方案详情
文
生物界中绝大多数反应发生在由磷脂双分子层构成的细胞膜,或者细胞内部。细胞膜会影响到蛋白质的折叠,并且会创造出适合细胞反应生存的微环境。为了进一步了解和模拟真实的细胞膜结构,使用KSV NIMA LB膜分析仪学习细胞生物分子的相互作用。
方案详情

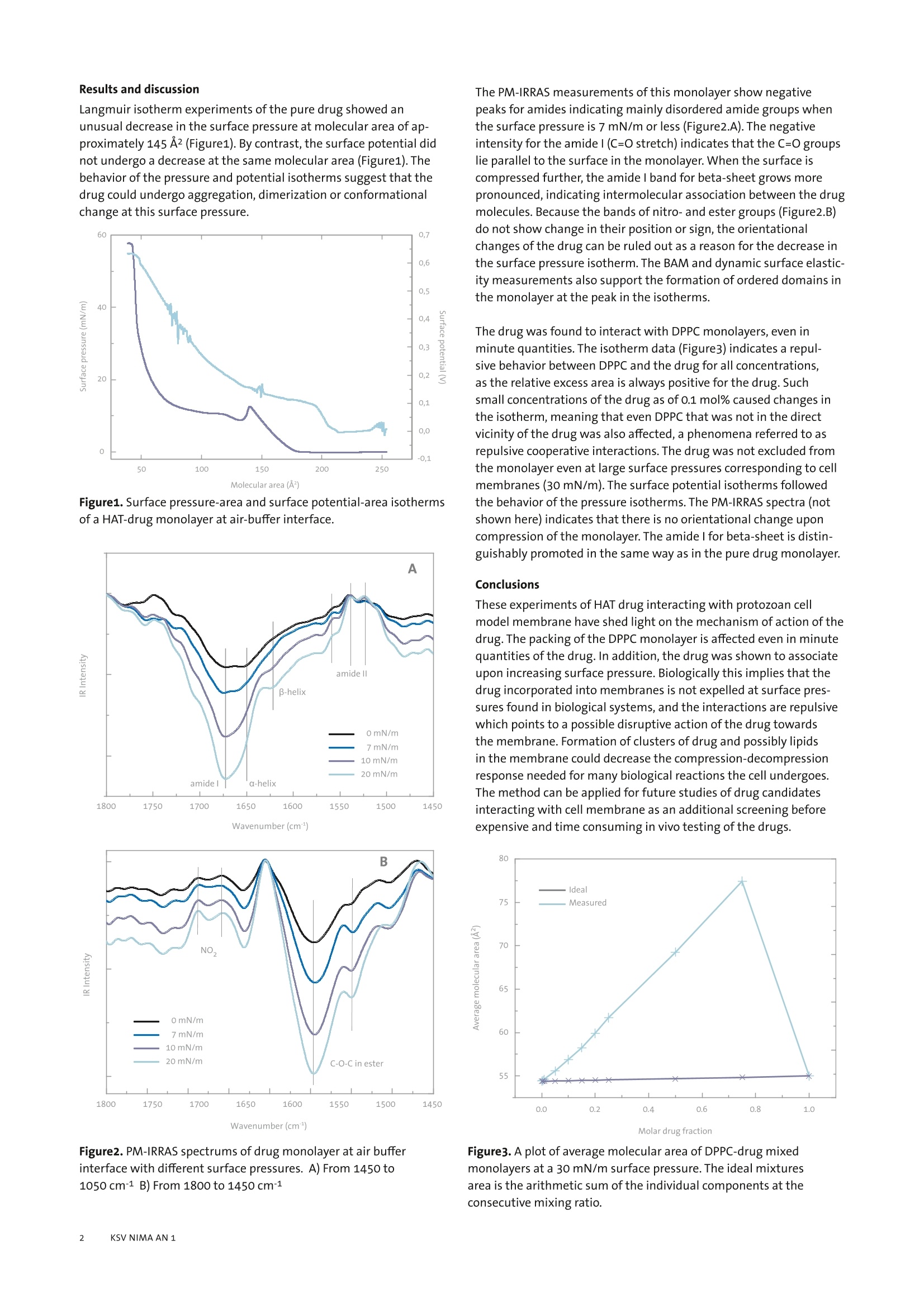
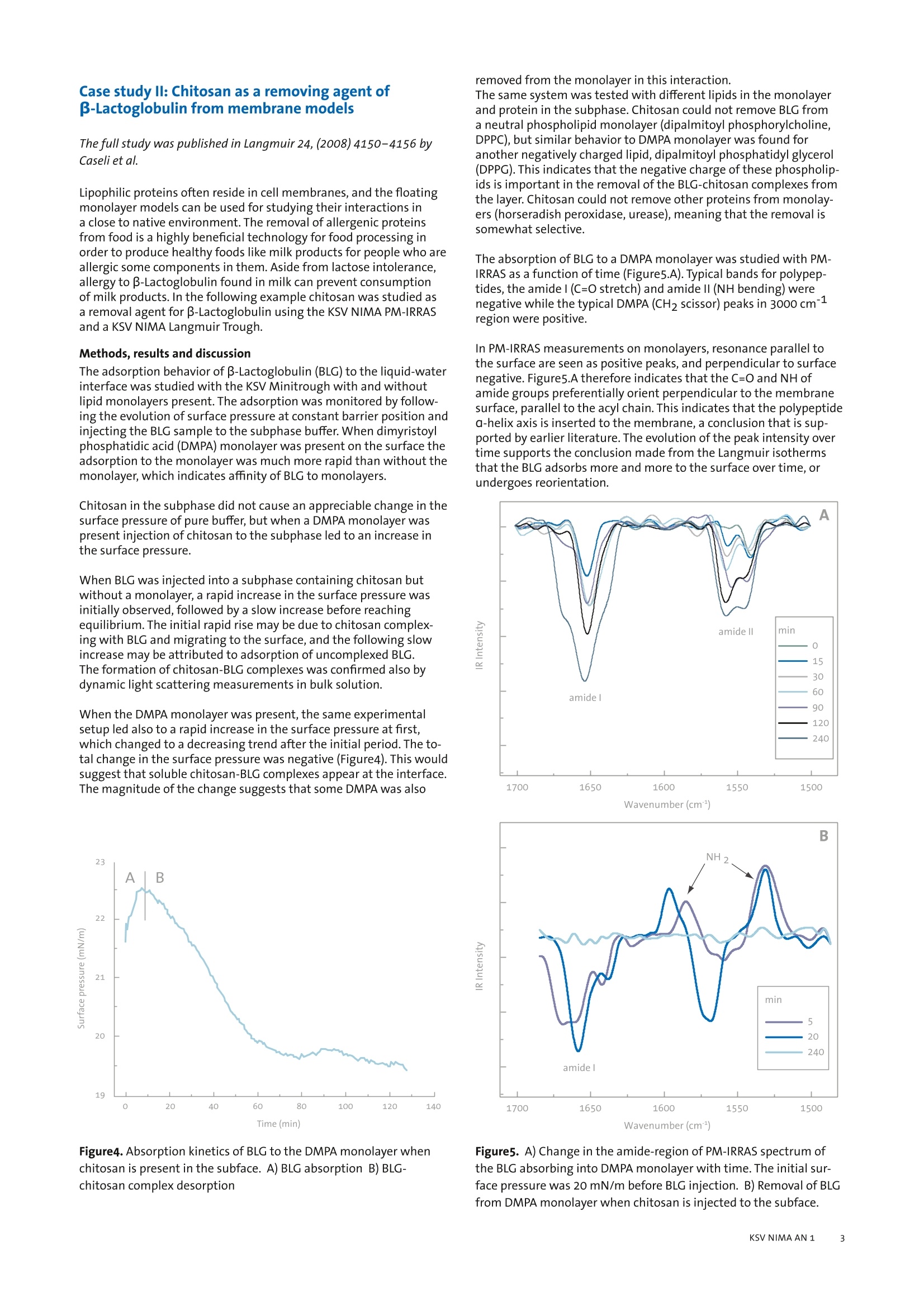
KSV NIMA APPLICATION NOTE 1 Interactions of biomolecules in cellmembrane models This application note illustrates how the analytical instruments fromKSV NIMA can be used to create cell membrane models and studvbiomolecularinteractions in these models. Introduction Most biochemical reactions in nature take place at membranescomposed of phospholipid bilayers on or inside cells. The membraneaffects protein folding and creates specific microenvironmentswhere the reactions take place. In order to understand and mimicreal biological systems, it is essential that interactions are studied inan environment as close as possible to nature. Langmuir monolayers of membrane phospholipids have been veri-fied as excellent model systems for biological membranes [1]. Themain alternative method is the supported lipid bilayer (SLB), wherea bilayer is created on a solid support by the Langmuir-Blodgett,Langmuir-Schaefer or self assemblytechnique. Disadvantageswith these techniques include unwanted interactions of lipids andbiomolecules with the support, where the diffusion of moleculesis hindered. In the worst case, this can lead to denaturation of thebiomolecules in question [2]. By contrast, no such interactions occurfor floating monolayers. These model systems are employed in several application areas, suchas drug development, food technology,and biological and biochemi-cal research. We present two case studies to show how modelmembranes are utilized. The first case is about the development ofantiparasitic drug and the second is about research into methodsfor removal of lipophilic allergenic compounds from food.Other ap-plication areas that could be studied with model membranes includeinteractions of drugs in cell membranes with proteins, nucleic acids,polysaccharides, and the membrane itself. This method can also beused to study migration of drugs or targeted drug carriers into mem-branes, native synthesis of biomolecules and drugs in membranes,and biosensor applications with naturally occurring proteins. Case study l: The interaction of an antiparasitic pep-tide with cell membrane models The full study was published in Colloids and Surfaces B: Biointerfaces74 (2009) 504-510 by Pascholati etal. The interactions of molecules in membranes are important for manyapplications outside basic research in biochemistry.In drug discoverythe permeation of the drug into cells through cell walls and the reac-tion of the drug within the cell membraneare important factors fordrug efficiency. The KSV NIMA PM-IRRAS can be used for screeningdrug candidates in vitro before embarking on expensive in vivo tests.The following example is a drug candidate for the Human AfricanSleeping Sickness (HAT - African