农用工业废料大麦皮、麦麸和燕麦皮的再生行为 :煎炸油的研究Regeneration behaviour of agro-industrial based waste materials. Barley husk, wheat bran and oat husk a frying oil study
方案详情

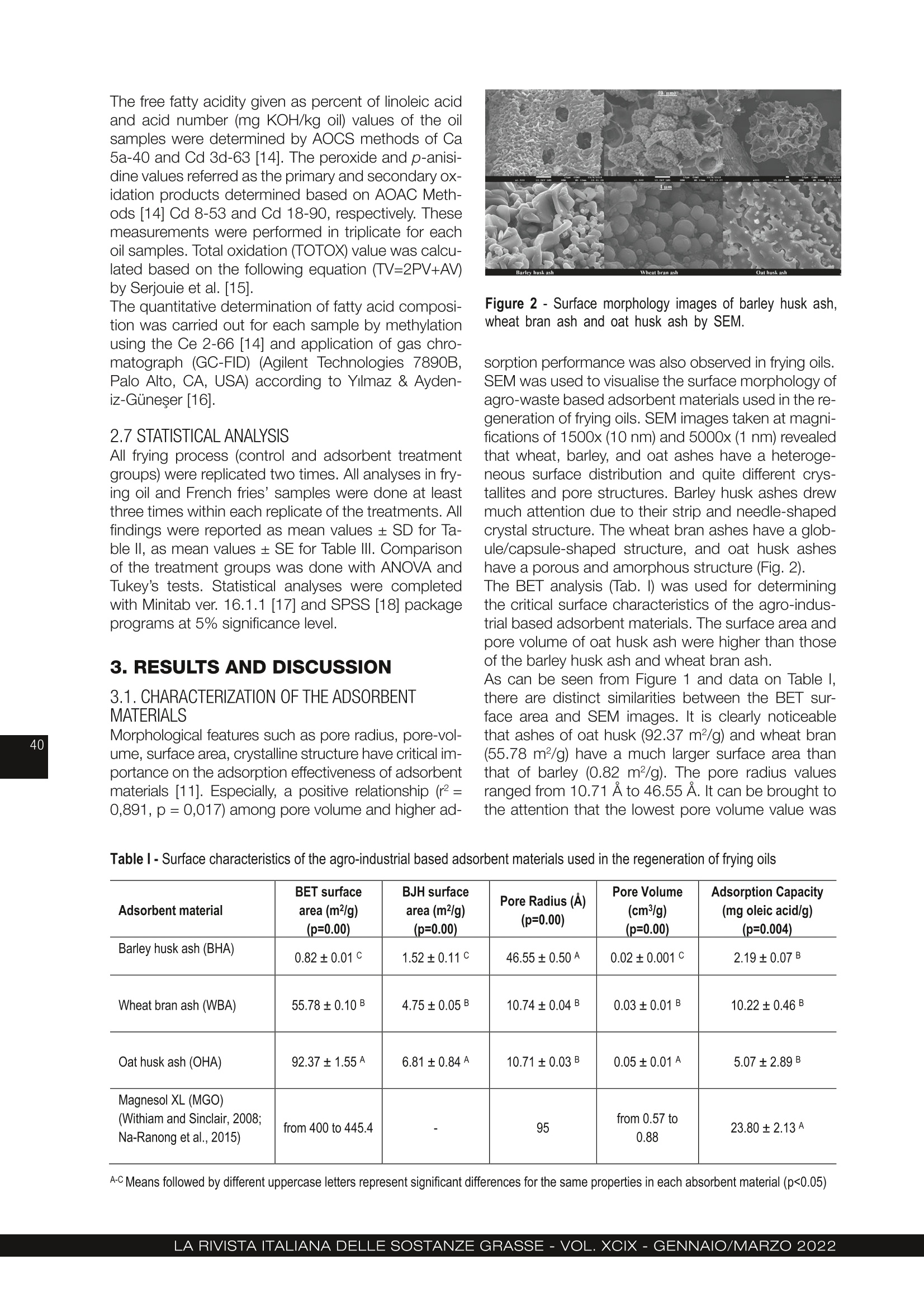
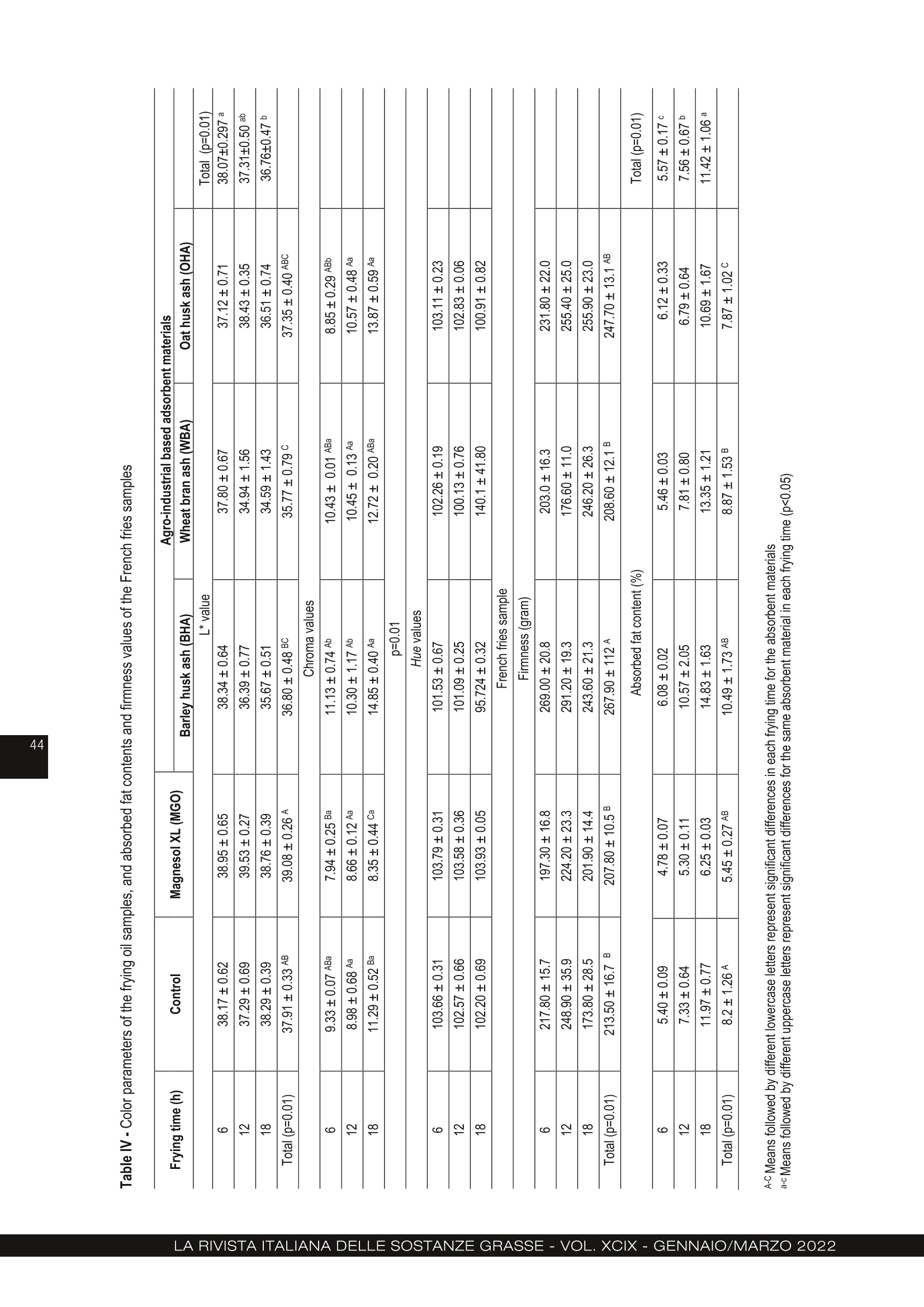
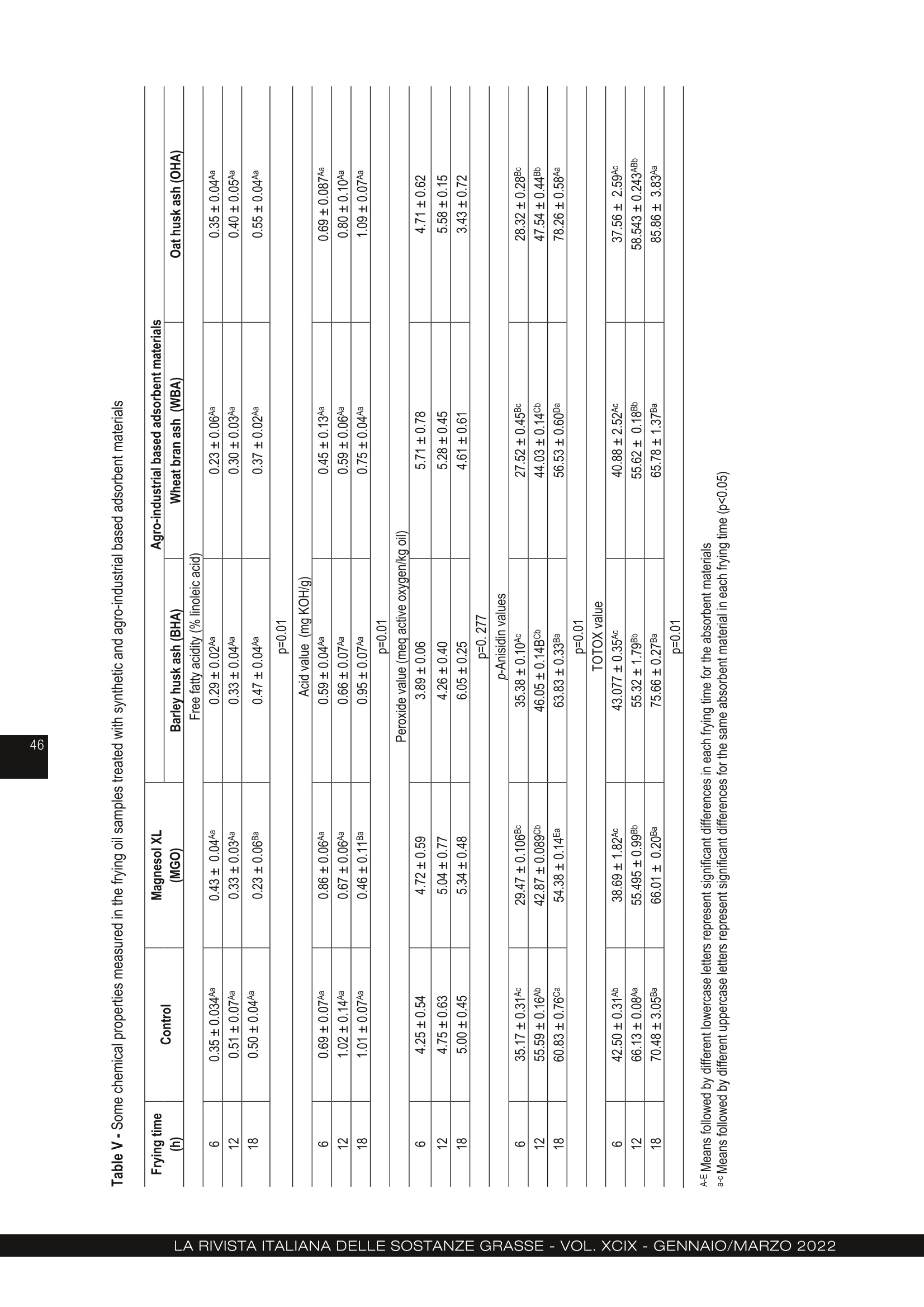
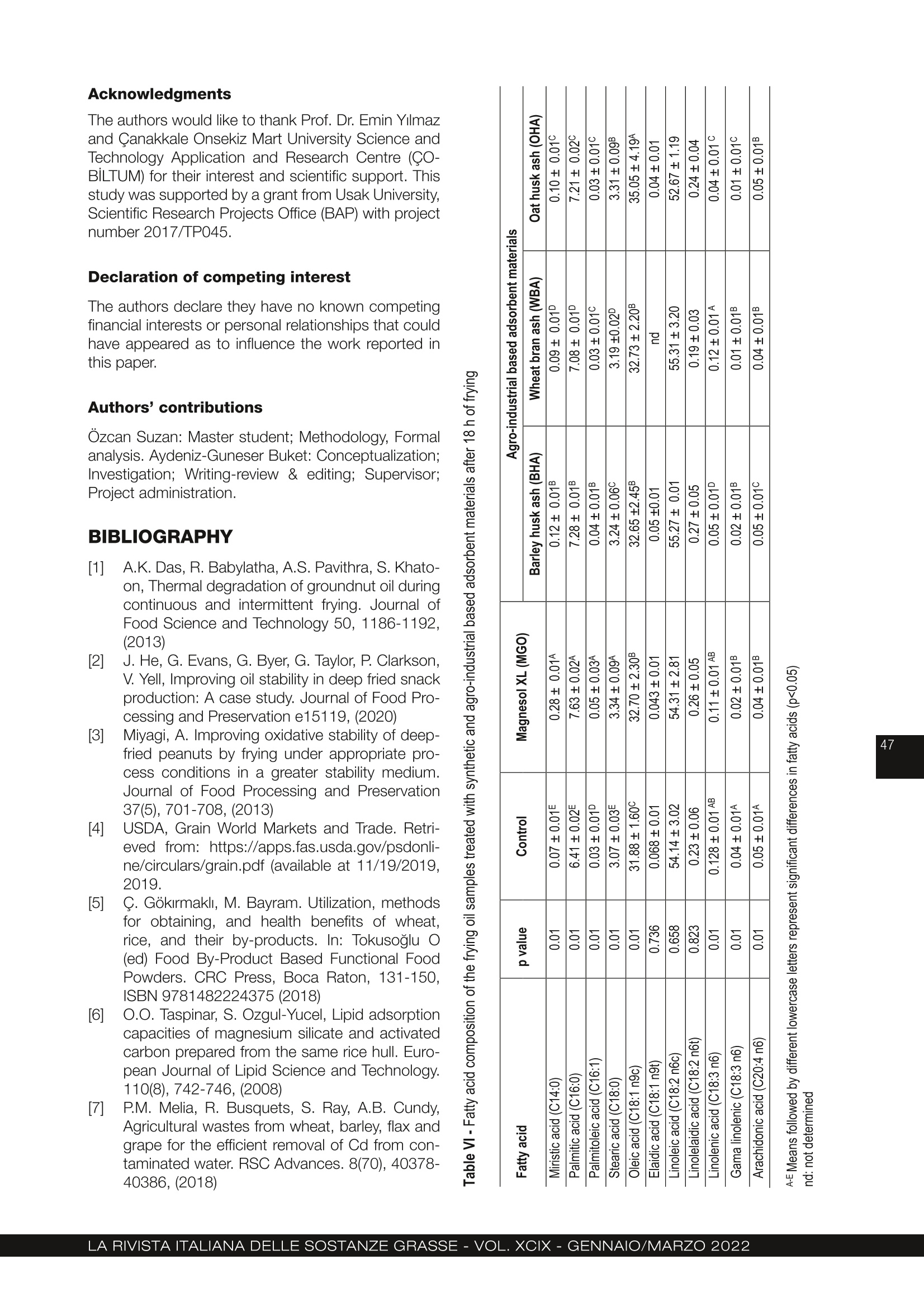
农用工业废料大麦皮、麦麸和燕麦皮的再生行为 :煎炸油的研究Regeneration behaviour of agro-industrial based waste materials. Barley husk, wheat bran and oat husk a frying oil study农用工业废料的再生行为。大麦皮、麦麸和燕麦皮:煎炸油的研究 Regeneration behaviour of agro-industrial based waste materials. Barley husk, wheat bran and oat husk: a frying oil study 土耳其乌萨木大学工程学院食品工程系 Suzan Özcan Buket Aydeniz-Guneser Department of Food Engineering, Faculty of Engineering, Usak University, Usak, Türkiye CORRESPONDENCE AUTHOR: Assistant Prof. Buket Aydeniz-Guneser, Department of Food Engineering, Faculty of Engineering, Usak University, Türkiye Tel: +90 276-221 21 21/2727 Email: buket.guneser@usak.edu.tr https://orcid.org/0000-0003-2197-5504 Suzan Özcan: https://orcid.org/0000-0002-7536-5915 Received: February 18, 2021Accepted: July 2, 2021 This study aims to experimentally evaluate the influence of agro-industrial based wastes on improving the quality of sunflower oil. A synthetic adsorbent and the ashes of barley husk, wheat bran, and oat husk were selected and utilised as flitration-aid adsorbents for the regeneration of used frying oil. The surface properties, crystal morphology, and framework structures of the ash-based adsorbentswerecharacterized,andthemineralcompositionsofadsorbentswere also determined. The results indicate that the crystal structure of adsorbent materials is responsible for its adsorption capacity and removal of impurities from the frying oil. Wheat bran ash was more effective in the adsorption of degradation products from oil, in reducing free fatty acid, p -anisidine values and also in preventing trans-fatty acid formation since it has the highest adsorption capacity and SiO2 level. Oat husk ash drew more attention due to its largest surface area and ability to reduce the peroxide value of oils. Treatment of frying oil with agro-industrial based ashes have been found to improve the oil quality and exhibit an effect like Magnesol XL. Keywords: Agricultural waste, Regeneration, Barley husk, Wheat bran, Oat husk 1. INTRODUCTION Cereal by-products (husk, germ, bran etc.) have attracted industrial and ac-ademic researchers interested in agro-food based waste valorisation and management. In this research, barley husk, wheat bran, and oat husk derived silica-rich ashes were used as sustainable and eco-friendly regeneration ma-terials for frying oils. Compared with Magnesol XL, a commercial adsorbent, wheat bran ash has many advantages, including lower free fatty acid content and p -anisidine values, lowest trans -fatty acid formation, and low cost (only needs combustion processes). Agro-industrial based adsorbent treatment exhibits innovative approach and an excellent performance for frying oil re-generation and has natural-derived and sustainable adsorption applications. This study contributes significantly to the existing literature because it pro-vides data regarding BET surface area, SEM images, and XRD graphs of wheat bran and oat husk ashes for the frist time. Deep-fat frying is a complex and critical process, as well as a popular food preparation technique. Briefly, the frying process can be defined as a quick-cooking method of immersing the food into oil at around 180°C [1]. Desirable and undesirable physico-chemical reactions occur in frying oil de-pending on the type of oil, presence of oxygen, and frying temperature-time interactions. These reactions play a notable role in the compositional and organoleptic attributes of fried foodstufsf and frying oil stability [2, 3]. Wheat (Triticum aestivum L.), barley (Hordeum vulgare L.) and oat (Avena sativa L.) have a growing global demand. Wheat, barley, and oat harvest statistics were reported 765.2, 155.8 and 22.6 thousand metric tonnes ac-cording to 2019/20 forecast, respectively [4]. Not only cereals, but also their by-products (husk, germ, bran, etc.) have become commercially and industrially important since their positive-health roles have been documented in detail[5]. Recent researches discussed the current technolo-gies used to remove solid and dissolved impurities, especiallyunwantedflavours,colours,andodour compounds from frying oils. Filtration with suitable fli-ter-aid materials is the most widely used regeneration technique. The adsorbent treatments involving syn-thetic magnesium silicate, activated carbon etc. are preferred for purifying used frying oils [6, 7]. Amongthepotentialadsorbentmaterialsusedto extend the shelf life of frying oils, silica-containing agro-industrial by-products or wastes have aroused much interest due to their low cost, framework struc-ture, and high adsorption capacity. Biogenic silica forms can be obtained from both agricultural- and in-dustrial-based wastes (furnace slag, coal fly etc) con-taining significant silica content [8, 9]. Choi et al. [10] reported that natural and powder forms of corncob as agricultural waste have effective adsorption capacities in aqueous solutions containing oil. According to researchers, the Langmuir model is more suitable to explain the spontaneous adsorption process, and the corncob is an inexpensive promis-ing adsorbent due to its high and rapid adsorption capacity. Choi and Gil [11] investigated the adsorption effec-tiveness of rice hull, barley hull, and soybean hull ash-es to remove impurities from the degummed soybean oil. Ash-type and carbon-type adsorbents prepared from the aforementioned agricultural wastes and their removal capacities were compared to one another. Ash-type adsorbents were shown to be more suc-cessful in reducing (86%) the free fatty acid values in soybean oil, and carbon-type adsorbents were more effective in reducing colour compounds compared