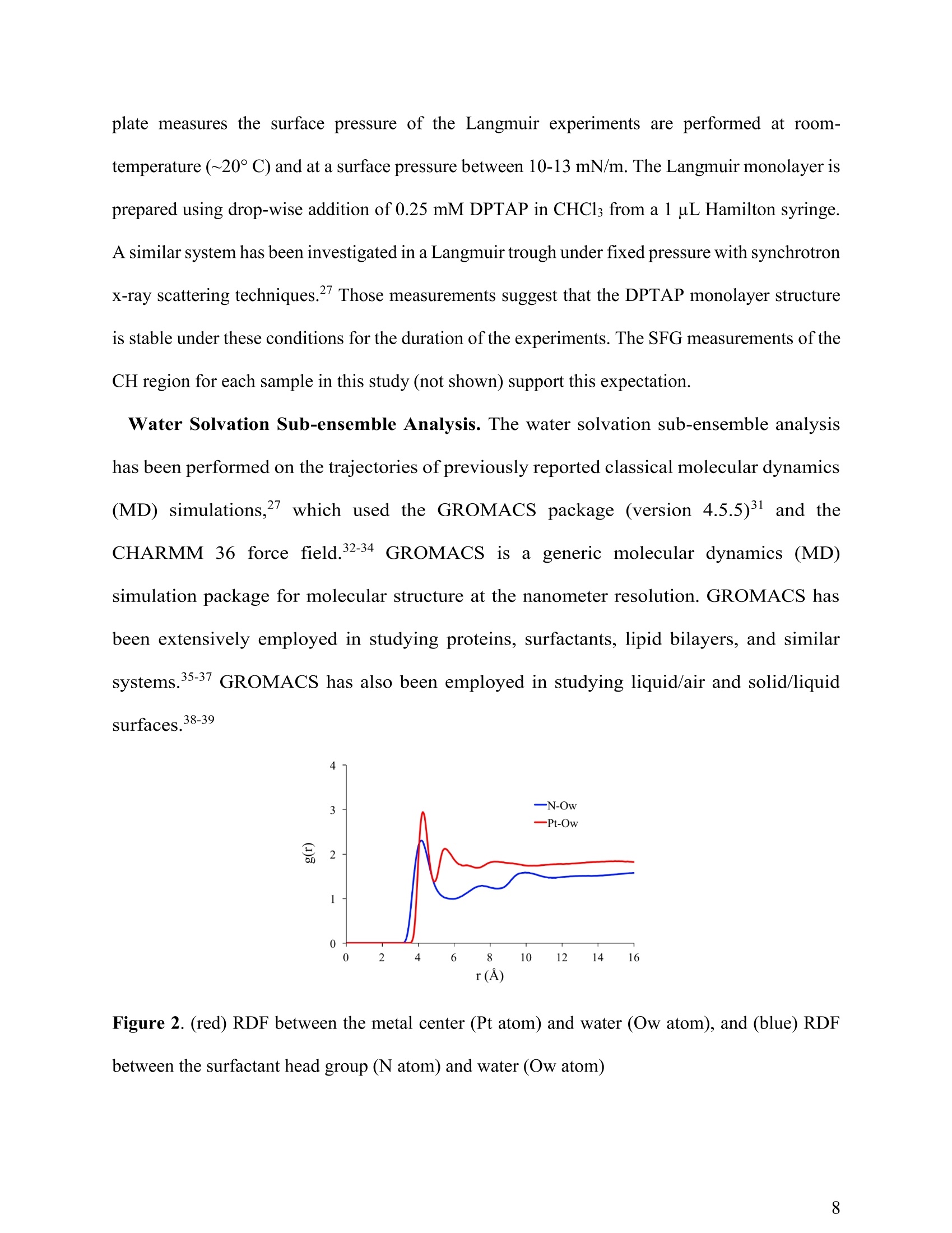
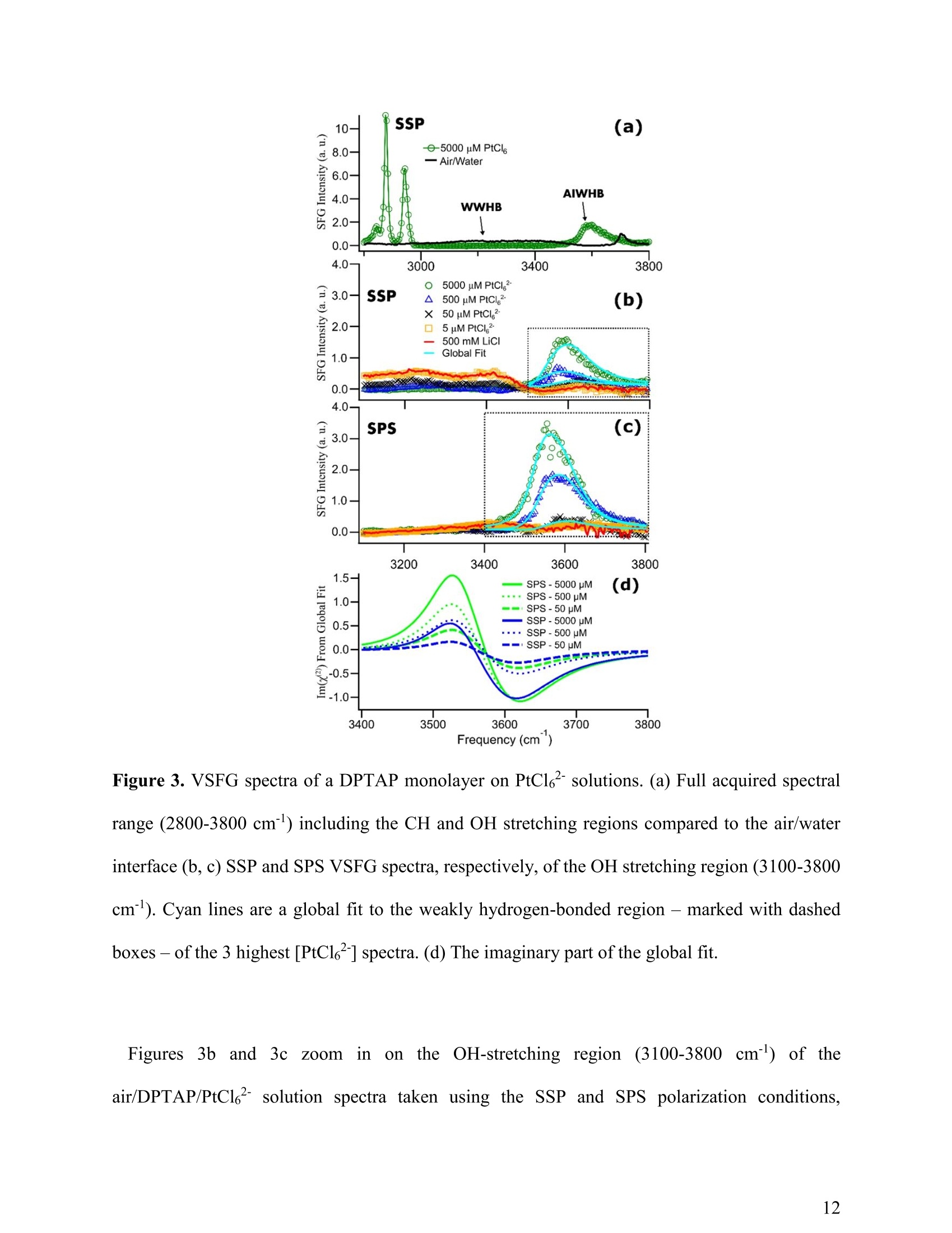
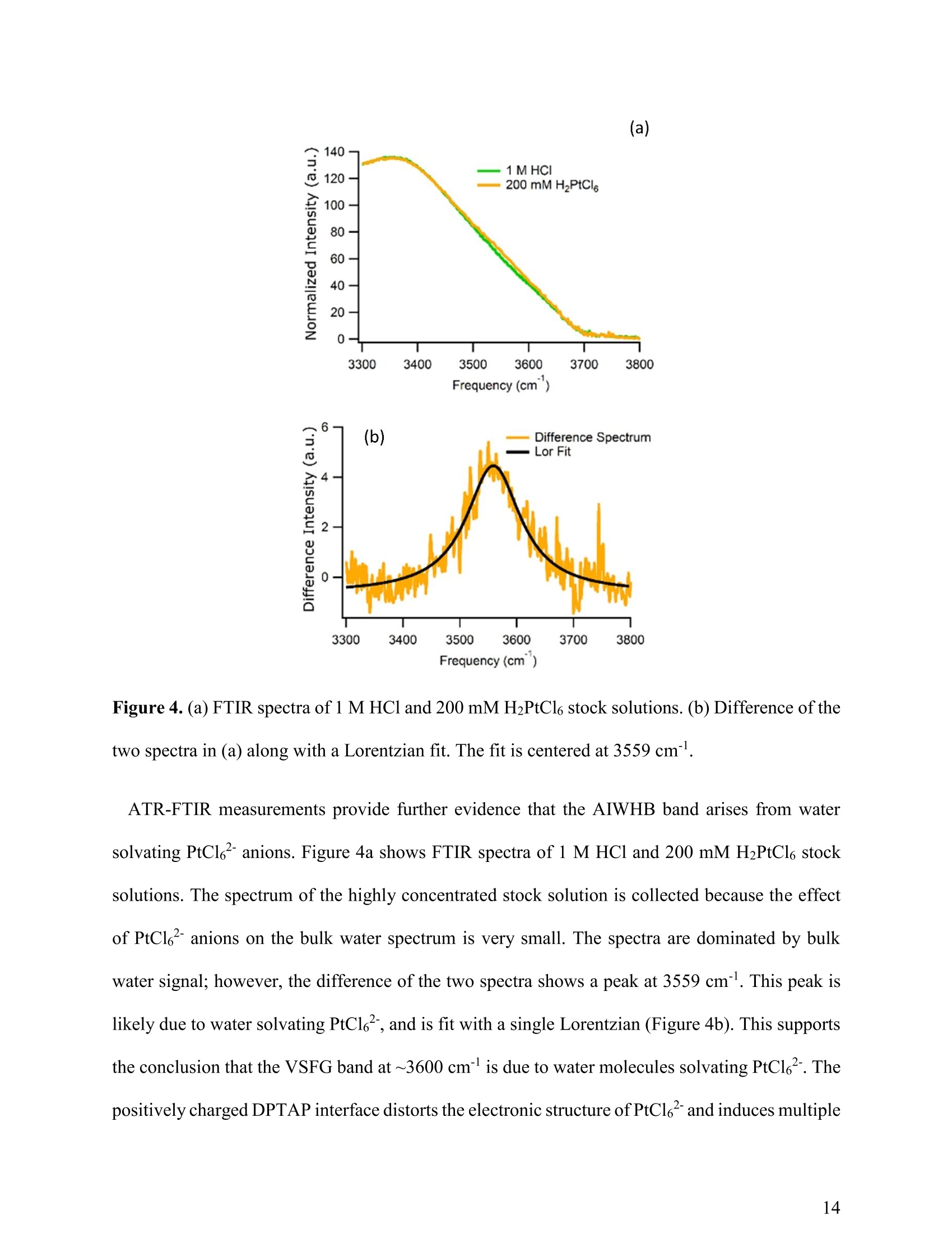
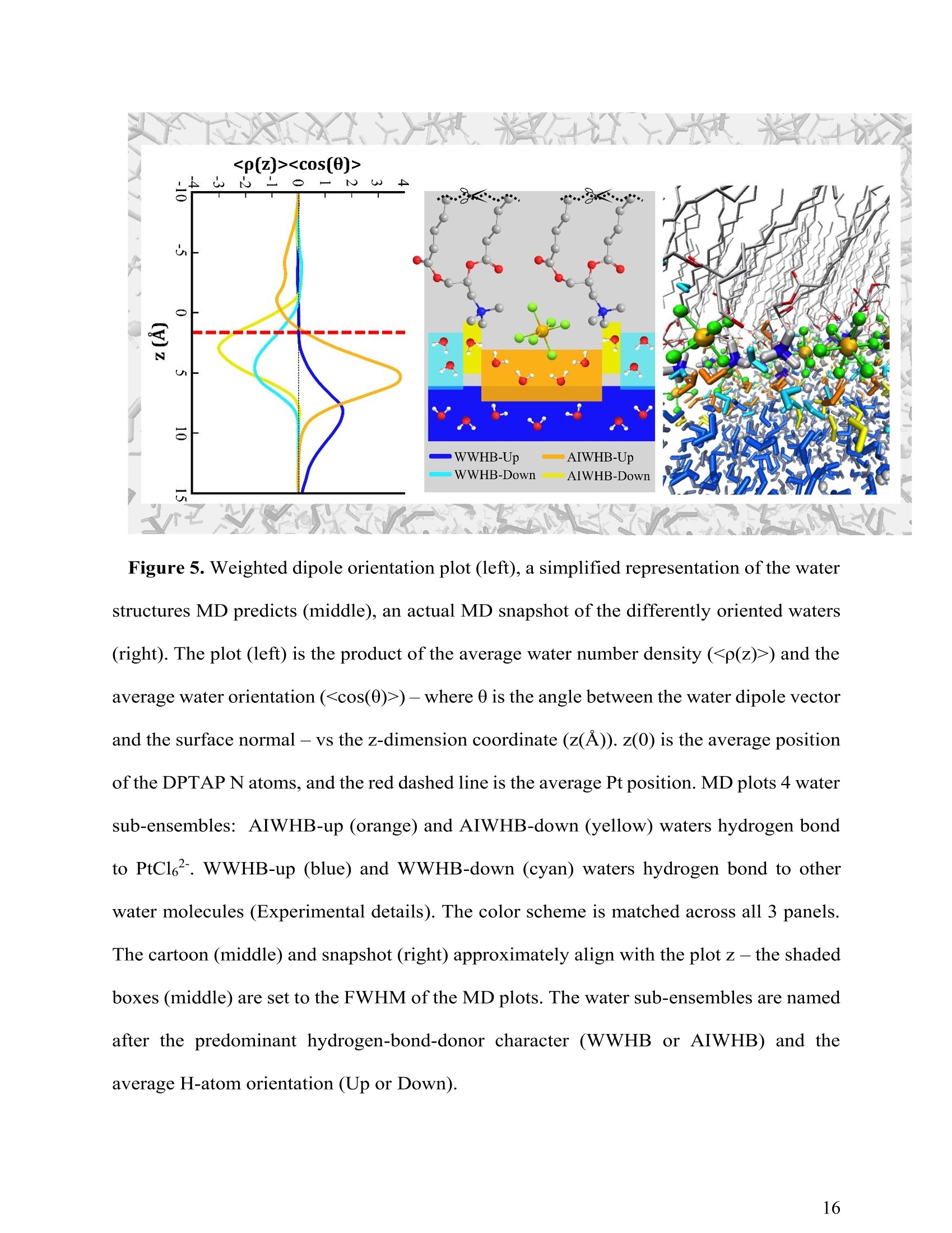
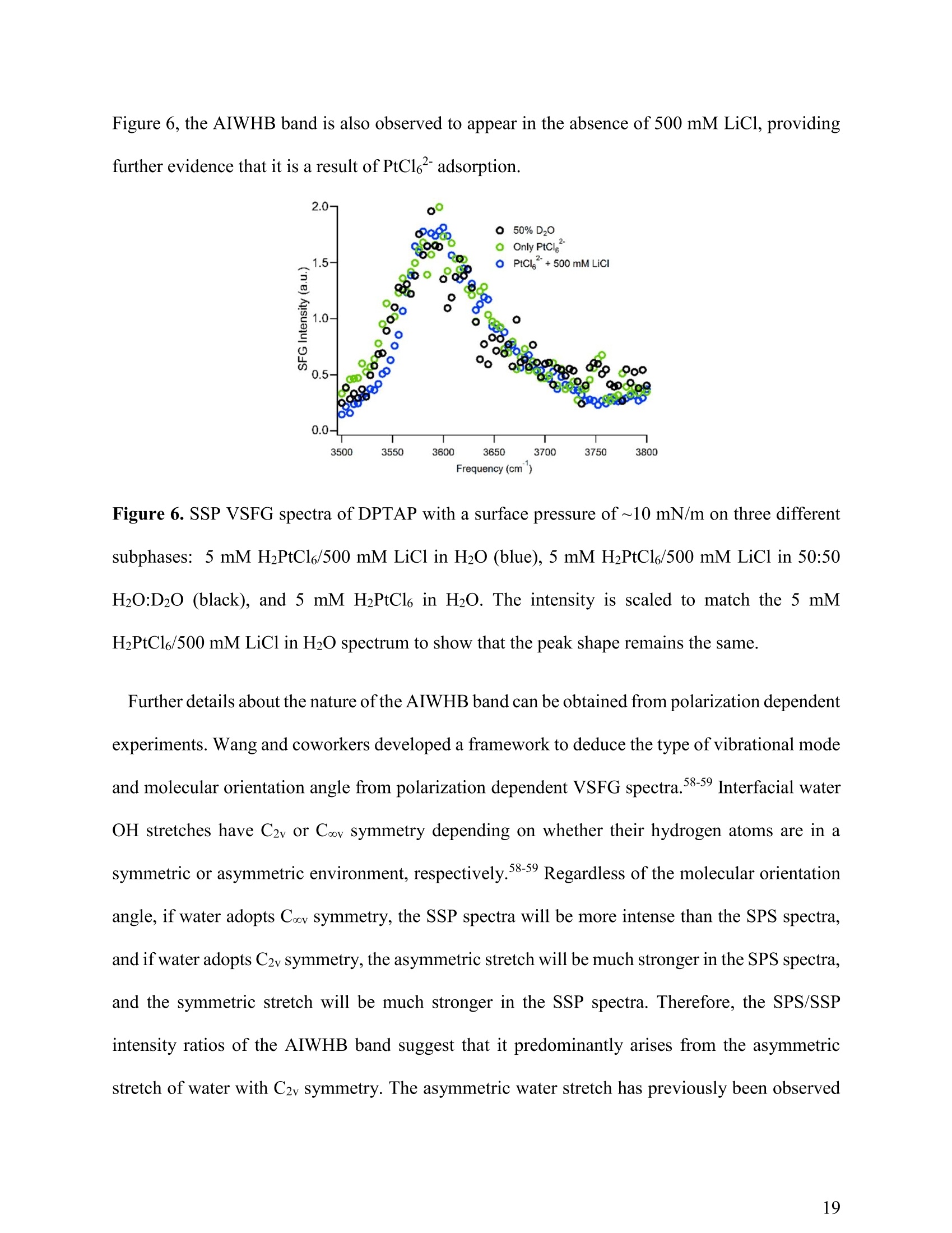
(a) Heavy Anionic Complex Creates a Unique WaterStructure at a Soft Charged Interface William Rock, t Baofu Qiao, Tiecheng Zhou, Aurora E. Clark,and Ahmet Uysalt,* T Chemical Sciences and Engineering Division, Argonne National Laboratory, Argonne, IL60439, United States Department of Chemistry and the Material Science and Engineering Program, WashingtonState University, Pullman, WA 99164, United States AUTHOR INFORMATION Corresponding Author *E-mail: ahmet@anl.gov. Web: www.ahmet-uysal.com. Phone:+1-630-252-9133 ABSTRACT Ion hydration and interfacial water play crucial roles in numerous phenomena ranging frombiological to industrial systems. Although biologically relevant (and mostly smaller) ions havebeen studied extensively in this context, very little experimental data exist about molecular scalebehavior of heavy ions and their complexes at interfaces, especially under technologicallysignificant conditions. It has recently been shown that PtCl? complexes adsorb at positivelycharged interfaces in a two-step process that cannot fit into well-known empirical trends, such asHofmeister series. Here, a combined vibrational sum frequency generation and moleculardynamics study reveals that a unique interfacial water structure is connected to this peculiaradsorption behavior. A novel sub-ensemble analysis of MD simulation results show that afteradsorption, PtCl6complexes partially retain their first and second hydration spheres, and it ispossible to identify three different types of water molecules around them based on theirorientational structures and hydrogen bonding strengths. These results have important implicationsfor relating interfacial water structure and hydration enthalpy to the general understanding ofspecific ion effects. This in turn influences interpretation of heavy metal ion distribution acrossand reactivity within, liquid interfaces. INTRODUCTION The adsorption of ions at aqueous charged interfaces drives many macroscopic processesincluding chemical separations, desalination, protein solvation, - atmospheric chemistry,-7 andgeochemistry. 9 Specific ion effects make modeling these processes difficult; a thoroughunderstanding of the molecular-scale interactions that govern them is necessary.Here,vibrational sum frequency generation (VSFG) spectra reveal a novel interfacial water structure dueto the adsorption of PtCle?complexes at a soft charged interface. MD simulations show that thisstructure is due to PtCl6partially retaining its hydration sphere after closely adsorbing to theinterface. This results is in stark contrast to the trends observed in Hofmeister anions, where easilyshedding the hydration sphere is correlated with a high surface affinity. A molecular-scaledescription of this unusual water structure is obtained through detailed sub-ensemble analysis ofMD simulation, and offers a new approach to the analysis of VSFG spectra. Electrostatics usually control the interaction of charged particles with surfaces; however, whena hydrated ion is close to a charged interface, short-range weak interactions become important.The interfacial chemistry is further complicated by the fact that the aqueous medium is not auniform continuum, but contains considerable structural organization.2 It is known that deviationsfrom ideal solvent behavior are related to specific ion effects.13-14 Although recent experimental, ,15-17 computational,18 and theoretical19-20 advances have improved our understanding considerably,the emerging view is that specific ion effects may not have a single unified explanation, especiallywhen ion-ion interactions and interfacial ion-binding sites are operative. The interfacial interactions of biologically relevant, and usually lighter, ions in aqueousenvironments have been extensively studied.15-16,21-22 Yet comparable work on heavier elements, including the platinum group metals (PGMs), lanthanides, and actinides, are scarce.23 This isdespite the fact that many modern lighting, displays, and carbon-free energy technologies rely onthe efficient refining and reprocessing of heavy metals based upon liquid:liquid extraction (LLE)technologies;1, 24 most of these processes involve the interaction of aqueous interfaces with theanionic complexes of heavy elements in highly concentrated solutions.24-26 In addition to its practical implications, a molecular scale understanding of interfacial water inthe presence of large ions is the best way to test the limits of the theories that explain specific-ioneffects. It is known that the thermochemical radii of ions plays an important role in their interfacialinteractions,23 and a recent study demonstrated qualitative differences in the adsorption behaviorof heavy and light anions at a charged interface."However, the surface x-ray scattering techniquesused in that study did not provide any information about the interfacial water structure. The currentwork leverages the well-established ability of VSFG to study interfacial water-ion interactions innumerous systems4, 16, 21-22,28-30 (including Langmuir monolayers on electrolyte solutions- Figure1), which allows for facile control of the interactions between organic functional groups and ions.,23,29 Using this approach we provide clear evidence, a unique signal in VSFG spectra at 3600 cm"appearing as a result of adsorbed ions, that heavy anions can adsorb tightly to a charged interfaceand remain strongly hydrated, indicating that adsorption behavior does not always followhydration strength. The main experiments are