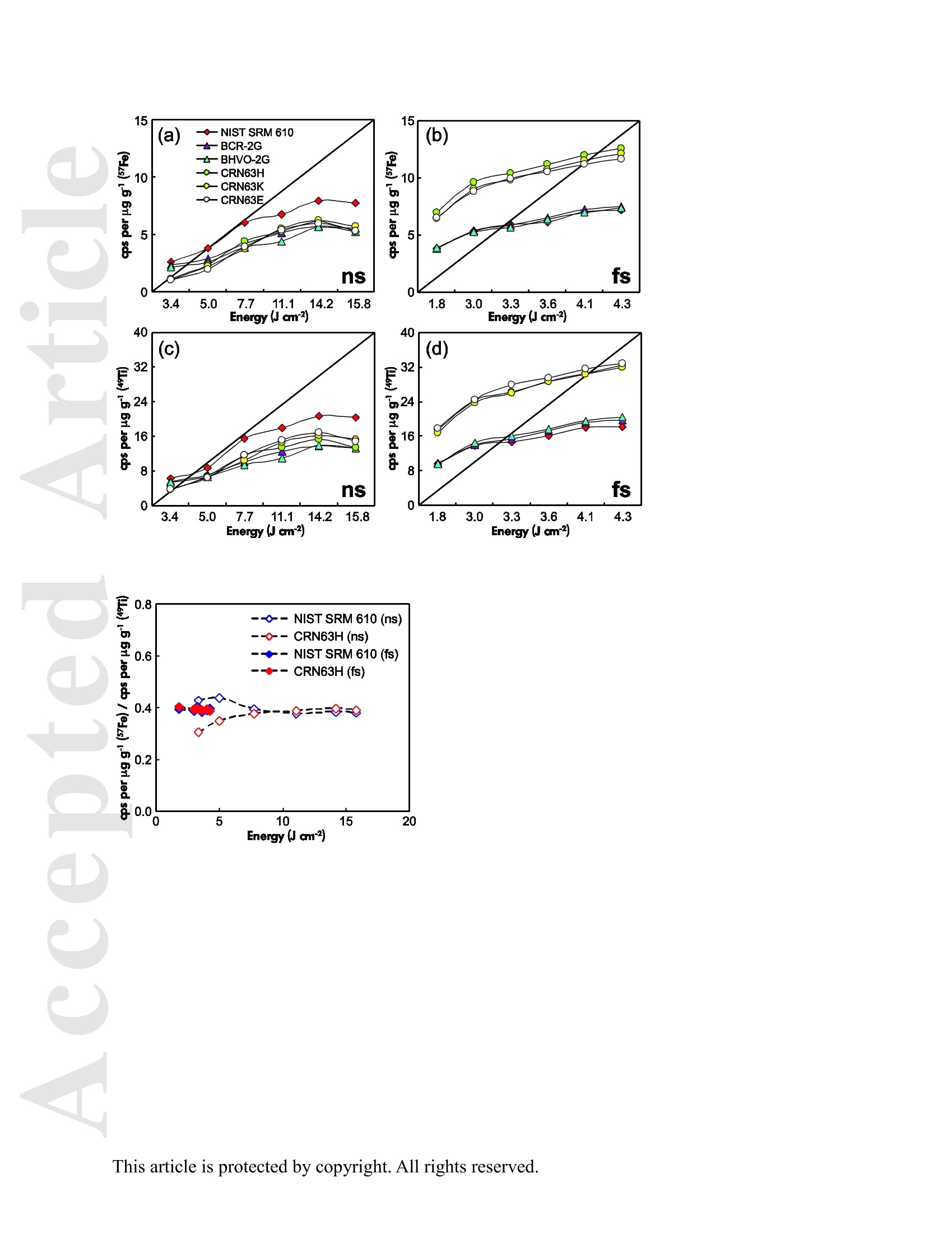
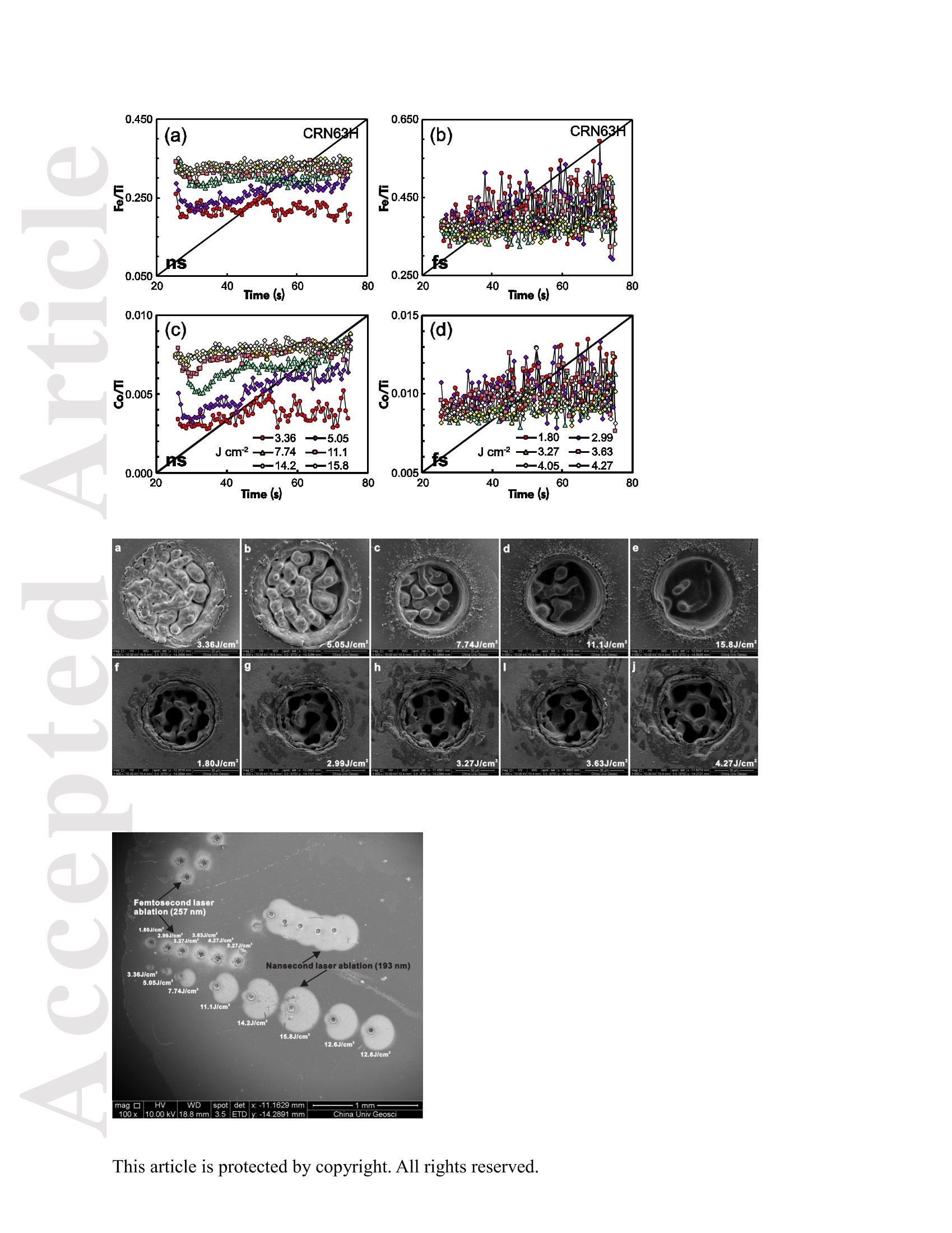
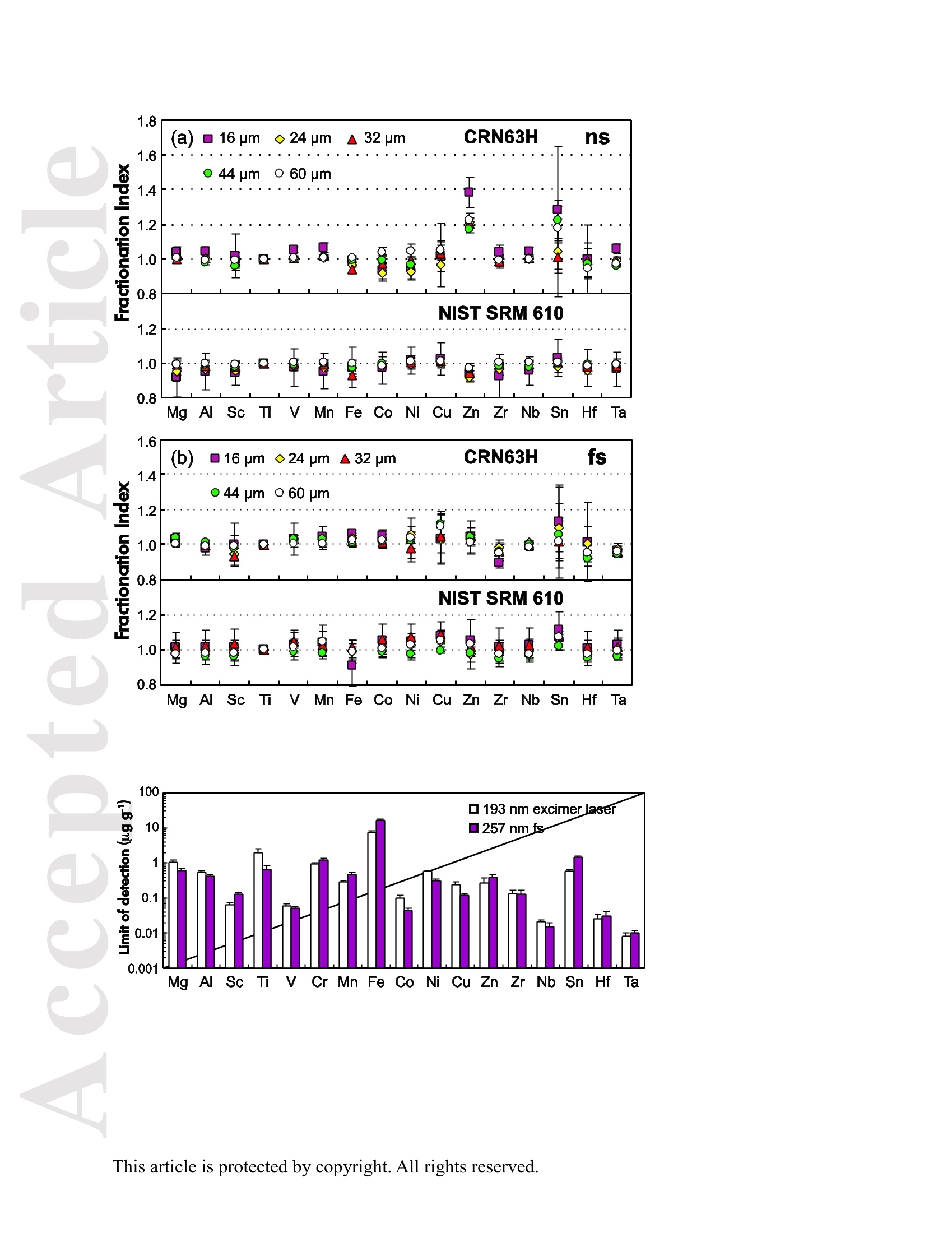
使用飞秒激光电感耦合等离子体质谱仪分析钛铁矿中57Fe和49Ti的浓度大约比NIST SRM 610高1.8倍。与193nm准分子激光器相比,257nm 飞秒激光器的元素分离量较小。采用193nm准分子激光剥蚀时,激光能量密度的选择对钛铁矿元素分离有显著影响。与飞秒激光相比,纳秒激光生成的剥蚀坑和沉积气溶胶形貌的扫描电镜图像显示了更大的熔化效应,烧蚀坑周围颗粒沉积面积更大。在纳秒剥蚀坑周围喷出物主要由大滴再凝固的熔融物质组成;然而,在飞秒剥蚀坑周围的喷出物是由形状“粗糙”的微粒团块组成。这是纳秒激光和飞秒激光不同剥蚀机制的结果。使用NIST SRM 610作为193nm准分子LA-ICP-MS和fs-LA-ICP-MS的参考材料,可以对钛铁矿样品进行非基体匹配条件下的定量分析。采用193nm准分子LA-ICP-MS 在12.7 J cm-2高激光能量密度条件和采用fs-LA-ICP-MS对钛铁矿样品中的大部分元素进行分析,得到的结果一致。
方案详情

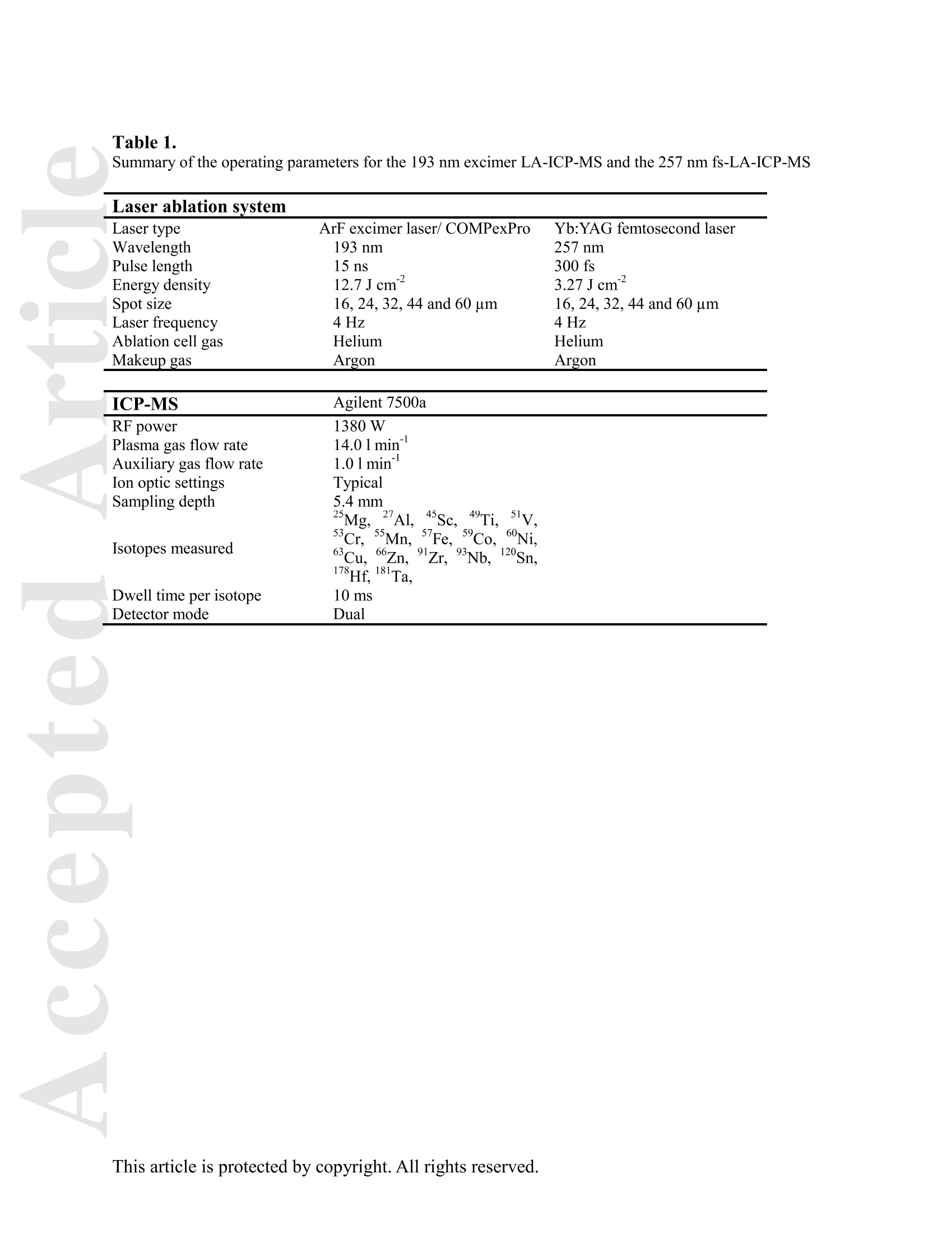
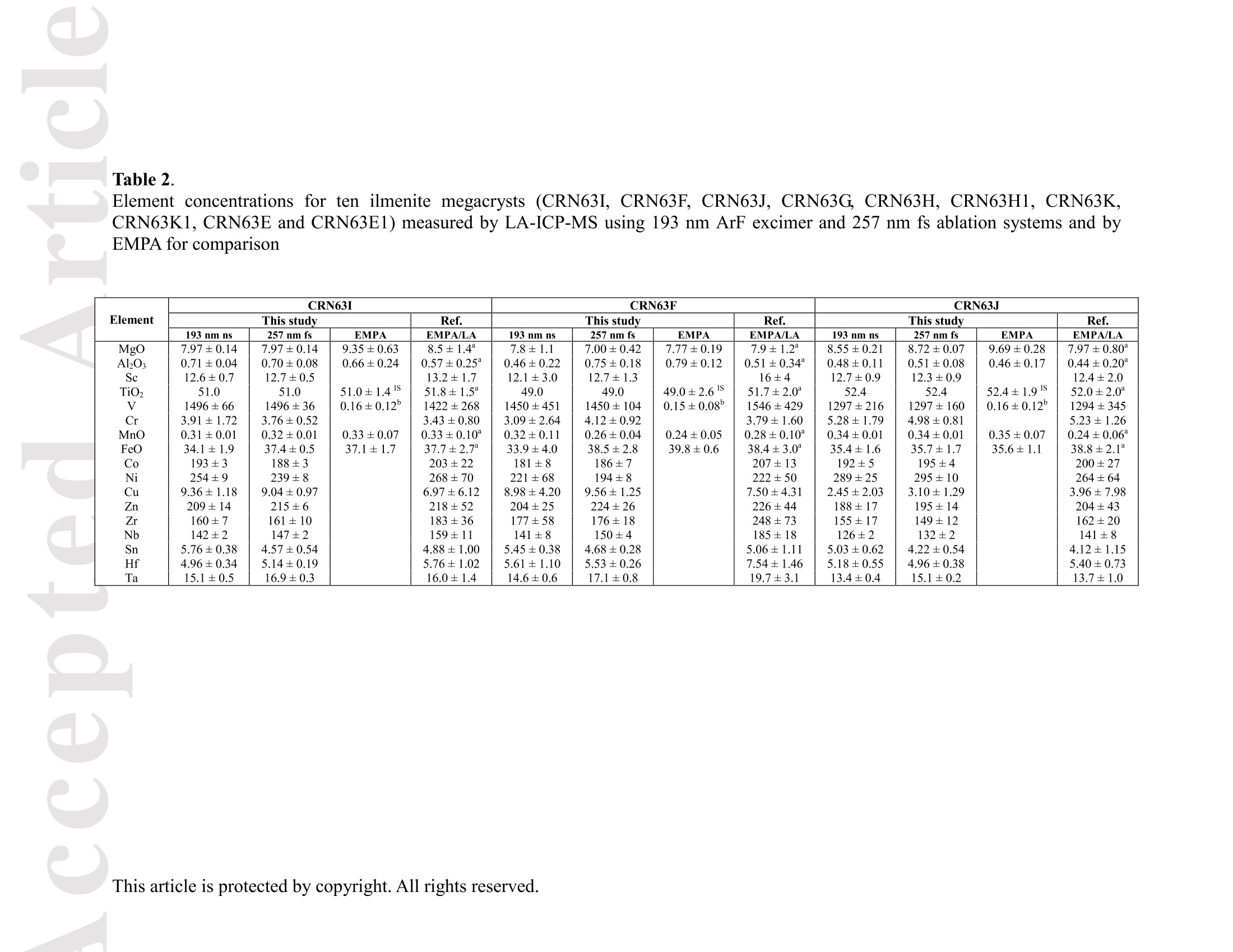
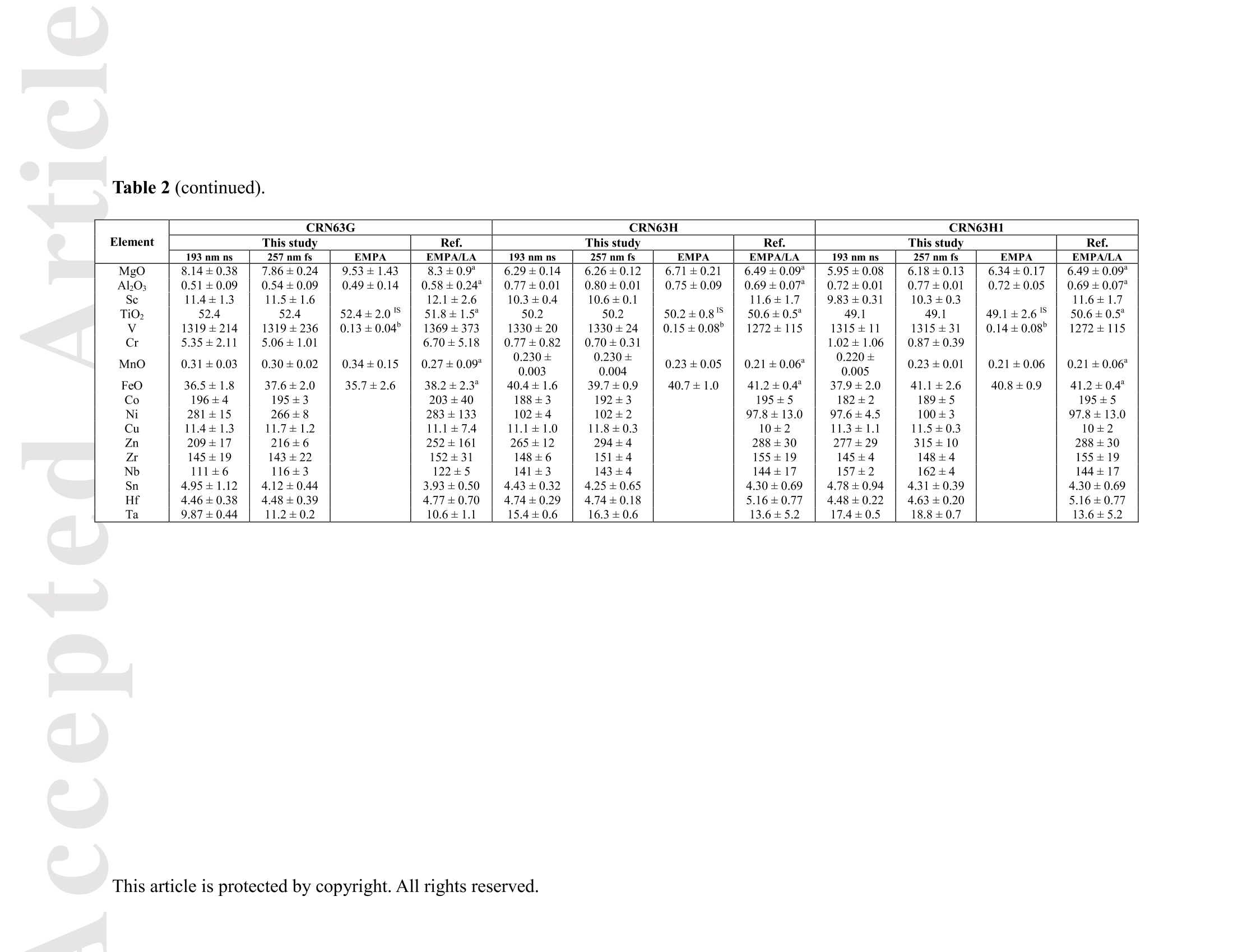
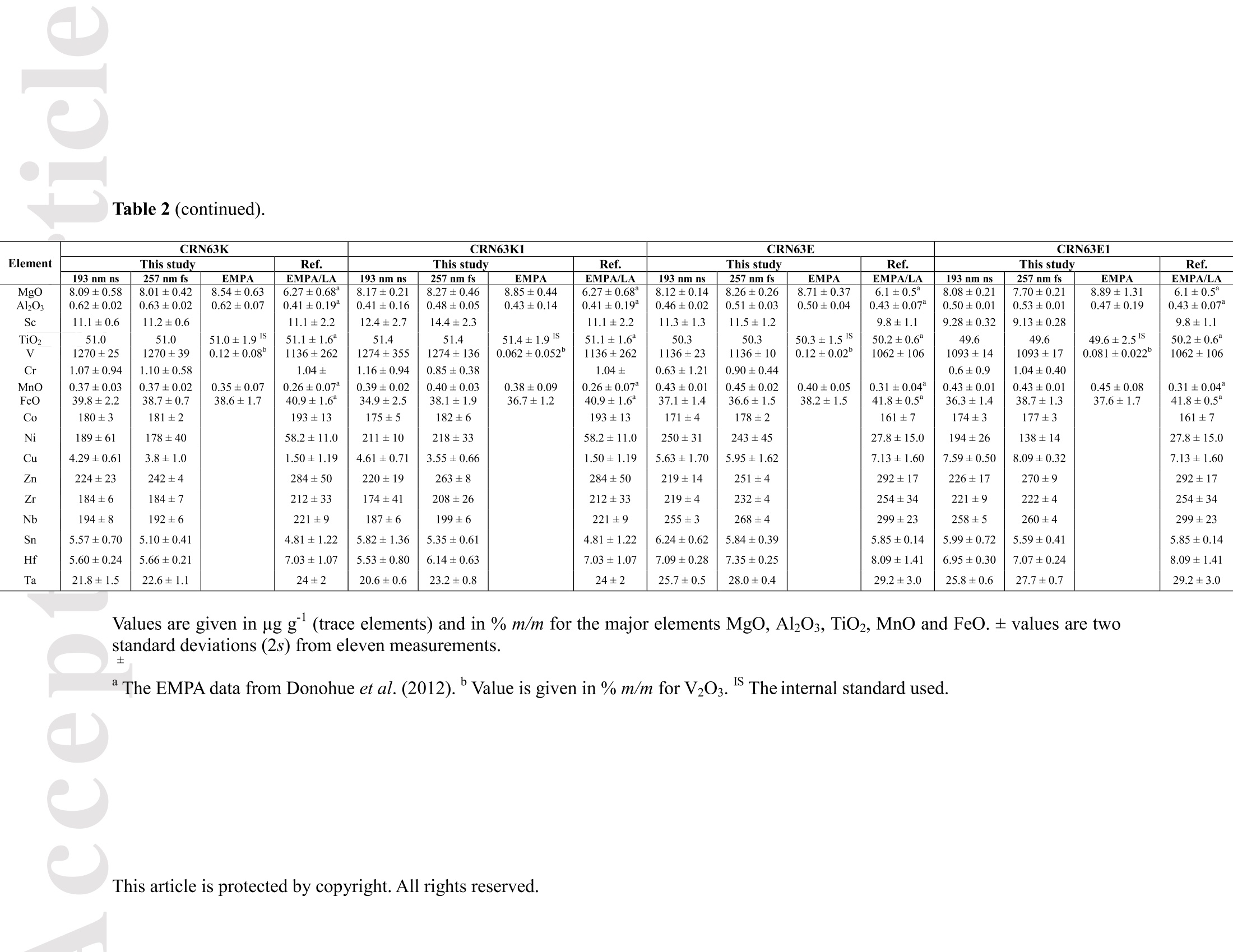
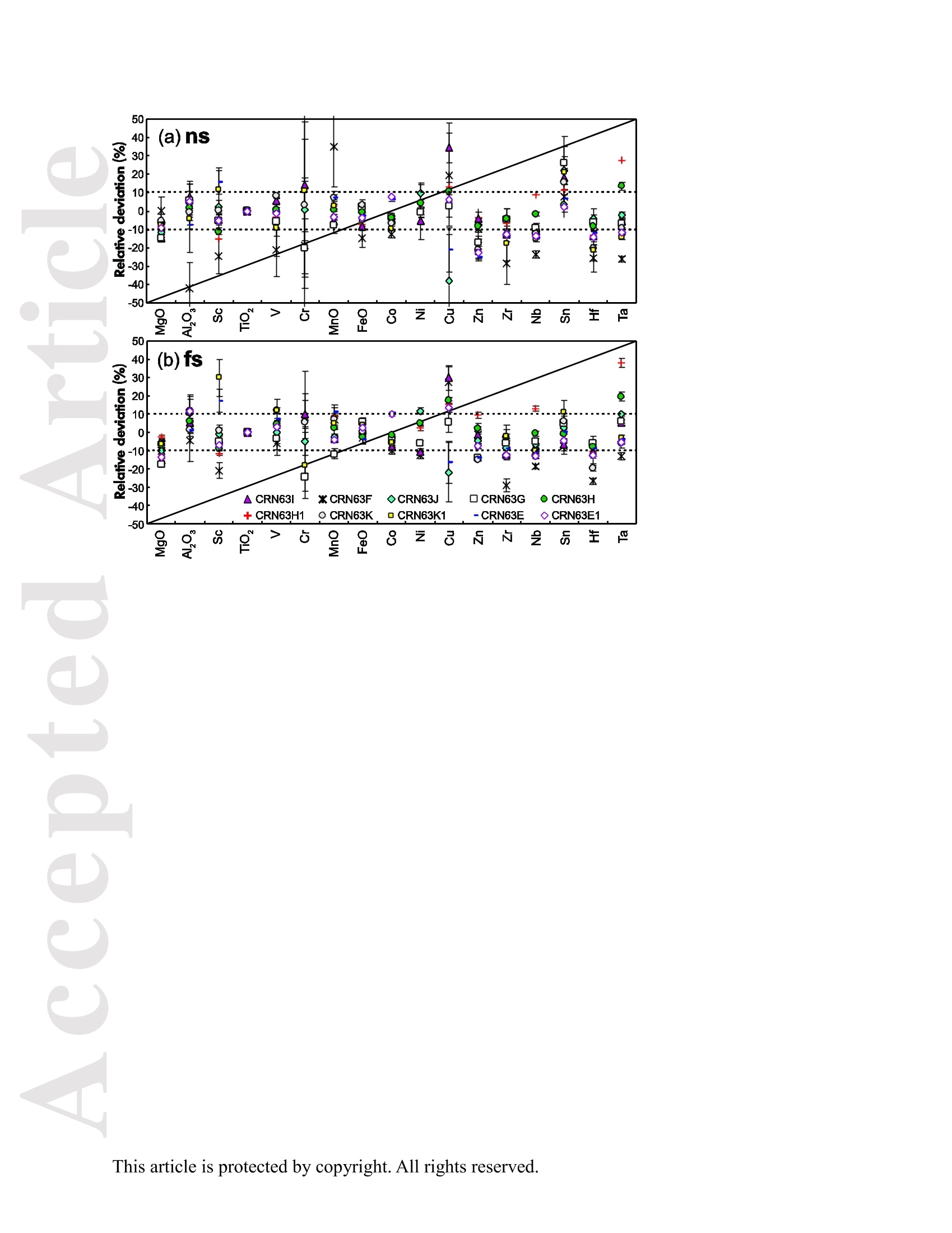
Received Date :19-Dec-2015 Revised Date :14-Mar-2016 Accepted Date :15-Mar-2016 Article type : Original Article Ablation Characteristic of Ilmenite using UV Nanosecond andFemtosecond Lasers: Implications for Non-Matrix-MatchedQuantification Zhen Li (1), Zhaochu Hu (1, 2) , Detlef Giinther (1, 3), Keqing Zong (1), Yongsheng Liu (1),Tao Luo (1), Wen Zhang(1), Shan Gao (1) and Shenghong Hu (1) (1) State Key Laboratory of Geological Processes and Mineral Resources, Faculty of EarthSciences, China University of Geosciences, Wuhan 430074, PR China (2) The Beijing SHRIMP Center, Institute of Geology, Chinese Academy of Geological Sciences,Beijing 102206, PR China (3) ETH Zurich, Laboratory for inorganic Chemistry, CH-8093 Zurich, Switzerland* Corresponding author. e-mail: zchu@vip.sina.com Ilmenite (FeTiO3) is a common accessory mineral and has been used as a powerful petrogeneticindicator in many geological settings. In this study, elemental fractionation and matrix effects inilmenite (CRN63E-K) and silicate glass NIST SRM 610 were investigated using 193 nm ArFexcimer nanosecond (ns) laser and 257 nm femtosecond (fs) laser ablation systems coupled to an This article has been accepted for publication and undergone full peer review but has not beenthrough the copyediting, typesetting, pagination and proofreading process, which may lead todifferences between this version and the Version of Record. Please cite this article as doi:10.1111/j.1751-908X.2016.00408.x This article is protected by copyright. All rights reserved. inductively coupled plasma-mass spectrometer. The concentration-normalisedFe and 4Tiresponses in ilmenite were approximately higher than that of NIST SRM 610 by a factor of 1.8using fs-LA. Compared with the 193 nm excimer laser, smaller elemental fractionation wasobserved using the 257 nm fs laser. When using 193 nm excimer laser ablation, the selectedrange of the laser energy density had a significant effect on the elemental fractionation inilmenite. Scanning electron microscopy images of ablation craters and the morphologies of thedeposited aerosol materials showed more melting effects and an enlarged particle deposition areaaround the ablation site of the ns-LA-generated crater when compared with those using fs-LA.The ejected material around the ns crater predominantly consisted of large droplets ofre-solidified molten material; however, the ejected material around the fs crater consisted ofagglomerates of fine particles with“rough"shapes. These observations are a result of thedifferent ablation mechanisms for ns- and fs-LAs. Non-matrix matched calibration was appliedfor the analysis of ilmenite samples using NIST SRM 610 as a reference material for both 193nm excimer LA-ICP-MS and fs-LA-ICP-MS. Similar analytical results for most elements inilmenite samples were obtained using both 193 nm excimer LA-ICP-MS at a high laser energydensity of 12.7 J cmand fs-LA-ICP-MS. Keywords: LA-ICP-MS, elemental fractionation, melting effects, ilmenite, non-matrix matchedcalibration. Received 19 Dec 15- Accepted 15 Mar 16 This article is protected by copyright. All rights reserved. Laser ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS) is a rapidlydeveloping analytical technique for trace element and isotopic determination with a wide rangeof geological applications (Arrowsmith 1987, Jackson et al. 2004, Gunther and Hattendorf 2005,Kosler 2007, Sylvester 2008, Russo et al. 2011, Orellana et al. 2013, Lin et al. 2014). With abetter understanding of the ablation mechanism and the application of a suitable calibrationstrategy, the precision and accuracy of trace element measurements has successively improved.This is particularly reported for LA-ICP-MS analysis of silicates and carbonates with the use ofultraviolet (UV) pulse wavelength lasers (Liu et al. 2008, Jochum et al. 2012, Jochum et al. 2014,Ohata et al. 2014, Li et al. 2015, Zhao et al. 2015). However, LA-ICP-MS has not become awidely applied analytical technique for the routine analysis of natural metal oxide minerals. Themain limitations for accurate analyses of trace elements in natural metal oxide minerals has beenthe lack of matrix-matched homogeneous reference materials as well as a poor understanding ofthe ablation behavior of metal oxide matrices. Therefore, the exploitation of a non-matrixmatched calibration strategy for such types of minerals remains of major interest. Ilmenite (FeTiO3) is the most important iron-titanium oxide on Earth and a common accessorymineral in igneous and metamorphic rocks, e.g., ref. (Papike et al. 1976, Mitchell 1977, Moore etal. 1992, Charlier et al. 2007). It is an excellent tracer of crystallisation and fractionationprocesses, because it contains both compatible (Cr and V) and incompatible (Mn, Nb, Ta, Zr andHf) elements (Moore et al. 1992, Klemme et al. 2006, Charlier et al. 2007).Klemme et al.(2006)measured the trace elements in synthetic ilmenite calibrated against NIST SRM 610 with Ti asan internal standard by 193 nm excimer LA-ICP-MS at a spot size of20 um. In another study,both synthetic titanite glass (TIT-200)(Odegard et al. 2005) and NIST SRM 612 have been This article is protected by copyright. All rights reserved. simultaneously used as external calibration materials for the analysis of the different traceelements in ilmenite using a 266 nm Nd:YAG laser at a spot size of20 um (Charlier et al.2007).The two standard calibration procedure has been applied because the synthetic titanite glass lackscertain elements of interest (e.g., Ta) (Charlier et al. 2007), thus requiring the use of anotherreference material (NIST SRM 612) for the ilmenite abundance determination. Klemme et al.