方案详情
文
无需额外样本制备;内置多个应用模块,结果显示直观;可实现高通量,一台电脑可控制四台设备同时进行分析。
方案详情

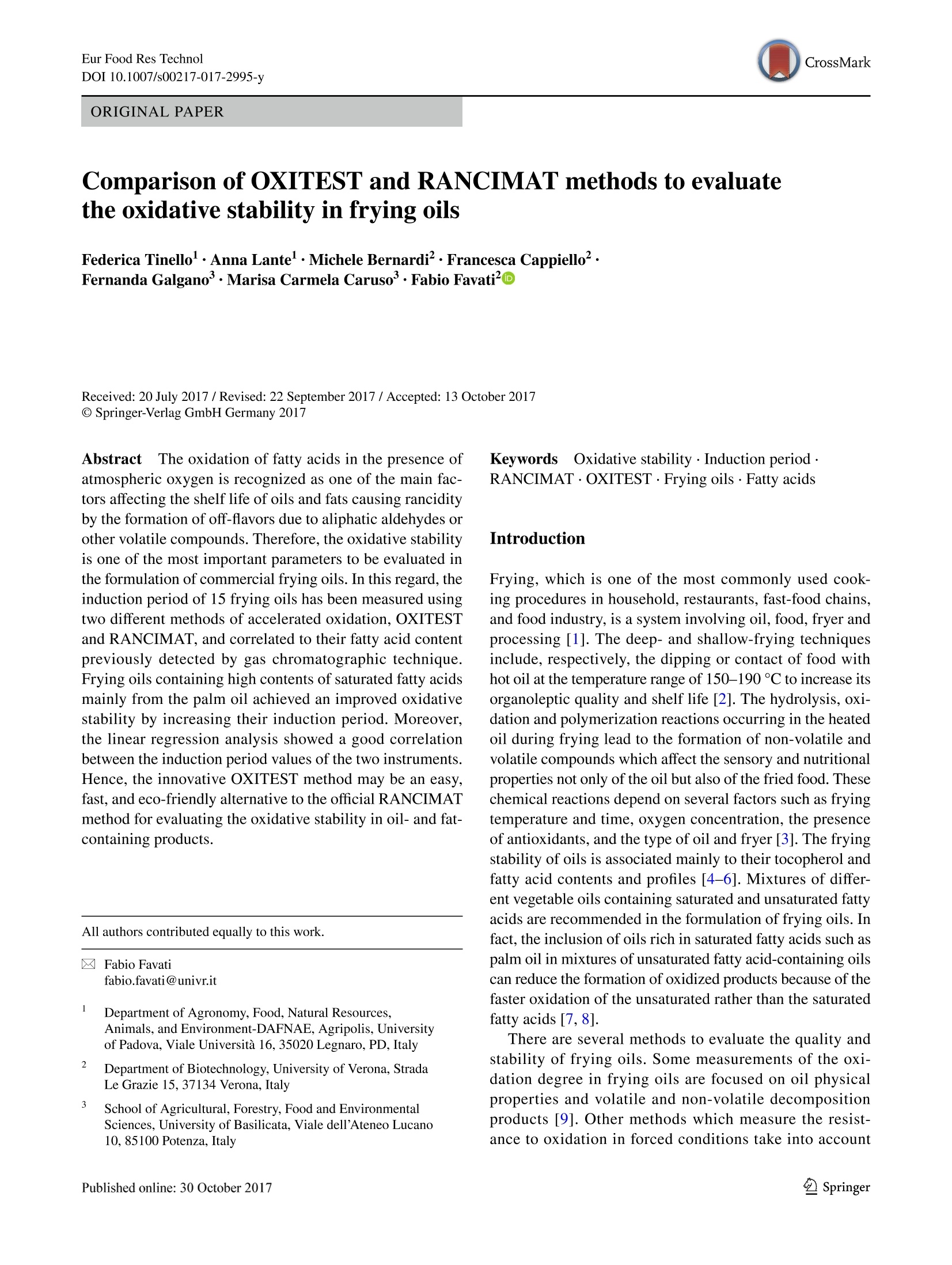
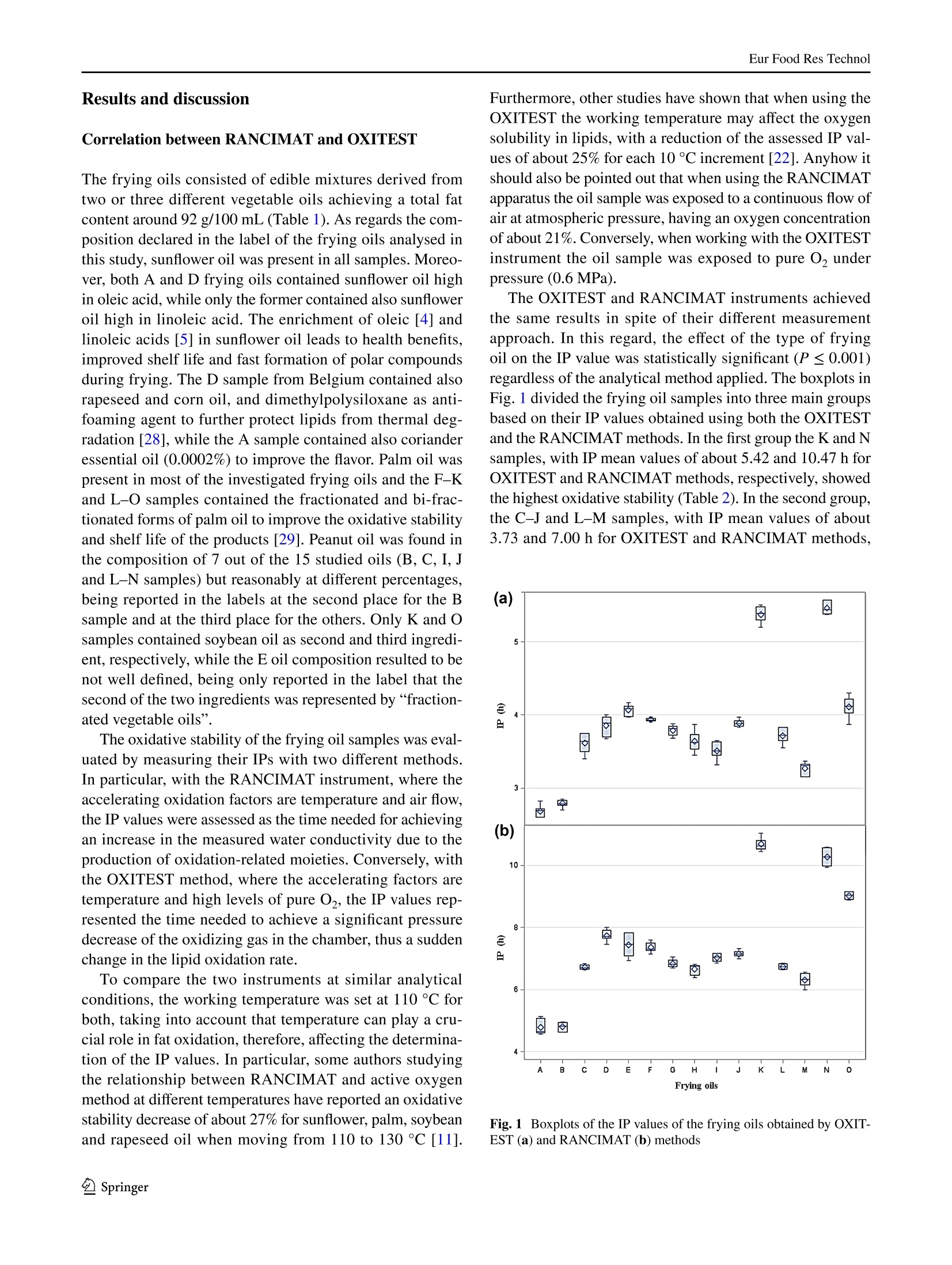
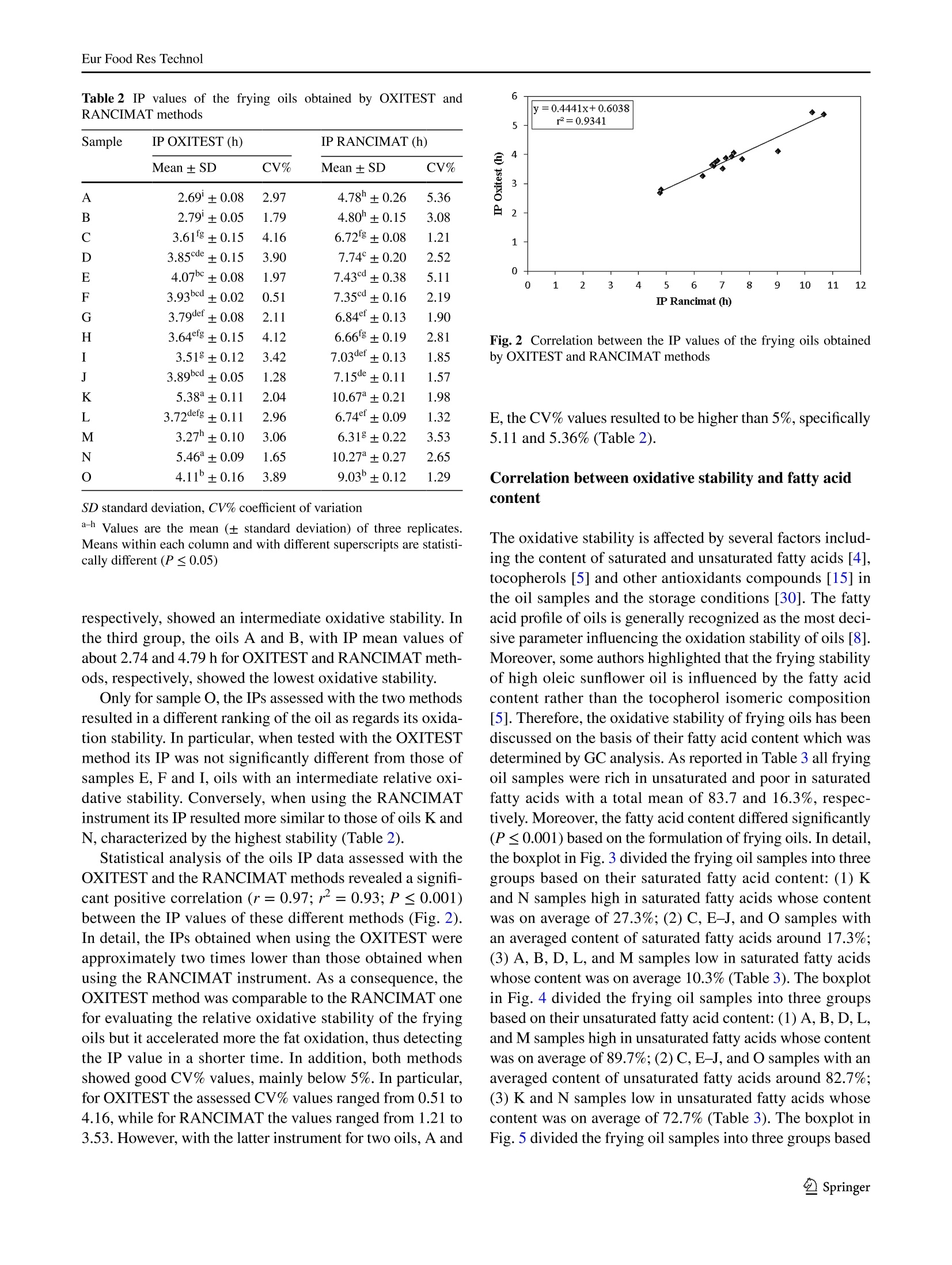
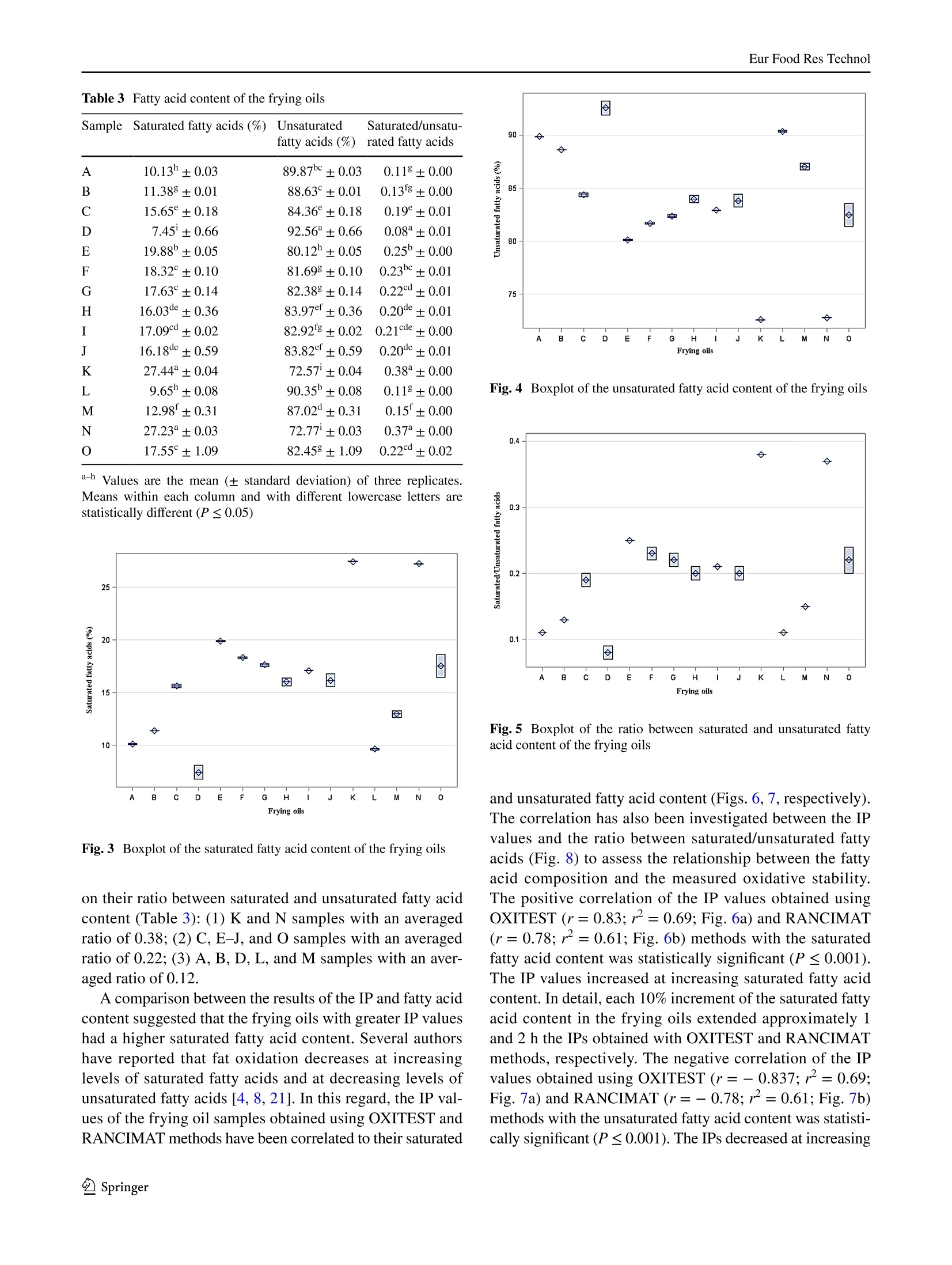
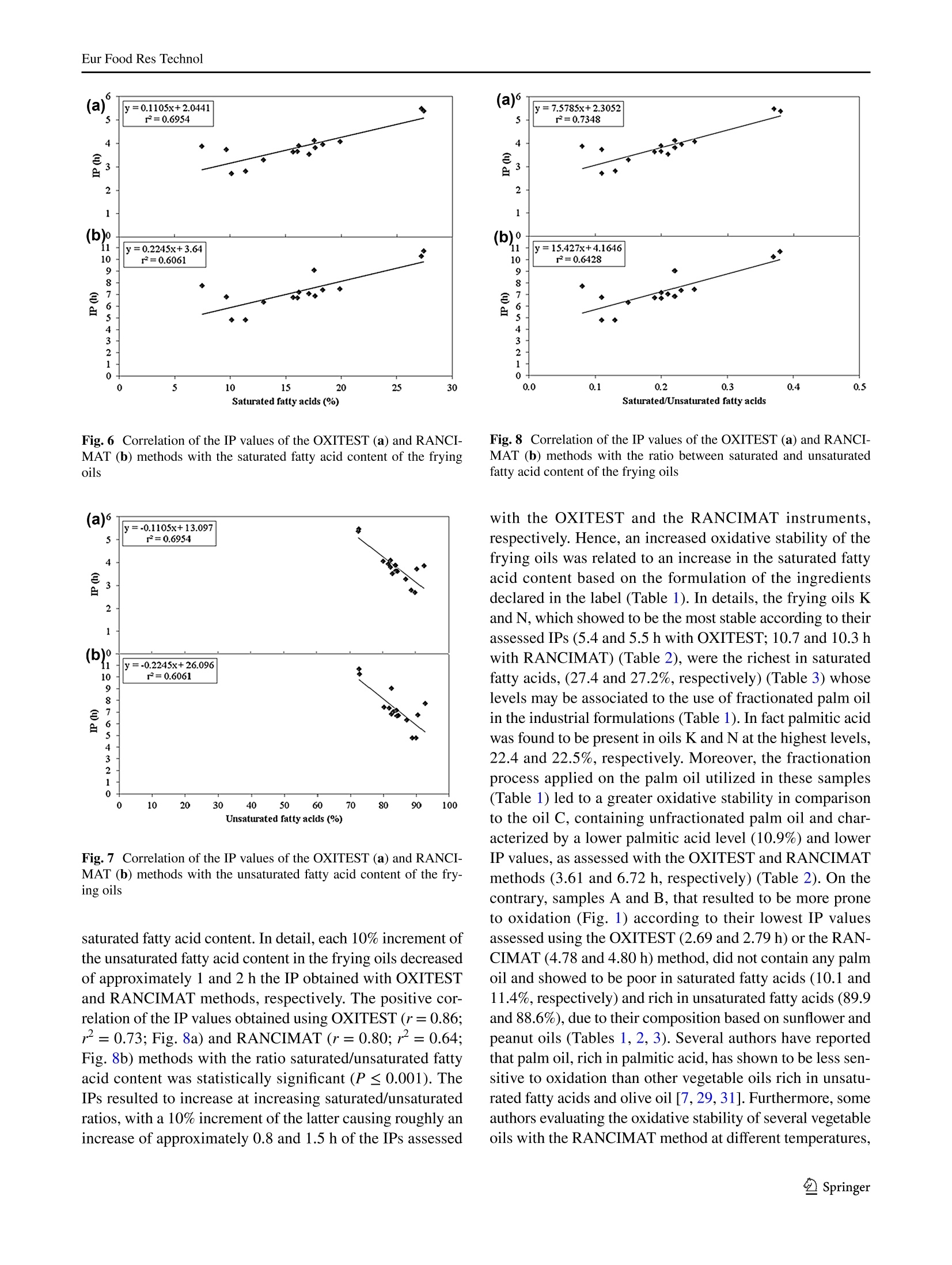
Eur Food Res TechnolDOI 10.1007/s00217-017-2995-yCrossMarkORIGINAL PAPER Eur Food Res Technol Comparison of OXITEST and RANCIMAT methods to evaluatethe oxidative stability in frying oils Federica Tinellol· Anna Lante·Michele Bernardi· Francesca Cappiello?· Fernanda Galgano·Marisa Carmela Caruso’Fabio Favati'o Abstract:t The oxidation of fatty acids in the presence ofatmospheric oxygen is recognized as one of the main fac-tors affecting the shelf life of oils and fats causing rancidityby the formation of off-flavors due to aliphatic aldehydes orother volatile compounds. Therefore, the oxidative stabilityis one of the most important parameters to be evaluated inthe formulation of commercial frying oils. In this regard, theinduction period of 15 frying oils has been measured usingtwo different methods of accelerated oxidation, OXITESTand RANCIMAT, and correlated to their fatty acid contentpreviously detected by gas chromatographic technique.Frying oils containing high contents of saturated fatty acidsmainly from the palm oil achieved an improved oxidativestability by increasing their induction period. Moreover,the linear regression analysis showed a good correlationbetween the induction period values of the two instruments.Hence, the innovative OXITEST method may be an easy,fast, and eco-friendly alternative to the official RANCIMATmethod for evaluating the oxidative stability in oil- and fat-containing products. All authors contributed equally to this work. 区Fabio Favatifabio.favati@univr.it Department of Agronomy, Food, Natural Resources,Animals, and Environment-DAFNAE, Agripolis, Universityof Padova, Viale Universita 16, 35020 Legnaro, PD, Italy ( 2 Department o f Biotechnology, University of Verona, StradaLe Grazie 15, 37134 Verona, Italy ) ( 3 S chool of Agricultural, Forestry, Food and Environmental Sciences, University of Basilicata, Viale dellAteneo Lucano 10,85100 Potenza, Italy ) Keywords Oxidative stability·Induction periodRANCIMAT·OXITEST· Frying oils· Fatty acids Introduction Frying, which is one of the most commonly used cook-ing procedures in household, restaurants, fast-food chains,and food industry, is a system involving oil, food, fryer andprocessing [1]. The deep- and shallow-frying techniquesinclude, respectively, the dipping or contact of food withhot oil at the temperature range of 150-190 ℃ to increase itsorganoleptic quality and shelf life [2]. The hydrolysis, oxi-dation and polymerization reactions occurring in the heatedoil during frying lead to the formation of non-volatile andvolatile compounds which affect the sensory and nutritionalproperties not only of the oil but also of the fried food. Thesechemical reactions depend on several factors such as fryingtemperature and time, oxygen concentration, the presenceof antioxidants, and the type of oil and fryer [3]. The fryingstability of oils is associated mainly to their tocopherol andfatty acid contents and profiles [4-6]. Mixtures of differ-ent vegetable oils containing saturated and unsaturated fattyacids are recommended in the formulation of frying oils. Infact, the inclusion of oils rich in saturated fatty acids such aspalm oil in mixtures of unsaturated fatty acid-containing oilscan reduce the formation of oxidized products because of thefaster oxidation of the unsaturated rather than the saturatedfatty acids [7, 8]. There are several methods to evaluate the quality andstability of frying oils. Some measurements of the oxi-dation degree in frying oils are focused on oil physicalproperties and volatile and non-volatile decompositionproducts [9]. Other methods which measure the resist-ance to oxidation in forced conditions take into account the oxidative stability as one of the most important factorsto evaluate the shelf life, palatability, nutritional qualityand toxicity of oils and fats by determining the inductionperiod (IP), a parameter representing the time needed toreach the starting point of lipid oxidation. Therefore, alonger IP can be associated