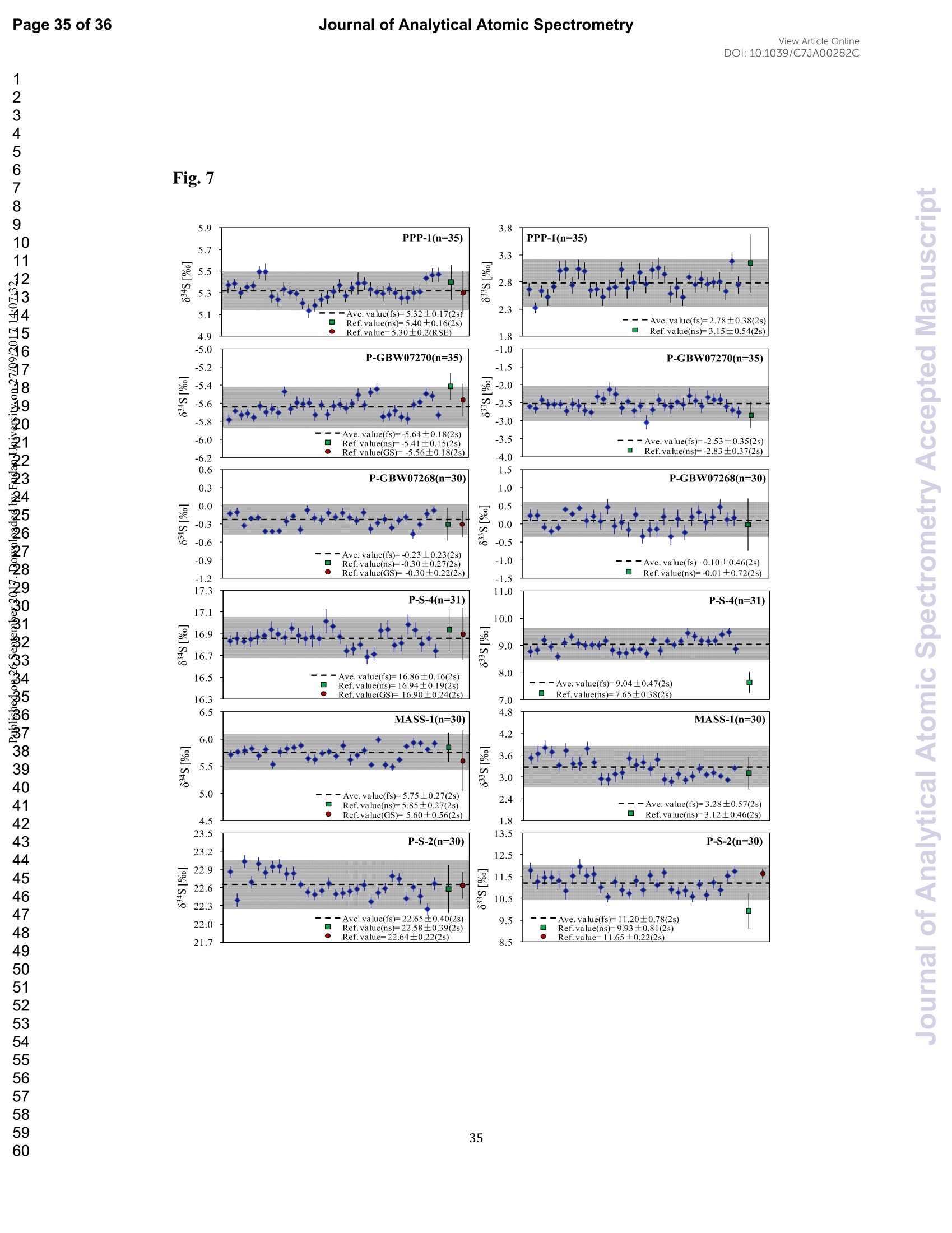
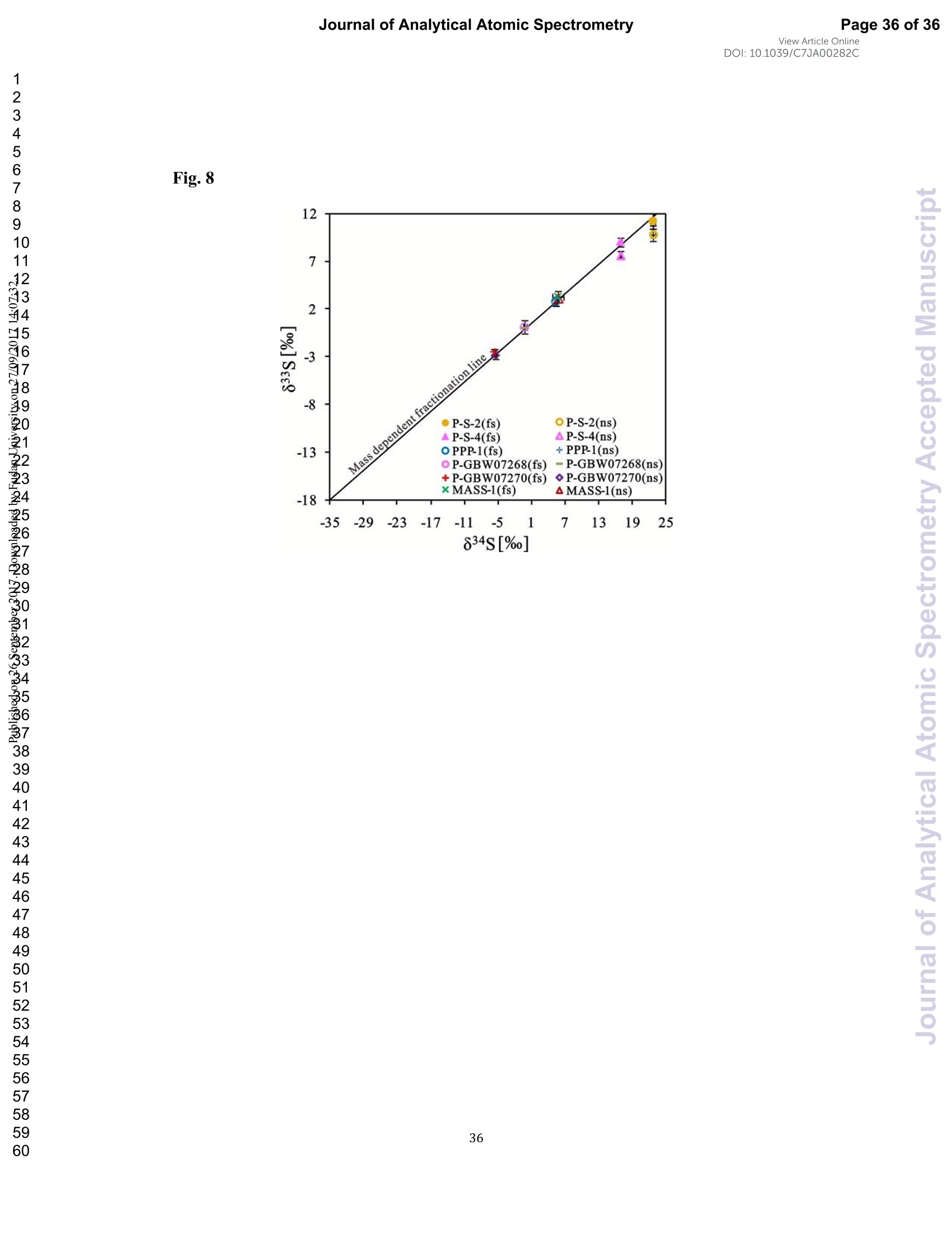
硫同位素在地球科学的多个领域中是一种重要的地球化学示踪剂。在这项研究中,采用257nm飞秒(fs)和193nm ArF准分子纳秒(ns)激光剥蚀系统结合Neptune Plus MC-ICP-MS,研究了不同基质富硫矿物(硫化物和元素S)中激光和等离子体等离子体诱导的同位素分离法。与ns-LA-MC-ICP-MS相比,在相似的仪器条件下,fs-LA-MC-ICP-MS具有更高的灵敏度(1.4-2.4倍),在相同的信号强度条件下,具有更好的精度(~1.6倍)。此外,与ns激光相比,fs激光对S同位素分离的影响更小,对基质的依赖性更小,瞬态同位素比更稳定。由于更小的粒子尺寸和飞秒激光更低的热效应,使用fs-LA-MC-ICP-MS可以得到更佳的测定结果。这一点可以通过P-S-1(IAEA-S-1压粉球团)和PPP-1(苏霍伊原木矿床中黄铁矿单晶)的剥蚀坑和喷射的气溶胶来证明。在*灵敏度条件下,fs-LA-MC-ICP-MS仍然存在等离子体诱导的同位素分离(基体效应)。然而,针对S同位素分析,在低较低的组成气体流速(0.52-0.54Lmin-1)稳定等离子体条件较*灵敏度条件(0.6Lmin-1)下,基体效应显著降低。这可以归结为粒子不仅在较高的温度下以较低的组成气体流速进入ICP,停留时间更长,从而使粒子雾化效率更高,同时在等离子体中加入4-6mL min-1 N2也能增强稳定性。此外,在稳定的等离子体条件下,对六种不同基体的参考材料使用fs-LA-MC-ICP-MS在20-44 µm光斑处不使用基体匹配校准进行测定,测定结果与参考值一致。验证了该方法非常适用于在高空间分辨率条件下利用非基体匹配分析提供高质量的硫元素和硫化物原位微区同位数据。
方案详情

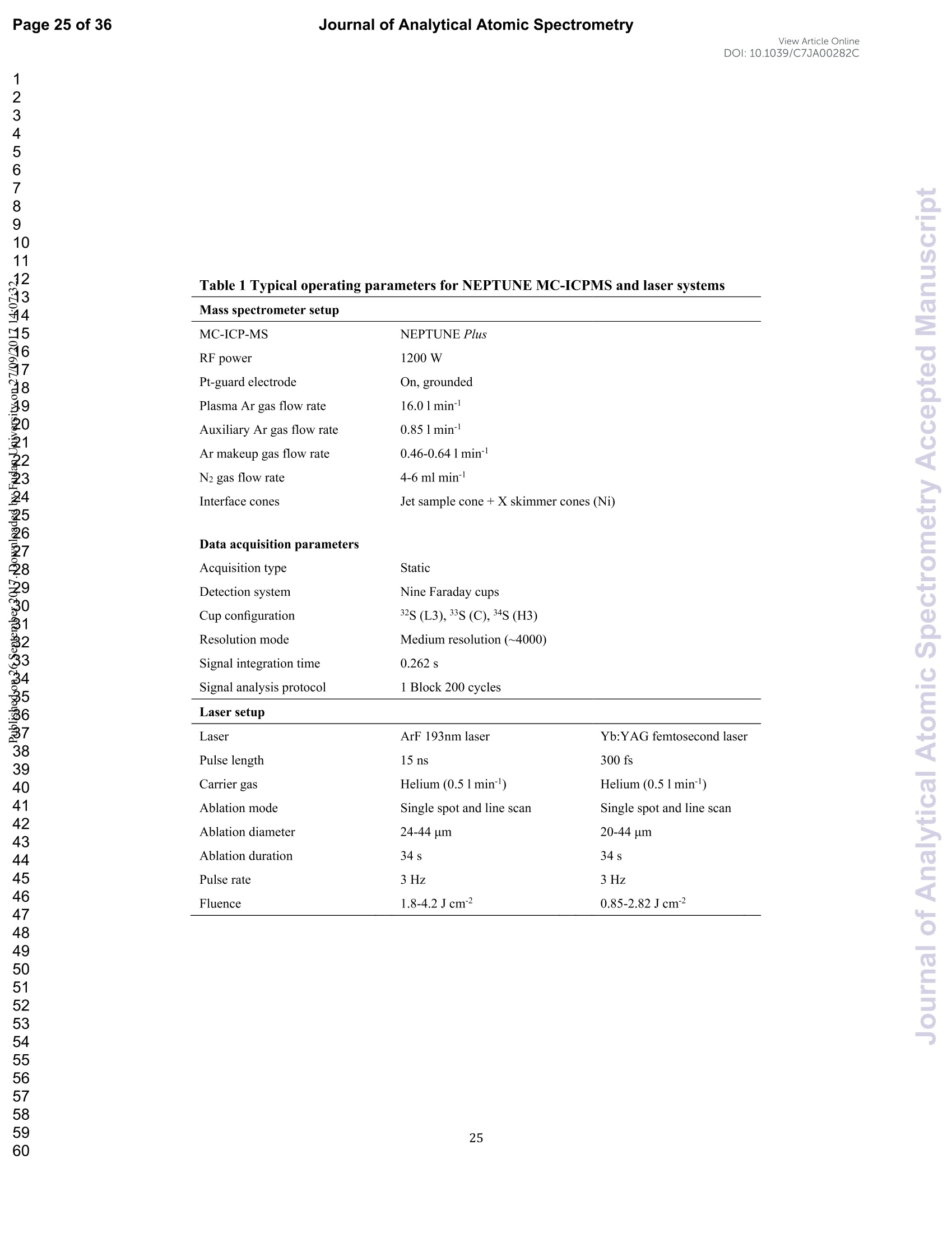
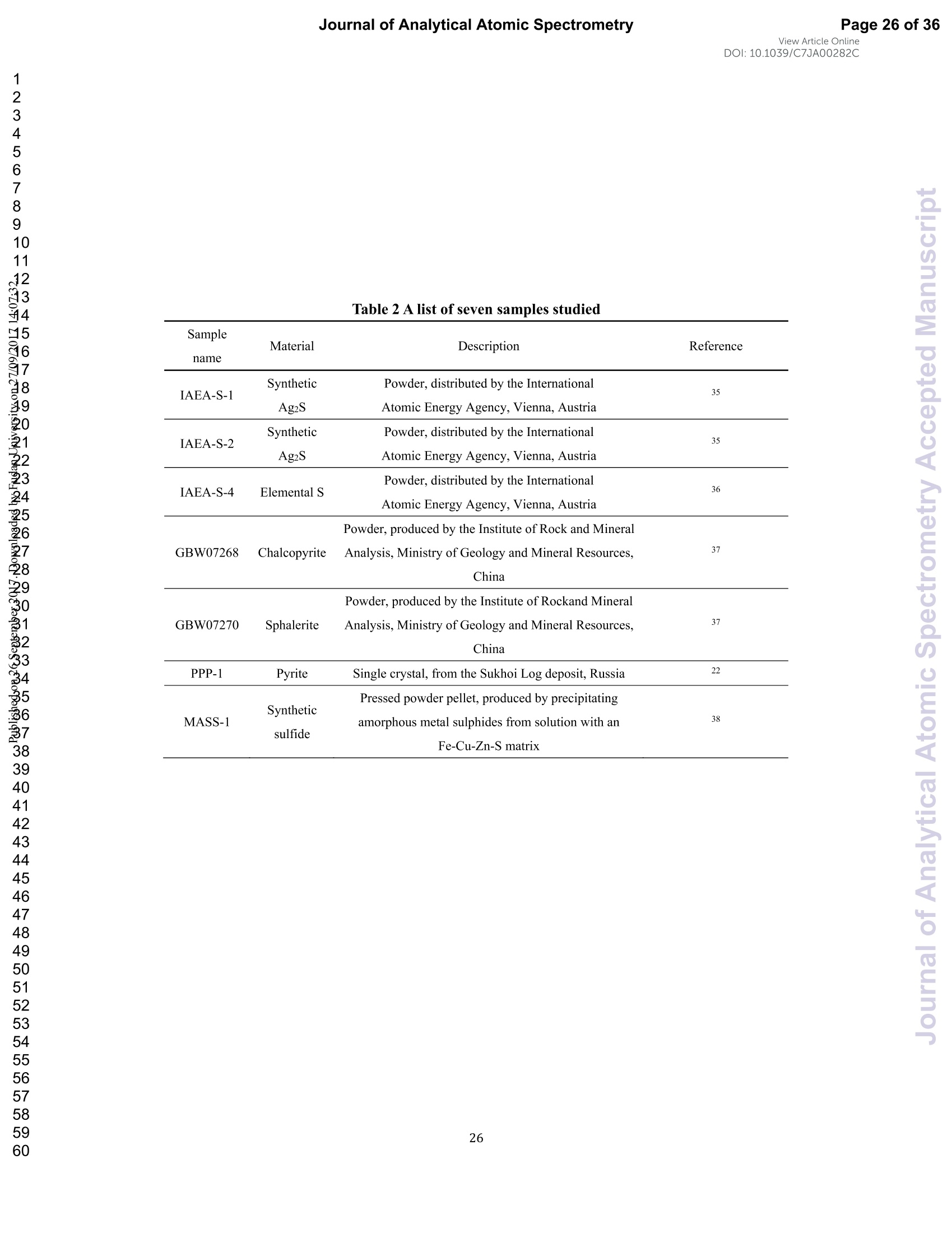
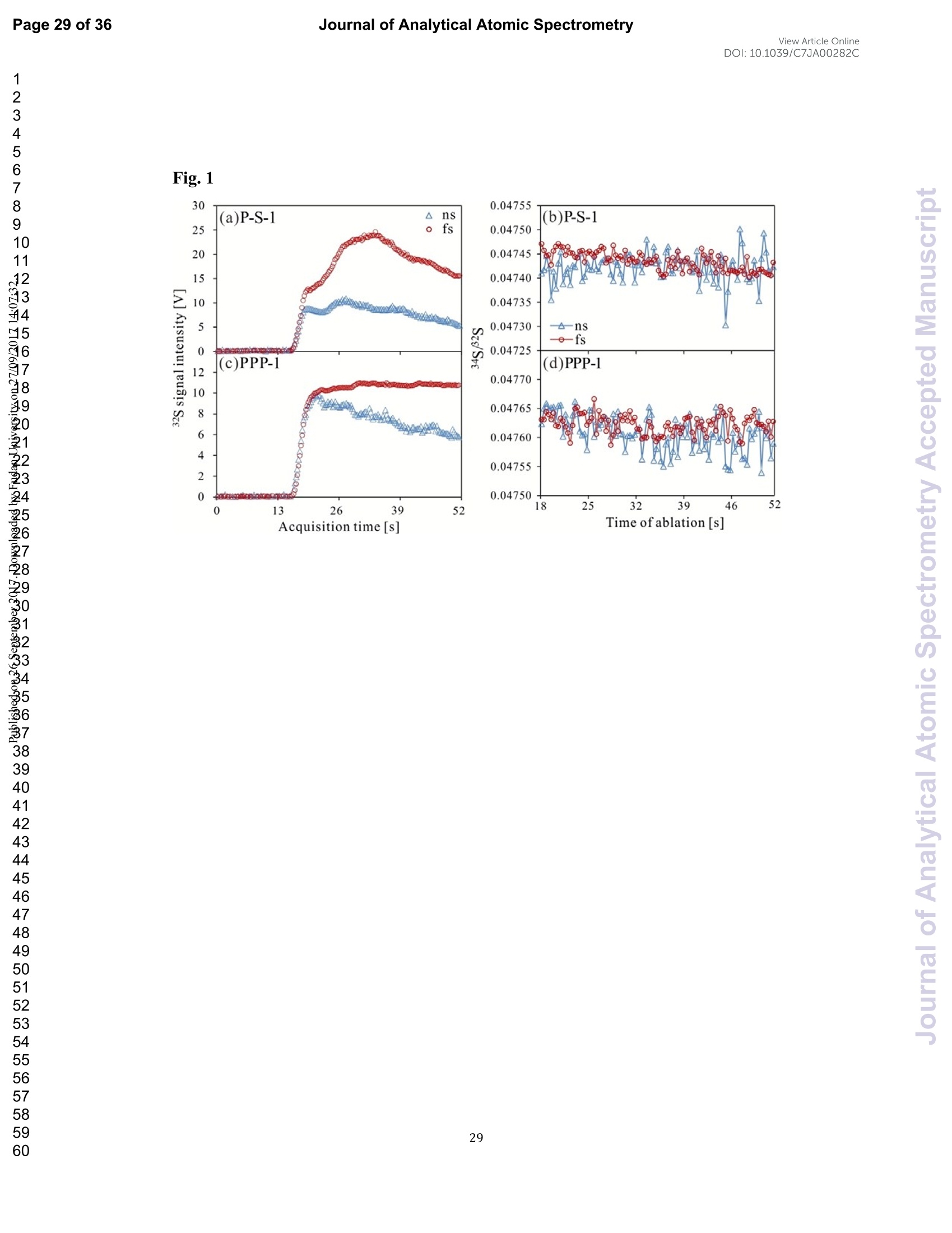
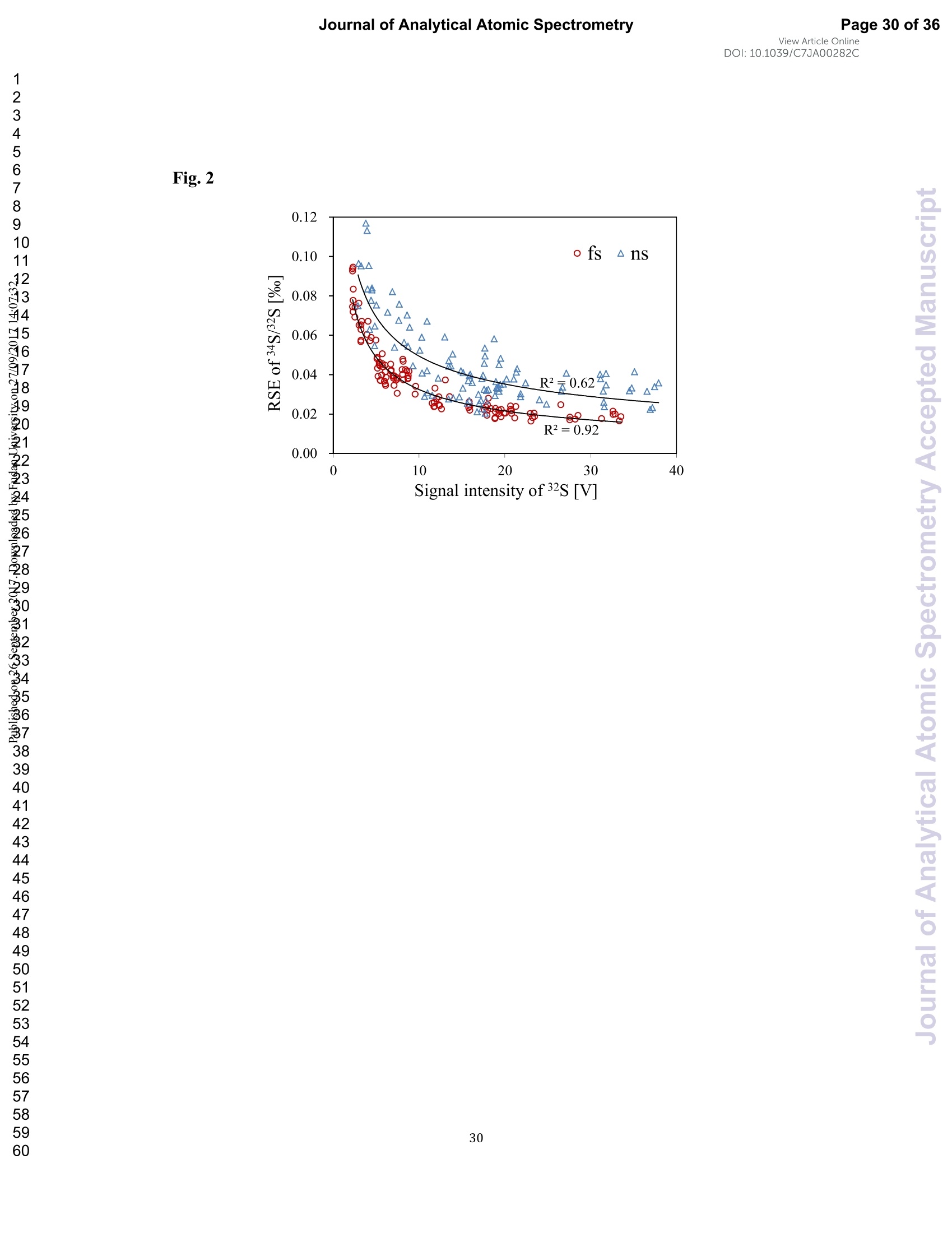
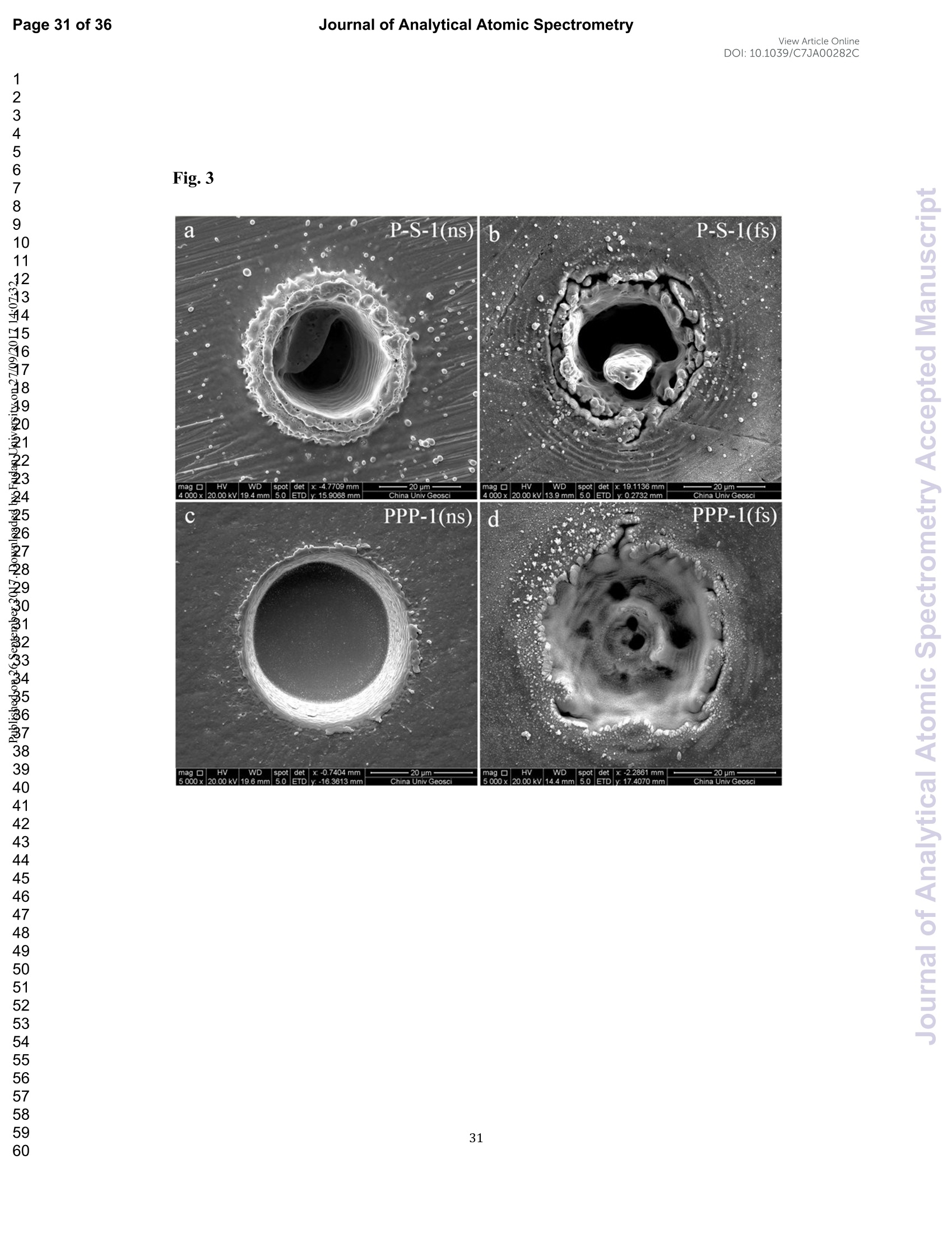
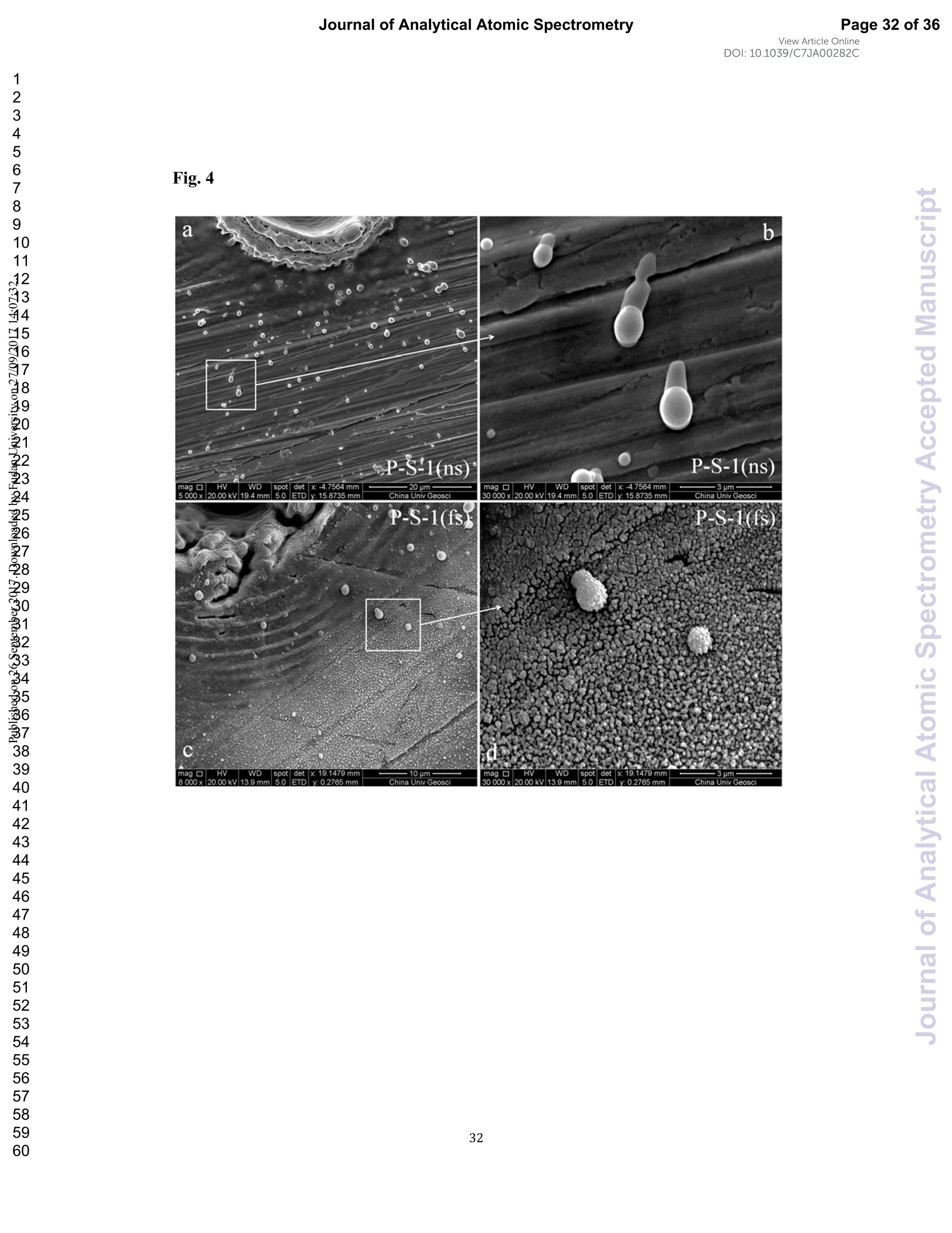
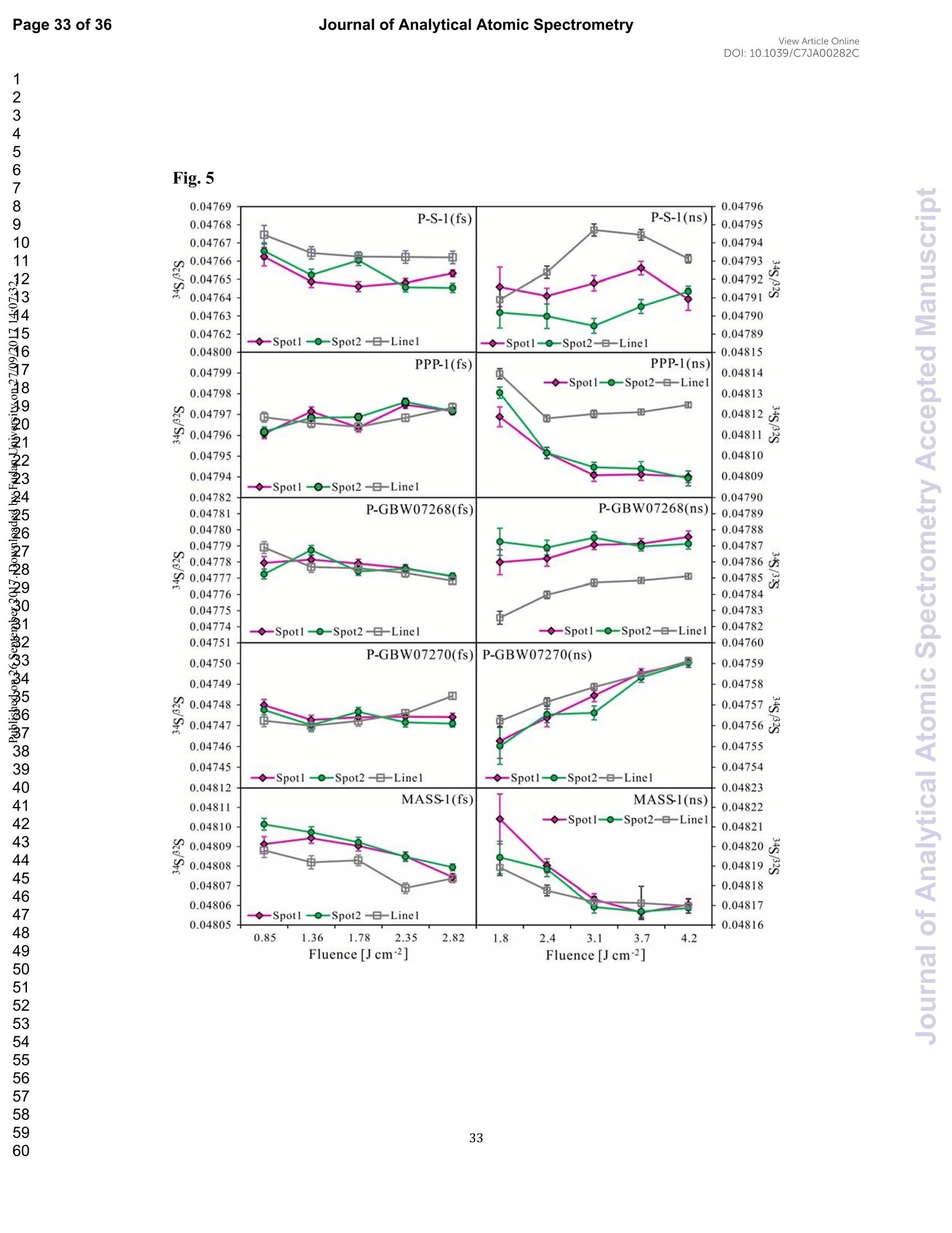
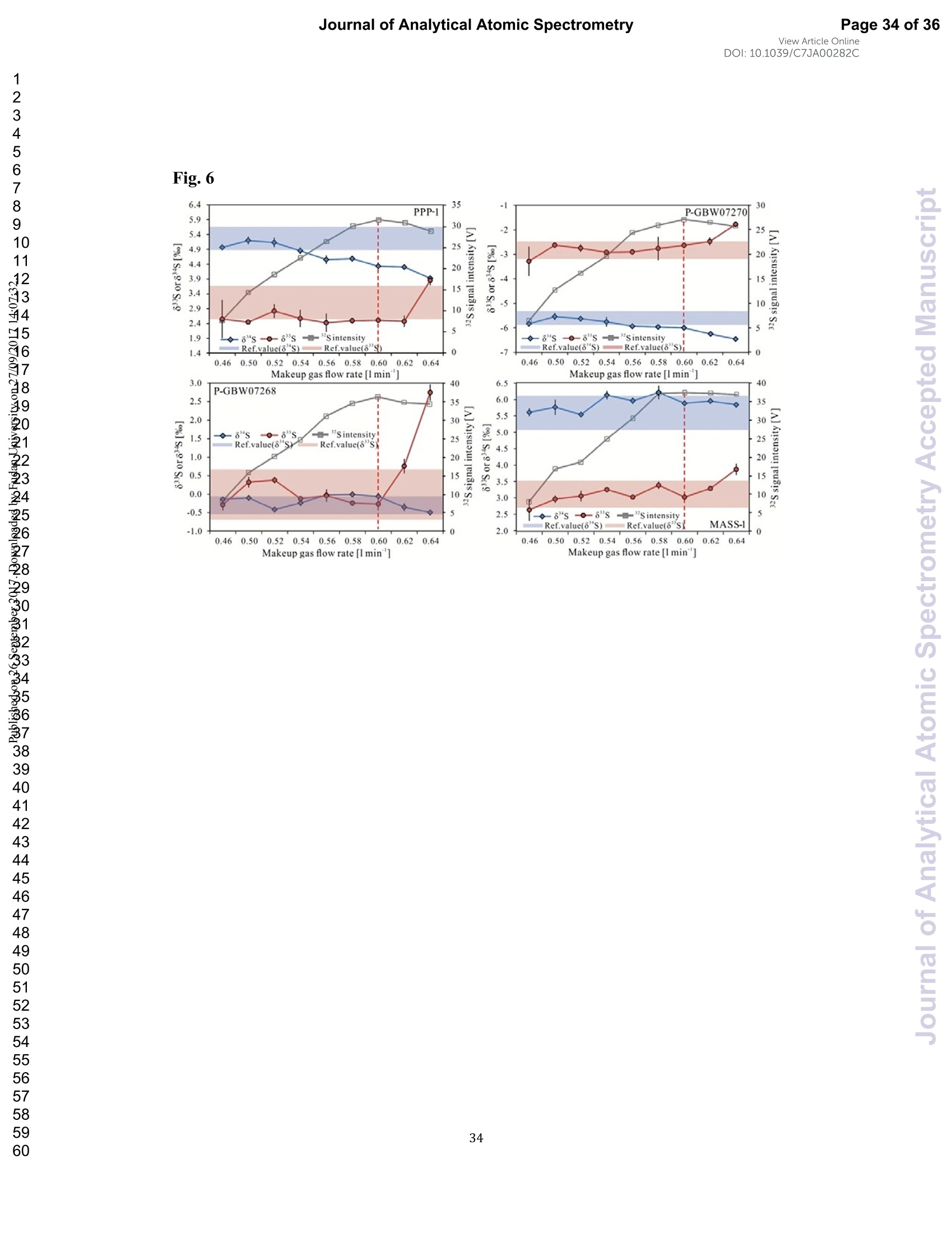
View Article OnlineView JournalCheck for updates8 Journal of Analytical Atomic SpectrometryPage 1 of 36View Article OnlineDOI: 10.1039/C7JA00282C JAAS Accepted Manuscript This article can be cited before page numbers have been issued, to do this please use: J. Fu, Z. hu, J. Li,L. Yang, W. Zhang,Y. liu, Q. Li, K. Zong and S. H. Hu, J. Anal. At. Spectrom., 2017,DOI:10.1039/C7JA00282C. This is an Accepted Manuscript, which has been through theRoyal Society of Chemistry peer review process and has beenaccepted for publication. Accepted Manuscripts are published online shortly afteracceptance, before technical editing, formatting and proof reading.Using this free service, authors can make their results availableto the community, in citable form, before we publish the editedarticle. We will replace this Accepted Manuscript with the editedand formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in theauthor quidelines. Please note that technical editing may introduce minor changesto the text and/or graphics, which may alter content.The journal'sstandard Terms 8 Conditions and the ethical guidelines,outlinedin our author and reviewer resource centre, still apply. In noevent shall the Royal Society of Chemistry be held responsiblefor any errors or omissions in this Accepted Manuscript or anyconsequences arising from the use of any information it contains. Accurate determination of sulfur isotopes (833S and 834S)in sulfides and elemental sulfur by femtosecond laserablation MC-ICP-MS with non-matrix matchedcalibration Jiali Fu, Zhaochu Hua*, Jianwei Li, Lu Yang, Wen Zhang, Yongsheng Liu, QiuliLi, Keqing Zong, and Shenghong Hu “ State Key Laboratory ofGeological Processes and Mineral Resources,Faculty ofEarth Resources, China University ofGeosciences, Wuhan 430074, ChinabNational Research Council Canada, 1200, Montreal Rd.,Ottawa, Ontario,K1A 0R6,Canada ‘ State Key Laboratory ofLithospheric Evolution, Institute of Geology andGeophysics, Chinese Academy of Sciences, Beijing 100029, China *Author to whom correspondence should be sent. E-mail: zchu@vip.sina.com Tel.: +86 27 61055600, Fax: +86 27 67885096 In this study, the laser- and ICP-induced S isotopic fractionation in different S-richminerals was investigated by using femtosecond (fs) and nanosecond (ns) laserablation MC-ICP-MS. 30 000x20.00 kV 13.9 mm 5.0ETD y:0.2765 mm China Univ Geosc ABSTRACT The isotopic composition of sulfur is a vital tracer used in the Earth and planetarysciences. In this study, the laser- and ICP-induced isotopic fractionation in S-richminerals (sulfides and elemental S) with different matrices was investigated by using257 nm femtosecond (fs) and 193 nm ArF excimer nanosecond (ns) laser ablationsystems coupled to a Neptune Plus MC-ICP-MS. Compared to ns-LA-MC-ICP-MS,higher sensitivity (1.4-2.4 times))aat similar instrumental conditions and betterprecision (~1.6-fold)) at the same signal intensity condition were achieved byfs-LA-MC-ICP-MS. In addition, fs-laser provides less fluence and matrix dependentof S isotopic fractionation, and more stable transient isotopic ratios compared tons-laser. Better results acquired by fs-LA-MC-ICP-MS were attributed to the smallersize of particles and less thermal effect produced by fs-laser, which were evidencedby the morphologies of the ablation craters and ejected aerosol particles of P-S-1 (thepressed powder pellet of IAEA-S-1) and PPP-1 (a pyrite single crystal from theSukhoi Log deposit). The ICP-induced isotopic fractionation (matrix effect) was stillfound in fs-LA-MC-ICP-MS at the maximum sensitivity condition. However, asignificant reduction of matrix effect was obtained using a robust plasma condition atlower makeup gas flow rate (0.52-0.541 min-l) relative to the maximum sensitivitycondition (0.6 1 min-) for S isotopes analysis. These could be ascribed to the particlesnot only pass into the higher temperature ICP for a longer residence time at lowermakeup gas flow rate that resulted in more efficient vaporization of the particles, but also experience a more robust plasma induced by adding 4-6 ml min N2 into theplasma. Furthermore, under the robust condition, the results of six reference materialswith different matrices obtained by fs-LA-MC-ICP-MS with non-matrix matchedcalibration at the spot size of 20-44 um showed excellent agreement with thereference values (the accuracy of 0.01-0.15 %o for 834S and 0.11-0.45 % for 833S andthe precision of 0.16-0.40 %o (2s) for 834S and 0.35-0.78 % (2s) for 833S) and themass-dependent fractionation line, validating the applicability of the