(2008) created a new titanite glass; however, it still lacked of several elements commonly ofinterest in ilmenite (V, Co, Ni, Cu). Thus, these synthetic titanite glasses are not ideal externalreference materials for multi-element determination of ilmenite, which adds to the complexity ofthe calibration process. Donohue et al. (2012) determined thirteen trace elements in ilmenitemegacrysts using Ti as an internal standard and NIST SRM 610 as a calibration material at a spotsize of 80 um using a 213 nm laser for ablation and reported good agreement between the resultsdetermined by LA-ICP-MS and solution nebulisation (SN)-ICP-MS for most elements, except incases where megacrysts with a titano-magnetite exsolution phase were present. UV-ns-LA hasalso been successfully applied for the analyses of other metal oxides, such as Fe-Mn oxides (Xuet al. 2005, Huelin et al. 2006) and magnetite (Nadoll and Koenig 2011). As shown in previous studies, fs lasers might be less prone to matrix-related problems whencompared with ns lasers (Poitrasson et al. 2003, Hirata and Kon 2008), because vaporisation andfragmentation mechanisms dominate in fs lasers (D'Abzac et al. 2012), which can efficientlycircumvent thermal influence on the ablated matrix (Seydoux-Guillaume et al. 2010) and helpsshrinking of the heat-affected zone (Fernandez et al.2007). Glaus et al. (2010) investigated themorphology of aerosols from ns- and fs-LAs using different matrices and laser fluences. Theyobserved different ablation mechanisms and particle formation mechanisms for both laser types, This article is protected by copyright. All rights reserved. but similar morphologies of fs-LA-generated aerosols for non-conducting materials (glass andzircon), metallic samples (steel and brass) and semiconductors (sulfides). Mozna et al. (2006)compared the performance of ns-UV-LA-ICP-MS and fs-UV-LA-ICP-MS for Fe-based samplesand showed that the fs-LA provided more accurate results using non-matrix-matched calibrationprocedures. Wiltsche and Giinther (2011) investigated the capabilities of near infrared (NIR) fs-LA-ICP-MS for the quantification of major, minor, and trace elements in unalloyed, alloyed,and highly alloyed steels and super alloys. Calibration with matrix-matched andnon-matrix-matched reference materials provided similar results within the uncertainty of thecertified values. Velasquez et al. (2012) determined Au and Cu concentrations in natural pyriteusing NIR-fs-LA-ICP-MS with sulfide SRMs (pyrrhotite Po-726-SRM and an in-house naturalchalcopyrite Cpy-RM) and NIST SRM 610 served as reference materials, respectively. Theirresults showed similar calculated concentrations that were independent of the external referencematerial used, indicating that there was no measurable matrix effect over a wide range of majorto trace element concentrations in silicate and sulfide materials. In addition, Horn et al.(2006)successfully determined Fe isotopes in a variety of matrices including native iron, iron alloys,iron sulfides, iron carbonates, iron oxides and iron hydroxides using the isotopically certifiediron reference material IRMM-014 for calibration by UV-fs-LA-MC-ICP-MS. Theaforementioned determination of Fe-based samples, metal alloys and sulfides by fs-LA-ICP-MS confirmed that there is no dependence on the matrix with this technique. The major aim of this study was to investigate the extent of elemental fractionation and matrixeffects on the quantification capabilities of the composition of ilmenite. UV 193 nm ArF excimerLA-ICP-MS analyses and 257 nm fs-LA-ICP-MS analyses were performed in parallel for direct This article is protected by copyright. All rights reserved. comparison of the results. For quantification calibration, the glasses NIST SRM 610 and 612were used as primary reference materials because they contain many trace elements at highconcentrations. Furthermore, most of the trace elements are homogenously distributed and wellcharacterised (Eggins and Shelley 2002, Jochum et al. 2011). Here, we tested the applicability ofNIST SRM 610 as an external reference material for ilmenite analysis under different ablationconditions. Moreover, to better understand the ablation mechanisms for ilmenite, themorphologies of the ablation craters and the deposited aerosols surrounding the craters wereanalysed by scanning electron microscopy (SEM). Experimental Instrumentation Ilmenite grains mounted on 1 inch epoxy rounds and polished were used for the EPMA andLA-ICP-MS analyses. The major elemental compositions were determined using a JXA-8230electron probe microanalyser (EPMA) at the Institute of Mineral Resources, Chinese Academyof Geological Sciences. The operating parameters for EPMA were as follows: acceleratingvoltage 15.0 kV, beam current 20 nA and beam diameter 5 um. Two different pulsed laser systems were used: an ArF excimer laser (=193 nm) with a 15 nspulse duration (GeoLas 2005, MicroLas, Gottingen, Germany) and a Yb fs laser (A=257 nm)with a 300 fs pulse duration (NWR-Femto,USA). The 193 nm excimer laser system wasequipped with a homogeniser. This optical configuration allowed selection of a constant fluencethat was independent of the crater diameter. Unlike in excimer laser ablation systems, there is nohomogeneous lens in fs laser, which has a Gaussian energy beam profile across its diameter. This article is protected by copyright. All rights reserved. Each laser system was coupled to an Agilent 7500a ICP-MS (Japan). Helium is advantageous as a carrier gas (Eggins et al. 1998, Giinther and Heinrich 1999) and was thus applied in this study.Argon was used as the make-up gas and mixed with the carrier gas via a T-connector beforeentering the ICP. Detailed operating conditions for the lasers and the ICP-MS instrument arelisted in Table 1. Prior to in situ analysis, the carrier and make-up gas flows were optimised byablating NIST SRM 610 to obtain maximum La signal intensities, while maintaining lowThO/Th <0.3% and Ca /Ca <0.4%ratios to reduce the oxide and doubly charged ioninterferences. The 238u/232Th ratio used as an indicator for complete vaporisation of particles(Guinther and Hattendorf 2005) was kept at approximately 1 during the ablation of NIST SRM610. Each single ablation comprised approximately 20 s of acquisition of the background signalsrepresenting the gas blank, followed by 50 s of data acquisition from the sample. Signals fromthe first 5 s and last 5 s time intervals of laser ablation were not integrated in sample analysis.The caculated data were based on the middle time interval of 40 s. The analyses ofevery fivesamples were followed by two analyses of NIST SRM 610 to correct for time-dependent drift ofsensitivity and mass discrimination. The off-line selection, integration of background andanalytical signals, time-drift correction and calibration were carried out by an in-house programICPMSDataCal (Liu et al. 2008,2010). 