designed to keep all parameters constant except thebulk concentration of PtCl6, to make the interpretation of the data relatively simple. The sub-ensemble analysis of MD simulations, and other supportive experiments also independentlystrengthen this interpretation. Figure 1. A schematic of VSFG measurements at the air/water/DPTAP interface. Tunable IR andfixed 532 nm laser pulses overlap spatially and temporally at the interface to generate VSFG signal EXPERIMENTAL AND COMPUTATIONAL METHODS VSFG Setup. Experiments were done with an EKSPLA VSFG spectrometer. The VSFG setupbegins with a picosecond laser (PL2231-50) containing a master oscillator with a Nd:YVO4 laserrod and a regenerative amplifier using a diode-pumped Nd:YAG rod. The amplified 1064 nmoutput is ~28 mJ with a pulse width of ~29 ps and a 50 Hz repetition rate. A Harmonics Unit(SFGH500-H/2H) splits the laser output into three paths; KDP crystals frequency-double twopaths. The 1064 nm path and one of the 532 nm paths pump an OPA/DFG (PG501-DFG1P) usingBBO/AgGaS2 crystals that produces narrowband infrared (IR) pulses tunable from ~1000-4000cm. The 532 nm polarization is adjusted with a /2 waveplate, and the VSFG signal is selectedusing a Glan polarizer. The IR polarization is selected by adjusting a pair of motorized mirrors thatcontrol the beam path through a periscope. After the sample, the visible and IR excitations areblocked with an iris, and are further attenuated with a 532 nm notch filter and a 510 nm shortpassfilter. The SFG signal is then directed to a monochromator (Sol, MS2001) and collected with aPMT (Hamamatsu, R7899). The VSFG spectrometer employs a reflection geometry; the visible and IR excitation angles,with respect to the sample normal, are 0vis = 60°and 0n =55°,respectively. The visible and IRenergies at the sample are~800 uJ and ~65 pJ,respectively. A motorized piezoelectric rotationstage (ThorLabs,ELL8K) rotates the sample after each frequency step to avoid sample damage.Each spectrum was collected with a 4 cmincrement over the range 2800-3800 cm, and averaged300 laser shots per point. The spectra were normalized against the SFG spectrum of z-cut quartz.Both the SSP and SPS polarization conditions were collected for each sample. PPP polarizationwas also collected for the highest concentration (not shown) sample and consistent with theinterpretation presented below. The polarization conditions are defined using the industry standard,S(S)-VSFG signal, S(P)- VIS excitation, and P(S)- IR excitation. The electric field of P-polarizedlight is parallel to the plane of incidence, and the electric field of S-polarized (from the Germansenkrecht) light is perpendicular to the plane of incidence. VSFG Data Global Fit. The AIWHB band cannot be fit by a single Lorentzian (SupportingInformation). Therefore, it is globally fit to a two-Lorentzian model, which suggests that at leasttwo different water environments contribute strongly to the AIWHB band: The nonresonant amplitude and phase (ANR,PNR), the peak location (ω1,ω2), and the peakwidth (T,I2) are linked in the global fit, and the peak amplitudes (A1n, A2n) are allowed to floatin each spectrum. A high-quality two-peak global fit requires one positive and one negative peak;however, which of the peaks is positive or negative depends on the initial guess (the absolute phase cannot be determined from this experiment). The imaginary part of the fit is given by the followingequation: The peaks were found to be centered at w1=3534 cmand w2=3606 cm. The SPS intensity ismuch stronger than the SSP intensity in the 3534 cm"peak, and SPS and SSP intensities areapproximately equal in the 3606 cmpeak. The fit parameters and the comparison of the variousfits based on different models can be found in the supporting information. FTIRMeasurements.。The spectra were collected with a Nicolet Nexus 870 FTIRspectrometer with an attenuated total reflectance accessory. Sample Preparation. Anhydrous lithium chloride (LiCl, 99%), chloroplatinic acid solution(H2PtCl6,8 wt.% in H2O), and HPLC grade chloroform (CHCl3, ≥99.9%) were purchased fromSigma-Aldrich. 1 N hydrochloric acid (HCl) was purchased from Fisher Scientific. 1,2-dioleoyl-3-trimethylammonium-propane (DPTAP) chloride salt was purchased in powder form from AvantiPolar Lipids and stored at -20°C. All chemicals were used as received. Aliquots of 0.25 mMDPTAP in CHCl3 were prepared and stored at -20°C. DPTAP aliquots are discarded in ≤ 5 daysand are not subject to any freeze-thaw cycles. All subphase solutions are 20 mL, contain 500 mMLiCl, and are adjusted to pH 2 using 1 N HCl. The 5 mM PtCle’solution is at pH~2 without theaddition of HCl. Ultrapure water with a resistivity of 18.2 MQcm (Barnstead, Nanopure TOC-UV)was used to prepare each subphase. Langmuir monolayer samples are prepared in a 60x20 mm flat-form PTFE dish. A Nimapressure sensor (from a model 601A Langmuir trough) using a chromatography paper Wilhelmy plate measures the surface pressure of the Langmuir experiments are performed at room-temperature (~20°C) and at a surface pressure between 10-13 mN/m. The Langmuir monolayer isprepared using drop-wise addition of 0.25 mM DPTAP in CHCl3 from a 1 uL Hamilton syringe.A similar system has been investigated in a Langmuir trough under fixed pressure with synchrotronx-ray scattering techniques." Those measurements suggest that the DPTAP monolayer structureis stable under these conditions for the duration of the experiments. The SFG measurements of theCH region for each sample in this study (not shown) support this expectation. Water Solvation Sub-ensemble Analysis. The water solvation sub-ensemble analysishas been performed on the trajectories of previously reported