
FtsZ is an attractive target for screening of anti-bacterial
agents. Inhibitors of FtsZ have been reported.Edeine B1
is a potentially useful tool for studying the mechanism of
bacterial cell division.
方案详情

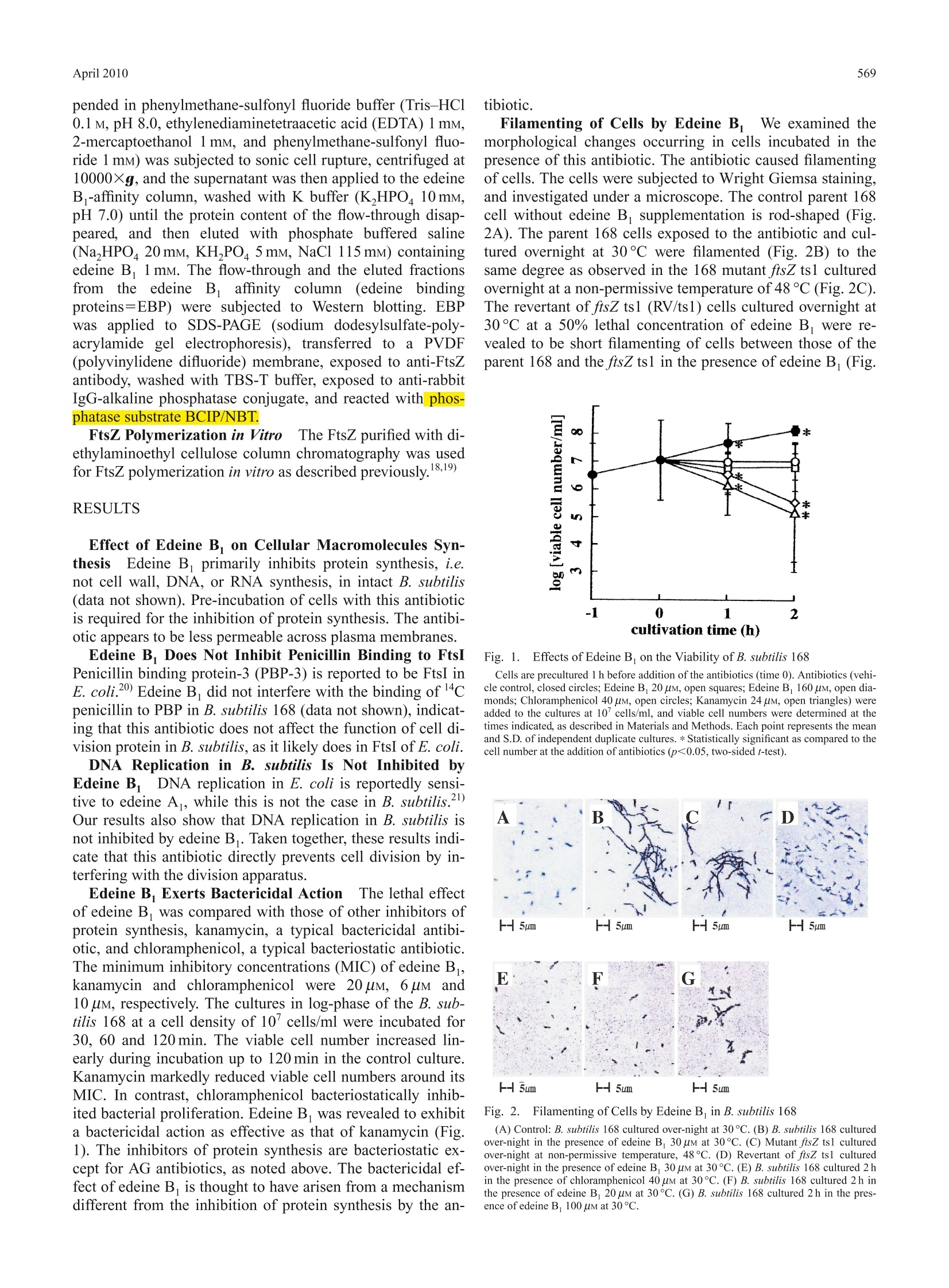
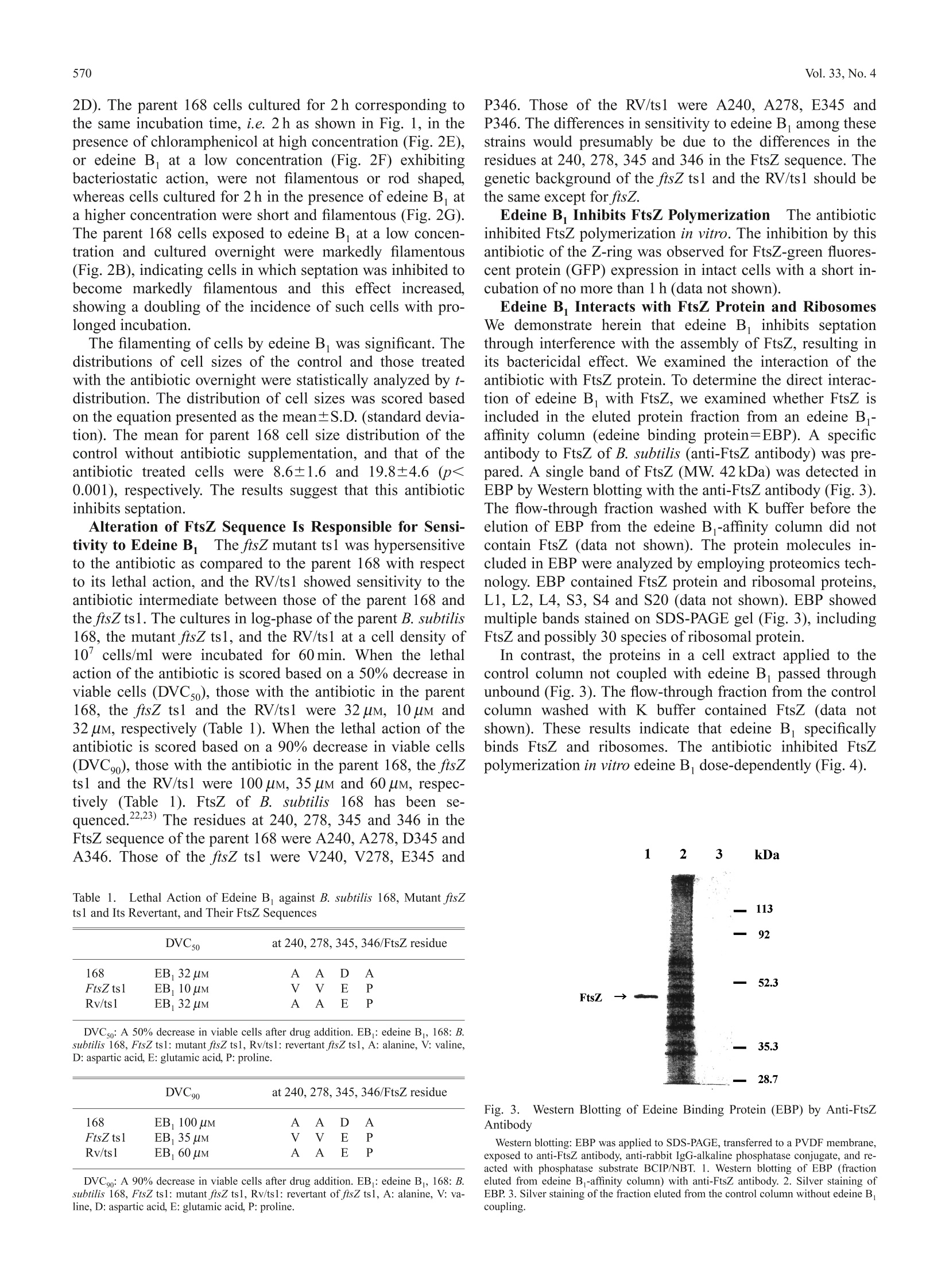
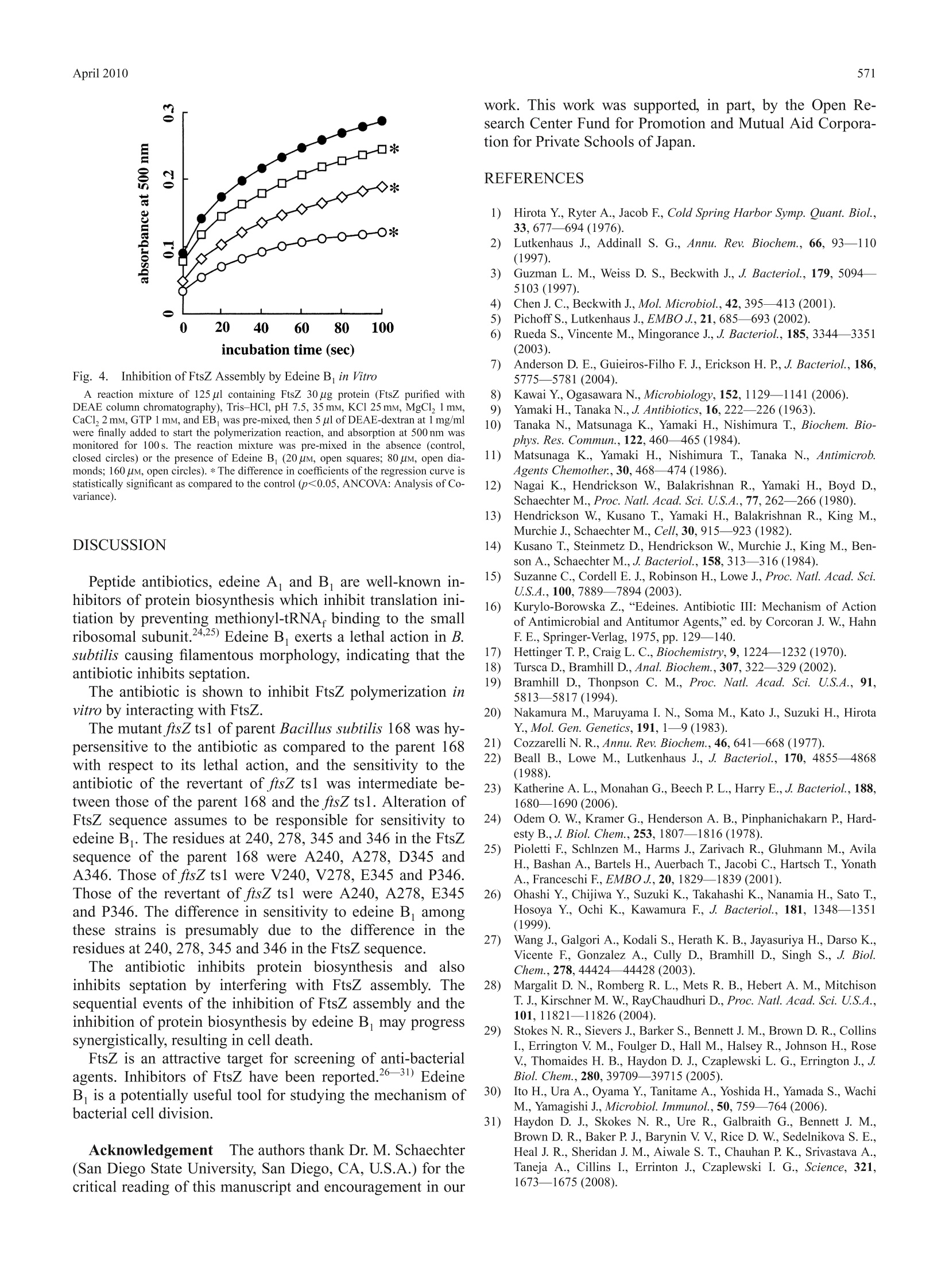
568Biol. Pharm. Bull. 33(4) 568—571(2010)Vol. 33, No. 4 569April 2010 *To whom correspondence should be addressed.l.e-mail: h_yamaki@fk2.so-net.ne.jpO2010 Pharmaceutical Society of Japan Inhibition of Septation in Bacillus subtilis by a Peptide Antibiotic, Edeine B Kumiko W. SHIMOTOHNO," Fujio KAWAMURA, Yousuke NATORI, Hideaki NANAMIYA, Junji MAGAE,Hiromitsu OGATA, Toyoshige ENDO," Takeshi SuzuKI," and Hiroshi YAMAKI*,4, aKeio University, Faculty of Pharmacy; Minato-ku, Tokyo 105-8512,Japan: Department of Biotechnology, Institute ofResearch and Innovation; Kashiwa, Chiba 277-0861, Japan: ‘Department of Life Science, College of Science, RikkyoUniversity; Toshima-ku, Tokyo 171-8501, Japan: and “National Institute of Public Health; Wako, Saitama 351-0197,Japan. Received October 29, 2009; accepted January 27,2010; published online February 1, 2010 A peptide antibiotic, edeine B, exerts a lethal action in Bacillus subtilis causing filamentous morphology.This antibiotic assumes to inhibit cell division by interacting with FtsZ and inhibiting FtsZ polymerization. Thetemperature-sensitive mutant ftsZ ts1 was shown to be hypersensitive to the antibiotic as compared to the parent168 with respect to its lethal action and the sensitivity to the antibiotic of the revertant of ftsZ ts1 was shown tobe intermediate between those of the parent 168 and the ftsZ ts1. Alteration of FtsZ sequence may be responsiblefor sensitivity to edeine B. The residues at 240, 278, 345 and 346 in the FtsZ sequence of the parent 168 wereA240, A278, D345 and A346. Those of ftsZ ts1 were V240, V278, E345 and P346. Those of the revertant of ftsZts1 were A240, A278, E345 and P346. The difference in sensitivity to edeine B. among these strains is presumablydue to the difference in the residues at 240, 278,345 and 346 in the FtsZ sequence. The sequential events of theinhibition of FtsZ assembly and the inhibition of protein biosynthesis by edeine B may progress synergistically,resulting in cell death. The antibiotics, edeine Aand B, are well-known in-hibitors of protein biosynthesis in both prokaryote andeukaryote cells. Edeine A and B show almost the samebiological activity. We show herein that edeine B exhibitsa lethal action in Bacillus subtilis, inhibiting septation via amechanism different from the inhibition of protein synthesisby this antibiotic. The complex process of cell division is closely intercon-nected with other cellular processes. Chromosomal DNAreplication, nuclear division, and cell division must be coor-dinated in time and space. DNA metabolism plays a