方案详情
文
延长食用油使用寿命是食品科学技术的巨大挑战之一。如果能加入合适的一泡剂避免泡沫的产生,食用油的寿命将会提高。Kruss提供的动态张力测量方法解释了静态测量方法无法得到的结论。
方案详情

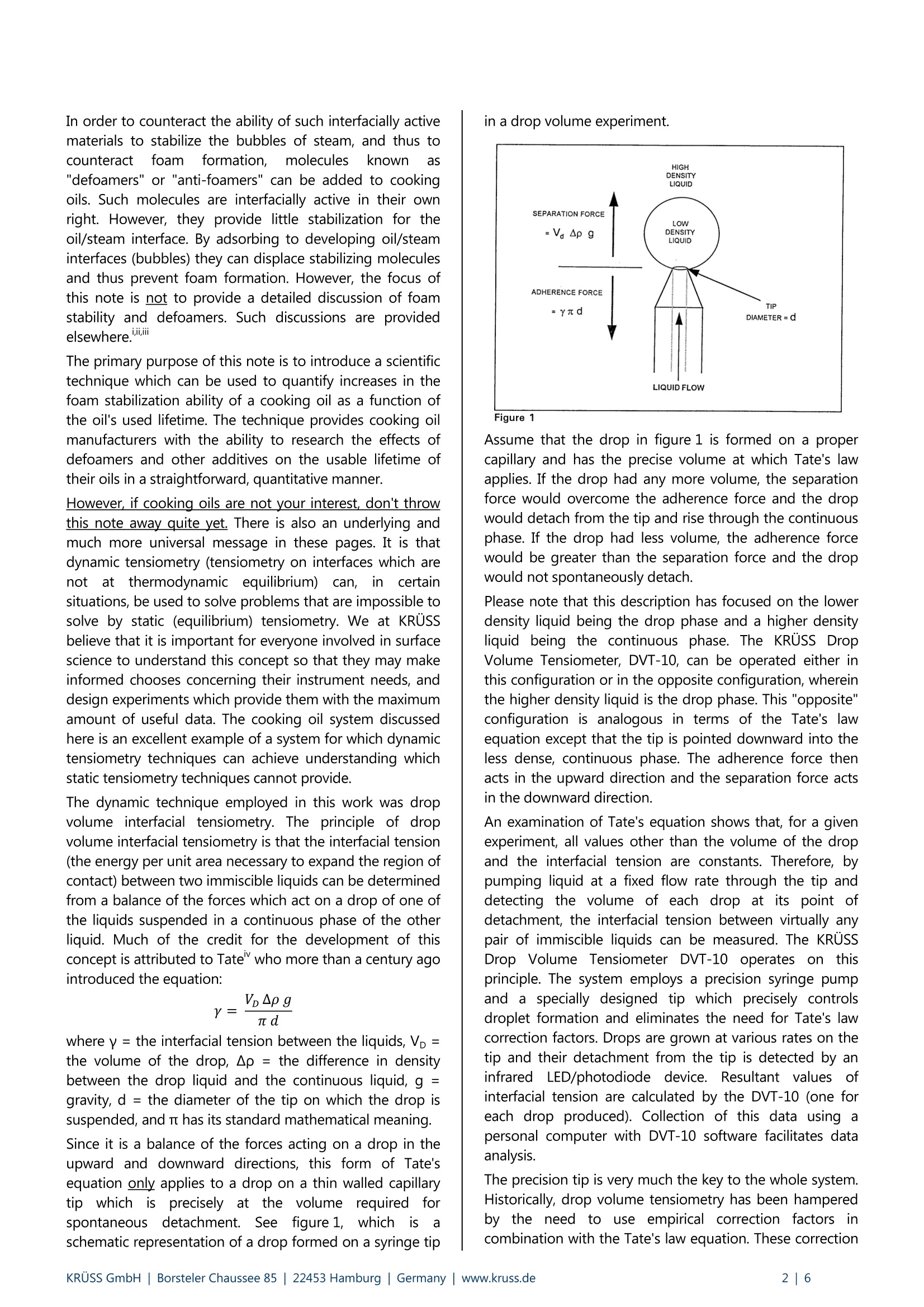
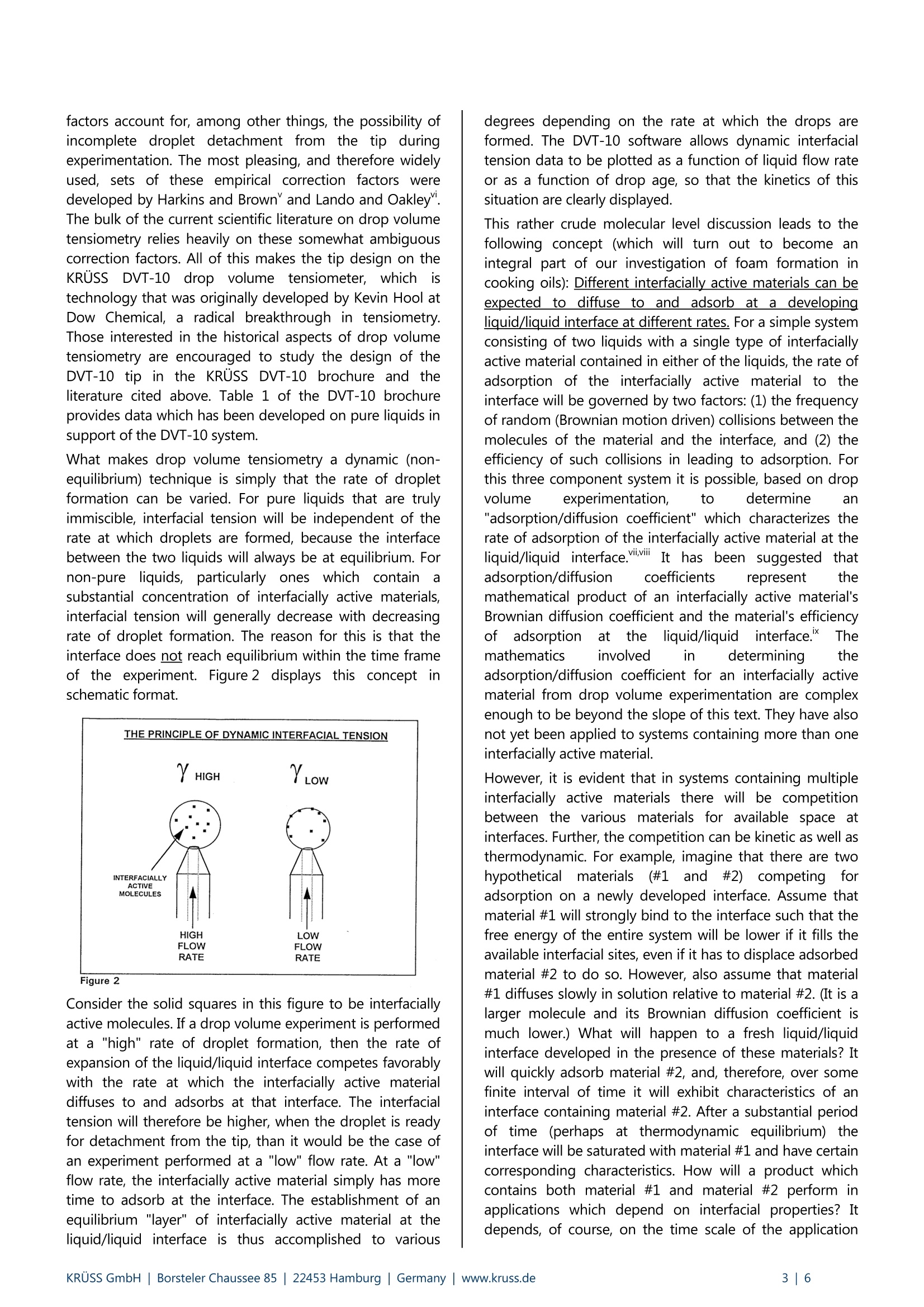
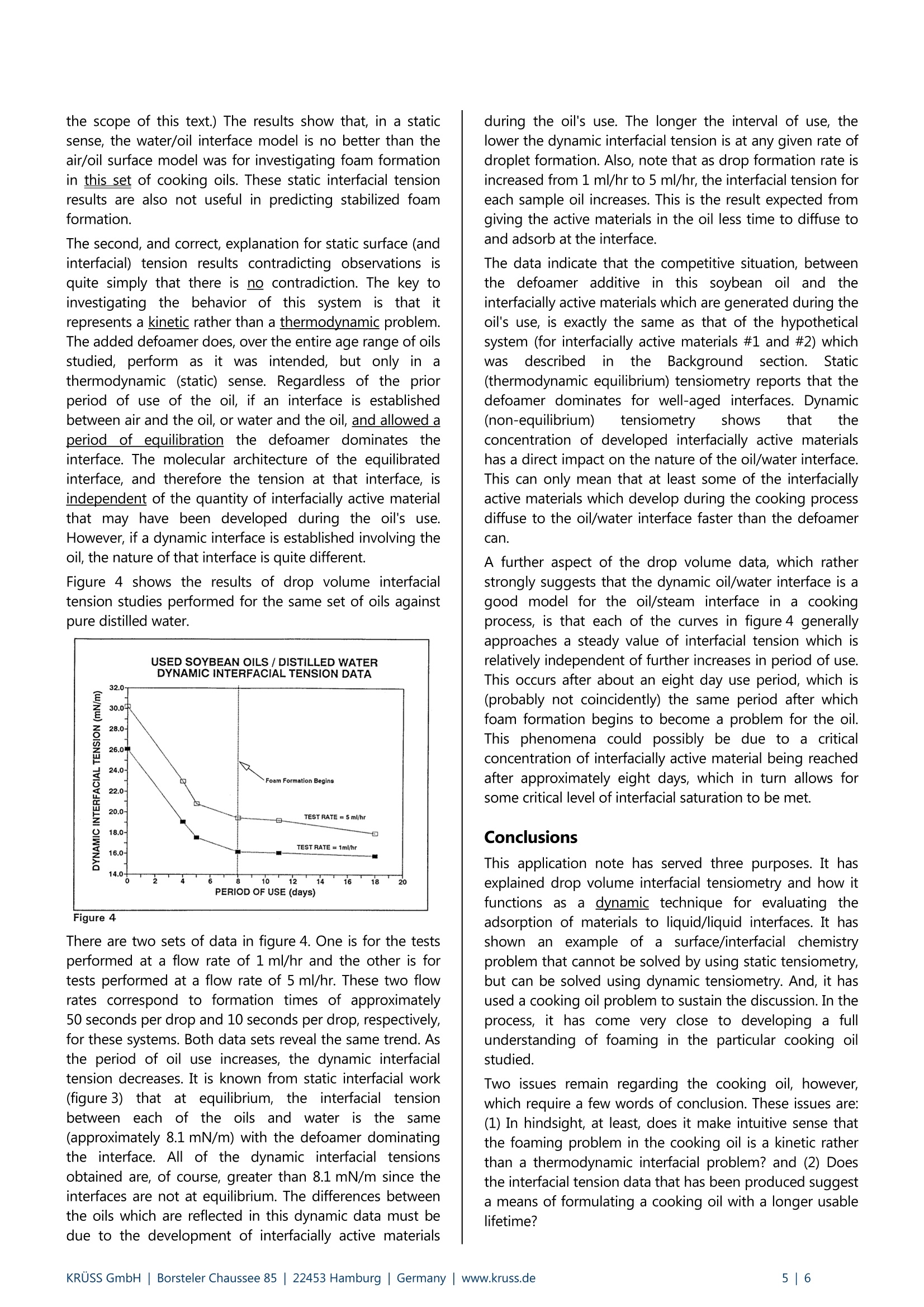
KRUSS Application Report The effects of ageing on the properties of soybean based cooking oils An Example of the Application of Dynamic Tensiometry to Problems that Cannot Be Solved byStatic Tensiometrv Background One of the large challenges in food science technology is the development of cooking oils with extended usable lifetimes.Extended lifetime oils decrease: (1) the necessity for hot-oil cooker downtime, (2) the overall quantity of oil used per itemcooked, and (3) the amount of used oil that subsequently needs disposal. The advantages are economic as well asenvironmental. After cooking oil has been used for a period of time, a stable froth (or foam) begins to develop on the surface of the oilduring the cooking process. Soon thereafter, controlling this surface layer becomes a problem. The esthetics of cookedfood, which has passed through a layer of foam during removal from the cooker, also becomes a consideration.It thenbecomes time to shut down the cooker and change the oil. However, if foaming can be prevented, then the oil would beuseable over a longer period. The "foam" is primarily stabilized bubbles of steam which result from the vaporization of water that is either containedwithin the food being cooked or, in the case of frozen foods, attached to the food in the form of ice. The foam isstabilized by interfacially active (surfactant like) molecules in the oil. This implies that as the oil ages, in terms of use, theamount of interfacially active materials present increases. This is, in fact, the case. During the cooking process, interfaciallyactive materials are often extracted from the foods being cooked. Many fish proteins and potato starches, for example,are highly interfacially active. Also, at cooking temperatures,which are typically 175° to 200℃ (350°to 400°F), interfaciallyactive molecules may be formed due to oxidation, hydrolysis, and/or polymerization reactions involving the oil itself. In order to counteract the ability of such interfacially activematerials to stabilize the bubbles of steam, and thus tocounteract toamfformation,), molecules known as"defoamers" or "anti-foamers" can be added to cookingoils. Such molecules are interfacially active in their ownright. However, they provide little stabilization for theoil/steam interface. By adsorbing to developing oil/steaminterfaces (bubbles) they can displace stabilizing moleculesand thus prevent foam formation. However, the focus ofthis note is not to provide a detailed discussion of foamstability and defoamers. Such discussions are providedelsewhere.ii The primary purpose of this note is to introduce a scientifictechnique which can be used to quantify increases in thefoam stabilization ability of a cooking oil as a function ofthe oil's used lifetime. The technique provides cooking oilmanufacturers with the ability to research the effects ofdefoamers and other additives on the usable lifetime oftheir oils in a straightforward, quantitative manner. However, if cooking oils are not your interest, don't throwthis note away quite yet. There is also an underlying andmuch more universal message in