proposedapproach for providing high-quality in situ isotope data (833S and 834S) of sulfides andelemental sulfur at high spatial resolution using non-matrix matched analysis.Keywords: fs-LA-MC-ICP-MS; sulfur isotopes; isotopic fractionation; non-matrix matched calibration; high spatial resolution analysis 1.Introduction Laser ablation multi-collector inductively coupled plasma massspectrometry(LA-MC-ICP-MS) allows for in situ analysis of isotopic composition at high spatialresolution, offering a powerful tool for measuring geological samples with small sizesorr complextextures such as mineralgrowth zonation, recrystallizationandmetasomatism preserved at the microscale.1-4 Sulfur isotopes are of particular interestbecause sulfur isotopes can be served as a vital tracer in the Earth and planetarysciences. Sulfur is a widely distributed element on the Earth and in the solar system.Its multiple valence states of-22 to + 6 allow it to participate in numerousgeochemical and biochemical processes. Naturally occurring sulfur has four stableisotopes, 32S, 33S, 34S and 36S, with abundances of 94.99, 0.75, 4.25 and 0.01 %,respectively.Studies have shown that considerable sulfur isotope fractionation occursin the natural environment and the overall isotopic variability of 834S generally rangesfrom at least-- 50 too++ 40.8 Sensitive analytical technique is vital to advanceapplications of S stable isotopes (32S, 33S, 34S) as a sensitive tracer in diverse fields ofgeosciences.9,10 However, bulk analysis methods (e.g., GS-MS, MC-ICP-MS)11,12would be unsatisfactory in applications for sulfur isotope analyses in tiny individualminerals or zones within minerals to provide key constraints on the sulfur cycle inrelevant studies.9,10,13 Thus, in situ analytical approach for isotopes determination ispreferred for many applications. In general, in situ analytical techniques for S isotopes include LA-MC-ICP-MS (<0.45 %o, 2s)14,15 and secondary ion mass spectrometry (SIMS) (<0.2 %o, 2s),16 allowing high precision isotopic analysis with a high spatial resolution of 10-60 um.Although SIMS analysis provides higher spatial resolution than LA-MC-ICP-MS, thistechnique is not widely available and itsiinstrumentalt Imassfractionation isintrinsically linked to the composition and crystallographic orientation of the materialbeing analyzed which leads to serious matrix effects.16,17 LA-MC-ICP-MS allows forin situ and rapid analysis at high spatial resolution with a lower detection limit andhas been recognized as an efficient, less sample matrix dependent and relativelywidespread tool for in situ analysis of isotopes.4,18 However, serious matrix effects ons isotopicanalysis occurred in ns-LA-(MC)-ICP-MS between differentmatrices,14,19,20 which is particularly significant at high spatial resolution analysis.Therefore, matrix-matched standard is usually necessary for accurate determination ofS isotope ratios by ns-LA-(MC)-ICP-MS. Bendall et al. (2006)2 used an in-housestandard of pyrite as a matrix-matched external standard to calibrate the instrumentalmass fractionation, achieving an external precision of 0.32 % (2s) for 834S measuredby ns-LA-MC-ICP-MS. Craddock et al. (2008)20 proposed an approach to correct theinstrumental mass fractionation by introducing a matrix-matched solution standardduring laser ablation sampling in ns-LA-MC-ICP-MS, and achieved an externalprecision of 0.45 %o (2s) for 834S of anhydrite standard Sch-M-2. However, thismethod is more complicated and requires time-consuming sample preparation process.Gilbert et al. (2014) developed an in-house pyrite reference material (PPP-1) for S isotopes analysis. Their results showed that significant matrix effects between pyriteand bornite can be reduced with increasing fluence using ns-LA-ICP-MS at a certainextent, and a precision of ~1 %o (RSE) for 834S was obtained. Fu et al. (2016)15adopted a line scan ablation mode at appropriate fluence for the reduction of sulfurisotopic fractionation during ablation process and matrix effect among certain S-richsamples (IAEA-S-1, pyrite, chalcopyrite, sphalerite, elemental S, molybdenite,MASS-1). Ai pprecision of 0.15-0.45 (2s))for 834S was obtained withns-LA-MC-ICP-MS. For high spatial resolution analysis in single spot ablation mode,a matrix-matched reference material was highly recommended. Zhu et al. (2016)reported up to 2 %o fractionation for 834S with different laser parameters duringanalysis of pyrite using a 193nm ArF excimer ns-laser, and the ablated particles ofpyrite decomposed into two phases with different 834S values during ablation process.Their results reinforce the importance of matrix-matched calibration for sulfurisotopes analysis using ns-LA-MC-ICP-MS. Many1 previous studiess have shown that fs-laser might be less1proneet(tolaser-induced(elemental/isotopic fractionationandmatrix-relatedproblems incomparison with ns-laser.24-26 This is because the pulse width of fs-laser is shorterthan the electron-phonon relaxation time, eliminating heating effects and avoidinglaser plasma interaction during ablation process. On the contrary, ns-laser ablationtends to cause significant heating and melting, because the pulse width of ns-laser istoo long to prevent electron-lattice heat transfer.27,28 For example, Mozna et al. (2006)26 compared the performance of UV-fs-LA-ICP-MS and UV-ns-LA-ICP-MSfor iron-based samples, higher sensitivity (25-30%), more stable elemental ratios(10 %%,. TRSD) and more accurate results (5-15 %) with non-matrix matchedcalibration for elemental analysis were obtained by UV-fs-LA-ICP-MS. Horn andBlanckenburg (2007)24 reportedthhaatlaser-inducedisotopic fractionationwasundetectable for Fe isotopes and the accuracy of the results was independent of matrixwhen using fs-LA-MC-ICP-MS, while an isotopic ratio drift up to several %o wasevident during ns-laser ablation process. Horn et al. (2006) and Chmeleff et al.