玉米棒中高植物甾醇水平表明其作为营养来源可持续利用High phytosterol levels in corn cobs point to their sustainable use as a nutritional source
方案详情

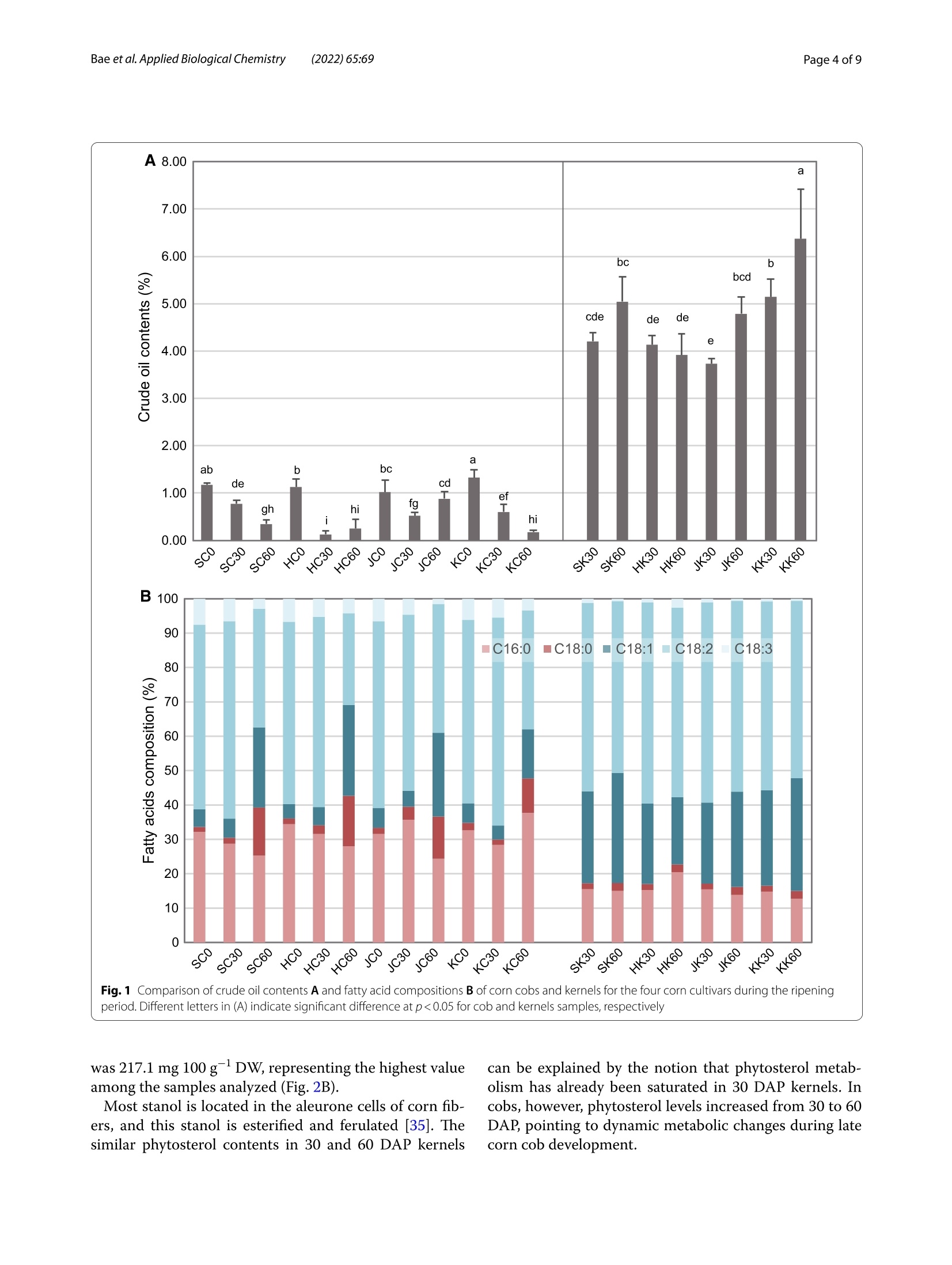
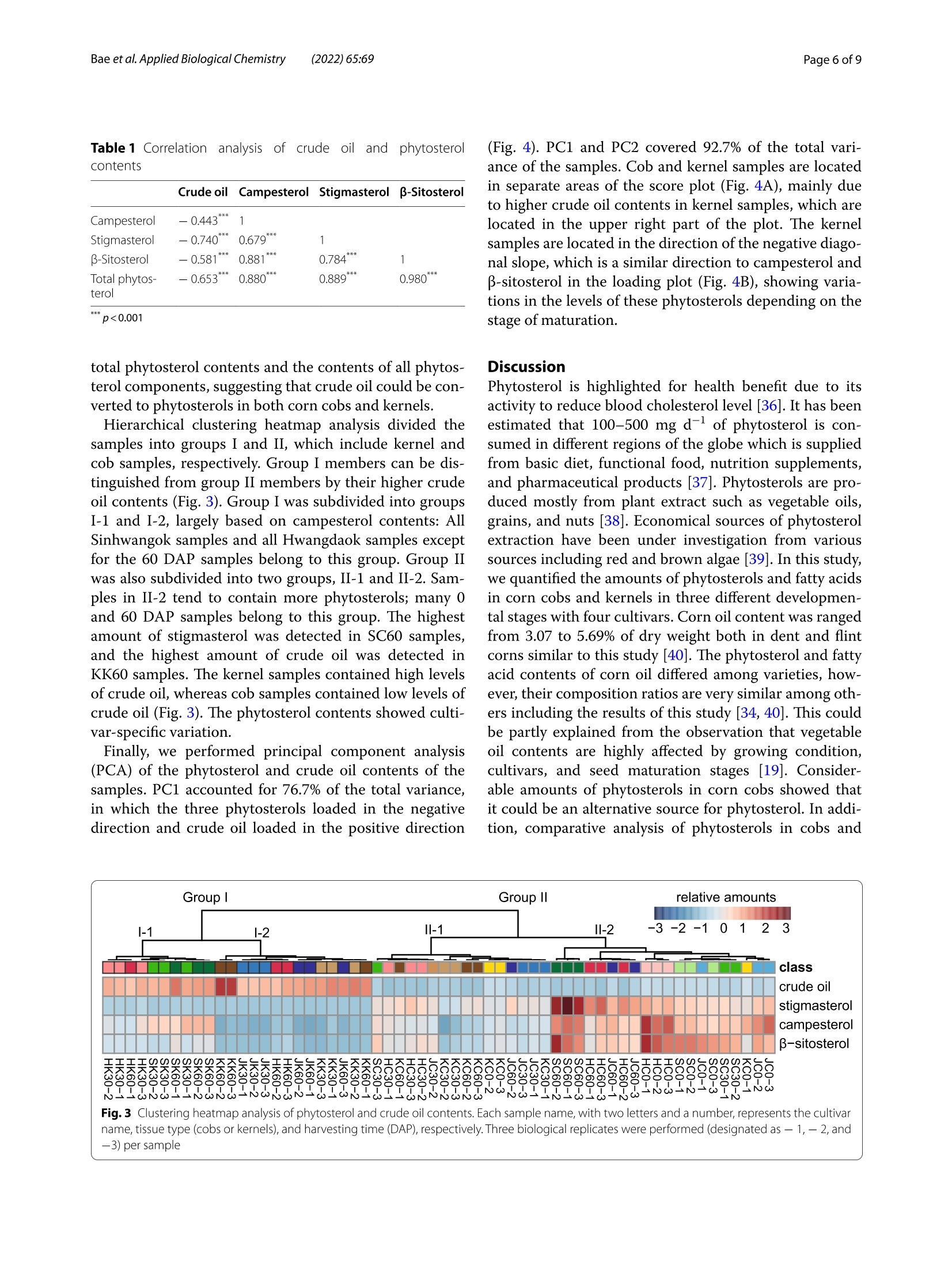
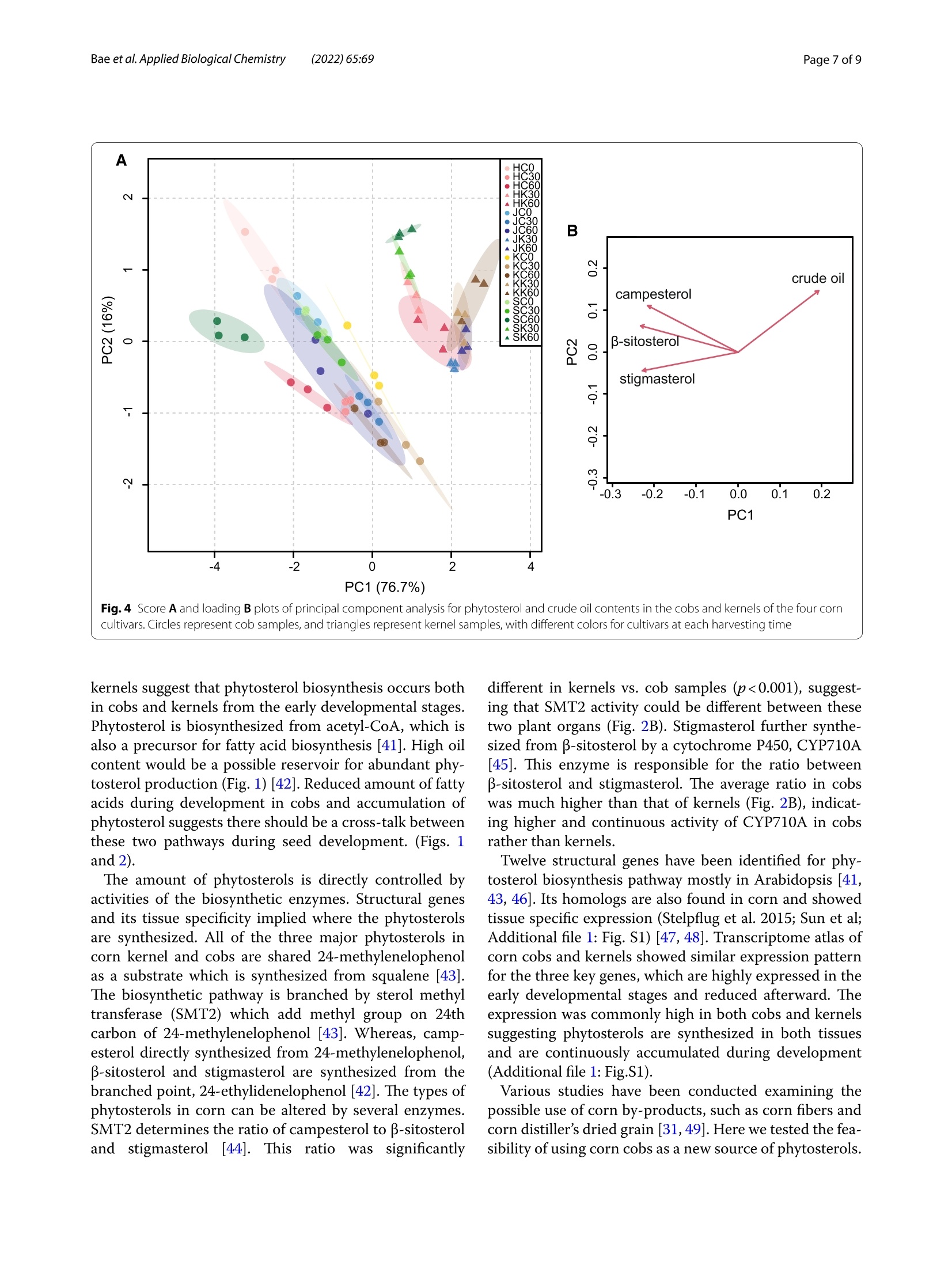
玉米棒中高植物甾醇水平表明其作为营养来源可持续利用High phytosterol levels in corn cobs point to their sustainable use as a nutritional sourceBae et al. Applied Biological Chemistry (2022)65:69https://doi.org/10.1186/s13765-022-00736-4KSABCThe Korean Society for Applied Biological Chemistry玉米棒中高植物甾醇水平表明其作为营养来源可持续利用ARTICLE Bae et al. Applied Biological Chemistry(2022) 65:69Page 2 of 9 Op en A cc e ss Check forupdates High phytosterol levels in corn cobs point to their sustainable use as a nutritional source Hwan-Hee Baelt , J un Young Ha't, Young Sam Go' , Jae-Han Son1, Beom-Young Son', Jae-Hong Kim²,Seonghyu Shin ,Tae-Wook J ung and Gibum Yi D Abstract Phytosterols are important structural component s of plant cel l s tha t affec t membrane f luidity, permeability,and membrane-related metabolic regulat i on. These compounds, which are abundant in vegetable oils and corn kernel oil , are also beneficial f or human heal t h. Cultivation of corn (Zea mays L.) prod u ces huge amounts of cobs as a by-product , but effort s to ut i lize cobs are still limited. Here, we invest i gated phytosterol, crude oil , and fatty acid contents in the kernels and cobs of four major corn cult i vars in South Korea and explored the potential use of cobs as a source of phytosterols. Total phytosterol levels were two t imes higher in cobs (68.0-217.1 mg 100 g-DW) than in kernels (43.8-89.5 mg 100 g-DW) and were highest i n the kernels and cobs of Sinhwangok at 60 days after poll i nation. We showed that not only kernels but also cobs can be a rich source of phytosterols . The results also revealed that the amount of phytosterol i s depending on a genetic background as well as developmental stages suggesting further investigation would enhance the ut i lization of corn cobs as a phytosterol source. Keywords: Maize, Cob, Phytosterol, B-sitosterol, Campesterol, Stigmasterol Introduction Phytosterols are not only important structural compo-nents of plant cells involved in regulating membrane f lu-idity, permeability, and membrane-related metabolism but also signaling molecules regulating diverse devel-opment processes [1-3]. Phytosterols are derived from squalene 2, 3-oxide and are found in a variety of terres-trial and aquatic plants [4]. Most phytosterols are 4-des-methylsterols; β-sitosterol, campesterol , and stigmasterol are particularly abundant in plants [2]. Different plant species have their own balance of phytosterols, depend-ing on their needs during growth and development [5,6]. For example, total phytosterol contents are high in oi l seeds such as nuts and rapeseed. Vegetable oils from soybean (Glycine max (L.) Merr.), wheat germ (Triticum H w an-H ee B ae and Jun You ng Ha h a ve c ont r ib ut ed e q ually t o thi s wor k *Corr e s p onde nc e: g ib um yi@cn u .ac.kr D epar t me nt of Bi o -Env i ronme nta l Che m i s t r y , Co llege of Agricu lt u r e a nd L i fe Scie nc es ,Chu ng nam Natio nal U ni versity , Daejeon, Repub lic of Ko r ea F ul l list of author i n format i on i s avai l a bl e at t he e nd of the a rt i cle aestivum L.), and corn (Zea mays L.) also contain high amounts of phytosterols [7]. Cereals are the main sources of phytosterols for the human diet, even though their phytosterol contents are not high, the large amount of cereal consumption in the average human diet provides more than 40% of daily phytosterol requirements [2, 8]. Corn, one of three major cereal crops, has the highest production among cereals worldwide, reaching 1,162 Mt of grain in 2020 (FAO, http://www.fao.org /fa ostat ). How-ever, for every 1 kg of dry corn grain produced, approxi-mately 0.15 kg of cobs i s also produced; therefore, about 174.3 Mt of corn cobs can be estimated to be produced in 2020. Corn cobs are mainly composed of cellulose (45%-55%), hemicellulose (25%-35%), and l ignin (20%-30%) [9, 10]. Corn cobs are l argely considered to be waste products, and efforts to use this agricultural waste have thus far been limited [11]. Studies on the use of corn cobs would enhance agricultural sustainability. Phytosterols are also beneficial for human health,especially for liver health, lowering cholesterol, and for preventing diabetes and