不同浓度施用生长激素吲哚乙酸(IAA)和细胞分裂素6-苄氨基嘌呤(BAP)对小葵子产量和油质的影响Effect of IAA and BAP application in varying concentration on seed yield and oil quality of Guizotia abyssinica (L.f.) Cass
方案详情

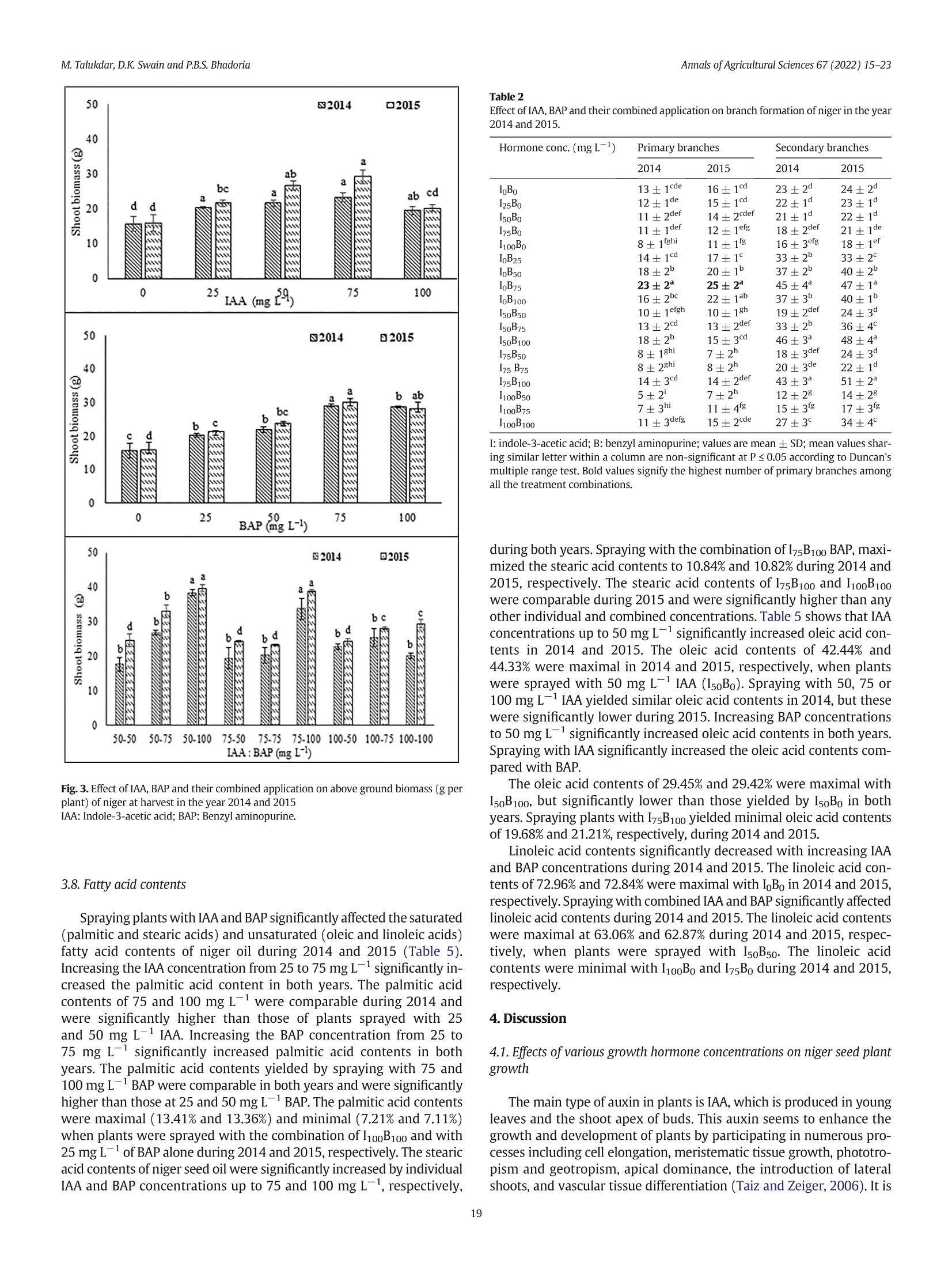
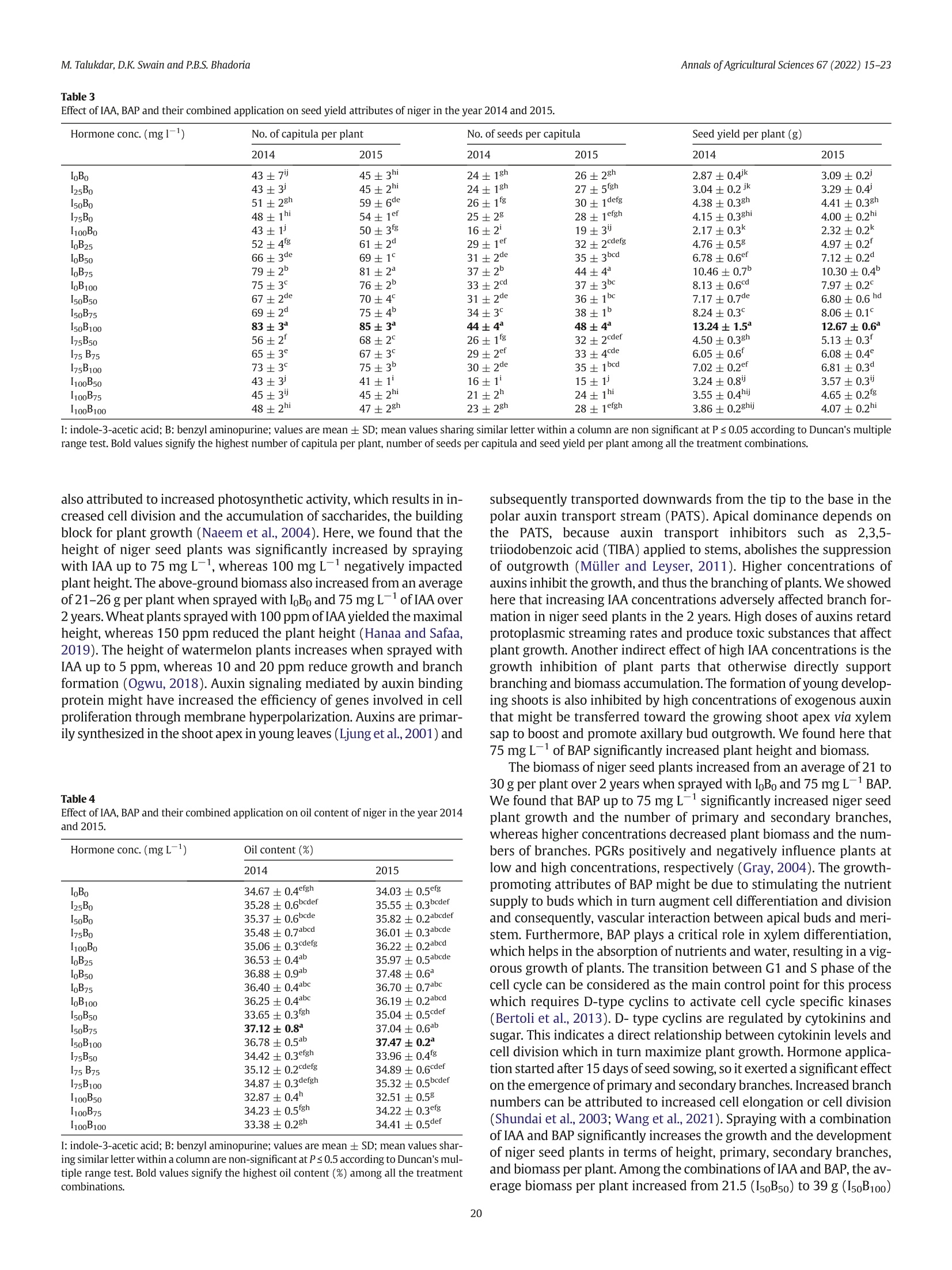
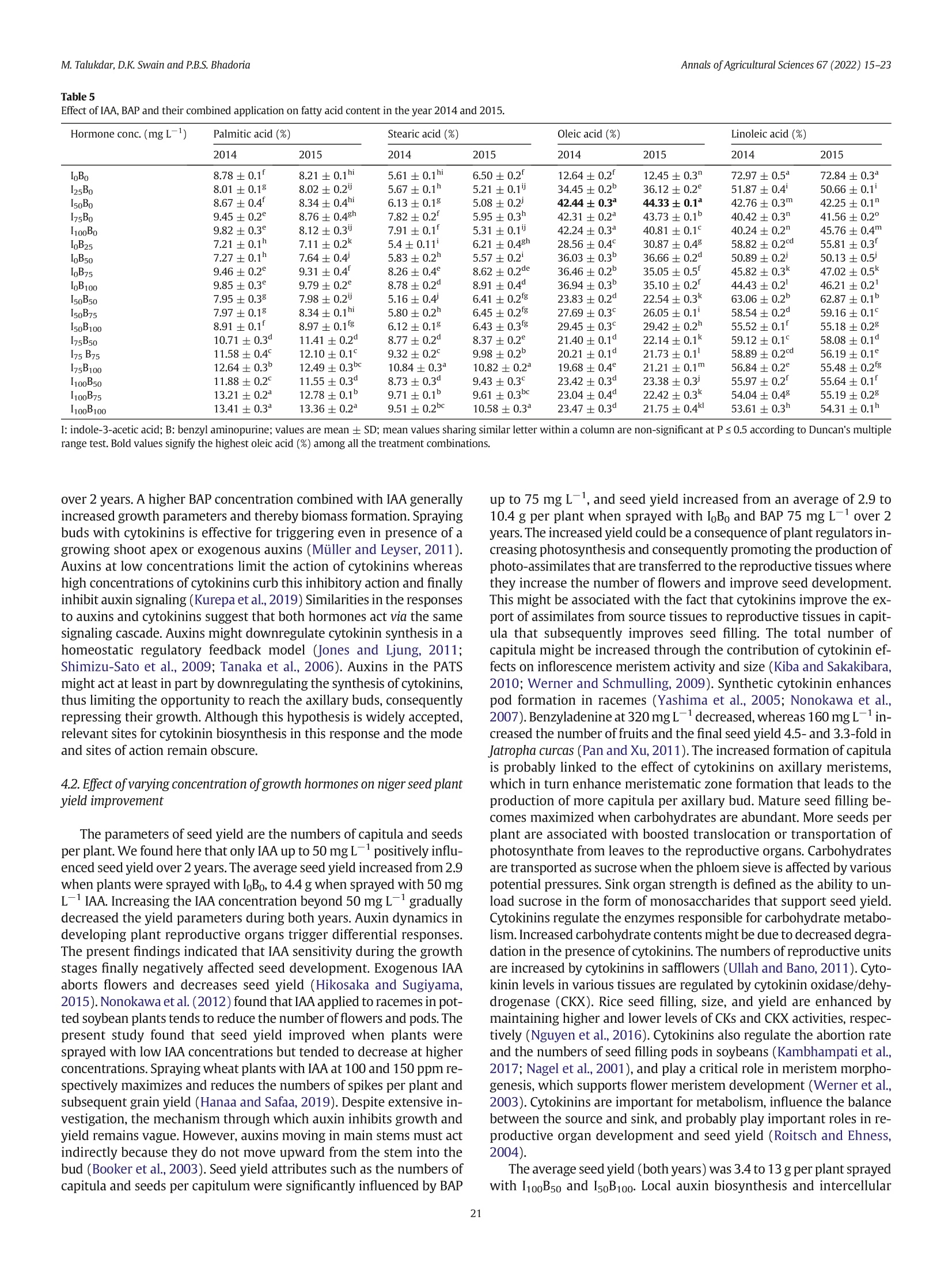
不同浓度施用生长激素吲哚乙酸(IAA)和细胞分裂素6-苄氨基嘌呤(BAP)对小葵子产量和油质的影响Effect of IAA and BAP application in varying concentration on seed yield and oil quality of Guizotia abyssinica (L.f.) CassAnnals of Agricultural Sciences 67(2022) 15–23journal homepage: www.elsevier.com/locate/aoas M.Talukdar, D.K. Swain and P.B.S. BhadoriaAnnals of Agricultural Sciences 67(2022) 15–23 Contents lists available at ScienceDirect Annals of Agricultural Sciences 不同浓度施用生长激素吲哚乙酸(IAA)和细胞分裂素6-苄氨基嘌呤(BAP)对小葵子产量和油质的影响 Original Article Effect of IAA and BAP application in varying concentration on seed yield and oil quality of Guizotia abyssinica(L.f.) Cass Monaswita Talukdar, Dillip Kumar Swain*, Pratap Bhanu Singh Bhadoria Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur, India a r t i c l e i n f o Article history: Received 8 August 2021Received in revised form9 February 2022Accepted 24 February 2022 Available online 16March 2022 Keywords: Fatty acid Niger seed yield Indole-3-acetic acid 6-Benzyl aminopurine Oil content a b s t r a c t A lowproduction of oil seed crops, pairedwith their increased demand, has becomeachallenge for the edible oil industry in India. Guizotia abyssinica(L.f.) Cass.(niger seed plant) is an annual, dicotyledonous,multipurpose oil seed crop belonging to the family of Asteraceae. Although this plant produces abundant dowers, it fails to pro-ducemature seedswith vigor due to poor vascularization.We investigated the effects of the plant growth-promoting hormone auxin, indole-3-acetic acid(IAA), and the cytokinin, 6-benzyl aminopurine(BAP) individu-ally and together on the growth and oil quality of niger in lateritic soil. Pot experimentswere conducted during thewet seasons of 2014 and 2015 in subtropical eastern India. The plantswere sprayedwith 25, 50, 75, and100mg L−1 each of IAA and BAP, 50, 75, and 100mg L−1 of IAA+BAP, orwater(control).When applied indi-vidually—IAA or BAP at 75mg L−1(I75B0 or I0B75)—the biomass productionwasmaximized. The combination of IAA(50mg L−1) and BAP(100mg L−1; I50B100) yielded signiocantly high biomass(38 and 40 g plant−1) and seed yield(13.24 and 12.67 g plant−1) in 2014 and 2015, respectively. Themost effective combinations for increasing niger seed oleic acid contentwere I50B100 and I50B75 as compared to other combinations of growth hormone. These results suggest that exogenous phytohormones can improve the seed yield and oil quality of niger seed plants in acid lateritic soil. © 2022 Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). 1. Introduction The exponential growth of the global population is increasing the pressure on agricultural productivity. The present food supply com-prises a fewmain crops grown undermaintained agricultural condi-tions, alongwith variousminor crops. Theseminor crops should not be underestimated, especiallywhen they are equally useful for food and industrial purposes and can be cultivatedwithminimal input. Human dietary input generally consists of carbohydrates, proteins, and numerousmicronutrients such as vitamins,minerals, and antioxi-dants. As rich sources of energy and nutrition, oilseeds can provide an adequate amount ofmicronutrients. Thus, they play important roles in maintaininghumanhealth.Interest in novel sources of edible oil has re-cently increased because no oil fromany single source has been found suitablefor all edible and non-edible applications. Amongnovel sources of edible oil,niger(Guizotia abyssinica(L.f.)Cass.) seed oilmightplayan important role in human nutrition and health owing to its fatty acid composition and abundant fat-soluble bioactive components (Ramadan andMoersel, 2002a, 2002b). Guizotiaabyssinica(L.f.)Cass.,alsoknownasnigerorramtil,is anan-nual dicotyledonous oil seed crop belonging to the family of Asteraceae. ItoriginatedinEthiopiaandisnowestablished inIndia.Theplantgrows to an average height of 0.5–1.5mand reachesmaturity in 95–110 days. Niger seed plant lowers are yellowwith a head diameter of 15–50mm andrayloretsof5–10mm.Theloweringofanindividualwith40seeds takes about 8 days(Getinet and Sharma, 1996). Peripheral shiny black seeds generally becomemore vigorous andmature than central and in-termediary seeds andweigh 3–5 g. The typical developmental order might occur due to poor vascularization in the central lower head. Nigerseedscontain32–38%oiland18–22%protein,andalongwithpro-vidingnutritiontohumansandanimals,it is usedin thepharmaceutical and biofuel industries, among others. The fatty acid composition of the niger seed plant resembles that of saflower(Carthamus tinctorius) and sunlower(Helianthus annuus),which contain up to 85% linoleic acid(C18:2n-6)(Ramadan andMoersel, 2003). Other predominant fattyacidsincludepalmitic(C16:0),stearic(C18:0),andoleic (C18:1n-9) acids. Niger seed plants are usually grownin the Kharif sea-son(June to the end of October) and are cross-pollinated via a loral mechanism and honeybees. Niger seed plants require a full dry spell during lowering and seed setting; rainfall at this stage is detrimental and can lower seed yield(Dalei et al., 2014). Abscission induction and several other physiological factors can also lower lower production, emptyachenesandresultinpoorseedsetting.Cropyieldisgenerallyin-creased by preventing losses caused byweather or insects, accelerating crop production cycles, or applying various hormones that promote plantgrowth.Recentstudieshaverevealedthatplantgrowthregulators (PGRs) play important roles in lower development and consequent seed yield(Krizek and Fletcher, 2005; Santner et al., 2009). Non-toxic auxins are versatile hormones that can be used as PGRs. Very low auxin concentrationsmight regulate cell division, elongation, adventi-tious root formation, callus initiation, and induction of embryogenesis. Indole 3 acetic acid(IAA) is themostprevalent auxin in plants; it affects cell division, root initiation, lowering, fruit setting, ripening, senes-cence, and gravitropism. The cytokinin 6-benzyl aminopurine(BAP) promotes plant growthand plays critical roles inwhole plant ontogeny, including cell division, photomorphogenic development,metabolism modulation, seed, and pod setting, senescence, death, and responses to environmental stimulants(Chernyad'ev, 2000; Hirose et al., 2007). Sprayingthefoliagewith2.5ppmofIAAresultsintallersummersquash plants and improved fruit yield. In contrast, IAA at 5 and 10 ppm re-duced both the height and yield of this plant(Nassef and El-Aref,2018). Exogenous IAA up to 1mg L−1 increases the growth and seed yield of linseed(Linumusitatissimum L.),whereas 2mgL−1 has the op-posite effects(Rastogi et al., 2013). At 50mg L−1, IAAmaximizes the growth of Epipremnumaureumwhereas 5 and 100mg L−1 both result in smaller plants(Di Benedetto et al., 2015).Mungbean branching is negatively affected by increasing concentrations of exogenous auxin (El Karamany et al., 2019). Exogenous CKs, especially BAP, increase the activity of inlorescences in plants(Werner and Schmulling, 2009). Exogenous cytokinin promotes plant growth and yield characteristics. Leaf area development is promoted by BAP up to 100mg L−1. Spraying E. aureumwith BAP at any concentration signiicantly increases the rate ofrelativeleafareaexpansion(RLAE)andplantgrowthaswellasdevel-opmentfromcuttings(Di Benedetto et al., 2013). Gandolfo et al.