(2008)29 successfully analyzed Fe and Si isotopes in sulfides, oxides and silicatesusing non-matrix-matched calibration (IRMM-014 and IRMM-017 as the externalcalibrators for Fe and Si, respectively) by UV-fs-LA-MC-ICP-MS. To date, onlyChen et al. (2016)30 has applied fs-laser coupled to Nu Plasma MC-ICP-MS for in situanalysis of S isotopes, and found that matrix effects were significant when usingIAEA-S-1 (even using chalcopyrite glass as calibrator) as a standard to calibratenatural chalcopyrite with the spot size of 20 um and laser fluence of 0.4 J cm.Therefore, the nature of matrix-related effects is still enigmatic for in situ analysis ofS isotopes using fs-LA-MC-ICP-MS at high spatial resolution. It is well known that isotopic fractionation not only originates during laser ablation,but also occurs within the ICP during vaporization, atomization and ionization.31-33Jackson and Giinther (2003)3l reported a large bias for the isotopic composition of thetarget sample for Cu isotope ratios by on-line ns-LA-MC-ICP-MS at high laser fluence, but this isotopic fractionation can be significantly reduced by filtering largerparticles (>0.5 um) from the ablation aerosol. These observations suggested thatisotopic fractionation was predominantly induced by the differential volatilization andionization of Cu isotopes during insufficient vaporization of large particles in the ICP.Kimura et al. (2016)33 reported that increased ICP sample loading through increasinglaser repetition rates and ablation diameters led to increased fractionation of -8 %o forboth 8Li and 8B using 266 nm fs-laser and 193 nm excimer ns-laser, and suggestedthe origin of isotope fractionation was primarily induced by the insufficient aerosolvaporization in the ICP resulted from mass load effect, as well as by thermal effect atthe laser site for Li and B isotopes. Further studies are necessary to investigate theoriginn oaf sulfurisotopicfractionationduringin situanalysis procesS byLA-MC-ICP-MS, especially at high spatial resolution. In this study, we systematically investigated the effects of laser fluence, spot sizeand ablation mode on S isotopic fractionation between fs-laser and ns-laser, and theeffects of makeup gas flow rate on matrix effect for sulfur isotopes analysis byfs-LA-MC-ICP-MS. In addition, the signal intensity, the stability of transient isotopicratios and the precision of sulfur isotopes ratios between fs-laser and ns-laser werecompared in detail, along with the morphologies of the ablation craters and thedeposited particles produced by fs-laser and ns-laser. The final optimized method wasvalidatedby analyzingsix referencenmaterials of differentt rmatrices withnon-matrix-matched calibration using fs-LA-MC-ICP-MS. 2. Experimental 2.1 Instrumentation All isotopic measurements were conducted on a Neptune Plus multiple collectorinductively coupled plasmamass spectrometerr (MC-ICP-MS,. ThermoFisherScientific, Germany) in combination with a :2577 nm Yb femtosecond (fs) laserablation system (NWR-Femto"C, USA) and a 193 nm ArF excimer nanosecond (ns)laser ablation system (GeoLas 2005, Lambda Physik, Gottingen, Germany) in theState Key Laboratory of Geological Processes and Mineral Resources, ChinaUniversity of Geosciences in Wuhan. The ns-laser is equipped with an opticalconfiguration producing a constant lateral energy distribution leading to pan-shapedablation pits on the sample, while there is a Gaussian energy beam profile across itsablation diameter in fs-laser.25 The Neptune Plus is a double-focusing MC-ICP-MSwith a movable multi-collector array of Faraday cups which permitted simultaneousdetection of 32S,33S and 34S signals collected in the L3, center and H3 Faraday cups,respectively. Approximately 0.5 1 min helium was flushed into the ablation cell,which was then mixed with argon (makeup gas) and nitrogen (4-6 ml min-) via a Tconnector downstream prior to the plasma torch. The amount of additional nitrogenwas controlled by a mass flow controller (Aalborg DFC 26 mass flow controller). Toachieve a smooth signal during isotopic ratio determination, a “wave”signal stabilizerdevice was used in this study.34 Based on previous study, the use of the Jet sample cone in combination with the X skimmer cone improved the S sensitivity by a factorof 3.6 compared to the standard sample cone combined with the H skimmer cone inns-LA-MC-ICP-MS. Furthermore, the addition of 4 ml minN2 to the central gasflowwasfound tto significantly suppres,s the oxide and hydride polyatomicinterferences on sulfur isotopes and improve the mass bias stability of sulfur isotoperatios at the optimum makeup gas flow rate.15 The instrumental and data acquisitionparameters of the lasers and the MC-ICP-MS are listed in Table 1. The morphologiesof the ablation craters and the aerosols deposited around the ablation holes wereimaged by a scanning electron microscopy (SEM, Quanta 450 FEG, FEI, USA). 2.2 Preparation of samples The well characterized reference materials are listed in Table 2. All the pulverizedreference materials including IAEA-S-1, IAEA-S-2, IAEA-S-4, GBW07268, andGBW07270 were analyzed as cold pressed powder pellets prepared by using a presserwithout the addition of a binder applying 300 kN (~1×10°kPa) for 5 min. ForGBW07268 and GBW07270, the original particles were further ground using agatemilling with the particle size of 90 % of each sample less than 10 um.15 All thesamples were fixed with epoxy resin in a plastic tube independently, and thenpolished with 2 um diamond paste to give a flat surface prior to LA-MC-ICP-MSanalyses. 