these pages. It is thatdynamic tensiometry (tensiometry on interfaces which arenot aitt thermodynamic equilibrium)can,in certainsituations, be used to solve problems that are impossible tosolve by static (equilibrium) tensiometry. We at KRUssbelieve that it is important for everyone involved in surfacescience to understand this concept so that they may makeinformed chooses concerning their instrument needs, anddesign experiments which provide them with the maximumamount of useful data. The cooking oil system discussedhere is an excellent example of a system for which dynamictensiometry techniques can achieve understanding whichstatic tensiometry techniques cannot provide. The dynamic technique employed in this work was dropvolume interfacial tensiometry. The principle of dropvolume interfacial tensiometry is that the interfacial tension(the energy per unit area necessary to expand the region ofcontact) between two immiscible liquids can be determinedfrom a balance of the forces which act on a drop of one ofthe liquids suspended in a continuous phase of the otherliquid. Much of the credit for the development of thisconcept is attributed to Tate" who more than a century agointroduced the equation: where y = the interfacial tension between the liquids, Vp =the volume of the drop, Ap= the difference in densitybetween the drop liquid and the continuous liquid, g =gravity, d=the diameter of the tip on which the drop issuspended, and nt has its standard mathematical meaning. Since it is a balance of the forces acting on a drop in theupward and downward directions, this form of Tate'sequation only applies to a drop on a thin walled capillarytip which sprecisely at the volume rrequired forspontaneous detachment. See figure 1,whichnis aschematic representation of a drop formed on a syringe tip in a drop volume experiment. Figure 1 Assume that the drop in figure 1 is formed on a propercapillary and has the precise volume at which Tate's lawapplies. If the drop had any more volume, the separationforce would overcome the adherence force and the dropwould detach from the tip and rise through the continuousphase. If the drop had less volume, the adherence forcewould be greater than the separation force and the dropwould not spontaneously detach. Please note that this description has focused on the lowerdensity liquid being the drop phase and a higher densityliquid being the continuous phase. The KRUSS DropVolume Tensiometer, DVT-10, can be operated either inthis configuration or in the opposite configuration, whereinthe higher density liquid is the drop phase. This "opposite"configuration is analogous in terms of the Tate's lawequation except that the tip is pointed downward into theless dense, continuous phase. The adherence force thenacts in the upward direction and the separation force actsin the downward direction. An examination of Tate's equation shows that, for a givenexperiment, all values other than the volume of the dropand the interfacial tension are constants. Therefore, bypumping liquid at a fixed flow rate through the tip anddetecting the volume of each drop at its point ofdetachment, the interfacial tension between virtually anypair of immiscible liquids can be measured. The KRUSSDrop Volume Tensiometer DVT-10operates on thisprinciple. The system employs a precision syringe pumpand a