pivotalrole in the cell division events controlled by the partitioningof newly replicated chromosomes to daughter cells. This in-volves chromosomal DNA replication, the positioning of aseptum at the midpoint of the cell, the assembly of cytoskele-tal elements, and the cleavage of the septum after chromo-some segregation. Genetic studies have unraveled the processof cell division by the analysis of filamenting temperature-sensitive mutants. In prokaryotes, filamenting temperature-sensitive (fts) genes produce the proteins essential for cell di-vision. Known fts mutants include ftsA, ftsI, ftsK, ftsL, ftsN,fts W and ftsZ.) Inhibitors of protein synthesis exhibit bacteriostatic actionexcept for the aminoglycoside (AG) antibiotics, suggestingthat the inhibition of protein synthesis by AG antibiotics isnot directly involved in their bactericidal action. The bacteri-cidal action of AG antibiotic is assumed to be based on theirinhibition of DNA replication. AG antibiotic blocks the initi-ation of DNA replication,1 and interrupts oriC-membraneattachment.11) The oriC-membrane attachment12-14) is cru-cial for subsequent initiation of DNA replication, chromo-some segregation, and septation of bacterial cells. BlockingDNA replication is known to lead to the save our ships(SOS) response in Escherichia coli, thereby inhibiting celldivision.15) We demonstrate herein that edeine B inhibits cell divi-sion, exerting its bactericidal action by inhibiting FtsZ as--s sembly, independently of its inhibitory effect on protein syn-thesis. MATERIALS AND METHODS Purification of Edeine B,Edeine B was isolated froma culture filtrate of Bacillus brevis TTO2-8. The purifiededeine B yielded a single TLC spot, a single HPLC peakand the predicted structure based on NMR and FAB-MASanalysis. Edeine B is a linear basic oligopeptide antibioticconsisting of five amino acid residues (isotyrosine, isoserine,o,B-diaminopropionic acid, 2,6-diamino-7-hydroxyazelanicacid, and glycine) and an organic base (guanylspermidine)produced by B. brevis.16,17 Bacterial Strains B. subtilis 168 and mutant FtsZ ts1 (atemperature sensitive mutant of B. subtilis 168) were used. Viablee Cell NumberThe: viable cell number wascounted by plating on LB (Luria-Bertani) agar, and record-ing the number of colonies that developed after 24 h incuba-tion. Anti-rabbit IgG-alkaline phosphatase conjugate was ob-tained from Sigma Co., U.S.A. BCIP/NBT was from KPLCo.,U.S.A. Edeine B-Affinity Column Chromatography A Hi-Trap NHS-activated HP column from Amersham-BioscienceCo. was coupled with edeine B. The log-phase culture of B.subtilis 168 cells was centrifuged, and the cell pellet sus- pended in phenylmethane-sulfonyl fluoride buffer (Tris-HCl0.1 M, pH 8.0, ethylenediaminetetraacetic acid (EDTA) 1 mM,2-mercaptoethanol 1 mM, and phenylmethane-sulfonyl fluo-ride 1 mM) was subjected to sonic cell rupture, centrifuged at10000Xg, and