2.3 Isotope ratio measurements and data reduction The sulfur isotopes were measured on the shoulder of interference-free plateau of peak of 32S, 33S, 34S (see ESI Fig. S1), which were obtained at medium massresolution of MC-ICP-MS (Rpower(5, 95%)=4707 as defined in a previous study).Each measurement was performed in static mode consisting of one block of 200cycles with an integration time of 0.262 s per cycle. All data were collected for 18 sbefore firing the laser to acquire average background signal that were subsequentlysubtracted from the ablation signals collected for the next 34 s during offline dataprocessing.The standard-sample bracketing approachwasappliedtocorrectinstrumental mass bias by repeatedly measuring the standard before and after everyfive samples. The final sulfur isotope ratios (34S/2S, 33S/32S) were calculated bycorrection of instrument mass bias using linear interpolation between the biasescalculated from four neighboring standard analyses. All results are expressed in %ousing delta notation as follows: 8*Sv-cDT[%]=[(*S/32S)sample/(XS/32S)v-cDT-1]×1000 (Eq.1) where x isis 33 and 34, (XS/32S)sample is the measured *S/2S of the sample and(XS/32S)v-cDT is defined as (34S/32S)v-CDT= 0.044163 and (33S/2S)v-CDT=0.00788.40 3. Results and discussion 3.1 Comparison of signal intensity and precision of between ns- and fs-laser Fig. 1 illustrates the changes of the 32S signal intensity and 34S/32S isotopic ratio asa function of the signal acquisition time obtained at identical MC-ICP-MS conditionsfor P-S-1 (the pressed powder pellet of IAEA-S-1) and PPP-1 using fs- and ns-LA-MC-ICP-MS. Compared with the average 32S signal intensity of the ns-laser(2.4 J cm²), fs-laser (1.36 J cm²) ablation produced higher 32S signal intensities ofP-S-1 and PPP-1 by a factor of 2.4 and 1.4, respectively. The 32S signal responseswith time between P-S-1 and PPP-1 are also different when using the same laser (Fig.la, 1c). This should be attributed to their different physical and chemical properties.Fig. 1b and Fig. 1d show transient 34S/32S ratios of P-S-1 and PPP-1 from 18 to 52 s(time of ablation) in Fig. 1a and Fig. 1c acquired by fs- and ns-laser, respectively. Thefluctuations of transient 34S/32S ratios of PPP-1 and P-S-1 achieved by ns-laser weresignificantly larger than those obtained by fs-laser, suggesting that fs-laser was moresuitable for high precision of sulfur isotopes analysis in sulfides. In general, the precision of the measured isotopic ratio is strongly connected to itssensitivity. The 32S signal intensity of ns- and fs-laser versus the correspondingprecision (“Relative Standard Error”instead of“precision"here) of34S/32S ratio for insitu S isotope analyses was shown in Fig. 2. Clearly, the correlation between the 32Ssignal intensity and the precision of 34S/32S ratio obtained by fs-laser fits well into anexponential function with R²=0.92, much higher than the correlation coefficient (R”= 0.62) acquired by ns-laser, illustrating the relationship between the 32S sensitivitiesof fs-laser and the corresponding precisions conforming to the Poisson distribution.The significant discrete data points acquired by ns-laser may be attributed to theincomplete vaporization of large particles produced by thermal effect of ns-laser.Compared to ns-laser, the corresponding average precision of 34S/2S ratios achieved by fs-laser is improved by a factor of ~1.6 at the same signal intensity condition. Thebetter internal precision is the result of less fluctuated transient isotopic ratio obtainedin single spot analysis by fs-laser. For a given analytical precision of 0.04 % for34S/32S, the 32S signal intensity required by fs-laser is only 5 V, while that for ns-laseris 8V. Therefore, a higher spatial resolution may be achieved by fs-laser compared tons-laser at the same analytical precision. 3.2 Morphology of ablation craters and aerosol particles by ns-and fs-laser Fig. 3 shows the morphologies of ablation craters and deposited aerosol particlesfrom P-S-1 and PPP-1 generated by using ns- and fs-laser. These craters were ablatedwith a 32 um spot size at a repetition rate of 3 Hz for 200 pulses by ns-laser (2.4 Jcm²) and fs-laser (1.36 Jcm2), respectively. As seen in the Fig. 3a, there areabundant melted ejecta around the ns-laser crater, and a raised rim around the craterperimeter caused by resolidification of molten material of P-S-1. The ns-laser hasenough time for thermal wave to propagate into the target and can create a recoilpressure by the expend vapor plume that expels the molten material to form the largedroplets and the raised rim around the crater 41,42 Due to the higher melting point,hardness and stronger bond strengths of PPP-1 than those of P-S-1, the amount ofejecta from PPP-1 ablated by ns-laser is significantly less than that of P-S-1,whereas the melting phenomenon can still be readily seen on the crater profiles ofPPP-1 (Fig. 3c). In contrast, melting is significantly reduced using fs-laser regardlessof P-S-1 or PPP-1, illustrating the dramatically reduced thermal effects. Moreover, fs-laser crater rims present border deformation, which could be related to theinterference between the scattering light and the incident laser light.25,43Due tons-laser (193 nm excimer laser) beam with a homogeneous energy distribution profile,pan-shaped ablation pits were produced on P-S-1 and PPP-1 (Fig. 3a and 3c). Unlikein 193 nm excimer laser ablation systems, there is no homogeneous lens in fs-laser,which has a Gaussian energy beam profile across its diameter. Some small holes andcolumnswithinntthee ffs-laser craters areeobserved(Fig.3band 3d). Theself-focusing/defocusing and the highly nonlinear processes of spatial and temporalsplitting of fs-laser pulses during propagating inside the samples have been proposedto explain these phenomena.