specially designed tip which precisely controlsdroplet formation and eliminates the need for Tate's lawcorrection factors. Drops are grown at various rates on thetip and their detachment from the tip is detected by aninfrared LED/photodiode device. Resultant values ofinterfacial tension are calculated by the DVT-10 (one foreach drop produced). Collection of this data using apersonal computer with DVT-10 software facilitates dataanalysis. The precision tip is very much the key to the whole system.Historically,drop volume tensiometry has been hamperedby the need to use empirical correction factors incombination with the Tate's law equation. These correction factors account for, among other things, the possibility ofincomplete droplet detachment from the tip duringexperimentation. The most pleasing, and therefore widelyused, sets of these empirical correction factors weredeveloped by Harkins and Brown and Lando and Oakley".The bulk of the current scientific literature on drop volumetensiometry relies heavily on these somewhat ambiguouscorrection factors. All of this makes the tip design on theKRUSS DVT-10 drop volume tensiometer, whichistechnology that was originally developed by Kevin Hool atDow Chemical, a radical breakthrough in tensiometry.Those interested in the historical aspects of drop volumetensiometry are encouraged to study the design of theDVT-10 tip in the KRUSS DVT-10 brochure and theliterature cited above. Table 1 of the DVT-10 brochureprovides data which has been developed on pure liquids insupport of the DVT-10 system. What makes drop volume tensiometry a dynamic (non-equilibrium) technique is simply that the rate of dropletformation can be varied. For pure liquids that are trulyimmiscible, interfacial tension will be independent of therate at which droplets are formed, because the interfacebetween the two liquids will always be at equilibrium. Fornon-pure liquids,particularly ones:s whichh containasubstantial concentration of interfacially active materials,interfacial tension will generally decrease with decreasingrate of droplet formation. The reason for this is that theinterface does not reach equilibrium within the time frameof the experiment. Figure 2 displays this concept inschematic format. Figure 2 Consider the solid squares in this figure to be interfaciallyactive molecules. If a drop volume experiment is performedat a "high" rate of droplet formation, then the rate ofexpansion of the liquid/liquid interface competes favorablywith the rate at which the interfacially active materialdiffuses to and adsorbs at that interface. The interfacialtension will therefore be higher, when the droplet is readyfor detachment from the tip, than it would be the case ofan experiment performed at a "low" flow rate. At a "low"flow rate, the interfacially active material simply has moretime to adsorb at the interface. The establishment of anequilibrium "layer" of interfacially active material at theliquid/liquid interface is thus accomplished to various degrees depending on the rate at which the drops areformed. The DVT-10 software allows dynamic interfacialtension data to be plotted as a function of liquid flow rateor as a function of drop age, so that the kinetics of thissituation are clearly displayed. This rather crude molecular level discussion leads to thefollowing concept (which