Trypanosomiasis) caused by proto-zoan parasite and studied in a model cell membrane. Methods The interactions of an oligopeptide-based drug that has shownactivity in the treatment of HAT, S-(2,4-dinitrophenyl)glutath-ione di-2-propyl ester, was studied in Langmuir monolayer modelmembrane of DPPC (dipalmitoyl phosphorylcholine), an abundantlipid in the protozoan membrane. The monolayer properties of thedrug itself and its interactions with DPPC monolayers were studiedwith Langmuir equipment (KSV NIMA Langmuir Trough) with asurface potential meter (KSV NIMA SPOT) and polarization modula-tion surface infrared reflection absorption spectrometer (KSV NIMAPM-IRRAS). Additionally Brewster angle microscopy (KSV NIMA BAM)and dynamic surface elasticity experiments were performed on thesystem. Langmuir isotherm experiments of the pure drug showed anunusual decrease in the surface pressure at molecular area of ap-proximately 145 A2(Figure1). By contrast, the surface potential didnot undergo a decrease at the same molecular area (Figure1). Thebehavior of the pressure and potential isotherms suggest that thedrug could undergo aggregation, dimerization or conformationalchange at this surface pressure. Figure1. Surface pressure-area and surface potential-area isothermsof a HAT-drug monolayer at air-buffer interface. 1650Wavenumber (cm) Figure2.PM-IRRAS spectrums of drug monolayer at air bufferinterface with different surface pressures. A) From 1450 to1050cm-1 B) From 1800 to 1450 cm-1 The PM-IRRAS measurements of this monolayer show negativepeaks for amides indicating mainly disordered amide groups whenthe surface pressure is 7 mN/m or less (Figure2.A). The negativeintensity for the amidel (C=O stretch) indicates that the C=0 groupslie parallel to the surface in the monolayer. When the surface iscompressed further, the amidel band for beta-sheet grows morepronounced, indicating intermolecular association between the drugmolecules. Because the bands of nitro-and ester groups (Figure2.B)do not show change in their position or sign, the orientationalchanges of the drug can be ruled out as a reason for the decrease inthe surface pressure isotherm. The BAM and dynamic surface elastic-ity measurements also support the formation of ordered domains inthe monolayer at the peak in the isotherms. The drug was found to interact with DPPC monolayers, even inminute quantities. The isotherm data (Figure3)indicates a repul-sive behavior between DPPC and the drug for all concentrations,as the relative excess area is always positive for the drug.Suchsmall concentrations of the drug as of 0.1 mol% caused changes inthe isotherm, meaning that even DPPC that was not in the directvicinity ofthe drug was also affected, a phenomena referred to asrepulsive cooperative interactions. The drug was not excluded fromthe monolayer even at large surface pressures corresponding to cellmembranes (30 mN/m). The surface potential