to an ash-type adsorbent. Farag et al. [12] evaluated ashes obtained from rice, wheat, and barley hull to regenerate fried oil at 180°C for 20 h. Researchers indicated that treatment with all-natural adsorbent materials might reduce red-co-lour, viscosity, acidity, peroxide content, TBA and con-jugated diene/triene values, and polar compounds compared to the non-treated frying oil. The highest refractive index and smoke point might be observed in oil treated with the synthetic adsorbent. As mentioned above, different agro-industrial by-products or wastes were evaluated for the remov-al of undesired compounds from non-refined or fried oils. Moreover, there are no data on the adsorption ef-fectiveness, surface morphology and characteristics by BET and SEM, X-ray difrfaction spectrum of wheat bran ash and oat hull ash in literature to compare. The aim of this study is to prepare adsorbent materi- als from agro-industrial wastes (barley husks, wheat bran and oat hulls) by controlled ashing and subse-quently characterize the structure and morphology of adsorbent materials. Findings obtained from this study includes comparison of the adsorption effec-tiveness of new adsorbent materials with commercial Magnesol XL for the regeneration of used frying oil. 2. MATERIAL AND METHODS 2.1. MATERIALS Refined sunflower oil used as the frying media was kindly provided by Gümüş Gıda Industry and Trade Co. Inc. (İstanbul, Türkiye). Some important phys-ico-chemical properties of the fresh oil were deter-mined as 6.5% of total polar materials (TPM), 0.08%of free fatty acidity, and 1 meq active oxygen perox-ides per kilogram of oil. Pre-fried and frozen French fries (Nimet, Türkiye) used in this study were pur-chased from local markets and stored at −18°C until frying and analyses. 2.2 PREPARATION OF THE ADSORBENT MATERIALS FROM AGRO-INDUSTRIAL BASED WASTE Magnesol XL® (amorphous and odourless) was kind-ly provided by The Dallas Group of America (White-house, NJ, USA). Barley (Hordeum vulgare L .) husk, wheat (Triticum aestivum L .) bran, and oat (Avena sa-tiva L.) husk were obtained from Akın Aklif Agricultural Products Industry (Bursa, Türkiye) and Saglık Agri-culturalProductsIndustry(Konya,Türkiye).Firstly, barley husk, oat husk, and wheat bran were cleaned to remove foreign materials, and then placed into the high temperature-resistant ceramic crucible. Burning process was performed in a programmable furnace(Nabertherm N500 LE, Germany) at 700°C for 12 h until it was completely ash (Fig. 1). Then, the ashes were cooled down to room temperature and stored in an airtight container. 2.3 CHARACTERIZATION OF THE ADSORBENT MATERIALS Scanning electron microscopy (SEM), Brunau-er-Emmett-Teller (BET), X-ray difrfaction (XRD) and Figure 1 - Agro-industrial based wastes and their ashes used in the regeneration of frying oils. X-Ray Fluorescence (XRF) analyses were performed to characterize the agro-industrial based adsorbent materials. Themorphologiesofagro-industrialbasedadsor-bents were carried out by using a field mission scan-ningelectronmicroscope(FE-SEM,JEOLJSM-7100F, JEOL Ltd., Japan) ftited with energy-dispersive X-ray (EDX) spectroscopy (Oxford Instruments, EDS X-Max). All samples were coated with a gold-palla-dium layer (80:20 wt. %) to prevent surface charges under the 8 × 10-1 mbar/Pa vacuum with an applied voltage of 10 mA. SEM images were acquired at an acceleration voltage of 15 kV. The specific surface area and pore distribution were performed by QuadraSorb SI (Quantachrome Instru-ments, FL, USA). The adsorbent materials were frist degassed under a vacuum at 150°C for 2 h, then the surface area, average pore size and total pore volume were determined using physical adsorption of N2 at −77K. Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda(BJH)methodswereused to calculate the mesopore distribution with Quanta-chrome Quadrawin 5.12 software (Quantachrome In-struments, FL, USA). Adsorption capacity measurement of synthetic (Mag-nesol XL) and agro-industrial based adsorbents (ash-es of barley husk, wheat bran and oat husk) were performed according to the technique of Taşpınar and Ozgul-Yucel [6] expressed as mg oleic acid/g ad-sorbent. The crystalline phases of all-natural adsorbent ma-terials were measured by X-ray difrfaction (Panalyti-cal Empyrean, Netherlands) from 10° to 60o (2θ). The XRD patterns were generated using CuK α radiation (λ =1.54056 Å) with at 45 kV and 40 mA. Data col-lection was analysed using X’Pert HighScore Plus software. Elemental composition of the prepared ashes was analysed with X-ray fluorescence spectrometer (SPECTRO xSORT, SPECTRO Analytical Instruments GmbH). 2.4 FRYING PROCESS Frying process was performed using a household fry-er (Premiuer PDF 5615, Premier Electronics Ltd., İs-tanbul, Türkiye). Both the control oils (non-treatment) and treatment group oil samples (treated with Magne-sol XL and agro-industrial based adsorbent materials) were heated up to 180° for totally 18h. The frying pro-cess was repeated for 6 h each day for three consec-utive days. Before starting the frying process, enough frozen pre-fried French fries were defrosted entirely, and excess water was removed with a clean cloth. At the beginning of the experiment, all fryers were fliled with 2 L of fresh sunflower oil. The frying process of totally 250 g French fries were performed for each day in each frying group. The fryers were stopped at the end of the sixth frying process on each frying day, and the adsorbent treatment process was carried out to all treatment groups. 2.5 TREATMENT OF FRIED OILS WITH ADSORBENT MATERIALS For this purpose, the oil remaining in the fryer was weighed at the end of each frying day (6 h). All adsor-bent materials [Magnesol XL (MGO), barley husk ash(BHA), wheat bran ash (WBA), oat husk ash (OHA)] were added at 2% (w/w) composition into the oil at100°C and stirred at 700 rpm for 30 min. Then the oil + adsorbent mixture was flitered through regular fliter paper (Whatman no:1, 110 mm diameter) to remove adsorbent materials. Filtered oil was put back into the fryer and restarted the next day without any oil re-plenishment and then, 100 mL of flitered frying oil and100 grams of French fries’ samples were collected, labelled and kept frozen until the analyses. 