will turn out to become anintegral part of our investigation of foam formation incooking oils): Different interfacially active materials can beexpected to diffuse to and adsorb at a developingliquid/liquid interface at different rates. For a simple systemconsisting of two liquids with a single type of interfaciallyactive material contained in either of the liquids, the rate ofadsorption of the interfacially active material to theinterface will be governed by two factors: (1) the frequencyof random (Brownian motion driven) collisions between themolecules of the material and the interface, and (2) theefficiency of such collisions in leading to adsorption. Forthis three component system it is possible, based on dropvolume experimentation, to determine an"adsorption/diffusion coefficient" which characterizes therate of adsorption of the interfacially active material at theliquid/liquid interface.iviiItIt has been suggested thatadsorption/diffusion coefficients represent themathematical product of an interfacially active material'sBrownian diffusion coefficient and the material's efficiencyofadsorptionat the liquid/liquid interface.TThemathematics involved in determining theadsorption/diffusion coefficient for an interfacially activematerial from drop volume experimentation are complexenough to be beyond the slope of this text. They have alsonot yet been applied to systems containing more than oneinterfacially active material. However, it is evident that in systems containing multipleinterfacially active materials there will be competitionbetween the various materials for available space atinterfaces. Further, the competition can be kinetic as well asthermodynamic. For example, imagine that there are twohypotheticalmaterials(#1 and #2)) competingforadsorption on a newly developed interface. Assume thatmaterial #1 will strongly bind to the interface such that thefree energy of the entire system will be lower if it fills theavailable interfacial sites, even if it has to displace adsorbedmaterial #2 to do so. However, also assume that material#1 diffuses slowly in solution relative to material #2. (It is alarger molecule and its Brownian diffusion coefficient ismuch lower.) What will happen to a fresh liquid/liquidinterface developed in the presence of these materials? Itwill quickly adsorb material #2, and, therefore, over somefinite interval of time it will exhibit characteristics of aninterface containing material #2. After a substantial periodof time (perhaps at thermodynamic equilibrium) theinterface will be saturated with material #1 and have certaincorresponding characteristics. How will a product whichcontains both material #1 and material #2 perform inapplications which depend on interfacial properties? Itdepends, of course, on the time scale of the application relative to the time scale for the establishment ofthermodynamic interfacial equilibrium. Would static tensiometric measurements on such a productbe expected to provide any insight into the productsperformance, if the product is used in the time frame overwhich the interface is dominated by material #2? No, staticmeasurementswill insteadbe veryyeffective incharacterizing an interface which is saturated by material#1 and, therefore, has little to do with the product'sapplication. It will be shown that the cooking oil which