(2014) reported that BAP up to 50mg L−1 positively inluenced the growth of Impatienswallerana,whereas concentrations up to 100mg L−1 nega-tively inluenced growth. Cytokinins support female reproductive organ development in some plants(Yuan et al., 2016; Cheng et al.,2013). Auxins and cytokinins associatewith each other in a complex way to support plant growth and differentiation. Di Benedetto et al.(2015) found that IAA and BAP up to 50mg L−1 similarly promote plant growth. Simultaneously applied auxins and cytokinins exert an-tagonistic effects(Jones et al., 2010; Tanaka et al., 2006; Shimizu-Sato et al., 2009). However, themechanism of interpreting the effects of these combined hormones on the entire plant is unclear. The present study aimed to determine the effects of an auxin and a cytokinin on niger seed plant growth, seed yield, and oil qualitywhen cultivated in lateritic soil. 2.Materials andmethods 2.1. Experimental site Niger seed plantswere cultivated in pots at the Experimental Farm ofAgricultural and Food EngineeringDepartmentat the Indian Institute of Technology, Kharagpur, India(22°19′ N and 87°19′ East longitude) during thewet seasons(June to September) of 2014 and 2015. The climate of this region is humid and subtropical. The average annual rainfall is 1640mm, 70% ofwhich falls during the rainy season(June–September). The average annual daily temperature ranges from21 °C to 38 °C. The daily average temperatures varied from 35 °C to 29 °C and 33 °C to 30 °C between June and September in 2014 and 2015, re-spectively, and the amount of rainfallwas 780 and 1173mm, respec-tively. The acid lateritic(Haplustalf) soil at the site has a sandy loam texture comprising 62% sand, 24% silt, and 14% claymixedwith lateritic pebbles. Table 1 shows themost important soil properties. 2.2. Pot experiments Weinvestigated theeffectsof sprayingnigerseedplantswith25,50,75, and 100mg L−1 each of IAA and BAP individuallywith 50, 75, and Table 1 Physical and chemical properties of soil of experimental site (0–20 cm). Parameters Value Physical properties Bulk density,mgm−3 1.64 Chemical properties: pH 5.49 Organic C,% 0.36 Available N, kg ha−1 143.03 Available P, kg ha−1 10.87 Available K, kg ha−1 93.51 100 mg L−1 of both combined, on plant growth and the quality of niger oil. Control plantswere sprayedwithwater. A total of 18 applica-tions with 3 replications proceeded in 54 earthen pots(diameter,30 cm; depth, 60 cm) using a Complete Randomized Design(CRD). About 10 seedswere sown in each pot under natural conditions at a depthof3cmtoensurethreeplantsperpotatharvest.Thesoilmoisture in each potwasmaintained at a constant level. 2.3. Application of IAA and BAP Weapplied 25, 50, 75, and 100mgL−1 each of IAA and BAP, and 50,75, and 100mg L−1 of both in combination. Stock solutions of IAA and BAP(both 25mgmL−1)were prepared by dissolving 1 g each in 5mL of 1 N NaOH and bringing the volumeup to 40mLwithmolecular biol-ogy gradewater. Tween 20(Polysorbate 20; 0.05% v/v)was added as a wetting agent to allworking solutions of BAP. The hormones(~100mL) at the described concentrationswere hand-sprayed onto the plants every3daysstartingfrom15daysaftersowing(DAS)duringtheadvent of vegetative growth, for 90 DASwhen the plants lowered. The plants were temporarily coveredwith transparent plastic sheets to avoid hor-mones drifting to nearby plants. Control plantswere sprayedwith 100mL of distilledwater. No other type of fertilizerwas added to the pots. 2.4. Crop growth assessment Plant height, drymatter production,the numberof branches and ca-pitula per plant, and the number of seeds per capitulumwere assessed at the time of harvest. The biomass obtained fromeach oven-dried pot wasweighed to calculate the dry yield. 2.5. Oil extraction 小葵子 WeobtainednigerseedoilbySOXTHERM®extraction(induplicate) using hexane(Bhatnagar and Krishna, 2013). Seedswere pulverized into a ine powder using an electrical grinder, then packed(3 g) in cellulose extraction thimbles(Whatman,Maidstone, UK). Duplicate thimbleswereplacedinanextractorandhot(60–70°C) solventhexane (1:4w/v)was percolated continuously for 2 h. 2.6. Fatty acid composition The fatty acid composition of oil sampleswas analyzed by gas chro-matography using aModel GC-2010 Plus-02 instrument(Shimadzu Corp., Kyoto, Japan), equippedwith a hydrogen lame ionization detec-tor(FID). The oil was trans-esteriied into fatty acid methyl esters (FAMEs) usingmethanolic KOH according to AOCSMethod No: Ce 1-62(Firestone,1998)andseparatedusinga column(30m×0.32mmdi-ameter) at aninitial temperature of 140 °Cthatwasgradually increased to 240 °C at 4 °Cmin−1 intervals. The lowrate of the carrier gas, nitro-gen,wasmaintained 3mL/min. Fatty acids in the sampleswere identi-ied based on the retention time of the reference standard FAMEmix C8–C24(Supelco In., Bellefonte, PA, USA) and are expressed as relative areas%. 2.7. Statistical analysis Datawere statistically analyzed as described by Gomez and Gomez (1984). All parameterswere analyzed using R software. Differences be-tweenmeanswere compared using Duncanmultiple range tests(P ≤0.05). 3. Results 3.1. Height Plant height signiicantly increased alongwith IAA concentrations from25 to 75mg L−1 in 2014 and 2015(Fig. 1),whereas 100mg L−1signiicantly reduced(p ≤ 0.05) plant height in both years to a level thatwas belowthat of control plants(I0B0). Plants reach amaximum height of 121 and 124 cmwhen sprayedwith 75mg L−1 of IAA in 2014 and 2015, respectively. Fig. 1. Effect of IAA, BAP and their combined application on plant height of niger atmatu-rity in the year 2014 and 2015 We also found that BAP(25–75mg L−1) signiicantly increased the height of niger seed plants in 2014 and 2015(Fig. 1). Plant height reachedmaxima of 140 and 156 cmwhen sprayedwith 75mg L−1BAP in 2014 and 2015, respectively. Plant heightwas statistically the same both yearswhen sprayedwith 25, 50, and 100mg L−1 BAP. Con-trol plant heightwasminimalat 89and88 cm.Sprayingwith combined IAA and BAP differently affected plant height in 2014 and 2015(Fig. 1). Signiicant difference in plant heightwas observed between control (sprayedwithwater) and combined hormonal treatments(Fig. 2). In general, plant height signiicantly increased as the concentration of BAP increased,while that of IAA remained constant. Spraying the plants with combinations of IAA 75mgL−1 and BAP 100mgL−1(I75B100), and IAA 50mg L−1 and BAP 100mg L−1(I50B100), respectively achieved maximumheights of 153(2014) and 147(2015) cm. Combinations of IAA and BAP at 75, 50, and 100 and 75 and 100mg L−1(I50B75, I50B100, I75B100) similarly achievedmaximal plant heights in both years,which were signiicantly higher than those achieved by sprayingwith any other combinations. Plant height reached 84 and 73 cm in 2014 and 2015, respectively,when sprayedwith I100B50. 