with a higher oxidation stabil-ity. The active oxygen method (AOM) and the analysis ofcarbonyl value (CV) have been the mostly used tests todetect the oxidative stability of oils by measuring primaryor secondary oxidation products, such as hydroperoxides,aldehydes and ketones, respectively [10]. However, thesechemical techniques, which are non-reproducible, timeconsuming, expensive and involve the use of toxic rea-gents, have been then replaced with accelerated oxidationmethods [11]. In this regard, the official oil stability index(OSI) method of American Oil Chemists’ Society (AOCS)requires the use of different analytical instruments thatcan be utilized as an alternative, RANCIMAT (Metrohm,Herisau, Switzerland) being one of these [12]. The RAN-CIMAT method allows to evaluate not only the oxidativestability of edible [13] and non-edible oils [14], fats [11],nanoemulsions [15], encapsulated extract within nanoe-mulsion and multiple emulsions [16] but also the antioxi-dant performance of plant extracts [17, 18] by monitor-ing over time the water conductivity, which increases as aconsequence of volatile acids formed after heating samplesunder atmospheric pressure and constant values of temper-ature and air flow. The OXITEST-Oxidation Test Reactor(VELP, Usmate,MB, Italy)-is an innovative instrumentthat can be utilized to investigate the oxidation stability of solid [19-21] or liquid [22, 23] samples, without priorpreparation. The instrument monitors the oxygen uptakeof the reactive components present in food/feed samples toevaluate the oxidative stability under accelerated oxidationconditions, and the reactor temperature can be regulatedfrom room temperature to 110°C, while the oxygen pres-sure can reach up to 0.8 MPa. A few papers have investi-gated its use for evaluating the oxidation stability of oils,chia seeds, and bakery products [20, 22-25] and recentlythe AOCS has approved a new analytical procedure (Cd12c-16) based on the use of this instrument. Aim of this work was to compare the OXITEST andthe RANCIMAT methods in evaluating the oxidative sta-bility of commercial frying oils, taking also into accountthe relationship between the IP values and the fatty acidcomposition of the oils. Materials and methods Samples A total of 15 frying oils from different commercial brandswere purchased from a local market and stored at roomtemperature under dark conditions until the analyses. Allof the commercial oils were manufactured and packagedin Italy with the exception of sample D, coming from Bel-gium (Table 1). Table 1 Composition and fat content declared in the label of the frying oil samples analysed in the study Code Composition Fat (g/100 mL) A Sunflower oil high in linoleic acid, sunflower oil high in oleic acid, cori- 92.0 ander essential oil (0.0002%) B Sunflower, peanut oils 100 C Sunflower, palm, peanut oils 92.0 D Sunflower oil, rapeseed oil, sunflower oil high in oleic acid, corn oil, 92.0 dimethylpolysiloxane (anti-foaming agent) E Sunflower, fractionated vegetable oils NR F Sunflower, fractionated palm oils 91.6 G Sunflower, fractionated palm oils 91.4 H Sunflower, fractionated palm oils 91.4 Sunflower, fractionated palm, peanut oils 91.6 J Sunflower, fractionated palm, peanut oils 92.C K Sunflower, soybean, fractionated palm oils 92.0 L Sunflower, bi-fractionated palm, peanut oils 92.0 M Sunflower, bi-fractionated palm, peanut oils 91.4 N Sunflower, bi-fractionated palm, peanut oils 91.6 O Sunflower (70%), bi-fractionated palm (20%), soybean (5%) oils 92.0 Oxidative stability measurement using the RANCIMATinstrument The oxidative stability of the frying oils was evaluated usinga RANCIMAT apparatus (Metrohm, model 743, Herisau,Switzerland) and measuring over time the water conductiv-ity [18]. A fixed amount of oil (3.0±0.1 g) was added toeach reaction tube and subjected to accelerated oxidationby raising the temperature to 110 C under a 20 L h-airflow. The volatile oxidation products formed under forcedconditions were transported by the air stream into the con-ductometric cell, previously filled with 60 mL of distilledwater, and detected by continuously measuring the waterelectrical conductivity (uS cm-) over time (h). The oxi-dative stability was expressed as the IP corresponding tothe