isthe subject of this note is an example of this type ofsystem, albeit an even more complex one. Experimental & Discussion A soybean based cooking oil, containing defoamingadditives that shall remain proprietary, was studied. Actualcooking tests suggested that, despite the defoamingadditive, this soybean oil had a useable lifetime of onlyapproximately 8 to 10 days under rigorous cookingconditions. Our initial theory was that the amount ofinterfacially active materials developed over this period ofuse became substantial enough to dominate the defoamer.In other words, the defoamer began to become the loser inthe ongoing thermodynamic competition for adsorptionsites at steam/oil interfaces. At periods of use less thanabout 8 days, the quantity of interfacially active materialproduced was not substantial enough to dominate thesituation, but near this time the equilibrium shifted. In investigating this initial theory, our first efforts focusedon static surface tension measurements by Wilhelmy platetechniques. (For those who are unfamiliar which suchtechniques, KRUSS would be happy to supply literature onthe subject.) The idea was that if the interfacially activematerials which develop during cooking actually competed,thermodynamically, with the defoamer additive for thesteam/oil interface, then a similar competition would existfor the air/oil interface. In other words, static surfacetension measurements on a series of oils, each having beenpreviously used in actual cooking for a certain period,would reflect the development of the interfacially activematerials by revealing that static surface tension wasdependent on the period of use. A result that would nothave been surprising might be that the static surfacetension of the oil increases with period of use. This wouldhave indicated that the interfacially active materials beingdeveloped were taking up adsorption sites at the air/oilsurface thus displacing the defoamer. It then might havebeen possible to use static surface tensiometry as aresearch tool in the investigation of the effects of variousdefoaming additives on the oil properties. Static surfacetensiometry could have acted as a guide to improvedcooking oil formulation. This, however, was not found to be the case. The followingoil samples were tested by static surface tensiometry:unused oil, 4-day oil, 5-day oil, 8-day oil, 11-day oil, and18-day oil. The results in figure 3 show no change in staticsurface tension with increased period of oil use Figure 3 Especially considering that an unused oil was included inthis testing, these results indicate that interfacially activematerials developed during the cooking process do not atall compete with the defoamer additive for adsorption atthe cooking oil/air surface, at least not in a thermodynamicsense. It is possible that the composition of the air/oilsurface did change in a static (thermodynamic) sense, andyet this change went undetected by the surface tensionmeasurements. This would be the case if the developedinterfacially active materials replaced the defoamer at thesurface and yet left the surface tension constant. Forcompleteness of understanding this must be considered. Itcould explain why the oil foams during cooking for periodsof use greater than 8 to 10 days. However, in reality, it is atrivial explanation. For this to actually