4Ti was used as the internal standard element, and NIST SRM 610 served as the reference material. Following ablation, the ilmenite samples were carefully handled to preserve the thin layer ofparticles (the ejecta blanket) on the sample surface adjacent to the laser crater for SEM imagingto obtain information about the craters and the morphology of the aerosols deposited around eachcrater. This article is protected by copyright. All rights reserved. Samples Ten natural ilmenite megacrysts provided by Donohue et al. (2012) and designated namesCRN63E to K were investigated in this study. Among these ilmenite megacrysts, replicatesamples for three ilmenite megacrysts (CRN63H, CRN63E,CRN63K) are named CRM63H1,CRN63E1, CRN63K1, respectively. In addition, the following widely used external calibrationreference materials were selected for analysis in this study: USGS basaltic reference glasses(BCR-2G and BHVO-2G) and synthetic NIST SRM soda-lime reference glass (NIST SRM 610).All of the reference glass materials were cleaned in a 2% HNO3 bath with ultrasonic washingprior to LA-ICP-MS analyses.To avoid corrosion from nitric acid, these ilmenite megacrystswere ultrasonically treated first in alcohol and then in ultrapure water. The working values forNIST SRM 610 used in this study were taken from Jochum et al. (2011). Results and discussion Comparison of the ablation behavior between ilmenite and glass Figure 1 shows the concentration-normalised Fe and 4Ti responses (cps/ugg) in referenceglasses and ilmenite samples with changing laser energy density by 193 nm excimer LA-ICP-MS(a)(c) and 257 nm fs-LA-ICP-MS (b) (d). There are obviously different concentration-normalisedFe and 4Tiresponses between NIST SRM 610 and the USGS glasses using ns-LA. This could be related to the very different major element compositions of NISTSRM 610 from USGS glasses. In contrast, NIST SRM 610 and USGS glasses suggest a similarresponse trend with increasing laser fluence for both "Fe and 4Ti when the fs-LA is adopted. Itseems that the fs laser is less prone to matrix-related ablation yield problems when compared This article is protected by copyright. All rights reserved. with ns lasers for the glasses analysed. Contrary to those observed in these glasses, the concentration-normalisedFe and 4Ti responses (cps/ugg) between ilmenite samples andglasses are much more different by fs-LA than ns-LA. For example, theconcentration-normalisedFe and 4Ti responses in ilmenite (CRN63H) are higher than those inNIST SRM 610 by a factor of 1.8 using fs LA (Figure 1b, d). For ns-LA, the differences aresignificantly smaller. Except for the lowest energy typically the concentration-normalisedFeand 4Ti responses in ilmenite are about 3/4 of the one for the NIST SRM 610 (Figure 1a, c).Figure 2 shows the ratio of concentration-normalised signal response (’Fe/4Ti) for both NISTSRM 610 and ilmenite as a function of laser fluence in fs-LA and ns-LA. Although thesignificant different concentration-normalisedFe and 4Tiresponses between NIST SRM 610and ilmenite using fs-LA, the ratios of concentration-normalised signal response (Fe/4Ti) areexactly the same at different laser fluences. In contrast, the ratios of concentration-normalisedsignal response(Fe/4Ti) between NIST SRM 610 and ilmenite are significant different byusing ns-LA at low laser fluences, which close to each other at high laser fluences (exceeding7.74 J cm). This result suggests that the similar concentration-normalised signal responsesamong different samples cannot guarantee the good analytical results, and vice versa. Figure 3 shows the variation of the Fe/Ti and Co/Ti ratios with time during the single holeablation of ilmenite (CRN63H) at different laser energy densities using a spot size of 44 um. Ascan be seen, the ns laser energy density had a significant effect on these elemental ratios.Increasing the laser fluence from 3.36 J cmto 11.1 J cm", the Fe/Ti and Co/Ti ratios wereincreased by a factor of 1.3 and 1.7, respectively. Above a laser fluence of 11.1 J cm, theseelemental ratios remained constant (Figure 3a, c). Further investigations are needed to reveal the This article is protected by copyright. All rights reserved. exact mechanism. In contrast, the Fe/Ti and Co/Ti ratios showed a slight positive slope duringthe single hole ablation of ilmenite samples at different laser energy densities of 1.80-4.27 J cmwhen using the fs laser for ablation. Morphology of ablation craters and aerosol particle structures of ilmenites A series of ablation craters produced on ilmenite (CRN63H) under different laser energydensities were imaged by SEM (Figure 4). These craters were ablated with a 44 um spot size for200 laser pulses over a range of fluence from 3.36 to 15.8 J cm"for the 193 nm excimer laserand 1.80 to 4.27J cm"for the 257 nm fs laser. The craters created by the ns-LA are significantlymore influenced by melting than those created by the fs-LA. As seen in this figure, there areabundant molten ejecta around the ns laser-generated craters , especially at a high laser energydensity, that are essentially absent around the fs craters. Another noticeable feature is that the fslaser ablation crater rims display some border deformation and ripples (Figure 4f, g, h, i, j),which are typical of interference between the incident laser light and the scattering light (Bonseet al. 2002). However, these ripples are largely shaded on the ns crater rims, and even thesecrater rims are smooth. This is a result of subsequent melting after initial ablation (cyclic melting)or the re-deposition of molten material. A similar phenomenon was observed and reported byPoitrasson et al. (2003). In addition, with increasing energy density, the bottom of the ns cratersbecome flatter, and the number of large molten spherical particles at the crater bottom becomereduced. Unlike ns craters, the morphologies of fs craters produced by different laser ablationenergy densities are similar. There are some small holes in the fs crater bottoms, which is causedby energy irregularities of the laser beam profile and lens aberrations, where different rays incident at the focusing lens at different angles can be focused at different points (Shaheen et al. This article is protected by copyright.All rights reserved. 