2.6 QUALITY ASSURANCE TESTS ON ALL FRYING OIL AND FRIED POTATO SAMPLES Total Polar Materials (TPM) levels (%) were measured using a commercial quick measuring device (Tes-to 270, Testo Inc., Germany) in the frying oils held at min 45°C temperature. The apparent viscosity values of the oil samples were measured using a Brookfield Viscometer (model DV II+Pro, Brookfield Eng. Lab., Inc., MA, USA) at 25ºC with no:18 spindle rotating at 30 rpm. Hach 2100 AN Turbidimeter (USA) was used to measure the turbidity values of frying oil samples. Instrumental colour values were recorded as L *, a *, b * from both oil and potato samples with a Minol-ta Colormeter CR-400 (Minolta Camera Co., Osaka, Japan). Four replications were read on different lo-cations of each sample. In addition, the hue angle, which describes the hue as well as the saturation in-dex or chroma (C*), which describes the brightness or vividness of colour, were also calculated as described by Manjunatha et al. [13]. All the determinations were performed in triplicate and reported as mean values. Texture measurements of the French fries samples expressed as hardness value were assessed with a texture analyser (Brookfield CT3, Brookfield Engi-neeringLabsIbc,Massachusetts,USA)equipped with TA39 probe (2 mm D, 20 mm L, motion speed1.5 mm/s) on five different points on each fried pota-to sample. Texture measurements were carried out immediately after the end of each frying cycle. Be-fore the absorbed fat content analysis, all fried potato samples were ground using a blender (Waring blend-er 8011EG, Waring Commercial, USA) and absorbed fat contents were quantified using petroleum ether solventwithautomaticSoxthermapparatus(Ger-hardt Soxtherm Manager SX, Germany). The free fatty acidity given as percent of linoleic acid and acid number (mg KOH/kg oil) values of the oil samples were determined by AOCS methods of Ca5a-40 and Cd 3d-63 [14]. The peroxide and p -anisi-dine values referred as the primary and secondary ox-idation products determined based on AOAC Meth-ods [14] Cd 8-53 and Cd 18-90, respectively. These measurements were performed in triplicate for each oil samples. Total oxidation (TOTOX) value was calcu-lated based on the following equation (TV=2PV+AV) by Serjouie et al. [15]. The quantitative determination of fatty acid composi-tion was carried out for each sample by methylation using the Ce 2-66 [14] and application of gas chro-matograph(GC-FID)(AgilentTechnologies7890B, Palo Alto, CA, USA) according to Yılmaz & Ayden-iz-Güneşer [16]. 2.7 STATISTICAL ANALYSIS All frying process (control and adsorbent treatment groups) were replicated two times. All analyses in fry-ing oil and French fries’ samples were done at least three times within each replicate of the treatments. All findings were reported as mean values ± SD for Ta-ble II, as mean values ± SE for Table III. Comparison of the treatment groups was done with ANOVA and Tukey’s tests. Statistical analyses were completed with Minitab ver. 16.1.1 [17] and SPSS [18] package programs at 5% significance level. 3. RESULTS AND DISCUSSION 3.1. CHARACTERIZATION OF THE ADSORBENT MATERIALS Morphological features such as pore radius, pore-vol-ume, surface area, crystalline structure have critical im-portance on the adsorption efefctiveness of adsorbent materials [11]. Especially, a positive relationship (r2 =0,891, p = 0,017) among pore volume and higher ad- Figure 2 - Surface morphology images of barley husk ash, wheat bran ash and oat husk ash by SEM. sorption performance was also observed in frying oils. SEM was used to visualise the surface morphology of agro-waste based adsorbent materials used in the re-generation of frying oils. SEM images taken at magni-fications of 1500x (10 nm) and 5000x (1 nm) revealed that wheat, barley, and oat ashes have a heteroge-neous surface distribution and quite different crys-tallites and pore structures. Barley husk ashes drew much attention due to their strip and needle-shaped crystal structure. The wheat bran ashes have a glob-ule/capsule-shaped structure, and oat husk ashes have a porous and amorphous structure (Fig. 2). The BET analysis (Tab. I) was used for determining the critical surface characteristics of the agro-indus-trial based adsorbent materials. The surface area and pore volume of oat husk ash were higher than those of the barley husk ash and wheat bran ash. As can be seen from Figure 1 and data on Table I, there are distinct similarities between the BET sur-face area and SEM images. It is clearly noticeable that ashes of oat husk (92.37 m2/g) and wheat bran (55.78 m2/g) have a much larger surface area than that of barley (0.82 m2/g). The pore radius values ranged from 10.71 Å to 46.55 Å. It can be brought to the attention that the lowest pore volume value was Table I - Surface characteristics of the agro-industrial based adsorbent materials used in the regeneration of frying oils Adsorbent material BET surfacearea (m2/g)(p=0.00) BJH surfacearea (m2/g)(p=0.00) Pore Radius (Å)(p=0.00) Pore Volume(cm3/g)(p=0.00) Adsorption Capacity (mg oleic acid/g)(p=0.004) Barley husk ash (BHA) 0.82 ± 0.01 C 1.52 ± 0.11 C 46.55 ± 0.50 A 0.02 ± 0.001 C 2.19 ± 0.07 B Wheat bran ash (WBA) 55.78 ± 0.10 B 4.75 ± 0.05 B 10.74 ± 0.04 B 0.03 ± 0.01 B 10.22 ± 0.46 B Oat husk ash (OHA) 92.37 ± 1.55 A 6.81 ± 0.84 A 10.71 ± 0.03 B 0.05 ± 0.01 A 5.07 ± 2.89 B Magnesol XL (MGO) (Withiam and Sinclair, 2008;Na-Ranong et al., 2015) from 400 to 445.4 - 95 from 0.57 to0.88 23.80 ± 2.13 A A-C Means followed by different uppercase letters represent significant differences for the same properties in each absorbent material (p<0.05) recorded with barley husk ash despite its 4-fold larger pore radius than wheat bran ash and oat husk ash. Choi & Gil [11] compared the surface area, pore vol-ume, pore diameter of ash-type and carbon-type of rice husk, barley husk, and soybean husk ashes. Ac-cording to research, rice husk and barley husk car-bon-type adsorbents have 100-times larger surface areas and 7-8 times larger pore volume than those of the rice hull and wheat bran ash-type adsorbents. The surface area, total pore volume, and pore radius for barley husk ash were determined to be 20 m2/g,0.08 cm3/g, and 165.4 Å, respectively. Moreover, re-searchers also reported 345 m2/g surface area, 0.37cm3/g pore volume, and 101.4 Å pore radius for com-mercial silica adsorbents. Barley husk ash used in this study mainly yielded a smaller surface area (0.82 m2/g), pore volume (0.0019cm3/g), and pore radius (46.55 Å) compared to the findings of Choi and Gil [11]. It was believed that the differences in surface properties was caused by the ashing temperature preferred by researchers. The crystal structure of the barley husk ash, wheat bran ash, and oat husk ash were characterized in-dividually and comparatively by XRD (Fig. 3). Some crystal structures existed in the XRD pattern of barley husk ash and oat husk ash. SEM images also sup-ported the fact that barley and oat husk ashes contain similar strip-shaped structures. Other impurity peaks and different X-ray broken positions were detected in the XRD patterns of wheat bran ashes. Moreover, wheat bran ashes have more intense crystal struc-tures (Fig. 3). Regarding the d-spacing of all ash sam-ples, BHA has a bigger d-spacing with 4.10269 Å than WBA with 3.12844 Å and OHA with 4.07894Å. Compared to the other two ashes, it can be con- cluded that the spherical-shaped crystal structure of WBA is responsible for its higher adsorption capacity and effective removal of impurities from the frying oil. In a recent study [19], spherical-shaped and non-spherical biochar were developed and tested to remove paracetamol from water. The researchers re-ported that spherical biochar’s X-ray difrfaction inten-sity and degree of crystallinity were higher than that of non-spherical biochar, spherical-shaped biochar exhibited higher porosity, surface area and total pore volume than non-spherical biochar. All these char-acteristics mentioned above prove that the spheri-cal-shaped crystal structure has an increasing effect of adsorption capacity. There is no BET data, SEM or XRD image data avail-able, especially for wheat bran and oat husk ashes in the literature to compare. Nevertheless, there are rel-atively limited studies in literature on barley husk and wheat husk. It was thought that the adsorption per-formance of the wheat bran layer used in this study might exhibit remarkable differences compared with wheat husk reported in the literature. Themaincrystallinephasesofallagro-industri-al-based adsorbent materials were listed in Table II. All tested agricultural-based adsorbent materials can bedistinguishedbytheirelementalcompositions. The five major elements identified among the three ash-type adsorbents are SiO2, P2O5, K2O, MgO, and CaO. Although the BHA and OHA exhibited similar crystal structure, their SiO2, P2O5, K2O, MgO con-tents and adsorption behaviours toward degradation products in used frying oil were statistically different. Among all agro-industrial-based adsorbent materials, WBA contained the highest SiO2 and CaO levels with a value of 87% and 3.33%, respectively. Figure 3 - X-ray diffraction spectras of agro-industrial based adsorbent materials (XRD-1: barley husk ash, XRD-2: wheat bran ash, XRD-3: oat husk ash) Table II - Crystalline composition of the barley husk, wheat bran and oat husk ashes as adsorbent materials used in the regeneration of frying oils Constituent (%) Barley husk ash (BHA) Wheat bran ash (WBA) Oat husk ash (OHA) MgO 11.64 A <3.60 B <1.39 C Al2O3 <0.26 A <0.33 A <0.13 B SiO2 1.27 B 87.00 A 75.8 A P2O5 41.57 A 16.65 B 6.98 C SO3 1.07 B 3.04 A 1.82 B Cl 0.23 0.31 0.41 K2O 23.35 A 13.12 B 6.08 C CaO 1.78 B 3.33 A 1.93 B TiO2 <0.020 0.04 <0.017 MnO 0.33 0.09 0.11 Fe2O3 0.28 0.77 0.25 NiO 0.008 0.016 0.006 CuO 0.03 0.02 0.01 ZnO 0.13 0.07 0.01 Rb2O <0.01 0.01 0.01 SrO 0.007 0.005 0.013 A-C Means followed by different uppercase letters represent significant differences for the same properties in each absorbent material (p<0.05) SiO2 content is accepted as an excellent diagnostic parameter because of its high chemical resistance and high stability at temperatures as high as 500-600°C. Hegedüs et al. [20] announced a new mod-ifiedzeolite-typeadsorbentcalledE4(Ebsorb-4), which has a high silicon content (73%) and a specif-ic surface of 40 m2/g. Based upon their high silicon content, researchers claimed that it could lead to im-provements of adsorption ability to small molecules such as water, ammonia, methanol, or hydrogen sul-phide, and it was claimed as environmentally-friendly, non-toxic and reusable material. Similar to the findings reported by Hegedus [20], our results demonstrated that the highest SiO2 content in WBA contributes to achieving the highest adsorption capacity and the effective removal of free fatty acids, trans-form fatty acids and peroxides from used frying oil. According to Farag et al. [12], Mn (429–352 ppm), Si(310–295 ppm) and Ca (129–107 ppm) were deter-mined as major minerals in wheat bran and barley hull ashes, respectively. Moreover, their Fe and Al con-tents were detected close to those of the Magnesol XL. Wangetal.