the supernatant was then applied to the edeineB -affinity column, washed with K buffer (K,HPO4 10 mM,pH 7.0) until the protein content of the flow-through disap-peared, and then eluted with phosphate buffered saline(Na,HPO 20mM, KH,PO4 5 mM, NaCl 115mM) containingedeine B1 mM. The flow-through and the eluted fractionsfrom the edeine Baffinity column(edeine bindingproteins=EBP) were subjected to Western blotting. EBPwasapplied to SDS-PAGE (sodium dodesylsulfate-poly-acrylamide gel electrophoresis), transferred to a PVDF(polyvinylidene difluoride) membrane, exposed to anti-FtsZantibody, washed with TBS-T buffer, exposed to anti-rabbitIgG-alkaline phosphatase conjugate, and reacted withi phos-phatase substrate BCIP/NBT. FtsZ Polymerization in VitroTThe FtsZ purified with di-ethylaminoethyl cellulose column chromatography was usedfor FtsZ polymerization in vitro as described previously.18,19) RESULTS Effect of Edeine B. on Cellular Macromolecules Syn-thesisEdeine B primarily inhibits protein synthesis, i.e.not cell wall, DNA, or RNA synthesis, in intact B. subtilis(data not shown). Pre-incubation of cells with this antibioticis required for the inhibition of protein synthesis. The antibi-otic appears to be less permeable across plasma membranes. Edeine B. Does Not Inhibit Penicillin Binding to FtsIPenicillin binding protein-3 (PBP-3) is reported to be FtsI inE. coli.2)Edeine B did not interfere with the binding of 4cpenicillin to PBP in B. subtilis 168 (data not shown), indicat-ing that this antibiotic does not affect the function of cell di-vision protein in B. subtilis, as it likely does in FtsI ofE. coli. DNA Replication in B. subtilis Is Not Inhibited byEdeine B DNA replication in E. coli is reportedly sensi-tive to edeine A, while this is not the case in B. subtilis.2Our results also show that DNA replication in B. subtilis isnot inhibited by edeine B. Taken together, these results indi-cate that this antibiotic directly prevents cell division by in-terfering with the division apparatus. Edeine B Exerts Bactericidal Actioni The lethal effectof edeine B was compared with those of other inhibitors ofprotein synthesis, kanamycin, a typical bactericidal antibi-otic, and chloramphenicol, a typical bacteriostatic antibiotic.The minimum inhibitory concentrations (MIC) of edeine B,kanamycin and chloramphenicol were 20 uM, 6uM and10 uM, respectively. The cultures in log-phase of the B. sub-tilis 168 at a cell density of 10cells/ml were incubated for30, 60 and 120 min. The viable cell number increased lin-early during incubation up to 120 min in the control culture.Kanamycin markedly reduced viable cell numbers around itsMIC. In contrast, chloramphenicol bacteriostatically inhib-ited bacterial proliferation. Edeine B was revealed to exhibita bactericidal action as effective as that of kanamycin (Fig.1). The inhibitors of protein synthesis are bacteriostatic