2012). Visual inspection of the ns and fs craters indicates that far more particles were depositedaround the craters produced by the ns-LA (Figure 5). There is a significant positive correlationbetween the laser output energy of the ns-LA and the deposited particle amount. Figure 6 shows SEM images of the ns-LA (at 11.1 J cm2) and fs-LA (at 3.63 J cm²) crater’s rims and particles in ilmenite (CRN63H). The morphologies of ns-LA-and fs-LA-generatedaerosols are significantly different for the ilmenite. In Figure 6a, the ns crater is mainlysurrounded by large spherical droplets of re-solidified molten material (approximately 0.3-2 um).Moving away from the ns-LA crater rim, there are elliptical holes in many droplets, most likelycaused by the rapid movement of unsolidified droplets when they sputter on the clean samplesurface. These typical melting patterns indicate that thermal processes are the dominantmechanisms of ilmenite ablation in ns-LA. The ns-LA-generated particles are formed by liquidejection, as a result of hydrodynamic sputtering, as well as by vapor condensation (Shaheen et al.2012). Large droplets are ejected by the expulsion of the molten material as a result of the impactof the recoil pressure from the expanding vapor plume in the background gas (Bogaerts et al.2003). In contrast, primarily separated agglomerates of fine particles are formed in case of fs-LA(approximately 0.05-0.1 um) (Figure 6b). Notably, these fine particles have“rough”shapes(approximately 70 nm) (Figure 6c), suggesting that they may condense quickly and not besubject to heating (Poitrasson et al. 2003). These features indicate that fs-LA is less prone tothermal effects. Fragmentation and vaporisation-condensation mechanisms are the mainprocesses in fs-LA (D'Abzac et al.2012), which is confirmed by the generation of fine particles with“rough” shapes (Figure 6c). This article is protected by copyright. All rights reserved. Elemental fractionation To examine the potential differences concerning elemental fractionation during the analysis by LA-ICP-MS, the elemental fractionation indices were calculated for CRN63H and NIST SRM 610. Fractionation as temporal drifts of elemental ratios (relative to Ti as the internal standard)was quantified using Fryer’s definition (Fryer et al. 1995). To simulate typical analysisconditions, the elemental fractionation indices were calculated by dividing the 50 s transientsignals into two equal time intervals, instead of 240 s as reported in Fryer et al. (1995). Figure 7shows the effect of changing the laser energy densities on the 16 calculated elementalfractionation indices with respect to Ti at a spot size of 44 um using the 193 nm excimer and the257 nm fs laser for sampling. For ns-LA analysis, the calculated elemental fractionation indicesof transition group elements (Fe, Co, Ni, Cu) significantly increase when the laser energy densityis less than 7.74 J cm. At high laser energy densities of 11.1-15.8 J cm", these calculatedelemental fractionation indices were significantly reduced. Therefore, we recommend the use ofhigh laser energy density for the analysis of ilmenites by ns-LA-ICP-MS. Gaboardi andHumayun (2009) also reported that the volatility effects (especially for transparent minerals orglasses) could be minimised and possibly negated by an increase of the laser energy density. Incontrast, the calculated elemental fractionation indices for most elements remain constant ataround 1 when using the 257 nm fs laser at different laser ablation energy densities between1.80-4.27 J cm’. It is worth noting that the calculated elemental fractionation indices for volatileelements such as Zn are significantly lower by fs-LA when compared with ns-LA. This is relatedto the significantly reduced thermal effects thus leading to a shrinking of the heat-affected zone during the fs-LA process (Fernandez et al.2007). The significant random change in the elemental fractionation index for Sn could be attributed to the heterogeneous distribution of this This article is protected by copyright. All rights reserved. element in ilmenite CRN63H and is therefore not further discussed. For NIST SRM 610. thecalculated elemental fractionation indices were very close to each other under these differentconditions, which is irrespective of the laser used for ablation. These results suggest that matrixdependent fractionation between ilmenite and NIST SRM 610 maybe reduced when using fslaser ablation for sampling. It should be noted, however, that the elemental fractionation indicesobtained in NIST SRM 610 using fs-LA are slightly larger than ns-LA at the spot size of 44 um.This could be related the heterogeneous distribution of energy density in the fs laser beam (Li etal. 2015). The interpretation of primary ilmenite compositions is sometimes complicated by postcumulusre-equilibration (trapped-liquid crystallisation, exsolution, reaction with magnetite and silicates)(Charlier et al.2007). Hence,spatially resolved analysis using a laser crater as small as possibleis required, especially for small ilmenite samples. Figure 8 shows the effect of changing thecrater diameters from 60 um to 16 um on the calculated elemental fractionation indices at highlaser energies (15.8 J cm’and 4.27 J cm’for ns- and fs-laser pulses, respectively). Thediscrepancies among the calculated elemental fractionation indices for these elements are notsignificant for the selected crater diameter range. In ns-LA, it is well documented that a largecrater diameter with a small depth/diameter ratio could reduce elemental fractionation (Mank andMason 