方案详情
文
当前科研研究趋势是采用多种仪器技术对一个实验进行综合的检测。对于生物分子来说,结构和功能信息对于我们掌握一个生物分子的性状有着至关重要的作用。本文中作者使用了QCM-D和电化学技术对磷脂双分子层进行了检测。
方案详情

APPLICATION NOTEQS 405-29-1 IMPROVED UNDERSTANDING OF LIPID MEMBRANES THROUGHCOMBINED QCM-D AND EIS MEASUREMENTS A current trend in the field of sensing is to use instruments in which several detection principlescan be combined in one experiment. Methods that provide a combination of structural and functionalinformation on biomolecules can provide crucial insights into the understanding of biomolecularfunctions. INTRODUCTION It is of great interest in many fieldsto investigate how peptide and pro-tein interactions change the prop-erties of a lipid membrane. It iswell known that membrane damageis fatal to cells and bacteria. There-fore, a common mechanism of toxinsis to disrupt the membrane or inter-fere with the transport of ions acrossthe membrane. Typically, antibacte-rial peptides incorporate into bacte-rial membranes and destroy the iongradient between the inside and theoutside of the bacterium. APPROACH ANDEXPERIMENTALSETUP This application note reviews two ex-amples of experiments where QCM-Dand Electrical Impedance Spectros-copy (ElS) were combined. The firstexample deals with the disruption of alipid membrane through the catalyticaction of the enzyme PLA2, foundin insect and snake venom. PLA2cleaves phospholipids into the corre-sponding lysolipids and fatty acids bycatalyzing the hydrolysis of a phos-phoester bond in the lipid molecule.This cleavage disrupts the; mem-brane. The second example demonstratestheeiirnsertion1of trans-membranepores in a model membrane by theaddition of the peptide Gramicidin D.The combination of QCM-D and elec-trochemistry is especially attractivein these examples. The effect on themembrane can be sensitively detect-ed by the electrochemical method,whereas the strength of the QCM-Dmethod lies in its ability to character-ize the membrane's viscoelastic (softor rigid) properties. Dissipation is related toviscoelastic properties 一 0 b Addition Rinseof PLA2* B) During this period theflow was stopped *0.1 mg/mL, in Tris buffer Figure 1 A) QCM-D and ElS results obtained for the formation of a supported lipid mem-brane on the SiO,-coated sensor surface in real-time. Figure adopted after [1] with permis-sion from the authors and Reproduced by permission of The Royal Society of Chemistry.B) The effect of addition of the enzyme PLA2 to the lipid membrane. Courtesy of E. Briand. RESULTS AND DISCUSSION The formation of good quality lipidmembranes was first ascertained byboth QCM-D and EIS. The forma-tion of a lipid bilayer on the QCM-Dsensor was detected in real time byboth frequency and dissipation shiftsand by single frequency impedancespectroscopy, as demonstrated inFigure 1A. Bilayer formation followsthe typical pathway via the adsop-tion of a critical mass of liposomes,which eventually ruptures and formsan extended lipid bilayer on the sur- face with the characteristic QCM-Dresponses Af=-26 Hz and AD<0.5,indicating that a layer a few nanome-ters thick (typically 5 nm) and rigid (asindicated by the low AD) has formedon the sensor surface. Note that bilay-er formation as monitored by QCM-Dis already complete long before theimpedance amplitude stabilizes. Aplausible explanation for this obser-vation is that the fusion and ruptureof adsorbed liposomes is followed bya slower annealing process, result-ing from increased ordering of thelipid molecules in the lipid membrane and improved electrical sealing of themembrane. The first example, involv-ing membrane disruption, is shownin