3.2. Above-ground biomass Above-groundbiomasswassigniicantlyincreasedbyIAA75mgL−1overcontrols(Fig.3).Thebiomasswasmaximalat23and29gperplant sprayedwith 75mgL−1 of IAA. The effects of 25, 50, and 75mgL−1 IAA on biomassformationwere similar in both years. The biomass per plant wasminimal in control plants sprayedwith I0B0. Spraying with BAP(75 mg L−1) signiicantly increased above-ground biomass formation to maxima of 29 and 30 g per plant comparedwith any other concentrations(Fig. 3). Biomass formation was similar in both years when plants were sprayed with 25 and50mg L−1 of BAP. However, biomass formation decreasedmore by BAP100mgL−1thanby75mgL−1.Thebiomasswasminimalincontrol plants sprayedwith I0B0. Fig. 3 shows the effects of spraying plantswith combined IAA and BAP on above-ground biomass in 2014 and 2015. Biomass production per plant increasedwith increasing concentrations of BAP and a con-stant concentration of IAA. The biomass of 38 and 40 g per plantwas maximalwhen sprayedwith I50B100 in 2014 and 2015, respectively. The amounts of biomass per plant generated by sprayingwith I50B100and I75B100were comparable and signiicantly higher than other combinations in both years. 3.3. Number of branches SprayingwithIAAingeneralinhibitedbranchformationin2014and 2015(Table 2). Increasing IAA concentrations signiicantly decreased the numbers of primary branches formed in both years. The numbers of primary branches per plantweremaximal at 12 and 15 in 2014 and 2015 respectively,when sprayedwith IAA I25B0, andminimal both years when sprayed with I100B0. Secondary branch formation was comparable in I25B0 and I50B0 and signiicantly higher than I100B0. In contrast to IAA, spraying plantswith BAP up to 75mg L−1 in-creased primary and secondary branch formation(Table 2). The num-ber of primary branches reached amaximumof 23 and 25 per plant when sprayedwith I0B75 in 2014 and 2015, respectively. Secondary branch formation in I0B25, I0B50, and I0B100was comparable in 2014and signiicantly lower than I0B75. Spraying the plantswith combined IAA and BAP signiicantly af-fected branch formation(Table 2). Increasing the BAP concentration from50 to 100mg L−1 signiicantly increased branch formationwhen IAA remained constant in both years. The number of primary branches of 18 and 15 per plantwasmaximalwhen the plantswere sprayed with I50B100 in 2014 and 2015, respectively. Secondary branches formed in I0B75, I50B100 and I75B100were comparable and signiicantly Fig. 2. Effects of hormones on niger plant growth at 30 DAS. Plants sprayedwith(a)water(control) and(b) hormones. higher than the other concentrations. Spraying the plantswith I100B50resulted inminimal primary and secondary branch formation. 3.4. Capitulumformation The numbers of capitula per plantwere signiicantly inluenced by IAA in 2014 and 2015(Table 3). The maximal numbers of capitula formed per plant sprayedwith IAA I50B0were 51 and 59 during 2014and 2015, respectively. Capitula formed in plantswere comparable during 2015 and signiicantly lowerwhen sprayedwith I0B0 and I25B0than any other IAA concentrations. Increasing concentrations of BAP up to 75 mg L−1 during 2014and2015signiicantlyincreasedcapitulum formation, whereas100 mg L−1(I0B100) signiicantly lowered it in 2015, despite being comparable during 2014. Among the BAP concentrations, I0B75 yielded maximalcapitulumformation(79and81in2014and2015,respectively). Spraying the plantswith IAA and BAP signiicantly affected capitu-lumformationduring2014 and2015(Table3).Thenumbersof capitula weremaximal at 83 and 85when sprayedwith I50B100 in 2014 and 2015, respectively. The numbers were comparable between I50B100and I0B75 in 2015, but signiicantly better than any other hormone concentrations in both years. Capitulum formation wasminimal in plants sprayedwith I100 B50 in both years. 3.5. Number of seeds per capitulum Sprayingwith IAA signiicantly affected seed formation(Table 3). Themaximumnumber of seeds per capitulumwasmaximal at 26 and 30 in 2014 and 2015, respectively when sprayed with I50B0. Seed formation in I50B0 and I75B0 was comparable in both years and signiicantly higher than any other hormone concentrations.Minimal numbers of seeds formedwith I100B0. Seed formation gradually increasedwith increasing BAP concentra-tions up to 75mg L−1 during 2014 and 2015(Table 3). The numbers ofseedsweremaximalat37 and44during2014and2015,respectively, when plantswere sprayedwith I0B75 and signiicantly higher than any other BAP concentrations. SprayingwithcombinedIAAandBAPsigniicantlyaffectedseedpro-duction(Table 3). The numbers of seedsweremaximal at 44 and 48during 2014 and 2015, respectively,when plantswere sprayedwith I50B100. The numbers of seeds per capitulum were comparable between I50B100 and I0B75 during 2015, and signiicantly higher than any other combinations. Minimal numbers of seeds formed when plantswere sprayedwith I100B50 in both years. 3.6. Seed yield Table 3 shows the effects of IAA and BAP on niger seed yield. The maximum seed yields of plants sprayed onlywith IAA(I50B0)were4.38 and 4.41 g plant−1 in 2014 and 2015, respectively. Increasing the BAP concentration from0 to 25, 50, and 75mg L−1signiicantly increased seed yield during both years(Table 3). The seed yield of 10.46 and 10.30 g plant−1wasmaximalwith I0B75 in2014 and 2015, respectively. The seed yieldwas signiicantly lower for I0B100 than I0B75. Spraying plantswith a combination of IAA and BAP signiicantly af-fected seed yield in 2014 and 2015(Table 3). Seed yields of 13.24 and12.67 g plant−1were maximal for I50B100 during 2014 and 2015, respectively. Moreover, the seed yield of I50B100was signiicantly higher than that of any other concentrations during both years. The seed yieldwas the lowestwith I100 B0 in both years. 