[21]comparedashcompositionand characteristics of barley straw and its husk by XRD and XRF analyses. Researchers observed that barley husk has higher ash content (4.9%) and SiO2, Al2O3, P2O5, CI levels (31.77, 1.55, 23.21, and 2.26%, re-spectively) compared to that of barley straw ash. Ma-jor crystalline compounds in barley husk and straw ashes obtained by combustion at 550°C were iden-tified as SiO2, KCI, and K2SO4. The barley husk ash used in this study contained lower SiO2 and CaO than indicated by Wang et al. [21] and its K2O and SO3 contents were similar to the findings reported. Wang et al. [21] drew attention to the fact that only sil-icates and potassium phosphates were identified as main crystalline phases in barley husk at 800°C com-bustions and that potassium and calcium salts could be released from the main ash structure after 550°C. In another study [22], the increment of temperature from 524°C to 962°C caused 10.5-18.5% weight loss for rice straw ash and 5.5-14.6% weight loss for wheat straw ash. Moreover, SiO2 content in wheat straw ash rose from 57.5% to 66.9% with the prog-ress in temperature. The increase in Al2O3, Fe2O3, MgO, CaO, Na2O and P2O5 contents were also posi-tively correlated with temperature. Thistemperature-weightlossphenomenonwas agreed upon in many publications. It was thought that the differences in the crystal structure might be associated with different combustion temperatures used in literature. 3.2 PHYSICO-CHEMICAL PROPERTIES OF THE TREATED FRYING OILS Polar substances with amphiphilic characters are the most reliable and critical indicators for detecting the degradation reactions in frying oils [23]. In this study, measured TPM values (Tab. III) in all frying oil samples increased with frying time. Treatment with oat husk ash resulted in the highest TPM values, followed by barley husk ash and wheat bran ash treated oil sam-ples at the end of 18 h of frying. According to frying oil regulations (FDA), the maximum percentage of po-lar compounds in used frying oil should not exceed 25%; on reaching that level; the oil must be changed or refreshed [24]. Under these frying conditions, it was determined that adsorbent materials obtained from agro-industrial based waste and Magnesol XL did not exceed the legal limit even at the end of 18 h of frying (Tab. III). Magnesol XL treatment resulted in the best reductions in the TPM contents of the fried oil samples. Wheat and barley hull ashes exhibited similar performances in adsorbing the polar materials from fried sunflower oil. It was observed that the viscosity values also followed a similar trend to TPM values for deep frying. Howev-er, both commercial (Magnesol XL) and agro-indus-trial-based adsorbent materials were not effective in preventingfrying-inducedviscosityenhancements. The viscosity values of oil samples treated with barley husk and wheat bran ashes were almost half of that of the values of oil samples treated with oat husk ash. It was considered that the higher initial viscosity value(81.65 cP) of oat husk ash treated oil samples result-ed in a higher-end viscosity value (100.60 cP). According to Chatzilazarou et al. [25], the increase in viscosity of used frying oils was due to the polym-erizationreactions,whichproduceshigh-molecu-lar-weight components through carbon-carbon and/or carbon-oxygen-carbon bridges among the fatty acids. The changes in viscosity values of fried sunflower oil were examined by Farag et al. [12] and a gradual and significant increase was detected in the viscosity of oil samples taken at the end of the frying process (20 h). Sunflower oils treated with barley hull ash and wheat hull ash have a relative flow time of 7-8 min as mea-sured by Ostwald viscometer. Chroma and hue angle values can be defined as the intensity of colour and combination of colour co-ordi-nate parameters, respectively [13]. Formation of red, yellow, and blue coloured pigments during the frying process was highly correlated with oxidized fatty ac-ids, pyrolytic condensation products, peroxides, al- dehydes, and oil-soluble particles, according to Blu-menthal & Stier [26]. The experimental data of the CIE colour parameters such as lightness (L ), chroma (C *), and hue angle (h *) values are presented in Table IV. The L values of ad-sorbent treated oil samples decreased linearly; on the contrary, chroma values of adsorbent treatment oil samples increased with frying time. The most expect-ed results on L values were obtained in oils treated with agro-industrial based adsorbent materials. OHA, which have the largest surface area, is more success-ful in reaching lighter (the highest L* value) than that of BHA and WBA at the end of frying. It is believed that the larger surface area will be effective in removing colour substances. Jacobson [27] concluded that degradation reactions occurred in frying oils due to an increase in the non-polar or unsaturated carbonyl compound contents and resulted in the accumulation of the compounds in the fried foods leading to the formation of darker pigments in the frying oils. Under these experimen-tal conditions, control and Magnesol XL treatment groups exhibited similar L* and hue values. The hue angle value of WBA was 102.260 at the ini-tial frying time (6 h), and this value reached 140.10 at the end of frying time (18 h). Also, the hue angles of BHA, OHA, and control groups decreased during the deep-frying period. It was considered that the varia-tions in Hue angle values were due to the increase in redness value of the oil and loss of moisture and pig-ment content, especially due to the Maillard reaction. French fries, donuts, fried fish chips, potato chips, and others are popular frying products liked by consum-ers worldwide. Textural characteristics like frimness, hardness, and crispness were accepted as critical quality attributes of the fried foods [28]. In this study, the frimness values and adsorbed fat contents of the Table III - Some physical properties measured in the frying oil samples treated with synthetic and agro-industrial based adsorbent materials Agro-industrial based adsorbent materials Frying time(h) Control Agro-industrial based adsorbent materials Magnesol XL (MGO) Barley husk ash(BHA) Wheat bran ash(WBA) Oat husk ash (OHA) TPM (%) 6 10.00 ± 0.46 Aa 8.62 ± 0.24 Aa 9.25 ± 0.72 Aa 9.38 ± 0.12 Ab 11.88 ± 1.38 Ab 12 