1999). However, the calculated elemental fractionation indices for Zn in ilmenite(CRN63H) still deviate significantly from unity at large spot sizes of 44-60 um as well as thoseat small spot sizes of 16-32 um, whereas they were closer to 1 in NIST SRM 610 (Figure 8a) atthese crater diameters. Thus, a matrix-matched calibration material is essential for the accuratedetermination of the volatile elements, e.g. Zn, in ilmenite by ns-LA-ICP-MS. For fs-LA This article is protected by copyright. All rights reserved. measurements, these calculated elemental fractionation indices were in the range of 1.0 ± 0.1 formost elements between ilmenite (CRN63H) and NIST SRM 610 at different crater diameters.The result implies that accurate analysis should be feasible for the analysis of ilmenite withnon-matrix matched calibration by fs-LA-ICP-MS. Quantitative analyses with non-matrix matched calibration Due to the experimental finding that the laser energy density has a major influence on the ns-LAprocess, the optimised laser energy densities were used in this study (e.g., 12.7 J cmfor ns-LAand 3.27 J cm’ for fs-LA). We determined 16 major and trace element concentrations in 10natural ilmenite megacrysts using NIST SRM 610 as an external reference material and Ti as aninternal standard using both laser ablation systems (Table 2). The calculated LODs (limit ofdetection) for most of these elements in NIST SRM 610 are similar for both fs-andns-LA-ICP-MS systems according to the protocol of Longerich et al. (1996). As illustrated inFigure 9, both fs- and ns-LA-ICP-MS could achieve 0.1-1 uggLODs for most of thesedetermined elements at a spot size of 44 um. Figure 10 shows the relative deviations of theaverage concentrations determined in these ilmenite megacrysts from our major elementmeasurements by EPMA and the working trace element values provided by Donohue et al.(2012). It can be seen that the relative deviations of major elements and transition-groupelements V, Co and Ni are in agreement with the 10% uncertainty for most ilmenite samples(Figure 10a). The large relative deviations of Al2O3 and MnO in ilmenite CRN63F should berelated to their heterogeneous distributions. The concentrations of the volatile element Zn have asystematic bias and were generally 4%-25% lower than the working value (Figure 10a), whichcould be a result of the matrix dependent fractionation with 193 nm excimer LA-ICP-MS. The This article is protected by copyright. All rights reserved. reference materials GSD-1G and BCR-2G with high TiO2 content could be a useful alternative toNIST SRM 610 as a reference material for ilmenite sample analysis. For fs-LA analysis, theaccuracy of Zn is obviously improved compared with the results obtained using 193 nm excimerLA-ICP-MS, which implies that matrix effects for Zn are reduced by fs-LA-ICP-MS (Figure10b). The relative deviations of most elements in these ilmenite megacrysts are lower than 10%for fs-LA analysis. It needs to be noted that the determined values of MgO, Sc, Zr, Nb and Hf inmost ilmenite samples are lower than the literature values by approximately 10%. Comparison ofthe results obtained by 193 nm excimer LA-ICP-MS at a high laser energy density of 12.7 Jcmand fs-LA-ICP-MS indicates that, except for Zn and Sn, the accuracies of most elementsdetermined by both two laser systems are not significantly different. The excellent agreementbetween the determined values for most elements by ns- and fs-LA-ICP-MS guarantees thequality of our data to a certain extent. Conclusions Our results indicate that non-matrix-matched calibration can be applied for the analysis ofilmenite using NIST SRM 610 as an external reference material for both 193 nm excimerLA-ICP-MS and fs-LA-ICP-MS. For ns-LA analyses, the elemental fractionation for transitiongroup elements is mostly significant at low laser energy densities (less than 7.74 J cm). Asshown in our study, they could be remarkably reduced by using high laser energy densities(exceeding 11.1 J cm). Zinc is an exception that could not be reduced by using high laserenergy densities for ns-LA analysis. The images of ablation craters reveal that there aresignificant differences in the morphologies of ablation craters and in the ejected materials aroundthe ablation craters. For ns-LA, a typical pattern of melting (structure of a re-solidified melt with This article is protected by copyright. All rights reserved. a number of re-deposited droplets) indicates that thermal processes are the dominant mechanismsof ilmenite ablation. The absence or reduced thermal effects in fs laser ablation is demonstratedby the generation of fine particles with“rough"shapes. The use of fs-LA systems or ns-LA at ahigh laser energy density is recommended when analysing ilmenite samples using NIST SRM610 as an external reference material. Acknowledgements We would like to thank Patrick H. Donohue for providing ilmenite megacrysts. This research issupported by the National Natural Science Foundation of China (Grants 41403017,41273030,41322023, and 41573015), the Fundamental Research Funds for National Universities, theCERS-China Equipment and Education Resources System (Grant CERS-1-81), the StateAdministration of the Foreign Experts Affairs of China (B07039), the Opening Foundation of theBeijing SHRIMP Center (National Science and Technology Infrastructure), and the MOSTSpecial Fund from the State Key Laboratories of Geological Processes and Mineral Resources. References Arrowsmith P. (1987) Laser ablation of solids for elemental analysis by inductively coupled plasma-mass spectrometry. AnalyticalChemistry,59, 1437-1444. Bogaerts A., Chen Z.Y., Gijbels R. and Vertes A. (2003) Laser ablation for analytical sampling: What can we learn from modeling? Spectrochimica Acta Part B,58,1867-1893. Bonse J., Baudach S., Kriger J., Kautek W. and Lenzner M. (2002) Femtosecond laser ablation of silicon-modification thresholds and morphology. Applied Physics A, 74, 19-25. Charlier B., Skar O., Korneliussen A., Duchesne J.-C. and Vander Auwera J. (2007) Ilmenite composition in the Tellnes Fe-Ti deposit, SW Norway: Fractional crystallization, postcumulus evolution andilmenite-zircon relation. Contributions to Mineralogy and Petrology, 154, 119-134. D'Abzac F.-X.,Seydoux-Guillaume A.