3.7. Oil content Spraying with IAA signiicantly inluenced the oil content of niger seed during 2014 and 2015(Table 4). Up to 100 mg L−1 of IAA did not signiicantly change oil content, whereas an increase in BAP up to 50mg L−1 signiicantly increased the oil content dur-ing both years. Themaximumoil content of 36.88% and 37.48%was achieved with BAP50 mg L−1(I0B50)in 2014 and 2015, respectively. The combination of I50B75 and/or I50B100 yielded maximal oil content comparable with I0B50 in both years. The oil contents of 32.87% and 32.51% wereminimalwith I100B50 in 2014and 2015, respectively. Fig. 3. Effect of IAA, BAP and their combined application on above ground biomass(g per plant) of niger at harvest in the year 2014 and 2015IAA: Indole-3-acetic acid; BAP: Benzyl aminopurine. 3.8. Fatty acid contents SprayingplantswithIAAandBAPsigniicantlyaffectedthesaturated (palmitic and stearic acids) and unsaturated(oleic and linoleic acids) fatty acid contents of niger oil during 2014 and 2015(Table 5). Increasing the IAA concentration from25 to 75mgL−1 signiicantly in-creased the palmitic acid content in both years. The palmitic acid contents of 75 and 100 mg L−1were comparable during 2014 and were signiicantly higher than those of plants sprayed with 25and 50 mg L−1 IAA. Increasing the BAP concentration from 25 to75 mg L−1 signiicantly increased palmitic acid contents in both years. The palmitic acid contents yielded by spraying with 75 and100mg L−1 BAPwere comparable in both years andwere signiicantly higher than those at 25 and 50mg L−1 BAP. The palmitic acid contents weremaximal(13.41% and 13.36%) andminimal(7.21% and 7.11%) when plantswere sprayedwith the combination of I100B100 andwith25mgL−1 of BAP alone during 2014 and 2015, respectively. The stearic acid contents of nigerseed oilwere signiicantly increased by individual IAA and BAP concentrations up to 75 and 100mg L−1, respectively, Table 2 Effect of IAA,BAP and their combinedapplication on branch formation of niger in the year 2014 and 2015. Hormone conc.(mg L−1) Primary branches Secondary branches 2014 2015 2014 2015 I0B0 13±1cde 16±1cd 23±2d 24±2d I25B0 12±1de 15±1cd 22±1d 23±1d I50B0 11±2def 14±2cdef 21±1d 22±1d IB750 11±1def 12±1efg 18±2def 21±1de I100B0 8±1fghi 11±1fg 16±3efg 18±1ef I0B25 14±1cd 17±1c 33±2b 33±2c I0B50 18±2b 20±1b 37±2b 40±2b I0B75 23±2a 25±2a 45±4a 47±1a I0B100 16±2bc 22±1ab 37±3b 40±1b I50B50 10±1efgh 10±1gh 19±2def 24±3d I50B75 13±2cd 13±2def 33±2b 36±4c IB50100 18±2b 15±3cd 46±3a 48±4a I75B50 8±1ghi 7±2h 18±3def 24±3d IB7575 8±2ghi 8±2h 20±3de 22±1d IB75100 14±3cd 14±2def 43±3a 51±2a IB10050 5±2i 7±2h 12±2g 14±2g IB10075 7±3hi 11±4fg 15±3fg 17±3fg IB100100 11±3defg 15±2cde 27±3c 34±4c I: indole-3-acetic acid; B: benzyl aminopurine; values aremean±SD;mean values shar-ing similar letterwithin a column are non-signi cant at P ≤ 0.05 according to Duncan's multiple range test. Bold values signify the highest number of primary branches among all the treatment combinations. during both years. Sprayingwith the combination of I75B100 BAP,maxi-mized the stearic acid contents to 10.84% and 10.82% during 2014 and 2015, respectively. The stearic acid contents of I75B100 and I100B100were comparable during 2015 andwere signiocantly higher than any other individual and combined concentrations. Table 5 shows that IAA concentrations up to 50mg L−1 signincantly increased oleic acid con-tents in 2014 and 2015. The oleic acid contents of 42.44% and 44.33% weremaximal in 2014 and 2015, respectively,when plants were sprayed with 50 mg L−1 IAA(I50B0). Spraying with 50, 75 or100mg L−1 IAA yielded similar oleic acid contents in 2014, but these were signiLcantly lower during 2015. Increasing BAP concentrations to 50mg L−1 signiicantly increased oleic acid contents in both years. Sprayingwith IAA signiicantly increased the oleic acid contents com-paredwith BAP. The oleic acid contents of 29.45% and 29.42%weremaximalwith I50B100, but signiicantly lower than those yielded by I50B0 in both years. Spraying plantswith I75B100 yieldedminimal oleic acid contents of 19.68% and 21.21%, respectively, during 2014 and 2015. Linoleic acid contents signiicantly decreasedwith increasing IAA and BAP concentrations during 2014 and 2015. The linoleic acid con-tents of 72.96% and 72.84%weremaximalwith I0B0 in 2014 and 2015, respectively.Sprayingwithcombined IAA andBAPsigniicantlyaffected linoleic acid contents during 2014 and 2015. The linoleic acid contents weremaximal at 63.06% and 62.87% during 2014 and 2015, respec-tively, when plants were sprayed with I50B50. The linoleic acid contentswereminimalwith I100B0 and I75B0 during 2014 and 2015, respectively. 4. Discussion 4.1. Effects of various growth hormone concentrations on niger seed plant growth Themain type of auxin in plants is IAA,which is produced in young leaves and the shoot apex of buds. This auxin seems to enhance the growth and development of plants by participating in numerous pro-cesses including cell elongation,meristematic tissue growth, phototro-pism and geotropism, apical dominance, the introduction of lateral shoots, and vascular tissue differentiation(Taiz and Zeiger, 2006). It is Table 3 Effect of IAA, BAP and their combined application on seed yield attributes of niger in the year 2014 and 2015. Hormone conc.(mg l−1) No. of capitula per plant No. of seeds per capitula Seed yield per plant(g) 2014 2015 2014 2015 2014 2015 I0B0 43±7ij 45±3hi 24±1gh 26±2gh 2.87±0.4jk 3.09±0.2j I25B0 43±3j 45±2hi 24±1gh 27±5fgh 3.04±0.2 jk 3.29±0.4j I50B0 51±2gh 59±6de 26±1fg 30±1defg 4.38±0.3gh 4.41±0.3gh I75B0 48±1hi 54±1ef 25±2g 28±1efgh 4.15±0.3ghi 4.00±0.2hi IB1000 43±1j 50±3fg 16±2i 19±3ij 2.17±0.3k 2.32±0.2k I0B25 52±4fg 61±2d 29±1ef 32±2cdefg 4.76±0.5g 4.97±0.2f I0B50 66±3de 69±1c 31±2de 35±3bcd 6.78±0.6ef 7.12±0.2d I0B75 79±2b 81±2a 37±2b 44±4a 10.46±0.7b 10.30±0.4b I0B100 75±3c 76±2b 33±2cd 37±3bc 8.13±0.6cd 7.97±0.2c I50B50 67±2de 70±4c 31±2de 36±1bc 7.17±0.7de 6.80±0.6hd I50B75 69±2d 75±4b 34±3c 38±1b 8.24±0.3c 8.06±0.1c IB50100 83±3a 85±3a 44±4a 48±4a 13.24±1.5a 12.67±0.6a I75B50 56±2f 68±2c 26±1fg 32±2cdef 4.50±0.3gh 5.13±0.3f I75B75 65±3e 67±3c 29±2ef 33±4cde 6.05±0.6f 6.08±0.4e IB75100 73±3c 75±3b 30±2de 35±1bcd 7.02±0.2ef 6.81±0.3d IB10050 43±3j 41±1i 16±1i 15±1j 3.24±0.8ij 3.57±0.3ij IB10075 45±3ij 45±2hi 21±2h 24±1hi 3.55±0.4hij 4.65±0.2fg IB100100 48±2hi 47±2gh 23±2gh 28±1efgh 3.86±0.2ghij 4.07±0.2hi I: indole-3-acetic acid; B: benzyl aminopurine; values aremean±SD;mean values sharing similar letterwithin a column are non signiicant at P ≤ 0.05 according to Duncan'smultiple range test. Bold values signify the highest number of capitula per plant, number of seeds per capitula and seed yield per plant among all the treatment combinations. also attributed to increased photosynthetic activity,which results in in-creased cell division and the accumulation of saccharides, the building block for plant growth(Naeem et al., 2004). Here,we found that the height of niger seed plants was signiicantly increased by spraying with IAA up to 75mg L−1,whereas 100mg L−1 negatively impacted plant height. The above-groundbiomassalsoincreased fromanaverage of 21–26 g per plantwhen sprayedwith I0B0 and 75mgL−1 of IAA over 2years.Wheatplantssprayedwith100ppmofIAAyieldedthemaximal height,whereas 150 ppmreduced the plant height(Hanaa and Safaa,2019). The height ofwatermelon plants increaseswhen sprayedwith IAA up to 5 ppm,whereas 10 and 20 ppm reduce growth and branch formation(Ogwu, 2018). Auxin signalingmediated by auxin binding proteinmight have increased the eficiency of genes involved in cell proliferation throughmembrane hyperpolarization. Auxins are primar-ilysynthesizedintheshootapexinyoungleaves(Ljungetal.,2001)and Table 4 Effect of IAA, BAP and their combined application on oil content of niger in the year 2014and 2015. Hormone conc.