time (h) at the intersection point between the horizontal(conductivity, uS min-l) and vertical (time, h) tangents ofthe fitted exponential oxidation curves. At this break pointthe water conductivity increased over time because of thefat oxidation. Two samples per frying oil were placed in theequipment and analysed simultaneously in triplicate. Oxidative stability measurement using the OXITESTinstrument The oxidative stability of the frying oils was also assessedusing the OXITEST apparatus (Velp Scientifica, Usmate,Italy), featuring two thermostated and hermetically sealedtitanium chambers,each one able to contain up to threeround 35-mL titanium sample holders, that can be piled upwhen working with large sample amounts. When the samplesize requires the use of less than three holders, the unusedones are replaced with titanium spacers, so to keep constantthe void volume in each chamber [22]. An amount equal to10 g of frying oil, thoroughly mixed immediately beforebeing sampled, was loaded in each chamber and due to thelimited sample amount only one holder was utilized, beingthen necessary the use oftwo spacers. The working tempera-ture was set at 110 °C, while the initial O2 pressure (grade5.0; SAPIO, Monza, Italy) was set at 0.6 MPa. The instru-ment was controlled by a specific software (OXISoftTM,Velp Scientifica, Usmate, Italy) that allowed to measureover time the absolute pressure change in the two chambers,monitoring the oxygen uptake of the active components ofthe samples. For each chamber at the end of the test, theprogram automatically calculated the IP from the resultantoxidation curve, by means of a graphical method (two tan-gent methods). Each analysis was repeated three times for atotal of six data acquired for each oil. The saturated and unsaturated fatty acid content in the fry-ing oils was assessed by gas chromatographic analysis ofthe respective methyl esters (FAMEs) [26]. The analyseswere carried out using an Agilent 7890A GC system (Agi-lent Technologies, Cernusco sul Naviglio, Milano, Italy)equipped with an Agilent 7683 autosampler and a FlameIonization Detector (FID). FAMEs were prepared by weight-ing 40 mg of each frying oil in a small glass test tube andadding 2 mL of hexane and 200 uL of 2 M methanolic KOH.The mixture was then vortexed at room temperature for2 min and then let to stand after the addition of about 0.5 gof anhydrous Na,SO to eliminate any water formed duringthe hydrolysis process. After the phase separation occurred,an aliquot of the upper hexane layer (1 pL) was injectedin split mode (1:50) into a SLB-IL111 capillary column(100 m × 0.25 mm ×0.20 um) (Sigma-Aldrich, Milano,Italy). Helium (grade 6.0, SOL, Monza, Italy) was used ascarrier gas (1 mL min-), while nitrogen (grade 5.0, SOL,Monza, Italy) was used as make-up gas. The injector andFID temperatures were set at 250 C and separation of thevarious FAMEs was achieved using an initial oven tempera-ture of 100 °C, which after being kept constant for 13 minwas then raised to 240 °C at 4C min-. The various fattyacids were identified according to their retention time andby spiking the samples, using a commercial mixture of purestandards (Supelco 37 Component FAME Mix CRM47885,Sigma-Aldrich, Milano, Italy). The fatty acid content wasexpressed as percentage based on the sum of the detectedpeak areas. Statistical analysis The data concerning the IP values and fatty acid contentwere analysed by a one-way analysis of variance (ANOVA),after verifying the normal distribution and homogeneity ofvariance, using the PROC GLM of Statistical Analysis Sys-tem (SAS) [27]. The model included the type of frying oil asfixed effect. Least-square means were chosen for presentingall data and compared using the Tukey’s multiple range test.Differences among means with P≤ 0.05 were accepted asrepresenting statistically significant differences. The box-plots were computed using the SAS software package [27]. Linear regression analysis was carried out using thePROC REG of SAS [27]. The OXITEST IPs were correlatedwith those obtained using the RANCIMAT instrument, andthe assessed IPs of both instruments were correlated withthe fatty acid content. Results and discussion Correlation between RANCIMAT and OXITEST The frying oils consisted