10.25 ± 0.43 Aa 9.00 ± 0.20 Aa 9.25 ± 0.72 Aa 10.00 ± 0.29 Aab 8.50 ± 0.29 Ac 18 11.50 ± 0.20 Ba 10.75 ± 0.14 Ba 12.25 ± 0.14 Ba 12.75 ± 0.14 Ba 16.50 ± 1.44 Aa p=0.01 Viscosity (25°C, cP) 6 57.94 ± 1.53 Ba 49.75 ± 1.78 Ba 49.705 ± 0.36 Bb 54.71 ± 2.72 Ba 81.65 ± 4.99 Ab 12 41.68 ± 0.96 Cb 45.97 ± 2.00 Ca 63.62 ± 1.47 Ba 57.17 ± 1.44 BCa 86.80 ± 4.10 Ab 18 47.06 ± 1.29 Cab 46.35 ± 0.782 Ca 52.46 ± 0.50 BCab 62.54 ± 2.72 Ba 100.60 ± 0.92 Aa p=0.01 A-C Means followed by different uppercase letters represent significant differences for the same absorbent material in each frying time a-c Means followed by different lowercase letters represent significant differences in each frying time for the absorbent materials (p<0.05) Table IV - Color parameters of the frying oi l samples, and absorbed fat contents and firmness values of the French fries samples Frying time (h) Control Magnesol XL (MGO) Agro-industrial based adsorbent materials Barley husk ash (BHA) Wheat bran ash (WBA) Oat husk ash (OHA) L*value Total (p=0.01) 6 38.17±0.62 38.95±0.65 38.34±0.64 37.80±0.67 37.12±0.71 38.07±0.297a 12 37.29±0.69 39.53±0.27 36.39±0.77 34.94±1.56 38.43±0.35 37.31±0.50ab 18 38.29±0.39 38.76±0.39 35.67±0.51 34.59±1.43 36.51±0.74 36.76±0.47b Total (p=0.01) 37.91±0.33 AB 39.08±0.26A 36.80±0.48 BC 35.77±0.79C 37.35±0.40 ABC Chroma values 6 9.33±0.07ABa 7.94±0.25Ba 11.13±0.74Ab 10.43± 0.01ABa 8.85±0.29 ABb 12 8.98±0.68 Aa 8.66±0.12 Aa 10.30±1.17Ab 10.45± 0.13 Aa 10.57±0.48 Aa 18 11.29±0.52 Ba 8.35±0.44Ca 14.85±0.40 Aa 12.72±0.20 ABa 13.87±0.59Aa p=0.01 Hue values 6 103.66±0.31 103.79±0.31 101.53±0.67 102.26±0.19 103.11±0.23 12 102.57±0.66 103.58±0.36 101.09±0.25 100.13±0.76 102.83±0.06 18 102.20±0.69 103.93±0.05 95.724±0.32 140.1±41.80 100.91±0.82 French fries sample Firmness (gram) 6 217.80±15.7 197.30±16.8 269.00±20.8 203.0±16.3 231.80±22.0 12 248.90±35.9 224.20±23.3 291.20±19.3 176.60±11.0 255.40±25.0 18 173.80±28.5 201.90±14.4 243.60±21.3 246.20±26.3 255.90±23.0 Total (p=0.01) 213.50±16.7B 207.80±10.5B 267.90±112A 208.60±12.1B 247.70±13.1AB Absorbed fat content (%) Total (p=0.01) 6 5.40±0.09 4.78±0.07 6.08±0.02 5.46±0.03 6.12±0.33 5.57±0.17c 12 7.33±0.64 5.30±0.11 10.57±2.05 7.81±0.80 6.79±0.64 7.56±0.67b 18 11.97±0.77 6.25±0.03 14.83±1.63 13.35±1.21 10.69±1.67 11.42±1.06a Total (p=0.01) 8.2±1.26A 5.45±0.27AB 10.49±1.73AB 8.87±1.53B 7.87±1.02C A-C Means followed by different lowercase letters represent significant differences in each frying time for the absorbent materials a c Means followed by different uppercase letters represent significant di f ferences f or the same absorbent material in each frying t ime (p<0.05) French fries were analysed during the whole frying period. Table IV shows that the adsorbent treatments significantly influenced the frimness and adsorbed fat content of French fries. Under these frying conditions, fried potato samples have different frimness proflies in all frying groups. However, frimness values of potato samples fried in control and BHA groups decreased, and frimness values of potato samples fried in MGO, WBA, and OHA did not follow the same trend and increased up to 18 h. Similarly, intermittent frying led to a gradual decrease infatabsorptionvalues,rangingfrom4.78%to 14.83% for all frying groups. The potato samples fried in BHA absorbed higher oil content than the other frying oils, followed by the WBA and OHA groups. French fries fried in sunflower oil treated with barley husk ash were characterized with the highest content of frimness (g) and absorbed fat content (%). Changes in the chemical properties of all frying oil samples can be examined in Table V. The formation of free fatty acids and peroxides can result in the accu-mulation of unhealthy compounds as well as provide a rancid taste and unpleasant sensory properties to fried food [29]. Free fatty acid and acid number values in the con-trol sample, BHA, WBA and OHA samples, increased with frying time. In contrast, the lowest increments for free fatty acid and acid number values were record-ed in oils treated with Magnesol XL. All agro-indus-trial-based adsorbent experimental groups demon-strated higher levels of free fatty acid compared to Magnesol XL treated sample. Nevertheless, WBA has the closest and lowest free fatty acidity value to Mag- nesol XL when compared to the other two ashes. According to Ofcfiail Notification of the Control Cri-teria of Frying Fats/Oils [30], the maximum tolerable acid number value is 2.5%, and samples in this study had acid values lower than the limit value throughout 18 h of frying. Oxidation indicators such as peroxide and p -anisidine valuesdevelopedduringdeep-fryinghaveimpor-tance due to the possibility of using them to predict the shelf-life of oil and to monitor the behaviour of oil degradation [31]. Although the initial peroxide values (6 hours) for WBA and OHA samples were higher than the other frying groups, the peroxide values for all frying groups ex-cept WBA and OHA samples increased with the fry-ing time. Compared to BHA, OHA and WBA, which have a much higher surface area, are thought to have a significant effect (lowest peroxide values) in the re-duction of primary oxidation products. Especially, the relationship between spherical-shaped crystal struc-ture and high adsorption capacity supports the pos-itive effect of WBA in the removal of oxidation and hydrolysis products. The p -anisidine value, which is accepted as the indi-cator of secondary oxidation products, can monitor oil degradation [32]. At the end of the frying period, p -anisidine values reached the highest point in all frying oil samples. WBA had the lowest TOTOX val-ue than the other frying oil samples and prevented trans -fatty acid formation during frying. Faraq et al. [12] reported that the acid and peroxide values gradually increased and reached unaccept-able values during the deep-frying period. Acid and peroxide values of sunflower oils treated with barley hull ash and