classical molecular dynamics(MD) simulations,27·which used the GROMACS package (version 4.5.5)3l and theCHARMM 36 force field.32-34(GROMACS is ageneric molecular dynamics (MD)simulation package for molecular structure at the nanometer resolution. GROMACS hasbeen extensively employed in studying proteins, surfactants, lipid bilayers, and similarsystems.35-37 GROMACS has also been employed in studying liquid/air and solid/liquidsurfaces.38-39 Figure 2. (red) RDF between the metal center (Pt atom) and water (Ow atom), and (blue) RDFbetween the surfactant head group (N atom) and water (Ow atom) The simulations were done at full PtClsurface coverage with 0.5 M LiCl backgroundto represent the highest concentration experiments.The molecular area per DPTAPmolecule in the simulations was set to 48 A2, as determined by grazing incidence X-raydiffraction measurements.27 The radial distribution functions in Figure 2 are calculatedfrom the MD simulations in order to determine the solvation environment of PtCl6?- andDPTAP surfactant head group. The first peak of the Pt-Ow RDF at r~4.25 A correspondsto the first solvation shell of PtCl6, with a first minimum at r ~ 5.00 A. Thus the firstsolvation shell of PtCl6can be defined as row..Pt <5.00 A. The second peak of the Pt-OwRDF at r ~ 5.45 A corresponds to the second solvation shell of PtCle, with a secondminimum at r~ 6.50 A. Therefore, the second solvation shell of PtCl6’ can be defined as5.00 < row..Pt <6.50 A. Similarly, in Figure 2 the first peak in the N-Ow RDF occurs at r~4.15 A with its minimum at r~ 6.00 A, corresponding to the solvation shell of DPTAPsurfactant head group. Therefore, the solvation shell of DPTAP head group can be definedas row..N <6.00 A. Considering the solvation environment of water around the metallate and surfactant headgroup, as shown in the radial distribution functions in Figure 2, we decompose the watermolecules in our simulation box into four groups: AIWHB-Up: water molecules in the 1st solvation shell of PtCl6, i.e. row..Pt <5.00 A; AIWHB-Down: water molecules in the 2nd solvation shell of PtCl6,and also within thesolvation shell of the DPTAP head group, i.e. 5.00 5.00 A and row..N<6.00 A; WWHB-Up: water molecules outside the 1st solvation shell of PtCl6and also outsidethe solvation shell of the DPTAP head group, i.e. row..Pt> 5.00 A and row..N > 6.00 A RESULTS AND DISCUSSION Observation of Anion Induced Interfacial Water Structure. A typical VSFG spectrum at theneat air/water interface has two significant features: a sharp peak at 3700 cmcorresponding tothe free OH stretch, and a broad band between ~3050-3500 cm"corresponding to the OH modesof water:water hydrogen bonds (WWHB).16 Spreading a charged Langmuir monolayer on purewater leads to the disappearance of the free OH and to significant enhancement of the WWHBsignal.28,40 When ions are introduced to the subphase, the WWHB signal decreases or completelydisappears depending on effect of the ion.21, 41-44 These effects usually correlate with theHofmeister series and hydration strength. Larger, more polarizable ions easily shed their hydrationshells and strongly associate with charged interfaces. For instance, the affinity of halide anions forthe positively charged 1,2-dioleoyl-3-trimethylammonium-propane (DPTAP) interface follows theHofmeister series;45 larger I anions adsorb close enough to completely eliminate the WWHBVSFG signal. This is usually interpreted as the loss oforientational order of interfacial water 15,45While many different salts and acids have been similarly investigated, the observed effects of theseions were limited solely to the changes in the intensity and shape of the WWHB peak. In thisstudy, VSFG spectroscopy, MD simulations and sub-ensemble analysis reveal a unique interfacialwater structure after the adsorption of PtCl6to a DPTAP monolayer. One aspect of this unusualstructure is the emergence of a weakly hydrogen-bonded interfacial water band at ~3600 cm. Thewater VSFG signal at 3600 cm", caused by the presence of interfacial ions, has not been previouslyobserved. In addition, the emergence of the 3600 cmsignal coincides with the disappearance of the WWHB signal (~3050-3500 cm"). In contrast to the straightforward interpretation - a loss oforientation order - sub-ensemble analysis of MD simulation trajectories reveals that, in thissystem, the vanishing VSFG signal is a result of oppositely oriented regions of ordered watermolecules. Figure 3a presents the VSFG spectra of the air/water interface and the air/DPTAP/5000 uMPtCle solution interface from 2800-3800 cmtaken using SSP polarization. VSFG spectra arecollected using the SSP and SPS polarization conditions (see Experimental Details). All PtCl6subphase solutions contain 500 mM LiCl and are adjusted to pH 2 using HCl to mimic industrialseparations conditions. This also helps to fix the PtCl6?speciation and control the PtCladsorption at the interface as a function of the bulk concentration. Henceforth, the solutions willbe delineated using only the [PtCl6]. The air/water VSFG spectrum is included as a benchmark., 46-48 Frequency (cm) Figure 3. VSFG spectra ofa DPTAP monolayer on PtCle’ solutions. (a) Full acquired spectralrange (2800-3800 cm) including the CH and OH stretching regions compared to the air/waterinterface (b,c) SSP and SPS VSFG spectra, respectively, of the OH stretching region (3100-3800cm). Cyan lines are a global fit to the weakly hydrogen-bonded region- marked with dashedboxes - of the 3 highest [PtCl6"] spectra. (d) The imaginary part of the global fit. Figures3bb aand 33czoom1in onthe OH-stretching region (3100-3800 cm) of theair/DPTAP/PtCl6²solution spectra taken using the SSP and SPS polarization conditions, respectively. The spectra span three orders of magnitude of PtCl?