occur, the developedinterfacially active materials would need to fill adsorptionsites at the air/oil interface in precisely the same manner asthe defoamer (in order to keep the surface tensionconstant). This is extremely unlikely, since the defoamerand the interfacially active material can be expected tohave radically different chemical structures. Static surface tensiometry has thus lead to the conclusionthat the nature of the air/oil interface is unchanged by thedevelopment ofinterfacially active materials duringcooking oil use. The defoamer dominates the interfaceeven after 18 days of oil use. This is, apparently, completelycontradictory to the direct observation that the steam/oilinterface is readily stabilized by some component of thecooking oil, after 8 to 10 days of oil use. Foaming doesbecome a problem after this period of use. Two possibleexplanations for this contradiction exist. The first is that the static air/oil surface is not a goodexperimental model for the steam/oil interface. If this is thecase, then since steam is water in the gaseous state, thenext logical room temperature experimental model wouldbe the water/oil interface. Figure 3 also shows results ofstatic interfacial tension studies between the series of oilsamples discussed previously and water. These results wereobtained using Du Nouy ring tensiometry. (KRUSS will alsoprovide those interested with a detailed explanation of DuNouy ring based tensiometry, but as was the case forWilhelmy plate tensiometry, such an explanation is beyond the scope of this text.) The results show that, in a staticsense, the water/oil interface model is no better than theair/oil surface model was for investigating foam formationin this set of cooking oils. These static interfacial tensionresults are also not useful in predicting stabilized foamformation. The second, and correct, explanation for static surface (andinterfacial) tension results contradicting observations isquite simply that there is no contradiction. The key toinvestigating the behavior of this system is that itrepresents a kinetic rather than a thermodynamic problem.The added defoamer does, over the entire age range of oilsstudied, perform as it was iintended, but only in athermodynamic (static) sense. Regardless of the priorperiod of use of the oil, if an interface is establishedbetween air and the oil,or water and the oil, and allowed aperiod of equilibration the defoamer dominates theinterface. The molecular architecture of the equilibratedinterface, and therefore the tension at that interface, isindependent of the quantity of interfacially active materialthat may have been developed during the oil's use.However, if a dynamic interface is established involving theoil, the nature of that interface is quite different. Figure 4 shows the results of drop volume interfacialtension studies performed for the same set of oils againstpure distilled water. Figure 4 There are two sets of data in figure 4. One is for the testsperformed at a flow rate of 1 ml/hr and the other is fortests performed at a flow rate of 5 ml/hr. These two flowrates correspond to formation times of approximately50 seconds per drop and 10 seconds per drop, respectively,for these systems. Both data sets reveal the same trend. Asthe period of oil use increases, the dynamic interfacialtension decreases. It