wheat hull ash changed between 1–1.5mg KOH/g oil and 2–4 meq active oxygen/kg oil. The increment trends for viscosity, TPM, acid, and perox-ide values reported by Faraq et al. [12] were similar to the findings of this study. The changes in the fatty acid composition of all treat-ment groups and the control group at the end of the frying period are given in Table VI. As expected, pal-mitic acid and linoleic acid were the primary saturated and monounsaturated fatty acids in sunflower oil, re-spectively [33]. The highest saturated fatty acid (C14-C16-C18) content was determined in MGO samples, and it was seen that this situation was statistically sig-nificant. Linoleic acid, as the major unsaturated fatty acid was shown to have very close results in all frying groups, even though OHA had the lowest linoleic acid content. There were no statistical differences in oleic acid contents among MGO, BHA, and WBA. The fatty acid content, and especially trans fatty acid formation, was accepted as one of the most signif-i cant indicators for evaluating the usage life of frying oils. The occurrence of trans fatty acids depends on the hydrogenation or thermal refining processing, fry-ing process, frying oil type, fatty acid composition, frying time, and the applied temperature [34]. It can be noted that the WBA sample had no elaidic acid (C18:1 n9t ) formation and also contained the lowest amount of linolelaidic acid (C18:2 n6t ). The high ad-sorption capacity of WBA may be related to the re-moval of more trans acids from frying oil compared to BHA and OHA. Moreover, agro-industrial based adsorbent materials were as effective as commercial adsorbent Magnesol XL in reducing the formation of trans fatty acid. The regenerative effect of tested ad-sorbents in the oil is quite remarkable. It is possible to say that WBA and OHA have a positive impact on reducing trans fatty acid formation. 4. CONCLUSION Treatment of used frying oils with the agro-industrial based adsorbents is getting special attention due to their high SiO2 contents and adsorption performanc-es. The valorisation of agro-industrial based adsor-bent materials for frying oil regeneration could be a sustainable solution for waste management and a re-duction of the environmental burden. This study can be appreciated as an important contribution to the existing literature because it provides data on the BET surface area, SEM images, and XRD graphs of wheat bran and oat husk ashes for the frist time. Table V- Some chemical properties measured in the frying oil samples treated with synthetic and agro-industrial based adsorbent materials Frying time(h) Control Magnesol XL(MGO) Agro-industrial based adsorbent materials Barley husk ash (BHA) Wheat bran ash (WBA) Oat husk ash (OHA) Free fatty acidity (%linoleic acid) 6 0.35±0.034Aa 0.43±0.04Aa 0.29±0.02Aa 0.23±0.06Aa 0.35±0.04Aa 12 0.51±0.07Aa 0.33±0.03Aa 0.33±0.04Aa 0.30±0.03Aa 0.40±0.05Aa 18 0.50±0.04Aa 0.23±0.06Ba 0.47±0.04Aa 0.37±0.02Aa 0.55±0.04Aa p=0.01 Acid value (mg KOH/g) 6 0.69±0.07Aa 0.86±0.06Aa 0.59±0.04Aa 0.45±0.13Aa 0.69±0.087Aa 12 1.02±0.14Aa 0.67±0.06Aa 0.66±0.07Aa 0.59±0.06Aa 0.80±0.10Aa 18 1.01±0.07Aa 0.46±0.11Ba 0.95±0.07Aa 0.75±0.04Aa 1.09±0.07Aa p=0.01 Peroxide value (meq active oxygen/kg oil) 6 4.25±0.54 4.72±0.59 3.89±0.06 5.71±0.78 4.71±0.62 12 4.75±0.63 5.04±0.77 4.26±0.40 5.28±0.45 5.58±0.15 18 5.00±0.45 5.34±0.48 6.05±0.25 4.61±0.61 3.43±0.72 D=0.277 p-Anisidin values 6 35.17±0.31Ac 29.47±0.106Bc 35.38±0.10Ac 27.52±0.45Bc 28.32±0.28Bc 12 55.59±0.16Ab 42.87±0.089Cb 46.05±0.14BCb 44.03±0.14Cb 47.54±0.44Bb 18 60.83±0.76Ca 54.38±0.14Ea 63.83±0.33Ba 56.53±0.60Da 78.26±0.58Aa p=0.01 TOTOX value 6 42.50±0.31Ab 38.69±1.82Ac 43.077±0.35Ac 40.88±2.52Ac 37.56±2.59Ac 12 66.13±0.08Aa 55.495±0.99Bb 55.32±1.79Bb 55.62±0.18Bb 58.543±0.243ABb 18 70.48±3.05Ba 66.01±0.20Ba 75.66±0.27Ba 65.78±1.37Ba 85.86±3.83Aa p=0.01 A-E Means followed by different lowercase letters represent significant differences i n each frying t ime for the absorbent materials ac Means f ollowed by different uppercase letters represent s i gnificant differences f or the same absorbent material in each frying t ime (p<0.05) Acknowledgments The authors would like to thank Prof. Dr. Emin Yılmaz and Çanakkale Onsekiz Mart University Science and Technology Application and Research Centre (ÇO-BİLTUM) for their interest and scientific support. This study was supported by a grant from Usak University, Scientific Research Projects Ofcfei (BAP) with project number 2017/TP045. Declaration of competing interest The authors declare they have no known competing financial interests or personal relationships that could have appeared as to influence the work reported in this paper. Authors’ contributions Özcan Suzan: Master student; Methodology, Formal analysis. Aydeniz-Guneser Buket: Conceptualization; Investigation;Writing-review&editing;Supervisor; Project administration. BIBLIOGRAPHY [1] A.K. Das, R. Babylatha, A.S. Pavithra, S. Khato-on, Thermal degradation of groundnut oil during continuous and intermittent frying. Journal of Food Science and Technology 50, 1186-1192,(2013) [2] J. He, G. Evans, G. Byer, G. Taylor, P. Clarkson, V. Yell, Improving oil stability in deep fried snack production: A case study. Journal of Food Pro-cessing and Preservation e15119, (2020) [3] Miyagi, A. Improving oxidative stability of deep‐fried peanuts by frying under appropriate pro-cess conditions in a greater stability medium. Journal of Food Processing and Preservation 37(5), 701-708, (2013) [4] USDA, Grain World Markets and Trade. Retri-evedfrom:https://apps.fas.usda.gov/psdonli-ne/circulars/grain.pdf (available at 11/19/2019,2019. [5] Ç. Gökırmaklı, M. Bayram. Utilization, methods forobtaining,andhealthbeneftisofwheat, rice,andtheirby-products.In:TokusoğluO(ed) Food By-Product Based Functional Food Powders. CRC Press, Boca Raton, 131-150, ISBN 9781482224375 (2018) [6] O.O. Taspinar, S. Ozgul‐Yucel, Lipid adsorption capacities of magnesium silicate and activated carbon prepared from the same rice hull. Euro-pean Journal of Lipid Science and Technology.110(8), 742-746, (2008) [7] P.M. Melia, R. Busquets, S. Ray, A.B. Cundy, Agricultural wastes from wheat, barley, flax and grape for the efcfieint removal of Cd from con-taminated water. RSC Advances. 