ex-cept for AG antibiotics, as noted above. The bactericidal ef-fect of edeine B is thought to have arisen from a mechanismdifferent from the inhibition of protein synthesis by the an- Filamenting of Cells by Edeine BWe examined themorphological changes occurring in cells incubated in thepresence of this antibiotic. The antibiotic caused filamentingof cells. The cells were subjected to Wright Giemsa staining,and investigated under a microscope. The control parent 168cell without edeine B supplementation is rod-shaped (Fig.2A). The parent 168 cells exposed to the antibiotic and cul-tured overnight at 30°C were filamented (Fig. 2B) to thesame degree as observed in the 168 mutant ftsZts1 culturedovernight at a non-permissive temperature of 48℃ (Fig. 2C).The revertant of ftsZ ts1 (RV/ts1) cells cultured overnight at30℃ at a 50% lethal concentration of edeine B were re-vealed to be short filamenting of cells between those of theparent 168 and the ftsZ ts1 in the presence of edeine B(Fig. Fig. 1. Effects of Edeine B on the Viability of B. subtilis 168 Cells are precultured 1 h before addition of the antibiotics (time 0). Antibiotics (vehi-cle control, closed circles; Edeine B 20 uM, open squares; Edeine B 160 uM, open dia-monds; Chloramphenicol 40 uM, open circles; Kanamycin 24 uM, open triangles) wereadded to the cultures at 10’ cells/ml, and viable cell numbers were determined at thetimes indicated, as described in Materials and Methods. Each point represents the meanand S.D. of independent duplicate cultures. * Statistically significant as compared to thecell number at the addition of antibiotics (p<0.05, two-sided t-test). Fig. 2.FFilamenting of Cells by Edeine B in B. subtilis 168 (A) Control: B. subtilis 168 cultured over-night at 30℃. (B) B. subtilis 168 culturedover-night in the presence of edeine B 30 uM at 30C. (C) Mutant ftsZ tsl culturedover-night at non-permissive temperature, 48C. (D) Revertant of ftsZ ts1 culturedover-night in the presence of edeine B 30 uM at 30℃. (E) B. subtilis 168 cultured 2 hin the presence of chloramphenicol 40 uM at 30℃. (F) B. subtilis 168 cultured 2 h inthe presence of edeine B 20 uM at 30℃. (G) B. subtilis 168 cultured 2 h in the pres-ence of edeine B 100 uM at 30C. 2D). The parent 168 cells cultured for 2 h corresponding tothe same incubation time, i.e.2 h as shown in Fig. 1, in thepresence of chloramphenicol at high concentration (Fig.2E),or edeine B at a low concentration (Fig. 2F) exhibitingbacteriostatic action, were not filamentous or rod shaped,whereas cells cultured for 2 h in the presence of edeine B ata higher concentration were short and filamentous (Fig. 2G).The parent 168 cells exposed to edeine B at a low concen-tration and cultured overnight were markedly filamentous(Fig.2B), indicating cells in which septation was inhibited tobecome markedly filamentous and this effect increased,showing a doubling of the