other chronic diseases O T h e A u th o r (s ) 2022.Open Access T h is a rti c l e i s lice n sed under a Cre ati ve Co m mons At tri but i on 4.0 Intern a t i o n al L icense, w h ic h p ermits u se, sharin g , a d aptatio n , d istr ib utio n and re p rodu c tio n in an y m ed iu m or f ormat , as long a s you giv e a ppropri a te credi t t o t h e orig in a l author(s ) a n d t he s o urc e, provide a l in k to t h e C r e a ti v e Com m o n s l i c en ce , a n d i ndicate i f ch a nges w er e m ade. T he im a ges o r o t h e r t h i r d p ar t y mat e r ia l i n this a r t i c l e are i n c l uded i n t h e art i c l e 's Cre a tive Com m o n s l i ce n ce, unl e ss indicated oth e rwise in a cre d i t li n e to th e m ater i al . l f mate r i al is not i nc lu ded in t h e a r t i c l e's Creative Commons lic ence and y ou r in tended use i s not per m itted by s t atutory regul at ion or e xce eds t h e p ermi tte d u se, yo u will n e e d to ob ta i n p e rmissi o n di re c t l y from the cop yrigh t ho ld e r. To vie w a c opy of this l icence , v i s i t htt p ://c rea ti vecom m o n s .org/l ic e n s es/b y/4.0/. [12]. Promising evidence for the anticancer activities of phytosterols has also been reported [13, 14]. In tra-ditional Korean medicine, the water from boiling corn cobs is used for gargling to treat periodontal disease.Indeed, some studies have shown that phytosterols from corn cobs are beneficial for treating gum disease [15]. Recently, phytosterol s from an unsaponified frac-tion of corn oil are used as periodontal health sup-plementals [16, 17]. Phytosterol contents in corn cobs depending on developmental stages and genetic back-grounds are very limited compared to those in ker-nels [18]. In addition, vegetable oil contents are highly affected by growing condition, cultivars, and seed maturation stages [19]. The major sources of commer-cially available phytosterols are tal l oil (derived from wood pulp) and vegetable oi l s (corn oil, canola oil)[20]. However, these sources cannot satisfy increas-ing commercial demands [21]. Therefore, i n this study,we analyzed phytosterol contents in cobs and kernels from four corn cultivars at three time points to explore the possibility of uti l izing corn cobs as a source of phytosterols. Materials and methods Plant materials Determination of crude oil contents The crude oil content was quant i fied using a Soxtherm Automatic System (Gerhardt ; Bonn, Germany)[25].Three grams of freeze-dried tissue in 140 mL hexane with boiling stones was placed in an extraction thimble and heated at 180°℃ for 30 min to elute the crude oil from the sample. Extraction was repeated 5 times for 80 min, and the solvent was collected. After drying at 105℃ for 1 h,the extraction thimble containing the eluted crude oil was cooled, and the weight was measured to determine the crude oil content. Analysis of fatty acid composition The fatty acid composition was analyzed as described by Garces and Mancha[26]. Each 0.5gpow-dered sample was heated at 80℃ in 2 mL of the mixed solvent methanol:heptane:benzene:2,2-dimethoxypropane:H,SO4(37:36:20:5:2, by volume).After the reac t ion, the sample was cooled to room temperature, and the supernatant, including fatty acid methyl esters (FAMEs), was collected and analyzed by gas chromatography (GC). GC analysis was per-formed using an Agilent HP-Innowax capillary column (30 mx0.25 mmx0.25 um; Palo Alto, CA, USA) on a Shimadzu GC-2010 plus (Kyoto, Japan). The oven pro-gram was set at an initial temperature of 150℃ to a final temperature 240℃ with an increase of 3℃ min.The injector and FID temperatures were set at 250 and 260℃, respectively, and the analysis was performed in split mode with a split ratio of 10:1. The FAME standard mixture (C8-C22) was purchased from Supelco FAME (Bellefonte, PA,USA). Quantitative analysis of phytosterols The phytosterol contents of corn cobs and kernels were quantified by GC following saponification of the sam-ple [27]. Each freeze-dried powdered sample (100 mg)was combined with 4 mL of ethanol and 1 mL of 0.1 N ethanolic KOH and saponified at 95℃ for 1 h. After the saponification reaction, the samples were cooled to room temperature, and 5 mL of saturated NaCl and 10 mL of hexane were added to each sample. The supernatant was recovered after vigorous stirring. Extraction was repeated three times, and the recovered supernatants were