is known from static interfacial work(figure 3)thatat equilibrium, the iinterfacialltensionbetweeneachof the oils and water is the same(approximately 8.1 mN/m) with the defoamer dominatingthe interface. All of the dynamic interfacial tensionsobtained are, of course, greater than 8.1 mN/m since theinterfaces are not at equilibrium. The differences betweenthe oils which are reflected in this dynamic data must bedue to the development of interfacially active materials during the oil's use. The longer the interval of use, thelower the dynamic interfacial tension is at any given rate ofdroplet formation. Also, note that as drop formation rate isincreased from 1 ml/hr to 5 ml/hr, the interfacial tension foreach sample oil increases. This is the result expected fromgiving the active materials in the oil less time to diffuse toand adsorb at the interface. The data indicate that the competitive situation, betweenthe defoamer additive in this soybean oil andtheinterfacially active materials which are generated during theoil's use, is exactly the same as that of the hypotheticalsystem (for interfacially active materials #1 and #2) whichwasdescribed in the Backgroundsection. Static(thermodynamic equilibrium) tensiometry reports that thedefoamer dominates for well-aged interfaces. Dynamic(non-equilibrium) tensiometry shows that theconcentration of developed interfacially active materialshas a direct impact on the nature of the oil/water interface.This can only mean that at least some of the interfaciallyactive materials which develop during the cooking processdiffuse to the oil/water interface faster than the defoamercan. A further aspect of the drop volume data, which ratherstrongly suggests that the dynamic oil/water interface is agood model for the oil/steam interface in a cookingprocess, is that each of the curves in figure 4 generallyapproaches a steady value of interfacial tension which isrelatively independent of further increases in period of use.This occurs after about an eight day use period, which is(probably not coincidently) the same period after whichfoam formation begins to become a problem for the oil.This phenomena could possibly be due to a criticalconcentration of interfacially active material being reachedafter approximately eight days, which in turn allows forsome critical level of interfacial saturation to be met. Conclusions This application note has served three purposes. It hasexplained drop volume interfacial tensiometry and how itfunctions as a dynamic technique for evaluating theadsorption of materials to liquid/liquid interfaces. It hasshown an example of a surface/interfacial chemistryproblem that cannot be solved by using static tensiometry,but can be solved using dynamic tensiometry. And, it hasused a cooking oil problem to sustain the discussion. In theprocess, it has come very close to developing a fullunderstanding of foaming in the particular cooking oilstudied. Two issues remain regarding the cooking oil, however,which require a few words of conclusion. These issues are:(1) In hindsight, at least, does it make intuitive sense thatthe foaming problem in the cooking oil is a kinetic ratherthan a thermodynamic interfacial problem? and (2) Doesthe interfacial tension data that has been produced suggesta means of formulating a cooking oil with a longer