of edible mixtures derived fromtwo or three different vegetable oils achieving a total fatcontent around 92 g/100 mL (Table 1). As regards the com-position declared in the label of the frying oils analysed inthis study, sunflower oil was present in all samples. Moreo-ver, both A and D frying oils contained sunflower oil highin oleic acid, while only the former contained also sunfloweroil high in linoleic acid. The enrichment of oleic [4] andlinoleic acids [5] in sunflower oil leads to health benefits,improved shelf life and fast formation of polar compoundsduring frying. The D sample from Belgium contained alsorapeseed and corn oil, and dimethylpolysiloxane as anti-foaming agent to further protect lipids from thermal deg-radation [28], while the A sample contained also corianderessential oil (0.0002%) to improve the flavor. Palm oil waspresent in most of the investigated frying oils and the F-Kand L-O samples contained the fractionated and bi-frac-tionated forms of palm oil to improve the oxidative stabilityand shelf life of the products [29]. Peanut oil was found inthe composition of 7 out of the 15 studied oils (B, C, I, Jand L-N samples) but reasonably at different percentages,being reported in the labels at the second place for the Bsample and at the third place for the others. Only K and Osamples contained soybean oil as second and third ingredi-ent, respectively, while the E oil composition resulted to benot well defined, being only reported in the label that thesecond of the two ingredients was represented by “fraction-ated vegetable oils”. The oxidative stability of the frying oil samples was eval-uated by measuring their IPs with two different methodsS.In particular, with the RANCIMAT instrument, where theaccelerating oxidation factors are temperature and air flow,the IP values were assessed as the time needed for achievingan increase in the measured water conductivity due to theproduction of oxidation-related moieties. Conversely, withthe OXITEST method, where the accelerating factors aretemperature and high levels of pure 02, the IP values rep-resented the time needed to achieve a significant pressuredecrease of the oxidizing gas in the chamber, thus a suddenchange in the lipid oxidation rate. To compare the two instruments at similar analyticalconditions, the working temperature was set at 110 °C forboth, taking into account that temperature can play a cru-cial role in fat oxidation, therefore, affecting the determina-tion of the IP values. In particular, some authors studyingthe relationship between RANCIMAT and active oxygenmethod at different temperatures have reported an oxidativestability decrease of about 27% for sunflower, palm, soybeanand rapeseed oil when moving from 110 to 130℃ [11]. Furthermore, other studies have shown that when using theOXITEST the working temperature may affect the oxygensolubility in lipids, with a reduction of the assessed IP val-ues of about 25% for each 10 C increment [22]. Anyhow itshould also be pointed out that when using the RANCIMATapparatus the oil sample was exposed to a continuous flow ofair at atmospheric pressure, having an oxygen concentrationof about 21%. Conversely, when working with the OXITESTinstrument the oil sample was exposed to pure O2 underpressure (0.6 MPa). The OXITEST and RANCIMAT instruments achievedthe same results in spite of their different measurementapproach. In this regard, the effect of the type of fryingoil on the IP value was statistically significant (P≤0.001)regardless of the analytical method applied. The boxplots inFig. 1 divided the frying oil samples into three main groupsbased on their IP values obtained using both the OXITESTand the RANCIMAT methods. In the first group the K and Nsamples, with IP mean values of about 5.42 and 10.47 h forOXITEST and RANCIMAT methods, respectively, showedthe highest oxidative stability (Table 2). In the second group,the C-J and L-M samples, with IP mean values of about3.73 and 7.00 h for OXITEST and RANCIMAT methods, Fig. 1 Boxplots of the IP values of the frying oils obtained by OXIT-EST (a) and RANCIMAT (b) methods Table 2 IP values of the frying oils obtained by OXITEST andRANCIMAT methods Mean ± SD CV% Mean ±SD CV% A 2.69±0.08 2.97 4.78h±0.26 5.36 B 2.79'±0.05 1.79 