con-centrated with nitrogen. After adding 1 mL of hexane and filtration through a 0.45 um syringe filter, the samples were analyzed by GC using a Shimadzu GC-2010 plus (Kyoto, Japan) and an Agilent DB-5MS UI (30 mx0.25mmx0.25 um; Palo Alto, CA, USA) column. The injector and FID temperatures were set to 270 and 300℃,respec-tively, and the oven temperature was increased from 105 to 272℃ at a rate of 20℃ min -. Campesterol, stigmas-terol, and B-sitosterol, which were used as standards,were obtained f rom Sigma-Aldrich (St. Louis, MO, USA). Statistical analysis All measurements S were performed withh three bio-logical replicates , and the values were expressed as mean±standard deviation. ANOVA 2and correlation analysis were performed with JAMOVI v1.2.27 (jamovi.org). Pr i ncipal components analysis (PCA) was per-formed on the autoscaled phytosterol profiles and crude oil contents. Hierarchical cluster analysis (HCA)Was based on Euclidean distance. PCA and HCA were per-formed with MetaboAnalyst (metaboanalyst.ca) [28]. Results Corn kernels have higher crude oi l contents than cobs Corn kernels had 5 to 36 times more crude oi l than corn cob samples, depending on the genetic background and developmental stage. The crude oil contents of cobs were 127.8-1331.1 mg 100 gDW, and those of kernels were 3735.6-6372.2 mg 100 gDW. The crude oil content was lowest i n HC30 (127.78 mg 100 gDW) and high-est i n KK60 (6372.22 mg 100 gDW). On average, cob samples contained 0.69% crude oil of total dry weight,whereas kernel samples contained 4.67% crude oil, which is 6.7 times more crude oil compared to cobs (Fig.1A). In three of the four cultivars examined, 60 DAP kernels con-tained more crude oil than 30 DAP kernels, and Hwang-daok showed a slight decrease in crude oi l contents in 60 DAP kernels. Since embryos contain more oil than endosperm, these changes in oil content in kernels might be related to the timing of embryo development [29]. In corn cob samples, the amount of oil was highest at 0 DAP and decreased after pollination as the cobs aged.Two cultivars (Sinhwangok and Kwangpyeongok) showed continuous decreases in oil contents, and the two other cultivars showed the lowest oil levels at 30 DAP. These results might be related to the differences in developmen-tal programs of the cultivars. Previously reported oil contents in corn kernels range from 3 to 4% [29, 30]. The cultivars used in this study showed a substantial range of genetic variation in crude oil contents. Since oil content in corn cobs during devel-opment has not been studied, the information from this study should be helpful for understanding the cob and kernel interaction during seed development. Corn cobs undergo significant changes in fatty acid composition during maturation There were c l ear distinctions in fatty ac i d composition between corn cobs and kernels. The proportions of pal-mitic (C16:0) and linolenic (C18:3) acids were markedly higher i n cobs than i n kernels. The palmitic acid contents ranged from 23.4%-37.7% (average 30.9%) in cobs and 12.7%-20.5% (average 15.4%) in kernels, and the l i nolenic acid contents were 1.5%-7.6% (average 5.1%) in cobs but only 0.6-2.6% (average 1.1%) i n kernels. The changes in fatty acid composition appeared to be more dynamic in cob samples compared to kernels. The cobs underwent signif i cant changes in fatty acid composi t ion, especially between 30 and 60 DAP, with stearic (C18:0) and oleic acids levels increasing by an average of 5.3- and 4.1-fold,respectively. No consistent changes in fatty acid com-position in kernels were observed in any of the cultivars examined. Commercial corn oil extracted from corn germ is rich in l i noleic acid (C18:2), with concentrations of up to 57.3% [31]. In this study, the l inoleic acid content was 26.7%-60.5% (average 47.7%) in cobs and 49.9%-58.6%(average 54.8%) in kernels (Fig. 1B), indicating that l in-oleic acid is the most abundant fatty acid in both corn cobs and kernels. Increasing levels of stearic and oleic acids from 30 to 60 DAP in corn cobs coincided with decreases in palmitic acid levels. This observation i s rea-sonable, since palmitic acid i s elongated to stearic acid,which is then converted to oleic acid by stearoyl-CoA desaturase [32]. Linolenic acid levels in kernels are a determinant of the quality of corn oils [33]. Therefore,the higher level of linolenic acid in cobs is notable and should