usablelifetime? Fortunately, the answer to both questions is yes.Anyonewho has watched a hot oil cooker in operation hasundoubtedly observed that the bubbles formed duringcooking pass through the oil to the surface quite rapidly.Therefore, it seems reasonable that there is little time forthe adsorption of interfacially active materials to thedeveloping steam/oil interfaces. Further, once the bubblesreach the surface and become part of the "froth", they areno longer exposed to the bulk of the oil. The steam/oilinterface is simply a thin film between each of the bubbles.It seems logical that, in this situation, exchange of theinterfacially active material at the steam/oil interfaceswould be slowed dramatically. In other words, it is more"difficult" for the defoamer molecules to even get to thestream/oil interface to displace the other interfacially activematerials, and thereby cause bubble collapse once thebubbles have separated from the bulk of the oil. Thisundoubtedly causes substantial increases in the stablelifetime of the foam. Obviously, at the point when bubblesare being formed much more rapidly than theyarecollapsing, foaming becomes a problem. This non-equilibrium explanation is further supported bythe observation that, in general, if the hot oil cooker isturned off and the oil is allowed to sit, the foam willeventually completely collapse. The molecular explanationfor this, based on the data presented here, would be thatthe defoamer eventually acts to displace the otherinterfacially active materials 1from the interfaces andprovides for the collapse of the foam. However, in realitythis is probably only one of the factors controlling collapseof the foam. Other factors surely include film drainage andcondensation of the steam once the temperature of the oilbegins to decrease. The findings reported here suggestthat the useable lifetime of the soybean oil under studywould be improved by changing defoamers. A defoamershould be sought which, not only thermodynamically, butalsookineticallyydominatestheinterfaciallvy materialsproduced during cooking in1yterms of adsorptionitsteam/oililinterfaces. Finding or.synthesizingsuchadefoamer may not be an easy task. However, we wouldsuggest that dynamic interfacial tension experimentationcould be of assistance in this quest. Literature Schramm, L. L., editor; Foams, The AmericanChemical Society Advances inChemistrySeries No.242, (1994). ii. Myers, D.; Surfaces, Interfaces, and Colloids-Principles and Applications, VCH Publishers,New York, (1991). ( i ii. Rosen. M .J.; S urfactants a nd I n terfacial Phenomena, 2nd ed., John W iley and S ons,New York, (1989). ) iv. Tate, T.; Phil. Mag., 27, 176,(1864). ( V. Harkins, W.D.; Brown, F.E.; J . Amer. Chem. Soc., 41, 499, ( 1919). ) ( v i. Lando, J .L.; Oakley, H.T.; J. Coll. and I nter.Sci., 25,526,(1967). ) ( vii. Van Voorst Vader, F.; Erkens, Th.F.; Van Den Tempel, M .; Trans. F araday Soc., 6 0, 1 170, (1964). ) ( v iii . Joos, P . ; R i llaerts, E.; Jour. Co l l. Inter. Sc i ., 79 , 1 ,96,(1981) ) IX Rulison, C.J.; Ph.D. Dissertation, Univ. ofSouthern Miss., (1994). KRUSS GmbH| Borsteler Chaussee Hamburg| Germany| www.kruss.de
确定



还剩4页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《大豆油中老化检测方案(表面张力仪)》,该方案主要用于食用植物油中食品添加剂检测,参考标准--,《大豆油中老化检测方案(表面张力仪)》用到的仪器有KRUSS DVT50型滴体积法张力仪
推荐专场
相关方案
更多
