4.80h±0.15 3.08 C 3.61fg±0.15 4.16 6.72g±0.08 1.21 D 3.85cde±0.15 3.90 7.74°±0.20 2.52 E 4.07bc±0.08 1.97 7.43d±0.38 5.11 F 3.93bcd±0.02 0.51 7.35d±0.16 2.19 G 3.79def ±0.08 2.11 6.84±0.13 1.90 H 3.64efg±0.15 4.12 6.668±0.19 2.81 3.518±0.12 3.42 7.03def ±0.13 1.85 J 3.89bcd±0.05 1.28 7.15de±0.11 1.57 K 5.38+0.11 2.04 10.67±0.21 1.98 L 3.72defg±0.11 2.96 6.74±0.09 1.32 M 3.27h±0.10 3.06 6.31±0.22 3.53 N 5.46°±0.09 1.65 10.27±0.27 2.65 O 4.11±0.16 3.89 9.03±0.12 1.29 SD standard deviation, CV% coefficient of variation a-h Values are the mean (± standard deviation) of three replicates.Means within each column and with different superscripts are statisti-cally different (P≤0.05) respectively, showed an intermediate oxidative stability. Inthe third group, the oils A and B, with IP mean values ofabout 2.74 and 4.79 h for OXITEST and RANCIMAT meth-ods, respectively, showed the lowest oxidative stability. Only for sample O, the IPs assessed with the two methodsresulted in a different ranking of the oil as regards its oxida-tion stability. In particular, when tested with the OXITESTmethod its IP was not significantly different from those ofsamples E, F and I, oils with an intermediate relative oxi-dative stability. Conversely, when using the RANCIMATinstrument its IP resulted more similar to those of oils K andN, characterized by the highest stability (Table 2). Statistical analysis of the oils IP data assessed with theOXITEST and the RANCIMAT methods revealed a signifi-cant positive correlation (r=0.97; r²=0.93; P≤ 0.001)between the IP values of these different methods (Fig.2).In detail, the IPs obtained when using the OXITEST wereapproximately two times lower than those obtained whenusing the RANCIMAT instrument. As a consequence, theOXITEST method was comparable to the RANCIMAT onefor evaluating the relative oxidative stability of the fryingoils but it accelerated more the fat oxidation, thus detectingthe IP value in a shorter time. In addition, both methodsshowed good CV% values, mainly below 5%. In particular,for OXITEST the assessed CV% values ranged from 0.51 to4.16, while for RANCIMAT the values ranged from 1.21 to3.53. However, with the latter instrument for two oils, A and 6 Fig. 2 Correlation between the IP values of the frying oils obtainedby OXITEST and RANCIMAT methods E, the CV% values resulted to be higher than 5%, specifically5.11 and 5.36%(Table 2). Correlation between oxidative stability and fatty acidcontent The oxidative stability is affected by several factors includ-ing the content of saturated and unsaturated fatty acids [4],tocopherols [5] and other antioxidants compounds [15] inthe oil samples and the storage conditions [30]. The fattyacid profile of oils is generally recognized as the most deci-sive parameter influencing the oxidation stability of oils [8].Moreover, some authors highlighted that the frying stabilityof high oleic sunflower oil is influenced by the fatty acidcontent rather than the tocopherol isomeric composition[5]. Therefore, the oxidative stability of frying oils has beendiscussed on the basis of their fatty acid content which wasdetermined by GC analysis. As reported in Table 3 all fryingoil samples were rich in unsaturated and poor in saturatedfatty acids with a total mean of 83.7 and 16.3%, respec-tively. Moreover, the fatty acid content differed significantly(P≤0.001) based on the formulation of frying oils. In detail,the boxplot in Fig. 3 divided the frying oil samples into threegroups based on their saturated fatty acid content: (1) Kand N samples high in saturated fatty acids whose contentwas on average of 27.3%; (2)C, E-J, and Osamples withan averaged content of saturated fatty acids around 17.3%;(3) A, B, D, L, and M samples low in saturated fatty acidswhose content was on average 10.3% (Table 3). The boxplotin Fig. 4 divided the frying oil samples into three groupsbased on their unsaturated fatty acid content: (1) A, B, D, L,and M samples high in unsaturated fatty acids whose contentwas on average of 89.7%;(2) C, E-J, and O samples with anaveraged content of unsaturated fatty acids around 82.7%;(3) K and N samples low in unsaturated fatty acids whosecontent was on average of 72.7% (Table 3). The boxplot in Fig. 5 divided the frying oil samples into