+4 Fig. 4 shows magnified views of ns- and fs-laser crater rims and ejected particles inFig. 3a and 3b. There is a significant difference in the size and structure of the aerosolparticles generated by the ns- and fs-laser. Most of the large spherical aerosolparticles (~1-2 um) deposited around the ns crater rims equip with a small tail (Fig. 4aand 4b), caused by the rapid movement of unsolidified droplets when they sputteredon the sulfide sample surface. These typical melting phenomena indicate that thermalprocesses are the dominant mechanisms of sulfide ablation in ns-laser. In contrast, thefs-laser crater is mainly surrounded by fine aerosol particles (~85 nm) (Fig 4c). Thereare also some large aerosol particles (0.5-1 um) around the fs-laser crater. Magnifiedview of such large aerosol particles showed that they were formed by agglomerationof fine particles (Fig. 4d). The rough shapes of these fine particles (Fig. 4d) suggest that they may condense quickly and not be affected by heating. These results confirmthat fs-laser is less prone to thermal effects, and produces smaller aerosol particles forthe ablation of sulfide samples in comparison with ns-laser. Thus, the improved sulfursignal sensitivity and 34S/32S ratio precision (Fig. 1 and 2) by fs-laser should bemainly attributed to the higher aerosol particle transportation efficiency and theirimproved ionization efficiency in ICP due to the significantly reduced particle sizes. 3.3 Effects of ns- and fs-laser fluence on isotopic fractionation In general, the characteristics of laser ablation and aerosol particle generation arestrongly linked with the fluence deposited at the target sample.45,46,47 The fluence hasa significant effect on the degree of laser-induced fractionation.45,46,48 Fig. 5 shows thevariation of the measured 34S/32S ratios as a function of laser fluence from P-S-1,PPP-1, P-GBW07268, P-GBW07270 and MASS-1 at both single spot and line scanablation modes using ns- and fs-laser, respectively. For fs-laser, there was almost nofluence dependent isotopic fractionation for these sulfides over a range of fluencefrom 0.85 to 2.82 J cm, except for MASS-1 whose determined 34S/2S ratios wereslightly decreased with increase of the laser fluence. However, significant fluencedependent sulfur isotopic fractionations were observed with ns-laser at fluences of1.8-4.2 J cm². For example, the determined 34S/2S ratios of P-GBW07268 andP-GBW07270 are increased with increasing the laser fluences from 1.8 to 4.2 J cm ,while those of PPP-1 and MASS-1 are significantly decreased with increasing thelaser fluences. Moreover, the 34S/32S ratios of all the samples measured at all laser fluences with single spot ablation mode were almost identical to those by line scanablation mode for fs-laser. In contrast, the determined 34S/32S ratios of P-S-1, PPP-1,P-GBW07268 were significantly different for these two different ablation modes byns-laser at these laser fluences. The analytical precisions were deteriorated for both nsand fs laser systems at low fluences. This is particularly true for ns-laser due to itslower and less stable signal intensity. The above results confirm that ns-laser samplingis more susceptible to laser-induced fractionation effects relative to fs-laser for sulfideanalysis. The much less dependency on laser fluence and matrix properties of Sisotopic fractionation by fs-laser may be attributed to the dramatically depressedthermal effects and the produced smaller aerosol particles compared to ns-laser (Fig. 3and 4). 3.4 Effect of makeup gas flow rate on matrix effect The incomplete aerosol or particle excitation in the ICP was one of the dominantprocesses that affected elemental/isotopic fractionation during laser ablation ICP-MSanalysis.33,49 Nebulizer gas flow rate (makeup gas flow rate) is one of the mostimportant parameters that have a significant effect on ICP properties.50,51,52 Themeasured 32S signal intensity, 834S and 833S as a function of makeup gas flow ratefrom four different matrix sulfides (PPP-1, P-GBW07270, P-GBW07268,MASS-1)by using fs-laser are illustrated in Fig. 6. P-S-1 was used as the external calibrationstandard for these sulfides. The 32S maximum sensitivities of these four sulfidesoccurred at the same makeup gas flow rate of 0.60 1 min, suggesting that the locations of zones of maximum 32S ion density formed in the ICP are matrixindependent, in agreement with the result that the lightest or most volatile elementsdid not shift the optimum gas flow rate induced by different matrices reported byRodushkin et al. (2002).53 The measured 834S or 833S values exhibited significantdeviation from their reference values at high makeup gas flow rates (≥0.60 1 min),especially for the PPP-1 and P-GBW07270, indicating that matrix effect is severe athigh makeup gas flow rate, even at the optimum makeup gas flow rate correspondingto the maximum sensitivity of 32S for PPP-1 and P-GBW07270. However, at lowmakeup gas flow rates (0.52-0.54 1 min-l), all the measured 834S and 833S values ofthese four sulfides were generally in good agreement with their reference values. Thesensitivity of 32S was reduced by up to ~30-40 % at the selected low makeup gas flowrates. Compared to the obtained signal enhancement factor of 3.6 by the use of the Jetsample cone in combination with the X skimmer cone,l5 this reduction of the Ssensitivity by adopted the low makeup gas flow rates is minimal. Generally, a lowmakeup gas flow rate means a robust plasma condition.50,54 This study suggests thatthe ICP induced S isotopic