isotherms followedthe behavior of the pressure isotherms. The PM-IRRAS spectra (notshown here) indicates that there is no orientational change uponcompression of the monolayer. The amide l for beta-sheet is distin-guishably promoted in the same way as in the pure drug monolayer. Conclusions These experiments of HAT drug interacting with protozoan cellmodel membrane have shed light on the mechanism of action of thedrug. The packing of the DPPC monolayer is affected even in minutequantities of the drug. In addition, the drug was shown to associateupon increasing surface pressure. Biologically this implies that thedrug incorporated into membranes is not expelled at surface pres-sures found in biological systems, and the interactions are repulsivewhich points to a possible disruptive action of the drug towardsthe membrane.Formation of clusters of drug and possibly lipidsin the membrane could decrease the compression-decompressionresponse needed for many biological reactions the cell undergoes.The method can be applied for future studies of drug candidatesinteracting with cell membrane as an additional screening beforeexpensive and time consuming in vivo testing of the drugs. Molar drug fraction Figure3. A plot of average molecular area of DPPC-drug mixedmonolayers at a 30mN/m surface pressure. The ideal mixturesarea is the arithmetic sum of the individual components at theconsecutive mixing ratio. Case study ll: Chitosan as a removing agent ofB-Lactoglobulin from membrane models The full study was published in Langmuir 24, (2008)4150-4156 byCaseli et al. Lipophilic proteins often reside in cell membranes, and the floatingmonolayer models can be used for studying their interactions ina close to native environment. The removal of allergenic proteinsfrom food is a highly beneficial technology for food processing inorder to produce healthy foods like milk products for people who areallergic some components in them. Aside from lactose intolerance,allergy to B-Lactoglobulin found in milk can prevent consumptionof milk products. In the following example chitosan was studied asa removal agent for B-Lactoglobulin using the KSV NIMA PM-IRRASand a KSV NIMA Langmuir Trough. Methods, results and discussion The adsorption behavior of B-Lactoglobulin (BLG) to the liquid-waterinterface was studied with the KSV Minitrough with and withoutlipid monolayers present. The adsorption was monitored by follow-ing the evolution of surface pressure at constant barrier position andinjecting the BLG sample to the subphase buffer. When dimyristoylphosphatidic acid (DMPA) monolayer was present on the surface theadsorption to the monolayer was much more rapid than without themonolayer, which indicates affinity of BLG to monolayers. Chitosan in the subphase did not cause an appreciable change in thesurface pressure of pure buffer, but when a DMPA monolayer waspresent injection of chitosan to the subphase led to an