incidence of such cells with pro-longed incubation. The filamenting of cells by edeine B was significant. Thedistributions of cell sizes of the control and those treatedwith the antibiotic overnight were statistically analyzed by t-distribution. The distribution of cell sizes was scored basedon the equation presented as the mean±S.D. (standard devia-tion). The mean for parent 168 cell size distribution of thecontrol without antibiotic supplementation, and that of theantibiotic treated cells were 8.6±1.6 and 19.8±4.6 (p<0.001), respectively. The results suggest that this antibioticinhibits septation. Alteration of FtsZ Sequence Is Responsible for Sensi-tivity to Edeine B The ftsZ mutant ts1 was hypersensitiveto the antibiotic as compared to the parent 168 with respectto its lethal action, and the RV/ts1 showed sensitivity to theantibiotic intermediate between those of the parent 168 andthe ftsZ ts1. The cultures in log-phase of the parent B. subtilis168, the mutant ftsZ ts1, and the RV/ts1 at a cell density of107 cells/ml were incubated for 60 min. When the lethalaction of the antibiotic is scored based on a 50% decrease inviable cells (DVC50), those with the antibiotic in the parent168, the ftsZ ts1 and the RV/ts1 were 32 uM, 10 uM and32 uM, respectively (Table 1). When the lethal action of theantibiotic is scored based on a 90% decrease in viable cells(DVC90), those with the antibiotic in the parent 168, the ftsZtsl and the RV/ts1 were 100 uM, 35 uM and 60 uM, respec-tively (Table 1). FtsZ of B. subtilis 168 has been se-quenced.22,23) The residues at 240, 278, 345 and 346 in theFtsZ sequence of the parent 168 were A240, A278,D345 andA346. Those of the ftsZ ts1 were V240, V278, E345 and Table 1. Lethal Action of Edeine B against B. subtilis 168, Mutant ftsZts1 and Its Revertant, and Their FtsZ Sequences DVC50 at 240,278, 345, 346/FtsZ residue 168 EB, 32 uM A A D A FtsZ ts1 EB, 10 uM V V E P Rv/ts1 EB, 32 uM A A E P DVC50: A 50% decrease in viable cells after drug addition. EB : edeine B, 168: B.subtilis 168, FtsZ ts1: mutant ftsZ ts1, Rv/ts1: revertant ftsZ ts1, A: alanine, V: valine,D: aspartic acid, E: glutamic acid, P: proline. DVC90 at 240,278, 345, 346/FtsZ residue 168 EB 100uM A A D A FtsZ ts1 EB, 35 uM V V E P Rv/ts1 EB 60 uM A A E P DVC9o: A 90% decrease in viable cells after drug addition. EB : edeine B, 168: B.subtilis 168, FtsZ ts1: mutant ftsZ ts1, Rv/ts1: revertant of ftsZ ts1, A: alanine, V: va-line,D: aspartic acid, E: glutamic acid, P: proline. P346. Those of the RV/ts1 were A240, A278, E345 andP346. The differences in sensitivity to edeine B among thesestrains would presumably be due to the differences in theresidues at 240, 278, 345 and 346 in the FtsZ sequence. Thegenetic background of the ftsZ ts1 and the RV/ts1 should bethe same except for ftsZ. Edeine B. Inhibits FtsZ Polymerization The antibioticinhibited FtsZ polymerization in vitro. The