- concentration (5 to 5000 uM),and include a background spectrum without PtCl6-(500 mM LiCl at pH 2). The air/DPTAP/waterVSFG spectrum has been reported previously.40,45 The VSFG spectrum of DPTAP on 500 mM LiCl is comparable to previous results at lowerconcentrations,45 showing some WWHB signal, suggesting that Cl partially shields the interfacialwater molecules from the DPTAP surface charge. Also, the monolayer blocks the free-OH groups,eliminating the narrow 3700 cm peak. The addition of 5000 uM PtCle’ eliminates the WWHBsignal, and gives rise to a new band around 3600 cm. The intensity and structure of the new banddepends on the [PtCl6]. Hydrogen-bonding red-shifts the water OH vibration (from ~3700 cm°in free-OH to ~3050-3500 cmin WWHB); since the magnitude of the red-shift depends on thestrength of the intermolecular interaction, this new peak is due to a weakly hydrogen-bonded waterstructure. Although some previous water studies have reported weakly hydrogen-bonded peaksaround 3600 cm due to water molecules trapped in the hydrophobic tail regions of Langmuirmonolayers,29, 49-52 this is the first evidence of an anion-induced weakly hydrogen-bonded(AIWHB) interfacial water structure. Although previous studies attribute water stretches around 3600 cm" to water moleculesinteracting with the hydrophobic tails of Langmuir monolayers, that is likely not the case here. Aprevious surface x-ray study on the same system shows that the area per DPTAP molecule (at aconstant surface pressure) decreases with increasing [PtCl6].27 Therefore, the addition of PtCle’should not increase the amount of water molecules between the lipid tails. In addition, as it isexplained in the next section in detail, our MD simulations show that only a very little amount ofwater molecules are present above the DPTAP head groups, and all of these water molecules arein the hydration shell of the PtCl6. Frequency(cm) Figure 4. (a) FTIR spectra of 1 M HCl and 200 mM H2PtCl6 stock solutions. (b) Difference of thetwo spectra in (a) along with a Lorentzian fit. The fit is centered at 3559 cm. ATR-FTIR measurements provide further evidence that the AIWHB band arises from watersolvating PtCle anions. Figure 4a shows FTIR spectra of 1 M HCl and 200 mM H2PtCl6 stocksolutions. The spectrum of the highly concentrated stock solution is collected because the effectof PtCleanions on the bulk water spectrum is very small. The spectra are dominated by bulkwater signal; however, the difference of the two spectra shows a peak at 3559 cm. This peak islikely due to water solvating PtCl6, and is fit with a single Lorentzian (Figure 4b). This supportsthe conclusion that the VSFG band at ~3600 cm"is due to water molecules solvating PtCl. Thepositively charged DPTAP interface distorts the electronic structure ofPtCland induces multiple water sub-ensembles, which causes the VSFG peaks to shift and induces the appearance of a bandrather than a single peak. This is also in agreement with previous infrared pump-probemeasurements that found the O-H---C1stretch centered at 3450 cmand the O-H---Istretchcentered at 3500 cm.3 It is reasonable to expect a smaller red-shift for larger, more polarizablePtClanions. The AIWHB band can be best fit by two oppositely signed Lorentzians (Figure 3d), whichsuggests that the spherically symmetric hydration shell in the bulk is changed at the interface andat least two different water environments contribute strongly to the AIWHB band. The peaks werefound to be centered at ωi=3534 cm"and ω2=3606 cm. The SPS intensity is much stronger thanthe SSP intensity in the 3534 cm°peak, and SPS and SSP intensities are approximately equal inthe 3606 cm peak. The sign of the peaks cannot be determined directly without heterodynedetected VSFG (HD-VSFG) measurements, and is instead obtained by sub-ensemble analysis ofMD simulation. Figure 5. Weighted dipole orientation plot (left), a simplified representation of the waterstructures MD predicts (middle), an actual MD snapshot of the differently oriented waters(right). The plot (left) is the product of the average water number density () and theaverage water orientation ()-where 0 is the angle between the water dipole vectorand the surface normal - vs the z-dimension coordinate (z(A)). z(0) is the average positionof the DPTAP N atoms, and the red dashed line is the average Pt position. MD plots 4 watersub-ensembles: AIWHB-up (orange) and AIWHB-down (yellow) waters hydrogen bondto PtCl6.WWHB-up (blue) and WWHB-down (cyan) waters hydrogen bond to otherwater molecules (Experimental details). The color scheme is matched across all 3 panels.The cartoon (middle) and snapshot (right) approximately align with the plotz - the shadedboxes (middle) are set to the FWHM of the MD plots. The water sub-ensembles are namedafter the predominant hydrogen-bond-donor character (WWHB or AIWHB) and theaverage H-atom orientation (Up or Down). Water Solvation Sub-ensemble Analysis. We next analyze the dipole orientation and waterorganization with MD simulations. The product , the average water numberdensity weighted by its orientation, has been shown to be a useful estimate of VSFG intensity.The orientational angle (0) of individual H2O molecules is defined as the angle that the dipolemoment vector of H2 makes with the surface normal. Figure 5 presents of fourdifferent interfacial water environments versus the z-dimension; the gas phase is on the top (z<-10 A), and the water bulk is on the bottom (z> 15 A). The total may obscurestructural details if there are multiple water environments (with different vibrational frequencies)at the same distance (z) from the interface. As