方案详情
文
某些多肽拥有集聚成薄且长纤维状,被称为淀粉样结构。这种结构与多种蛋白质折叠错乱相关,并且也是在生物技术方面和药物应用中主要的问题。本文中使用了QCM-D技术原位检测这种淀粉状多肽的的厚度和粘弹性变化,这也为研究蛋白质纤维化提供了一种新的方式。
方案详情

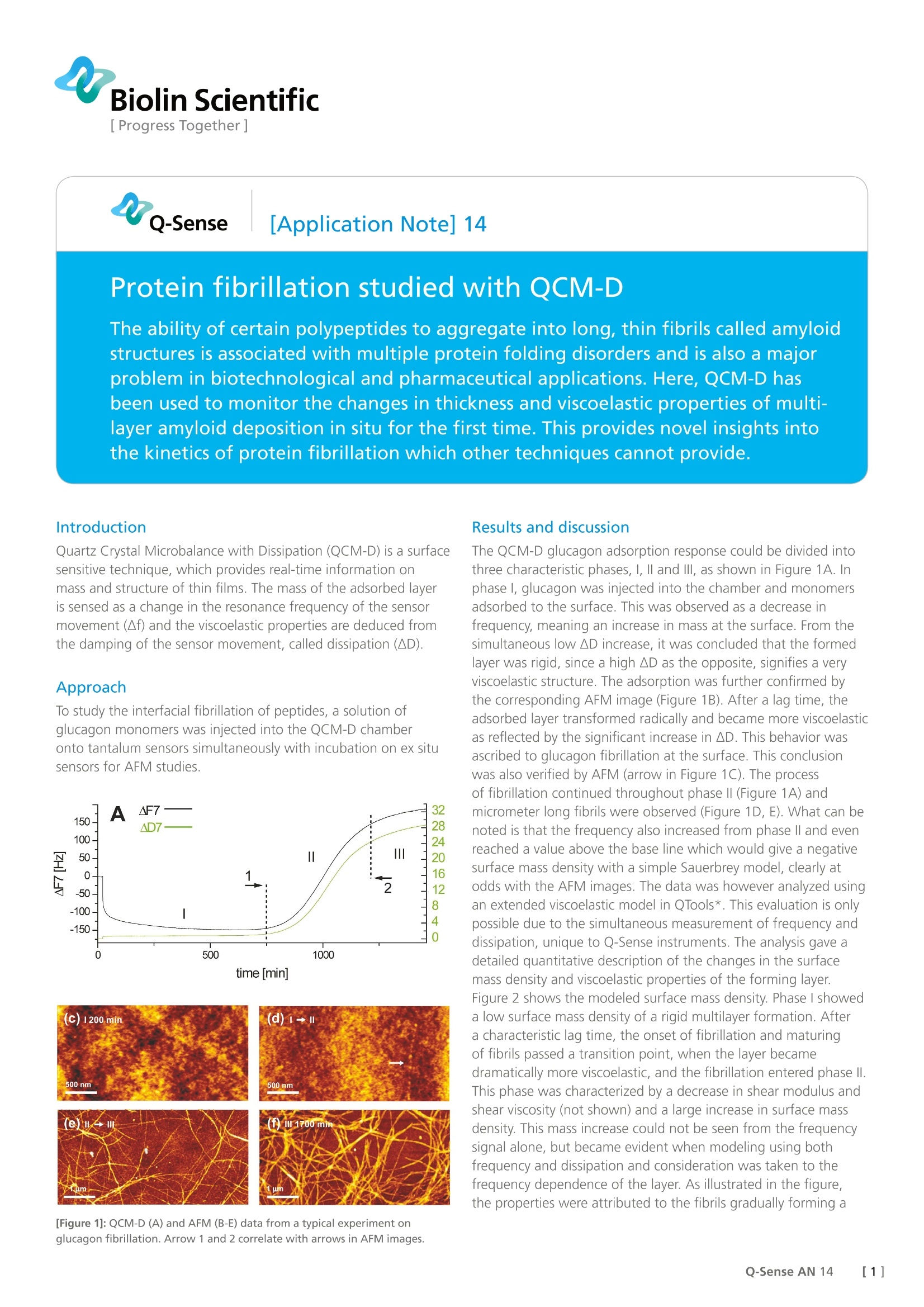
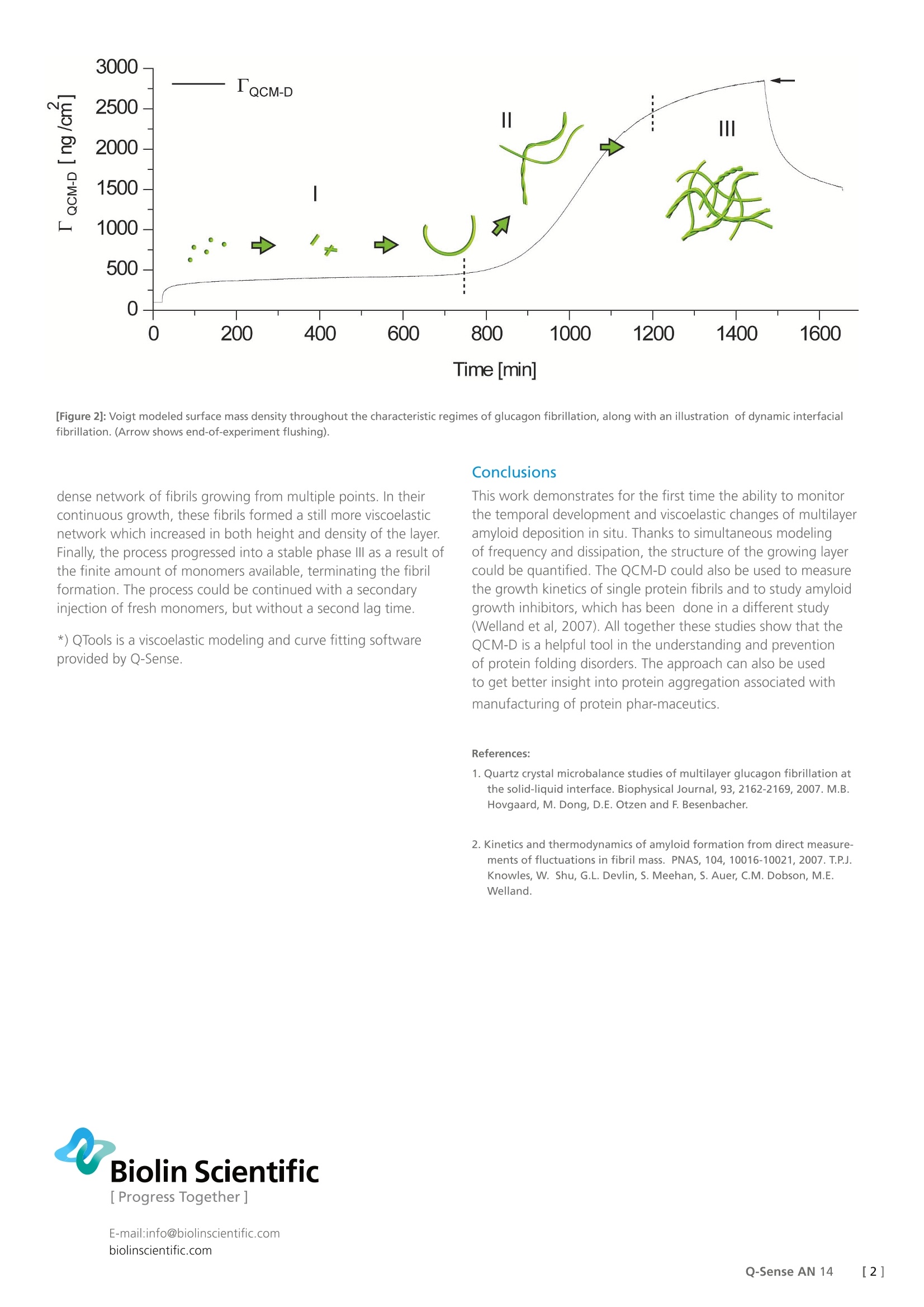
Biolin Scientific[ Progress Together] [Application Note] 14 Protein fibrillation studied with QCM-D The ability of certain polypeptides to aggregate into long, thin fibrils called amyloidstructures is associated with multiple protein folding disorders and is also a majorproblem in biotechnological and pharmaceutical applications. Here, QCM-D hasbeen used to monitor the changes in thickness and viscoelastic properties of multi-layer amyloid deposition in situ for the first time. This provides novel insights intothe kinetics of protein fibrillation which other techniques cannot provide. Quartz Crystal Microbalance with Dissipation (QCM-D) is a surfacesensitive technique, which provides real-time information onmass and structure of thin films. The mass of the adsorbed layeris sensed as a change in the resonance frequency of the sensormovement (Af) and the viscoelastic properties are deduced fromthe damping of the sensor movement, called dissipation (AD). Approach To study the interfacial fibrillation of peptides, a solution ofglucagon monomers was injected into the QCM-D chamberonto tantalum sensors simultaneously with incubation on ex situsensors for AFM studies. Results and discussion The QCM-D glucagon adsorption response could be divided intothree characteristic phases, l, Il and IIl, as shown in Figure 1A. Inphasel, glucagon was injected into the chamber and monomersadsorbed to the surface. This was observed as a decrease infrequency, meaning