(mg L−1) Oil content(%) 2014 2015 IB00 34.67±0.4efgh 34.03±0.5efg I25B0 35.28±0.6bcdef 35.55±0.3bcdef I50B0 35.37±0.6bcde 35.82±0.2abcdef I75B0 35.48±0.7abcd 36.01±0.3abcde I100B0 35.06±0.3cdefg 36.22±0.2abcd I0B25 36.53±0.4ab 35.97±0.5abcde I0B50 36.88±0.9ab 37.48±0.6a IB075 36.40±0.4abc 36.70±0.7abc I0B100 36.25±0.4abc 36.19±0.2abcd I50B50 33.65±0.3fgh 35.04±0.5cdef IB5075 37.12±0.8a 37.04±0.6ab IB50100 36.78±0.5ab 37.47±0.2a I75B50 34.42±0.3efgh 33.96±0.4fg I75B75 35.12±0.2cdefg 34.89±0.6cdef IB75100 34.87±0.3defgh 35.32±0.5bcdef IB10050 32.87±0.4h 32.51±0.5g IB10075 34.23±0.5fgh 34.22±0.3efg IB100100 33.38±0.2gh 34.41±0.5def I: indole-3-acetic acid; B: benzyl aminopurine; values aremean±SD;mean values shar-ingsimilarletterwithinacolumnarenon-signi cantat P≤ 0.5accordingtoDuncan'smul-tiple range test. Bold values signify the highest oil content(%) among all the treatment combinations. subsequently transported downwards from the tip to the base in the polar auxin transport stream(PATS). Apical dominance depends on the PATS, because auxin transport inhibitors such as 2,3,5-triiodobenzoic acid(TIBA) applied to stems, abolishes the suppression of outgrowth(Müller and Leyser, 2011). Higher concentrations of auxinsinhibit the growth,and thus thebranchingof plants.Weshowed here that increasing IAA concentrations adversely affected branch for-mation in niger seed plants in the 2 years. High doses of auxins retard protoplasmic streaming rates and produce toxic substances that affect plant growth. Another indirect effect of high IAA concentrations is the growth inhibition of plant parts that otherwise directly support branching and biomass accumulation. The formation of young develop-ing shoots is also inhibited by high concentrations of exogenous auxin thatmight be transferred toward the growing shoot apex via xylem sap to boost and promote axillary bud outgrowth.We found here that75mg L−1 of BAP signiicantly increased plant height and biomass. The biomass of niger seed plants increased froman average of 21 to30 g per plant over 2 yearswhen sprayedwith I0B0 and 75mgL−1 BAP. We found that BAP up to 75mg L−1 signiicantly increased niger seed plant growth and the number of primary and secondary branches, whereas higher concentrations decreased plant biomass and the num-bers of branches. PGRs positively and negatively inluence plants at low and high concentrations, respectively(Gray, 2004). The growth-promoting attributes of BAPmight be due to stimulating the nutrient supply to budswhich in turn augment cell differentiation and division and consequently, vascular interaction between apical buds andmeri-stem. Furthermore, BAP plays a critical role in xylem differentiation, which helps in the absorption of nutrients andwater, resulting in a vig-orous growth of plants. The transition between G1 and S phase of the cell cycle can be considered as themain control point for this process which requires D-type cyclins to activate cell cycle speciic kinases (Bertoli et al., 2013). D- type cyclins are regulated by cytokinins and sugar. This indicates a direct relationship between cytokinin levels and cell divisionwhich in turnmaximize plant growth. Hormone applica-tionstartedafter15daysofseedsowing,soitexertedasigniicanteffect ontheemergenceof primaryandsecondarybranches. Increasedbranch numbers can be attributed to increased cell elongation or cell division (Shundai et al., 2003;Wang et al., 2021). Sprayingwith a combination of IAA and BAP signiicantly increases the growth and the development of niger seed plants in terms of height, primary, secondary branches, and biomassper plant. Amongthecombinations of IAA and BAP, theav-erage biomass per plant increased from21.5(I50B50) to 39 g(I50B100) Table 5 Effect of IAA, BAP and their combined application on fatty acid content in the year 2014 and 2015. Hormone conc.(mg L−1) Palmitic acid(%) Stearic acid(%) Oleic acid(%) Linoleic acid(%) 2014 2015 2014 2015 2014 2015 2014 2015 I0B0 8.78±0.1f 8.21±0.1hi 5.61±0.1hi 6.50±0.2f 12.64±0.2f 12.45±0.3n 72.97±0.5a 72.84±0.3a I25B0 8.01±0.1g 8.02±0.2ij 5.67±0.1h 5.21±0.1ij 34.45±0.2b 36.12±0.2e 51.87±0.4i 50.66±0.1i I50B0 8.67±0.4f 8.34±0.4hi 6.13±0.1g 5.08±0.2j 42.44±0.3a 44.33±0.1a 42.76±0.3m 42.25±0.1n I75B0 9.45±0.2e 8.76±0.4gh 7.82±0.2f 5.95±0.3h 42.31±0.2a 43.73±0.1b 40.42±0.3n 41.56±0.2o IB1000 9.82±0.3e 8.12±0.3ij 7.91±0.1f 5.31±0.1ij 42.24±0.3a 40.81±0.1c 40.24±0.2n 45.76±0.4m I0B25 7.21±0.1h 7.11±0.2k 5.4±0.11i 6.21±0.4gh 28.56±0.4c 30.87±0.4g 58.82±0.2cd 55.81±0.3f I0B50 7.27±0.1h 7.64±0.4j 5.83±0.2h 5.57±0.2i 36.03±0.3b 36.66±0.2d 50.89±0.2j 50.13±0.5j I0B75 9.46±0.2e 9.31±0.4f 8.26±0.4e 8.62±0.2de 36.46±0.2b 35.05±0.5f 45.82±0.3k 47.02±0.5k I0B100 9.85±0.3e 9.79±0.2e 8.78±0.2d 8.91±0.4d 36.94±0.3b 35.10±0.2f 44.43±0.2l 46.21±0.21 I50B50 7.95±0.3g 7.98±0.2ij 5.16±0.4j 6.41±0.2fg 23.83±0.2d 22.54±0.3k 63.06±0.2b 62.87±0.1b I50B75 7.97±0.1g 8.34±0.1hi 5.80±0.2h 6.45±0.2fg 27.69±0.3c 26.05±0.1i 58.54±0.2d 59.16±0.1c IB50100 8.91±0.1f 8.97±0.1fg 6.12±0.1g 6.43±0.3fg 29.45±0.3c 29.42±0.2h 55.52±0.1f 55.18±0.2g I75B50 10.71±0.3d 11.41±0.2d 8.77±0.2d 8.37±0.2e 21.40±0.1d 22.14±0.1k 59.12±0.1c 58.08±0.1d I75B75 11.58±0.4c 12.10±0.1c 9.32±0.2c 9.98±0.2b 20.21±0.1d 21.73±0.1l 58.89±0.2cd 56.19±0.1e IB75100 12.64±0.3b 12.49±0.3bc 10.84±0.3a 10.82±0.2a 19.68±0.4e 21.21±0.1m 56.84±0.2e 55.48±0.2fg IB10050 11.88±0.2c 11.55±0.3d 8.73±0.3d 9.43±0.3c 23.42±0.3d 23.38±0.3j 55.97±0.2f 55.64±0.1f IB10075 13.21±0.2a 12.78±0.1b 9.71±0.1b 9.61±0.3bc 23.04±0.4d 22.42±0.3k 54.04±0.4g 55.19±0.2g IB100100 13.41±0.3a 13.36±0.2a 9.51±0.2bc 10.58±0.3a 23.47±0.3d 21.75±0.4kl 53.61±0.3h 54.31±0.1h I: indole-3-acetic acid; B: benzyl aminopurine; values aremean±SD;mean values sharing similar letterwithin a column are non-signiicant at P ≤ 0.5 according to Duncan'smultiple range test. Bold values signify the highest oleic acid(%) among all the treatment combinations. over 2 years. A higher BAP concentration combinedwith IAA generally increased growth parameters and thereby biomass formation. Spraying budswith cytokinins is effective for triggering even in presence of a growing shoot apex or exogenous auxins(Müller and Leyser, 2011). Auxins at low concentrations limit the action of cytokininswhereas high concentrations of cytokinins curb this inhibitory action and inally inhibit auxinsignaling(Kurepaetal., 2019) Similaritiesintheresponses to auxins and cytokinins suggest that both hormones act via the same signaling cascade. Auxinsmight downregulate cytokinin synthesis in a homeostatic regulatory feedback model(Jones and Ljung, 2011; Shimizu-Sato et al., 2009; Tanaka et al., 2006). Auxins in the PATS might act at least in part by downregulating the synthesis of cytokinins, thus limiting the opportunity to reach the axillary buds, consequently repressing their growth. Although this hypothesis iswidely accepted, relevant sites for cytokinin biosynthesis in this response and themode and sites of action remain obscure. 