such, we decomposed the total intosub-ensembles of different intermolecular interactions. This approach is a good alternative tocalculating the VSFG spectra explicitly using MD simulations, which is resource intensive andrequires parameter optimization for the non-ideal solutions under study. Four primary water environments were ascertained based on their nearest possible interactions(either the N* of DPTAP or PtCle) and explained in Figure 5. A negative indicates that water is oriented with its H-atoms pointing away from the surface (H-down), and apositive value indicates that the water-hydrogens point towards the surface (H-up); this is the samesign convention used in Im(x) in Figure 3d. The MD simulations predict that the first hydration shell of PtCl6has~10 water molecules inthe bulk, and this number drops to ~5 at the interface. These AIWHB-up waters mainly stayunderneath the PtClions (Figure 5) and give rise to the positive peak in Figure 3d. Also a verylittle amount of water is observed to remain above PtCl? which create the small and broadnegative peak at z<0. The second hydration shell also exhibits an asymmetric structure at theinterface. AIWHB-down waters are defined as being in the second hydration shell of PtCl6and in the first hydration shell of DPTAP N*. Therefore, they are closer to the interface, where the firsthydration shell of PtClis less dense (as can be seen by comparing the AIWHB-up (orange) andAIWHB-down (yellow) curves in Figure 5). This allows AIWHB-down water molecules tohydrogen bond to PtCle, but with a weaker intermolecular interaction than AIWHB-up waters inthe first hydration shell. (The first and second hydration shells are defined by water distances fromPtCl6?, as explained in experimental details). These AIWHB-down waters are responsible for theless red shifted negative peak in Figure 3d. The rest of the second hydration shell is found in theWWHB-up sub-ensemble, underneath the PtCl. These waters feel the negative charge fromPtClabove and adopt an H-up orientation, hydrogen bonding to the water molecules in the firsthydration shell, and should have a peak in the WWHB region.However, WWHB-down waters -which are outside the 2nd solvation shell of PtCle but within the solvation shell of Nt, and adoptan H-down orientation due to the positive charge from DPTAP N+-create an equal but oppositesignal in WWHB region. Therefore, these oppositely oriented sub-ensembles cancel and noWWHB signal is observed in the VSFG spectrum. This is in stark contrast to other systems, suchas I, in which the WWHB peak disappears because the strongly adsorbed ions destroy theorientational ordering of water molecules.+ Detailed Investigation of AIWHB Peak. The interpretation above depends on the assumptionthat the two peaks in the AIWHB band in Figure 3 actually originate from waters in two differentenvironments, and not from a splitting of a single peak due to vibrational coupling, as is observedin the VSFG spectra of the air/water interface.’ We repeated the 5mM PtCl6’ measurement in a50:50 volume % of H2O:D20 to address this issue (Figure 6). The intensity of the AIWHB banddecreased approximately by a factor of two as expected, but the spectral shape did not change,supporting the premise that couplings do not complicate the lineshape of the AIWHB band. In Figure 6, the AIWHB band is also observed to appear in the absence of 500 mM LiCl, providingfurther evidence that it is a result of PtCl6adsorption. Figure 6. SSP VSFG spectra of DPTAP with a surface pressure of ~10 mN/m on three differentsubphases: 5 mM H2PtCl6/500 mM LiCl in H2O (blue), 5 mM H2PtC16/500 mM LiCl in 50:50H2O:D2O (black), and 5 mM H2PtCl6 in H2O. The intensity is scaled to match the 5 mMH2PtCl6/500 mM LiCl in H2O spectrum to show that the peak shape remains the same. Further details about the nature ofthe AIWHB band can be obtained from polarization dependentexperiments. Wang and coworkers developed a framework to deduce the type of vibrational modeand molecular orientation angle from polarization dependent VSFG spectra.58-59 Interfacial waterOH stretches have C2v or Cov symmetry depending on whether their hydrogen atoms are in asymmetric or asymmetric environment, respectively.58-59 Regardless of the molecular orientationangle, if water adopts Coov symmetry, the SSP spectra will be more intense than the SPS spectra,and if water adopts C2v symmetry, the asymmetric stretch will be much stronger in the SPS spectra,and the symmetric stretch will be much stronger in the SSP spectra. Therefore, the SPS/SSPintensity ratios of the AIWHB band suggest that it predominantly arises from the asymmetricstretch of water with C2v symmetry. The asymmetric water stretch has previously been observed in Langmuir monolayer systems, and was attributed to water molecules interacting with the lipidtails 5l, although this is not the first observation of the asymmetric water stretch, the fact that it isobserved suggests that the adsorbed ions create a unique water environment, because the WWHBstretch at the air/water interface is dominated by the symmetric stretch. Here, we presented a detailed investigation of AIWHB peak with all the tools available to us atthe moment.Nevertheless, further investigations of AIWHB peak with HD-VSFG will beimportant to elucidate all aspects of its orientational structure, since the phase information is lostin the presented results. It is also important to expand these studies to other heavy anioniccomplexes, such as PdCl, to see how AIWHB peak depends on the detailed structure of thecomplex. CONCLUSIONS The results presented here provide evidence of an unexpected orientational structure ofinterfacial water due to adsorbed PtCl6ions