fractionation among different sulfides can be significantlysuppressed when the operating parameters (e.g., makeup gas flow rate) are optimizedto generate a sufficient gas temperature in the central channel of the ICP. These resultsalso indicate that the robust plasma condition is a prerequisite for the accuratedetermination of sulfur isotopes (833S and 834S) in sulfides by femtosecond laserablation MC-ICP-MS with non-matrix matched calibration. Note that nitrogen (4-6 ml min-) was added to the central channel gas of ICP in this study. Fu et al. (2016)15reported that the addition of 4 ml min-N2 to the central gas flow in LA-MC-ICP-MScan efficiently reduce polyatomic interferences and stabilize the mass bias for sulfurisotopes analysis. It was found that N2 has a higher thermal conductivity than that ofargon by a factor of 32 at 7000 K.55,56 It is therefore possible that the reduced ICPinduced S isotopic fractionation among different sulfides partly results from theincreased thermal conductivity which causes a higher plasma temperature, and thushigher ionization efficiency of the aerosol particles. 3.5 Results of reference materials analyzed with non-matrix matched calibration To verify non-matrix matched calibration capability for S isotopes analysis ofS-rich samples at high spatial resolution under the robust condition (at the lowmakeup gas flow rate of 0.54 1 min-l) by fs-LA-MC-ICP-MS, six reference materialsof different matrices were analyzed at a fluence of ~1.36 J cm with spot sizes of20-44 um at the single spot ablation mode. The samples were calibrated againstPPP-1, except for PPP-1, which was calibrated against P-S-1. Fig. 7 shows acompilation of the measured 833S and 834S (n=30-35) values that were obtained forthese six S-rich reference materials over the course of this study (approximately sixmonths) by fs-LA-MC-ICP-MS. The measured 833S and 834S of these samples are inexcellent agreement with the reference values.15,22,35,36 The determined 833S values inP-S-2 and P-S-4 are exceptions, which significantly deviated from the referencevalues by ns-laser.15 The determined 833S value of P-S-2 by fs-laser is consistent with the reference value by gas-source mass spectrometer,35 confirming our value is correct. Tofurther evaluatetthe accuracyy Oof thedetermined values by ns- andfs-LA-MC-ICP-MS, the analytical results of these six reference materials are plottedin a 833S versus 834S diagram to show deviations from the trend expected formass-dependent fractionation line (MDF)(Fig. 8). This three-isotope plot is oftenused in stable isotope studies to confirm analytical accuracy.19 As these referencematerials are all young samples, their expected results should be fall on the MDFbased on the previous studies.14,57 Clearly, all the results broadly fit to the MDF,except for the results of P-S-2 and P-S-4 measured by ns-LA-MC-ICP-MS15 whichshow distinctly negative deviation from the MDF, suggesting the biased 833S resultsof P-S-2 and P-S-4 by ns-LA-MC-ICP-MS. These results show that fs-laser issuperior to ns-laser for sulfur isotopes analysis. The excellent long-term accuracies(0.01-0.15 % for 834S and 0.11-0.45 %o for 833S) and precisions (0.16-0.40 %o for 834Sand 0.35-0.78 %o for 833S, 2s) of the determined values in these different S-richreference: 1materialsdemonstrateid thatit ttheproposed analyticalprotocolwithnon-matrix matched calibration for sulfur isotopes analysis by fs-LA-MC-ICP-MS athigh spatial resolutions of 20-44 um is feasible. 4. Conclusions The results presented here indicate that non-matrix matched calibration for accuratesulfur isotopes analysis in S-rich samples is feasible by fs-LA-MC-ICP-MS in combination with the robust plasma conditions. Compared to ns-LA-MC-ICP-MS,higher sensitivity (1.4-2.4 times) under similar instrumental conditions and betterprecision (~1.6-fold) at the same signal intensity condition were achieved byfs-LA-MC-ICP-MS. Moreover, the less thermal effect and the smaller particles infs-laser were observed from the morphologies of ablation craters and ejected particlesproduced by fs- and ns-laser at similar instrumental conditions. No significant fluenceand matrix dependent sulfur isotopic fractionation for these sulfides with differentmatrices Werree observedwiththeefs-laser.Thus.;. better resultsobtainedbyfs-LA-MC-ICP-MS are attributed to the less thermal effect and the smaller particlesgenerated in fs-laser which resulted in more efficient transportation and ionization.Matrix effect was still observed in fs-LA-MC-ICP-MS at the maximum sensitivitycondition. However, application of the robust plasma conditions (e.g., at lowermakeup gas flow rate) can significantly reduce matrix effect for S isotopes analysis.The results obtained from sixx reference materials with (different matricesbyfs-LA-MC-ICP-MS using non-matrix matched calibration show excellent agreementwith the reference values and the mass-dependent fractionation line at spot sizes of20-44 um, demonstrating the capability of this developed method for achievingreliable in situ S isotope measurements in S-rich samples at high spatial resolution. Conflicts of interest There are no conflicts of interest to declare. Acknowledgments We would like to thank Sarah Gilbert for providing the pyrite PPP-1 referencematerial. Thisresearch is supported byy ttheNational KeyR&P Plan(2016YFE0203000), the National Science Fund for Distinguished Young Scholars andthe National Natural Science Foundation of China (Grants 41573015 and 41322023),the Science Fund for Distinguished Young Scholars of Hubei Province (2016CFA047),and the MOST Special Fund from the State Key Laboratories of Geological Processesand Mineral Resources. ( References ) 1. R. E. Russo, X. Mao, H. Liu, J. Gonzalez and S. S. Mao, Talanta, 2002, 57,425-451. ( 2. D. Giinther, I .Horn and B. Hattendorf, Fresenius’J. Anal. Chem., 2000, 368, 4. ) ( 3. I. Horn, F. von Blanckenburg, R. Schoenberg, G . Steinhoefel and G. M a rkl, Geochim. Cosmochim. Acta, 2006,70,3677-3688. ) ( 4. Z. C. Hu, Y. S. Liu, S. Gao, W . G. Liu, W. Zhang, X. R. Tong, L. Lin, K. Q. Zong, M. Li,H. H. Chen, L.Zhou and L. Yang,J. Anal. At. Spectrom., 2012,27,1391-1399. 