be further i nvestigated. Higher phytosterol levels accumulate in corn cobs vs.kernels Corn oil contains free phytosterols and esterified (fatty acyl) phytosterols. Among the various phytosterols, we analyzed three phytosterols, campesterol, stigmasterol,and β-sitosterol, representing the three major phytoster-ols found in corn oil [17, 31, 34]. These three phytosterols were clearly separated by GC under our conditions, using retention times (RTs) of 19.663, 20.119, and 21.084 min (Fig.2A). The cobs contained higher levels of phytosterols than kernels in all four cultivars analyzed, which ranged from 86.5 to 217.1 (average 131.4) mg 100 g -DW i n cobs and 43.8 to 89.5 (average 61.2) mg 100 g -DW in kernels. The phytosterol contents in corn cobs were the lowest in 30DAP samples, which fluctuated during the 0 to 60 DAP period. The increases i n phytosterol content s in 60 DAP cobs were strongly related to the increases in stigmas-terol levels; this tendency was not observed in kernels. Although the distributions of the three phytosterols varied based on genetic and developmental differences,β-sitosterol was the most abundant phytosterol in both cobs and kernels. The total phytosterol content of SC60 a ab b bc工de 工T cd fgeTgh hihi Fig.1 Compar i son of cr u d e oil co n tents A and fa t t y a c i d composi t io n s B of corn co b s a n d k ernels f or t h e f o u r co rn c u l t iva r s du r i n g t h e ri pe n i i r ng period . Di f feren t l etters in (A) i nd i cat e sign if ica n t di f ference at p<0.05 f or cob an d kern el s samples, r es p ect i v el y was 217.1 mg 100 g-DW, representing t he highest value among the samples analyzed (Fig. 2B). Most stanol is located in the aleurone cells of corn fib-ers, and this stanol is esterified and ferulated [35]. The similar phytosterol contents in 30 and 60 DAP kernels can be explained by the notion that phytosterol metab-olism has already been saturated in 30 DAP kernels. In cobs, however, phytosterol levels increased from 30 to 60DAP, pointing to dynamic metabolic changes during late corn cob development . Fig. 2 Quant i f icat i on of p h yt o sterols in cor n sample s . A chromatogr am s of p hytos t erols in cob a nd kernel sa m p le s of co rn cu l ti var Si n hw a ngd obta in ed by GC a n alysis. Cob and kerne l s am p les are colored ba s ed on harvest ti m e. B c o mparison of phytostero l contents b etween corn cobs and ke rn el s du r ing ri p e n ing. Different l e tt ers in dic a te s i gni fi cant dif f ere n ce in to t al phytosterol conte n ts at p<0.05 Four cultivars are distinguished by their oil contents and phytosterol profiles The crude oil content showed a negative correlation with campesterol , stigmasterol, B-sitosterol, and total phytos-terol contents (Table 1) in the corn samples. The total phytosterol content had the most significant correlation with p-sitosterol content (r=0.980) among the three phytosterol components, since B-sitosterol comprises the largest proportion of total phytosterols. The crude oil content showed signi f icant negative correlations with Table 1 Corre l ation 12analysis of cr u de oil 12and phytosterol contents Campesterol -0.443*** 1 Stiqmasterol -0.740*** 0.679** 1 B-Sitosterol -0.581*** 0.881*** 0.784*** Total phytos- -0.653*** 0.880*** 0.889*** 0.980*** terol ***p<0.001 total phytosterol contents and the contents of all phytos-terol components, suggesting that crude oil could be con-verted to phytosterols in both corn cobs and kernels. Hierarchical clustering heatmap analysis divided the samples into groups I and II, which include kernel and cob samples, respectively. Group I members can be dis-tinguished from group II members by their higher crude oil contents (Fig.3). Group I was subdivided into groups I-1 and I-2, l argely based on campesterol contents: All Sinhwangok samples and all Hwangdaok samples except for the 60 DAP samples belong to this group. Group II was also subdivided into two groups, II-1 and II-2. Sam-ples in II-2 tend to contain more phytosterols; many 0and 60 DAP samples belong to this group. The highest amount of stigmasterol was detected in SC60 samples,and the highest amount of crude oil was detected in KK60 samples. The kernel samples contained high l evels of crude oil, whereas cob samples contained low levels of crude oil (Fig.3). The phytosterol contents showed culti-var-specific variation. Finally, we performed