4.2. Effect of varying concentration of growthhormones on niger seed plant yield improvement The parameters of seed yield are the numbers of capitula and seeds per plant.Wefound here that only IAA up to 50mgL−1 positively inlu-enced seedyield over 2 years.The average seed yield increased from2.9when plantswere sprayedwith I0B0, to 4.4 gwhen sprayedwith 50mg L−1 IAA. Increasing the IAA concentration beyond 50mg L−1 gradually decreased the yield parameters during both years. Auxin dynamics in developing plant reproductive organs trigger differential responses. The present indings indicated that IAA sensitivity during the growth stages inally negatively affected seed development. Exogenous IAA aborts lowers and decreases seed yield(Hikosaka and Sugiyama,2015).Nonokawaetal.(2012)foundthatIAAappliedtoracemesinpot-ted soybean plantstends toreducethenumberoflowersand pods. The present study found that seed yield improved when plants were sprayedwith lowIAA concentrations but tended to decrease at higher concentrations. Sprayingwheatplantswith IAA at100 and 150 ppmre-spectivelymaximizes and reduces the numbers of spikes per plant and subsequent grain yield(Hanaa and Safaa, 2019). Despite extensive in-vestigation, themechanismthroughwhich auxin inhibits growth and yield remains vague. However, auxinsmoving inmain stemsmust act indirectly because they do notmove upward from the stem into the bud(Booker et al., 2003). Seed yield attributes such as the numbers of capitula and seeds per capitulumwere signiicantly inluenced by BAP up to 75mg L−1, and seed yield increased from an average of 2.9 to 10.4 g per plantwhen sprayedwith I0B0 and BAP 75mg L−1 over 2years.Theincreasedyieldcouldbea consequenceofplantregulators in-creasing photosynthesis and consequently promoting the production of photo-assimilatesthataretransferredtothereproductivetissueswhere they increase the number of lowers and improve seed development. Thismight be associatedwith the fact that cytokinins improve the ex-port of assimilates fromsource tissues to reproductive tissues in capit-ula that subsequently improves seed illing. The total number of capitulamight be increased through the contribution of cytokinin ef-fects on inlorescencemeristemactivity and size(Kiba and Sakakibara,2010;Werner and Schmulling, 2009). Synthetic cytokinin enhances pod formation in racemes(Yashima et al., 2005; Nonokawa et al.,2007).Benzyladenineat320mgL−1decreased,whereas160mgL−1in-creased the number of fruits and the inal seed yield 4.5- and 3.3-fold in Jatropha curcas(Pan and Xu, 2011). The increased formation of capitula is probably linked to the effect of cytokinins on axillarymeristems, which in turn enhancemeristematic zone formation that leads to the production ofmore capitula per axillary bud.Mature seed illing be-comesmaximizedwhen carbohydrates are abundant.More seeds per plant are associatedwith boosted translocation or transportation of photosynthate fromleaves to the reproductive organs. Carbohydrates are transported as sucrosewhen the phloemsieve is affected by various potential pressures. Sink organ strength is deined as the ability to un-load sucrose in the form ofmonosaccharides that support seed yield. Cytokinins regulate the enzymes responsible for carbohydratemetabo-lism.Increasedcarbohydratecontentsmightbeduetodecreaseddegra-dation in the presence of cytokinins. The numbers of reproductive units are increased by cytokinins in saflowers(Ullah and Bano, 2011). Cyto-kinin levels in various tissues are regulated by cytokinin oxidase/dehy-drogenase(CKX). Rice seed illing, size, and yield are enhanced by maintaining higher and lower levels of CKs and CKX activities, respec-tively(Nguyen et al., 2016). Cytokinins also regulate the abortion rate and the numbers of seed illing pods in soybeans(Kambhampati et al.,2017; Nagel et al., 2001), and play a critical role inmeristemmorpho-genesis,which supports lowermeristemdevelopment(Werner et al.,2003). Cytokinins are important formetabolism, inluence the balance between the source and sink, and probably play important roles in re-productive organ development and seed yield(Roitsch and Ehness,2004). Theaverageseedyield(bothyears)was3.4to13gperplantsprayed with I100B50 and I50B100. Local auxin biosynthesis and intercellular transport render auxins perfect signalingmolecules that can coordinate the development of reproductive processes(Figueiredo and Köhler,2018). Fourteen putative Gm IPT and 17 GmCKX cytokininmetabolic genes in lowers are involved in these processes. Exogenous BAP can overcome a homeostatic regulatory feedback mechanism in which auxins downregulatecytokinin synthesis(Jones and Ljung, 2011). Cyto-kinins and auxins act antagonistically; thus, the need for nutrients in-creases when cytokinins increase in sink organs and this leads to heightened sink strength and accelerated the translocation of photo-assimilates(Goetz et al., 2007). Themajor sink organs are seeds, and stronger seeds facilitate good setting and illing that is relected in ele-vated seed yield at higher concentrations of combined IAA and BAP (Brugière et al., 2008). 4.3. Effects of growth hormone application on seed oil quality of niger seed plants Seeddevelopmentissensitivetoabioticfactorsthatcanleadtoqual-itative and quantitative changes in the oil. The biological roles of hor-mones in oil accumulation have not been investigated in detail. The present study found that IAA did not signiicantly affect the oil content of niger seed,whereas BAP up to 50mgL−1 increased the seed oil con-tent. Spraying niger seed plantswith combined I50B75 and/or I50B100maximally increased the seed oil content,which is generally increased by BAP, which promotes protein ratios(%) and total carbohydrate (Bartrina et al., 2011). Both RNA and protein synthesis are promoted by BAP via the inhibition of proteolytic enzymes. Seeds contain the mostcytokinins(Emery et al., 2000; Nguyen et al., 2016) and seedmat-uration is critical for fatty acid and oil accumulation,whichwill inally deinethequalityof seeds.Thedevelopmentoffattyacids inconnection with plant hormones has not been studied in detail despite their critical role in seed development. Gavelient et al.