three groups based Table 3 Fatty acid content of the frying oils fatty acids (%) rated fatty acids A 10.13h±0.03 89.87bc±0.03 0.118±0.00 B 11.388±0.01 88.63°±0.01 0.13g±0.00 C 15.65°±0.18 84.36°±0.18 0.19°±0.01 D 7.45±0.66 92.56°±0.66 0.08°±0.01 E 19.88±0.05 80.12h±0.05 0.25±0.00 F 18.32°±0.10 81.698±0.10 0.23±0.01 G 17.63°±0.14 82.388±0.14 0.22d±0.01 H 16.03de±0.36 83.97±0.36 0.20de±0.01 17.09d±0.02 82.92g±0.02 0.21cde±0.00 16.18de±0.59 83.82°±0.59 0.20de±0.01 27.44±0.04 72.57±0.04 0.38±0.00 L 9.65h±0.08 90.35°±0.08 0.118±0.00 M 12.98±0.31 87.02°±0.31 0.15±0.00 N 27.23°±0.03 72.77±0.03 0.37±0.00 O 17.55°±1.09 82.458±1.09 0.22±0.02 a-hValues are the mean (± standard deviation) of three replicates.Means within each column and with different lowercase letters arestatistically different (P≤0.05) Fig.3 Boxplot of the saturated fatty acid content of the frying oils on their ratio between saturated and unsaturated fatty acidcontent (Table 3):(1) K and N samples with an averagedratio of 0.38; (2) C, E-J, andO samples with an averagedratio of 0.22; (3) A, B,D, L, and M samples with an averaged ratio of 0.12. A comparison between the results of the IP and fatty acidcontent suggested that the frying oils with greater IP valueshad a higher saturated fatty acid content. Several authorshave reported that fat oxidation decreases at increasinglevels of saturated fatty acids and at decreasing levels ofunsaturated fatty acids [4, 8, 21]. In this regard, the IP val-ues of the frying oil samples obtained using OXITEST andRANCIMAT methods have been correlated to their saturated Fig. 4 Boxplot of the unsaturated fatty acid content of the frying oils Fig.5 Boxplot of the ratio between saturated and unsaturated fattyacid content of the frying oils and unsaturated fatty acid content (Figs. 6, 7,respectively).The correlation has also been investigated between the IPvalues and the ratio between saturated/unsaturated fattyacids (Fig. 8) to assess the relationship between the fattyacid composition and the measured oxidative stability.The positive correlation of the IP values obtained usingOXITEST (r=0.83; r²=0.69; Fig.6a) and RANCIMAT(r=0.78; r²=0.61; Fig. 6b) methods with the saturatedfatty acid content was statistically significant(P≤0.001).The IP values increased at increasing saturated fatty acidcontent. In detail, each 10% increment of the saturated fattyacid content in the frying oils extended approximately land 2 h the IPs obtained with OXITEST and RANCIMATmethods, respectively. The negative correlation of the IPvalues obtained using OXITEST (r=-0.837;r²=0.69;Fig. 7a) and RANCIMAT (r=-0.78;r²=0.61; Fig.7b)methods with the unsaturated fatty acid content was statisti-cally significant (P≤0.001). The IPs decreased at increasing Fig. 6 Correlation of the IP values of the OXITEST (a) and RANCI-MAT (b) methods with the saturated fatty acid content of the fryingoils Fig.7 Correlation of the IP values of the OXITEST (a) and RANCI-MAT (b) methods with the unsaturated fatty acid content of the fry-ing oils saturated fatty acid content. In detail, each 10% increment ofthe unsaturated fatty acid content in the frying oils decreasedof approximately 1 and 2 h the IP obtained with OXITESTand RANCIMAT methods, respectively. The positive cor-relation of the IP values obtained using OXITEST (r=0.86;r=0.73; Fig.8a) and RANCIMAT (r= 0.80; r²=0.64;Fig. 8b) methods with the ratio saturated/unsaturated fattyacid content was statistically significant (P≤0.001). TheIPs resulted to increase at increasing saturated/unsaturatedratios, with a 10% increment of the latter causing roughly anincrease of approximately 0.8 and 1.5 h of the IPs assessed Fig. 8 Correlation of the IP values of the OXITEST (a) and RANCI-MAT (b) methods with the ratio between saturated and unsaturatedfatty acid content of the frying oils with the OXITEST and the RANCIMAT instruments.respectively. Hence, an increased oxidative stability of thefrying oils was related to an increase in the saturated fattyacid content based on the formulation of the ingredientsdeclared in the label (Table 1). In details, the frying oils Kand N, which showed to be the most stable according to theirassessed IPs (5.4 and 5.5 h with OXITEST; 10.7 and 10.3 hwith RANCIMAT) (Table 2), were the