principal component analysis (PCA) of the phytosterol and crude oil contents of the samples. PC1 accounted for 76.7% of the total variance,in which the three phytosterols loaded in the negative direction and crude oil loaded in the positive direction (Fig. 4). PC1 and PC2 covered 92.7% of the total vari-ance of the samples. Cob and kernel samples are located in separate areas of the score plot (Fig. 4A), mainly due to higher crude oil contents i n kernel samples, which are located in the upper right part of the plot. The kernel samples are located in the direction of the negative diago-nal slope, which i s a similar direction to campesterol and B-sitosterol in the loading plot (Fig. 4B), showing varia-tions i n the l evels of these phytosterols depending on the stage of maturation. Discussion Phytosterol is highlighted for health benefit due to i ts activity to reduce blood cholesterol level [36]. I t has been estimated t hat 100-500 mg d-of phytosterol is con-sumed in different regions of the globe which is supplied from basic diet, functional food, nutrition supplements,and pharmaceutical products [37]. Phytosterols are pro-duced mostly from plant extract such as vegetable oils,grains, and nuts [38]. Economical sources of phytosterol extraction have been under investigation from various sources i ncluding red and brown algae [39]. In this study,we quantified the amounts of phytosterols and fatty acids in corn cobs and kernels in three different developmen-tal stages with four cultivars. Corn oil content was ranged from 3.07 to 5.69% of dry weight both in dent and flint corns similar to this study [40]. The phytosterol and fatty acid contents of corn oil differed among varieties, how-ever, their composition ratios are very similar among oth-ers including the results of this study [34,40]. This could be partly explained from the observation that vegetable oil contents are highly affected by growing condition,cultivars, and seed maturation stages [19]. Consider-able amounts of phytosterols in corn cobs showed that it could be an alternative source for phytosterol . In addi-tion, comparative analysis of phytosterols in cobs and n a me, ti s s u e type (cobs or k ernel s ), and harvesti n g t im e (DAP), re s pec t ively. Three b io l ogic a l replicates were per f ormed (des i gnated a s - 1, - 2,a nd -3) per sample Fig.4 Score A and loading B plo ts of pr inc i p al component an al ysis f or phytos ter ol a n d crude oil c onte n t s in th e cobs a n d k er nels of t h e fou r corn c u ltiva r s . C i rcles re p resent cob samples, a n d tr i angles rep re sent k ernel samples, with dif f e r e n t colors f or cul t i v ars at each h a r vesting time kernels suggest that phytosterol biosynthesis occurs both in cobs and kernels from the early developmental stages.Phytosterol is biosynthesized from acetyl-CoA, which is also a precursor for fatty acid biosynthesis [41]. High oil content would be a possible reservoir for abundant phy-tosterol production (Fig. 1) [42]. Reduced amount of fatty acids during development in cobs and accumulation of phytosterol suggests there should be a cross-talk between these two pathways during seed development . (Figs. 1and 2). The amount of phytosterols i s directly controlled by activit i es of the biosynthetic enzymes. Structural genes and its t issue specificity implied where the phytosterols are synthesized. All of the three major phytosterols in corn kerne l and cobs are shared 24-methylenelophenol as a substrate which is synthesized from squalene [43].The biosynthetic pathway is branched by sterol methyl transferase (SMT2) which add methyl group on 24th carbon of 24-methylenelophenol [43]. Whereas, camp-esterol directly synthesized from 24-methylenelophenol,B-sitosterol and stigmasterol are synthesized from the branched point, 24-ethylidenelophenol [42]. The types of phytosterols in corn can be altered by several enzymes.SMT2 determines the ratio of campesterol to β-sitosterol and stigmasterol [44]. Thi 1s Sratio Was significantly different in kernels vs. cob samples (p<0.001), suggest-ing that SMT2 activity could be different between these two plant organs (Fig. 2B). Stigmasterol further synthe-sized f rom β-sitosterol by a cytochrome P450, CYP710A [45]. This enzyme is responsible for the ratio between B-sitosterol and stigmasterol. The average ratio in cobs was much higher than that of kernels (Fig. 