inhibition by thisantibiotic of the Z-ring was observed for FtsZ-green fluores-cent protein (GFP) expression in intact cells with a short in-cubation of no more than 1 h (data not shown). Edeine B Interacts with FtsZ Protein and RibosomesWe demonstrate herein that edeine B inhibits septationthrough interference with the assembly of FtsZ, resulting inits bactericidal effect. We examined the interaction of theantibiotic with FtsZ protein. To determine the direct interac-tion of edeine B with FtsZ, we examined whether FtsZ isincluded in the eluted protein fraction from an edeine B.-affinity column (edeine binding protein=EBP). A specificantibody to FtsZ of B. subtilis (anti-FtsZ antibody) was pre-pared. A single band of FtsZ (MW. 42kDa) was detected inEBP by Western blotting with the anti-FtsZ antibody (Fig. 3).The flow-through fraction washed with K buffer before theelution of EBP from the edeine B-affinity column did notcontain FtsZ (data not shown). The protein molecules in-cluded in EBP were analyzed by employing proteomics tech-nology. EBP contained FtsZ protein and ribosomal proteins,L1, L2, L4, S3, S4 and S20 (data not shown). EBP showedmultiple bands stained on SDS-PAGE gel (Fig. 3), includingFtsZ and possibly 30 species of ribosomal protein. In contrast, the proteins in a cell extract applied to thecontrol column not coupled with edeine B passed throughunbound (Fig. 3). The flow-through fraction from the controlcolumn washed with K buffer contained FtsZ (data notshown). These results indicate that edeine B specificallybinds FtsZ and ribosomes. The antibiotic inhibited FtsZpolymerization in vitro edeine B dose-dependently (Fig. 4). Fig. 3.Western Blotting of Edeine Binding Protein (EBP) by Anti-FtsZAntibody Western blotting: EBP was applied to SDS-PAGE, transferred to a PVDF membrane,exposed to anti-FtsZ antibody, anti-rabbit IgG-alkaline phosphatase conjugate, and re-acted with phosphatase substrate BCIP/NBT. 1. Western blotting of EBP (fractioneluted from edeine B -affinity column) with anti-FtsZ antibody. 2. Silver staining ofEBP. 3. Silver staining of the fraction eluted from the control column without edeine Bcoupling. Fig. 4. Inhibition of FtsZ Assembly by Edeine B in Vitro A reaction mixture of 125 ul containing FtsZ 30 ug protein (FtsZ purified withDEAE column chromatography), Tris-HCl, pH 7.5, 35 mM, KCl 25 mM, MgCl,1 mM,CaCl, 2 mM, GTP1 mM, and EB, was pre-mixed, then 5 ul of DEAE-dextran at 1 mg/mlwere finally added to start the polymerization reaction, and absorption at 500 nm wasmonitored for 100s. The reaction mixture was pre-mixed in the absence (control,closed circles) or the presence of Edeine B (20 uM, open squares; 80 uM, open dia-monds; 160 uM, open circles). * The difference in coefficients of the regression curve isstatistically significant as compared to the control (p<0.05, ANCOVA: Analysis of Co-variance). DISCUSSION