with important implications to both the role of theinterfacial water molecules in amphiphile-ion interactions, and their detection through vibrationalspectroscopy. Hofmeister trends are often explained using hydration energies - ions that easilyshed their hydration shell adsorb at interfaces much more easily than strongly hydrated ions.14,42-45 However, PtClanions both adsorb tightly to the DPTAP interface and are strongly hydrated.2The present work demonstrates that PtCl6retains part of its hydration shell after adsorption andcan keep the second hydration shell oriented (WWHB-Up), a behavior that is showing that largermultivalent anions behave significantly differently than their lighter Hofmeister anions. This system also leads to an interesting interfacial water structure with a complex profile ofpositively and negatively charged patches (Figure 5). Averaging these structures within theexcitation volume, as is done in VSFG measurements, may lead to the loss of information. The most common reason for the absence of VSFG signal is a lack of interfacial orientational order.However, these results highlight the fact that the absence of VSFG signal can also indicate thepresence of oppositely ordered sub-ensembles. Therefore, integrating MD simulation and sub-ensemble analysis with VSFG experiments is an important approach for interpretation. This newly observed interfacial water structure may play a crucial role in chemical processes(i.e. LLE) involving heavy metal anions, which usually rely on a few kBT of free energy differencebetween different phases for interfacial ion transfer.60 Interfacial water structures and hydrationeffects may create energetic barriers comparable to such small free energy differences. Forinstance, it has recently been shown that the competitive adsorption of PtCle ions on aminefunctionalized silicon surfaces6l and at DPTAP monolayers2do not follow the predictions of meanfield theories, and the differences are caused by hydration effects and ion-ion correlations. In abroader perspective, these experiments show that the generalizations that are based on lighter andsimpler ions cannot be easily extended to predict the behavior of heavier and more complex ionsat interfaces, and specific experiments, combined with appropriate computational tools, arenecessary to address them. ASSOCIATED CONTENT The supporting information is available free of charge on the ACS Publications website. Details of the VSFG Data global fit and determining the interfacial hydrogen bondingenvironment; wider range VSFG spectrum of D2O dilution studies. AUTHOR INFORMATION Corresponding Author *E-mail: ahmet@anl.gov. Web: www.ahmet-uysal.com. Phone: +1-630-252-9133 Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS We thank Lynda Soderholm for her valuable comments, and Richard Wilson for FTIRmeasurements. This work is supported by the U.S. Department of Energy, Office of Basic EnergyScience,Division of Chemical Sciences, Geosciences, and Biosciences, under contract DE-AC02-06CH11357. T.Z. and A.E.C. are supported by the same program under contract DE-SC0001815.The MD simulations were done at the computing resources provided on Blues, a high-performancecomputing cluster operated by the Laboratory Computing Resource Center at Argonne NationalLaboratory. REFERENCES 1. Sholl, D. S.; Lively, R. P. Seven chemical separations to change the world. Nature 2016,532 (7600), 435-437. 2. Rica,R. A.;Ziano, R.; Salerno, D.; Mantegazza, F.; Brogioli, D. Thermodynamic relationbetween voltage-concentration dependence and salt adsorption in electrochemical cells. Phys.Rev. Lett. 2012,109(15),156103. 3. Lo Nostro, P.; Ninham, B. W. Hofmeister phenomena: an update on ion specificity inbiology. Chem. Rev. 2012,112(4),2286-322. 4. Sung,W.; Avazbaeva, Z.; Kim, D. Salt promotes protonation of amine groups atair/water interface. J. Phys. Chem. Lett.2017,8(15),3601-3606. 5. Casillas-Ituarte, N. N.; Callahan, K. M.; Tang, C. Y.; Chen, X.; Roeselova,M.; Tobias,D. J.; Allen, H. C. Surface organization of aqueous MgCl2 and application to atmosphericmarine aerosol chemistry. Proc. Natl. Acad. Sci. USA 2010,107(15), 6616-21. 6. Hua, W.; Verreault, D.;Allen, H. C. Surface prevalence of perchlorate anions at theair/aqueous interface. J. Phys. Chem. Lett.2013,4(24), 4231-4236. ( 7. H ua, W.; Jubb, A . M.; Allen, H. C. Electric field rever s al of Na2SO4,(NH4)2SO4, andNa2CO3 relative to CaCl2 and NaCl at the air/aqueous interface revealed by heterodyne detectedphase-sensitive sum frequency. J. Phys. C hem. Lett. 2011,2(20),2515-2520. ) ( 8. Fenter, P . ; Lee, S. S. Hydration layer structure at solid-water interfaces. Mrs. Bull . 2014,39(12),1056-1061. ) ( 9. DeWalt-Kerian, E . L . ; Kim, S.; Azam, M. S.;Zeng,H.; Liu, Q.; Gibbs, J. M . pH-dependent inversion of Hofmeister trends in the water structure of the electrical d ouble layer. J.Phys. Chem. Lett.2017,8(13),2855-2861. ) ( 10. M azzini, V.; Craig, V. S. J. What is the fundamental i on-specific series for anions andcations? I on specificity in standard partial molar volumes of electrolytes and electrostriction inwater and non-aqueous solvents. Chemical Science 2017,8(10),7052-7065. ) ( 11. Wang, W . J.; Park, R. Y.; T r avesset, A.; Vaknin, D. Ion-specific induced charges ataqueous soft interfaces. Phys. Rev. Lett. 2011, 106 (5),056102. ) ( 12. Marcus, Y. Effect of ions on t h e structure of water: s tructure m aking and breaking. Chem.Rev.2009, 109 (3), 1 346-70. ) ( 13. McCaffrey, D. L. ; Nguyen, S. C.; Cox, S. J.; Weller, H.; Alivisatos, A. P.; Geissler, P. L.;Saykally, R. J. Mechanism of ion adsorption to aqueous i n terfaces: Graphene/water vs. air/water.Proc. Natl. Acad. Sci. USA 2017,114(51),13369-13373. ) ( 14. Okur, H. I.; Hladilkova,J.; R embert, K. B.;Cho, Y.; H e yda, J.; Dzubiella, J.; Cremer, P .S.; Jungwirth, P . Beyond t h e H o fmeister series: Ion-specific effects on proteins and theirbiological functions. J. Phys. Chem. B2017,121(9) , 1997-2014. ) 15. Nihonyanagi, S.; Yamaguchi, S.; Tahara, T. Counterion effect on interfacial water atcharged interfaces and its relevance to the Hofmeister series. J. Am.Chem. Soc. 2014, 136 (17),6155-8. 