-M., Chmeleff J., Datas L. and Poitrasson F. (2012) In situ characterization of infra red femtosecond laser ablation in geological samples. Part B: the laser inducedparticles. Journal of Analytical Atomic Spectrometry,27, 108-119. This article is protected by copyright. All rights reserved. Donohue P.H., Simonetti A. and Neal C.R. (2012) Chemical characterisation of natural ilmenite: A possible new reference material. Geostandards and GeoanalyticalResearch,36, 61-73. Eggins S.M., Kinsley L.P.J. and Shelley J.M.G. (1998) Deposition and element fractionation processes during atmospheric pressure laser sampling for analysis by ICP-MS.Applied Surface Science, 127,278-286. Eggins S.M. and Shelley J.M.G. (2002) Compositional heterogeneity in NIST SRM 610-617 glasses. Geostandards Newsletter: The Journal ofGeostandards and Geoanalysis,26,269-286. Fernandez B., Claverie F.,Pecheyran C. and Donard O.F. (2007) Direct analysis of solid samples by fs-LA-ICP-MS. TrAC Trends in Analytical Chemistry, 26, 951-966. Fryer B.J., Jackson S.E. and Longerich H.P. (1995) The design, operation and role of the laser-ablation microprobe coupled with an inductively coupled plasma-massspectrometer (LAM-ICP-MS) in the earth sciences. Canadian Mineralogist, 33,303-303. Gaboardi M. and Humayun M. (2009) Elemental fractionation during LA-ICP-MS analysis of silicate glasses: Implications for matrix-independentstandardization. Journal of Analytical Atomic Spectrometry,24, 1188-1197. Glaus R., Kaegi R., Krumeich F. and Giinther D. (2010) Phenomenological studies on structure and elemental composition of nanosecond and femtosecond laser-generatedaerosols with implications on laser ablation inductively coupled plasma mass spectrometry. Spectrochimica ActaPart B, 65,812-822. Giinther D. and Heinrich C.A. (1999) Enhanced sensitivity in laser ablation-ICP mass spectrometry using helium-argon mixtures as aerosol carrier. Journalof Analytical Atomic Spectrometry, 14,1363-1368. Giinther D. and Hattendorf B. (2005) Solid sample analysis using laser ablation inductively coupled plasma mass spectrometry. TrAC Trends inAnalytical Chemistry,24,255-265. Hirata T. and Kon Y.(2008) Evaluation of the analytical capability of NIR femtosecond laser ablation-inductively coupled plasma massspectrometry. Analytical Sciences, 24,345-354. Horn I., von Blanckenburg F., Schoenberg R., Steinhoefel G. and Markl G. (2006) In situ iron isotope ratio determination using UV-femtosecond laser ablation with application to hydrothermal oreformation processes. Geochimica et Cosmochimica Acta, 70, 3677-3688. Huelin S.R., Longerich H.P., Wilton D.H. and Fryer B.J. (2006) The determination of trace elements in Fe-Mn oxide coatings on pebbles using LA-ICP-MS. Journal ofGeochemical Exploration, 91,110-124. Jackson S.E.,Pearson N.J., Griffin W.L. and Belousova E.A. (2004) The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U-Pb zircon geochronology.Chemical Geology, 211, 47-69. Jochum K.P., Weis U., Stoll B., Kuzmin D., Yang Q.C., Raczek I., Jacob D.E.,Stracke A., Birbaum K. andFrick D.A. (2011) Determination of reference values for NIST SRM 610-617 glasses following ISO guidelines. Geostandards and This article is protected by copyright. All rights reserved. Geoanalytical Research, 35,397-429. Jochum K.P., Scholz D., Stoll B., Weis U., Wilson S.A., Yang Q.C., Schwalb A., Borner N., Jacob D.E. andAndreae M.O. (2012) Accurate trace element analysis of speleothems and biogenic calcium carbonates by LA-ICP-MS. Chemical Geology,318,31-44. Jochum K.P., Stoll B.,Weis U., Jacob D.E., Mertz-Kraus R. and Andreae M.O. (2014) Non-matrix-matchedcalibration for the multi-element analysis of geological and environmental samples using 200 nm femtosecondLA-ICP-MS: A comparison with nanosecond lasers. Geostandards and Geoanalytical Research, 38, 265-292. Klemme S., Giinther D., Hametner K., Prowatke S. and Zack T. (2006) The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications forthe early differentiation of the moon. Chemical Geology, 234,251-263. Klemme S.,Prowatke S., Miinker C.,Magee C.W., Lahaye Y., Zack T., Kasemann S.A., Cabato E.J.A. andKaeser B. (2008) Synthesis and preliminary characterisation of new silicate, phosphate and titanite reference glasses. Geostandardsand Geoanalytical Research,32,39-54. Kosler J. (2007) Laser ablation ICP-MS- A new dating tool in Earth science. Proceedings of the Geologists' Association, 118,19-24. Li Z., Hu Z.C., Liu Y.S., Gao S., Li M.,Zong K.Q., Chen H.H. and Hu S.H. (2015) Accurate determination of elements in silicate glass by nanosecond and femtosecond laser ablation ICP-MS at highspatial resolution. Chemical Geology, 400, 11-23. Lin L., HuZ.C., Yang L.,Zhang W., Liu Y.S., Gao S. and Hu S.H. (2014) Determination of boron isotope compositions of geological materials by laser ablation MC-ICP-MS using newlydesigned high sensitivity skimmer and sample cones. Chemical Geology,386, 22-30. Liu Y.S.,Hu Z.C., Gao S., Giinther D., Xu J., Gao C.G. and Chen H.H. (2008) In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internalstandard. Chemical Geology,257,34-43. Liu Y.S., Hu Z.C., Zong K.Q., Gao C.G., Gao S., Xu J. and Chen H.H. (2010) Reappraisement and refinement of zircon U-Pb isotope and trace element analyses by LA-ICP-MS. Chinese ScienceBulletin, 55, 1535-1546. Longerich H.P., Jackson S.E. and Giinther D. (1996) Laser ablation inductively coupled plasma-mass spectrometric transient signal data acquisition and analyteconcentration calculation. Journal of Analytical Atomic Spectrometry, 11, 899-904. Mank A.J. and Mason P.R. (1999) A critical assessment of laser ablation ICP-MS as an analytical tool for depth analysis in silica-based glass samples.Journal of Analytical Atomic Spectrometry,14, 1143-1153. Mitchell R.H.