increase inthe surface pressure. When BLG was injected into a subphase containing chitosan butwithout a monolayer, a rapid increase in the surface pressure wasinitially observed, followed by a slow increase before reachingequilibrium. The initial rapid rise may be due to chitosan complex-ing with BLG and migrating to the surface, and the following slowincrease may be attributed to adsorption of uncomplexed BLG.The formation of chitosan-BLG complexes was confirmed also bydynamic light scattering measurements in bulk solution. When the DMPA monolayer was present, the same experimentalsetup led also to a rapid increase in the surface pressure at first,which changed to a decreasing trend after the initial period. The to-tal change in the surface pressure was negative (Figure4). This wouldsuggest that soluble chitosan-BLG complexes appear at the interface.The magnitude of the change suggests that some DMPA was also Figure4.Absorption kinetics of BLG to the DMPA monolayer whenchitosan is present in the subface. A) BLG absorption B) BLG-chitosan complex desorption removed from the monolayer in this interaction. The same system was tested with different lipids in the monolayerand protein in the subphase. Chitosan could not remove BLG froma neutral phospholipid monolayer (dipalmitoyl phosphorylcholine,DPPC), but similar behavior to DMPA monolayer was found foranother negatively charged lipid, dipalmitoyl phosphatidyl glycerol(DPPG). This indicates that the negative charge of these phospholip-ids is important in the removal of the BLG-chitosan complexes fromthe layer. Chitosan could not remove other proteins from monolay-ers (horseradish peroxidase, urease), meaning that the removal issomewhat selective. The absorption of BLG to a DMPA monolayer was studied with PM-IRRAS as a function of time (Figure5.A). Typical bands for polypep-tides, the amidel (C=O stretch) and amide II (NH bending) werenegative while the typical DMPA (CH2 scissor) peaks in 3000 cm-region were positive. In PM-IRRAS measurements on monolayers,resonance parallel tothe surface are seen as positive peaks, and perpendicular to surfacenegative. Figure5.A therefore indicates that the C=0 and NH ofamide groups preferentially orient perpendicular to the membranesurface, parallel to the acyl chain. This indicates that the polypeptidea-helix axis is inserted to the membrane, a conclusion that is sup-ported by earlier literature. The evolution of the peak intensityovertime supports the conclusion made from the Langmuir isothermsthat the BLG adsorbs more and more to the surface over time, orundergoes reorientation. cc一一 Wavenumber (cm1) c Figure5. A) Change in the amide-region of PM-IRRAS spectrum ofthe BLG absorbing into DMPA monolayer with time. The initial sur-face pressure was 20 mN/m before BLG injection. B) Removal of BLGfrom DMPA monolayer when chitosan is injected to the subface. Figure5.B shows the evolution of the