2B), indicat-ing higher and continuous activity of CYP710A in cobs rather than kernels. Twelve structural genes have been identified for phy-tosterol biosynthesis pathway mostly in Arabidopsis [41,43, 46]. Its homologs are also found in corn and showed tissue specific expression (Stelpf l ug et al. 2015; Sun et al;Additional f ile 1: Fig. S1) [47, 48]. Transcriptome atlas of corn cobs and kernels showed similar expression pattern for the three key genes, which are highly expressed i n the early developmental stages and reduced afterward. The expression was commonly high in both cobs and kernels suggesting phytosterols are synthesized in both t issues and are cont i nuously accumulated during development (Addi t ional file 1: Fig.S1). Various studies have been conducted examining the possible use of corn by-products, such as corn fibers and corn distiller’s dried grain [31, 49]. Here we tested the fea-sibility of using corn cobs as a new source of phytosterols. The total phytosterol content of cobs ranged from 68.0 to 217.1 mg 100 g -DW, which is higher t han that of ker-nels (43.8-89.5 mg 100 g -DW). The total phytosterol content differed depending on the genetic background of the plant and the developmental stage, but the total phy-tosterol content of cobs was always higher than that of kernels (Fig. 2B). Supplementary Information The onl i ne ve r s i on con t ains sup pl em e nta r y m a te r ia l avail a bl e a t http s ://do i .or g /10.1186/s 13765-022-00736-4. Additional file 1. Supple m ent a ry Fig . S1. Ex pr e s sio n leve l s o f the thre e key genes f or phytoste r ol biosynt h esis in c obs an d kernels in di ff erent deve l opme nt a l stages . Data from qTell e r in m a i zeg d b (ht t p s ://q t el l er .maize g db.o r g /) were red r awn f o r s elected t i ssue s and devel op mental stages . Acknowledgements The a uthors a ppreci a t e anonymous r eviewers. Author contributions Conceptua l iz a tio n: B H H , H J Y , YG; Formal analys i s a nd Data c ur at i on: BH H ,HJ Y ,Y G ; Resources: GYS , SJ H , SBY, SS,JTW;Validatio n a n d V i sualiza t ion: BH H ,HJY, KJH , Y G ; W r it i ng: BH H , HJY , YG. Al l authors read and approved t he final m an usc r ipt . Funding Th i s wo r k was car r ied out wi t h the suppo r t of t he'Cooperative Research Prog r am for Agr i cu l ture Scie nc e & Te c hn o logy De v e l opment in Rur al D evelop-m e nt Administration (Pro j ect No.PJ01429201), Repu b li c of K orea. Availability of data and materials The d a tase ts used a nd analy z ed in t hi s s tudy are ava i l a ble f r om t he c or r e-sp o nd i ng author upon reasonable req ue st. The pl ant materials are commer -c ia lly a vai l a b le . Declarations Competing i nterests The authors d eclare t ha t t he y have no competing interest s . Author details D epart m ent of C e ntr a l Are a C r o p Scie nc e, Nat i onal I ns ti tute o f Cro p S cience,R u ral D evelo pm ent Adm in istration, Suwon, Repub l ic of Korea.De pa rtm e nt of Bio-E n v i r o n me nta l Chemistry, Col l ege of Agr i cult u re a nd Lif e Sc i enc e s,Chung nam Nat i onal Univer sit y , Dae j eon, R e pu blic of Korea. Received: 7 July 2022 Accepted: 14 October 2022Published online: 01 November 2022 References 1. B outteY, J ai llais Y (2020) Metabolic cel l ular commu n ic at ions: feedback mechanisms between mem b rane l i pid homeos t as i s a nd plant d e v e lop-ment . Dev Ce ll 54:171-182 2. Mo r eau RA, Ny s t r om L , Whit a ker BD , Winkler-Moser J K, B aer DJ, Gebauer SK et al (2018) Phytosterols a n d t heir d e r i vativ e s: S t ruct ur al diver s ity,d i st r ibutio n, m e tabol i sm, analysis , and health-promoting uses . Prog Lip id R e s 70:35-61 3. P riyadarsini VR, Nagi ni S (2012) C a ncer chemopreve nti on by dietary phyt ochemicals: pr omises a nd pit f a l ls . Cu r r Pharm B iotec h nol 13:125-136 4 Tr a utwei n EA, Demontyl (2007) Ph ytoster o ls: na tur a l c omp o unds wi t h establ i shed and emerging he a lt h b enefits . Oleag i neux, Corp s Gras,Lipides 14:259-266 5. Valitova J N, S ulk a rnayeva AG , Min i bayeva F V (2016) P l ant sterols : diver s i t y,biosynt he s is , and phy s i o l o gic a l f un c t ions . B iochem 81:819-834 6 Sc ha l l er H (2003) The ro l e of ste r ols i n pla nt growth and developm e nt.Progres s Li pi d Res 42:163175 7 Normen L , E ll e g ard L, Br ant s H, D u tta P ,A nderss o n H (2007) A phytosterol d a ta ba se: f a tt y fo od s consu m e d i n Swe de n and the Net h er la nd s. J Food Compos Ana l 20:193-201 8. Valsta LM,Lemst r om A,Ovas k ainen ML, Lampi AM, T oivo J, K o r hon e n T,P i irone n V et al (2004) E s timat i on of plant s t erol and chole s terol i ntake in Fi nland: q u a l ity of new values a nd t hei r effe ct o n int a ke. B r J N ut r 92(4):671-678 9 Kanengo n i A T, Chimonyo M ,N dim b a BK , D zama K (2015) Potentia l of us i ng maize cobs in p i g d iets -A r e v iew. A si a n-A us t ra l asian J A n i m Sc i 28:1669 10.Zhang Y , Gh a ly AE, Li B (2012) Physical pr operties of c orn residues . Am J Biochem Biot ec hnol 8:44-53 11.Ja nse n C. (2012). Breedi ng for cob t ra its in maize. Disse r t at ion, lowa State U n iver s ity. A m es , IA 12.Br u f a u G , C anel a MA, Ra f ecas M (2008) Phy t os t erols: physio l ogic and meta b olic aspects rel at ed to c ho les t e r ol -l ow e ring pr opert i es. N u t r Res 28:217-225 13.S ha h z ad N , K han W, Sha d ab MD, Al i A, S a lu ja SS, Sharm a S et al (2017)Phyto s terols as a na t u r al anti ca ncer agent : c ur rent status and f uture perspect i ve. Biomed Phar m acothe r 88:786-794 14.Ramp ra s at h VR, A wad A B (2015) Role of phyto s terols in cancer pr eve nt ion a nd tr e atment . J AOAC I nt 98:735-738 15.Othman R A, Moghadasian MH (2011) Beyond choles t erol -l owe r ing effects o f plant s t e r ols: clin i cal and e xpe r ime nt a l e videnc e o f a nt i -i nf lam-ma t ory prope rt ie s . Nutr R ev 69:371-382 16.Kim D , Par k JB , Choi WK , L ee SJ, L i m l , Bae SK (2016) Simu lt aneous deter mi nat i on of B -sitostero l , campeste r ol ,and sti g master ol i n rat plasma by using L C-APC I -MS/MS: application in a pharmacokinetic study of a t i trated extract of t he u nsa poni fi a bl e fr a ction of Ze a m ay s L. J Se p S c i 39:4060-4070 17. Kim SL , K im MJ, Ju n g GH,Lee YY , Son BY , K i m J T et al (2018) I d entificat i on a nd qu ant if i cati o n of phy t os t e r ols i n maize k e rnel and cob. K or ean J Crop Sc i 63:131-139 18.Dee p ak T S , J aya d e ep P A (2021) Pr osp ect s o f m a i z e (c or n) we t mil l ing by prod uc ts as a so u rce of f unct i onal f o od ingred i ents a nd nu t ra ceuticals .Food T echnol Biote c hnol 60:109-120 19.Phillips KM, Ruggio DM, Ash raf -K ho rassa n i M (2005) Phy t os t e r ol composi-t i on of nut s and s ee d s commo n ly co nsumed in t he U n ited S tat es. J Agri c Foo d Chem 53:9436-9445 20.Randhi r A, Laird DW, Maker G, Tre ngov R, Moh ei m ani N R (2020) Micro -a l g ae: a potent i a l sust a inable commer c ia l sour c e of s t erols . Al g a l Res 46:101772 21.Bacc he t t i T, Masciangelo S, Bicch i ega V, Bertoli E, Fe r ret t i G (2011) Phy-tosterols, phy tostanols and the ir este r s: f r om nat ur al t o f u nct i ona l foods.Med J Nu t ri t ion Metab 4:165-172 22.Son BY, B ae k S B, Kim JT, Lee JS , Bae HH, Par k C H et a l (2017) S ing le c r oss maize hybr i d f o r gra i n,Si n hwangok. Kor e an J Breed Sci 49:109-112 23.Son BY, Ba e k SB, Kim JT, Lee JS , Bae HH, Go YS e t a l (2019) Single c r oss maiz e hybrid'H w angdaok'for hig h g rain yie l d . Korean J Breed S c i 51:105-109 24.Ri t ch ie SW, H anway J J , Benson GO.(1993). How a cor n plant develops (s p eci a l repor t No.48). lowa S tate U n ivers i ty. Ames, IA 25.Lee Y Y , P ar k H M, Hwang TY, Kim S L, K i m MJ, L ee S K et al (2015) A cor r ela-t i on betwe e n t oc o phe r o l content and ant iox i dant ac t i vit y i n seeds a nd g erminating seeds of soybean cul t ivar s . J S c i Food Agr ic 95:819-824 26.Garc es R , Mancha M (1993) One -ste p li pid extract i on and f at t y a c id methyl es t e rs pr epara t ion fro m fresh p l ant t i ssues . Ana l Bi o chem 211:139-143 27. J eke l AA, V a essen HAMG, Schothorst RC (1998) C a pillary gas-chromato-gr a phic method for det e rmin i ng non-derivat i zed sterols—s ome r e sults for duplicate 24 h d i et samples col l ec t ed in 1994. Frese ni us J Ana l Chem 360:595-600 28.Pang Z, Z ho u G , E w al d J , Chang L , H a ca r iz O, B a su N e t a l (2022) U s in g MetaboAnaly s t 5 0 f o r LC -H R MS spect r a processing,mu lt i-o mi cs integr a-tio n a nd covar i at e a d jus t me nt o f globa l m e t abo l omics data. N at Protoc 17:1735-1761 29. S he n B, Allen WB, Z he n g P, L i C , G las sman K, R anch J e t a l (2010) Expres-sion of