an increase in mass at the surface. From thesimultaneous low AD increase, it was concluded that the formedlayer was rigid, since a high AD as the opposite, signifies a veryviscoelastic structure. The adsorption was further confirmed bythe corresponding AFM image (Figure 1B). After a lag time, theadsorbed layer transformed radically and became more viscoelasticas reflected by the significant increase in AD. This behavior wasascribed to glucagon fibrillation at the surface. This conclusionwas also verified by AFM (arrow in Figure 1C). The processof fibrillation continued throughout phase II (Figure 1A) andmicrometer long fibrils were observed (Figure 1D, E). What can benoted is that the frequency also increased from phase Il and evenreached a value above the base line which would give a negativesurface mass density with a simple Sauerbrey model, clearly atodds with the AFM images. The data was however analyzed usingan extended viscoelastic model in QTools*. This evaluation is onlypossible due to the simultaneous measurement of frequency anddissipation, unique to Q-Sense instruments. The analysis gave adetailed quantitative description of the changes in the surfacemass density and viscoelastic properties of the forming layer.Figure 2 shows the modeled surface mass density. Phase I showeda low surface mass density of a rigid multilayer formation. Aftera characteristic lag time, the onset of fibrillation and maturingof fibrils passed a transition point, when the layer becamedramatically more viscoelastic, and the fibrillation entered phase ll.This phase was characterized by a decrease in shear modulus andshear viscosity (not shown) and a large increase