5. C. W. Mandeville and C. W. Mandeville, Elements,2010,6,75-80. ) ( 6. B. Mayer and H . R. Krouse, Handb. Stable Isot. Anal. Tech., 2004,538-596. ) ( 7. M . Berglund and M. E . Wieser, Pure Appl. C hem., 2011 , 83,39 7 -410. ) ( 8.R. R. Seal, Rev. Mineral. Geochem., 2006,61,633-677. ) ( 9 . S. Ono, B. Wing, D. Johnston,J. Farquhar and D . R umble, Geochim. Cosmochim. Acta, 2006,70,2238-2252. ) ( 10. D. Tanner, R. W. Henley, J . A . Mavrogenes and P. Holden, C ontrib. Mineral. Petrol,2016,171,33. ) ( 11. R. Clough, P. Evans, T. Catterick and E . H . Evans, Anal. C h em., 2 0 06, 78 , 6126-6132. ) ( 12. M. Yun, M. A. Wadleigh a nd A. Pye, Chem. G eol., 2004,204,369-376. ) 13. M. Peters, H. Strauss,J. Farquhar, C. Ockert, B. Eickmann and C. L. Jost, Chem.Geol,2010,269,180-196. ( 14. B. Buhn, R. V.Santos, M. A. D ardenne and C. G. de Oliveira, C h em. Geol.,20 1 2, 312-313,163-176. ) ( 15. J . F u, z. Hu, W. Zhang, L. Yang, Y. Liu, M. Li, K. Zong, S . Gao and S. Hu, Anal Chim Acta,2016,911,14-26. ) ( 16. T.Ushikubo, K. H . Williford, J. Farquhar, D. T.Johnston, M . J. Van Kranendonkand J. W. Valley, Chem. Geol.,201 4 ,383,86-99. ) ( 17. J. M. Eiler, C. Graham and J . W. Valley, Chem. Geol.,1997,138,221-244. ) ( 18. M. Shaheen and B. J. Fryer,J. Anal. At. Spectrom . , 2010,25 , 1006. ) ( 19. P. R. D. Mason, J. Kosler, J. C. M. de Hoog, P. J . Sylvester and S. Meffan-Main,J.Anal. At. Spectrom., 2006,21,177-186. ) ( 20. P. R. Craddock, O . J . Rouxel, L. A. Ball and W. Bach, Chem. Geol., 2008, 253, 102-113. ) ( 21. C. B endall, Y. L ahaye, J. F iebig, S . Weyer and G. P. Brey, Appl. Geochem., 200 6 , 21,782-787. ) ( 22. S. E. Gilbert , L.V . Danyushevsky, T. Rodemann, N. S himizu, A. Gurenko, S.Meffre, H. Thomas, R. R. Large and D . Death, J. Anal. At. Spectrom., 2 014, 29, 1042-1051. ) ( 23. Z.-Y. Zhu, S.-Y. Jiang, C. L. Ciobanu, T. Yang and N. J. Cook, Chem. Geol., 2016. ) ( 24. I. Horn and F . von B lanckenburg, Spectrochim. Acta, P art B, 2 007, 6 2 , 410-422. ) ( 25. Z. L i, Z. C. Hu, D. Gunther, K. Q. Zong, Y. S. Liu, T. Luo, W. Z hang, S. Gao and S. H. Hu, Geostand. Geoanal. Res. , 2016,40,477-491. ) ( 26. V. Mozna, J. Pisonero, M . H olá, V. Kanicky and D. Gunther, J. Anal. At. Spectrom., 2006,21,1194-1201. ) ( 27. B. F ernandez, F. Claverie, C. P echeyran, O. F. X. Donard an d F. Cl a verie, Tr A C, Trends Anal. Chem., 2007,26,951-966. ) 28. M. E. Shaheen, J. E. Gagnon and B. J. Fryer, Chem. Geol., 2012, 330-331,260-273. ( 29. J. Chmeleff, I . Horn, G. Steinhoefel a nd F. von Blanckenburg, Chem. Geol., 2008, 249,155-166. ) ( 30. L. C hen, K. Chen, Z . Bao, P . Liang, T. Sun a n d H. 1. Yuan, J. Anal. At. Spectrom., 2017,32,107-116. ) ( 31. S. E. Jackson and D. Gunther, J. Anal. At. Spectro m ., 2003,18,205-212. ) ( 32. H .-R. K uhn, N . J. Pearson and S. E. Ja c kson, J. Anal. At. Spectrom., 2007, 22, 547. ) ( 33.J.-I.Kimura, Q. Chang, T. Ishikawa and T . Tsujimori, J. Anal. At. Spectrom., 2016, 31,2305-2320. ) ( 34. Z . Hu, W . Zhang, Y . Liu, S. Gao, M. L i, K. Zong, H. Chen and S. Hu, Anal. C h em., 2015,87,1152-1157. ) ( 35. T.P . Ding, S. Valkiers, D . F . Wan, R. M. Bai, X.Q. Zou, Y. H. Li, Q. L. Zh a ng and P. De Bievre,Mineral. Petrol.Geochem.,2001,20,425-427. ) ( 36. H . P. Qi and T. B. Coplen, Chem. Geol., 2003,1 9 9,183-187. ) ( 37. Research G roup of Sulphide Mineral Reference M a terials, R ock Miner Anal, 1995,14,81-113. ) Journal of Analytical Atomic Spectrometry ( 38. S. A. Wilson, W. I . Ridley and A . E . K oenig, J. Anal. At. Spectrom., 2002, 17, 406-409. ) ( 39. S.Weyer and J. B. Schwieters, Int.J. Mass spectrom., 2003,226,355-368. ) ( 40. T. P. Ding, S. Valkiers, H. Kipphardt, P . D e B i evre, P. D. P. Taylor and R. Gonfiantini, Geochim. Cosmochim.Acta,2001,65,2433-2437. ) ( 41. C. L iu, X. L. Mao, S. S. Mao, X. Zeng, R. Greif and R. E. Russo, Anal. Chem., 2004,76,379-383. ) ( 42. B. N. Chichkov,C. Momma, S . Nolte, F. Von Alvensleben and A. Tinnermann, Appl. Phys. A: Mater. Sci. Process., 1996, 63,109-115. ) ( 43. F. Poitrasson,X. Mao, S. S. Mao, R. Freydier and R. E. Russo, Anal. Chem.,2003, 75,6184-6190. ) ( 44. S. S. M ao,F. Q u ére, S. Guizard,X. Mao,R. E . Russo, G. Petite and P. Martin,Appl.Phys. A: Mater. Sci.Process . , 2004,79,1695-1709. ) ( 45. P. K. Diwakar, J. J. Gonzalez, S. S . Harilal,R. E . Russo and A. Hassanein, J. Anal.At. Spectrom.,2014,29,339-346. ) ( 46. Q. Bian, C. C . G arcia, J. Koch and K. Niemax, J. Anal. At. Spectrom., 2006, 21, 187-191. ) ( 47. N. L. Lahaye, M. C. Phillips, A. M. Duffin, G. C. Eiden and S. S. Harilal, J. Anal. At.Spectrom., 2015,31,515-522. ) ( 48. Z.Li, Z. Hu, Y. Liu, S. Gao, M. L i , K. Zong, H. Chen and S. Hu, Chem. Geol., 2015,400,11-23. ) ( 49.M. Guillong and D. Gunther,J. A nal. At. Spectrom, 2002,17,831-837. ) ( 50. I. I . Stewart and J. W. Olesik,J. Anal. At . Spectrom., 1998,13,1313-1320. ) ( 51.S. H. Tan and G. H orlick,J. Anal. At. Spectrom., 1987,2,745-763. ) ( 52. H. A ndren,I. R o dushkin, A. Stenberg, D. Ma l inovsky and D. C. Baxter, J. Anal. At. Spectrom.,2004,19,1217-1224. ) ( 53. I. Rodushkin, M. D . Axelsson, D . M alinovsky and D. C . Baxter, J. Anal. At. Spectrom.,2002,17,1223-1230. ) ( 54. C. Agatemor and D. Beauchemin, Spectrochim. Acta, Part B, 2011,66,1-11. ) ( 