16. Johnson, C. M.; Baldelli, S. Vibrational sum frequency spectroscopy studies of theinfluence of solutes and phospholipids at vapor/water interfaces relevant to biological andenvironmental systems. Chem. Rev. 2014, 114 (17),8416-46. Jungwirth,P.; Cremer, P. S. Beyond Hofmeister. Nat. Chem. 2014, 6(4), 261-3.Benjamin, I. Reaction dynamics at liquid interfaces. Annu. Rev. Phys.Chem. 2015,66,165-88. 19. Salis, A.; Ninham, B. W. Models and mechanisms of Hofmeister effects in electrolytesolutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43 (21), 7358-77.20. Levin, Y.; dos Santos, A. P.; Diehl, A. Ions at the air-water interface: An end to ahundred-year-old mystery? Phys.Rev. Lett.2009,103(25),257802. 21. Gurau, M. C.; Lim, S.M.; Castellana,E. T.; Albertorio, F.; Kataoka, S.; Cremer, P. S. Onthe mechanism of the hofmeister effect.J. Am. Chem. Soc. 2004, 126(34),10522-3. 22. Darlington, A. M.; Jarisz, T. A.;DeWalt-Kerian, E. L.; Roy, S.; Kim, S.; Azam, M. S.;Hore,D. K.; Gibbs, J. M. Separating the pH-dependent behavior of water in the Stern and diffuselayers with varying salt concentration. J. Phys. Chem. C 2017, 121(37),20229-20241. 23. Leontidis, E. Investigations of the Hofmeister series and other specific ion effects usinglipid model systems. Adv. Colloid. Interface. Sci. 2017,243,8-22. 24. Doidge, E. D.; Carson, I.; Tasker, P. A.; Ellis, R. J.; Morrison, C. A.; Love, J. B. ASimple primary amide for the selective recovery of gold from secondary resources. Angew.Chem. Int. Ed. 2016, 55 (40), 12436-9. 25. Tasker,P. A.; Plieger, P. G.; West, L. C. Metal complexes for hydrometallurgy andextraction, Compr. Coord. Chem. II. 2003,759-808. 26. Fowler,C. J.; Haverlock, T. J.; Moyer, B. A.; Shriver, J. A.; Gross, D. E.; Marquez, M.;Sessler, J. L.; Hossain, M. A.; Bowman-James, K. Enhanced anion exchange for selective sulfateextraction: Overcoming the Hofmeister bias. J. Am. Chem. Soc.2008, 130 (44), 14386-14387. ( 27. U ysal, A.; R ock, W.; Qiao, B.; Bu, W.; Lin, B. T wo-step adsorption of PtCl6complexesat a charged Langmuir monolayer: Ro l e of hydration an d ion co r relations. J. Phys. Chem. C2017,121(45),25377-25383. ) 28. Wen, Y. C.; Zha, S.; Liu, X.; Yang, S.; Guo, P.; Shi, G.; Fang, H.; Shen, Y. R.; Tian, C..srUnveiling microscopic structures of charged water interfaces by surface-specific vibrationalspectroscopy. Phys. Rev. Lett.2016,116(1),016101. 29. Ma, G.; Chen, X.; Allen, H. C. Dangling OD confined in a Langmuir monolayer. J. Am.Chem. Soc. 2007,129(45),14053-7. 30. Du, Q.; Superfine, R.; Freysz, E.; Shen, Y. R. Vibrational spectroscopy of water at thevapor/water interface. Phys. Rev. Lett. 1993,70(15),2313-2316. 31. Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms forhighly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory. Comput.2008,4(3),435-47. ( 32. Best,R. B.; Zhu, X.; Shim, J.; Lopes, P. E.; Mittal, J.; Feig, M.; Mackerell, A. D., Jr.Optimization of the additive CHARMM all-atom protein force field tar g eting improved samplingof the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. T h eory.Comput. 2012,8(9),3257-3273. ) 33. Bjelkmar, P.; Larsson, P.; Cuendet, M. A.; Hess, B.; Lindahl, E. Implementation of theCHARMM Force field in GROMACS: Analysis of protein stability effects from correctionmaps, virtual interaction sites, and water models. J. Chem. Theory. Comput. 2010,6(2), 459-66.34. Lienke, A.; Klatt, G.; Robinson,D. J.; Koch, K. R.; Naidoo, K. J. Modeling platinumgroup metal complexes in aqueous solution. Inorg. Chem.2001,40(10),2352-7. ( 35. Guardado-Calvo, P.; Atkovska, K.; Jeffers,S. A.; Grau, N.; Backovic, M.; Perez-Vargas,J.; de Boer, S.M.; Tortorici,M. A.; Pehau-Arnaudet, G.; Lepault,J.; England, P.; Rot t ier,P. J.;Bosch, B. J.; Hub,J. S.;R e y, F. A . A glycerophospholipid-specific pocket in the RVFV class IIfusion protein drives target membrane insertion. Science 2017,358 (6363),663-667. ) 36. Cai,J.;Townsend, J. P.; Dodson,T. C.; Heiney,P. A.;Sweeney, A. M. Eye patches:Protein assembly of index-gradient squid lenses. Science 2017, 357 (6351),564-569. 37. Nagano, T.; Lubling, Y.; Varnai, C.;Dudley, C.; Leung, W.; Baran, Y.; MendelsonCohen, N.; Wingett, S.; Fraser, P.; Tanay, A. Cell-cycle dynamics of chromosomal organizationat single-cell resolution. Nature 2017, 547, 61. 38, Chen, S.;Itoh, Y.; Masuda, T.; Shimizu, S.; Zhao, J.; Ma, J.; Nakamura,S.; Okuro, K.;Noguchi, H.; Uosaki, K.; Aida, T. Subnanoscale hydrophobic modulation of salt bridges inaqueous media. Science 2015,348(6234),555-559. 39. Gonzalez-Rubio, G.; Diaz-Nunez, P.; Rivera, A.; Prada, A.; Tardajos, G.; Gonzalez-Izquierdo, J.; Banares, L.; Llombart, P.; Macdowell, L.G.; Alcolea Palafox,M.; Liz-Marzán, L.M.; Pena-Rodriguez, O.; Guerrero-Martinez, A. Femtosecond laser reshaping yields goldnanorods with ultranarrow surface plasmon resonances. Science 2017, 358 (6363), 640-644. 40. Sovago,M.; Vartiainen, E.; Bonn, M. Observation of buried water molecules inphospholipid membranes by surface sum-frequency generation spectroscopy. J. Chem. Phys.2009,131(16),161107. 41. Chen,X.; Yang, T.; Kataoka, S.; Cremer,P. S. Specific ion effects on interfacial waterstructure near macromolecules. J. Am. Chem. Soc. 2007,129(40),12272-9. 