in surface massdensity. This mass increase could not be seen from the frequencysignal alone, but became evident when modeling using bothfrequency and dissipation and consideration was taken to thefrequency dependence of the layer. As illustrated in the figure,the properties were attributed to the fibrils gradually forming a This work demonstrates for the first time the ability to monitorthe temporal development and viscoelastic changes of multilayeramyloid deposition in situ. Thanks to simultaneous modelingof frequency and dissipation, the structure of the growing layercould be quantified. The QCM-D could also be used to measurethe growth kinetics of single protein fibrils and to study amyloidgrowth inhibitors, which has been done in a different study(Welland et al, 2007). All together these studies show that theQCM-D is a helpful tool in the understanding and prevention.of protein folding disorders. The approach can also be usedto get better insight into protein aggregation associated withmanufacturing of protein phar-maceutics. References: 1. Quartz crystal microbalance studies of multilayer glucagon fibrillation atthe solid-liquid interface. Biophysical Journal, 93, 2162-2169,2007.M.B.Hovgaard, M. Dong, D.E. Otzen and F. Besenbacher. 2. Kinetics and thermodynamics of amyloid formation from direct measure-ments of fluctuations in fibril mass. PNAS, 104, 10016-10021,2007. T.P.J.Knowles, W. Shu, G.L. Devlin, S. Meehan, S. Auer, C.M. Dobson,M.E.Welland. []Q-Sense AN
确定
还剩1页未读,是否继续阅读?
瑞典百欧林科技有限公司为您提供《使用QCM-D技术研究蛋白质纤维化》,该方案主要用于其他中--检测,参考标准--,《使用QCM-D技术研究蛋白质纤维化》用到的仪器有QSense卓越版四通道石英晶体微天平、QSense Explorer扩展版石英晶体微天平、QSense全自动八通道石英晶体微天平
推荐专场
相关方案
更多
该厂商其他方案
更多