richest in saturatedfatty acids, (27.4 and 27.2%, respectively) (Table 3) whoselevels may be associated to the use of fractionated palm oilin the industrial formulations (Table 1). In fact palmitic acidwas found to be present in oils K and N at the highest levels,22.4 and 22.5%, respectively. Moreover, the fractionationprocess applied on the palm oil utilized in these samples(Table 1) led to a greater oxidative stability in comparisonto the oil C, containing unfractionated palm oil and char-acterized by a lower palmitic acid level (10.9%) and lowerIP values, as assessed with the OXITEST and RANCIMATmethods (3.61 and 6.72 h, respectively)(Table 2). On thecontrary, samples A and B, that resulted to be more proneto oxidation (Fig.1) according to their lowest IP valuesassessed using the OXITEST (2.69 and 2.79 h) or the RAN-CIMAT (4.78 and 4.80 h) method, did not contain any palmoil and showed to be poor in saturated fatty acids (10.1 and11.4%, respectively) and rich in unsaturated fatty acids (89.9and 88.6%), due to their composition based on sunflower andpeanut oils (Tables 1, 2, 3). Several authors have reportedthat palm oil, rich in palmitic acid, has shown to be less sen-sitive to oxidation than other vegetable oils rich in unsatu-rated fatty acids and olive oil [7, 29, 31]. Furthermore, someauthors evaluating the oxidative stability of several vegetableoils with the RANCIMAT method at different temperatures, confirmed the greatest resistance of palm oil to fat oxida-tion, being characterized by higher IP values (19.95, 10.89,and 5.70 h) than rapeseed (7.38, 4.10, and 1.85 h), soybean(5.24, 2.61, and 1.51 h) and sunflower oils (3.70, 1.89, and0.88 h) at 110, 120, and 130 ℃,respectively [11]. Conclusions The detection of IP value represents an important parameterfor evaluating the oxidative stability of frying oils. Acceler-ated tests such as OXITEST and RANCIMATare able tospeed up the fat oxidation and monitor the oxidative stabilityin a relatively short period of time without using expen-sive and environmental hazardous reagents. The regressionanalysis carried out on the IP values showed a linear correla-tion between RANCIMAT and OXITEST instruments whichachieved the same comparative results following differentprocedures. Hence, the OXITEST method may representa valid alternative to the RANCIMAT method and couldbe a useful tool for the edible oil industry in formulatingoil mixtures to be utilized for food frying. Furthermore, themethod does not necessitate any specific sample preparationand requires less time for carrying out oxidative stabilitystudies, therefore, allowing higher throughputs. AcknowledgementsDThe authors would like to thank VELP Scienti-fica (Usmate, MB,Italy) for lending the OXITEST instrument and Dr.Paola Ornaghi for technical assistance. Compliance with ethical standards Conflict of interest The authors declare that they have no conflictof interest. Compliance with ethics requirements This article does not containany studies with human or animal subjects. References ( 1. Stier RF(2004) Frying as a science-an introduction. Eur J Lipid Sci Technol 106:715-721 ) ( 2. O ( reopoulou V, Krokida M, Marinos-Kouris D (2006) In: Mujum-dar AS (ed) Handbook of industrial drying, 3rd edn. CRC Press, Boca Raton ) ( 3. Choe E, Min D B (2007) Chemistry of deep-fat f r ying oils. J Food Sci72:77-86 ) 4.Marmesat S, Morales A, Velasco J, Dobarganes MC (2012) Influ-ence of fatty acid composition on chemical changes in blendsof sunflower oils during thermoxidation and frying. Food Chem135:2333-2339 ( 5. Aladedunye F, Przybylski R(2013) Fry i ng stability of high oleic sunflower oils as affected by composition of tocopherol i s omers and linoleic acid content. Food Chem 141:2373-2378 ) 6.).Gliszczynska-Swiglo A, Sikorska E, Khmelinskii I, Sikorski M(2007) Tocopherol content in edible plant oils. Pol JFood NutrSci 57:157-161 7.1De Marco E, Savarese M, Parisini C, Battimo I, Falco S, Sac-chi R (2007) Frying performance of a sunflower/palm oil blendin comparison with pure palm oil. Eur J Lipid Sci Technol109:237-246 8.De Leonardis A, Macciola V (2012) Heat-oxidation stabil-ity of palm oil blended with extra virgin olive oil. Food Chem135:1769-1776 9.Melton SL, Jafar S, Sykes D, Trigiano MK (1994) Review