55. S.F. Durrant, Fresenius'J. Anal. Chem., 1994, 349,768-771. ) ( 56. S. Greenfield,I. L. Jones, H.McGreachin and P.B. Smith, Anal. Chim. Acta,1975, 74,225-245. ) ( 57. H. Ohmoto, Y. Watanabe, H. Ikemi, S. R. Poulson and B . E. Taylor, Nature,2006, 442,908-911. ) Table 1 Typical operating parameters for NEPTUNE MC-ICPMS and laser systems Mass spectrometer setup MC-ICP-MS NEPTUNE Plus RF power 1200 W Pt-guard electrode On, grounded Plasma Ar gas flow rate 16.01min Auxiliary Ar gas flow rate 0.851min Ar makeup gas flow rate 0.46-0.641 min 4-6 ml min- N2 gas flow rateInterface cones Jet sample cone +Xskimmer cones (Ni)Data acquisition parametersAcquisition type StaticDetection system Nine Faraday cupsCup configuration 2S (L3), 33S (C),34S (H3)Resolution mode Medium resolution (~4000)Signal integration time 0.262 sSignal analysis protocol 1 Block 200 cyclesLaser setupLaser Yb:YAG femtosecond laser Laser ArF 193nm laser Yb:YAG femtosecond laser Pulse length 15 ns 300 fs Carrier gas Helium (0.5 1 min) Helium (0.51 min) Ablation mode Single spot and line scan Single spot and line scan Ablation diameter 24-44 um 20-44 um Ablation duration 34 s 34 s Pulse rate 3 Hz 3 Hz Fluence 1.8-4.2Jcm- 0.85-2.82 Jcm View Article OnlineDOI: 10.1039/C7JA00282C Table 2 A list of seven samples studied Sample Material Description Reference name IAEA-S-1 Synthetic Powder,distributed by the International 35 AgS Atomic Energy Agency, Vienna, Austria IAEA-S-2 Synthetic Powder, distributed by the International 35 Ag2S Atomic Energy Agency, Vienna, Austria IAEA-S-4 Elemental S Powder, distributed by the International 36 Atomic Energy Agency, Vienna, Austria Powder, produced by the Institute of Rock and Mineral GBW07268 Chalcopyrite Analysis, Ministry of Geology and Mineral Resources, 37 China Powder, produced by the Institute of Rockand Mineral GBW07270 Sphalerite Analysis, Ministry of Geology and Mineral Resources, 37 China PPP-1 Pyrite Single crystal, from the Sukhoi Log deposit, Russia 22 MASS-1 Synthetic sulfide Pressed powder pellet, produced by precipitating amorphous metal sulphides from solution with an Fe-Cu-Zn-S matrix 38 Fe-Cu-Zn-S matrix Figure Captions Fig. 1 Transient signal intensity of 32S (a, c) and transient isotopic ratio of 34S/32S (b,d) obtained from P-S-1 (44um) and PPP-1 (24 um) at repetition rate of 3 Hz usingfs-laser (1.36 J cm²) and ns-laser (2.4 J cm²) in single spot ablation mode,respectively. Fig. 2 Comparison of the relationships between the relative standard error (RSE) of34S/32S and the signal intensity of 32S from PPP-1 and P-S-1 measured by using ns-and fs-laser. Fig. 3 SEM images of ablation craters in P-S-1 and PPP-1 produced with (a, c)ns-laser, 32 um and 2.4 J cm; (b, d) fs-laser, 32 um and 1.36 J cm. Fig. 4 SEM images of ablation crater rims and ejected particles in P-S-1 producedwith (a, b) ns-laser, 32 um and 2.4 J cm; (c, d,) fs-laser, 32 um and 1.36 J cm2. b isthe detail of a, and d is the detail of c. Fig. 5 Comparison of the measured 34S/32S ratios from five sulfides as a function oflaser fluence at different ablation mode between ns- and fs-laser. Spot1, Spot2 andLinel represent continuous two measurements in the single spot ablation mode, andfollowed by a line scanning measurement. Error bars represent within-run precision(2s). Fig. 6 Effects of makeup gas flow rate on the measured 834S, 833S and 32S signalintensity from four different matrix sulfides (PPP-1, P-GBW07270, P-GBW07268,MASS-1) using P-S-1 as the calibration standard by fs-laser (44 um, 1.21 J cm, 3Hz). The red vertical dashed line in each panel represents the value is achieved underthe condition obtained the maximum signal intensity for 32S. The horizontal red andgrey shadows in each panel represent the reference values of833S and 834S and 2serror of each sample, respectively. Error bars represent the standard deviation (2s) ofthree measurements. Fig.7 The measured 833S and 834S of six reference materials by using fs-laser. Theblack horizontal dash line and grey area in each panel represent the determinedaverage 833S or 834S value and its 2s error in the study, respectively. The green squareand red circle represent the value measured by ns-laser reported in Fu et al. (2016)15and the recommended values in literatures22,35,36for these reference materials.respectively. Fig. 8 A plot of the measured 833S and 834S for six reference materials using ns-laserin Fu et al. (2016)15 and fs-laser in this study. The full line is the mass-dependentfractionation line. Error bars represent the standard deviation (2s). Fig. 1 (a)P-S-1 Ansofs A (c)PPP-1 Journal of Analytical Atomic Spectrometry Fig.2 Fig.4 kV13.9mm5.0E D.2765 mm hina Univ Geosci Fig.5 Fig.6 Fig.7 Journal of Analytical Atomic Spectrometry Fig.8 硫同位素在地球科学的多个领域中是一种重要的地球化学示踪剂。在这项研究中,采用257nm飞秒(fs)和193nm ArF准分子纳秒(ns)激光剥蚀系统结合Neptune Plus MC-ICP-MS,研究了不同基质富硫矿物(硫化物和元素S)中激光和等离子体等离子体诱导的同位素分离法。与ns-LA-MC-ICP-MS相比,在相似的仪器条件下,fs-LA-MC-ICP-MS具有更高的灵敏度(1.4-2.4倍),在相同的信号强度条件下,具有更好的精度(~1.6倍)。此外,与ns激光相比,fs激光对S同位素分离的影响更小,对基质的依赖性更小,瞬态同位素比更稳定。由于更小的粒子尺寸和飞秒激光更低的热效应,使用fs-LA-MC-ICP-MS可以得到更佳的测定结果。这一点可以通过P-S-1(IAEA-S-1压粉球团)和PPP-1(苏霍伊原木矿床中黄铁矿单晶)的剥蚀坑和喷射的气溶胶来证明。在最大灵敏度条件下,fs-LA-MC-ICP-MS仍然存在等离子体诱导的同位素分离(基体效应)。然而,针对S同位素分析,在低较低的组成气体流速(0.52-0.54Lmin-1)稳定等离子体条件较最大灵敏度条件(0.6Lmin-1)下,基体效应显著降低。这可以归结为粒子不仅在较高的温度下以较低的组成气体流速进入ICP,停留时间更长,从而使粒子雾化效率更高,同时在等离子体中加入4-6mL min-1 N2也能增强稳定性。此外,在稳定的等离子体条件下,对六种不同基体的参考材料使用fs-LA-MC-ICP-MS在20-44 µm光斑处不使用基体匹配校准进行测定,测定结果与参考值一致。验证了该方法非常适用于在高空间分辨率条件下利用非基体匹配分析提供高质量的硫元素和硫化物原位微区同位数据。
确定



























还剩35页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《硫化物和硫单质中硫同位素检测方案(激光剥蚀进样)》,该方案主要用于其他中硫同位素检测,参考标准--,《硫化物和硫单质中硫同位素检测方案(激光剥蚀进样)》用到的仪器有ESLfemto 飞秒激光剥蚀系统
推荐专场
相关方案
更多
该厂商其他方案
更多