Peptide antibiotics, edeine A and B are well-known in-hibitors of protein biosynthesis which inhibit translation ini-tiation by preventing methionyl-tRNA. binding to the smallribosomal subunit.24,25) Edeine B, exerts a lethal action in B.subtilis causing filamentous morphology, indicating that theantibiotic inhibits septation. The antibiotic is shown to inhibit FtsZ polymerization invitro by interacting with FtsZ. The mutant ftsZ tsl of parent Bacillus subtilis 168 was hy-persensitive to the antibiotic as compared to the parent 168with respect to its lethal action, and the sensitivity to theantibiotic of the revertant of ftsZ ts1 was intermediate be-tween those of the parent 168 and the ftsZ ts1. Alteration ofFtsZ sequence assumes to be responsible for sensitivity toedeine B. The residues at 240, 278, 345 and 346 in the FtsZsequence of the parent 168 were A240, A278, D345 andA346. Those of ftsZ ts1 were V240, V278, E345 and P346.Those of the revertant of ftsZ ts1 were A240, A278, E345and P346. The difference in sensitivity to edeine B amongthese strains is presumably due to the difference in theresidues at 240, 278,345 and 346 in the FtsZ sequence. The antibiotic inhibits protein biosynthesis and alsoinhibits septation by interfering with FtsZ assembly. Thesequential events of the inhibition of FtsZ assembly and theinhibition of protein biosynthesis by edeine B may progresssynergistically, resulting in cell death. FtsZ is an attractive target for screening of anti-bacterialagents. Inhibitors of FtsZ have been reported.2631) EdeineB is a potentially useful tool for studying the mechanism ofbacterial cell division. Acknowledgement The authors thank Dr.M. Schaechter(San Diego State University, San Diego, CA, U.S.A.) for thecritical reading of this manuscript and encouragement in our work. This work was supported, in part, by the Open Re-search Center Fund for Promotion and Mutual Aid Corpora-tion for Private Schools of Japan. ( REFERENCES ) ( 1) H irota Y ., Ryter A., Jacob F, Cold Spring Harbor Symp. Quant. Bi o l.,33,677—694(1976). ) ( 2) Lutkenhaus J, Addinall S. G., Annu. Rev . Biochem. , 66 , 9 3— 110 (1997). ) ( 3) G uzman L. M., Weiss D. S . , Be c kwith J., J. B a cteriol., 1 79, 50945103 (1997). ) ( Chen J. C . , Beckwith J.,Mol. Microbiol.,42,395—413(2001). ) ( P ichoff S. , Lutkenhaus J., EMBOJ, 21,685 — 693 (2002). ) ( R ueda S., Vincente M ., M ingorance J . , J. Bacteriol., 185,3344— 3 351(2003). ) ( 7) A nderson D. E., Guieiros-Filho F. J., Erickson H. P, J. B a cteriol., 186, 5775—5781( 2 004). ) ( K awai Y., Ogasawara N ., Microbiology, 152,1129—1141 (2006). ) ( Y amaki H., Tanaka N., J. Antibiotics , 16, 22 2 — 2 26 (1963). ) ( T anaka N., M atsunaga K . , Yamaki H. , Ni s himura T., Bi o chem. Bio- phys. Res . Commun.,122,460— 4 65(1984). ) ( 11) M atsunaga K . , Y a maki H. , Nishimura T, T anaka N., Ant i microb.Agents Chemother,30,468—474 (1986). ) 12)Nagai K., Hendrickson W, Balakrishnan R., Yamaki H., Boyd D.,Schaechter M., Proc. Natl. Acad. Sci. U.S.A.,77,262—266(1980). 