8(70), 40378-40386, (2018) c o o c c a E o c 8 o 二 0 C.01 C.02 C.01o B.09 A4.19 十寸 、十9N 丈十寸一 C.01寸 C.01 B.01 、Da.0160 D.01 、C.01O口 aD026、m B2.20 LO 6 A.01 B.01口 B.01寸0 工的c的 B.01 B.01 B.01寸十 C.06寸m B寸.45m 0 出 D.01 口 B.01 C.01 一三o A.01 A.02 A.03 A6.09寸 OB2.30O 口 L寸O 一 AB.01 B.01 B.01寸 oco E.01 E.02十、 D.01 oE.03om C91.60、 十寸、寸 O A0.01 A.01 A.01 enjeAd 三 喜 喜 喜 喜 喜 喜 喜 山 一E 一c石山 coc 三 co v 芒 a 芒 30c [8] Y.Y. Chang, C.I. Lin, H.K. Chen, Rice hull ash structure and bleaching performance produced by ashing at various times and temperatures. Journal of the American Oil Chemists’ Society 78, 657-660, (2001) [9] H.Nguyen,M.J.Moghadam,H.Moayedi, Agricultural wastes preparation, management, and applications in civil engineering: a review. Journal of Material Cycles and Waste Manage-ment 1-13, (2019) [10] H.J. Choi, Agricultural bio-waste for adsorptive removal of crude oil in aqueous solution. Journal ofMaterialCyclesandWasteManagement21(2), 356-364, (2019) [11]E.S. Choi, B.I. Gil, Development of adsorbents for edible oil refining using agricultural bypro-ducts. Journal of the East Asian Society of Die-tary Life 20(3), 396-401, (2010) [12]S. Farag, A.M. Basuny, S.M. Arafat, S.A. Arafa, Use of some agricultural waste hull ashes for the regeneration of fried sunflower oil. International Journal of Food Science & Technology 44(9),1850-1856, (2009) [13]S.S. Manjunatha, A.T. Mathews, P.E. Patki, Mo-delling the kinetics of mass transfer and change in colour during deep fat frying of green peas(Pisum sativum L.) at different frying temperatu-res. Heat and Mass Transfer, 1-16, (2019) [14]AOCS,Of cfiailMethodsandRecommended Practices of the American Oil Chemists’ Society 5th ed., AOCS Press, Champaign, Ca 5a-40, Cd 3d-63, Cd 8-53 and Cd, 18-90, (2004) [15]A. Serjouie, C.P. Tan, H. Mirhosseini, Y.B. Che Man, Effect of vegetable-based oil blends on physicochemical properties of oils during deep-fat frying. American Journal of Food Technology 5(5), 310-323, (2010) [16]E. Yilmaz, B. Güneşer-Aydeniz, Cold pressed versus solvent extracted lemon (Citrus limon L.) seed oils: yield and properties. Journal of Food scienceandTechnology54(7),1891-1900,(2017) [17]Minitab,MinitabStatisticalSoftware(Version16.1.1). Minitab, State College SPSS (2010) [18]SPSS,ProfessionalStatistics(Version10.1). SPSS, Chicago, (1994) [19]H.N. Tran, F. Tomul, N.T.H. Ha, D.T. Nguyen, E.C. Lima, G.T. Le, C.T. Chang, V. Masindi, S.H. Woo, Innovative spherical biochar for pharma-ceutical removal from water: Insight into adsorp-tion mechanism. Journal of Hazardous Mate-rials, 394, 122255, (2020) [20]A.Hegedüs,Z.Hell,A.Potor,Simpleenvi-ronmentally-friendlymethodfortheselective synthesisof1,5-benzodiazepinederivatives using zeolite catalyst. Catalysis Letters 105(3-4), 229-232, (2005) [21]L. Wang, J.E. Hustad, O. Skreiberg, G. Skjevrak, M. Grønli, A critical review on additives to redu- ce ash related operation problems in biomass combustion applications. Energy Procedia 20,20-29, (2012). [22]P. Thy, B. M. Jenkins, S. Grundvig, R. Shiraki, C.E. Lesher, High temperature elemental losses and mineralogical changes in common biomass ashes. Fuel 85(5-6), 783-795, (2006) [23]X. Li, G. Wu, Y. Wu, E. Karrar, J. Huang, Q. Jin, H. Zhang, X. Wang, Effectiveness of the rapid test of polar compounds in frying oils as a fun-ction of environmental and compositional varia-bles under restaurant conditions. Food Chemi-stry 126041, (2019) [24] S. Lalas, Quality of frying oil, in: Sumnu S.G., Sahin S., (Eds.), Advances in Deep-Fat Frying of Foods, CRC Press, Boca Raton, 57-81. ISBN-13: 978-1420055580 (2008) [25] A. Chatzilazarou, O. Gortzi, S. Lalas, E. Zoidis, J. Tsaknis, Physicochemical changes of olive oilandselectedvegetableoilsduringfrying. Journal of Food Lipids 13, 27-35, (2006) [26]M.M. Blumenthal, R.F. Stier, Optimization of de-ep-fat frying operations. Trends in Food Science & Technology 2, 144-148, (1991) [27] G.A.Jacobson,Qualitycontrolindeep-fat frying operations. Food Technology 45(2), 72-74, (1991) [28] J.S. Utomo, Y.B. Che Man, R.A. Rahman, M. Said Saad, The effect of shape, blanching me-thods and flour on characteristics of restructu-red sweetpotato stick. International Journal of Food Science & Technology 43(10), 1896-1900,(2008) [29]R. Farhoosh, S. Einafshar, P. Sharayei, The ef-fect of commercial refining steps on the ranci-dity measures of soybean and canola oils. Food Chemistry 115(3), 933-938, (2009) [30]TFC, Codex, Turkish Food. “41: Ofcfiail Notifica-tion of the Control Criteria of Frying Fats.” Oils, Türkiye (2007) [31]S. Nas, H .Y. Gökalp, M. Ünsal, Bitkisel YağTeknolojisi.PamukkaleUniversityPublishing, Denizli 291- 303, (2001) [32]K. Sahin Ozkan, O. Ketenoglu, A. Yorulmaz, A. Tekin, Utilization of molecular distillation for de-termining the effects of some minor compounds on the quality and frying stability of olive poma-ce oil. Journal of Food Science and Technology56(7), 3449-3460, (2019) [33]Codex Alimentarius: Standard for named vege-table oils (CODEX STAN 210-1999) Retrieved from http://www.fao.org/3/y2774e/y2774e04. htm (available at 01/14/2020) (1999) [34]W. Gong, R. Shi, M. Chen, J. Qin, X. Liu, Quanti-fication and monitoring the heat-induced forma-tion of trans fatty acids in edible oils by Raman Spectroscopy. Journal of Food Measurement and Characterization, 1-8, (2019)
确定





还剩10页未读,是否继续阅读?
中国格哈特为您提供《预炸薯条和冷冻炸薯条脂肪含量的检测》,该方案主要用于薯类和膨化食品中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《预炸薯条和冷冻炸薯条脂肪含量的检测》用到的仪器有格哈特全自动快速索氏提取SOXTHERM、格哈特自动升降凯氏定氮电热消解仪KT-L 20s、格哈特快速干燥仪STL56、格哈特带自动进样器凯氏定氮仪VAP500C、格哈特强力高重现振荡器LS500/RO500、滤纸筒、德国移液器MM
相关方案
更多
该厂商其他方案
更多


