(1977) Geochemistry of magnesian ilmenites from kimberlites in South Africa and Lesotho. Lithos,10, 29-37. Moore R., Griffin W., Gurney J., Ryan C., Cousens D., Sie S.and Suter G. (1992) Trace element geochemistry of ilmenite megacrysts from the Monastery kimberlite, South Africa. Lithos, 29, 1-18. Mozna V., Pisonero J., Hola M., Kanicky V. and Giinther D. (2006) Quantitative analysis of Fe-based samples using ultraviolet nanosecond and femtosecond laser ablation-ICP-MS. This article is protected by copyright.All rights reserved. Journal of Analytical Atomic Spectrometry, 21,1194-1201. Nadoll P. and Koenig A.E. (2011) LA-ICP-MS of magnetite: Methods and reference materials. Journal of Analytical Atomic Spectrometry,26,1872-1877. Odegard M., Skar O., Schiellerup H. and Pearson N.J. (2005) Preparation of a synthetic titanite glass calibration material for in situ microanalysis by direct fusion in graphiteelectrodes: A preliminary characterisation by EPMA and LA-ICP-MS. Geostandards and Geoanalytical Research,29,197-209. Ohata M., Tabersky D., Glaus R., Koch J., Hattendorf B. and Giinther D. (2014) Comparison of 795 nm and 265 nm femtosecond and 193 nm nanosecond laser ablation inductively coupledplasma-mass spectrometry for the quantitative multi-element analysis of glass materials. Journal of AnalyticalAtomic Spectrometry, 29,1345-1353. Orellana F.A., Galvez C.G., Roldan M.T. and Garcia-Ruiz C. (2013) Applications of laser ablation-inductively coupled plasma-mass spectrometry in chemical analysis of forensicevidence. TrAC Trends in Analytical Chemistry, 42,1-34. Papike J., Hodges F., Bence A., Cameron M. and Rhodes J.(1976) Mare basalts: Crystal chemistry, mineralogy, and petrology. Reviews of Geophysics,14, 475-540. Poitrasson F., Mao X.L., Mao S.S., Freydier R. and Russo R.E. (2003) Comparison of ultraviolet femtosecond and nanosecond laser ablation inductively coupled plasma-mass spectrometryanalysis in glass, monazite, and zircon. Analytical Chemistry,75, 6184-6190. Russo R.E., Suen T.W., Bol'shakov A.A., Yoo J., Sorkhabi O.,Mao X.L., Gonzalez J., Oropeza D. and Zorba V.(2011) Laser plasma spectrochemistry. Journal of Analytical Atomic Spectrometry, 26, 1596-1603. Seydoux-Guillaume A.-M., Freydier R., Poitrasson F.,D’Abzac F.-X., Wirth R. and Datas L. (2010) Dominance of mechanical over thermally induced damage during femtosecond laser ablation of monazite. EuropeanJournal of Mineralogy,22,235-244. Shaheen M., Gagnon J. and Fryer B. J.(2012) Femtosecond (fs) lasers coupled with modern ICP-MS instruments provide new and improved potential for in situelemental and isotopic analyses in the geosciences. Chemical Geology, 330,260-273. Sylvester P.J. (2008) Laser ablation-ICP-MS in the Earth sciences: Current practices and outstanding issues. Mineralogical Association ofCanada, Short Course Series, 40, 1-348. Velasquez G., Borisova A.Y., Salvi S. and Beziat D. (2012) In situ determination of Au and Cu in natural pyrite by near-infrared femtosecond laser ablation-inductively coupledplasma-quadrupole mass spectrometry: No evidence for matrix effects. Geostandards and Geoanalytical Research,36,315-324. Wiltsche H. and Giinther D. (2011) Capabilities of femtosecond laser ablation ICP-MS for the major, minor, and trace element analysis of high alloyedsteels and super alloys. Analytical and bioanalytical chemistry, 399, 2167-2174. Xu H.Z., Hu S.H., Hu Z.C., Liu X.M., Yuan H.L. and Wang X.H. (2005) Determination of rare earth elements in rich-Co crust by 193 nm ArF excimer laser ablation-inductively coupledplasma-mass spectrometry. Journal of Analytical Science, 21, 119-122. This article is protected by copyright. All rights reserved. Zhao H.X., Jiang S.Y., Dai B.Z., Ma L. and Li J.W. (2015) Geochronology and Hf isotope study of pegmatite in the Xiaoqinling area ofNW China: Implication for petrogenesisand regional metamorphism. Journal ofEarth Science, 26, 295-305. Figure captions Figure 1. The concentration-normalised Fe’ and Ti4responses (cps/ugg) in reference glassesand ilmenites with changing laser energy density by 193 nm excimer LA-ICP-MS (a) (c) and 257nm fs-LA-ICP-MS (b) (d). The normalised concentrations of NIST SRM 610, the USGS basaltreference glasses and ilmenite samples were taken from Jochum et al.(2011), the GeoReMdatabase (http://georem.mpch-mainz.gwdg.de/) and Donohue et al. (2012), respectively. Figure 2. The ratio of the concentration-normalised signal response (’’Fe/4Ti) in NIST SRM610 and ilmenite (CRN63H) with changing laser energy density by 193 nm excimer LA-ICP-MSand 257 nm fs-LA-ICP-MS. Figure 3. Variation of the Fe/Ti and Co/Ti ratios with time during single hole ablation ofilmenite (CRN63H) at different laser energy densities with the spot size of 44 um in 193 nmexcimer LA-ICP-MS (a) (c) and 257 nm fs-LA-ICP-MS (b) (d). The data points were from themean of every group of three original data points. Figure 4. Scanning electron microscope images of ns-LA craters (a) (b) (c) (d) (e) and fs-LAcraters (f) (g) (h) (i) (j) in ilmenite (CRN63H) at five laser energy densities, respectively. Figure 5. Visual observations of all scanning electron microscope images of ns- and fs-LAcraters in ilmenite (CRN63H). This article is protected by copyright. All rights reserved. Figure 6. Scanning electron microscope images of ns-LA (a) and fs-LA (b) (c) crater rims andparticles in ilmenite (CRN63H). The laser energy densities used for ablation were 11.1 J cm° forthe ns laser and 3.63 Jcm" for the fs laser, respectively. Figure 7. Fractionation indices for sixteen elements normalised to Ti for the ablation of ilmenite(CRN63H) and NIST SRM 610 in 193 nm excimer LA-ICP-MS (a) and 257 nm fs-LA-ICP-MS(b) at six selected laser energy densities, respectively. Error bars represent the standard deviation(1s) of three measurements. Figure 8. Ti-normalised fractionation indices determined for ilmenite (CRN63H) and NISTSRM 610 at spot sizes of 16-60 um with a relatively high laser energy density (15.8 J cm’and4.27 J cmfor ns- or fs-laser pulses, respectively) using 193 nm excimer LA-ICP-MS (a) and257 nm fs-LA-ICP-MS (b). Error bars represent the standard deviation (1s) of threemeasurements. Figure 9. Limits of detection (LOD) for both in fs- and ns-LA-ICP-MS systems at a spot size of44 um. The LOD was calculated according to the protocol of Longerich et al. (1996). Figure 10. Relative deviations of average concentrations in ilmenite samples measured in thisstudy from our EPMA measurements and the working trace element values provided byDonohue et al. (2012) calibrated against NIST SRM 610 with Ti as the internal standard elementat a spot size of 44 um in 193 nm excimer LA-CP-MS (a) and fs-LA-CP-MS (b). Error barsrepresent the standard deviation (1s) of eleven measurements. This article is protected by copyright. All rights reserved. Table 1. Summary of the operating parameters for the 193 nm excimer LA-ICP-MS and the 257 nm fs-LA-ICP-MS Laser ablation system Laser type ArF excimer laser/ COMPexPro Yb:YAG femtosecond laser Wavelength 193 nm 257 nm Pulse length 15 ns 300 fs Energy density 12.7Jcm 3.27Jcm Spot size 16, 24,32, 44 and 60 um 16, 24, 32, 44 and 60 um Laser frequency 4 Hz 4Hz Ablation cell gas Helium Helium Makeup gas Argon Argon ICP-MS Agilent 7500a RF power 1380 W Plasma gas flow rate 14.01 min Auxiliary gas flow rate 1.01 min-1 Ion optic settings Typical Sampling depth 5.4 mm Mg,52. 