Z mLE C1 a nd ZmW RI 1 increases seed oil produc t ion in m ai ze.Pl a n t physio l 153:980-987 30.Yang X, Ma H , Zhang P , Yan J , Guo Y, Song T et al (2012) Characterizat i on of QTL for oil c o n te n t i n m aiz e k e rnel. Theor Appl Gene t 125:1169-1179 31.Morea u RA, La mp i AM, H ic ks KB (2009) Fat t y ac i d , phytos t e r ol, a n d poly -ami ne c on j ugate profiles of edibl e oils extracte d from cor n g e rm , cor n f i ber, and corn k er n els. J Am Oil Chem Soc 86:1209-1214 32.K im J T , Yi G, Chu n g IM, Son BY, B a e H H , Go YS et a l (2020) Timing an d p attern of a nt hocyani n a ccumu la tio n during g r ai n f illi ng in pur ple waxy c or n (Ze a may s L.) sugges t optimal har vest dates . AC S Omega 5:15702-15708 33.W hi te PJ , Web er E J . 2003 L i pids o f th e k e rne l, in Co r n: Che mistr y an d Tech no logy,ed . By Whi t e PL and Jo h nson LA. Am e r i ca n Associat i on o f Ce r eal Chemists, St Pau l, MN 355-406 34.H arrabi S, St-A m and A , Sa k ou hi F, Sebei K , Kallel H, Ma y e r PM e t al (2008)Phyt os t anol s and phy tostero ls d i st r ibuti ons in cor n k e r ne l. Fo o d Che m 111:115-120 35.Morea u R A, Singh V, N unez A, Hicks KB (2000) Phytoster ol s in the ale u-r o ne laye r of c orn kernel s . Biochem Soc Trans 28:803-806 36.O s t l un d RE J r (2002) Phy tos t e r o ls in human n u t r i t i on. A nn u R ev N u t r 22:533-549 37.Jie F, Ya ng X, Wu L,Wang M, L u B (2022) Linki n g phytosterols a nd oxy -phy t osterols f r om food to bra i n heal t h: origins , ef f ect s , a nd under l ying m ec ha ni s ms. Cr i t Rev F o o d Sc i N u t r 62:3613-3630 38 Zhan g R, Han Y, McClement s DJ , Xu D , Chen S (2022) P r oduct i on,charac -t e rizati o n,delivery, a nd chol e st e r ol-lower ing mechan i sm of phytost e ro l s :a review. J Agric Food Chem 70:2483-2494 39.H annan MA, Sohag AAM, D a s h R, Haqu e MN, Mo hi bbul l ah M, Okt av i a ni D F et a l (2020) Phyto s terol s o f ma rine algae: ins i ght s into t he potential he al t h b enefits and mol e cu lar pharm a cology. Phyto m edicine 69:153201 40.E sche R, Scho lz B, Enge l KH (2013) A nalysi s of f r ee phytosterols/s tano l s and thei r i nt a c t f a t t y a c i d a nd pheno li c ac i d e s ter s in var i ous co r n cul t i-vars. J Cerea l Sc i 58:333-340 41D e V r i e se K, Pollier J, G o oss e ns A, Be e ckman T ,Van ne ste S (2021) Dissect-ing cholesterol and ph ytosterol biosynthe sis vi a muta nts and inh i bi t or s.J E xp B o t 72:241-253 42.Sonawane P D , Pollie J , P anda S, Szymansk i J , Massalha H, Y o n a M et al (2016) Pl an t chol e st e r ol bi osyntheti c pa t hway ov er laps wi t h phy tosterol metabo li sm.Nat Pl a nts 3:16205 43.N e s WD (2000) S terol methyltr ansf erase : e nz ymo lo gy and i n hi b it i o n.B iochim B iophys Ac t a 1529:6388 44.N akamoto M, Schmit AC, H e int z D , Sc ha l l er H, Ohta D (2015) Dive r si -f i cati o n of s terol me thy l t ra nsferase enzym e s i n plant s a nd a rol e for β -si t o s t e r ol in or i ented cell p la te for m ati o n and polari z e d growth. PlantJ 84:860-874 45.Mo r ika w a T , Mizutani M, Aoki N , Watanabe B, Saga H, Saito S et a l (2006)Cytochro me P450 C Y P710A encod e s t he ster ol C-22 d e s aturase in A ra bi -dopsi s a nd t om a t o . Pl a nt Cel l 18:1008-1022 46.B e nv enis t e P (2004) B iosynthe s is a nd accum u l at ion of s t erols . Annu Rev Pl a n t Bio l 55:429-457 49 R y a n E , G al vin K,O'Connor TP , Mag u ir e A R ,O'Brie n NM (2007) P h y t osterol ,s qualene, t ocophe r ol c ontent and fatty ac i d profile of s elec t ed seeds ,grains, an d leg u me s . Pl a nt F o o ds Hu m Nu t r 62:85-91 Publisher's Note Spr i nger N a ture r e m ai ns neut r al wit h regard t o jurisdic tio nal c l ai ms in p u b-lished maps and i n st i tutio na l a f f l i at ions . Su b m it yo u r m a n u s cri pt to a Sp r i n ger Op e n°回 i o urna l an d be n ef i t from: Convenie n t onli n e submission R igorous peer review Open access: articles freely available onl in e H igh visibility within the f ie l d R etaining the copyright to your a r t i c l e S ub mi t you r next ma nus cript at D sp ring e rop e n.com
确定






还剩7页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《4个玉米品种玉米棒含油率和脂肪酸含量的检测》,该方案主要用于食用植物油中营养成分检测,参考标准《GB/T 5512 粮油检验 粮食中粗脂肪含量测定》,《4个玉米品种玉米棒含油率和脂肪酸含量的检测》用到的仪器有格哈特全自动快速索氏提取SOXTHERM、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多