42. Chen, X.; Flores, S. C.; Lim, S. M.; Zhang, Y.; Yang, T.; Kherb, J.; Cremer, P. S.Specific anion effects on water structure adjacent to protein monolayers. Langmuir 2010, 26(21),16447-54. 43. Flores, S. C.; Kherb, J.; Cremer, P. S. Direct and reverse Hofmeister effects on interfacialwater structure. J. Phys. Chem. C 2012,116(27),14408-14413. 44. Flores, S. C.; Kherb, J.; Konelick, N.; Chen, X.; Cremer,P. S. The effects of Hofmeistercations at negatively charged hydrophilic surfaces. J. Phys. Chem. C 2012,116(9),5730-5734.45. Sung, W.;Wang, W.; Lee,J.; Vaknin, D.; Kim, D. Specificity and variation of lengthscale over which monovalent halide ions neutralize a charged interface. J. Phys. Chem. C 2015,119(13),7130-7137. 46. Raymond, E. A.; Tarbuck, T. L.; Brown, M. G.; Richmond, G. L. Hydrogen-bondinginteractions at the vapor/water interface investigated by vibrational sum-frequency spectroscopyofHOD/H2O/D2O mixtures and molecular dynamics simulations. J. Phys. Chem. B 2003, 107(2),546-556. 47. Nihonyanagi, S.; Ishiyama, T.; Lee, T.K.; Yamaguchi, S.; Bonn, M.; Morita,A.; Tahara,T. Unified molecular view of the air/water interface based on experimental and theoretical chi(2)spectra of an isotopically diluted water surface. J. Am. Chem. Soc. 2011,133 (42),16875-80. 48. Bonn,M.; Nagata, Y.; Backus, E. H. G. Molecular structure and dynamics of water at thewater-air interface studied with surface-specific vibrational spectroscopy. Angew. Chem. Int. Ed.2015,54(19),5560-5576. 49. Scatena, L. F.; Brown, M. G.; Richmond, G. L. Water at hydrophobic surfaces: weakhydrogen bonding and strong orientation effects. Science 2001,292(5518),908-12. 50. Mondal,J. A.; Nihonyanagi,S.; Yamaguchi, S.; Tahara, T. Three distinct water structuresat a zwitterionic lipid/water interface revealed by heterodyne-detected vibrational sum frequencygeneration. J. Am. Chem. Soc.2012, 134(18),7842-7850. 51. Tyrode, E.; Johnson, C. M.; Kumpulainen, A.; Rutland, M. W.;Claesson, P. M.Hydration state of nonionic surfactant monolayers at the liquid/vapor interface: Structuredetermination by vibrational sum frequency spectroscopy.J. Am. Chem. Soc. 2005, 127 (48),16848-16859. ( 52. Livingstone, R . A.; Nagata, Y.; Bonn, M.; Backus, E. H. G. Two types of water at thewater-surfactant interface revealed by time-resolved vibrational spectroscopy. J. Am. Chem . Soc.2015, 1 37(47),14912-14919. ) 53. Kropman, M. F.; Bakker, H. J. Dynamics of water molecules in aqueous solvation shells.Science 2001,291(5511),2118-20. 54. Ishihara, T.; Ishiyama, T.; Morita, A. Surface structure of methanol/water solutions viasum frequency orientational analysis and molecular dynamics simulation. J. Phys. Chem. C2015,119(18),9879-9889. 55. Ishiyama,T.; Terada, D.;Morita, A. Hydrogen-bonding structure at zwitterioniclipid/water interface.J. Phys. Chem. Lett. 2016,7(2),216-20. 56. Nagata, Y.; Mukamel, S. Vibrational sum-frequency generation spectroscopy at thewater/lipid interface: Molecular dynamics simulation study. J. Am. Chem. Soc.2010, 132 (18),6434-6442. 57. Sovago,M.; Campen, R. K.; Wurpel, G. W. H.; Miiller, M.; Bakker, H. J.; Bonn, M.Vibrational response ofhydrogen-bonded interfacial water is dominated by intramolecularcoupling. Phys. Rev. Lett. 2008, 100 (17),173901. 58. Gan, W.; Wu, D.; Zhang, Z.; Feng, R. R.; Wang, H. F. Polarization and experimentalconfiguration analyses of sum frequency generation vibrational spectra, structure, andorientational motion of the air/water interface. J. Chem. Phys. 2006,124(11),114705. 59. Wang, H.-F.; Gan, W.; Lu, R.;Rao, Y.; Wu, B.-H. Quantitative spectral and orientationalanalysis in surface sum frequency generation vibrational spectroscopy (SFG-VS). Int. Rev. Phys.Chem. 2005, 24(2),191-256. 60. Knight, A. W.; Qiao, B.; Chiarizia, R.; Ferru, G.; Forbes, T.; Ellis, R. J.; Soderholm, L.Subtle effects of aliphatic alcohol structure on water extraction and solute aggregation inbiphasic water/n-dodecane. Langmuir 2017, 33 (15), 3776-3786. 61. Rock, W.; Oruc, M. E.; Ellis, R. J.; Uysal, A. Molecular scale description of anioncompetition on amine-functionalized surfaces. Langmuir 2016, 32 (44),11532-11539. TOC GRAPHICS Ion hydration and interfacial water play crucial roles in numerous phenomena ranging from biological to industrial systems. Although biologically relevant (and mostly smaller) ions have been studied extensively in this context, very little experimental data exist about molecular scale behavior of heavy ions and their complexes at interfaces, especially under technologically significant conditions. It has recently been shown that PtCl62- complexes adsorb at positively charged interfaces in a two-step process that cannot fit into well-known empirical trends, such as Hofmeister series. Here, a combined vibrational sum frequency generation and molecular dynamics study reveals that a unique interfacial water structure is connected to this peculiar adsorption behavior. A novel sub-ensemble analysis of MD simulation results show that after adsorption, PtCl62- complexes partially retain their first and second hydration spheres, and it is possible to identify three different types of water molecules around them based on their orientational structures and hydrogen bonding strengths. These results have important implications for relating interfacial water structure and hydration enthalpy to the general understanding of specific ion effects. This in turn influences interpretation of heavy metal ion distribution across and reactivity within, liquid interfaces.
确定






















还剩25页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《水界面中和频光谱检测方案(其它光谱仪)》,该方案主要用于其他中可靠性能检测,参考标准--,《水界面中和频光谱检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、Ekspla PL2230型高能量皮秒激光器
推荐专场
该厂商其他方案
更多
