(2013) found that the physi-ological auxin analogs TA-12 and TA-14 participate in rapeseed produc-tivity, exert stabilizing effects on crude fat content, and affect plant cells through the IAA receptor system. Exogenous TA-12 forms complexes with proteins in cell plasmalemma. Gaveliene et al.(2013) also showed thatauxinsaffectthefattyacidcomposition.Ourresultsshowedthatin-creasing the IAA and BAP concentrations enhanced the saturated fatty acid content over 2 years. The average palmitic acid content increased from 7.16%(I0B25) to 13.38%(I100B100) over 2 years. The content of oleic acid generally increased but that of linoleic acid decreased over the same period. The average in oleic acid content over 2 years increased from12%(I0B0) to 43%(I50B0). Although a potential link has not yet been identiied between IAA and fatty acidmetabolism, genetic studies have identiied some potential effects of cytokinins on the fatty acid development of the seed. Cytokinins aremajormanipulators of seed fatty acids. The fatty acid synthesis starts at an early stage in seeds, and exogenous cytokinins are proven key factors in altering fatty acid contents in oil. Early seed development requires auxin trans-port and signaling for pattern formation and cytokinins are required for nutritive effects and interactions that inally determine the lipid andfatty acid contents ofseedoil. Lemonbalmandthymerespondpos-itively to auxins(Affonso et al., 2009).We showed thatmanipulating fatty acids to increase their poly-unsaturated fatty acid contents using phytohormonesisa promisingstrategythatcouldallowplantoilstobe-come integrated into food and other industries. 5. Conclusions Exogenous phytohormones can alter the ratios of saturated and unsaturated fatty acids, directly affecting oil quality. Spraying plants with phytohormones increased seed yield, oil contents, and mono-unsaturated fatty acid(oleic acid) contents. Plants with IAA(50mgL−1) combinedwith BAP(100mgL−1) increased yield compo-nents and seed yield in lateritic soil compared with control plants sprayedwithwater. Spraying leaveswith phytohormones improved niger seed quality and quantity thatwill be useful for food and other in-dustrial purposes. Transferring thismethod to ieldswill be economi-cally beneicial and should be critically evaluated under various socio-environmental conditions. However, further genetic studies are needed to identify the underlying roles of cytokinins in plantmorphology and reproduction. Funding This research did not receive any speciic grant fromfunding agen-cies in the public, commercial, or not-for-proit sectors. The data pre-sented in this article is a part of Ph.D.work of the irst author. Declaration of competing interest The authors declare that they have no known competing inancial interests or personal relationships that could have appeared to inlu-ence thework reported in this paper. References Affonso, V.R., Bizzo, H.R., Lage, C.L.S., Sato, A., 2009. Inluence of growth regulators in bio-mass production and volatile proile of in vitro plantlets of Thymus vulgaris L. J. Agric. Food Chem. 57, 6392–6395. Bartrina, I., Otto, E., Strnad,M.,Werner, T., Schmülling, T., 2011. Cytokinin regulates the activity of reproductivemeristems, lower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69–80. Bertoli, C., Skotheim,J.M., de Bruin, R.A.M., 2013. Control of cell cycle transcription during G1 and S phases. Nat. Rev.Mol. Cell Biol. 14, 518–528. Bhatnagar, A.S., Krishna, A.G.G., 2013. Effect of extraction solvent on oil and bioactives composition of commercial Indian niger(Guizotia abyssinica(L.F.) Cass.) seed. J. Am. Oil Chem. Soc. 90, 1203–1212. Booker, J., Chatield, S., Leyser, O., 2003. Auxin acts in xylem-associated ormedullary cells tomediate apical dominance. Plant Cell 15, 495–507. Brugière, N., Humbert, S., Rizzo, N., Bohn, J., Habben, J.E., 2008. Amember of themaize isopentenyl transferase gene family, Zeamays isopentenyl transferase 2(ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. PlantMol. Biol. 67, 215–229. Cheng, C.Y.,Mathews, D.E., Eric Schaller, G., Kieber, J.J., 2013. Cytokinin-dependent speci-ication of the functionalmegaspore in the Arabidopsis female gametophyte. Plant J.73, 929–940. Chernyad'ev, I.I., 2000. Ontogenetic changes in the photosynthetic apparatus and effects of cytokinins – review. Appl. Biochem.Microbiol. 36, 527–528. Dalei, B.B., Kheroar, S.,Mohapatra, P.M., Panda, S., Deshmukh,M.R., 2014. Effect of foliar sprays on seed yield and economics of niger[Guizotia abyssinica(L.F.) Cass]. J. Agric. Sci. 6, 143–147. Di Benedetto, A., Galmarini, C., Tognetti, J., 2013. Contribution of changes in leaf size and leaf production rate to cytokinin-mediated growth promotion in Epipremnum aureumL. J. Hortic. Sci. 88, 179–186. Di Benedetto, A., Galmarini, C., Tognetti, J., 2015. Effects of combined or single exogenous auxin and/or cytokinin applications on growth and leaf area development in Epipremnum aureum. J. Hortic. Sci. Biotechnol. 90, 643–654. El Karamany,M.F., Sadak,M.S., Bakry, B.A., 2019. Improving quality and quantity of mungbean plant via foliar application of plant growth regulators in sandy soil condi-tions. Bull. Natl. Res. Cent. 43, 61. https://doi.org/10.1186/s42269-019-0099-5. Emery, R.J.N.,Ma,Q., Atkins, C.A., 2000. The formsand sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol. 123, 1593–1604. Figueiredo, D.D., Köhler, C.C., 2018. Auxin: amolecular trigger of seed development. Genes Dev. 32, 479–490. Firestone, D., 1998. Oficialmethods and recommended practices of the American Oil Chemists Society, AOCS method No. Ai 2-75, AOCS method No. Ca 5a-40, AOCS method No. Cd 8-53, AOCSmethod No. Cd 3c-91, AOCSmethod No. Cd 1-25, AOCS method No. Ca 6a-40, AOCSmethod No. Ce 1-62, AOCSmethod No. Ce 5b-89, AOCS method No. Ce8-89. 5th edn. AOCS press, Illinois. Gandolfo, E., De Lojo, J., Gómez, D., Pagani, A.,Molinari, J., Di Benedetto, A., 2014. Anatom-ical changes involved in the response of impatiens wallerana to different pre-transplant plug cell volumes and BAP sprays. Eur. J. Hortic. Sci. 79, 226–232. Gaveliene, V., Novickienl, L., PakalniškytA, L., 2013. Effect of auxin physiological analogues on rapeseed(Brassica napus) cold hardening, seed yield and quality. J. Plant Res. 126,283–292. Getinet, A., Sharma, S.M., 1996. Niger. Guizotia abyssinica(L.F.) Cass. International Plant Genetic Resources Institute. https://www.bioversityinternational.org/ileadmin/user_upload/online_library/publications/pdfs/136.pdf. Goetz,M., Hooper, L.C., Johnson, S.D., Rodrigues, J.C.M., Vivian-Smith, A., Koltunow, A.M.,2007. Expression of aberrant forms of auxin response factor 8 stimulates partheno-carpy in Arabidopsis and tomato. Plant Physiol. 