PM-IRRAS signal in the amide-region after chitosan was introduced to the subphase of DMPA-BLGmonolayer. After 20 minutes both amidel and Il adsorptions hadshifted, indicating a change in the BLG conformation. After 240minutes from the chitosan addition the amide adsorptions hadcompletely disappeared, meaning that the chitosan had removedthe BLG from the membrane completely. Other experiments on the system such as rheological measurementsand fluorescence from deposited LB films support the findingsabove. Conclusions The Langmuir isotherm studies indicate that both chitosan andBLG adsorb to the DMPA monolayer. Chitosan is able to remove BLGfrom the monolayer,and even some of the DMPA is removed at thesame time. From the tested phospholipid monolayers, chitosan wasable to remove BLG only from those made with anionic charge. ThePM-IRRAS measurements made it possible to see the complexingbehavior between BLG and chitosan in the monolayer prior to theremoval of the complex, and is, to our knowledge, the first molecularlevel evidence for the protein-removal mechanism of chitosan. Summary Model membranes provide means to study molecular interactionsin an environment closely resembling natural cell membranes.Floating monolayers are thought to be the best model for real cellmembranes in terms of molecular packing, mobility and dynamics.The two case studies highlight well the key research areas wherefloating model membranes can be used. Applications of particularinterest include design and development of new peptide drugs anddrug carriers that interact with cell membranes where the Langmuirfilm method can be used as a screen to find effective derivatives ofmolecules before going to in vivo tests. [1] Brockman, H., Curr. Opin. Biol., 1999, 9,438-443 [2]Castellana, E.T., and Cremer, P. S., Solid supported lipid bilayers:From biophysical studies to sensor design, Surface Science Reports,2006 ,61, 429-444 KSV NIMA KSV NIMA - at the creativeinterface of people andtechnology We create value for ourcustomers by providingadvanced,innovative instru-ments for thin film fabrica-tion and characterisation.by constantly exchangingknowledge with our custom-ers and through buildingopen,trusting relationshipswith customers and partners. Availability KSV NIMA products and services areprovided to customers all over the worldthrough Biolin Scientific in co-operationwith a highly competent network ofDistribution Partners. For a list of relevantcontact details, visit www.ksvnima.com Contactinformation KSV NIMA Biolin ScientificTietajantie 2 FIN-02130 Espoo,Finland Tel +358 9 5497 3300 Fax +358 9 5497 3333 info@ksvnima.comwww.ksvnima.com KSV NIMA AN
确定

还剩2页未读,是否继续阅读?
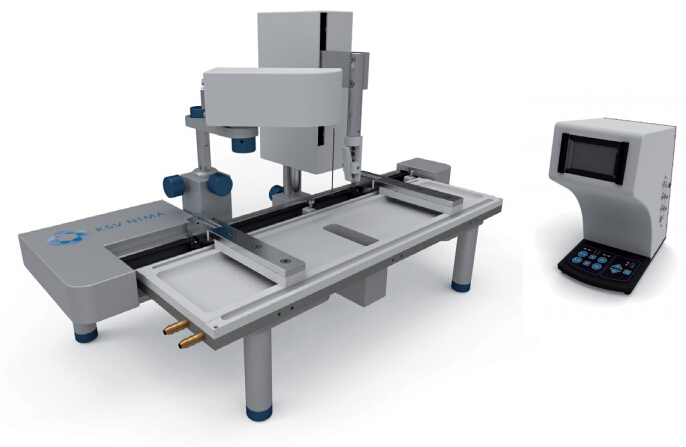
瑞典百欧林科技有限公司为您提供《使用KSV NIMA LB膜分析仪研究细胞模型内生物分子相互作用》,该方案主要用于其他中检测,参考标准--,《使用KSV NIMA LB膜分析仪研究细胞模型内生物分子相互作用》用到的仪器有KSV NIMA LB膜分析仪、KSV NIMA 缎带型LB膜分析仪、独立式KSV NIMA布鲁斯特角显微镜、KSV NIMA Langmuir膜分析仪
推荐专场
相关方案
更多
该厂商其他方案
更多