227A1. 45Sc, 49Ti. 5lvV, Cr,55Mn, 57Fe, 59Co, Ni, Isotopes measured 6Cu,66Zn, 91zr. 93Nb, 120Sn, 178Hf.t,181Ta. Dwell time per isotope 10 ms Detector mode Dual This article is protected by copyright. All rights reserved. Table 2. Element concentrations for ten ilmenite megacrysts (CRN63I, CRN63F, CRN63J, CRN63G, CRN63H, CRN63H1, CRN63K,CRN63K1, CRN63E and CRN63E1) measured by LA-ICP-MS using 193 nm ArF excimer and 257 nm fs ablation systems and byEMPA for comparison Element CRN63I CRN63F CRN63J This study Ref. This study Ref. This study Ref. 193nm ns 257 nm fs EMPA EMPA/LA8.5±1.4 193 nm ns 257 nm fs EMPA EMPA/LA 193 nm ns 257 nm fs EMPA EMPA/LA MgOAl2O3ScTiOVCrMnOFeOCoNiCuZnZrNbSnHf Ta 7.97±0.140.71±0.0412.6±0.751.01496±663.91±1.720.31±0.0134.1±1.9193±3254±99.36±1.18209±14160±7142±25.76±0.384.96±0.3415.1±0.5 7.97±0.140.70±0.0812.7±0.551.01496±363.76±0.520.32±0.0137.4±0.5188±3239±89.04±0.97215±6161±10147±24.57±0.545.14±0.19 16.9±0.3 9.35±0.630.66±0.24 51.0±1.40.16±0.12° 0.33±0.0737.1±1.7 0.57±0.25° 7.8±1.1 7.00±0.420.46±0.22 0.75±0.18 7.77±0.190.79±0.12 7.9±1.2 8.55±0.21 8.72±0.070.51±0.34 0.48±0.11 0.51±0.08 13.2±1.7 12.1±3.0 12.7±1.3 16±4 0.48±0.1112.7±0.9 0.51±0.0812.3±0.9 51.8±1.5 49.0 49.0 49.0±2.6 51.7±2.0* 52.4 52.4 52.4±1.9 IS 1422±268 1450±451 1450±104 0.15±0.08” 1546±429 1297±216 1297±160 3.43±0.80 3.09±2.64 4.12±0.92 3.79±1.60 5.28±1.79 4.98±0.81 0.33±0.10* 0.32±0.11 0.26±0.04 0.24±0.05 0.34±0.01 0.34±0.01 37.7±2.7° 33.9±4.0 38.5±2.8 203±22 181±8221±68 186±7194±8 39.8±0.6 38.4±3.0°207±13222±50 35.4±1.6192±5 35.7±1.7195±4295±10 268±70 289±25 6.97±6.12218±52 8.98±4.20 9.56±1.25 222±507.50±4.31 2.45±2.03 3.10±1.29 183±36 204±25177±58 224±26176±18 226±44 188±17 195±14 248±73 149±12 155±17 159±114.88±1.00 141±85.45±0.38 150±44.68±0.28 185±185.06±1.11 126±25.03±0.62 132±24.22±0.54 5.76±1.02 5.61±1.10 5.53±0.26 5.18±0.55 13.4±0.4 4.96±0.38 16.0±1.4 14.6±0.6 17.1±0.8 Table 2 (continued). Element CRN63G CRN63H CRN63H1 This study Ref. This study Ref. This study Ref. 193 nm ns 257 nm fs EMPA EMPA/LA 8.3±0.9° 193 nm ns6.29±0.14 257 nm fs EMPA EMPA/LA 193 nm ns 257 nm fs EMPA EMPA/LA MgOAl23ScTiO2VCrMnOFeOCoNiCuZnZrNb Sn Hf Ta 7.86±0.240.54±0.09 9.53±1.430.49±0.14 0.58±0.24 0.77±0.01 6.71±0.210.75±0.09 6.49±0.09°0.69±0.07 5.95±0.080.72±0.01 6.18±0.13 0.77±0.01 6.34±0.170.72±0.05 6.49±0.09*0.69±0.07° 11.5±1.652.4 52.4±2.0IS 12.1±2.651.8±1.5° 10.3±0.450.2 10.6±0.150.2 50.2±0.8 9.83±0.31 10.3±0.349.1 49.1±2.6 11.6±1.7 1319±2365.06±1.01 1369±3736.70±5.18 1330±200.77±0.82 1330±240.70±0.31 0.14±0.08” 50.6±0.5°1272±115 0.21±0.06°41.2±0.4195±597.8±13.010±2288±30155±19144±174.30±0.695.16±0.7713.6±5.2 0.30±0.02 0.34±0.15 0.27±0.09° 0.230±0.003 0.230±0.004 0.23±0.05 0.21±0.06° 0.220± 0.00537.9±2.0 0.23±0.01 0.21±0.06 35.7±2.6 38.2±2.3°203±40 40.4±1.6188±3 40.7±1.0 41.2±0.4195±5 182±2 266±8 283±133 102±4 102±2 97.8±13.0 97.6±4.5 100±3 11.1±7.4 11.1±1.0 11.8±0.3 10±2 11.3±1.1 216±6 252±161 265±12 294±4 288±30 277±29 143±22 152±31 148±6 151±4 155±19 145±4 116±3 122±53.93±0.50 141±3 143±4 144±17 157±2 162±4 4.31±0.39 4.12±0.444.48±0.39 4.77±0.70 4.43±0.324.74±0.29 4.25±0.654.74±0.18 4.30±0.695.16±0.77 4.78±0.944.48±0.22 4.63±0.20 9.87±0.44 11.2±0.2 10.6±1.1 15.4±0.6 16.3±0.6 13.6±5.2 17.4±0.5 18.8±0.7 Table 2 (continued). CRN63K CRN63K1 CRN63E CRN63E1 This study Ref. This study Ref. This study Ref. This study Ref. 257 nm fs 8.01±0.420.63±0.02 EMPA/LA 6.27±0.68° 0.41±0.19° 0.43±0.07°9.8±1.150.2±0.6° EMPA 8.89±1.310.47±0.19 49.6±2.5IS EMPA/LA6.1±0.5°0.43±0.07°9.8±1.150.2±0.6°1062±106 51.0±1.9IS0.12±0.08° 0.35±0.0738.6±1.7 49.61093±171.04±0.400.43±0.01 FeO 40.9±1.6° Co 180±3 181±2 193±13 175±5 38.1±1.9182±6 36.7±1.2 NiCuZn 189±614.29±0.61 178±403.8±1.0 36.6±1.5 38.7±1.3 58.2±11.0 211±10 218±33 171±4 178±2 161±7 174±3 177±3 161±7 ZrNb 224±23184±6194±8 27.8±15.0 194±26 27.8±15.0 242±4184±7192±6 1.50±1.19284±50212±33221±9 1.50±1.19 5.63±1.70219±14219±4 5.95±1.62251±4232±4 7.13±1.60 7.59±0.50 7.13±1.60 SnHf 5.57±0.705.60±0.24 21.8±1.5 5.10±0.41 5.66±0.2122.6±1.1 292±17 226±17 292±17 5.60±0.24 21.8±1.5 5.66±0.2122.6±1.1 4.81±1.227.03±1.07 254±34 221±9258±5 254±34 299±23 255±36.24±0.627.09±0.28 25.7±0.5 268±45.84±0.39 7.35±0.25 299±23 5.85±0.14 8.09±1.41 29.2±3.0 5.99±0.726.95±0.30 5.59±0.41 7.07±0.24 5.85±0.14 7.03±1.07 7.09±0.28 7.35±0.25 24±2 25.7±0.5 28.0±0.4 8.09±1.41 29.2±3.0 25.8±0.6 7.07±0.24 27.7±0.7 Values are given in ug g(trace elements) and in % m/m for the major elements MgO, Al203, TiO2, MnO and FeO.± values are twostandard deviations (2s) from eleven measurements. 40 (d) 32 =9 624 16su 8 fs 0 1.8 3.0 3.3 3.6 4.1 4.3 Energy (J cm²) Energy (J cm2) This article is protected by copyright. All rights reserved. 0.450 0.650 (a) CRN63H (b) CRN63H fs 0.050 0.250 20 40 60 80 20 40 60 80 Time (s) Time (s) 0.010 0.015 (c) (d) This article is protected by copyright. All rights reserved. This article is protected by copyright. All rights reserved. Mg AlScTijvMn Fe Co Ni Cu Zn Zr Nb Sn HfTa This article is protected by copyright. All rights reserved. This article is protected by copyright. All rights reserved. This article is protected by copyright. All rights reserved. 使用飞秒激光电感耦合等离子体质谱仪分析钛铁矿中57Fe和49Ti的浓度大约比NIST SRM 610高1.8倍。与193nm准分子激光器相比,257nm 飞秒激光器的元素分离量较小。采用193nm准分子激光剥蚀时,激光能量密度的选择对钛铁矿元素分离有显著影响。与飞秒激光相比,纳秒激光生成的剥蚀坑和沉积气溶胶形貌的扫描电镜图像显示了更大的熔化效应,烧蚀坑周围颗粒沉积面积更大。在纳秒剥蚀坑周围喷出物主要由大滴再凝固的熔融物质组成;然而,在飞秒剥蚀坑周围的喷出物是由形状“粗糙”的微粒团块组成。这是纳秒激光和飞秒激光不同剥蚀机制的结果。使用NIST SRM 610作为193nm准分子LA-ICP-MS和fs-LA-ICP-MS的参考材料,可以对钛铁矿样品进行非基体匹配条件下的定量分析。采用193nm准分子LA-ICP-MS 在12.7 J cm-2高激光能量密度条件和采用fs-LA-ICP-MS对钛铁矿样品中的大部分元素进行分析,得到的结果一致。
确定






















还剩28页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《钛铁矿中非基体匹配定量检测方案(激光剥蚀进样)》,该方案主要用于金属矿产中非基体匹配定量检测,参考标准--,《钛铁矿中非基体匹配定量检测方案(激光剥蚀进样)》用到的仪器有ESLfemto 飞秒激光剥蚀系统
推荐专场
相关方案
更多
该厂商其他方案
更多