145, 351–366. Gomez, K.A., Gomez, A.A., 1984. Statistical procedures for agricultural research. An Inter-national Rice Research Institute Book. JohnWiley& Sons. Publication. Gray,W.M.,2004. Hormonalregulation of plantgrowthand development.PLoS Biol.2(9), e311. https://doi.org/10.1371/journal.pbio.0020311. Hanaa, H., Safaa, A., 2019. Foliar application of IAA at different growth stages and their in-luenced on growth and productivity of breadwheat(Triticumaestivuml.). J. Phys. Conf. Ser. 1294, 092029. https://doi.org/10.1088/1742-6596/1294/9/092029. Hikosaka, S., Sugiyama, N., 2015. Effects of exogenous plant growth regulators on yield, fruitgrowth,andconcentrationofendogenoushormonesingynoeciousparthenocarpic cucumber(Cucumis sativus L.). Hort. J. 84, 342–349. Hirose, N., Takei, K., Kuroha, T., Kamada-Nobusada, T., Hayashi, H., Sakakibara, H., 2007. Regulation of cytokinin biosynthesis, compartmentalization, and translocation. J. Exp. Bot. 59, 75–83. Jones, B., Ljung, K., 2011. Auxin and cytokinin regulate each other's levels via ametabolic feedback loop. Plant Signal. Behav. 6, 901–904. Jones, B., Andersson, S.G., Petersson, S.V., Tarkowski, P., Graham, N.,May, S., Dolezal, K., Sandberg, G., Ljung, K., 2010. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal trans- duction. Plant Cell 22, 2956–2969. Kambhampati, S., Kurepin, L.V., Kisiala, A.B., Bruce, K.E., Cober, E.R.,Morrison,M.J., 2017. Yield associated traits correlate with cytokinin proiles in developing pods and seeds of ield-grown soybean cultivars. Field Crops Res. 214, 175–184. Kiba, T., Sakakibara, H., 2010. Role of cytokinin in the regulation of plant development. In:Pua,E.C.,Davey,M.R.(Eds.),PlantDevelopmentalBiology-BiotechnologicalPerspectives. Springer, NewYork, pp. 237–254. Krizek, B.A., Fletcher, J.C., 2005.Molecularmechanisms of lower development: an arm-chair guide. Nat. Rev. Genet. 6, 688–698. Kurepa, J., Shull, T.E., Smalle, J.A., 2019. Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct 3, e00121. https://doi.org/10.1002/pld3.121. Ljung, K., Bhalerao, R.P.,Sandberg, G., 2001. Sitesand homeostatic control ofauxin biosyn-thesis in Arabidopsis during vegetative growth. Plant J. 28, 465–474. Müller, D., Leyser, O., 2011. Auxin, cytokinin and the control of shoot branching. Ann. Bot.107, 1203–1212. Naeem,M., Bhatti, I., Ahmad, R.H., Ashraf, Y.M., 2004. Effect of some growth hormones (GA3, IAA and kinetin) on themorphology and early or delayed initiation of bud of lentil(Lens culinarisMedik). Pak. J. Bot. 36, 801–809. Nagel, L., Brewster, R., Riedell,W.E., Reese, R.N., 2001. Cytokinin regulation of lower and pod set in soybeans(Glycinemax(L.)Merr.). Ann. Bot. 88, 27–31. Nassef,D.,El-aref,H.,2018.EffectoffoliarspraywithIAAandGA3onproductionandprotein synthesis of two summer squash hybrid cultivars. Egypt. J. Hort. 45, 121–143. Nguyen,Q.T.,Kisiala,A.,Andreas,P.,Neil-Emery,R.J.,Narine,S.,2016.Soybeanseeddevelop-ment: fatty acid andphytohormonemetabolismand theirinteractions. Curr.Genomics17, 241–260. Nonokawa, K., Kokubun,M., Nakajima, T., Nakamura, T., Yoshida, R., 2007. Roles of auxin and cytokinin in soybean pod setting. Plant Prod. Sci. 10, 199–206. Nonokawa,K., Nakajima,T., Nakamura, T.,Kokubun,M.,2012. Effectofsyntheticcytokinin application on pod setting of individual loretswithin raceme in soybean. Plant Prod. Sci. 15, 79–81. Ogwu,M.C., 2018. Effects of Indole-3-Acetic Acid on the growth parameters of Citrullus lanatus(Thunberg)Matsum and Nakai.Momona Ethiop. J. Sci. 10, 109–125. Pan, B.Z., Xu, Z.F., 2011. Benzyladenine treatment signiicantly increases the seed yield of the biofuel plant Jatropha curcas. J. Plant Growth Regul. 30, 166–174. Ramadan, M.F., Moersel, J.T., 2002a. Proximate neutral lipid composition of niger (Guizotia abyssinica Cass.) seed. Czech J. Food Sci. 20, 98–104. Ramadan,M.F.,Moersel, J.T., 2002b. Direct isocratic normal-phase HPLC assay of fat- soluble vitamins and beta-carotene in oilseeds. Eur. Food Res. Technol. 214, 521–527. Ramadan,M.F.,Moersel, J.T., 2003. Determination of lipid classes and fatty acid proile of niger(Guizotia abyssinica Cass.) seed oil. Phytochem. Anal. 14, 366–370. Rastogi, A., Siddiqui, A.,Mishra, B.K., Srivastava,M., Pandey, R.,Misra, P., Singh,M.,Shukla, S., 2013. Effect of auxin and gibberellic acid on growth and yield components of lin-seed(LinumusitatissimumL.). Crop. Breed. Appl. Biotechnol. 13, 136–143. Roitsch, T., Ehness, R., 2004. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 32, 359–367. Santner, A., Calderon-Villalobos, L.I.A., Estelle,M., 2009. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 5, 301–307. Shimizu-Sato, S., Tanaka,M.,Mori, H., 2009. Auxin–cytokinin interactions in the control of shoot branching. PlantMol. Biol. 69, 429–435. Shundai, Li, Blanchoin, L., Yang, Z., Elizabeth,M.L., 2003. The putative Arabidopsis arp2/3complex controls leaf cellmorphogenesis. Plant Physiol. 132, 2034–2044. Taiz, L., Zeiger, E., 2006. Plant Physiology. 3rd edition. Sinauer Associates, Inc., Publishers, USA. Tanaka,M., Takei, K., Kojima,M., Sakakibara, H.,Mori, H., 2006. Auxin controls local cyto-kinin biosynthesis in the nodal stemin apical dominance. Plant J. 45, 1028–1036. Ullah, F., Bano, A., 2011. Effect of plant growth regulators on oil yield and biodiesel pro-duction of saflower(Carthamus tinctorius L.). Braz. J. Plant Physiol. 23, 27–31. Wang,M., Le Gourrierec, J., Jiao, F., Demotes-Mainard, S., Perez-Garcia,M.D., Ogé, L., Hamama, L., Crespel, L., Bertheloot, J., Chen, J., Grappin, P., Sakr, S., 2021. Convergence and divergence of sugar and cytokinin signaling in plant development. Int. J.Mol. Sci.22, 1282. https://doi.org/10.3390/ijms22031282. Werner, T., Schmulling, T., 2009. Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527–538. Werner, T.,Motyka, V., Laucou, V., Smets, R., Van Onckelen, H., Schmulling, T., 2003. Cytokinin-deicient transgenic Arabidopsis plants showmultiple developmental al-terations indicating opposite functions of cytokinins in the regulation of shoot and rootmeristemactivity. Plant Cell 15, 2532–2550. Yashima, Y., Kaihatsu, A., Nakajima, T., Nakamura, T., Kokubun,M., 2005. Effects of source/sink ratio and cytokinin application on pod set in soybean.Plant Prod. Sci.8, 139–144. Yuan, L., Liu, Z., Song, X., Johnson, C., Yu, X., Sundaresan, V., 2016. The CKI1 histidine ki-nase speciies the female gametic precursor of the endosperm. Dev. Cell 37, 34–46Dev. Cell.
确定





还剩7页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《小葵子含油率和脂肪酸含量的检测》,该方案主要用于食用植物油中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《小葵子含油率和脂肪酸含量的检测》用到的仪器有格哈特全自动快速索氏提取SOXTHERM、德国加液器MM、滤纸筒
推荐专场
相关方案
更多
该厂商其他方案
更多