13) Hendrickson W, Kusano T., Yamaki H., Balakrishnan R., King M.,Murchie J., Schaechter M., Cell, 30,915—923(1982). 14) Kusano T., Steinmetz D., Hendrickson W., Murchie J, King M., Ben-son A., Schaechter M., J. Bacteriol., 158, 313—316(1984). 15) Suzanne C., Cordell E. J., Robinson H., Lowe J., Proc. Natl.Acad. Sci.U.S.A., 100,7889—7894 (2003). 16)Kurylo-Borowska Z.,“Edeines. Antibiotic III: Mechanism of Actionof Antimicrobial and Antitumor Agents,ed. by Corcoran J. W., HahnF.E., Springer-Verlag, 1975, pp. 129-140. Hettinger T. P, Craig L. C., Biochemistry, 9, 1224—1232(1970). Tursca D., Bramhill D., Anal. Biochem., 307,322—329(2002). Bramhill D., Thonpson C. M., Proc. Natl. Acad. Sci. U.S.A., 91,5813—5817(1994). 20) Nakamura M., Maruyama I. N., Soma M., Kato J, Suzuki H., HirotaY.,Mol. Gen. Genetics, 191,1—9 (1983). 21) Cozzarelli N. R., Annu. Rev. Biochem., 46,641—668 (1977). 22) Beall B., Lowe M., Lutkenhaus J., J. Bacteriol, 170, 4855—4868(1988). 23) Katherine A. L., Monahan G., Beech P. L., Harry E., J. Bacteriol., 188,1680—1690(2006). 24)Odem O. W, Kramer G., Henderson A. B., Pinphanichakarn P, Hard-esty B., J. Biol. Chem., 253,1807—1816(1978). 25)Pioletti F, Schlnzen M., Harms J., Zarivach R., Gluhmann M., AvilaH., Bashan A., Bartels H., Auerbach T., Jacobi C., Hartsch T., YonathA., Franceschi F, EMBO J,20,1829—1839(2001). ( 26) O hashi Y., Chijiwa Y ., Suzuki K., Takahashi K., Nanamia H., S ato T .,Hosoya Y ., Ochi K., Kawamura F, J. B acteriol. , 181 , 134 8— 1351 (1999). ) ( 27) Wang J, Galgori A . , Kodali S., Herath K. B., J ayasuriya H ., Darso K.,Vicente F , Gonzalez A. , Cu l ly D., Bra m hill D., S ing h S., J . Biol . Chem. ,2 78,44424- —44428 (2003). ) ( 28) M argalit D . N., Romberg R . L ., Mets R. B., Hebert A. M., Mitchison T .J.,Kirschner M. W. , RayChaudhuri D., Proc. Natl. Acad. Sci. U . S.A.,101,11821— 1 1826(2004). ) ( 29) S tokes N. R . , Sievers J. , Barker S., Bennett J . M., Brown D . R . , Collins I ., Errington V. M., F oulger D. , Hal l M . , Halsey R. , Johnson H ., Rose V , Th o maides H. B. , Ha y don D. J ., Cz a plewski L. G. , Errington J., J . Biol. Chem.,280,39709—39715 (2005). ) ( 30) I to H., Ura A . , Oyama Y ., Tanitame A., Yoshida H., Yamada S., Wachi M ., Yamagishi J., Microbiol. Immunol., 5 0 , 7 59— 764 ( 2006). ) ( 31) H aydon D . J., S k okes N. R. , Ur e R., Galbraith G., Ben n ett J. M ., B rown D. R., B a ker P. J. , Barynin V . V ., Rice D. W, S edelnikova S. E., H eal J. R., Sheridan J . M., Aiwale S. T ., Chauhan P . K., S rivastava A ., T aneja A ., C illins I ., Errinton J, Czaplewski I . G ., S c ience, 321,1673 —1 675( 2 008). )
确定

还剩2页未读,是否继续阅读?
海德创业(北京)生物科技有限公司为您提供《生物制品中有效成分含量分析检测方案 》,该方案主要用于预防类生物药品中含量测定检测,参考标准--,《生物制品中有效成分含量分析检测方案 》用到的仪器有
相关方案
更多