ofstability measurements for frying oils and fried food flavor. J AmOil Chem Soc 71:1301-1308 10.AOCS (1989) Official methods and recommended practices of theAOCS, 4th edn. AOCS, Champaign, Ill 11A.nwar F, Bhanger MI, Kazi TG (2003) Relationship between ran-cimat and active oxygen method values at varying temperatures. JAm Oil Chem Soc 80:151-155 12.AOCS (2012) Official methods and recommended practices of theAOCS, 6th edn. AOCS, Champaign, Ill 13.Mateos R, Uceda M, Aguilera MP, Escuderos ME, MazaGB (2006) Relationship of Rancimat method values at vary-ing temperatures for virgin olive oils. Eur J Lipid Sci Technol223:246-252 14.Peer MS, Kasimani R, Rajamohan S, Ramakrishnan P (2017)Experimental evaluation on oxidation stability of biodiesel/die-sel blends with alcohol addition by rancimat instrument and FTIRspectroscopy. J Mech Sci Technol 31:455-463 15.L]ante A, Friso D (2013) Oxidative stability and rheological prop-erties of nanoemulsions with ultrasonic extracted green tea infu-sion. Food Res Int 54:269-276 16.Mohammadi A, Jafari SM, Esfanjani AF, Akhavan S (2016)Application of nano-encapsulated olive leaf extract in controllingthe oxidative stability of soybean oil. Food Chem 190:513-519 17./.Lante A, Nardi T, Zocca F, Giacomini A, Corich V(2011) Evalu-ation of red chicory extract as a natural antioxidant by pure lipidoxidation and yeast oxidative stress response as model systems. JAgric Food Chem 59:5318-5324 18.. .Mihaylova DS, Lante A, Tinello F, Albert IK (2014) Study onthe antioxidant and antimicrobial activities of Allium ursinum L.pressurised-liquid extract. Nat Prod Res 28:2000-2005 19.1Riciputi Y, Caboni MF (2017) Assessing oil oxidative stability inTarallini by OXITEST. Ital J Food Sci 29:63-73 20.Caruso MC, Galgano F, Colangelo MA, Condelli N, Scarpa T,Tolve R, Favati F (2017) Evaluation of the oxidative stability ofbakery products by OXITEST method and sensory analysis. EurFood Res Technol 243:1183-1191 21.Verardo V, Riciputi Y, Sorrenti G, Ornaghi P, Marangoni B,Caboni MF (2013) Effect of nitrogen fertilisation rates on thecontent of fatty acids, sterols, tocopherols and phenolic com-pounds, and on the oxidative stability of walnuts. Food Sci Tech-nol 50:732-738 22.Comandini P, Verardo V,Maiocchi P, Caboni MF (2009) Acceler-ated oxidation: comparative study of a new reactor with oxidationstability instrument. Eur J Lipid Sci Technol 111:933-940 23. Caruso MC, Galgano F, Scarpa T, Ornaghi P, Favati F (2017)Accelerated shelf life studies of extra virgin olive oils using theOxitest method. Inform 28:26-29 24.Verardo V, Riciputi Y, Trivisonno MC, Marconi E, Caboni MF(2010) Effect of the addition of air-classified barley flours onthe lipid stability of bakery products. Eur Food Res Technol231:309-319 25..Amato M, Caruso MC, Guzzo F, Galgano F, Commisso M, Boch-icchio R, Labella R, Favati F (2015) Nutritional quality of seedsand leaf metabolites of Chia (Salvia hispanica L.) from SouthernItaly. Eur Food Res Technol 241:615-625 26.IIchihara K, Shibahara A, Yamamoto K, Nakayama T (1996) Animproved method for rapid analysis of the fatty acids of glyc-erolipids. Lipids 31:535-539 27. S$AS (2013). SAS/STAT(R) 9.2 User’s guide, 2nd edn. SAS Insti-tute Inc., Cary. http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#glm_toc.htm. Accessed29 June 2017 28.G(erde JA, Hammond EG, White PJ (2011) Influence of polydi-methylsiloxane on the oxygen concentration of oils at varioustemperatures.J Am Oil Chem Soc 88:925-929 29.MNba OI, Dumont MJ, Ngadi M (2015) Palm oil: processing,char-acterization and utilization in the food industry-a review. FoodBiosci 10:26-41 ( 30 . Mancebo-Campos V, Salvador MD, Fregapane G (2014) Anti-oxidant capacity of individual and combined virgin olive oilminor compounds evaluated at mild temperature (25 and 40°C)as compared to accelerated and a ntiradical assays. Food Chem150:374-381 ) ( 31. Romano R, Giordano A, Vitiello S, L e G r ottaglie L, Musso S S (2012) C omparison of the f r ying performance of olive oil a ndpalm superolein. J Food S ci 77:519-531 ) SpringerPublished online: October Springer
确定




还剩7页未读,是否继续阅读?
意大利VELP公司为您提供《煎炸油中油脂氧化稳定性检测方案(氧化分析仪)》,该方案主要用于食用植物油中理化分析检测,参考标准--,《煎炸油中油脂氧化稳定性检测方案(氧化分析仪)》用到的仪器有VELP Oxitest 油脂氧化分析仪
推荐专场
相关方案
更多
该厂商其他方案
更多