Figure 1B, where the action of theenzyme immediately alters the elec-trical properties of the membrane, asevidenced by the sharp decrease inthe impedance amplitude. By QCM-D, the process is first characterizedby mass uptake, followed by massremoval only after the system hasbeen rinsed with buffer. In the secondexample, impedance spectra wererecorded at different stages of an ex-periment involving a supported lipidmembrane and the pore-forming pep-tide Gramicidin D, as schematicallydepicted in Figure 3. Bode plot fromsuch experiments are shown in Fig-ure 3. As expected, the resistivity ofthe membrane decreased after expo-sure to Gramicidin D. The insertion ofthe peptides is seen in the EIS spec-tra, while no changes are observed in Figure 2. Schematic illustration of an electrochemistry module. Combined QCM-D and electrochemistry can also be performed in chronoamperio-metric mode. Three recent examples include: -Grieshaber, D.,Voros, J.,Zambelli, T., Ball,V., Schaaf, P., Voegel, J-C. and Boulme-dais, F., Swelling and Contraction of Ferrocyanide-Containing Polyelectrolyte Multi-layers upon Application of an Electric Potential, Langmuir,2008,24,13668-13676.-Wickman, B., Gronbeck, H., Hanarp, P. and Kasemo, B., Corrosion Induced Deg-radation of Pt/C Model Electrodes Measured with Electrochemical Quartz CrystalMicrobalance. Journal of The Electrochemical Society, 2010, 157, B592-B598.(Also see Application note 27 from Q-Sense). -Barry D. Fleming, Slavica Praporski, Alan M. Bond, and Lisandra L. Martin*,Elec-trochemical Quartz Crystal Microbalance study of azurin adsorption onto an alkane-thiol self-assembled monolayer on gold. Langmuir, 2008, 24, 323-327. Headquarters:BiolinScientificAB,Q-Sense,Hangpilsgatan 7, 426 77VFrolunda,Sweden,Phone+46317697690,Fax+4631698040,info@q-sense.com North America: BiolinScientific, Inc.,+1 (877)7736730, sales@q-sense.com Scandinavia:Q-Sense,+46 31769 7690,sales@q-sense.com UK/IE: BiolinScientific, +44-7530879037, daniel.slater@biolinscientific.com Germany/CH/AT:LOT-Oriel GmbH,+49 6151/8806-44,qsense@lot-oriel.de France: LOT-Oriel FR, +33 1 6919 4949, tcherbak@lot-oriel.fr Italy: Nordtests.r.l.,+39-0143-62422,mbruni@nordtest.it Benelux: LOT-Oriel Benelux, +32 57 363 954, struyve@lot-oriel.com Japan: MeiwafosisCoLtd,+81353790051,fukuda@meiwanet.co.jpChina:BiolinScientific, +862154661071,vanilla.chen@k-analys.seKorea:HucomSystems Inc., + 82 31 204 5030, hucomsys@korea.comAustralia: ATA Scientific+61 2 95430477 enquiries@atascientific.com.au The combination of QCM-D andelectrical impedance spectroscopyis a powerful tool to investigatestructure-function relationships ofproteins and peptides interactingwith lipid membranes. In particularthe function of ion channels canbe followed while at the same timemonitoring the viscoelastic proper-ties of the lipid membrane. Q-Sense equipment avaliable for simi-lar studies E1 or E4 together with the Electrochemis-try Module and a potentiostat. Sensors were fabricated by the authors. Reference[1] Elisabeth Briand, Michael Zach,Sofia Svedhem, Bengt Kasemo andSarunas Petronis. Combined QCM-Dand ElS study of supported lipid bilayerformation and interaction with pore-forming peptides. Analyst, 2010, 135,343-350. Acknowledgement Dr. Elisabeth Briand is acknowledgedfor valuable input during the prepara-tion of this note.
确定

还剩1页未读,是否继续阅读?
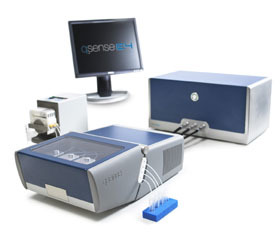
瑞典百欧林科技有限公司为您提供《QCM-D和电化学技术联用检测磷脂双分子层吸附》,该方案主要用于其他中--检测,参考标准--,《QCM-D和电化学技术联用检测磷脂双分子层吸附》用到的仪器有QSense卓越版四通道石英晶体微天平、QSense Explorer扩展版石英晶体微天平、QSense全自动八通道石英晶体微天平
推荐专场
相关方案
更多
该厂商其他方案
更多